Abstract
Background
Hypertensive disorders of pregnancy (HDP) is one of the most common pregnancy complications and causes of maternal morbidity and mortality. Assisted reproductive technology (ART) has been associated with adverse pregnancy outcomes, including HDP. However, the impact of multiple pregnancies, oocyte donation, as well as fresh and frozen embryo transfer needs to be further studied. We conducted a systematic review and meta-analyses to evaluate the association between ART and HDP or preeclampsia relative to spontaneous conception (SC).
Methods
We identified studies from EMBASE, MEDLINE, and Cochrane Library (up to April 8, 2020) and manually using structured search strategies. Cohort studies that included pregnancies after in vitro fertilization (IVF) with or without intracytoplasmic sperm fertilization (ICSI) relative to SC with HDP or preeclampsia as the outcome of interest were included. The control group was women who conceived spontaneously without ART or fertility medications. The pooled results were reported in odds ratios (OR) with 95% confidence intervals based on random effects models. Numbers needed to harm (NNH) were calculated based on absolute risk differences between exposure and control groups.
Results
Eighty-five studies were included after a screening of 1879 abstracts and 283 full text articles. Compared to SC, IVF/ICSI singleton pregnancies (OR 1.70; 95% CI 1.60–1.80; I2 = 80%) and multiple pregnancies (OR 1.34; 95% CI 1.20–1.50; I2 = 76%) were both associated with higher odds of HDP. Singleton pregnancies with oocyte donation had the highest odds of HDP out of all groups analyzed (OR 4.42; 95% CI 3.00–6.51; I2 = 83%). Frozen embryo transfer resulted in higher odds of HDP (OR 1.74; 95% CI 1.58–1.92; I2 = 55%) than fresh embryo transfer (OR 1.43; 95% CI 1.33–1.53; I2 = 72%). The associations between IVF/ICSI pregnancies and SC were similar for preeclampsia. Most interventions had an NNH of 40 to 100, while singleton and multiple oocyte donation pregnancies had particularly low NNH for HDP (16 and 10, respectively).
Conclusions
Our meta-analysis confirmed that IVF/ICSI pregnancies are at higher odds of HDP and preeclampsia than SC, irrespective of the plurality. The odds were especially high in frozen embryo transfer and oocyte donation pregnancies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-021-03938-8.
Keywords: Assisted reproductive technology, In vitro fertilization, Intracytoplasmic sperm injection, Hypertensive disorders of pregnancy, Preeclampsia, Frozen embryo transfer, Fresh embryo transfer, Oocyte donation, Meta-analysis
Background
In 2010, 48.5 million couples worldwide were estimated to be affected by infertility [1]. The use of in vitro fertilization (IVF) and other assisted reproductive technologies (ART) is expanding rapidly, accounting for more than seven million births worldwide [2]. The advancement of treatments and changes in protocols have also reshaped the landscape of fertility practice in recent years. For example, intracytoplasmic sperm injection (ICSI) is mainly indicated for male factor infertility or poor response to IVF [3]; cryopreservation has led to the rise of frozen embryo transfer (FET), which expands the scope of treatment and decreases the risk of ovarian hyperstimulation syndrome [4]; finally, oocyte donation (OD) allows women with decreased ovarian reserve or ovarian failure to achieve pregnancy [5].
While ART continues to benefit many couples around the world, it may be associated with adverse outcomes, including hypertensive disorders of pregnancy (HDP) [6–10]. HDP, including gestational hypertension and preeclampsia, occur in approximately 12–22% of all pregnancies and is associated with significant maternal and prenatal morbidity and mortality [11]. Preeclampsia is associated with a wide range of complications related to microangiopathy, vasoconstriction, and malperfusion. Women with a history of preeclampsia also continue to be at a high risk for cardiovascular disease, chronic kidney disease, and cardiovascular mortality even after pregnancy [12]. The pregnancy and postpartum complications as well as high mortality rates highlight the importance of prevention and early detection of HDP.
Although previous meta-analyses have shown that ART is associated with an increased risk of preeclampsia, the underlying mechanism is not well understood [13, 14]. Many included studies were based on singleton pregnancies or mixed cohorts, while studies specifically comparing the risk of HDP in ART and spontaneous multiple pregnancies often yielded inconsistent results [9, 15]. The types of ART and treatment protocols also appear to play a role in differences in maternal and perinatal outcomes [16]. For example, recent meta-analysis by Rogue et al. showed that FET is associated with a higher rate of low birth weight and preeclampsia when compared to fresh ET [17]. Currently, it remains unclear whether the differences in pregnancy outcomes, including HDP, were due to maternal factors, the procedure itself, or both. With the increasing number of literature over the past decade and changes in protocols, there is a need for an updated and comprehensive review of IVF/ICSI pregnancies in consideration of patient and treatment factors.
Our systematic review and meta-analysis aim to understand whether IVF/ICSI pregnancies are associated with increased odds of HDP and preeclampsia in comparison to spontaneous conception (SC); furthermore, we aim to understand whether the odds differ depending on types of procedure. Together, this review may inform clinical recommendations for women planning to achieve pregnancy through IVF/ICSI.
Methods
Search strategy
The study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist (Supplementary information, Additional file 1) and the protocol was registered and available on Open Science Framework (DOI: https://osf.io/562jr/). A search strategy was developed under the support of a research librarian to identify studies evaluating the incidence of HDP and/or preeclampsia in IVF or ICSI pregnancies compared to SC (Additional file 2). MeSH terms and selection criteria were based on the Patient, Intervention, Comparison and Outcome statement. Cohort studies published up to April 8, 2020 were retrieved from Medline, Embase, and Cochrane Central Register of controlled Trials using the OVID platform. A manual search of previously published systematic reviews and meta-analysis was also conducted to identify other eligible studies.
Selection of studies
Both abstract and full text screening were performed by two reviewers (HC, FTSE). In the first screening, articles were selected based on titles and abstracts. The second screening involved full-text reviews, where studies were evaluated based on a set of eligibility criteria. Any conflict was resolved by consensus or the involvement of a third team member (MPV).
Studies that compared pregnancies after IVF or ICSI and SC with HDP or preeclampsia as the outcome of interest were included. The control group consisted of women who conceived spontaneously without the use of ART or fertility medications. The exposure group consisted of singleton or multiple IVF/ICSI pregnancies. Non-randomized studies in the form of prospective and retrospective cohort studies were of interest; other study designs such as review articles, randomized control trials, case-control studies, conference abstracts, and case reports were excluded. Studies were excluded if they were not in English, French, Portuguese, or Chinese, included patients undergoing ART or fertility treatments other than IVF/ICSI, did not specify the type of ART used, or did not clearly separate patients into singleton or multiple pregnancies. Studies that included a subgroup of women (e.g. advanced maternal age, obesity) were not included in the general singleton and multiple gestation analyses as they were not representative of the general population. However, they were included in sub-analyses for type of embryo transfer (fresh embryo transfer (fresh ET) or FET) and OD. For studies with overlapping cohorts, where the same database was used for analyses, only the most recent study was included in the meta-analysis. A complete list of excluded studies after full text screening with their respective reasons of exclusion may be found in Additional file 3.
Outcomes of interest included HDP and preeclampsia. Hypertensive disorders of pregnancy describe any hypertensive effects that is observed during pregnancy, including pre-existing hypertension, gestational hypertension and preeclampsia. Preeclampsia was defined as hypertension that develops for the first time after 20 weeks of gestation with one or more of the following: proteinuria, adverse conditions, or severe complications [18].
Data extraction and quality assessment
Data was extracted manually and entered into an Excel spreadsheet by a reviewer (HC). The following characteristics of each study were collected: authorship, year of publication, country, study design, search database, time period of the cohort, matching factors, statistical analysis, outcome of interest, definition of outcome, mean maternal age, mean BMI, number of patients with chronic hypertension, type of ART, type of infertility, source of oocyte, method of embryo transfer, sample size, and crude data. If needed, percentages of HDP and preeclampsia were converted to crude data based on the sample size. Study quality was assessed using the Newcastle-Ottawa Scale for Cohort Studies [19]. Each study was scored out of nine based on eight items across three domains: the selection of study groups (4 items), comparability of groups (1 item), and ascertainment of exposure or outcome of interest in cohort studies, respectively (3 items). It was then determined to have either high quality (8 or 9), moderate quality (6 or 7), or low quality (less than 5) based on the total NOS score. A second reviewer (FTSE) reviewed all data extraction and quality assessment performed.
Statistical analysis
The meta-analyses were performed using Review Manager (RevMan) version 5.4. Cohort studies were included in the general meta-analyses by plurality. Studies that explicitly excluded ICSI pregnancies and those that included ICSI pregnancies only were included in the IVF and ICSI sub-analyses, respectively. In addition, separate analyses on fresh ET, FET, and OD were also conducted. Results were reported as odds ratios with corresponding 95% confidence intervals based on random effects models, which assumed heterogeneity of the data. The Mantel-Haenszel method was used to calculate overall odds ratios. Statistical significance was determined by a P value of equal or less than 0.05. Numbers needed to harm (NNH), which represented the number of patients needed to undergo IVF/ICSI for one patient to receive harm, were calculated based on the absolute risk differences between exposure and control groups [20]. Sensitivity analysis was carried out by removing one study at a time to assess the effect of the study on the results. If the measure of association without the chosen study fell outside of the confidence interval, the study was said to have a significant influence [21]. I-squared (I2) test was used to evaluate heterogeneity, with an I2 value of greater than 50% being considered as high heterogeneity [22]. Risk of publication bias was evaluated using funnel plots if the meta-analysis included 10 or more studies [23].
Results
Study selection
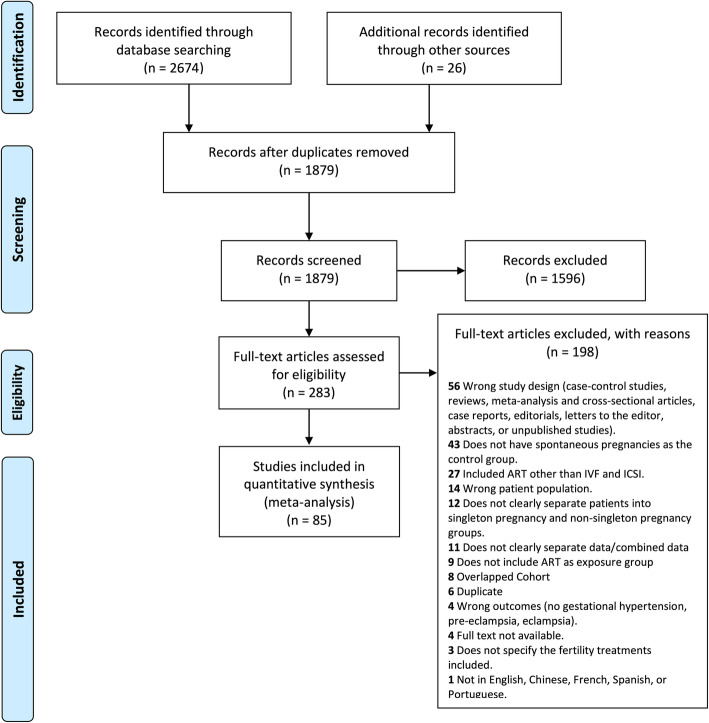
Our search strategy identified 2674 studies and 1879 citations were eligible for abstract and title screening after removing duplicates. Finally, 85 studies met the inclusion criteria and were included in the meta-analysis (Fig. 1). Eight studies had overlapping cohorts with more recently published studies and thus were excluded (Additional file 3).
Fig. 1.
PRISMA Flowchart. Flow diagram for study identification and inclusion according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines
Characteristics of included studies
The characteristics of all included studies, which involved 405,920 IVF/ICSI pregnancies and 8,122,210 SC, may be found in Table 1. The sizes of the exposure (IVF/ICSI) and control (SC) groups ranged from 19 to 83,582 pregnancies and from 21 to 1,382,311 pregnancies respectively. Out of the 85 studies included, 21 were population-based cohort studies conducted in Canada [39, 40, 88], Denmark [64], Finland [55, 78], Israel [73, 85], China [105], Japan [69], Netherlands [31], Norway [94], Slovenia [52], Sweden [43, 44, 71, 83], and the United States [62, 63]. Two studies were conducted across multiple European countries [75, 101]. Fifty-one studies looked at the incidences of preeclampsia or HDP in IVF/ICSI singleton pregnancies in comparison to SC, while 41 studies investigated the outcomes of multiple pregnancies in particular (Table 1). Based on their respective NOS scores, 15 studies had a high methodological quality, 61 studies had a moderate quality, and 9 had a low quality (Table 1, Additional file 4). Thirty-three studies were matched cohort studies using varying factors such as maternal age, birth year, parity, socioeconomic status, location (Table 1). Eight studies used chronic hypertension to adjust the comparability between exposure and control groups [65, 70, 75, 81, 88, 97, 100, 108]. One study calculated propensity scores that account for 27 maternal and paternal variables [100].
Table 1.
Characteristics of 78 included cohort studies. S = Singleton pregnancy, M = Multiple pregnancy
| First author, Publication year, Country | Type of cohort | Years of the cohort | Matching factors | Comparison groups | Pregnancies conceived by IVF/ICSI (n) | Spontaneous pregnancies (n) | NOS Score |
|---|---|---|---|---|---|---|---|
| Agarwal, 2005, Singapore [24] | Hospital-based Retrospective | 1998–1999 | Maternal age, sex, date of delivery, race, plurality and parity | IVF/ICSI, ICSI alone |
(S) 41 (M) 35 |
(S) 147 (M)114 |
7 |
| Ai, 2005, China [25] | Hospital-based Retrospective | 1998–2004 | No | IVF/ICSI | (M) 47 | (M) 98 | 6 |
| Apantaku, 2008, UK [26] | Hospital-based Retrospective | 1999–2004 | Maternal age, parity | IVF/ICSI | (S) 88 | (S) 88 | 8 |
| Aydin, 2016, Turkey [27] | Hospital-based Retrospective | 2007–2010 | Maternal age | IVF/ICSI | (M)137 | (M) 133 | 8 |
| Barda, 2017, Israel [28] | Hospital-based Retrospective | 2009–2015 | No | IVF/ICSI | (M) 449 | (M) 259 | 6 |
| Barua, 2016, Australia [29] | Hospital-based Retrospective | 2007–2010 | No | IVF/ICSI, ICSI alone | (S) 470 | (S) 48654 | 6 |
| Beltran Anzola, 2019, France [30] | Hospital-based Retrospective | 1995–2015 | Maternal age, exact year of birth, parity, sex | IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer | (S) 2327 | (S) 6981 | 7 |
| Bensdorp, 2016, Netherlands [31] (a) | Population-based Retrospective | 2000–2012 | Zygosity, parity, socioeconomic status, conception method | IVF/ICSI, ICSI alone | (M) 2437 | (M) 3276 | 7 |
| Beyer, 2016, Germany [32] | Hospital-based Retrospective | N/A (13-year period) | No | IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer | (S) 467 | (S) 6417 | 6 |
| Carbone, 2011, UK [33] | Hospital-based Prospective | 2006–2009 | No | IVF/ICSI | (S) 426 | (S) 26538 | 7 |
| Caserta, 2008, Italy [34] |
Hospital-based Prospective |
2004–2006 | Parity, age, height, weight, ethnic origin, smoking, history of infertility | IVF/ICSI, ICSI alone | (S) 364 | (S) 304 | 3 |
| Caserta, 2014, Italy [35] | Hospital-based Retrospective | 2007–2011 | No | IVF/ICSI | (M) 138 | (M) 207 | 7 |
| Choi, 2006, Korea [36] | Hospital-based Retrospective | 1994–2003 | No | IVF/ICSI | (M) 190 | (M) 347 | 6 |
| Daniel, 2000, Israel [37] | Hospital-based Retrospective | 1996–1997 | No | IVF/ICSI | (M) 104 | (M) 121 | 7 |
| Dayan, 2015, Canada [38] | Hospital-based Retrospective | 2001–2008 | No | IVF/ICSI | (S) 326 | (S) 9175 | 7 |
| Dayan, 2016, Canada [39] | Population-based Retrospective | 2006–2012 | No | IVF/ICSI | (S) 5371 | (S) 795997 | 7 |
| Dayan, 2018, Canada [40] | Population-based Retrospective | 2013–2014 | No | IVF/ICSI | (S) 1596 | (S) 112813 | 6 |
| Deltombe-Bodart, 2017, France [41] | Hospital-based Retrospective | 1997–2014 | No | IVF/ICSI, ICSI alone | (M) 360 | (M) 986 | 6 |
| Dior, 2018, Israel [42] | Hospital-based Retrospective | 1995–2012 | No | Oocyte Donation | (S) 135 | (S) 270 | 7 |
| Elenis, 2015, Sweden [43] | Population-based Retrospective | 2005–2008 | Age | IVF/ICSI, Oocyte Donation | (S) 139 | (M) 150 | 7 |
| Ernstad, 2019, Sweden [44] | Population-based Retrospective | 2005–2015 | No | IVF/ICSI, Fresh embryo transfer | (S) 34091 | (S) 1127566 | 6 |
| Fan, 2013, China [45] | Hospital-based Retrospective | 2010–2013 | No | IVF/ICSI, ICSI alone | (M) 162 | (M) 213 | 7 |
| Farhi, 2013, Israel [46] | Hospital-based Prospective | 2006–2008 | No | IVF/ICSI, ICSI alone | (S) 509 | (S) 587 | 5 |
| Geipel, 2001, Germany [47] | Hospital-based Retrospective | 1995–1999 | Maternal age, parity, plurality | IVF/ICSI, ICSI alone |
(S) 114 (M) 32 |
(S) 114 (M) 32 |
8 |
| Gocmen, 2015, Turkey [48] | Hospital-based Retrospective | 2011–2014 | No | IVF/ICSI | (M) 19 | (M) 65 | 8 |
| Gojnic, 2005, Serbia [49] | N/A | N/A | Age, education, parity | IVF/ICSI | (M) 120 | (M) 120 | 2 |
| Hessami, 2020, Iran [50] | Hospital-based Retrospective | 2013–2018 | No | IVF/ICSI | (M) 202 | (M) 449 | 6 |
| Howe, 1990, US [51] | Hospital-based Retrospective | N/A (first 100 clinical pregnancies conceived in the IVF program) | Age, race, parity, pre-existing medical problem, DES exposure, insurance status | IVF/ICSI | (S)54 | (S)54 | 7 |
| Jancar, 2018, Slovenia [52] | Population-based Retrospective | 2002–2015 | No | IVF/ICSI | (S) 5837 | (S) 261881 | 7 |
| Jeve, 2016, UK [53] | Hospital-based Retrospective | 2007–2014 | Age | IVF/ICSI, Oocyte donation | (S) 90 | (S) 45 | 8 |
| Katalinic, 2004, Germany [54] | Hospital-based Prospective | 1998–2000; 1993–2001 (control) | No | IVF/ICSI, ICSI alone, Fresh embryo transfer |
(S) 2055 (M) 632 |
(S) 7861 (M) 77 |
6 |
| Koivurova, 2002, Finland [55] | Population-based Retrospective | 1990–1995 | Sex of the child, birth year, area of residence, parity, maternal age, social class (defined by occupation) | IVF/ICSI, Fresh embryo transfer |
(S) 153 (M) 62 |
(S) 580 (M) 82 |
7 |
| Korosec, 2016, Slovenia [56] | Hospital-based Retrospective | 2004–2011 | Age, parity, hospital | IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer | (S)1127 | (S) 3381 | 7 |
| Kouhkan, 2018, Iran [57] | Hospital-based Prospective | 2014–2017 | No | IVF/ICSI | (S) 260 | (S) 314 | 7 |
| Kuivasaari- Pirinen, 2012, Finland [58] | Hospital-based Retrospective | 1996–2007 | No | IVF/ICSI | (S) 255 | (S) 26870 | 7 |
| Lee, 2015, US [59] | Hospital-based Retrospective | 2007–2009 | No | IVF/ICSI | (S) 108 | (S) 2284 | 7 |
| Lei, 2019, China [60] | Hospital-based Retrospective | 2013–2015 | No | IVF/ICSI |
(S) 1453 (M) 803 |
(S) 6667 (M) 101 |
6 |
| Li, 2015, China [61] | Hospital-based Retrospective | 2009–2011 | No | IVF/ICSI | (M) 108 | (M) 144 | 6 |
| Luke, 2019, US [62] | Population-based Retrospective | 2004–2013 (depending on states) | No | IVF/ICSI, Oocyte donation | (M) 58,920 | (M) 34,033 | 7 |
| Luke, 2020, US [63] | Population-based Retrospective | 2004–2013 (depending on states) | No |
IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer, Oocyte donation |
(S) 83582 | (S) 1382311 | 6 |
| Malchau, 2013, Denmark [64] | Population-based Retrospective | 1995–2010 | Date and year of birth | IVF/ICSI, ICSI alone, Oocyte donation |
(S) 15741 (M) 8564 |
(S) 31010 (M) 25,012 |
7 |
| Martinez-Varea, 2015, Spain [65] | Hospital-based Prospective | N/A | No | IVF/ICSI, Oocyte donation | (S) 50 | (S) 25 | 6 |
| Meyer, 2020, Israel [66] | Hospital-based Retrospective | 2011–2018 | Age | Oocyte donation | (S) 159 | (S) 73 | 7 |
| Mohammed, 2012, Qatar [67] | Hospital-based Retrospective | 2002–2011 | No | IVF/ICSI | (M) 145 | (M) 175 | 7 |
| Moini, 2012, Iran [68] | Hospital-based Prospective | 2008–2010 | No | IVF/ICSI, ICSI alone | (M) 230 | (M) 170 | 6 |
| Nagata, 2019, Japan [69] | Population-based Retrospective | 2011–2014 | No | IVF/ICSI, ICSI alone |
(S) 2993 (M) 129 |
(S) 88873 (M) 625 |
7 |
| Nassar, 2003, Lebanon [70] | Hospital-based Retrospective | 1995–2000 | Age, parity | IVF/ICSI | (M) 56 | (M) 112 | 9 |
| Nejdet, 2016, Sweden [71] | Population-based Retrospective | 2003–2012 | No | IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer, Oocyte donation | (S) 27084 | (S) 999804 | 7 |
| Ochsenkuehn, 2003, Germany [72] | Hospital-based Retrospective | 1991–1996 | Maternal age, gestational age, parity | IVF/ICSI |
(S) 163 (M) 65 |
(S) 322 (M) 78 |
8 |
| Okby, 2018, Israel [73] | Population-based Retrospective | 1988–2010 | No | IVF/ICSI | (M) 465 | (M) 3053 | 8 |
| Olivennes, 1993, France [74] | Hospital-based Retrospective | 1987–1989 | No | IVF/ICSI | (S) 162 | (S) 5096 | 6 |
| Opdahl, 2015, Sweden, Denmark, Norway [75] | Population-based Retrospective | 1988–2007 | Parity, birth year | IVF/ICSI |
(S) 47088 (M) 10,918 |
(S) 268599 (M) 46,674 |
9 |
| Poikkeus, 2007, Finland [76] | Hospital-based Retrospective | 1997–2003 | Year, place of residence | IVF/ICSI, Fresh embryo transfer | (S) 499 | (S) 15037 | 7 |
| Qin, 2017, China [77] | Hospital-based Prospective | 2013–2016 | No | IVF/ICSI | (S) 1260 | (S) 2480 | 6 |
| Raisanen, 2013, Finland [78] | Population-based Retrospective | 2006–2010 | No | IVF/ICSI | (S) 5647 | (S) 285357 | 7 |
| Reismullerova, 2015, Slovakia [79] | Hospital-based Retrospective | N/A | No | IVF/ICSI | (S) 526 | (S) 15874 | 7 |
| Reubinoff, 1997, Israel [80] | Hospital-based Retrospective | 1983–1993 | Maternal ethnic origin, age, parity, location and date of delivery | IVF/ICSI | (S) 260 | (S) 260 | 7 |
| Rizzo, 2016, Italy [81] | Hospital-based Prospective | 2007–2014 | Maternal age | IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer | (S) 266 | (S) 266 | 9 |
| Rizzo, 2016, Italy [2] [82] | Hospital-based Prospective | 2007–2014 | Maternal age | IVF/ICSI, Oocyte donation | (S) 109 | (S) 498 | 9 |
| Sazonova, 2012, Sweden [83] | Population-based Retrospective | 2002–2006 | No | IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer | (S) 11292 | (S) 571914 | 7 |
| Shi, 2018, China [84] | Hospital-based Retrospective | 2013–2016 | No | IVF/ICSI | (M) 850 | (M) 250 | 5 |
| Shiloh, 2019, Israel [85] | Population-based Retrospective | 1991–2014 | No | IVF/ICSI | (S) 2603 | (S) 237863 | 6 |
| Silberstein, 2014, Israel [86] | Hospital-based Retrospective | 1988–2006 | No | IVF/ICSI | (S) 1294 | (S) 171513 | 6 |
| Stojnic, 2013, Serbia [87] | Hospital-based Retrospective | 2006–2010 | Maternal age, parity, education, time and place of delivery, BMI | IVF/ICSI, Fresh embryo transfer | (S) 634 | (S)634 | 7 |
| Sun, 2009, Canada [88] | Population-based Retrospective | 2004–2007 | Maternal age, parity | IVF/ICSI | (S) 870 | (S) 3433 | 9 |
| Sun, 2016, China [89] | Hospital-based Retrospective | 2010–2014 | No | IVF/ICSI | (M) 411 | (M) 742 | 7 |
| Suzuki, 2010, Japan [90] | Hospital-based Retrospective | 2000–2007 | No | IVF/ICSI | (M) 64 | (M) 76 | 6 |
| Szymusik, 2012, Poland [91] | Hospital-based Retrospective | 2005–2009 | No | IVF/ICSI, Fresh embryo transfer | (M) 43 | (M)83 | 4 |
| Szymusik, 2018, Poland [92] | Hospital-based Prospective | 2013–2016 | No | IVF/ICSI | (S) 183 | (S) 368 | 4 |
| Tan, 1992, UK [93] | Hospital-based Retrospective | 1978–1987 | Maternal age | IVF/ICSI |
(S) 494 (M) 125 |
(S) 978 (M) 21 |
6 |
| Tandberg, 2015, Norway [94] | Population-based Retrospective | 1988–2009 | Parity | IVF/ICSI | (S) 12440 | (S) 1097084 | 8 |
| Tomic, 2011, Croatia [95] | Hospital-based Retrospective | 2006–2009 | Ethnicity, age, gravidity, smoking habits, BMI, weight gain in pregnancy, site and time of delivery | IVF/ICSI, Fresh embryo transfer | (S) 283 | (S) 283 | 7 |
| Valenzuela-Alcaraz, 2013, Spain [96] | Hospital-based Prospective | N/A | Age | IVF/ICSI | (S) 100 | (S) 100 | 6 |
| Valenzuela-Alcaraz, 2018, Spain [97] | Hospital-based Prospective | 2014–2016 | No | IVF/ICSI | (M) 50 | (M) 50 | 7 |
| Vasario, 2012, Italy [98] | Hospital-based Prospective | 2004–2008 | No | IVF/ICSI | (M) 84 | (M) 139 | 6 |
| Von Versen-Hoynck, 2019, US [99] | Hospital-based Prospective | 2011–2017 | No | IVF/ICSI, Fresh embryo transfer, Frozen embryo transfer | (S) 367 | (S)143 | 5 |
| Watanabe, 2014, Japan [100] | Hospital-based Retrospective | 2009–2011 | Closest propensity score (accounting for 27 maternal and paternal variables) | IVF/ICSI | (S) 474 | (S) 474 | 9 |
| Wennberg, 2016, Sweden, Denmark, Finland, Norway [101] | Population-based Retrospective | 1982–2007 | Parity, year and month of birth | IVF/ICSI, Fresh embryo transfer | (S) 39919 | (S) 260166 | 7 |
|
Wu, 2010, China [102] |
Hospital-based Retrospective | 2006–2008 | No | IVF/ICSI | (M) 204 | (M) 255 | 4 |
| Xu, 2005, China [103] | Hospital-based Retrospective | 2001–2003 | No | IVF/ICSI | (M) 41 | (M) 44 | 4 |
| Yang, 2011, Korea [104] | Hospital-based Retrospective | 1995–2008 | No | IVF/ICSI | (M) 67 | (M) 143 | 7 |
| Yang, 2014, China [105] | Population-based Retrospective | 2011 | No | IVF/ICSI |
(S) 825 (M) 314 |
(S) 109971 (M) 1473 |
6 |
| Zadori, 2003, Hungary [106] | Hospital-based Retrospective | 1995–2002 | Maternal age, parity, gravidity, previous obstetrics outcomes | IVF/ICSI |
(S) 185 (M) 36 |
(S) 185 (M) 36 |
6 |
| Zhang, 2015, China [107] | Hospital-based Retrospective | 2010–2014 | No | IVF/ICSI | (M) 53 | (M) 128 | 6 |
| Zhu, 2016, China [108] | Hospital-based Retrospective | 2006–2014 | Maternal age, birth year | IVF/ICSI |
(S) 1659 (M) 982 |
(S) 5193 (M) 89 |
9 |
Specific outcomes
IVF/ICSI singleton pregnancies
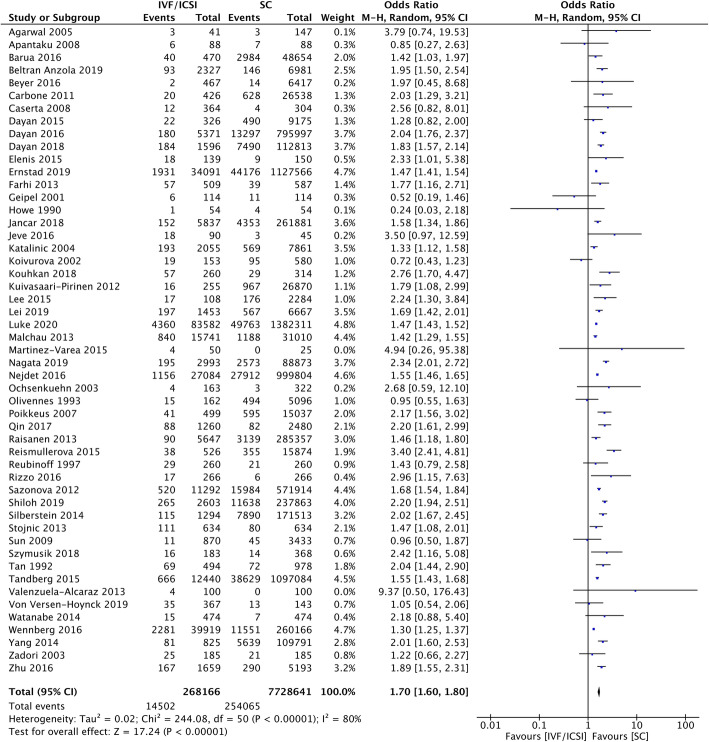
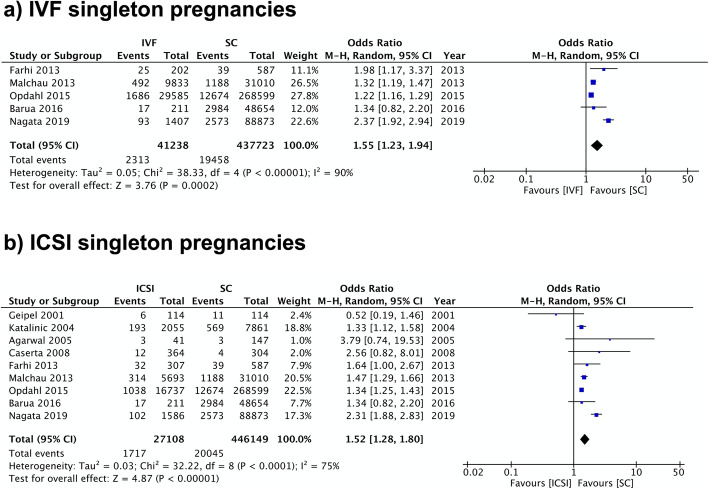
Fifty-one studies including 268,166 pregnancies in the IVF/ICSI group and 7.7 million pregnancies in the SC group were included in the analysis of HDP. The overall odds ratio (OR) was 1.70 (95% CI 1.60–1.80) with high heterogeneity (I2 = 80%) (Fig. 2). Almost all studies were of high or moderate quality according to their NOS scores; four were categorized as low quality. A separate analysis of 5 studies that included only IVF pregnancies yielded consistent findings with an OR of 1.55 (95% CI 1.23–1.94; I2 = 90%) (Fig. 3).
Fig. 2.
IVF/ICSI Singleton Pregnancies Meta-analysis. Forest plot comparing hypertensive disorders of pregnancy in IVF/ICSI singleton pregnancies in comparison to spontaneous pregnancies
Fig. 3.
IVF and ICSI Singleton Pregnancies Meta-analysis. Forest plot comparing hypertensive disorders of pregnancy in a) IVF singleton pregnancies and b) ICSI singleton pregnancies in comparison to spontaneous pregnancies
Of those studies above, 28 studies specifically reported the incidence of preeclampsia, resulting in a sample size of 118,787 pregnancies in the IVF/ICSI group and 4.5 million pregnancies in the SC group. Cumulative incidences found that the IVF/ICSI group had significantly higher odds of preeclampsia than the SC group with an OR of 1.59 (95% CI 1.46–1.74) with high heterogeneity (I2 = 70%) (Additional file 5). Twenty-seven studies were classified as high or moderate quality, and one study received an NOS score of low quality. Half of the included studies matched the control group with IVF/ICSI group by maternal factors such as age or parity. Only one study studying preeclampsia explicitly excluded ICSI pregnancies and therefore a sub-analysis for IVF could not be conducted.
The sub-analysis of nine studies that included ICSI pregnancies only found that this type of procedure had a higher rate of HDP in comparison to SC. The resulting OR was 1.52 (95% CI 1.28–1.80; I2 = 75%) (Fig. 3). In the case of preeclampsia, only two studies were eligible with no significant difference between the two groups (OR 0.98, 95% CI 0.38–2.51; I2 = 72%) (Additional file 5). Both pooled analyses showed high heterogeneity.
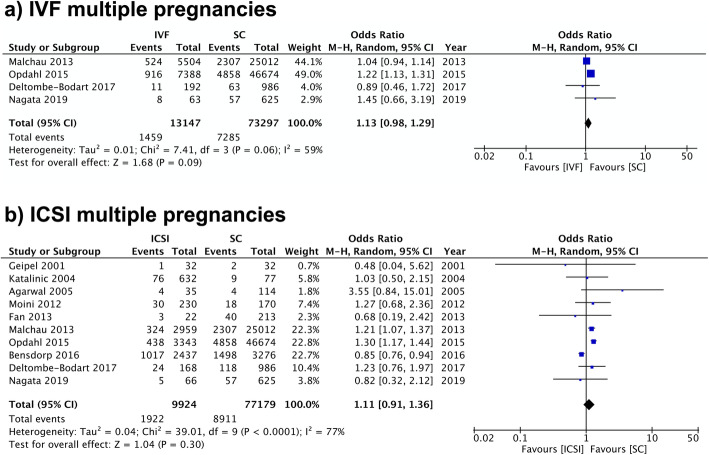
IVF/ICSI multiple pregnancies
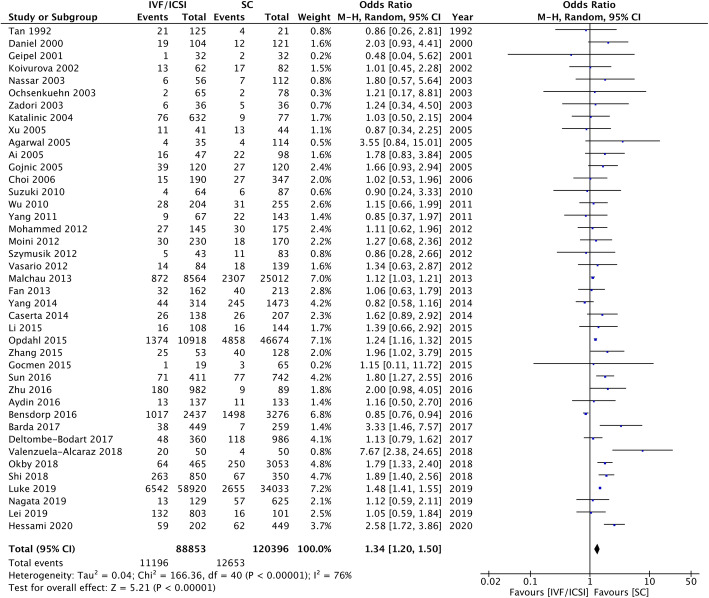
Forty-one studies assessed HDP in multiple pregnancies. Higher odds were observed in the IVF/ICSI group than the SC group, with an OR of 1.34 (95% CI 1.20–1.50) with high heterogeneity (I2 = 76%) (Fig. 4). The number of studies that were rated as high, moderate, and low quality by NOS scores were 8, 28, and 5 respectively.
Fig. 4.
IVF/ICSI Multiple Pregnancies Meta-analysis. Forest plot comparing hypertensive disorders of pregnancy in IVF/ICSI multiple pregnancies in comparison to spontaneous pregnancies
Seventeen studies assessed preeclampsia as the outcome of interest. The odds of preeclampsia were higher in pregnancies resulting from IVF/ICSI than SC (OR 1.24, 95% CI 1.08–1.43) with low heterogeneity (I2 = 32%) (Additional file 5). All studies either had moderate or high quality.
For IVF pregnancies, four and two studies looked at HDP and preeclampsia respectively. Both showed slightly increased odds in the ART groups in comparison to SC; however, the differences were insignificant (HDP: OR 1.13, 95% CI 0.98–1.29; I2 = 59%. Preeclampsia: OR 1.04, 95% CI 0.93–1.16; I2 = 0%) (Fig. 5, Additional file 5).
Fig. 5.
IVF and ICSI Multiple Pregnancies Meta-analysis Forest plot comparing hypertensive disorders of pregnancy in a) IVF multiple pregnancies and b) ICSI multiple pregnancies in comparison to spontaneous pregnancies
For ICSI multiple pregnancies, 10 studies were eligible for the analysis of HDP. Overall, no increase in the risk of HDP was observed in the exposure group (OR 1.11, 95% CI 0.91–1.36; I2 = 77%) (Fig. 3). Five studies reported data on preeclampsia in multiple pregnancies after ICSI. The odds of preeclampsia were slightly higher in pregnancies resulting from ICSI than SC (OR 1.11, 95% CI 1.00–1.24) in the pooled analysis with no heterogeneity (I2 = 0%) (Additional file 5).
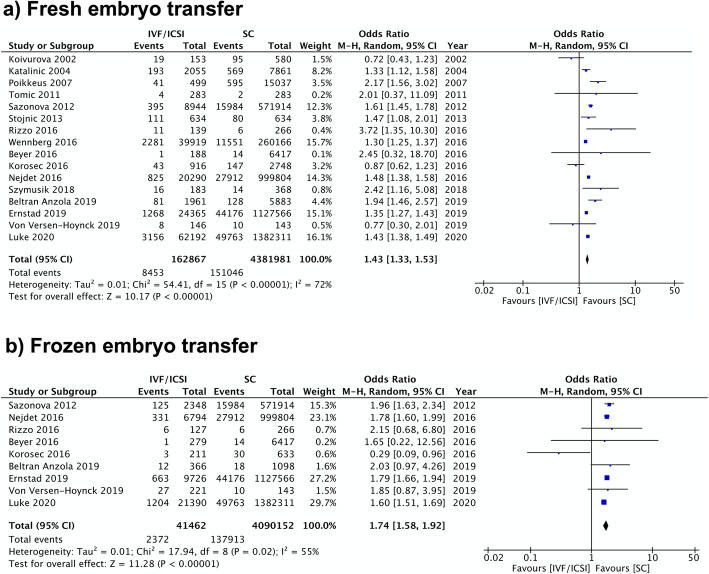
Fresh and frozen embryo transfer
Sixteen studies reported on the relationship between fresh ET and HDP. The pooled result showed that, when compared to SC, fresh ET is associated with increased odds of HDP with an OR of 1.43 (95% CI 1.33–1.53; I2 = 72%) (Fig. 6). A similar finding was also found in the pooled result of eight studies using preeclampsia as the outcome of interest (OR 1.48, 95% CI 1.37–1.60) with low heterogeneity (I2 = 39%) (Additional file 5).
Fig. 6.
Fresh and Frozen Embryo Transfer Meta-analysis. Forest plot comparing hypertensive disorders of pregnancy in singleton pregnancies resulting from a) fresh embryo transfer or b) frozen embryo transfer in comparison to spontaneous pregnancies
FET was also associated with higher odds of HDP and preeclampsia compared to SC. Nine studies were included resulting in a pooled OR of 1.74 (95% CI 1.58–1.92; I2 = 55%) for HDP (Fig. 6). Comparably, in five studies that studied preeclampsia, the OR was 1.82 (95% CI 1.71–1.95) with no heterogeneity between included studies (I2 = 0%) (Additional file 5).
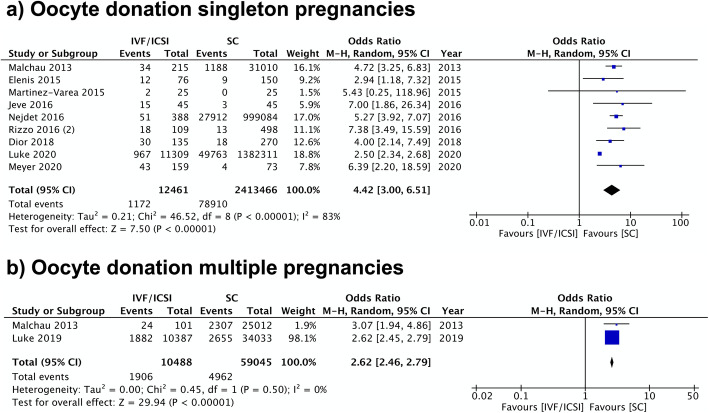
Oocyte donation
Pregnancies resulting from OD were found to have the highest risk of hypertensive complications of all analyses conducted for the study. For HDP, nine studies resulted in a pooled OR of 4.42 (95% CI 3.00–6.51; I2 = 83%) (Fig. 7). Similar findings were observed in multiple pregnancies, with an OR of 2.62 (95% CI 2.46–2.79) with no heterogeneity (I2 = 0%). However, only two studies were eligible (Fig. 7).
Fig. 7.
Oocyte Donation Meta-analysis. Forest plot comparing hypertensive disorders of pregnancy in a) singleton pregnancies or b) multiple pregnancies resulting from oocyte donation in comparison to spontaneous pregnancies
Seven studies studied preeclampsia as the outcome of interest. The resulting OR was 5.20 (95% CI 4.02–6.73) with low heterogeneity (I2 = 9%) (Additional file 5). All included studies had a moderate or high quality according to their NOS scores.
Overall, all IVF/ICSI groups were associated with increased odds of HDP in comparison to SC; however, the differences were insignificant when selected IVF and ICSI multiple pregnancies were analyzed separately. Similarly, all study groups except ICSI singleton pregnancies were associated with higher odds of preeclampsia. The difference between IVF multiple pregnancies and SC was small and insignificant. All findings described above were summarized in Table 2.
Table 2.
Summary of results by type of ART and outcome of interest
| Experimental | Number of studies | ART study size (n) | SC study size (n) | OR; 95% CI | I2 (%) | P value | NNH (n) |
|---|---|---|---|---|---|---|---|
| Hypertensive Disorders of Pregnancy | |||||||
| IVF/ICSI singleton | 51 | 268,166 | 7,728,641 | 1.70 (1.60–1.80) | 80 | < 0.01a | 47.2 |
| IVF singleton | 5 | 41,238 | 437,723 | 1.55 (1.23–1.94) | 90 | < 0.01a | 85.9 |
| ICSI singleton | 9 | 27,108 | 446,149 | 1.52 (1.28–1.80) | 75 | < 0.01a | 54.3 |
| IVF/ICSI multiple | 41 | 88,853 | 120,396 | 1.34 (1.20–1.50) | 76 | < 0.01a | 47.8 |
| IVF multiple | 4 | 13,147 | 73,297 | 1.13 (0.98–1.29) | 59 | 0.09 | 86.3 |
| ICSI multiple | 10 | 9924 | 77,179 | 1.11 (0.91–1.36) | 77 | 0.30 | 12.8 |
| Fresh embryo transfer singleton | 16 | 162,867 | 4,381,981 | 1.43 (1.33–1.53) | 72 | < 0.01a | 57.4 |
| Frozen embryo transfer singleton | 9 | 41,462 | 4,090,152 | 1.74 (1.58–1.92) | 55 | < 0.01a | 52.6 |
| Oocyte donation singleton | 9 | 12,461 | 2,413,466 | 4.42 (3.00–6.51) | 83 | < 0.01a | 16.3 |
| Oocyte donation multiple | 2 | 10,488 | 59,045 | 2.62 (2.46–2.79) | 0 | < 0.01a | 10.2 |
| Preeclampsia | |||||||
| IVF/ICSI singleton | 28 | 118,787 | 4,509,905 | 1.59 (1.46–1.74) | 70 | < 0.01a | 81.0 |
| ICSI singleton | 2 | 5807 | 31,124 | 0.98 (0.38–2.51) | 72 | 0.97 | 105.2 |
| IVF/ICSI multiple | 17 | 14,842 | 34,337 | 1.24 (1.08–1.43) | 32 | < 0.01a | 91.7 |
| IVF multiple | 2 | 5696 | 25,998 | 1.04 (0.93–1.16) | 0 | 0.47 | 364.0 |
| ICSI multiple | 5 | 5618 | 29,519 | 1.11 (1.00–1.24) | 0 | 0.05a | 52.1 |
| Fresh embryo transfer singleton | 8 | 55,300 | 2,715,647 | 1.48 (1.37–1.60) | 39 | < 0.01a | 85.5 |
| Frozen embryo transfer singleton | 5 | 19,216 | 2,699,693 | 1.82 (1.71–1.95) | 0 | < 0.01a | 45.6 |
| Oocyte donation singleton | 7 | 1107 | 1,031,110 | 5.20 (4.02–6.73) | 9 | < 0.01a | 10.1 |
a= Statistically significant. NNH Numbers needed to harm
Numbers needed to harm
The numbers needed to harm for each IVF/ICSI subgroups were shown in Table 2. While most interventions had an NNH of 40 to 100, OD pregnancies had particularly low NNH. Sixteen patients who achieved singleton pregnancies through OD were needed to have one case of HDP; similarly, only ten multiple pregnancy patients through OD were needed to have one case of HDP.
Sensitivity analysis and publication bias
Sensitivity analyses were performed to identify individual studies with large influences on the overall risk estimates. Exclusion of any study did not yield significantly different OR, with the exception of one study by Malchau et al. for the analysis of preeclampsia in IVF multiple pregnancies (Additional file 6). Funnel plots of meta-analyses involving more than 10 studies did not reveal any publication bias (Additional file 5).
Discussion
Principle findings
IVF/ICSI pregnancies, when compared to SC, carried higher odds of HDP and preeclampsia regardless of their plurality. While both fresh ET and FET were found to have odds of hypertensive complications, FET was associated with higher odds in comparison fresh ET. Pregnancies resulting from OD had the highest odds of HDP and preeclampsia out of all the groups analyzed.
Analyses on IVF and ICSI pregnancies specifically yielded mixed results and were limited by a relatively small number of studies. Although IVF pregnancies had higher incidences of HDP in comparison to SC, the difference was not statistically significant for multiple pregnancies. While ICSI singleton pregnancies resulted in higher odds of HDP compared to SC, the odds of preeclampsia were similar in both groups. In multiple pregnancy, although ICSI was associated with increased odds of preeclampsia, the difference with SC was small.
Comparison with existing literature
Our results of higher odds of HDP and preeclampsia in singleton pregnancies following IVF/ICSI were in accordance with other previous meta-analyses [7, 10, 13, 14]. The measures of association for HDP in IVF/ICSI pregnancies (OR 1.70; 95% CI 1.60–1.80) was comparable to meta-analyses by Panday et al. (RR 1.49; 95% CI 1.39–1.59) and Qin et al. (RR 1.30; 95% CI 1.04–1.62) with a significant increase in statistical power due to a high number of studies included (51 studies) [7, 14]. Although the most recent meta-analysis by Thomopoulos et al. did not report data for IVF/ICSI pregnancies, the estimated relative risk for HDP in IVF pregnancies (RR 1.45; 95% CI 1.26–1.68) was similar to our study result (RR 1.55; 95% CI 1.23–1.94) [10]. This comprehensive systemic review included some cohort studies with mixed gestational orders, which was a strict exclusion criterion for our meta-analysis to minimize confounding bias. Our study results could not be directly compared to those by Almasi-Hashiani et al. as the study also included fertility treatments other than IVF/ICSI in the exposure group [13].
Multiple pregnancy following IVF/ICSI was also found to be at higher odds for preeclampsia and HDP, although the differences were smaller. This was likely because HDP and preeclampsia are known have a higher prevalence in multiple pregnancies than singleton pregnancies, resulting in higher risks of hypertensive complications in both SC and IVF/ICSI groups [68]. The meta-analysis by Qin et al. published in 2015 comprehensively studied the risk of HDP in IVF/ICSI multiple pregnancy. However, the study included other fertility treatments, such as ovulation induction and intrauterine insemination, in the control group, potentially underestimating the risk of IVF/ICSI on the outcome of interest. This might explain the slightly lower relative risk (RR 1.13; 95% CI 1.02–1.26) in comparison to our findings (OR 1.34; 95% CI 1.20–1.50) [9].
When studying ICSI pregnancies separately, our study found varying results for preeclampsia and HDP, and the findings were consistent with those reported by Thomopoulos and colleagues. While the meta-analysis by Thomopoulos et al. was limited by the small number of available studies, our sub-analysis included more recent studies and yielded similar findings [10]. Further research on ICSI pregnancies, as well as their indications for the procedure, such as male infertility, should be conducted to identify potential contributing factors.
Although the mechanism by which IVF/ICSI increases the risk of preeclampsia remains unknown, there exists a great body of literature studying potential causes. First, baseline maternal characteristics such as advanced maternal age, obesity, and medical comorbidities vary between the exposure and control groups and have been shown to be associated with preeclampsia [38, 94]. The underlying infertility diagnosis may also lead to varying maternal and perinatal outcomes. A large-scale cohort study by Stern and colleagues found that women with infertility due to tubal factors and ovulation disorders may be at a particularly high risk of HDP in comparison to their respective SC groups with the same diagnoses [8]. Patients with endometriosis may carry a higher risk of HDP than the general population, although the risk is attenuated when specifically studying women with ART [109]. Together, these findings suggest that an individual’s risk of HDP must be evaluated based on the mode of conception, along with other patient factors and comorbidities.
The presence of significant differences in the outcomes between fresh ET, FET, and OD pregnancies highlighted the potential role of the procedure on the development of hypertensive complications. In recent years, the use of cryopreservation has expanded widely from women with medical indications (e.g. with medical conditions or treatments that impairs fertility) to social embryo and oocyte freezing, including women who prefer to defer childbearing and transgender people as a part of their medical transition process [110]. There is a growing interest in the “freeze-all” strategy as a result of its decreased incidence of ovarian hyperstimulation syndrome without compromising live birth rates [110]. While FET carries many unique advantages, it is still important to understand the associated perinatal outcomes with the procedure. Our review of all available literature showed that both fresh ET and FET were associated with increased risks of preeclampsia and HDP in comparison to SC; furthermore, the observed differences were greater in FET pregnancies than fresh ET pregnancies, which was consistent with earlier evidence [17, 111, 112]. It is worth noting that most past literature used fresh ET as a control group instead, making it difficult to directly compare the results between studies.
The observed difference between fresh ET and FET may be explained by the absence of a corpus luteum (CL), as suggested by five recently published cohort studies [44, 99, 113–115]. SC typically develop under the presence of one CL, while the number of CL for IVF pregnancies varies depending on the type of procedure. Fresh IVF cycles typically involve more than one CL, whereas frozen IVF cycles and OD are usually performed under programmed cycles with exogenous hormones in the absence of a CL [113, 115].
von Versen-Hoynck and colleagues performed a prospective cohort study showing that women who conceived without a CL had a higher risk of preeclampsia than women with one or more CL. In the same paper, further analysis also demonstrated that programmed FET pregnancies were associated with higher incidences of preeclampsia than natural FET pregnancies, which had a comparable risk as fresh IVF pregnancies [99]. This finding was again supported by Luke and colleagues [115]. These studies proposed that performing FET during a natural cycle or with supplementation of missing hormones, such as relaxin, may potentially reduce the risk of preeclampsia and HDP. However, this theory alone could not explain the increased risk in fresh ET in comparison to SC, suggesting that there may be other components of the procedure or unaccounted confounders that increased the risk of hypertensive complications. As fresh ET pregnancies were found to be associated with other perinatal complications such as low birth weight and small for gestational age, it is important to balance the risks and benefits with each patient’s health status when considering treatment options [116].
OD is becoming a common standard practice for patients with reproductive disorders, diminished ovarian reserve, or advanced maternal age due to its relatively high success rate and comparable live delivery rates in comparison to autologous IVF pregnancies [117, 118]. In our study, women who achieved singleton pregnancies from donated oocytes carried four- to five-fold odds of preeclampsia and HDP in comparison to women who achieved pregnancy through SC. Multiple pregnancies from OD also had higher odds, although the differences with spontaneous multiple pregnancies were smaller.
This finding was consistent with other previous systematic reviews and meta-analyses that used SC as the control group [5, 119, 120]. Pecks et al. reported the OR for HDP in OD pregnancies in comparison to SC to be 6.60 (95% CI 4.55–9.57). However, some included studies did not adjust for plurality, potentially leading to an overestimation due to the known risk of multiple pregnancy. Similarly, Masoudian et al. calculated an odds ratio of 4.34 (95% CI 3.10–6.06) with studies that included both singleton and multiple pregnancies. Storgaard et al. reported an odds ratio of 2.45 (95% CI 1.53–1.93) and an odds ratio of 2.95 (95% CI 2.29–3.76) for HDP and preeclampsia, respectively. Since the most recent literature, five other cohort studies were published and included in our analyses [42, 43, 53, 63, 66].
In addition to the potential role of CL on maternal circulation, the increased risk of preeclampsia and HDP observed in women who conceived via OD has also been suggested to be a result of a heighten immunologic response between the mother and the allogenic oocyte [121]. This was formed on the basis that normal placentation requires the development of immunologic tolerance of the mother and the fetus; studies reporting an increased risk of preeclampsia in primiparous women and after a change in paternity in multiparous women further support this immunologic theory [121, 122]. Lashley and colleagues found that among successful and uncomplicated OD pregnancies, there was a higher level of human leukocyte antigen (HLA) matching between mother and fetus in than expected by chance, suggesting the role of HLA gene in the development of preeclampsia [123]. However, it is still important to consider other patient factors such as advanced maternal age, which is very common in this patient population and may also play a role to the increased risk of hypertensive disorders.
Strengths and limitations
Our findings were generally consistent with previous literature; however, this study also carries many distinctive strengths. This is the most up-to-date, large-scale meta-analysis (including 85 studies and 8.5 million pregnancies) studying the association between IVF/ICSI pregnancies and HDP. Strict inclusion and exclusion criteria were employed to focus solely on IVF/ICSI pregnancies and SC (without any fertility treatment) to provide findings that are unique to this ART procedure. Recognizing the inherent risk of HDP in multiple pregnancies, all analyses stratified patients by plurality. Two outcomes, preeclampsia and HDP, were reported separately due to their differences in risk and prognosis. Furthermore, 89.4% of all included studies were of moderate to high quality (Table 1, Additional file 4). Finally, all publications in English, Chinese, Portuguese, and French were screened and reviewed to minimize language bias.
However, there were also some limitations. IVF-specific analyses were limited by the low number of studies that explicitly excluded ICSI pregnancies. The diagnostic criteria of preeclampsia differ depending on the study period and geographical location [75]. The lack of definition for the outcomes of interest among the cohorts (43% of all included studies) also made it difficult to create a uniform definition for the meta-analysis. This issue was solved by considering the following criteria: if a study used preeclampsia as an outcome without specifying the definition, it was included in analyses for preeclampsia. If a study used terminologies such as “pregnancy-induced hypertension”, “gestational hypertension”, or “hypertensive disorder”, it was only included in analyses for HDP. A high heterogeneity was reported in many pooled analyses, likely due to differences in study populations and geographical areas. Finally, uncontrolled confounders remained to be a concern due to the nature of the study design and could also influence heterogeneity. Some of the included studies did employ a matching method when selecting control subjects, while other studies accounted for potential confounders such as age, parity, medical comorbidities, year of birth, socioeconomic status, ethnic origin, location, and cause of infertility.
Conclusions and implications
There has been an increasing amount of literature studying the relationship between ART and pregnancy and perinatal outcomes over the past decade, but there is also a lack of clinical practice guidelines for women who conceived through ART [124]. Results of our meta-analyses confirmed that IVF/ICSI pregnancies were at high odds of preeclampsia and HDP than SC, irrespective of the plurality. In particular, the odds in FET and OD pregnancies were high. Further population-based research studying different IVF treatment protocols should be considered. Health care providers should be aware of these risks and develop specific care plans and interventions for pregnancies conceived by IVF/ICSI to decrease the incidence of hypertensive complications and subsequently the risks of maternal morbidity and mortality. For example, a systematic review of randomized controlled trials by Henderson et al. suggested that daily low-dose aspirin starting after the first trimester might reduce the risk of preeclampsia [125]. The relationship between preeclampsia and ICSI singleton pregnancies remains unclear due to insufficient literature that studies this population. Given that the use of ICSI is gaining popularity over time, more research studying the pregnancy outcomes after ICSI is warranted.
Supplementary Information
Additional file 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. A list of 27 items required under the PRISMA statement with their respective locations.
Additional file 2. Search strategy for the systematic review and meta-analysis. Complete Search Strategy for a) Embase (1947 to 2020 April 08) b) Ovid MEDLINE, MEDLINE Daily and Epub Ahead of Print, In-Process & Other Non-Indexed Citations (1947 to 2020 April 08) c) EBM Reviews - Cochrane Central Register of Controlled Trials (1947 to April 2020).
Additional file 3. Excluded full text studies, with reasons. A list of full text studies excluded after screening with reasons for removal.
Additional file 4. Newcastle-Ottawa Scale for quality assessment and publication bias. A breakdown of NOS scores assigned to each study included in the meta-analysis.
Additional file 5. Forest plots for preeclampsia and funnel plots. Forest plots comparing preeclampsia in IVF/ICSI pregnancies and spontaneous pregnancies and funnel plots for publication bias in meta-analyses with 10 or more studies.
Additional file 6. Sensitivity analysis. A list of highest and lowest overall odds ratios after removing individual studies.
Acknowledgements
The authors would like to thank Sandra McKeown from Queen’s University for assisting with the search strategy for the study.
Abbreviations
- ART
Assisted reproductive technologies
- IVF
In vitro fertilization
- ICSI
Intracytoplasmic sperm injection
- Fresh ET
Fresh embryo transfer
- FET
Frozen embryo transfer
- OD
Oocyte donation
- HDP
Hypertensive disorders of pregnancy
- SC
Spontaneous conception
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- NOS
Newcastle Ottawa Scale
- OR
Odds ratio
- CL
Corpus luteum
- HLA
Human leukocyte antigen
Authors’ contributions
HC: conception of study design, screening, data collection and analysis, manuscript draft and revision. FTSE: conception of study design, screening, data collection, manuscript revision. LG: manuscript revision. MPV: conception of study design, supervision, manuscript revision, and approval of the version to be published. All authors approved the final version of the manuscript.
Funding
This study was supported by the Canadian Institutes of Health Research Institute of Human Development, Child and Youth Health (IHDCYH), Clinician Investigators Program, Grant number MFM-146444 and the Department of Obstetrics and Gynecology, Queen’s University. The funding bodies had no role in the design of the study or the collection, analysis, and interpretation of data.
Availability of data and materials
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinborg A. Short- and long-term outcomes in children born after assisted reproductive technology. Bjog. 2019;126(2):145–148. doi: 10.1111/1471-0528.15437. [DOI] [PubMed] [Google Scholar]
- 3.Hamberger L, Lundin K, Sjögren A, Söderlund B. Indications for intracytoplasmic sperm injection. Hum Reprod. 1998;13(Suppl 1):128–133. doi: 10.1093/humrep/13.suppl_1.128. [DOI] [PubMed] [Google Scholar]
- 4.Paulson RJ. Introduction: frozen 2: an update on cryopreserved embryo transfer in the era of vitrification. Fertil Steril. 2020;113(2):239–240. doi: 10.1016/j.fertnstert.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Masoudian P, Nasr A, de Nanassy J, Fung-Kee-Fung K, Bainbridge SA, El Demellawy D. Oocyte donation pregnancies and the risk of preeclampsia or gestational hypertension: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(3):328–339. doi: 10.1016/j.ajog.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103(3):551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 7.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(5):485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 8.Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil Steril. 2015;103(6):1438–1445. doi: 10.1016/j.fertnstert.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J, Wang H, Sheng X, Liang D, Tan H, Xia J. Pregnancy-related complications and adverse pregnancy outcomes in multiple pregnancies resulting from assisted reproductive technology: a meta-analysis of cohort studies. Fertil Steril. 2015;103(6):1492–1497. doi: 10.1016/j.fertnstert.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos C, Salamalekis G, Kintis K, Andrianopoulou I, Michalopoulou H, Skalis G, et al. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: overview and meta-analysis. J Clin Hypertension (Greenwich, Conn) 2017;19(2):173–183. doi: 10.1111/jch.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckmann C, Ling F, Herbert W, Laube D, Smith R. Obstetrics and gynecology. 7th edition ed. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 12.Butalia S, Audibert F, Côté AM, Firoz T, Logan AG, Magee LA, et al. Hypertension Canada's 2018 Guidelines for the Management of Hypertension in Pregnancy. Can J Cardiol. 2018;34(5):526-31. [DOI] [PubMed]
- 13.Almasi-Hashiani A, Omani-Samani R, Mohammadi M, Amini P, Navid B, Alizadeh A, Khedmati Morasae E, Maroufizadeh S. Assisted reproductive technology and the risk of preeclampsia: an updated systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):149. doi: 10.1186/s12884-019-2291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril. 2016;105(1):73–85e6. doi: 10.1016/j.fertnstert.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Helmerhorst FM, Perquin DAM, Donker D, Keirse MJNC. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328(7434):261. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okun N, Sierra S. Pregnancy outcomes after assisted human reproduction. J Obstet Gynaecol Can. 2014;36(1):64–83. doi: 10.1016/S1701-2163(15)30685-X. [DOI] [PubMed] [Google Scholar]
- 17.Roque M, Valle M, Sampaio M, Geber S. Obstetric outcomes after fresh versus frozen-thawed embryo transfers: a systematic review and meta-analysis. JBRA Assisted Reprod. 2018;22(3):253–260. doi: 10.5935/1518-0557.20180049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416–441. doi: 10.1016/S1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. [Google Scholar]
- 20.Straus SE. Individualizing treatment decisions. The likelihood of being helped or harmed. Eval Health Prof. 2002;25(2):210–224. doi: 10.1177/016327870202500206. [DOI] [PubMed] [Google Scholar]
- 21.Patsopoulos NA, Evangelou E, Ioannidis JPA. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International Journal of Epidemiology. 2008;37:1148-57. [DOI] [PMC free article] [PubMed]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21:1539-58. [DOI] [PubMed]
- 23.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical research ed). 2011;343. [DOI] [PubMed]
- 24.Agarwal P, Loh S, Lim S, Sriram B, Daniel M, Yeo S, et al. Two-year neurodevelopmental outcome in children conceived by intracytoplasmic sperm injection: prospective cohort study. BJOG. 2005;112(10):1376-83. [DOI] [PubMed]
- 25.Ai W, Liu Z. Study on the adverse outcomes in twins conceived by IVF-ET. Matern Child Health Care China. 2005;23:3119-20.
- 26.Apantaku O, Chandrasekaran I, Bentick B. Obstetric outcome of singleton pregnancies achieved with in vitro fertilisation and intracytoplasmic sperm injection: experience from a district general hospital. J Obstetrics Gynaecol. 2008;28(4):398–402. doi: 10.1080/01443610802091792. [DOI] [PubMed] [Google Scholar]
- 27.Aydin CA, Aydin S, Serdaroglu H. Multifetal gestations with assisted reproductive technique before the single-embryo transfer legislation: obstetric, neonatal outcomes and congenital anomalies. J Maternal-fetal Neonatal Med. 2016;29(15):2475–2480. doi: 10.3109/14767058.2015.1090422. [DOI] [PubMed] [Google Scholar]
- 28.Barda G, Gluck O, Mizrachi Y, Bar J. A comparison of maternal and perinatal outcome between in vitro fertilization and spontaneous dichorionic-diamniotic twin pregnancies. J Maternal-fetal Neonatal Med. 2017;30(24):2974–2977. doi: 10.1080/14767058.2016.1270934. [DOI] [PubMed] [Google Scholar]
- 29.Barua S, Hng TM, Smith H, Bradford J, Mclean M. Ovulatory disorders are an independent risk factor for pregnancy complications in women receiving assisted reproduction treatments. The Australian & New Zealand journal of obstetrics & gynaecology. 2016;57(3):286-93. [DOI] [PubMed]
- 30.Beltran AA, Pauly V, Riviere O, Sambuc R, Boyer P, Vendittelli F, et al. Birthweight of IVF children is still a current issue and still related to maternal factors. Reprod BioMed Online. 2019;39(6):990–999. doi: 10.1016/j.rbmo.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Bensdorp AJ, Hukkelhoven CW, van der Veen F, Mol BWJ, Lambalk CB, van Wely M. Dizygotic twin pregnancies after medically assisted reproduction and after natural conception: maternal and perinatal outcomes. Fertil Steril. 2016;106(2):371–377. doi: 10.1016/j.fertnstert.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 32.Beyer DA, Amari F. Maternal risk factors and neonatal outcomes after ART treatment - a German monocenter experience. Middle East Fertil Soc J. 2016;21(3):155–160. doi: 10.1016/j.mefs.2015.12.001. [DOI] [Google Scholar]
- 33.Carbone IF, Cruz JJ, Sarquis R, Akolekar R, Nicolaides KH. Assisted conception and placental perfusion assessed by uterine artery Doppler at 11-13 weeks' gestation. Human reproduction. 2011;26(7):1659-64. [DOI] [PubMed]
- 34.Caserta D, Marci R, Tatone C, Schimberni M, Vaquero E, Lazzarin N, Fazi A, Moscarini M. IVF pregnancies: neonatal outcomes after the new Italian law on assisted reproduction technology (law 40/2004) Acta Obstet Gynecol Scand. 2008;87(9):935–939. doi: 10.1080/00016340802283681. [DOI] [PubMed] [Google Scholar]
- 35.Caserta D, Bordi G, Stegagno M, Filippini F, Podagrosi M, Roselli D, et al. Maternal and perinatal outcomes in spontaneous versus assisted conception twin pregnancies. Eur J Obstet Gynecol Reprod Biol. 2014;174(64-69). [DOI] [PubMed]
- 36.Choi SJ, Kim HS, Roh CR. Pregnancy outcomes of twins after in vitro and spontaneous fertilization. Int J Gynecol Obstet. 2006;94(1):49–51. doi: 10.1016/j.ijgo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Daniel Y, Ochshorn Y, Fait G, Geva E, Bar-Am A, Lessing JB. Analysis of 104 twin pregnancies conceived with assisted reproductive technologies and 193 spontaneously conceived twin pregnancies. Fertil Steril. 2000;74(4):683–689. doi: 10.1016/S0015-0282(00)01491-6. [DOI] [PubMed] [Google Scholar]
- 38.Dayan N, Pilote L, Opatrny L, Basso O, Messerlian C, El-Messidi A, et al. Combined impact of high body mass index and in vitro fertilization on preeclampsia risk: a hospital-based cohort study. Obesity (Silver Spring, Md) 2015;23(1):200–206. doi: 10.1002/oby.20896. [DOI] [PubMed] [Google Scholar]
- 39.Dayan N, Lanes A, Walker MC, Spitzer KA, Laskin CA. Effect of chronic hypertension on assisted pregnancy outcomes: a population-based study in Ontario, Canada. Fertil Steril. 2016;105(4):1003–1009. doi: 10.1016/j.fertnstert.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 40.Dayan N, Fell DB, Guo Y, Wang H, Velez MP, Spitzer K, et al. Severe maternal morbidity in women with high BMI in IVF and unassisted singleton pregnancies. Hum Reprod (Oxford, England) 2018;33(8):1548–1556. doi: 10.1093/humrep/dey224. [DOI] [PubMed] [Google Scholar]
- 41.Deltombe-Bodart S, Deruelle P, Drumez E, Cordiez S, Catteau-Jonard S, Garabedian C. Obstetrical and perinatal complications of twin pregnancies: is there a link with the type of infertility treatment? Acta Obstet Gynecol Scand. 2017;96(7):844–851. doi: 10.1111/aogs.13135. [DOI] [PubMed] [Google Scholar]
- 42.Dior UP, Laufer N, Chill HH, Granovsky-Grisaru S, Yagel S, Yaffe H, Gielchinsky Y. Increased incidence of preeclampsia in mothers of advanced age conceiving by oocyte donation. Arch Gynecol Obstet. 2018;297(5):1293–1299. doi: 10.1007/s00404-017-4616-1. [DOI] [PubMed] [Google Scholar]
- 43.Elenis E, Svanberg AS, Lampic C, Skalkidou A, Akerud H, Sydsjo G. Adverse obstetric outcomes in pregnancies resulting from oocyte donation: a retrospective cohort case study in Sweden. BMC Pregnancy Childbirth. 2015;15(100967799):247. doi: 10.1186/s12884-015-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernstad EG, Wennerholm U-B, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: Increased risks in programmed cycles. Am J Obstet Gynecol. 2019;221(2):126. doi: 10.1016/j.ajog.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Fan C, Sun Y, Yang J, Ye J, Wang S. Maternal and neonatal outcomes in dichorionic twin pregnancies following IVF treatment: a hospital-based comparative study. International journal of clinical and experimental pathology. 2013;6(10):2199-207. [PMC free article] [PubMed]
- 46.Farhi A, Reichman B, Boyko V, Hourvitz A, Ron-El R, Lerner-Geva L. Maternal and neonatal health outcomes following assisted reproduction. Reprod BioMed Online. 2013;26(5):454–461. doi: 10.1016/j.rbmo.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Geipel A, Ludwig M, Germer U, Katalinic A, Diedrich K, Gembruch U. Uterine artery Doppler velocimetry and the outcome of pregnancies resulting from ICSI. Human reproduction. 2001;16(7):1397-402. [DOI] [PubMed]
- 48.Gocmen A, Guven S, Bagci S, Cekmez Y, Sanlikan F. Comparison of maternal and fetal outcomes of IVF and spontaneously conceived twin pregnancies: three year experience of a tertiary hospital. Int J Clin Exp Med. 2015;8(4):6272–6276. [PMC free article] [PubMed] [Google Scholar]
- 49.Gojnic M, Jeremic K, Boskovic V, Fazlagic A, Stefanovic A, Pervulov M. Perinatal outcome in multiple pregnancies - spontaneous gestation versus. Clin Exp Obstet Gynecol. 2005;32(1):65–67. [PubMed] [Google Scholar]
- 50.Hessami K, Kasraeian M, Shoushtari S, Hessami A. Maternal and Neonatal Outcomes of Monochorionic and Dichorionic Twin Pregnancies Following Assisted Reproductive Technology in Southern Iranian Women. Shiraz E-Med J. 2020;21(3).
- 51.Howe RS, Sayegh RA, Durinzi KL, Tureck RW. Perinatal outcome of singleton pregnancies conceived by in vitro fertilization: a controlled study. Journal of perinatology : official journal of the California Perinatal Association. 1990;10(3):261-6. [PubMed]
- 52.Jancar N, Mihevc Ponikvar B, Tomsic S, Vrtacnik Bokal E, Korosec S. Is IVF/ISCI an Independent Risk Factor for Spontaneous Preterm Birth in Singletons? A Population-Based Cohort Study. BioMed Research International. 2018;7124362. [DOI] [PMC free article] [PubMed]
- 53.Jeve YB, Potdar N, Opoku A, Khare M. Three-arm age-matched retrospective cohort study of obstetric outcomes of donor oocyte pregnancies. Int J Gynaecol Obstetrics. 2016;133(2):156–158. doi: 10.1016/j.ijgo.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 54.Katalinic A, Rösch C, Ludwig M, Group. GIF-US. Pregnancy course and outcome after intracytoplasmic sperm injection: a controlled, prospective cohort study. Fertility and sterility. 2004;81(6):1604-16. [DOI] [PubMed]
- 55.Koivurova S, Hartikainen AL, Karinen L, Gissler M, Hemminki E, Martikainen H, Tuomivaara L, Jarvelin MR. The course of pregnancy and delivery and the use of maternal healthcare services after standard IVF in northern Finland 1990-1995. Hum Reprod. 2002;17(11):2897–2903. doi: 10.1093/humrep/17.11.2897. [DOI] [PubMed] [Google Scholar]
- 56.Korosec S, Frangez HB, Steblovnik L, Verdenik I, Bokal EV. Independent factors influencing large-for-gestation birth weight in singletons born after in vitro fertilization. J Assist Reprod Genet. 2016;33(1):9–17. doi: 10.1007/s10815-015-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kouhkan A, Khamseh ME, Pirjani R, Moini A, Arabipoor A, Maroufizadeh S, Hosseini R, Baradaran HR. Obstetric and perinatal outcomes of singleton pregnancies conceived via assisted reproductive technology complicated by gestational diabetes mellitus: a prospective cohort study. BMC Pregnancy Childbirth. 2018;18(1):495. doi: 10.1186/s12884-018-2115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuivasaari-Pirinen P, Raatikainen K, Hippelainen M, Heinonen S. Adverse outcomes of IVF/ICSI pregnancies vary depending on aetiology of infertility. ISRN Obstetrics and Gynecology. 2012;(451915). [DOI] [PMC free article] [PubMed]
- 59.Lee MS, Cantonwine D, Little SE, McElrath TF, Parry SI, Lim K-H, et al. Angiogenic markers in pregnancies conceived through in vitro fertilization. Am J Obstet Gynecol. 2015;213(2):212. doi: 10.1016/j.ajog.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 60.Lei L-L, Lan Y-L, Wang S-Y, Feng W, Zhai Z-J. Perinatal complications and live-birth outcomes following assisted reproductive technology: a retrospective cohort study. Chin Med J. 2019;132(20):2408–2416. doi: 10.1097/CM9.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Yang J, Xu WM, Cheng D, Zou YJ. Comparison of the perinatal outcome of twins conceived after assisted reproductive technologies versus those conceived naturally. J Reprod Med. 2015;60(1):37–42. [PubMed] [Google Scholar]
- 62.Luke B, Brown MB, Wantman E, Seifer DB, Sparks AT, Lin PC, Doody KJ, van Voorhis BJ, Spector LG. Risk of prematurity and infant morbidity and mortality by maternal fertility status and plurality. J Assist Reprod Genet. 2019;36(1):121–138. doi: 10.1007/s10815-018-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luke B, Brown MB, Eisenberg ML, Callan C, Botting BJ, Pacey A, et al. In vitro fertilization and risk for hypertensive disorders of pregnancy: associations with treatment parameters. Am J Obstet Gynecol. 2020;222(4):350. doi: 10.1016/j.ajog.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malchau SS, Loft A, Larsen EC, Aaris Henningsen A-K, Rasmussen S, Andersen AN, Pinborg A. Perinatal outcomes in 375 children born after oocyte donation: a Danish national cohort study. Fertil Steril. 2013;99(6):1637–1643. doi: 10.1016/j.fertnstert.2013.01.128. [DOI] [PubMed] [Google Scholar]
- 65.Martínez-Varea A, Pellicer B, Serra V, Hervás-Marín D, Martínez-Romero A, Bellver J, et al. The Maternal Cytokine and Chemokine Profile of Naturally Conceived Gestations Is Mainly Preserved during In Vitro Fertilization and Egg Donation Pregnancies. Journal of immunology research. 2015;128616. [DOI] [PMC free article] [PubMed]
- 66.Meyer R, Orvieto R, Timerman Y, Gorodesky T, Toussia-Cohen S, Kedem A, Simchen MJ, Machtinger R. Impact of the mode of conception on gestational hypertensive disorders at very advanced maternal age. Reprod BioMed Online. 2020;40(2):281–286. doi: 10.1016/j.rbmo.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 67.Mohammed A-BF, Abdel-Maaboud M. Obstetric and neonatal outcomes of IVF versus spontaneously conceived dichorionic twins. Middle East Fertil Soc J. 2012;17(4):231–235. doi: 10.1016/j.mefs.2012.02.008. [DOI] [Google Scholar]
- 68.Moini A, Shiva M, Arabipoor A, Hosseini R, Chehrazi M, Sadeghi M. Obstetric and neonatal outcomes of twin pregnancies conceived by assisted reproductive technology compared with twin pregnancies conceived spontaneously: a prospective follow-up study. European journal of obstetrics, gynecology, and reproductive biology. 2012;165(1):29-32. [DOI] [PubMed]
- 69.Nagata C, Yang L, Yamamoto-Hanada K, Mezawa H, Ayabe T, Ishizuka K, et al. Complications and adverse outcomes in pregnancy and childbirth among women who conceived by assisted reproductive technologies: a nationwide birth cohort study of Japan environment and children's study. BMC Pregnancy Childbirth. 2019;19(1):77. doi: 10.1186/s12884-019-2213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nassar AH, Usta IM, Rechdan JB, Harb TS, Adra AM, Abu-Musa AA. Pregnancy outcome in spontaneous twins versus twins who were conceived through in vitro fertilization. Am J Obstet Gynecol. 2003;189(2):513–518. doi: 10.1067/S0002-9378(03)00485-X. [DOI] [PubMed] [Google Scholar]
- 71.Nejdet S, Bergh C, Kallen K, Wennerholm U-B, Thurin-Kjellberg A. High risks of maternal and perinatal complications in singletons born after oocyte donation. Acta Obstet Gynecol Scand. 2016;95(8):879–886. doi: 10.1111/aogs.12904. [DOI] [PubMed] [Google Scholar]
- 72.Ochsenkühn R, Strowitzki T, Gurtner M, Strauss A, Schulze A, Hepp H, et al. Pregnancy complications, obstetric risks, and neonatal outcome in singleton and twin pregnancies after GIFT and IVF. Archives of gynecology and obstetrics. 2003;268(4):256-61. [DOI] [PubMed]
- 73.Okby R, Harlev A, Sacks KN, Sergienko R, Sheiner E. Preeclampsia acts differently in in vitro fertilization versus spontaneous twins. Arch Gynecol Obstet. 2018;297(3):653–658. doi: 10.1007/s00404-017-4635-y. [DOI] [PubMed] [Google Scholar]
- 74.Olivennes F, Rufat P, André B, Pourade A, Quiros MC, Frydman R. The increased risk of complication observed in singleton pregnancies resulting from in-vitro fertilization (IVF) does not seem to be related to the IVF method itself. Human reproduction. 1993(8):1297-300. [DOI] [PubMed]
- 75.Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod (Oxford, England) 2015;30(7):1724–1731. doi: 10.1093/humrep/dev090. [DOI] [PubMed] [Google Scholar]
- 76.Poikkeus P, Gissler M, Unkila-Kallio L, Hyden-Granskog C, Tiitinen A. Obstetric and neonatal outcome after single embryo transfer. Hum Reprod (Oxford, England) 2007;22(4):1073–1079. doi: 10.1093/humrep/del492. [DOI] [PubMed] [Google Scholar]
- 77.Qin J, Sheng X, Wu D, Gao S, You Y, Yang T, et al. Adverse Obstetric Outcomes Associated With In Vitro Fertilization in Singleton Pregnancies. Reprod Sci (Thousand Oaks, Calif) 2017;24(4):595–608. doi: 10.1177/1933719116667229. [DOI] [PubMed] [Google Scholar]
- 78.Raisanen S, Randell K, Nielsen HS, Gissler M, Kramer MR, Klemetti R, et al. Socioeconomic status affects the prevalence, but not the perinatal outcomes, of in vitro fertilization pregnancies. Hum Reprod (Oxford, England) 2013;28(11):3118–3125. doi: 10.1093/humrep/det307. [DOI] [PubMed] [Google Scholar]
- 79.Reismullerova L, Holoman K, Polackova-Borosova M, Luha J. Polycystic ovary syndrome - a risk factor of pre-eclampsia after in vitro fertilisation. Bratisl Lek Listy. 2015;116(5):311–315. doi: 10.4149/bll_2015_058. [DOI] [PubMed] [Google Scholar]
- 80.Reubinoff B, Samueloff A, Ben-Haim M, Friedler S, Schenker JLA. Is the obstetric outcome of in vitro fertilized singleton gestations different from natural ones? A controlled study. Fertility and sterility. 1997;67(6):1077-83. [DOI] [PubMed]
- 81.Rizzo G, Aiello E, Pietrolucci ME, Arduini D. Are There Differences in Placental Volume and Uterine Artery Doppler in Pregnancies Resulting From the Transfer of Fresh Versus Frozen-Thawed Embryos Through In Vitro Fertilization. Reproductive sciences. 2016;23(10):1381-6. [DOI] [PubMed]
- 82.Rizzo G, Aiello E, Pietrolucci ME, Arduini D. Placental volume and uterine artery Doppler evaluation at 11 + 0 to 13 + 6 weeks' gestation in pregnancies conceived with in-vitro fertilization: comparison between autologous and donor oocyte recipients. Ultrasound Obstetrics Gynecol. 2016;47(6):726–731. doi: 10.1002/uog.14918. [DOI] [PubMed] [Google Scholar]
- 83.Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm U-B, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod (Oxford, England) 2012;27(5):1343–1350. doi: 10.1093/humrep/des036. [DOI] [PubMed] [Google Scholar]
- 84.Shi J, Zhang Y, Luo H, Han Y, Du A. Clinical outcomes of test-tube versus naturally-conceived twins. Int J Clin Exp Med. 2018;11(3):2507–2512. [Google Scholar]
- 85.Shiloh SR, Sheiner E, Wainstock T, Walfisch A, Segal I, Landau D, Harlev A. Long-term cardiovascular morbidity in children born following fertility treatment. J Pediatr. 2019;204:84–88. doi: 10.1016/j.jpeds.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 86.Silberstein T, Levy A, Harlev A, Saphier O, Sheiner E. Perinatal outcome of pregnancies following in vitro fertilization and ovulation induction. J Maternal-fetal Neonatal Med. 2014;27(13):1316–1319. doi: 10.3109/14767058.2013.856415. [DOI] [PubMed] [Google Scholar]
- 87.Stojnic J, Radunovic N, Jeremic K, Kotlica BK, Mitrovic M, Tulic I. Perinatal outcome of singleton pregnancies following in vitro fertilization. Clinical and experimental obstetrics & gynecology. 2013;40(2):277-83. [PubMed]
- 88.Sun L-M, Walker MC, Cao H-L, Yang Q, Duan T, Kingdom JCP. Assisted reproductive technology and placenta-mediated adverse pregnancy outcomes. Obstet Gynecol. 2009;114(4):818–824. doi: 10.1097/AOG.0b013e3181b76bd1. [DOI] [PubMed] [Google Scholar]
- 89.Sun L, Zou G, Wei X, Chen Y, Zhang J, Okun N, et al. Clinical outcomes after assisted reproductive technology in twin pregnancies: chorionicity-based comparison. Scientific reports. 2016;6(26869). [DOI] [PMC free article] [PubMed]
- 90.Suzuki S, Miyake H. Perinatal outcomes of elderly primiparous dichorionic twin pregnancies conceived by in vitro fertilization compared with those conceived spontaneously. Arch Gynecol Obstet. 2010;281(1):87–90. doi: 10.1007/s00404-009-1083-3. [DOI] [PubMed] [Google Scholar]
- 91.Szymusik I, Kosinska-Kaczynska K, Bomba-Opon D, Wielgos M. IVF versus spontaneous twin pregnancies - which are at higher risk of complications. J Matern Fetal Neonatal Med. 2012;25(12):2725–2728. doi: 10.3109/14767058.2012.705398. [DOI] [PubMed] [Google Scholar]
- 92.Szymusik I, Kosinski P, Kosinska-Kaczynska K, Warzecha D, Karwacka A, Kaczynski B, Wielgos M. The first trimester aneuploidy biochemical markers in IVF/ICSI patients have no additional benefit compared to spontaneous conceptions in the prediction of pregnancy complications. J Perinat Med. 2018;46(9):953–959. doi: 10.1515/jpm-2017-0199. [DOI] [PubMed] [Google Scholar]
- 93.Tan SL, Doyle P, Campbell S, Beral V, Rizk B, Brinsden P, et al. Obstetric outcome of in vitro fertilization pregnancies compared with normally conceived pregnancies. American journal of obstetrics and gynecology. 1992;167(3):778-84. [DOI] [PubMed]
- 94.Tandberg A, Klungsoyr K, Romundstad LB, Skjaerven R. Pre-eclampsia and assisted reproductive technologies: consequences of advanced maternal age, interbirth intervals, new partner and smoking habits. BJOG. 2015;122(7):915–922. doi: 10.1111/1471-0528.13051. [DOI] [PubMed] [Google Scholar]
- 95.Tomic V, Tomic J. Neonatal outcome of IVF singletons versus naturally conceived in women aged 35 years and over. Archives of gynecology and obstetrics. 2011;284(6):1411-6. [DOI] [PubMed]
- 96.Valenzuela-Alcaraz B, Crispi F, Bijnens B, Cruz-Lemini M, Creus M, Sitges M, et al. Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation. 2013;128(13):1442–50. 10.1161/CIRCULATIONAHA.113.002428. [DOI] [PubMed]
- 97.Valenzuela-Alcaraz BA-O, Cruz-Lemini M, Rodríguez-López M, Goncé A, García-Otero L, Ayuso H, et al. Fetal cardiac remodeling in twin pregnancy conceived by assisted reproductive technology. Ultrasound Obstetrics Gynecol. 2018;51(1):94–100. doi: 10.1002/uog.17527. [DOI] [PubMed] [Google Scholar]
- 98.Vasario E, Borgarello V, Bossotti C, Libanori E, Biolcati M, Arduino S, et al. IVF twins have similar obstetric and neonatal outcome as spontaneously conceived twins: a prospective follow-up study. Reproductive biomedicine online. 2012;21(3):422-8. [DOI] [PubMed]
- 99.von Versen-Höynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, Stan Williams R, Rhoton-Vlasak A, Nichols WW, Fleischmann RR, Zhang W, Winn VD, Segal MS, Conrad KP, Baker VL. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a Corpus luteum. Hypertension. 2019;73(3):640–649. doi: 10.1161/HYPERTENSIONAHA.118.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watanabe N, Fujiwara T, Suzuki T, Jwa SC, Taniguchi K, Yamanobe Y, Kozuka K, Sago H. Is in vitro fertilization associated with preeclampsia? A propensity score matched study. BMC Pregnancy Childbirth. 2014;14(100967799):69. doi: 10.1186/1471-2393-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wennberg AL, Opdahl S, Bergh C, Aaris Henningsen A-K, Gissler M, Romundstad LB, et al. Effect of maternal age on maternal and neonatal outcomes after assisted reproductive technology. Fertil Steril. 2016;106(5):1142–1149. doi: 10.1016/j.fertnstert.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 102.Wu QF, Xu DF, Zhao Y, Xin CL. Outcomes of 223 twins born after assisted reproductive techniques compared with 281 twins born after natural conception. Jiangxi Med J. 2010;46:95–97. [Google Scholar]
- 103.Xu Z, Ye B, Lin J, Lin W, Lin L. Analysis of perinatal outcomes of twin pregnancies conceived by in vitro fertilization and embryo transfer (IVF-ET) and those conceived spontaneously. J Wenzhou Med Coll. 2005;35:34–36. [Google Scholar]
- 104.Yang H, Choi YS, Nam KH, Kwon JY, Park YW, Kim YH. Obstetric and perinatal outcomes of dichorionic twin pregnancies according to methods of conception: spontaneous versus in-vitro fertilization. Twin Res Hum Genetics. 2011;14(1):98–103. doi: 10.1375/twin.14.1.98. [DOI] [PubMed] [Google Scholar]
- 105.Yang X, Li Y, Li C, Zhang W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril. 2014;101(2):385–391. doi: 10.1016/j.fertnstert.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 106.Zádori J, Kozinszky Z, Orvos H, Katona M, Pál A, Kovács L. Dilemma of increased obstetric risk in pregnancies following IVF-ET. Journal of assisted reproduction and genetics. 2003;20(6):216-21. [DOI] [PMC free article] [PubMed]
- 107.Zhang H-Q, Fan R, Tian G-H, Li L, Zhang Z-H, Zhang Y-P. Clinical outcomes of twin pregnancies conceived by in vitro fertilization compared with spontaneous twin pregnancies. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(1):63–67. [PubMed] [Google Scholar]
- 108.Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, Liu F, Li M, Sun S, Xing L, Zhu Y, Chen Y, Xu L, Zhou L, Huang H, Zhang D. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: a retrospective cohort study. Sci Rep. 2016;6(101563288):35141. doi: 10.1038/srep35141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lalani S, Choudhry AJ, Firth B, Bacal V, Walker M, Wen SW, et al. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum Reprod (Oxford, England) 2018;33(10):1854–1865. doi: 10.1093/humrep/dey269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bosch E, De Vos M, Humaidan P. The Future of Cryopreservation in Assisted Reproductive Technologies. Front Endocrinol (Lausanne) 2020;11:67. doi: 10.3389/fendo.2020.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maheshwari A, Pandey S, Raja EA, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update. 2018;24(1):35–58. doi: 10.1093/humupd/dmx031. [DOI] [PubMed] [Google Scholar]
- 112.Sha T, Wang X, Cheng W, Yan Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod BioMed Online. 2019;39(2):281–293. doi: 10.1016/j.rbmo.2019.03.203. [DOI] [PubMed] [Google Scholar]
- 113.von Versen-Höynck F, Narasimhan P, Selamet Tierney ES, Martinez N, Conrad KP, Baker VL, Winn VD. Absent or excessive Corpus luteum number is associated with altered maternal vascular health in early pregnancy. Hypertension. 2019;73(3):680–690. doi: 10.1161/HYPERTENSIONAHA.118.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Versen-Höynck F, Strauch NK, Liu J, Chi YY, Keller-Woods M, Conrad KP, Baker VL. Effect of mode of conception on maternal serum Relaxin, creatinine, and sodium concentrations in an infertile population. Reprod Sci. 2019;26(3):412–419. doi: 10.1177/1933719118776792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luke B, Brown MB, Eisenberg ML, Callan C, Botting BJ, Pacey A, et al. In Vitro Fertilization and Risk for Hypertensive Disorders of Pregnancy: Associations with Treatment Parameters. American journal of obstetrics and gynecology. 2019;222(4):350.e1-.e13. [DOI] [PMC free article] [PubMed]
- 116.Elias FTS, Weber-Adrian D, Pudwell J, Carter J, Walker M, Gaudet L, Smith G, Velez MP. Neonatal outcomes in singleton pregnancies conceived by fresh or frozen embryo transfer compared to spontaneous conceptions: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;302(1):31–45. doi: 10.1007/s00404-020-05593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sauer MV. Revisiting the early days of oocyte and embryo donation: relevance to contemporary clinical practice. Fertil Steril. 2018;110(6):981–987. doi: 10.1016/j.fertnstert.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 118.Kupka MS, Ferraretti AP, de Mouzon J, Erb K, D'Hooghe T, Castilla JA, Calhaz-Jorge C, de Geyter C, Goossens V, Strohmer H, Obruca, Kreuz-Kinderwunschzentrum SPG, Bogaerts K, Biostat I, D'Hooghe T, Kyurkchiev S, Antonova I, Rezabek K, Markova J, Erb K, Gissler M, Tiitinen A, Royere D, Buhler K, Uszkoriet M, Loutradis D, Tarlatzis BC, Kosztolanyi G, Urbancsek J, Bjorgvinsson H, Mocanu E, Scaravelli G, Lokshin V, Ravil V, Gudleviciene Z, Matkeviciute G, Lazarevski S, Moshin V, Simic TM, Vukicevic D, Hazekamp JT, Kurzawa R, Calhaz--Jorge C, Laranjeira AR, Rugescu I, Korsak V, Radunovic N, Tabs N, Tomazevic T, Virant-Klun I, Hernandez JH, Castilla Alcala JA, Bergh C, Weder M, de Geyter C, Smeenk JMJ, Lambalk C, Veselovsky V, Baranowski R, The European IVF-monitoring (EIM) Consortium, for the European Society of Human Reproduction and Embryology (ESHRE) Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE†. Hum Reprod. 2014;29(10):2099–2113. doi: 10.1093/humrep/deu175. [DOI] [PubMed] [Google Scholar]
- 119.Storgaard M, Loft A, Bergh C, Wennerholm UB, Soderstrom-Anttila V, Romundstad LB, et al. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: a systematic review and meta-analysis. BJOG. 2017;124(4):561–572. doi: 10.1111/1471-0528.14257. [DOI] [PubMed] [Google Scholar]
- 120.Pecks U, Maass N, Neulen J. Oocyte donation: a risk factor for pregnancy-induced hypertension: a meta-analysis and case series. Deutsches Arzteblatt international. 2011;108(3):23-31. [DOI] [PMC free article] [PubMed]
- 121.Levron Y, Dviri M, Segol I, Yerushalmi GM, Hourvitz A, Orvieto R, et al. The 'immunologic theory' of preeclampsia revisited: a lesson from donor oocyte gestations. Am J Obstet Gynecol. 2014;211(4):383. doi: 10.1016/j.ajog.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 122.Tubbergen P, Lachmeijer AM, Althuisius SM, Vlak ME, van Geijn HP, Dekker GA. Change in paternity: a risk factor for preeclampsia in multiparous women? Journal of reproductive immunology. 1999;45(1):81-8. [DOI] [PubMed]
- 123.Lashley LE, Haasnoot GW, Spruyt-Gerritse M, Claas FH. Selective advantage of HLA matching in successful uncomplicated oocyte donation pregnancies. J Reprod Immunol. 2015;112:29–33. doi: 10.1016/j.jri.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 124.Velez MP, Hamel C, Hutton B, Gaudet L, Walker M, Thuku M, Cobey KD, Pratt M, Skidmore B, Smith GN. Care plans for women pregnant using assisted reproductive technologies: a systematic review. Reprod Health. 2019;16(1):9. doi: 10.1186/s12978-019-0667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Henderson J, Whitlock E, O'Connor E, Senger C, Thompson J, Rowland M. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. preventive services task force. Ann Intern Med. 2014;160(10):695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. A list of 27 items required under the PRISMA statement with their respective locations.
Additional file 2. Search strategy for the systematic review and meta-analysis. Complete Search Strategy for a) Embase (1947 to 2020 April 08) b) Ovid MEDLINE, MEDLINE Daily and Epub Ahead of Print, In-Process & Other Non-Indexed Citations (1947 to 2020 April 08) c) EBM Reviews - Cochrane Central Register of Controlled Trials (1947 to April 2020).
Additional file 3. Excluded full text studies, with reasons. A list of full text studies excluded after screening with reasons for removal.
Additional file 4. Newcastle-Ottawa Scale for quality assessment and publication bias. A breakdown of NOS scores assigned to each study included in the meta-analysis.
Additional file 5. Forest plots for preeclampsia and funnel plots. Forest plots comparing preeclampsia in IVF/ICSI pregnancies and spontaneous pregnancies and funnel plots for publication bias in meta-analyses with 10 or more studies.
Additional file 6. Sensitivity analysis. A list of highest and lowest overall odds ratios after removing individual studies.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.