Abstract
Background
Anemia is a common extraintestinal manifestation of Inflammatory Bowel Disease (IBD) affecting negatively the patients’ quality of life. The aim of this study was to determine the frequency and real-life management of anemia in IBD patients in Greece.
Methods
This study was conducted in 17 Greek IBD referral centers. Demographic, clinical, laboratory, IBD and anemia treatment data were collected and analyzed retrospectively.
Results
A total of 1394 IBD patients [560 ulcerative colitis (UC), 834 Crohn’s disease (CD)] were enrolled. Anemia at any time was reported in 687 (49.3%) patients of whom 413 (29.6%) had episodic and 274 (19.7%) had recurrent/persistent anemia. Anemia was diagnosed before IBD in 45 (6.5%), along with IBD in 269 (39.2%) and after IBD in 373 (54.3%) patients. In the multivariate analysis the presence of extraintestinal manifestations (p = 0.0008), IBD duration (p = 0.026), IBD related surgeries and hospitalizations (p = 0.026 and p = 0.004 accordingly) were risk factors of recurrent/persistent anemia. Serum ferritin was measured in 839 (60.2%) IBD patients. Among anemic patients, 535 (77.9%) received treatment. Iron supplementation was administered in 485 (90.6%) patients, oral in 142 (29.3%) and intravenous in 393 (81%).
Conclusions
The frequency of anemia in IBD patients, followed at Greek referral centers, is approximately 50%. Development of recurrent/persistent anemia may be observed in 20% of cases and is independently associated with the presence of extraintestinal manifestations, IBD duration, IBD related surgeries and hospitalizations. Anemia treatment is administered in up to of anemia IBD patients with the majority of them receiving iron intravenously.
Keywords: Anemia, Crohn’s disease, Iron, Iron deficiency, Ulcerative colitis
Background
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders that encompass mainly Crohn’s disease (CD) and ulcerative colitis (UC). The incidence of IBD in Greece is about 10 per 100,000 inhabitants per year (6.1–8.9 for UC and 2.75–3.0 for CD) (1).
IBD patients often present extra-intestinal manifestations with anemia to be the most common (2). There is a wide range of the reported prevalence of anemia in IBD possibly reflecting the heterogeneity of the study population (in-hospital or out-patient patients), of the severity of IBD, of the IBD treatment (5-ASA, anti-TNFs etc.) as well as of the time point of the assessment (at diagnosis or during the course of IBD).
Anemia is typically classified into iron deficiency anemia (IDA), anemia of chronic disease (ACD) and anemia due to B12 or folic acid deficiency based on the ECCO guidelines. However, there is a difficulty in determining the exact type of anemia in IBD patients, since chronic blood loss, inflammation, medication toxicity, surgical procedures, malabsorption or even malnutrition can be present at the same time in an IBD patient (3, 4). Another classification of anemia that has been suggested is based on the course of anemia as episodic (if presented only once), recurrent (if Hb was restored for a period of time and then dropped below normal again) and persistent anemia (if all available values of Hb were below normal) (5). Most of the anemia’s symptoms are very common (fatigue, dizziness, dyspnea, cold skin, glossitis, hair loss) while others (impairment of cognitive function, restless legs syndrome) are less frequently seen. Anemia is of a great importance not only because of its high frequency but also because of the negative impact on work productivity and quality of life (6, 7). It is well established that anemia is positively correlated with the IBD activity, risk of surgery and prolonged hospitalization but recent studies even correlate anemia with IBD severity (5, 8).
Although anemia diagnosis in IBD is often neglected, anemia treatment should be given to all patients with low hemoglobin (Hb) particularly in the form of iron replacement, since ID is the most frequent cause of anemia in IBD. Recent data suggest that intravenous iron administration is safe, well tolerated and efficient particularly when it comes to patients with severe anemia (Hb < 10.0 g/dl), intolerant or non-responding to oral iron supplementation, and those with active disease (9).
The aims of this study were: (i) to capture the frequency of anemia in a cohort of IBD patients followed at Greek referral centers, (ii) to investigate for potential risk factors for anemia and (iii) to register real life management of anemia.
Methods
This is a retrospective, multicenter observational study conducted in 17 IBD centers in Greece. Patients 18–80 years, with confirmed diagnosis of UC or CD for at least 3 months and having attended > 1 IBD clinic visits with available lab results permitting the evaluation of anemia were included. All patients with known comorbidities that could cause anemia independently (congestive heart failure, chronic kidney failure, active cancer, cirrhosis, Mediterranean anemia trait etc.) as well vegetarian patients were excluded. Gender, age at IBD diagnosis, disease duration at study entry for non-anemic patients and at first presentation of anemia for anemic patients, disease location and behavior for CD and disease extent for UC according to Montreal Classification, smoking habits, extra-intestinal manifestations, IBD-related hospitalizations and surgeries (right hemicolectomy, enterectomy, colectomy), IBD treatment and C-reactive protein (CRP) at entry for non-anemic and at first presentation of anemia for anemic patients were recorded in a pre-defined standardized report form. Clinical disease activity was evaluated with Harvey- Bradshaw score (HBI) and Simple Clinical Colitis Score (SCCAI) for CD and UC respectively and Short Inflammatory Bowel Disease Questionnaire (SIBDQ) was used to evaluate quality of life, at the study entry. Data was extracted retrospectively from the local IBD databases of 17 Greek centers for a period of 12 months (from February 2019 until January 2020). There has not been adopted a special policy of checking either the hemoglobin or the general blood count, specifically for this study protocol and the frequency of the lab tests was according to the clinical practice (usually every 3 months for those receiving biologics, immunomodulators or combination treatment and every 6 months for those on exclusively non-immunosuppressive treatment).
Anemia was defined according to World Health Organization (WHO) criteria when hemoglobin (Hb) was below 12.0 g/dl for non-pregnant women and below 13.0 g/dl for men (7). Anemia was characterized as episodic, if presented only once, recurrent, if Hb was restored for a period of time and then dropped below normal again, and persistent if all available values of Hb were below normal. Treatment of anemia was also captured. Hemopoietic response was also evaluated, in patients treated with iron, where there were available laboratory data. Responders to the treatment of anemia were considered those with an increase of Hb by > 2 g/dl or when Hb returned to normal levels within 4 weeks after iron administration (11, 12).
Statistical analysis
Data are presented either as mean (± SD) for normally distributed variables or as median (IQR, range) for non-normally distributed. Categorical data were analyzed with the chi-square or Fischer’s exact test. Independent factors associated with persistent/recurrent anemia were assessed by multivariate analysis based on binary logistic regression analysis and including those covariates with p < 0.10 in the univariate analysis. Odds ratios (OR) were calculated with 95% confidence intervals (CI). Tests were two-sided and P values < 0.05 were considered to be statistically significant. The statistical program used was MedCalc (MedCalc Software Ltd, Belgium).
Results
A total of 1394 patients met the inclusion criteria and formed the study population. Of those, 560 patients with UC and 834 with CD were included. Demographics and clinical characteristics of the population under investigation are shown in Table 1.
Table 1.
Demographics and clinical characteristics of study population (N = 1394)
| Total IBD | CD | UC | |
|---|---|---|---|
| Number (%) | 1394 (100) | 834 (59.8) | 560 (40.2) |
| Median diagnosis age (years, range) | 33 (23–46) | 32 (22–46) | 35 (24–46) |
| Sex (females, %) | 635 (45.6) | 389 (46.6) | 246 (43.9) |
| Median IBD durationa (years, range) | 3 (1–9) | 3 (1–8.25) | 3 (1–10) |
| Active smokers (%) | 442 (31.7) | 330 (39.5) | 112 (20) |
| Ex-smokers (%) | 366 (26.2) | 189 (22.7) | 177 (31.6) |
| Never smoked (%) | 586 (42.1) | 315 (37.8) | 271 (48.4) |
| Median BMI (range) | 24.5 (21.5–27.8) | 24.3 (21.5–27.9) | 24.7 (21.6–27.7) |
| Montreal classification for UC | |||
| Proctitis (E1, %) | 63 (11.25) | ||
| Left sided colitis (E2, %) | 237 (42.5) | ||
| Extensive colitis (E3, %) | 259 (46.25) | ||
| Montreal classification for CD | |||
| I nflammatory (B1, %) | 515 (61.8) | ||
| Stricturing (B2, %) | 190 (22.8) | ||
| Penetrating (B3, %) | 129 (15.4) | ||
| Perianal (p, %) | 165 (19.8) | ||
| Ileum (L1, N, %) | 376 (45.0) | ||
| Colon (L2, N, %) | 79 (9.5) | ||
| Ileocolonic (L3, N, %) | 380 (45.5) | ||
| Upper GI (L4, N, %) | 114 (13.7) | ||
| EIMs (%) | 469 (33.6) | 325 (38.9) | 144 (25.7) |
| Anti-TNFs (N, %) | 692 (49.6) | 495 (59.4) | 197 (35.2) |
| Immunomodulators (N, %) | 529 (37.9) | 380 (45.6) | 149 (26.6) |
| Other biologics (N, %) | 230 (16.5) | 131 (15.7) | 99 (17.7) |
| IBD-related surgery (N, %) | 176 (12.6) | 156 (18.7) | 20 (3.6) |
UC, Ulcerative Colitis; CD, Crohn’s Disease; BMI, Body Mass Index; IBD, Inflammatory Bowel Disease; GI, Gastrointestinal; EIMs, Exta-intestinal Manifestations; Anti-TNFs, Anti-Tumor Necrosis Factors
aAt first presentation of anemia for anemic patients or at entry for non-anemic patients
The median age at diagnosis was 32 (IQR 24, 22–46) years for CD and 35 (IQR 22, 24–46) years for UC. Females in CD were 389 (46.6%) whereas in UC were 246 (43.9%). Active smokers were more in CD (39.5%) than in UC (20%) (p < 0.0001). Extra-intestinal manifestations, other than anemia, were present in 325 (38.9%) CD and 144 (25.7%) UC patients (p < 0.0001). A history of major IBD-related surgery was reported by 176 (12.6%) patients, 156 (18.7%) with CD and 20 (3.6%) with UC (p < 0.0001). Anti-TNFs exposed were more in CD (59.4%) than in UC (35.2%) and use of immunomodulators (Azathioprine, Methotrexate, 6-Mercaptopurine) was also more frequent in CD than in UC patients (45.6% vs 26.6%) (both with p < 0.0001). The patients on newer biologics (Vedolizumab and Ustekinumab) were 230 (151 and 79 accordingly) are referred together as “other biologics” on the Tables (Table 1, 2, 4). Regarding Tofacitinib, at that timepoint when the study recruited patients, was not available in Greece.
Table 2.
Patients’ characteristics according to their anemia history (N = 1394)
| Group A No anemia |
Group B Episodic anemia |
Group C Recurrent/persistent anemia |
P | |
|---|---|---|---|---|
| Number (%) | 707 (50.7) | 413 (29.6) | 274 (19.7) | |
| Males/females (%) | 432/275 (61.1/38.9) | 208/205 (50.4/49.6) | 119/155 (43.4/56.6) | < 0.0001 |
| CD/UC (%) | 412/ 295 (58.3/41.7) | 236/177 (57.1/42.9) | 186/88 (67.9/32.1) | 0.0089 |
| Median IBD durationa (years, range) | 5 (2–11) | 1 (0–4) | 2 (0–8) | < 0.0001 |
| Median diagnosis age (years, range) | 35 (24–46) | 32 (23–45.5) | 30 (21–46) | < 0.0001 |
| Active smokers (%) | 238 (33.7) | 121 (29.3) | 83 (30.3) | 0.2356 |
| Ex-smokers (%) | 205 (29) | 96 (23.2) | 64 (23.3) | 0.0435 |
| Never smoked (%) | 264 (37.3) | 195 (47.2) | 127 (46.4) | 0.0008 |
| Median BMI (range) | 25.2 (22.5–28.6) | 24.2 (21.3–27.25) | 23.5 (20.7–27.0) | < 0.0001 |
| Montreal classification for UC | ||||
| Proctitis (E1, %) | 53 (18) | 7 (3.9) | 3 (3.4) | < 0.0001 |
| Left sided colitis (E2, %) | 129 (43.7) | 75 (42.4) | 33 (37.5) | 0.5834 |
| Extensive colitis (E3, %) | 113 (38.3) | 95 (53.7) | 52 (59.1) | 0.0002 |
| Montreal classification for CD | ||||
| Inflammatory (B1, %) | 272 (66) | 154 (65.2) | 89 (47.8)) | < 0.0001 |
| Stricturing (B2, %) | 84 (20.4) | 45 (19.1) | 61 (32.8) | 0.6111 |
| Penetrating (B3, %) | 56 (13.6) | 36 (15.2) | 36 (19.4) | 0.6050 |
| Perianal (p, %) | 80 (19.4) | 42 (17.8) | 42 (22.5) | 0.4901 |
| Ileum (L1, N, %) | 209 (50.7) | 97 (41.1) | 70 (37.6) | 0.0037 |
| Colon (L2, N, %) | 35 (8.5) | 23 (9.7) | 20 (10.8) | 0.6575 |
| Ileocolonic (L3, N, %) | 168 (40.8) | 116 (49.2) | 96 (51.6) | 0.0191 |
| Upper GI (L4, N, %) | 46 (11.2) | 41 (17.4) | 27 (14.5) | 0.0766 |
| Disease activity—clinically judged | 176 (24.9) | 238 (57.6) | 145 (52.9) | < 0.0001 |
| EIMs (%) | 199 (28.1) | 138 (33.4) | 131 (47.8) | < 0.0001 |
| Anti-TNFs (N, %) | 261 (36.9) | 242 (58.6) | 188 (68.6) | < 0.0001 |
| Immunomodulators (N, %) | 225 (31.8) | 175 (42.4) | 129 (47.1) | < 0.0001 |
| Other biologics (N, %) | 82 (11.6) | 90 (21.8) | 58 (21.2) | 0.0624 |
| IBD-related surgery (N, %) | 61 (8.6) | 51 (12.3) | 64 (23.4) | < 0.0001 |
Group A never anemic patients, Group B episodically anemic patients, Group C recurrently/persistently anemic patients
UC, Ulcerative Colitis; CD, Crohn’s Disease; BMI, Body Mass Index; IBD, Inflammatory Bowel Disease; GI, Gastrointestinal; EIMs, Exta-intestinal Manifestations; Anti-TNFs, Anti-Tumor Necrosis Factors
aAt first presentation of anemia for anemic patients or at entry for non-anemic patients
Table 4.
Risk factors for persistent/recurrent anemia (group C)
| Risk factors | Univariate analysis | P | Multivariate analysis | P |
|---|---|---|---|---|
| OR (95% CI) | OR (95% Cl) | |||
| Female sex | 1.32 (0.97–1.79) | 0.0804 | 0.78 (0.56–1.10) | 0.1555 |
| CD diagnosis | 1.59 (1.16–2.19) | 0.0044 | 0.77 (0.54–1.10) | 0.1519 |
| Median IBD duration | 1.0304 (1.01–1.05) | 0.0100 | 1.03 (1.00–1.05) | 0.0259 |
| Median diagnosis age | 0.9971 (0.99–1.01) | 0.5563 | ||
| Active smokers | 1.04 (0.75–1.45) | 0.8113 | ||
| CRP > 3 mg/dl | 1.11 (0.79–1.56) | 0.5512 | ||
| Extensive colitis | 1.28 (0.76–2.14) | 0.3568 | ||
| Penetrating or stricturing CD | 2.07 (1.40–3.07) | 0.0003 | ||
| Ileocolonic | 1.09 (0.74–1.61) | 0.6464 | ||
| EIMs | 1.82 (1.33–2.49) | 0.0002 | 1.79 (1.27–2.52) | 0.0008 |
| Major disease surgeries | 2.15 (1.43–3.23) | 0.0002 | 1.73 (1.07–2.78) | 0.0257 |
| IBD hospitalizationsa | 2.39 (1.56–3.66) | 0.0001 | 1.96 (1.25–3.09) | 0.0035 |
| Immmunomodulators | 1.20 (0.89–1.64) | 0.2346 | ||
| Anti-TNF any time | 1.53 (1.11–2.12) | 0.0090 | 0.79 (0.55–1.14) | 0.2062 |
| Other biologics at any time | 1.48 (0.82–2.68) | 0.1944 |
CD, Crohn’s Disease; IBD, Inflammatory Bowel Disease; CRP, C-Reactive Protein; EIMs, Exta-intestinal Manifestations; Anti-TNFs, Anti-Tumor Necrosis Factors
aIBD hospitalizations n = 5 the last 5 years
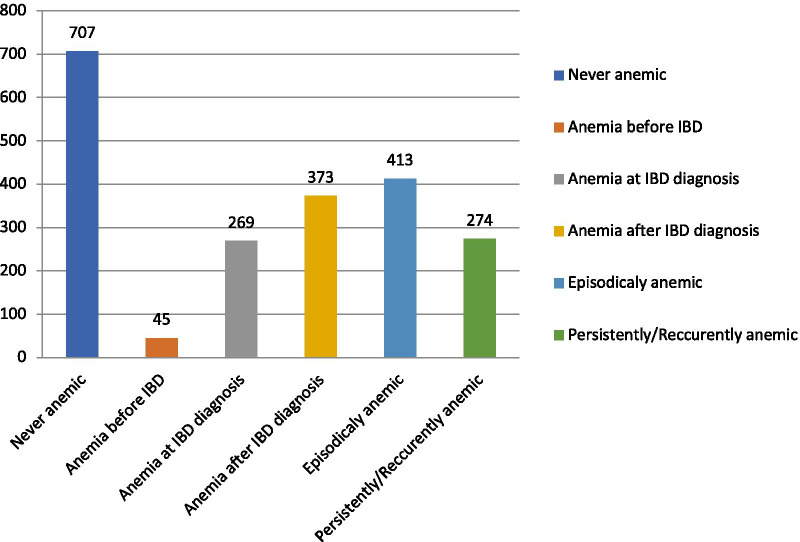
In total, 687/1394 (49.3%) had a diagnosis of anemia. Anemia was initially detected more than one year before IBD in 45 patients (6.5%, 31 CD and 14 UC), concurrently with IBD or less than 1 year before in 269 (39.2%, 156 CD and 113 UC) and after IBD diagnosis in 373 (54.3%, 236 CD and 137 UC) (Fig. 1). The study population was divided into three groups: non-anemic patients at any time (group A), episodically anemic (group B), and recurrently or persistently anemic patients (group C) (Table 2). There were more males than females in group A (61.1% vs 38.9%, p < 0.0001) whereas there was no significant difference in the other two groups regarding gender. CD was the more frequent diagnosis in groups B and C (p = 0.0039 and p = 0.0043).
Fig. 1.
Time point and status of Anemia in IBD patients (N = 1394)
Median age at diagnosis, median disease duration and median BMI were higher in group A compared to the other two groups (all with p < 0.0001, Table 2). UC proctitis was more common and extensive colitis less common in group A (p < 0.0001 and p = 0.0002 respectively). In CD, patients non-stricturing non-penetrating phenotype (B1) was more common in group A (p < 0.0001) as well as ileal (L1) disease (p = 0.0037) whereas ileocolonic disease (L3) was more common in group C (p = 0.0191, Table 2).
Clinically judged active disease was more frequently seen in groups B and C (p < 0.0001). Median CRP levels as well as median HBI (for CD) and SCCAI (for UC) were significantly higher in groups B and C (Table 3). Quality of life as measured with SIBDQ was worse in groups B and C (p < 0.0001). Furthermore, patients in these groups were more often under treatment with anti-TNFs or immunomodulators (p < 0.0001). IBD-related surgeries were also more often among anemic patients (p < 0.0001).
Table 3.
Disease activity in IBD anemic (Group B and C) and non-anemic patients (Group A)
| Characteristics | Anemic patients Group B and C (N = 687) |
Non-anemic patients Group A (N = 707) |
p |
|---|---|---|---|
| Disease activity (clinically judged)a (%) | 382 (55.6%) | 176 (24.9%) | < 0.0001 |
| Median CRPa mg/dl | 2.00 (0.50–7.00) | 0.40 (0.14–1.3) | < 0.0001 |
| Median SIBDQa | 50 (39–60) | 61 (51–67) | < 0.0001 |
| Median HBIa | 5 (3–7) | 3 (1–4) | < 0.0001 |
| Median SCCAIa | 5 (3–8) | 1 (0–4) | < 0.0001 |
| Median Ferritina ng/ml | 23.03 (10–65.9) | 70 (39–113.2) | < 0.0001 |
Group A never anemic patients, Group B episodically anemic patients, Group C recurrently/persistently anemic patients
CRP, C-Reactive Protein; SIBDQ, Simple Inflammatory Bowel Disease Questionnaire; HBI, Harvey-Bradshaw Index; SCCAI, Simple Colitis Activity Index
aAt entry
In the univariate analysis, persistent/recurrent anemia was found to be associated with CD diagnosis [OR 1.59 (1.16–2.19), p = 0.0044], median disease duration [1.03 (1.01–1.05), p = 0.0100], penetrating or stricturing CD [2.07 (1.40–3.07), p = 0.0003], presence of EIMs [1.82 (1.33–2.49), p = 0.0002] use of anti-TNF [1.53 (1.11–2.12), p = 0.0090], IBD related hospitalizations [2.39 (1.56–3.66), p = 0.0001] and IBD related surgeries [2.15 (1.43–3.23), p = 0.0002] (Table 4). In the multivariate analysis, parameters that remained significantly associated with persistent/recurrent anemia were median disease duration [1.03 (1.00–1.05), p = 0.0259], presence of EIMs [1.79 (1.27–2.52), p = 0.0008], IBD related hospitalizations [1.96 (1.25–3.09), p = 0.0035], and IBD related surgeries [1.73 (1.07–2.78), p = 0.0257] (Table 4).
Anemia and iron status were mainly assessed with Hb and ferritin measurement. Ferritin value was available in 839/1394 patients (60.2%) and the median value was 43 ng/ml (IQR 76.7, 13–89.7). Moreover, ferritin was measured in 523 of the 687 (76.1%) anemic patients [median value 23 ng/ml (IQR 55.9, 10–65.9)]. Ferritin below 30 ng/ml was detected in 377 patients (72%) [median value 8 ng/ml (IQR 8, 7–17)] whereas ferritin above 30 ng/ml and below 100 ng/ml with CRP above 1 mg/dl was measured in 61 patients (11.7%) [median value 55.2 ng/ml (IQR 28.5, 41–69.5)] which is, according to the ECCO guidelines, compatible with Iron Deficiency Anemia (IDA). Furthermore, anemia of chronic disease (ferritin > 100 ng/ml) was detected in 85 patients (16.3%) [median value 176 ng/ml (IQR 118, 134.8–252.8)].
From the 687 anemic IBD patients, 535 (77.9%, 327 CD, 208 UC) received treatment for their anemia. The vast majority (485/535, 90.6%) were treated with iron supplementation either as an oral in 142 (29.3%) or as an intravenous formulation in 393 (81%) with a small percentage of patients having received both successively. Of those received iron intravenously, 351 (89.3%) responded, whereas 77 (54.2%) responded of those received per os iron. Thirty one out of the 142 patients (21.8%) that received iron orally presented with adverse events (mostly gastrointestinal symptoms such as abdominal pain and constipation) that eventually led to cessation of the treatment. On the other hand, 31/393 patients (7.9%) that iron was given intravenously experienced adverse events during infusion (allergic reactions, skin rash) and 11/393 (2.8%) post infusion (headache, dizziness, hypophosphatemia). B12 vitamin was administered in 113 (21.1%) and folic acid in 182 (33.9%) patients based on laboratory findings compatible with deficiency. Sixty-eight patients (12.7%) needed blood transfusion. More details for the treatment of anemia are shown in Table 5.
Table 5.
Treatment of anemic IBD patients (N = 687)
| Anemia total (N = 687) | Episodic anemia Group B (N = 413) | Recurrent/Persistent anemia Group C (N = 274) |
P | ||
|---|---|---|---|---|---|
| Anemia treatment (%) | 535 (77.9) | 300 (72.6) | 235 (85.8) | < 0.0001 | |
| CD/UC (%) | 327/208 (61.2/38.8) | 167/133 (55.7/44.3) | 160/75 (68.1/31.9) | ||
| Iron Administration (%) | 485 (90.6) | 286 (95. 3) | 213 (90.6) | ||
| Iron PO/IVa (%) | 142/392 (29.3/ 81) | 72/214 (25.2/75) | 70/178 (32.9/83.6) | ||
| B12 Administration (%) | 113 (21.1) | 48 (16) | 65 (27.6) | 0.0014 | |
| Folic acid Administrationb (%) | 182 (33.9) | 80 (26.7) | 102 (43.4) | < 0.0001 | |
| Blood Transfusion (%) | 68 (12.7) | 36 (12) | 32 (13.6) | 0.0061 | |
Group A never anemic patients, Group B episodically anemic patients, Group C recurrently/persistently anemic patients
UC, Ulcerative Colitis; CD, Crohn’s Disease; PO, Per Os; IV, Intravenously
aPatients who were initially treated with oral iron, were subsequently treated with intravenous iron due to either lack of response or adverse events that led to discontinuation of the oral formulation
bApart from concurrent administration with MTX
Discussion
The present study showed that the frequency of anemia in Greek IBD patients followed at tertiary referral centers is 49.3% meaning that almost half of those patients experience anemia, which is similar to previous reports from other countries (2, 13–16). Anemia was present more frequently in CD patients and in females which is also in accordance to other studies (13, 15, 17). It is of notice that there were few patients (6.5%) with anemia diagnosed more than one year before IBD diagnosis, whereas anemia was diagnosed at IBD diagnosis or less than one year before in 39.2% of the patients. In newly diagnosed IBD patients of a population‐based inception cohort the prevalence of anemia has been reported to be 48.8% for CD and 20.2% for UC (18). Data on the prevalence of anemia before IBD diagnosis is limited (19).
Anemia was found to be episodic (presented once) in 29.6% and persistent/recurrent in 19.7% of the patients. Recurrent/persistent is considered the most serious type of anemia in IBD since it has been associated with severe and disabling disease (5). In our study recurrent/persistent anemia was found to be significantly and independently associated with disease duration, presence of EIMs, history of major IBD related surgeries and IBD related hospitalizations (n = 5 the last 5 years). It is of notice that EIMs (other than anemia) were found to be strongly correlated with the development of persistent/recurrent anemia (p = 0.0008). Even though anemia itself is one of the most common EIM, it seems that the presence of EIMs (arthritic, skin etc.) could independently be a causative factor for anemia of chronic disease, which is a possible explanation for the above-mentioned strong association between anemia and EIMs. On the other hand, both anemia and EIMs have been reported to be associated with worse disease outcome and severe disease (20).
There is a known association between IBD activity and the presence of anemia (21) something that is in accordance with our results since anemic patients had clinically judged active IBD more often than non-anemic patients. Moreover, median CRP as well as median HBI (for CD) and median SCAAI (for UC) were higher and median IBDQ was lower in patients with anemia. The IBD activity either expressed by clinical judgment or by clinical scores (HBI, SCAAI), accompanied by patients’ reported outcomes (IBDQ) reflects a snapshot of a particular moment during disease course. On the other hand, the IBD severity is a wider term incorporating the overall disabling course of the disease with worst outcome, including surgeries frequent hospitalizations etc. Recent reports (5) and the present study support that persistent or recurrent anemia correlates with more aggressive or disabling disease in patients with IBD.
Anemia in IBD patients is considered to be often neglected during the course and treatment of the primary disease, since according to literature review 68.6% of the anemic UC patients in USA were not further investigated (with ferritin etc.) and even 25% of IDA IBD patients remained untreated (22). Furthermore, in Switzerland anemia treatment (iron, B12 administration etc.) occurred only in 40% of patients in private practice and 43% in University Hospitals (23). On the other side, this study showed that the majority of the anemic IBD patients (77.9%) received proper treatment. In this context, most of the patients (90.6%) received iron supplementation with 81% of them receiving iron intravenously. This could be partially attributed to the fact that our study was conducted in referral centers and may not be representative of the real practice in the general IBD population. Our findings are in contrast with earlier studies in Germany (24) and other European countries, in which most of the IBD anemic patients (92%) are treated with iron, but in the vast majority (67%) with oral and only 28% with intravenous formulations (25). An exception to this strategy constitutes Sweden and Switzerland, where iron supplementation was intravenously administered in 72% and 52% accordingly. In the present study ferritin was measured in 60.2% of the study population and in 76.1% of the anemic patients. This percentage is higher than what was previously referred in the literature (22) and even better than what is recorded in pediatric IBD patients. Although a more meticulous approach is expected in pediatric patients, Miller et al. mentioned ferritin measurement only 1 out of 5 anemic pediatric IBD patients (26).
Currently there are no pre-existing data for the frequency, diagnosis and treatment of anemia in Greek IBD patients. Anemia and iron status are mainly assessed with Hb and ferritin since measurement of transferrin saturation (TSAT) or other blood markers of anemia are not widely available in the everyday clinical practice in Greece. On the contrary TSAT was available in 61% of the anemic patients in the study of Blumenstein et al. in Germany (24) whereas in other studies this percentage reached 25% (3). Our findings demonstrate that intravenous iron is implemented more frequently compared to oral administration in IBD patients in Greece. This is in contrast with previous European studies something probably attributed to the fact that these were conducted some years ago and before the development of recent guidelines.
According to ECCO guidelines screening for anemia should be implemented every 6 to 12 months for patients in remission or mild disease and at least every 3 months in those with active disease. Furthermore, a ferritin threshold of 30 ng/ml for patients with inactive disease and of 100 ng/ml for patients with active IBD with raised inflammation markers such as CRP has been proposed for the diagnosis of IDA. Moreover, in the presence of inflammation, the diagnostic criteria for ACD are serum ferritin > 100 ng/ml and TSAT < 20%. If serum ferritin level is between 30 and 100 ng/ml, a combination of true iron deficiency and ACD is more probable (11).
This study has several strengths but some limitations as well. First, this is a retrospective study, conducted in 17 different referral centers, where more severe IBD cases are followed up and further not all of them are abide by the anemia guidelines with the same way. Second, selection bias may exist because patients visit these centers and received treatment for anemia more frequently. What is more, the IBD activity assessment was made based on clinical scores (HBI, SCAAI) since the gold-standard, which is endoscopy, could not be applied in a retrospective study in 17 different IBD centers and furthermore fecal calprotectin is not yet widely available in Greece and the national health system does not reimburse IBD patients for this laboratory examination. On the other hand, this study included a large number of IBD patients and constitutes the first attempt to record anemia burden in Greek IBD patients and relevant treatment strategy. Moreover, a different classification of anemia based on the course of anemia (episodic, persistent and recurrent) together with the classic one (iron deficiency anemia, anemia of chronic disease and B12 or folic acid deficiency) were applied in order to depict more accurately the overall burden of anemia in IBD patients. Last, this study aimed to raise awareness towards anemia diagnosis and treatment that seems to be often neglected.
In conclusion, half of the Greek IBD patients present anemia during their disease course. One out of five patients present recurrent/persistent anemia despite the treatment for their primary disease. Risks factors for recurrent/persistent anemia are the presence of extraintestinal manifestations, IBD duration, IBD related surgeries and hospitalizations. In real life in referral Greek IBD centers, 77.9% of anemic patients receive anemia treatment with the majority of them receiving iron intravenously.
Conclusions
The frequency of anemia in IBD patients followed at Greek referral centers is approximately 50%. Development of recurrent/persistent anemia may be observed in 20% of cases and is independently associated with presence of extraintestinal manifestations, IBD duration and IBD related surgeries and hospitalizations. Anemia treatment is administered in up to of anemia IBD patients with the majority of them receiving iron intravenously.
Acknowledgements
The members of the Hellenic group for the study of IBD: Kalliopi Foteinogiannopoulou, Konstantinos Karmiris, Georgios Axiaris, Magdalini Velegraki, Antonios Gklavas, Christina Kapizioni, Charalabos Karageorgos, Christina Kateri, Anastasia Katsoula, Georgios Kokkotis, Evgenia Koureta, Charikleia Lamouri, Panagiotis Markopoulos, Maria Palatianou, Ploutarchos Pastras, Konstantinos Fasoulas, Olga Giouleme, Evanthia Zampeli, Aggeliki Theodoropoulou, Georgios Theocharis, Konstantinos Thomopoulos, Pantelis Karatzas, Konstantinos H. Katsanos, Andreas Kapsoritakis, Anastasia Kourikou, Nikoleta Mathou, Spilios Manolakopoulos, Georgios Michalopoulos, Spyridon Michopoulos, Alexandros Boubonaris, Giorgos Bamias, Vasileios Papadopoulos, George Papatheodoridis, Ioannis Papaconstantinou, Ioannis Pachiadakis, Konstantinos Soufleris, Maria Tzouvala, Christos Triantos, Eftychia Tsironi, Dimitrios K. Christodoulou, and Ioannis E. Koutroubakis.
Abbreviations
- IBD
Inflammatory bowel disease
- UC
Ulcerative colitis
- CD
Crohn’s disease
- IBDU
Inflammatory bowel disease unclassified
- 5-ASA
5-Sibdqaminosalicylic acid
- ID
Iron deficiency
- Hb
Hemoglobin
- CRP
C-reactive protein
- HBI
Harvey-Bradshaw score
- SCCAI
Simple clinical colitis score
- SIBDQ
Short inflammatory bowel disease questionnaire
- WHO
World health organization
- SD
Standard deviation
- IQR
Interquartile range
- CI
Confidence interval
- BMI
Body mass index
- EIMs
Extraintesinal manifestations
- IDA
Iron deficiency anemia
- TSAT
Transferin saturation
- ACD
Anemia of chronic disease
- ECCO
European Chron’s and colitis organization
Authors' contributions
KF: Study design, data analysis, patient recruitment, data collection and writing up of the first draft of the paper. KK: patient recruitment, data collection. GA: patient recruitment, data collection. MV: patient recruitment, data collection. AG: patient recruitment, data collection. CK: patient recruitment, data collection. CK: patient recruitment, data collection. CK: patient recruitment, data collection. AK: patient recruitment, data collection. GK: patient recruitment, data collection. EK: patient recruitment, data collection. LC: patient recruitment, data collection. PM: patient recruitment, data collection. MP: patient recruitment, data collection. PP: patient recruitment, data collection. KF: patient recruitment, data collection. OG: patient recruitment, data collection. EZ: patient recruitment, data collection. AT: patient recruitment, data collection. GT: patient recruitment, data collection. KT: patient recruitment, data collection. PK: patient recruitment, data collection. KK: patient recruitment, data collection. AK: patient recruitment, data collection. AK: patient recruitment, data collection. NM: patient recruitment, data collection. SM: patient recruitment, data collection. GM: patient recruitment, data collection. SM: patient recruitment, data collection. AB: patient recruitment, data collection. GB: patient recruitment, data collection. VP: patient recruitment, data collection. GP: patient recruitment, data collection. IP: patient recruitment, data collection. IP: patient recruitment, data collection. KS: patient recruitment, data collection. MT: patient recruitment, data collection. CT: patient recruitment, data collection. ET: patient recruitment, data collection. DC: patient recruitment, data collection. IK: Study design, data analysis and correcting the first draft of the paper. All authors have read and approved the manuscript.
Funding
The study was partially supported by a grant of Vifor Pharma and a grant of the Hellenic team for the study of inflammatory bowel disease (EMIFNE).
Availability of data and materials
Data are available on request. Data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee of University Hospital of Heraklion with the decision number 972/22-1-2019. For this type of study formal consent is not required.
Consent for publication
Not applicable.
Competing interests
Ioannis E. Koutroubakis: Αdvisory board member for Abbvie, Astelas, Genesis, Janssen, MSD, Pharmacosmos, Pfizer, Shire and Takeda; Speaker for AbbVie, Astelas, Genesis, Janssen, MSD, Takeda and Mylan; research support Abbvie and Ferring. Kalliopi Foteinogiannopoulou: no conflict of interest. Konstantinos Karmiris: received speaker fees from Abbvie, Aenorasis, Janssen, MSD, Pfizer and Takeda and consultancy or advisory board member fees from Abbvie, Amgen, Ferring, Galenica, Genesis, Janssen, MSD, Pfizer and Takeda. Konstantinos Thomopoulos: no conflict of interest. Olga Giouleme: no conflict of interest. Maria Tzouvala: no conflict of interest. Magdalini Velegraki: no conflict of interest. Nikoleta Mathou: no conflict of interest. Spilios Manolakopoulos: Gilead Sciences: research grands, Abbvie research grands, Pfizer: advisory, celltrion: research grands, shire plc/ Takeda pharmaceutical: research grands. Andreas Kapsoritakis: no conflict of interest. Christos Triantos: no conflict of interest. Ploutarchos Pastras: no conflict of interest. George Papatheodoridis: advisor/lecturer for Abbvie, Dicerna, Elpen, Gilead, GlaxoSmithKline, Ipsen, Janssen, Merck Sharp & Dohme, Roche, Spring Bank, Takeda; research grants Abbvie, Gilead; clinical trials: Abbvie, Astellas, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Janssen, Merck Sharp & Dohme, Noorik, Novartis, Novo Nordisk, Regulus, Roche, Takeda. Evgenia Koureta: no conflict of interest. Christina Kapizioni: no conflict of interest. Georgios Michalopoulos: no conflict of interest. Ioannis Papaconstantinou: no conflict of interest. Antonios Gklavas: no conflict of interest. Lamouri Charikleia: no conflict of interest. Evanthia Zampeli: no conflict of interest. Georgios Kokkotis: no conflict of interest. Dimitrios K Christodoulou: advisor/lecturer for Abbvie, Janssen, Merck Sharp & Dohme, Takeda, Enorasis, Genesis, Pfizer, Ferring. Spyridon Michopoulos: Advisor or Lecturer for Pfizer, Takeda, Abbvie, Ferring, MSD, Janssen. Giorgos Bamias: Advisor/lecturer for Janssen, Pfizer, Takeda, Abbvie, MSD, Mylan, Genesis Pharma, Adacyte Therapeutics, Amgen, Ferring, Cooper; Funding (Grants/Honoraria): Pfizer, Takeda, Abbvie, Aenorasis; Research/Clinical Trials: Abbvie. Konstantinos Soufleris: Advisory Board Member, Lecturer for Abbvie, Amgen, Genesis Pharma, Janssen, MSD, Mylan, Pfizer, Takeda. Funding/Research Support/Clinical Trials: Abbvie, Celgene, Faran, Ferring, Janssen, Roche, Shire, Takeda.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kalliopi Foteinogiannopoulou, Email: drfpopi@hotmail.com.
the Hellenic group for the study of IBD:
Kalliopi Foteinogiannopoulou, Konstantinos Karmiris, Georgios Axiaris, Magdalini Velegraki, Antonios Gklavas, Christina Kapizioni, Charalabos Karageorgos, Christina Kateri, Anastasia Katsoula, Georgios Kokkotis, Evgenia Koureta, Charikleia Lamouri, Panagiotis Markopoulos, Maria Palatianou, Ploutarchos Pastras, Konstantinos Fasoulas, Olga Giouleme, Evanthia Zampeli, Aggeliki Theodoropoulou, Georgios Theocharis, Konstantinos Thomopoulos, Pantelis Karatzas, Konstantinos H. Katsanos, Andreas Kapsoritakis, Anastasia Kourikou, Nikoleta Mathou, Spilios Manolakopoulos, Georgios Michalopoulos, Spyridon Michopoulos, Alexandros Boubonaris, Giorgos Bamias, Vasileios Papadopoulos, George Papatheodoridis, Ioannis Papaconstantinou, Ioannis Pachiadakis, Konstantinos Soufleris, Maria Tzouvala, Christos Triantos, Eftychia Tsironi, Dimitrios K. Christodoulou, and Ioannis E. Koutroubakis
References
- 1.ECCO Country Member Profiles: Greece. https://www.ecco-ibd.eu/publications/ecco-news/ecco-country-member-profiles/item/ecco-country-member-profiles-greece-2.html?category_id=545
- 2.Kulnigg S, Gasche C. Systematic review: managing anemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1507–1523. doi: 10.1111/j.1365-2036.2006.03146.x. [DOI] [PubMed] [Google Scholar]
- 3.Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599–610. doi: 10.1038/nrgastro.2010.151. [DOI] [PubMed] [Google Scholar]
- 4.Woźniak M, Barańska M, Małecka-Panas E, Talar-Wojnarowska R. The prevalence, characteristics, and determinants of anaemia in newly diagnosed patients with inflammatory bowel disease. Gastroenterology Rev. 2019;14:39–47. doi: 10.5114/pg.2019.83424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koutroubakis IE, Ramos-Rivers C, Regueiro M, et al. Persistent or recurrent anemia is associated with severe and disabling inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:1760–1766. doi: 10.1016/j.cgh.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12:123–130. doi: 10.1097/01.MIB.0000196646.64615.db. [DOI] [PubMed] [Google Scholar]
- 7.Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–688S. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- 8.Rieder F, Paul G, Schnoy E, et al. Hemoglobin and hematocrit levels in the prediction of complicated Crohn's disease behaviorea cohort study. PLoS ONE. 2014;9:e104706. doi: 10.1371/journal.pone.0104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–1553. doi: 10.1002/ibd.20285. [DOI] [PubMed] [Google Scholar]
- 10.WHO Haemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011
- 11.Dignass AU, Gasche C, Bettenworth D, et al. European Consensus on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. J Crohn's Colitis. 2015;9:211–222. doi: 10.1093/ecco-jcc/jju009. [DOI] [PubMed] [Google Scholar]
- 12.Okam MM, Koch TA, Tran MH. Iron Supplementation, Response in Iron-Deficiency Anemia: Analysis of Five Trials. Am J Med 2017; 130:991 e1–991 e8 [DOI] [PubMed]
- 13.Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936–945. doi: 10.1097/01.MIB.0000442728.74340.fd. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1299–1307. doi: 10.1111/j.1572-0241.2008.01846.x. [DOI] [PubMed] [Google Scholar]
- 15.Bengi G, Keyvan H, Durmaz SB, Akpınar H. Frequency, types, and treatment of anemia in Turkish patients with inflammatory bowel disease. World J Gastroenterol. 2018;24:4186–4196. doi: 10.3748/wjg.v24.i36.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzi LT, Weston CM, Goldfarb NI, et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(1):47–52. doi: 10.1097/01.MIB.0000191670.04605.e7. [DOI] [PubMed] [Google Scholar]
- 17.Bergamaschi G, Di Sabatino A, Albertini R, et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease Influence of anti-tumor necrosis factor alpha treatment. Haematologica. 2010;95:199–205. doi: 10.3324/haematol.2009.009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Høivik ML, Reinisch W, Cvancarova M, Moum B; IBSEN study group. Anaemia in inflammatory bowel disease: a population-based 10-year follow-up. Aliment Pharmacol Ther. 2014;39:69–76 [DOI] [PubMed]
- 19.Kwon YH, Kim YJ. Pre-diagnostic clinical presentations and medical history prior to the diagnosis of inflammatory bowel disease in children. Pediatr Gastroenterol Hepatol Nutr. 2013;16:178–184. doi: 10.5223/pghn.2013.16.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vegh Z, Kurti Z, Gonczi L, et al. Association of extraintestinal manifestations and anaemia with disease outcomes in patients with inflammatory bowel disease. Scand J Gastroenterol Gastroenterol. 2016;51:848–854. doi: 10.3109/00365521.2016.1140807. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez CM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematol. 2019;142:30–33. doi: 10.1159/000496728. [DOI] [PubMed] [Google Scholar]
- 22.Khan N, Patel D, Shah Y, Yang YX. Factors predicting testing and treatment of iron deficiency in a nationwide cohort of anemic UC patients. Inflamm Bowel Dis. 2016;22:2894–2901. doi: 10.1097/MIB.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 23.Voegtlin Μ, Vavricka SR, Schoepfer AM, et al. Swiss IBD Cohort Study Prevalence of anaemia in inflammatory bowel disease in Switzerland: a cross-sectional study in patients from private practices and university hospitals. J Crohns Colitis. 2010;4:642–648. doi: 10.1016/j.crohns.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Blumenstein I, Dignass A, Vollmer S, Klemm W, Weber-Mangal S, Stein J. Current practice in the diagnosis and management of IBD-associated anaemia and iron deficiency in Germany: The German AnaemIBD Study. J Crohns Colitis. 2014;8:1308–1314. doi: 10.1016/j.crohns.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 25.SteinJ B. P, Befrits R, Gasche C, Gudehus M, Lerebours E, Anaemia management in patients with inflammatory bowel disease: routine practice across nine European countries. Eur J Gastroenterol Hepatol. 2013;25:1456–1463. doi: 10.1097/MEG.0b013e328365ca7f. [DOI] [PubMed] [Google Scholar]
- 26.Miller SD, Cuffari C, Akhuemonkhan E, Guerrerio AL, Lehmann H, Hutfless S. Anemia screening, prevalence, and treatment in pediatric inflammatory bowel disease in the united states 2010–2014. Pediatr Gastroenterol Hepatol Nutr. 2019;22:152–161. doi: 10.5223/pghn.2019.22.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request. Data underlying this article will be shared on reasonable request to the corresponding author.