Abstract
Long non-coding (lnc)RNAs serve a role in a number of diseases, including different types of cancer and acute myocardial infarction. The aim of the present study was to investigate the protective role of lncRNA small nucleolar RNA host gene 8 (SNHG8) in hypoxia-ischemia-reoxygenation (HI/R)-induced myocardial injury and its potential mechanism of action. Cell viability, proliferation, creatine kinase myocardial band, cell apoptosis and protein expression levels were determined by Cell Counting Kit-8 assay, EdU assay, ELISA, flow cytometry and western blotting, respectively. The association between SNHG8 and microRNA (miR)-335 was confirmed using a dual-luciferase reporter gene assay. The effects of the miR-335 inhibitor transfections had on increasing apoptosis and decreasing H9C2 cell viability were reversed in cells co-transfected with SNHG8 small interfering (si)RNA. Furthermore, it was found that miR-335 could regulate RAS p21 protein activator 1 (RASA1) expression and that transfection with SNHG8 siRNA downregulated RASA1 expression. Silencing of RASA1 protected against HI/R-induced H9C2 cell injury. However, SNHG8 siRNA did not further reduce apoptosis, demonstrating that SNHG8 may act through RASA1, and RASA1 may mediate the protection of SNHG8 siRNA in HI/R myocardial injury. Thus, inhibition of lncRNA SNHG8 alleviated HI/R-induced myocardial damage by regulating miR-335 and RASA1.
Keywords: long non-coding, small nucleolar RNA host gene 8, hypoxia-ischemia-reoxygenation, microRNA-335, myocardial injury
Introduction
Ischemic heart disease is a major disease with the highest mortality and morbidity rate (1.655%) in the world, an estimated 31% of all deaths worldwide are due to cardiovascular diseases (1–3). At present, the most effective treatment for myocardial infarction is primary percutaneous coronary intervention, but there is no effective way to prevent myocardial ischemia-reperfusion (I/R) injury (4). Furthermore, continuous perfusion after ischemia can cause myocardial I/R injury, leading to reperfusion arrhythmia, myocardial shock and other pathological changes (5). Therefore, alleviating myocardial I/R injury is considered particularly important for the prevention and treatment of ischemic myocardial injury.
Long non-coding (lnc)RNAs are >200 nucleotides in length and have no significant protein-coding potential. lncRNAs are associated with different cellular biological processes, such as cell apoptosis, invasion and proliferation (6). Furthermore, it has been found that lncRNAs are involved in the pathogenesis of cardiovascular disease (7). Upregulation of lncRNA H19 imprinted maternally expressed transcript reduced myocardial infarction-induced myocardial injury through the regulation of KDM3A expression (7). Inhibition of lncRNA KCNQ1OT1 protected against oxygen glucose deprivation/reperfusion (OGD/R)-induced myocardial cell injury via the p38/MAPK/NF-κB signaling pathway (8). A recent study demonstrated that lncRNAs can act as competing endogenous (ce)RNAs to specifically bind to microRNAs (miRNAs) and reduce the regulatory effect of miRNAs on targeted mRNAs (9). For example, lncRNA downregulation of lncRNA MALAT1 inhibited OGD/R-induced myocardial cell injury by acting as a miRNA (miR)-20b sponge and regulating autophagy (10). Inhibition of lncRNA GAS5 can alleviate myocardial reperfusion injury by acting as a ceRNA for miR-335 and regulating Rho-associated coiled-coil containing protein kinase 1 expression (11). It has been reported that lncRNA SNHG8 acts as a potential biomarker and may participate in ischemia in myocardial cells (12). Results from the present study further identified the association between SNHG8 and miR-335 in protection from hypoxia-ischemia-reoxygenation (HI/R)-induced myocardial cell injury.
Heart failure, myocardial infarction and I/R injury are the main factors in the pathogenesis of heart disease that may result in cell death (13). Cardiomyocytes are lost as a result of apoptosis and necrosis in heart disease (14). Inhibition of lncRNA HOTAIR could increase the apoptotic rate of hypoxia-induced cardiomyocytes (15). LncRNA Gpr19 could protect OGD/R-induced myocardial cell injury by reducing cell apoptosis and activation of the miR-324-5p/mitochondrial fission regulator 1 axis (16). Bcl-2 and Bax proteins, members of the Bcl-2 family, and caspase-3, their downstream target, are considered to be the main regulators of cell apoptosis; they are expressed in the cytoplasm in the form of zymogens and subsequently activate cell apoptosis (17). Knockdown of lncRNA ZFAS1 expression could decrease I/R-induced myocardial cell apoptosis by regulating the changes in Bcl-2, Bax and cleaved caspase-3 expression (18). Overexpression of lncRNA SNHG8 was reported to inhibit chronic cerebral ischemia-induced neuron apoptosis (19). Therefore, the present study hypothesized that SNGH8 may protect against HI/R-induced myocardial injury by regulating apoptosis. Furthermore, it has been reported that the RAS signaling pathways serve a role in the development of a number of diseases, such as cancer and cardiac diseases (20,21). RAS p21 protein activator 1 (RASA1; also known as p120RasGAP) was the first RasGAP protein to be identified; it primarily acts by negatively regulating RAS signaling (22,23). RASA1 has been shown to serve a role in the cardiac myocyte growth induced by hypertrophic stimuli (24).
Therefore, the aim of the present study was to investigate the protective effects of lncRNA SNHG8 in HI/R-induced myocardial damage. SNHG8 may serve an important role by regulating miR-335 and RASA1 expression, suggesting that SNHG8 could be a novel therapeutic strategy for treatment of myocardial I/R injury.
Materials and methods
Cell culture and HI/R conditions
The embryonic rat cardiomyocyte-derived H9C2 cell line was purchased from the American Type Culture Collection and grown in Gibco Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.), which contained Gibco 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (MilliporeSigma) in a humidified incubator under standard conditions (5% CO2). After a 24-h incubation at 37°C, cells were washed three times with phosphate-buffered saline (PBS) and the growth media was replaced with glucose- and FBS-free DMEM. Cells were then cultured in a Thermo 3131 hypoxic incubator (<0.1% O2, 5% CO2 and 95% N2; Thermo Fisher Scientific, Inc.) at 37°C for 24 h (Hypoxia-ischemic; HI). Following treatment, the cells then were reoxygenated in an incubator in a 5% CO2 atmosphere for 2 h (Hypoxia-ischemic-reoxygenate; HI/R). H9C2 cells were used to HI/R conditions to induce a myocardial I/R injury model in vitro.
Transfection
H9C2 cells were seeded into 24-well plates at a density of 1×105 cells/well. The cells were cultured overnight before transfection at 37°C. Next, transfection with SNHG8 siRNA (100 nM), RASA1 siRNA (100 nM), negative control (NC; scrambled sequence) siRNA (Santa Cruz Biotechnology, Inc.), RASA1 plasmid, NC (empty plasmid) plasmid (OriGene Technologies, Inc.), miR-335 mimics, NC-mimics inhibitor or NC-inhibitor (Guangzhou RiboBio Co., Ltd.) into H9C2 cells was performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The transfection medium was replaced with complete medium 6 h after transfection, and the cells were incubated for the indicated times. All treatments were started 24 h after transfection. The siRNA sequences are presented in Table I and Fig. S1 demonstrates the transfection efficacy as determined by reverse transcription-quantitative PCR (RT-qPCR).
Table I.
siRNAs, miRNA, miRNA inhibitors and NC sequences used for transfections.
| Gene | Sequence (5′→3′) |
|---|---|
| NC siRNA | CATAGCGGTGTAGTAAAGCATAATA |
| SNHG8 siRNA | ATTACGATGGATGATGGAAACATA |
| RASA1 siRNA | TAGGATATTACAGTCACGT |
| NC-mimic | UUCUCCGAACGUGUCACGUTT |
| miR-335 mimics | UCAAGAGCAA UAACGAAAAAUGU |
| NC inhibitor | GTGTAACACGTCTATACGCCCA |
| miR-335 inhibitor | ACAUUUUUCGUUAUUGCUCUUGA |
miR, microRNA NC, negative control; RASA1, RAS p21 protein activator 1; si, small interfering RNA; SNHG8, small nucleolar RNA host gene 8.
Cell viability
Cell viability was examined using a Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.). The H9C2 cells transfected with SNHG8 siRNA, miR-335 mimics, inhibitor, RASA1 siRNA or NC were seeded into 96-well plates at a density of 3×103 cells/well incubation at 37°C for 24 h. Then, 10 µl CCK-8 solution was added to the cells (100 µl/well) and incubated at 37°C for 3 h. An MRX II microplate reader (Dynex Technologies, Inc.) was used to detect the absorbance at 450 nm.
Western blot analysis
The cells were lysed by the cell lysis buffer (Cell Signaling Technology, Inc.) and samples centrifuged at 300 × g for 5 min at 4°C after the lysis treatment. The supernatant was collected and the protein concentrations measured with a BCA Protein Assay kit (Sigma-Aldrich; Merck KGaA). The proteins (20 µg/lane) were separated by 10% SDS-PAGE and then transferred to polyvinylidene difluoride membranes (EMD Millipore). The membranes were blocked with Tris-buffered saline (TBS) and 0.1% Tween 20 (TBS-T) containing 5% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) at room temperature and then incubated with anti-Bax (cat. no. ab182734), anti-Bcl-2 (cat. no. ab194583) or anti-cleaved caspase-3 (cat. no. ab2302; all from Abcam; 1:1,000 in TBST containing 5% BSA) primary antibodies overnight at 4°C. The membranes were washed with TBST three times and then incubated with the appropriate horseradish peroxidase-labeled secondary antibody (1:2,000, Cell Signaling Technology, Inc.; cat. no. 7076) for 2 h at room temperature. Finally, the ECL Plus detection system (EMD Millipore) was used to examine the protein bands and the expression levels of the target proteins were semi-quantified by detecting the optical density value of each band. GAPDH (Cell Signaling Technology, Inc.; cat. no. 5174; 1:1,000) served as an internal control.
EdU assay
The H9C2 cells were seeded in 96-well plates at a density of 3×103 cells/well in culture media. The medium was replaced with serum-free medium to synchronize the cells. After 24 h, the serum-free medium was replaced with growth media for 48 h. Cell proliferation was assessed using a Click-iT EdU imaging kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions.
RT-qPCR analysis
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA from the H9C2 cells according to the manufacturer's instructions. Total RNA was then reverse transcribed to cDNA using a PrimeScript RT Reagent kit (Takara Biotechnology, Co., Ltd.; cat. no. RR047A). qPCR was then performed using a SYBR Green RT-PCR Kit (Takara Biotechnology, Co., Ltd.). The PCR conditions were as follows: 40 cycles of 95°C for 30 sec, 60°C for 34 sec and 72°C for 30 sec. U6 (Takara Biotechnology, Co., Ltd.; cat. no. 638315) and β-actin served as the internal controls. The U6 was used for miRNA, and mRNA and lncRNA was normalization by β-actin. The relative expression levels of lncRNA SNHG8, miR-335 and RASA1 expression were assessed using the comparative 2−ΔΔCq method (25) and primer sequences are presented in Table II and Table SI.
Table II.
Primer sequences used for reverse transcription-quantitative PCR.
| Gene | Primer sequence (5′→3′) |
|---|---|
| SNHG8 | F: GACACAAGGTGGCTATGGTGCTG |
| R: CATGGTGGTCGTCGCGCTAAC | |
| miR-335 | F: GCGGTCAAGAGCAATAACGAA |
| R: GTGCAGGGTCCGAGGTATTC | |
| RASA1 | F: CTGGAGATTATTCCCTGTATTTTCG |
| R: TGTTCTTTCCGATAGTGGTCTATGA | |
| β-actin | F: CCTCTATGCCAACACAGTGC |
| R: CATCGTACTCCTGCTTGCTG |
F, forward; miR, microRNA; R, reverse; RASA1, RAS p21 protein activator 1; SNHG8, small nucleolar RNA host gene 8.
Dual-luciferase reporter assays
The cDNA fragments of SNHG8 carrying the wild-type (WT) or mutated (MuT) binding sites of miR-335 and the 3′-untranslated region (UTR) of the amplified RASA1 fragment containing the predicated target sites for miR-335 were synthesized by Shanghai GenePharma Co., Ltd. H9C2 cells (1×105 cells/well) were seeded into 24-well plates and co-transfected with 50 ng recombinant luciferase vectors, 10 ng pGL3 vectors and 50 nM miR-335 mimics, miR-335 inhibitor or NC-mimic, NC-inhibitor using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.). After transfection for 48 h, the cells were lysed and luciferase activities were evaluated using the Dual-Luciferase Reporter Assay System (Promega Corporation) and a luminometer (Glomax20/20; Promega Corporation).
Lactate dehydrogenase (LDH) release analysis
The expression of LDH was determined by Cytotoxicity LDH Assay Kit-WST (Roche Diagnostics). Briefly, 50 µl cell suspension (2.5×104 cells) was added to each well of a 96-well plate after transfection with or without SNHG8 siRNA, RASA1 siRNA or miR-335 inhibitor and incubated at 37°C for 1 h. Lysis buffer (10 µl) was added to each well and incubated at 37°C for 30 min. Then, 100 µl working solution was added to each well. The plate was protected from light and incubated at room temperature for 30 min. Finally, 50 µl stop solution was added to each well and the absorbance was measured at 490 nm using a microplate reader.
Detection of cell apoptosis
The number of apoptotic cells was determined by flow cytometry using the Annexin V-FITC Apoptosis Detection Kit (Dojindo Molecular Technologies, Inc.). Different treatment groups of H9C2 cells were treated with a trypsin-EDTA (0.25%) solution and centrifuged at 300 × g for 3 min at 4°C. Annexin V Binding Solution, diluted 10-fold, was added to make a final cell concentration of 1×106 cells/ml. The cell suspension (100 µl) was transferred to a new tube, 5 µl Annexin V-FITC conjugate was added and then 5 µl PI solution was added to the cell suspension. The samples were protected from light and incubated for 15 min at room temperature. Finally, 10-fold diluted Annexin V binding solution (400 µl) was added and the percentage of early + late apoptotic cells was quantified by flow cytometry with a FACSCalibur system equipped with the CellQuest software (version 5.1; BD Biosciences).
Bioinformatics analysis
Bioinformatics analysis was performed using StarBase v 3.0 (http://starbase.sysu.edu.cn/index.php) and miRTarBase (http://mirtarbase.cuhk.edu.cn/php/index.php) to determine the association between miRNA and lncRNA (26,27).
Statistical analysis
Data are expressed as the mean ± SD, and statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software, Inc.). Statistical comparisons were conducted using unpaired Student's t-test or one-way analysis of variance with Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Protective effect of SNHG8 on HI/R myocardial injury
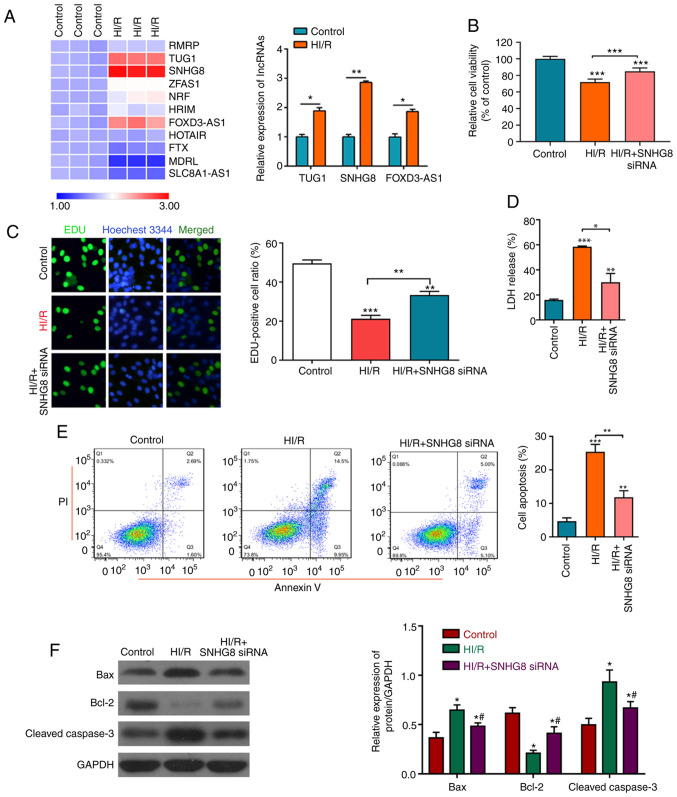
To determine the protective effect of lncRNA on HI/R myocardial injury, the expression levels of lncRNA were detected in the control group and HI/R myocardial cells. The results revealed that lncRNA SNHG8 was the most markedly upregulated lncRNA (Fig. 1A). It was also found that treatment with SNHG8 siRNA significantly increased HI/R-induced cell viability and proliferation, as analyzed by the CCK-8 assay and EdU analysis. LDH analysis demonstrated that treatment with SNHG8 siRNA reduced HI/R-induced cell damage (Fig. 1B-D). Subsequently, apoptotic rates and the change of apoptotic-related protein expressions following SNHG8 siRNA treatment in the HI/R myocardial cells was examined. As presented in Fig. 1E and F, HI/R-induction increased apoptosis and the protein expression levels of Bax and cleaved caspase-3 and reduced the expression of Bcl-2 protein, whereas HI/R treated cells transfected with SNHG8 siRNA exhibited decreased cell apoptosis and Bcl-2 and cleaved caspase-3 expression and increased the expression of Bcl-2 protein. These data indicated that inhibition of SNHG8 may protect against HI/R-induced myocardial cell injury.
Figure 1.
Protective effect of SNHG8 on HI/R myocardial injury. (A) Reverse transcription-quantitative PCR was used to determine the expression levels of various lncRNAs before or after HI/R injury in H9C2 myocardial cells. *P<0.05, **P<0.01. (B) Cell Counting Kit-8 assay was used to examine cell viability under different conditions. ***P<0.001 vs. Control. (C) Cell proliferation was determined by EdU analysis. **P<0.01, ***P<0.001 vs. Control. (D) LDH assay was used to detect LDH release. **P<0.01, ***P<0.001 vs. Control. *P<0.05. (E) Flow cytometric analysis of cell apoptosis in different groups. **P<0.01, ***P<0.001 vs. Control. (F) The expression levels of Bax, Bcl-2 and cleaved caspase-3 were determined by western blotting. *P<0.05 vs. Control. #P<0.05 vs. HI/R. FOXD3-AS1, forkhead box D3 antisense RNA1; HI/R, hypoxia-ischemia-reoxygenation; LDH, lactate dehydrogenase; siRNA, small interfering RNA; SNHG8, small nucleolar RNA host gene 8; TUG1, taurine upregulated 1.
SNHG8 regulates miR-335 expression
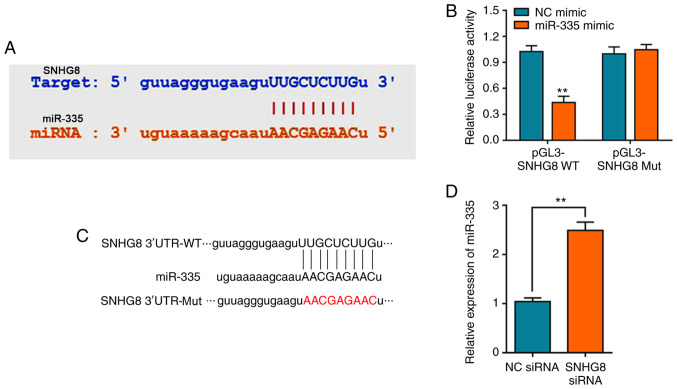
It has previously been reported that lncRNA mediates HI/R injury through the regulation of miRNAs, such as lncRNA MALAT1 which can regulate miRNA-20b after HI/R injury (9,28). Bioinformatics analysis was performed to screen potential miRNAs that have complementary base pairing with SNHG8. We firstly determined the expression of miRNA after under HI/R condition, showing that miR-335 was (Fig. S2A). StarBase analysis identified miR-335 as a potential target of SNHG8 (Fig. 2A). Consistent with our predication, the dual-luciferase reporter assay verified that miR-335 mimics could decrease the luciferase activity of pGL3-SNHG8-wild-type (WT), whereas no significant difference on pGL3-SNHG8-mutant (Mut) was observed (Fig. 2B). The schematic diagram of the relationship between SNHG8 and miR-335 was shown in Fig. 2C. Furthermore, silencing of SNHG8 resulted in upregulation of miR-335 expression (Fig. 2D).
Figure 2.
SNHG8 regulates miR-335 expression. (A) StarBase was used to predict miR-335 as a potential target of SNHG8B. (B) Relative luciferase activities of WT and Mut SNHG8 reporter plasmid in H9C2 cells after co-transfection with miR-335 mimics. **P<0.01 vs. NC mimic. (C) Schematic diagram of the relationship between SNHG8 and miR-335. (D) Reverse transcription-quantitative PCR was used to determine the expression level of miR-335 after treatment with SNHG8 siRNA. **P<0.01. miR, microRNA; Mut, mutant; NC, negative control; siRNA, small interfering RNA; SNHG8, small nucleolar RNA host gene 8; UTR, untranslated region; WT, wild-type.
SNHG8 serves a protective role in HI/R myocardial injury by regulating miR-335
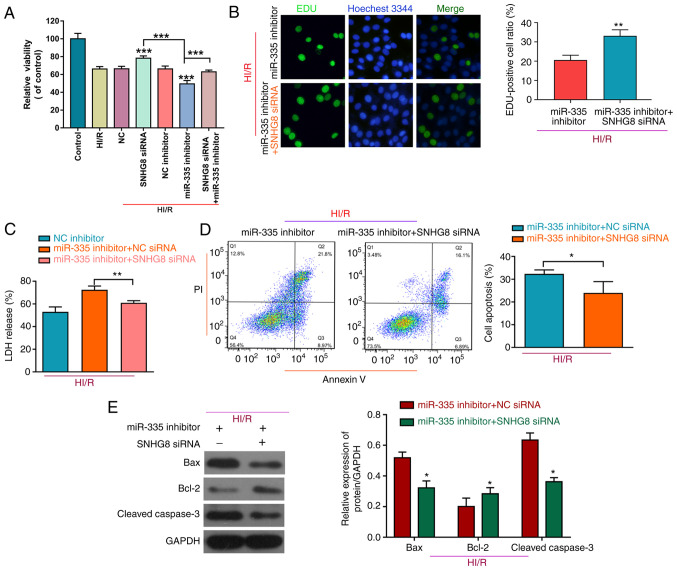
To further determine the role of miR-335 on HI/R myocardial injury, H9C2 cells were co-transfected with a miR-335 inhibitor and SNHG8 siRNA. As shown in Fig. 3A, treatment with the miR-335 inhibitor reduced cell viability compared with HI/R, whereas inhibition of SNHG8 increased cell viability after co-transfected with the miR-335 inhibitor on HI/R-induced H9C2 myocardial cell injury. Furthermore, SNHG8 siRNA combined with the miR-335 inhibitor was able to enhance cell proliferation and reduce LDH release compared with the miR-335 inhibitor alone group (Fig. 3B and C, respectively). SNHG8 siRNA reduced the miR-335 inhibitor-induced increase in the number of apoptotic cells under the HI/R condition in H9C2 cells (Fig. 3D). Western blot analysis of apoptosis-related proteins demonstrated that, Bax was decreased and Bcl-2 was increased following co-transfection with SNHG8 siRNA and miR-335 inhibitor (Fig. 3E).
Figure 3.
SNHG8 serves a protective role in HI/R myocardial injury by regulating miR-335. (A) The Cell Counting Kit-8 assay was used to examine cell viability in the different conditions. ***P<0.001 vs. HI/R. (B) Cell proliferation was determined by EdU analysis **P<0.01. (C) LDH assay was used to detect LDH release **P<0.01. (D) Flow cytometric analysis was used to determine cell apoptosis in the different groups *P<0.05. (E) The expression levels of Bax, Bcl-2 and cleaved caspase-3 were determined by western blotting. *P<0.05 vs. HI/R+ miR-335 inhibitor + NC siRNA. HI/R, hypoxia-ischemia-reoxygenation; LDH, lactate dehydrogenase; miR, microRNA; NC, negative control; siRNA, small interfering RNA; SNHG8, small nucleolar RNA host gene 8.
miR-335 negatively regulates the expression of RASA1
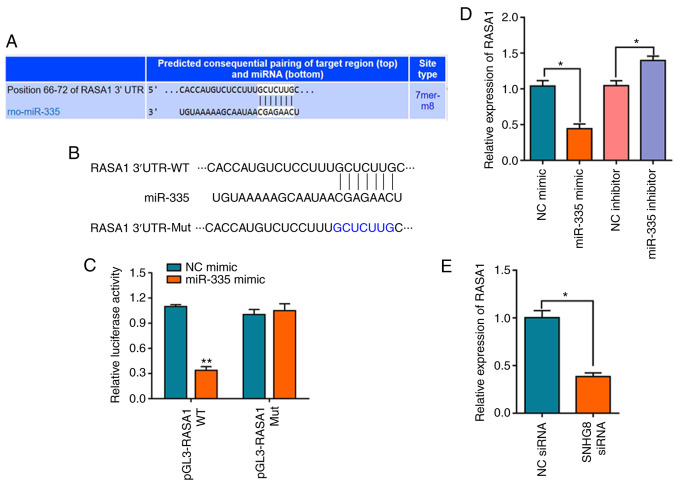
Based on the aforementioned results, TargetScan and miRTarBase were used to investigate the binding site of miR-335 (29,30). The overlapping analysis of the 196 differentially expressed mRNAs identified by TargetScan and the predicted single mRNA identified by miRTarBase indicated that only RASA1 interacted with miR-335 (Fig. S2B). The results indicated that miR-335 targets the 3′-UTR of the RASA1 mRNA (Fig. 4A). The schematic diagram of the relationship between miR-335 and RASA1 is shown in Fig. 4B. A dual-luciferase reporter assay was used to further verify this prediction, and the results indicated that miR-335 mimics decreased the luciferase activity of pGL3-RASA1-WT, whereas no effect on pGL3-RASA1-Mut was observed (Fig. 4C). In addition, it was demonstrated that overexpression of miR-335 significantly downregulated the expression level of RASA1 mRNA, whereas transfection with the miR-335 inhibitor had the opposite effect on the expression of RASA1 (Fig. 4D). The expression of RASA1 following transfection of HI/R-induced H9C2 cells with SNHG8 siRNA was also confirmed. The results demonstrated that SNHG8 siRNA treatment significantly downregulated the expression of RASA1 (Fig. 4E).
Figure 4.
miR-335 negatively regulates the expression of RASA1. (A) TargetScan analysis was used to predict matches between miR-335 and the RASA1 3′-UTR. (B) Schematic diagram of the relationship between miR-335 and RASA1. (C) Relative luciferase activities of WT and Mut RASA1 reporter plasmid in H9C2 cells after co-transfection with miR-335 mimics. **P<0.01 vs. NC mimic. (D) The expression level of RASA1 mRNA was determined by reverse transcription-quantitative PCR after transfection with the miR-335 mimic, inhibitor or NC. *P<0.05. (E) SNHG8 siRNA transfection downregulated RASA1 expression. *P<0.05. miR, microRNA; Mut, mutant; NC, negative control; RASA1, RAS p21 protein activator 1; rno, Rattus norvegicus; siRNA, small interfering RNA; SNHG8, small nucleolar RNA host gene 8; UTR, untranslated region; WT, wild-type.
SNHG8 protects against HI/R myocardial injury through regulation of miR-335 and RASA1
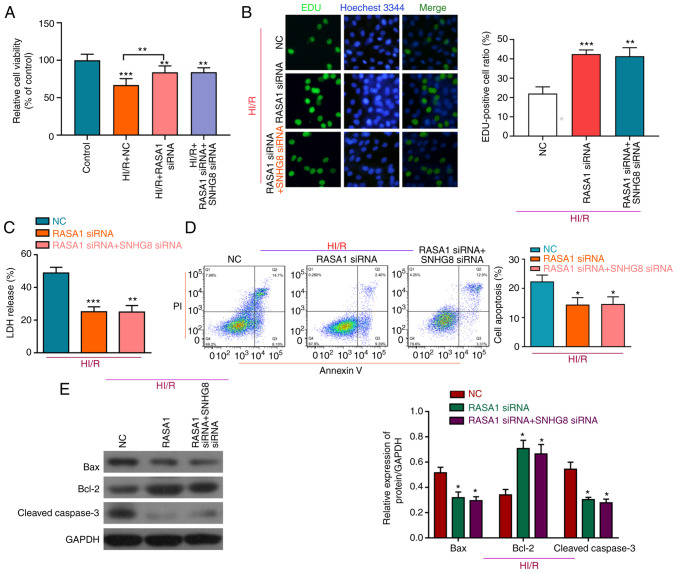
To investigate whether miR-335 and RASA1 mediated the protective effect of SNHG8 on HI/R-induced H9C2 cell injury, a series of experiments was performed. Knockdown of RASA1 protected HI/R-induced H9C2 cells by increasing cell viability and cell proliferation, and decreasing LDH release, whereas compared with RASA1 siRNA + HI/R group, there was no significant difference in cell viability, proliferation and LDH following treatment with SNHG8 siRNA and RASA1 siRNA (Fig. 5A-C). Moreover, following RASA1 silencing, SNHG8 did not further reduce apoptosis, again demonstrating that SNHG8 may act through RASA1 (Fig. 5D). Western blot analysis showed that, compared with the RASA1 siRNA group, there was no significant difference in Bax and Bcl-2 expression after combined treatment with RASA1 siRNA and SNHG8 siRNA (Fig. 5E).
Figure 5.
RASA1 mediates the protective effect of SNHG8 on HI/R myocardial injury. (A) Cell Counting Kit-8 viability assay and (B) EdU analysis of cell proliferation after transfection with RASA1 siRNA alone or after co-transfection with SNHG8 siRNA. **P<0.01, ***P<0.001 vs. Control. (C) LDH expression was determined following transfection with RASA1 siRNA alone or co-transfection with SNHG8 siRNA. **P<0.01, ***P<0.001 vs. NC. (D) The number of apoptotic cells was determined by flow cytometry. *P<0.05 vs. NC. (E) Western blot analysis of Bax, Bcl-2 and cleaved caspase-3 protein expression levels in H9C2 cells following transfection RASA1 siRNA alone or co-transfection of RASA1 siRNA and SNHG8 siRNA. *P<0.05 vs. NC. HI/R, hypoxia-ischemia-reoxygenation; LDH, lactate dehydrogenase; miR, microRNA; NC, negative control; RASA1, RAS p21 protein activator 1; siRNA, small interfering RNA; SNHG8, small nucleolar RNA host gene 8.
The protective effect of SNHG8 combined with RASA1 overexpression plasmid on HI/R induced H9C2 cells was analyzed further. The data demonstrated that RASA1 overexpression reduced cell viability, whereas SNHG8 siRNA combined with RASA1 overexpression increased viability (Fig. S2C). These results demonstrated that lncRNA SNHG8 may regulate the expression of RASA1 and that RASA1 may mediate the effects SNHG8 on the damage to cells. These data indicated that SNHG8 may protect HI/R-induced H9C2 cell injury through the regulation of miR-335 and RASA1 expression.
Discussion
Myocardial I/R injury is one of the major causes of mortality worldwide (31). Myocardial ischemia leads to hypoxia and cell apoptosis, further aggravating myocardial tissue damage (32). Therefore, investigating strategies to reduce myocardial injury during hypoxia are essential for treatment of heart failure and angina pectoris.
Studies have indicated that lncRNAs are related to cerebral ischemia and that these lncRNAs could regulate the development of ischemic cerebrovascular disease (33,34). They also act as ceRNAs, regulating specific RNA transcription by competitively binding with miRNAs. This lncRNA-miRNA crosstalk might be particularly important for the control and treatment of myocardial I/R injury and recovery after ischemia, and lncRNA-miRNA crosstalk have also emerged as new regulators in myocardial injury (31). Upregulation of lncRNA HULC could reduce HI/R-induced H9C2 cell apoptosis and inflammation through the miR-377-5p-NLRP3/caspase-1/IL-1β axis (35). Silencing of lncRNA PVT1 could reduce HI/R injury-induced cell apoptosis and autophagy via the miR-186/Beclin-1 signaling pathway (36). lncRNA SNHG8, located on 4qq26, belongs to the lncRNA family and is 1,062 nt in length (37). A recent study indicated that lncRNA SNHG8 was significantly upregulated in AMI and that it may be correlated with regulation of myocardial cell necrosis and apoptosis (12). The present study found that, after HI/R induction, SNHG8 exhibited the highest expression among the lncRNAs examined, and it was associated with ischemic myocardial cells, which was consistent with studies by Zhuo et al (12) and Zhang and Bian (38). Inhibition of SNHG8 could protect against HI/R-induced myocardial injury by increasing cell viability and proliferation, and by decreasing cell apoptosis.
In the present study, a bioinformatics approach was used to predict that the SNHG8 transcript contained a miR-335 binding region. It has previously been reported that miR-335 serves an important role in myocardial I/R injury (39), which is consistent with the results of the present study. The present findings also demonstrated that miR-335 inhibitor treatment could increase cell apoptosis and reduce cell viability or proliferation in HI/R-induced H9C2 cells, with co-transfection with SNHG8 siRNA, the effect of the miR-335 inhibitor on increasing apoptosis was also reversed.
miRNAs serve an important role in cardiovascular disease by interacting with downstream mRNAs (40,41). For example, upregulation of miR-149 protected against myocardial I/R damage by inhibiting forkhead box O3 expression (42). miR-7b overexpression reduced HI/R-induced H9C2 apoptosis via the hypoxia inducible factor-1α/p38 signaling pathway (43). The present study demonstrated that miR-335 negatively regulates the expression of RASA1. Combined with the aforementioned results, it was confirmed that knockdown of SNHG8 could decrease RASA1 expression. Moreover, the present study confirmed that RASA1 mediated the protective effect of SNHG8 in HI/R-induced myocardial cell injury. Therefore, it was demonstrated that SNHG8 may control HI/R-induced myocardial cell injury by regulating miR-335 and RASA1.
The main factors leading to reperfusion injury are oxidative stress, inflammation and apoptosis (44). Myocardial apoptosis is also related to ventricular dysfunction subsequent to cardiac surgery (45). Therefore, inhibition of myocardial cell apoptosis after ischemia might be considered an effective treatment to improve diastolic function after ischemia. The Bcl-2 family, including Bcl-2 and Bax, are related to the regulation of cell apoptosis (46). The present study revealed that SNHG8 could regulate apoptotic-related proteins Bax, Bcl-2 and cleaved caspase-3 by regulating miR-335 and RASA1. However, the present study also had certain limitations. The protective effect of SNHG8 was verified under HI/R-induced H9C2 cells in vitro; however, the experiment could be extended to demonstrate the effect in vivo. Additionally, further identification and confirmation of the precise mechanisms underlying RASA1 are required, such as whether RASA1 regulates Ras/MEK1/2/ERK1/2 and PI3K/Akt signaling.
In conclusion, the present study demonstrated that transfection with SNHG8 siRNA protected against HI/R-induced injury in H9C2 cells by mediating the regulation of miR-335 and RASA1, thus indicating that SNHG8 may be an effective target for treatment of myocardial I/R injury.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The current study was funded by The Traditional Chinese Medicine Research Project of Jiangxi Province (grant no. 2019A151).
Funding
The current study was funded by The Traditional Chinese Medicine Research Project of Jiangxi Province (grant no. 2019A151).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
DR and LH conceived the idea. YL and PZ performed the experiments. FW, XZ and DY analyzed the data. DR and YL wrote the manuscript. All authors have read and approved the final manuscript. DR and LH confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Severino P, D'Amato A, Pucci M, Infusino F, Adamo F, Birtolo LI, Netti L, Montefusco G, Chimenti C, Lavalle C, et al. Ischemic heart disease pathophysiology paradigms overview: From plaque activation to microvascular dysfunction. Int J Mol Sci. 2020;21:8118. doi: 10.3390/ijms21218118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389:197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux PJ, Szczeklik W. Myocardial injury after non-cardiac surgery: Diagnosis and management. Eur Heart J. 2020;41:3083–3091. doi: 10.1093/eurheartj/ehz301. [DOI] [PubMed] [Google Scholar]
- 4.Jokinen E. Coronary artery disease in patients with congenital heart defects. J Intern Med. 2020;288:383–389. doi: 10.1111/joim.13080. [DOI] [PubMed] [Google Scholar]
- 5.Khan MA, Hashim MJ, Mustafa H, Baniyas MY, Al Suwaidi SKBM, AlKatheeri R, Alblooshi FMK, Almatrooshi MEAH, Alzaabi MEH, Al Darmaki RS, Lootah SNAH. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Duan L, Li Y, Liu B. Long noncoding RNA/circular noncoding RNA-miRNA-mRNA axes in cardiovascular diseases. Life Sci. 2019;233:116440. doi: 10.1016/j.lfs.2019.04.066. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018;22:5768–5775. doi: 10.1111/jcmm.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Dai Y, Yan S, Shi Y, Han B, Li J, Cha L, Mu J. Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury following acute myocardial infarction. Biochem Biophys Res Commun. 2017;491:1026–1033. doi: 10.1016/j.bbrc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Sui C, Dong Z, Yang C, Zhang M, Dai B, Geng L, Lu J, Yang J, Xu M. LncRNA FOXD2-AS1 as a competitive endogenous RNA against miR-150-5p reverses resistance to sorafenib in hepatocellular carcinoma. J Cell Mol Med. 2019;23:6024–6033. doi: 10.1111/jcmm.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Yao T, Deng F, Yu W, Song Y, Chen J, Ruan Z. LncRNA MALAT1 promotes oxygen-glucose deprivation and reoxygenation induced cardiomyocytes injury through sponging miR-20b to Enhance beclin1-mediated autophagy. Cardiovasc Drugs Ther. 2019;33:675–686. doi: 10.1007/s10557-019-06902-z. [DOI] [PubMed] [Google Scholar]
- 11.Wu N, Zhang X, Bao Y, Yu H, Jia D, Ma C. Down-regulation of GAS5 ameliorates myocardial ischaemia/reperfusion injury via the miR-335/ROCK1/AKT/GSK-3β axis. J Cell Mol Med. 2019;23:8420–8431. doi: 10.1111/jcmm.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuo LA, Wen YT, Wang Y, Liang ZF, Wu G, Nong MD, Miao L. LncRNA SNHG8 is identified as a key regulator of acute myocardial infarction by RNA-seq analysis. Lipids Health Dis. 2019;18:201. doi: 10.1186/s12944-019-1142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shekhar A, Heeger P, Reutelingsperger C, Arbustini E, Narula N, Hofstra L, Bax JJ, Narula J. Targeted imaging for cell death in cardiovascular disorders. JACC Cardiovasc Imaging. 2018;11:476–493. doi: 10.1016/j.jcmg.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Xu T, Ding W, Ao X, Chu X, Wan Q, Wang Y, Xiao D, Yu W, Li M, Yu F, Wang J. ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol. 2019;20:414–426. doi: 10.1016/j.redox.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao L, Liu Y, Guo S, Yao R, Wu L, Xiao L, Wang Z, Liu Y, Zhang Y. Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cell Physiol Biochem. 2017;44:1497–1508. doi: 10.1159/000485588. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Guo B, Liu S, Miao C, Li Y. Inhibition of the LncRNA Gpr19 attenuates ischemia-reperfusion injury after acute myocardial infarction by inhibiting apoptosis and oxidative stress via the miR-324-5p/Mtfr1 axis. IUBMB Life. 2020;72:373–383. doi: 10.1002/iub.2187. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 18.Huang P, Yang D, Yu L, Shi Y. Downregulation of lncRNA ZFAS1 protects H9c2 cardiomyocytes from ischemia/reperfusion-induced apoptosis via the miR5903p/NFκB signaling pathway. Mol Med Rep. 2020;22:2300–2306. doi: 10.3892/mmr.2020.11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, An P, Xue Y, Che D, Liu X, Zheng J, Liu Y, Yang C, Li Z, Yu B. Mechanism of Snhg8/miR-384/Hoxa13/FAM3A axis regulating neuronal apoptosis in ischemic mice model. Cell Death Dis. 2019;10:441. doi: 10.1038/s41419-019-1631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soleimani A, Rahmani F, Saeedi N, Ghaffarian R, Khazaei M, Ferns GA, Avan A, Hassanian SM. The potential role of regulatory microRNAs of RAS/MAPK signaling pathway in the pathogenesis of colorectal cancer. J Cell Biochem. 2019;120:19245–19253. doi: 10.1002/jcb.29268. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Xu Y, Deng Y, Li H. MicroRNA-223 regulates cardiac fibrosis after myocardial infarction by targeting RASA1. Cell Physiol Biochem. 2018;46:1439–1454. doi: 10.1159/000489185. [DOI] [PubMed] [Google Scholar]
- 22.Tao H, Yang JJ, Chen ZW, Xu SS, Zhou X, Zhan HY, Shi KH. DNMT3A silencing RASSF1A promotes cardiac fibrosis through upregulation of ERK1/2. Toxicology. 2014;323:42–50. doi: 10.1016/j.tox.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Trial J, Entman ML, Cieslik KA. Mesenchymal stem cell-derived inflammatory fibroblasts mediate interstitial fibrosis in the aging heart. J Mol Cell Cardiol. 2016;91:28–34. doi: 10.1016/j.yjmcc.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diao X, Shen E, Wang X, Hu B. Differentially expressed microRNAs and their target genes in the hearts of streptozotocin-induced diabetic mice. Mol Med Rep. 2011;4:633–640. doi: 10.3892/mmr.2011.489. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 27.Huang HY, Lin YC, Li J, Huang KY, Shrestha S, Hong HC, Tang Y, Chen YG, Jin CN, Yu Y, et al. MiRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020;48(D1):D148–D154. doi: 10.1093/nar/gkz896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Lu Y, Ming J, Pan Y, Yu R, Wu Y, Wang T. SNHG16 accelerates the proliferation of primary cardiomyocytes by targeting miRNA-770-5p. Exp Ther Med. 2020;20:3221–3227. doi: 10.3892/etm.2020.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, LaVigne CA, Jones BT, Zhang H, Gillett F, Mendell JT. A ubiquitin ligase mediates target-directed microRNA decay independently of tailing and trimming. Science. 2020;370:eabc9546. doi: 10.1126/science.abc9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi CY, Kingston ER, Kleaveland B, Lin DH, Stubna MW, Bartel DP. The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science. 2020;370:eabc9359. doi: 10.1126/science.abc9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu S, Tan J, Li Q, Liu S, Ma J, Zheng Y, Liu J, Bi W, Sha P, Li X, et al. Downregulation of LAPTM4B contributes to the impairment of the autophagic flux via unopposed activation of mTORC1 signaling during myocardial ischemia/reperfusion injury. Circ Res. 2020;127:e148–e165. doi: 10.1161/CIRCRESAHA.119.316388. [DOI] [PubMed] [Google Scholar]
- 32.Liang S, Ren K, Li B, Li F, Liang Z, Hu J, Xu B, Zhang A. LncRNA SNHG1 alleviates hypoxia-reoxygenation-induced vascular endothelial cell injury as a competing endogenous RNA through the HIF-1α/VEGF signal pathway. Mol Cell Biochem. 2020;465:1–11. doi: 10.1007/s11010-019-03662-0. [DOI] [PubMed] [Google Scholar]
- 33.Ghafouri-Fard S, Shoorei H, Taheri M. Non-coding RNAs participate in the ischemia-reperfusion injury. Biomed Pharmacother. 2020;129:110419. doi: 10.1016/j.biopha.2020.110419. [DOI] [PubMed] [Google Scholar]
- 34.Bao MH, Szeto V, Yang BB, Zhu SZ, Sun HS, Feng ZP. Long non-coding RNAs in ischemic stroke. Cell Death Dis. 2018;9:281. doi: 10.1038/s41419-018-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang H, Li F, Li H, Wang R, Du M. Overexpression of lncRNA HULC attenuates myocardial ischemia/reperfusion injury in rat models and apoptosis of hypoxia/reoxygenation cardiomyocytes via targeting miR-377-5p through NLRP3/Caspase1/IL1β signaling pathway inhibition. Immunol Invest. 2020 Jul 17; doi: 10.1080/08820139.2020.1791178. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 36.Ouyang M, Lu J, Ding Q, Qin T, Peng C, Guo Q. Knockdown of long non-coding RNA PVT1 protects human AC16 cardiomyocytes from hypoxia/reoxygenation-induced apoptosis and autophagy by regulating miR-186/Beclin-1 axis. Gene. 2020;754:144775. doi: 10.1016/j.gene.2020.144775. [DOI] [PubMed] [Google Scholar]
- 37.Zhang P, Li S, Chen Z, Lu Y, Zhang H. LncRNA SNHG8 promotes proliferation and invasion of gastric cancer cells by targeting the miR-491/PDGFRA axis. Hum Cell. 2020;33:123–130. doi: 10.1007/s13577-019-00290-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Bian Y. Long non-coding RNA SNHG8 plays a key role in myocardial infarction through affecting hypoxia-induced cardiomyocyte injury. Med Sci Monit. 2020;26:e924016. doi: 10.12659/MSM.924016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu N, Zhang X, Du S, Chen D, Che R. Upregulation of miR-335 ameliorates myocardial ischemia reperfusion injury via targeting hypoxia inducible factor 1-alpha subunit inhibitor. Am J Transl Res. 2018;10:4082–4094. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Fang J, Ma H. Inhibition of miR-182-5p protects cardiomyocytes from hypoxia-induced apoptosis by targeting CIAPIN1. Biochem Cell Biol. 2018;96:646–654. doi: 10.1139/bcb-2017-0224. [DOI] [PubMed] [Google Scholar]
- 41.Xiao X, Lu Z, Lin V, May A, Shaw DH, Wang Z, Che B, Tran K, Du H, Shaw P. MicroRNA miR-24-3p reduces apoptosis and regulates Keap1-Nrf2 pathway in mouse cardiomyocytes responding to ischemia/reperfusion injury. Oxid Med Cell Longev. 2018;2018:7042105. doi: 10.1155/2018/7042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Lin H, Ma C, Dong F, Hu Y, Li H. MiR-149 aggravates pyroptosis in myocardial ischemia-reperfusion damage via silencing FoxO3. Med Sci Monit. 2019;25:8733–8743. doi: 10.12659/MSM.918410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng Z, Lu W, Zuo Z, Wang D, Zuo P, Yao Y, Ma G. MicroRNA-7b attenuates ischemia/reperfusion-induced H9C2 cardiomyocyte apoptosis via the hypoxia inducible factor-1/p-p38 pathway. J Cell Biochem. 2019;120:9947–9955. doi: 10.1002/jcb.28277. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Zhang L, Qin H, Han X, Zhang Z, Zhang Z, Qin SY, Niu J. Inhibition of TRAF3 expression alleviates cardiac ischemia reperfusion (IR) injury: A mechanism involving in apoptosis, inflammation and oxidative stress. Biochem Biophys Res Commun. 2018;506:298–305. doi: 10.1016/j.bbrc.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 45.Pereira RM, Mekary RA, da Cruz Rodrigues KC, Anaruma CP, Ropelle ER, da Silva ASR, Cintra DE, Pauli JR, de Moura LP. Protective molecular mechanisms of clusterin against apoptosis in cardiomyocytes. Heart Fail Rev. 2018;23:123–129. doi: 10.1007/s10741-017-9654-z. [DOI] [PubMed] [Google Scholar]
- 46.Banjara S, D Sa J, Hinds MG, Kvansakul M. The structural basis of Bcl-2 mediated cell death regulation in hydra. Biochem J. 2020;477:3287–3297. doi: 10.1042/BCJ20200556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.