Abstract
Background and aim
Astragalus membranaceus (AM) is a major Chinese herb used in the treatment of stroke. Astragaloside IV (AS)is a component of AM. This study investigated the effects of AM on the protein expression through proteomics analysis in ischemia-reperfusion injured Sprague Dawley rats.
Experimental procedure
An animal model of ischemia-reperfusion injury by occlusion of the right middle cerebral artery for 90 min followed by reperfusion for 24 h. The rats were intraperitoneally injected with AM or AS three times at 30 min, 1 day, and 2 days prior to the occlusion of the cerebral blood flow.
Results
Aldolase C was overexpressed in the cortex, and Dihydrolipoamide dehydrogenase and Triose-phosphate isomerase were overexpressed in the hippocampus.
Conclusion
Pretreatment with AM or AS can induce the overexpression of Aldolase C in the cerebral cortex and that of Dihydrolipoamide dehydrogenase and Triose-phosphate isomerase in the hippocampus, suggesting that both AM and AS may act as neuroprotectors through regulating the expression of Aldolase C, Dihydrolipoamide dehydrogenase and Triose-phosphate isomerase. However, the underlying neuroprotective mechanisms need more studies.
Keywords: Protein biomarker, Astragalus membranaceus, Astragaloside IV, Brain tissue, Ischemia-reperfusion injury
Graphical abstract
Highlights
-
•
Astragalus membranaceus(AM) is a famous Chinese herb for treating stroke.
-
•
This study used Proteomics analysis to investigate the protein biomarkers of brain tissue in AM and its component of Astragaloside (AS) treating ischemic stroke.
-
•
The present study found that Aldolase C in the cortex, and Dihydrolipoamide dehydrogenase and Triose- in the hippocampus were overexpression.
Abbreviations
- ALDOB
aldolase B
- ALDOC
aldolase C
- AM
Astragalus membranaceus
- AS
Astragaloside
- ATP
adenosine triphosphate
- CA
cornu ammonis
- CMU
China Medical University
- DNA
deoxyribonucleic acid
- CNS
central nervous system
- CORO1A
Coronin 1A
- DTE
dithioerythritol
- EEF1B2
Eukaryotic Translation Elongation Factor 1 Beta 2
- GADPH
Glyceraldehyde-3-phosphate Dehydrogenase
- GSH-PX
Glutathione peroxidase
- GSK 3
Glycogen synthase kinase 3
- IEF
isoelectric focusing
- iNOS
inducible nitric oxide synthase
- JNK3
c-jun N-terminal kinase 3
- LDH
Lactate Dehydrogenase
- DLD
Dihydrolipoamide dehydrogenase
- LC
liquid chromatography
- MCA
middle cerebral artery
- MDA
malondiadehyde
- nano-LC-MS/MS
nanoscale LC coupled to tandem mass spectrometry
- NO
nitric oxide
- PC
Purkinje cell
- Q-PCR
quantitative polymerase chain reaction
- RNA
Ribonucleic acid
- SD
Sprague Dawley
- SDS
sodium dodecyl sulfate
- SEM
standard error of the mean
- SOD
superoxidase dismutase
- Syn2
Synapsin II
- TNF
tumor necrosis factor
- TPI1
Triose-phosphate isomerase
- 2-DE
two-dimensional gel electrophoresis
1. Background
Astragalus membranaceus (AM) is a major traditional Chinese herb that tonifies Qi.1 AM has been used in large doses for the treatment of stroke since the time of the Qing Dynasty (i.e., the 1600s). In our previous study, we reported that compared with controls, the oral administration of AM increased the Functional Independence Measure scale score of patients with acute cerebral hemorrhage.2 The intraperitoneal injection of AM can reduce the neuronal apoptosis and infarction volume by downregulating the expression of c-jun N-terminal kinase 3 (JNK3) and protein and improve neurological behavior by providing neuroprotection in ischemia-reperfusion injured rats.3 Astragaloside IV (AS), a component of AM, can reduce the infarction volume and neurological deficit score in ischemia-reperfusion injured rats by increasing the activity of superoxide dismutase (SOD) and lactate dehydrogenase (LDH) and reducing the expression of inducible nitric oxide (NO) synthase (iNOS).4 However, the therapeutic effects and mechanisms of AM in stroke treatment remain unclear.
Proteomics is a powerful tool used to identify the differential expression of proteins in single tissues, and these proteins can be used as biomarkers.5, 6, 7, 8 In proteomics, two-dimensional gel electrophoresis (2-DE) is used to separate proteins, and mass spectrometry is used to analyze and identify the protein expression.8 Recently, proteomics has been widely applied in clinical studies to identify disease-relevant protein biomarkers.5,9,10
This study investigated the effect of AM on protein expression through proteomics analysis in ischemia-reperfusion injured Sprague Dawley (SD) rats. We established an animal model of ischemia-reperfusion injury through the occlusion of the right middle cerebral artery (MCA) for 90 min followed by reperfusion for 24 h. AM and AS may through regulating the expression of Aldolase C (ALDOC), Dihydrolipoamide dehydrogenase (DLD) and Triose-phosphate isomerase (TPI1) plays as the neuroprotection in human with cerebral ischemic infarction.
2. Materials and methods
2.1. Animals
Male SD rats weighing 300–350 g were purchased from BioLASCO Taiwan Co., Ltd and raised in the animal center of China Medical University (CMU). All experimental procedures followed the Care and Use of Laboratory Animals guidelines. The rats were maintained in a 12-h/12-h light and dark cycle and controlled room temperature of 25 °C and were provided adequate food and water. All the procedures were in accordance with the guide for the use of laboratory animals provided by the National Research Council and the ethical guidelines of the International Association for the Study of Pain. The number of animals used and their suffering were minimized. The protocol was approved by the Animal Care and Use Committee of CMU (Protocol No. 106-158-C).
2.2. Ischemia-reperfusion injury rat model
Cerebral ischemia was induced by the intraluminal suture occlusion of the MCA, as described in our previous study.11 Briefly, the rats were anesthetized using isoflurane (Aerrane, Canada) by using the MATRX VIP3000 vaporizing system (Midmark, USA). Then, the rats were placed on a stereotaxic apparatus in a prone position. The right parietal bone was thinned using a grinding machine to monitor the right MCA blood flow. The position of the rats was changed to the supine position, and a midline incision was made on the neck to expose the right common carotid artery. The right internal carotid artery and common carotid artery were clipped, and the external carotid artery was permanently ligated and incised. A 3.0 mm nylon filament suture, blunted at the tip by a flame and coated with poly-l-lysine (UNIK, Taiwan), was inserted from the incision on the external carotid artery through the common carotid artery into the internal carotid artery for a distance of approximately 23–25 mm to block the MCA origin. The right MCA blood flow was monitored using a laser Doppler blood-flow monitor (DRT4, Moor instrument Ltd., England); occlusion of the cerebral blood flow was confirmed when the on-screen blood-flow reading declined to 50 from 300. In this study, the cerebral blood flow of the right MCA was occluded for 90 min and reperfusion for 24 h.
2.3. Preparation of AM and AS
AM (Fisch.) Bge was purchased from China and was authenticated and extracted by Koda Pharmaceutical Company (Taoyuan, Taiwan), a Good Manufacturing Practice–certified company. Two kilograms of crude AM was used, one kg of AM was decocted in 7 L of water for 1 h, and then filtered. Add 5 L of water to the filter residue and decocted for 1 h, then filter, mix and concentrate the two filtrates. The other one kg of AM is decocted in 95% alcohol for 1 h, and then concentrated. The final product of AM was 407.24 g, and the yield was 20.36%. The AM extracts were identified through high-performance liquid chromatography (LC; Interface D-700, Pump L-7100, UV–Vis detector L-7420; Hitachi Instruments Service Co. Ltd., Ibaraki-ken, Japan). AS (Biotic Chemical Co., Ltd., New Taipei City, Taiwan) was used as the standard. The dose of AS was according to a previous study.12
2.4. Grouping
Twelve rats were equally divided into the following four groups. 1) sham group: the common carotid artery was exposed but the cerebral blood flow was not occluded; 2) control group: the cerebral blood flow of the right MCA was occluded for 90 min and reperfusion for 24 h; 3) AM group: the methods were identical to those of the control group; however, 1.0 g/kg of AM was intraperitoneal injected three times at 30 min, 1 day, and 2 days prior to the occlusion of the cerebral blood flow; and 4) AS group: the methods were identical to those of the AM group; however, 20 mg/kg of AS was intraperitoneal injected. The rats were sacrificed 24 h after the surgery and their brains were removed.
2.5. Protein extractions
All obtained tissues were minced into pieces of 2–3 mm3 and were homogenized using 0.5 mL of a lysis buffer (8 M urea and 4% CHAPS) containing a protease inhibitor cocktail (Sigma, St. Louis, MO, USA). The lysates were collected and purified through acetone precipitation, as described in our previous study.13 The final concentrations were measured using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA, USA) according to NenoDrop instructions.
2.6. 2-DE analysis
2-DE analysis was conducted according to the method reported by Lo et al.14 We used 250 μg of extracted proteins per sample for 2-DE separation. The rehydration solution containing the sample was then placed into a 17-cm immobilized pH gradient (IPG, pH 3–10) strip (ReadyStrip IPG strip; Bio-Rad) and left overnight. First-dimension electrophoresis was conducted for 60 kVh [Protean isoelectric focusing (IEF) cell, Bio-Rad] at 20 °C. The IPG strips were equilibrated using 3 mL of an equilibrium solution containing 50 mM Tris-HCl (pH 8.8), 6 M urea, 30% glycerol, 2% sodium dodecyl sulfate (SDS), bromophenol blue traces, and dithioerythritol (DTE) (1% w/v) for 20 min, followed by a second equilibration for 20 min in the same equilibrium solution containing iodoacetamide (2.5% w/v) instead of DTE. The strips were then transferred atop 12% polyacrylamide gels and held in the position by using molten 0.5% agarose in a running buffer containing 25 mM Tris, 0.192 M glycine, and 0.1% SDS. All gels were run at 16 mA/gel for 30 min, followed by 50 mA/per gel for 4–5 h. All experiments were performed at least three times.
2.7. Detection of protein spots and data analysis
The gels were stained with silver nitrate and scanned using the GS-800 imaging densitometer and PDQuest software (Version 7.1.1; Bio-Rad). To compare tissue protein distributions among the AM, AS, sham, and control groups (stroke group), we screened proteins that were overexpressed in the AM and AS groups compared with the sham group and those that were overexpressed by more than two-fold in the sham group compared with the control group (AM or AS > sham > 2-fold control) for advanced analysis through nanoscale liquid chromatography (LC) coupled to tandem mass spectrometry (nano-LC-MS/MS). All protein spots on the 2-DE gels were quantified and compared using PDQuest software.
2.8. Enzyme digestion and analysis through nano-LC-MS/MS
Each target protein spot from the AM or AS group (1–2 mm in diameter) was picked using a pipette tip and transferred into microcentrifuge tubes according to the method described in our previous study.13 Protein identification was performed using the Ultimate capillary LC system (LC Packings, Amsterdam, The Netherlands) coupled to a QSTARXL quadrupole time-of-flight mass spectrometer (Applied Biosystem/MDS Sciex, Foster City, CA). Nanoscale capillary LC separation was performed on an RP C18 column. Data acquisition was performed using an automatic information dependent acquisition system (Applied Biosystem/MDS Sciex). The product ion spectra generated through nano-LC-MS/MS were searched against NCBI databases for exact matches using the ProID program (Applied Biosystem/MDS Sciex) and the MASCOT search program.
2.9. Real-time Q-PCR
The overlapping overexpressed proteins in the AM and AS groups were screened as target biomarkers. The relative expression of these correlated genes was determined through real-time quantitative Polymerase Chain Reaction (Q-PCR). Total Ribonucleic acid (RNA) was extracted using the RNeasy Mini Kit (Qiagen). All PCR reactions were performed using the real-time fluorescence detection method and LightCycler System (Roche Diagnostics, Mannheim, Germany), with a FirstStart deoxyribonucleic acid (DNA) Master SYBR Green I kit (RocheMolecular Biochemicals, Indianapolis, IN). The primers used for real-time Q-PCR are listed in Supplemental data 1. Glyceraldehyde-3-phosphate Dehydrogenase (GAPD) was used to normalize the data.
2.10. Statistical analyses
Statistical analyses were performed using SPSS 18.0 statistical software for Windows. All data are presented as the mean ± standard error of the mean (SEM). The relative levels of expression of tissue samples in the sham, control, AM, and AS groups were compared using student t-test. p < 0.05 was considered as significant.
3. Results
3.1. Screening of overexpressed proteins in the frontal cortex of the rats in AM and AS groups
Fig. 1A presents the images of frontal cortex fractions of the sham group separated using 2-DE and stained with silver nitrate. The abundance ratios of proteins in the frontal cortex of the AM, AS, sham, and control groups were calculated using the 2-DE gels and PDQuest software. Compared with the sham group, in the AM and AS groups, individual spots 1–4 and spot 4 respectively showed protein overexpression. Furthermore, compared with the control group, these distinct spots exhibited an overexpression of proteins by more than two-fold in the sham group. Spot 4 demonstrated an overexpression of proteins in both the AM and AS groups. Spots 1, 2, 3, and 4 were matched and identified as Eukaryotic Translation Elongation Factor 1 Beta 2 (EEF1B2), Coronin 1A (CORO1A), TPI1, and ALDOC, respectively, through nano-LC-MS/MS. Table 1 summarizes the protein identification, sequence coverage, and characterization of these four proteins.
Table 1.
Q-TOF mass spectrum data and database searching for protein identification in the frontal cortex and hippocampus.
| Protein ID |
Accession No. |
Experimental Mr (kDa)/pI |
Theoretical Mr (kDa)/pI |
Sequence coverage |
Gene expression |
|---|---|---|---|---|---|
| Frontal cortex | |||||
| EEF1B2 | B5DEN5 | Mr = 37 kDa; pI = 5.6 | Mr = 24.8 kDa; pI = 4.6 | 35.3% | AM>(Sham>2 fold C) |
| CORO1A | Q91ZN1 | Mr = 56 kDa; pI = 6.4 | Mr = 55.4 kDa; pI = 6.1 | 21.2% | AM>(Sham>2 fold C) |
| TPI1 | P48500 | Mr = 26 kDa; pI = 6.3 | Mr = 26.8 kDa; pI = 6.9 | 24.6% | AM>(Sham>2 fold C) |
| ALDOC | P09117 | Mr = 39 kDa; pI = 6.8 | Mr = 39.3 kDa; pI = 6.7 | 16.2% | AM & AS >(Sham>2 fold C) |
| Hippocampus | |||||
| DLD | Q6P6R2 | Mr = 45.8 kDa; pI = 6.8 | Mr = 54 kDa; pI = 7.96 | 30.4% | AM & AS >(Sham>2 fold C) |
| TPI1 | P48500 | Mr = 26.5 kDa; pI = 7.0 | Mr = 26.8 kDa; pI = 6.9 | 17.5% | AM & AS >(Sham>2 fold C) |
| SYN2 | Q63537 | Mr = 65.5 kDa; pI = 8.0 | Mr = 63.4 kDa; pI = 8.7 | 12.3% | AM> (Sham>2 fold C) |
EEF1B2: Eukaryotic translation elongation factor 1 beta 2; CORO1A: Coronin 1A; TPI1: Triose-phosphate isomerase; ALDOC: Aldolase C; DLD: Dihydrolipoamide dehydrogenase; SYN2: Synapsin II; AM: AM group; AS: AS group; Sham: sham group; C: control group.
3.2. Screening of overexpressed proteins in the hippocampus of the rats in AM and AS groups
Fig. 1B presents the images of hippocampus fractions of the sham group separated through 2-DE and stained with silver nitrate. The abundance ratios of proteins in the hippocampus of the AM, AS, sham, and control groups were calculated using the 2-DE gels and PDQuest software. Compared with the sham group, in the AM and AS groups, individual spots 5–7 and spot 5–6 respectively showed protein overexpression. Furthermore, compared with the control group, these distinct spots exhibited an overexpression of proteins by more than two-fold in the sham group. Spots 5 and 6 demonstrated an overexpression of proteins in both AM and AS groups. Spots 5, 6, and 7 were matched and identified as DLD, TPI1, and Synapsin II (Syn2), respectively, through the nano-LC-MS/MS analysis. Table 1 summarizes the protein identification, sequence coverage, and characterization of these three proteins.
Fig. 1.
Images of frontal cortex and hippocampus fractions of the sham group separated using 2-DE and stained with silver nitrate. A: Spots 1, 2, 3, and 4 were matched and identified as Eukaryotic translation elongation factor 1 beta 2 (EEF1B2), Coronin 1A (CORO1A), Triose-phosphate isomerase (TPI1), and Aldolase C (ALDOC), respectively, through nano- LC-MS/MS. B: Spots 5, 6, and 7 were matched and identified as Dihydrolipoamide dehydrogenase (DLD), TPI1, and Synapsin II (Syn2), respectively, through nano-LC- MS/MS.
3.3. Quantitation of candidate gene expressions
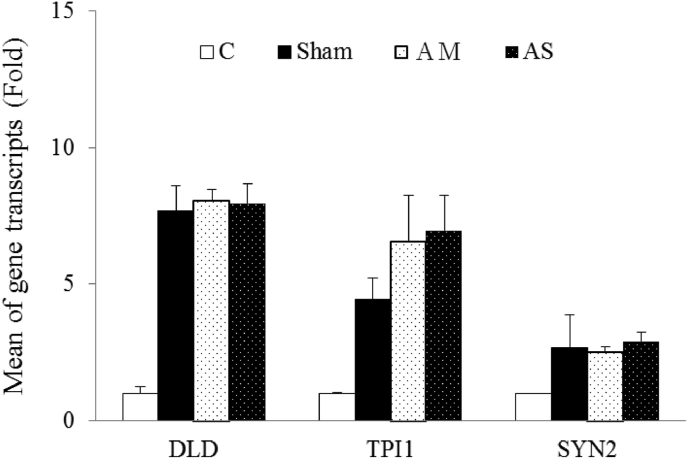
The dynamic results of the real-time Q-PCR analysis revealed that the candidate genes were not consistently overexpressed in the AM and AS groups. Compared with the sham group, only CORO1A was overexpressed by an average of 1.45-fold in the AM group in the frontal cortex (Fig. 2). Moreover, compared with the AM group, only ALDOC was overexpressed in the AS group in the frontal cortex. Furthermore, compared with the sham group, only TPI1 was overexpressed by an average of 1.73-fold in the AM group in the hippocampus (Fig. 3). These results indicate that protein expressions were not correlated with mRNA transcriptions.
Fig. 2.
Dynamic results of real-time quantitative-PCR (real-time Q-PCR) analysis in the cortex. Compared with the sham group, only Coronin 1A (CORO1A) was overexpressed by an average of 1.45-fold in the AM group. Compared with the AM group, only Aldolase C (ALDOC) was overexpressed in the AS group; C: control group; Sham: sham group; AM: Astragalus membraneus group; AS: Astraloside IV group; ∗, significantly overexpressed compared with the sham group; #, significantly overexpressed compared with the AS group.
Fig. 3.
Dynamic results of real-time Q-PCR analysis in the hippocampus. Compared with the sham group, only Triose-phosphate isomerase (TPI1) was overexpressed by an average of 1.73-fold in the AM group; C: control group; Sham: sham group; AM: Astragalus membraneus group; AS: Astraloside IV group. ∗, significantly overexpressed compared with the sham group.
4. Discussion
Stroke is the leading cause of death and causes serious long-term disabilities; it poses a major challenge to healthcare services and is associated with huge healthcare costs.15 The development of ischemic-reperfusion brain injuries in stroke associated with the depletion of oxygen and glucose (resulting in increased extracellular potassium and intracellular calcium levels, ATP depletion, protein synthesis inhibition, decreased pH, and free radical and lactic acid accumulation), the excessive production of reactive oxygen species (such as superoxide anions, hydroxyl radicals, hydrogen peroxide, and NO) and the inflammatory responses (mediate by iNOS, the infiltration of neutrophils, and the releasing of interleukin-1 and tumor necrosis factor (TNF)-alpha) which contribute to secondary brain injuries after ischemia and reperfusion as well. 12,16,4,17
AS, the active constituent of the Chinese herb AM, is a strong scavenger of superoxide radicals and hydroxyl radicals. The phenyl hydroxyl and cyclic propane groups are likely responsible for the antioxidant properties.18 On cerebrum of ischemic-reperfusion SD rats, several studies shows that AS could significantly reduce the neurological deficit score; infarct volume and water content; increase SOD (an antioxidant enzyme), Glutathione peroxidase (GSH-PX, an antioxidant enzyme) and LDH (an important metabolic enzyme) in brain cells and released into the blood by injured cells; decrease iNOS activity and malondiadehyde (MDA, an indicator of lipid peroxidation), LDH and NO content. Other studies also shows that AS could inhibits the release and production of the inflammatory mediators interleukin-1 and TNF-alpha in murine peritoneal macrophages. These studies show that the mechanism of neuroprotective effects on AS can be achieved by antagonize the anti-oxidation and anti-inflammatory properties. 12 4.
We observed the overexpression of DLD and TPI1 in the hippocampus of both AM and AS groups. DLD, a mitochondrial enzyme, is capable of using NO as an electron acceptor, with NADH as the electron donor, forming nitrate in the reaction. Therefore, DLD acts as an antioxidant by scavenging NO.19 Moreover, TPI1 is a crucial enzyme in the glycolytic pathway and provides electrostatic stabilization.20 Our research supports that AM and AS may provide neuroprotection for the hippocampus through regulating the DLD and TPI1 expression. We also observed the overexpression of ALDOC in the frontal cortex of both AM and AS groups. The enzyme aldolase is critical in the glycolytic pathway. Vertebrate aldolases exist as three isozymes: ALDOA, (expressed ubiquitously, the mutations on ALDOA is associated with hemolytic anemia and myopathy), aldolase B (ALDOB), expressed mainly in the liver and involved in utilizing the exogenous fructose, the mutations on ALDOB can cause an autosomal recessive disease called hereditary fructose intolerance), and ALDOC (expressed selectively in the central nervous system and tissue of neuronal origin. It catalyzes the reversible cleavage of fructose 1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde-3-phosphate.). 21,22 There are reports shows aldolase has various functions other than glycolysis. Aldolase can induce the expression of several endogenous Wnt target genes, activate Wnt signaling in a Glycogen synthase kinase 3 (GSK-3)β-dependent mechanism by disrupting the GSK-3β-axin interaction and targeting axin to disheveled-induced signalosomes that positively regulate the Wnt pathway, thus it is possible to act as a colorectal oncogene. 23,24 In cervical cancer, kidney cancer, lung cancer and endometrial cancer, the expression of aldolase is increased.25 Moreover, aldolase may interacts with proton pumps that involved in the vesicle intracellular trafficking or the proteins crutial for the proliferation of cancer cells, through a nonglycolytic pathway.26 In addition to its glycolytic function, human ALDOC has another brain-specific role in cornu ammonis (CA)3 hippocampal neurons and alternate Purkinje cell (PC) clusters.27 Reports shows that ALDOC provides marked neuroprotection to PC after trauma and excitotoxicity. The differences in ALDOC expression level among PC affects their tolerance for environmental changes, after acute ischemia or in a chronic pathological state, ALDOC-positive PCs are more likely to survive than ALDOC-negative PCs. These studies show that ALDOC has neuroprotective functions.28,29 Taken together, suggesting AM can be translated clinically to treat stroke in human.
In our study, compared with the sham and AM groups, ALDOC was significantly overexpressed in the AS group. Moreover, compared with the control group, ALDOC was overexpressed by more than two-fold in the sham group. These results indicate that AS may be a key ingredient in AM contributing to the neuroprotective effect of AM on ischemic brain injuries. Recently, researchers have developed many novel tools such as monoclonal antibody 9F against human ALDOC (9F has high affinity toward epitope-containing peptides thus may solve the dilemma that ALDOC is always coexpressed with ALDOA in the CNS in homologous aldolase isoforms) and knock-in Aldoc-Venus mice (ALDOC expression in cerebrum is visualized by expression of a fluorescent protein, Venus) 22 29. Although the role of ALDOC in ischemia-reperfusion brain injuries remains unclear, investigating the functions and specific molecular interactions of ALDOC in the brain by using a novel and powerful tool is feasible in the future.
In our real-time Q-PCR results, the protein expressions were not correlated with mRNA transcriptions. These result implying that there are post-transcriptional regulation occurred on the AS induced protein expression. We suggest that investigating of AS-induced expression on protein level will be more informative to where and how the regulation took place.
In conclusion, pretreatment with AM or AS can induce the overexpression of ALDOC in the cerebral cortex and that of DLD and TPI1 in the hippocampus, suggesting that both AM and AS may act as neuroprotectors through regulating the expression of ALDOC, DLD and TPI1. However, the underlying neuroprotective mechanisms remain unclear.
Consent for publication
This study has “Not applicable” any individual person's data.
Declaration of competing interest
We declare that there are no conflicts of interest associated with this manuscript and no significant financial support that would influence our findings.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.04.002.
Funding
This study was financially supported by the Chinese Medicine Research Center, China Medical University from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan (CMRC-CENTER-0).
Availability of data and materials
Materials and data in this study are available to other researchers upon request.
Author contributions
W–Y Lo performed the 2-DE and proteomic analysis and wrote the manuscript text; C–H Chen performed the AM extraction and HPLC analysis of the AM; C–H Liu participated in the discussion and provided experimental help; C-L Hsieh participated in protocol design, wrote and revised the manuscript text. All authors have reviewed and approved the manuscript.
Disclosures
None.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chen J., Chen T., Huang Qi (Radix Astragali) Chinese Medical Herbology and Pharmacology. 2004;17(1) [Google Scholar]
- 2.Chen C.C., Lee H.C., Chang J.H. Chinese herb Astragalus membranaceus enhances recovery of hemorrhagic stroke: double-blind, placebo-controlled, randomized study. Evid base Compl Alternative Med. 2012;2012:708452. doi: 10.1155/2012/708452. eCAM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu G., Song J., Guo Y., Wang T., Zhou Z. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct : BBF. 2013;9:36. doi: 10.1186/1744-9081-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin Y.Y., Li W.P., Gong H.L., Zhu F.F., Li W.Z., Wu G.C. Protective effect of astragaloside on focal cerebral ischemia/reperfusion injury in rats. Am J Chin Med. 2010;38(3):517–527. doi: 10.1142/S0192415X10008020. [DOI] [PubMed] [Google Scholar]
- 5.Liu X.Y., Yang J.L., Chen L.J. Comparative proteomics and correlated signaling network of rat hippocampus in the pilocarpine model of temporal lobe epilepsy. Proteomics. 2008;8(3):582–603. doi: 10.1002/pmic.200700514. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins M.R., Sanchez J.C., Gooley A.A. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1996;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins M.R. What do we want from proteomics in the detection and avoidance of adverse drug reactions. Toxicol Lett. 2002;127(1-3):245–249. doi: 10.1016/s0378-4274(01)00506-9. [DOI] [PubMed] [Google Scholar]
- 8.Butterfield D.A., Perluigi M., Sultana R. Oxidative stress in Alzheimer's disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545(1):39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Krapfenbauer K., Engidawork E., Cairns N., Fountoulakis M., Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003;967(1-2):152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]
- 10.Kischel P., Waltregny D., Castronovo V. Identification of accessible human cancer biomarkers using ex vivo chemical proteomic strategies. Expet Rev Proteonomics. 2007;4(6):727–739. doi: 10.1586/14789450.4.6.727. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C.Y., Su S.Y., Tang N.Y., Ho T.Y., Chiang S.Y., Hsieh C.L. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–150. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y., Qin Z., Hong Z. Astragaloside IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neurosci Lett. 2004;363(3):218–223. doi: 10.1016/j.neulet.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 13.Lo W.Y., Tsai F.J., Liu C.H. Uncaria rhynchophylla upregulates the expression of MIF and cyclophilin A in kainic acid-induced epilepsy rats: a proteomic analysis. Am J Chin Med. 2010;38(4):745–759. doi: 10.1142/S0192415X10008214. [DOI] [PubMed] [Google Scholar]
- 14.Lo W.Y., Tsai M.H., Tsai Y. Identification of over-expressed proteins in oral squamous cell carcinoma (OSCC) patients by clinical proteomic analysis. Clin Chim Acta. 2007;376(1-2):101–107. doi: 10.1016/j.cca.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auriel E., Bornstein N.M. Neuroprotection in acute ischemic stroke--current status. J Cell Mol Med. 2010;14(9):2200–2202. doi: 10.1111/j.1582-4934.2010.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Domenico F., Casalena G., Jia J. Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion. J Neurochem. 2012;121(4):680–692. doi: 10.1111/j.1471-4159.2012.07721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gui D., Guo Y., Wang F. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PloS One. 2012;7(6) doi: 10.1371/journal.pone.0039824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igamberdiev A.U., Bykova N.V., Ens W., Hill R.D. Dihydrolipoamide dehydrogenase from porcine heart catalyzes NADH-dependent scavenging of nitric oxide. FEBS Lett. 2004;568(1-3):146–150. doi: 10.1016/j.febslet.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Wierenga R.K., Kapetaniou E.G., Venkatesan R. Triosephosphate isomerase: a highly evolved biocatalyst. Cell Mol Life Sci. 2010;67(23):3961–3982. doi: 10.1007/s00018-010-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arakaki T.L., Pezza J.A., Cronin M.A. Structure of human brain fructose 1,6-(bis)phosphate aldolase: linking isozyme structure with function. Protein Sci. 2004;13(12):3077–3084. doi: 10.1110/ps.04915904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langellotti S., Romano M., Guarnaccia C. A novel anti-aldolase C antibody specifically interacts with residues 85-102 of the protein. mAbs. 2014;6(3):708–717. doi: 10.4161/mabs.28191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warburg O., Wind F., Negelein E. The metabolism OF tumors IN the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caspi M., Perry G., Skalka N. Aldolase positively regulates of the canonical Wnt signaling pathway. Mol Canc. 2014;13:164. doi: 10.1186/1476-4598-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mhawech-Fauceglia P., Wang D., Kesterson J. Aldolase mRNA expression in endometrial cancer and the role of clotrimazole in endometrial cancer cell viability and morphology. Histopathology. 2011;59(5):1015–1018. doi: 10.1111/j.1365-2559.2011.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritterson Lew C., Tolan D.R. Targeting of several glycolytic enzymes using RNA interference reveals aldolase affects cancer cell proliferation through a non-glycolytic mechanism. J Biol Chem. 2012;287(51):42554–42563. doi: 10.1074/jbc.M112.405969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buono P., Barbieri O., Alfieri A. Diverse human aldolase C gene promoter regions are required to direct specific LacZ expression in the hippocampus and Purkinje cells of transgenic mice. FEBS Lett. 2004;578(3):337–344. doi: 10.1016/j.febslet.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Slemmer J.E., Haasdijk E.D., Engel D.C., Plesnila N., Weber J.T. Aldolase C-positive cerebellar Purkinje cells are resistant to delayed death after cerebral trauma and AMPA-mediated excitotoxicity. Eur J Neurosci. 2007;26(3):649–656. doi: 10.1111/j.1460-9568.2007.05708.x. [DOI] [PubMed] [Google Scholar]
- 29.Fujita H., Aoki H., Ajioka I. Detailed expression pattern of aldolase C (Aldoc) in the cerebellum, retina and other areas of the CNS studied in Aldoc-Venus knock-in mice. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0086679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Materials and data in this study are available to other researchers upon request.