Graphical abstract
Keywords: Assisted reproductive technology, Embryo culture media, MicroRNAs, Reproductive outcome
Abstract
Introduction
MicroRNAs (miRNAs) are important regulators of many biological functions, including embryo implantation and development. Recently, it has been reported that miRNAs in biofluids are predictive for physiological and pathological processes.
Objectives
In this study, we aim to investigate whether the miRNAs secreted by human embryos in culture medium can be used as embryonic biomarkers.
Methods
The culture media were prospectively collected from embryos of patients at reproductive medicine center with informed consent. A high-throughput miRNA sequencing method was applied to detect the miRNA profiles in the human embryo culture media. After bioinformatics analysis and screening of differentially expressed miRNAs, quantitative real-time polymerase chain reaction (qRT-PCR) assay was subsequently performed to further confirm the sequencing results with mixed samples. Furthermore, we performed droplet digital PCR (ddPCR) to verify the target miRNAs at single sample level. Receiver operating characteristic (ROC) analyses were performed for differentially expressed miRNAs.
Results
Compared with embryos with failed pregnancy, the embryos with successful pregnancy secreted different miRNA profiles into the culture media, which were predicted to be involved in multiple biological processes. Validated by droplet digital polymerase chain reaction (ddPCR), the expression of hsa-miR-26b-5p and hsa-miR-21-5p in the culture media of cleavage embryos with successful pregnancy was significantly lower than that of embryos with failed pregnancy. Moreover, the Receiver Operating Characteristic (ROC) curve analysis indicated that hsa-miR-26b-5p and hsa-miR-21-5p could serve as potential biomarkers for reproductive outcomes.
Conclusion
Together, our findings highlight the important predictive potential of miRNAs secreted by human embryos in culture media, which is meaningful for non-invasive embryo selection in assisted reproductive technology.
Introduction
Infertility is an ongoing reproductive health issue worldwide, with an average prevalence of 9% in couples at reproductive age [1], [2]. There has been a significant development for assisted reproductive technology (ART) to better help the infertile couples over the past decades. ART consists of a series of fertility treatments, involving the manipulations of human oocytes, sperm, and embryos in vitro, which work to establish a successful pregnancy [3]. The most commonly performed ART procedures are in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) followed by embryo transfer (ET). The current trend in ART is to achieve a higher pregnancy rate per transfer and to reduce the rate of multiple gestations [4]. The pregnancy rate following an IVF/ICSI-ET cycle depends on multiple factors and embryonic defect is one of the main causes for implantation failure. Therefore, selection of the best embryo for transfer is crucial for embryo implantation and live birth rates.
Presently, assessment of embryo quality based on morphologic criteria is the predominant non-invasive technique for selecting viable embryos and this provides valuable information for the prediction of IVF/ICSI-ET outcomes [5], [6]. However, parameters such as cleavage rates and blastocyst formation as well as developmental kinetics are mostly evaluated subjectively and many morphologically normal embryos either do not implant or spontaneously abort early in pregnancy because of chromosome anomalies [7]. Notably, preimplantation genetic diagnosis/screening (PGD/PGS) has been increasingly performed to help couples lower the risks of transmitting genetic defects to their offspring and to improve pregnancy and live birth rates during IVF cycles [8]. However, conventional PGD/PGS procedures require the invasive biopsy of early embryos before transfer to the uterus, which might affect embryo quality and awaits long-term biosafety test [9]. Another limitation of PGD/PGS is the high overall costs due to the technical expertise including special training for embryologists to perform the biopsy as well as the geneticists to carry out the genetic analysis [10]. As a result, it is necessary to develop new non-invasive and easy-to-screen biomarkers for embryo selection in addition to morphological evaluation and PGD/PGS.
MicroRNAs (miRNAs) are a family of short (approximately 20–22 nucleotides), noncoding single-stranded RNAs, which participate in transcriptional or posttranscriptional regulation of gene expression by binding to complementary sites in the 3′-untranslated regions of the targeted messenger RNAs (mRNAs) [11], [12]. MiRNAs are bound to stabilizing proteins and packaged into membrane-bound exosomes and microvesicles before being secreted out of donor cells [13]. The encapsulated miRNAs are highly stable and can be detected in different biofluids, including serum, plasma, saliva, urine, semen, amniotic fluid, breast milk, bronchial lavage, cerebrospinal fluid, peritoneal fluid, and pleural fluid as well as the culture media collected from different cell lines [14], [15]. Recent studies have identified that human embryos could also secrete specific miRNAs in the extracellular environment [16], [17], [18]. In addition, it has been demonstrated that miRNAs are implicated in a variety of biologic processes, including cell proliferation, differentiation, apoptosis and early embryo development [19], [20], [21], [22]. Based on these characteristics, miRNAs in biofluids may be effective biomarkers for physiological and pathological process. Accumulating evidence indicate that the circulating miRNAs contain fingerprints for various diseases and can serve as an important approach for the blood-based detection of human diseases, especially for cancer [23], [24]. Recent studies have illustrated that the different miRNAs profiles in human blastocysts culture media were related to the fertilization method, chromosomal status, and pregnancy outcome and that miRNAs could be explored for non-invasive embryo selection in IVF cycles [16], [25]. However, the miRNA expression in these studies was evaluated using the array-based quantitative polymerase chain reaction (PCR) analysis. There is a need for more accurate miRNA data with better resolution, which could be achieved by next generation sequencing. Moreover, droplet digital PCR (ddPCR) is an end-point analysis based on limiting partition of the PCR sample into many micro-droplets, most of which contain one or zero reaction [26], [27]. After endpoint PCR reaction, the starting target concentration is estimated with the number of positive (containing amplified target) and negative (no amplified target detected) reactions by Poisson statistical analysis. Several studies had suggested that both reverse transcription-quantitative PCR (RT-qPCR) and ddPCR can be reliably used to quantify circulating miRNAs [26], [28], [29]. For the first few days of the IVF process, however, the human embryo is incubated in a single small droplet (about 20 l of culture medium) with low target copies. Compared with RT-qPCR, ddPCR enables the detection and quantification of low abundant targets with increased precision and sensitivity [30], [31], [32].
In the present study, we investigated the miRNA profiles in embryo culture media of patients who underwent routine IVF/ICSI-ET by a high throughput sequencing method, and then we validated the results by RT-qPCR and ddPCR to determine the potential miRNAs as non-invasive biomarkers for embryo selection during IVF cycles.
Material and methods
Ethics statement
All experiments involving samples from human patients were conducted according to the ethical policies and procedures approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology, China (Approval no. S1162). Written informed consents were obtained from all participants.
Patients and sample collection
We recruited patients who underwent routine IVF/ICSI-ET at Wuhan Tongji Reproductive Medicine Hospital and Wuhan Union Hospital. After fertilization assessment, the zygotes were washed extensively in G-1 PLUS (Vitrolife, Göteborg, Sweden) and cultured in 30 μL G-1 PLUS droplets until day 3 of development. After cleavage assessment, the day 3 embryos were washed extensively in equilibrated G-2 PLUS (Vitrolife, Göteborg, Sweden) and transferred to G-2 PLUS culture droplets for further culture to blastocyst stage. After another two days in culture, the development and quality of the day 5 blastocysts were evaluated according to the blastocyst scoring system. All the embryos were cultured under 6% CO2, 5% O2, and balance N2 at 37 °C in a tri-gas incubator (Cook, Bloomington, IN, USA). Culture media were prospectively collected from the in vitro cultured embryos for miRNA sequencing and validation. The miRNA profiles of embryo culture media collected from samples with successful and failed pregnancy were compared to identify the differentially expressed miRNAs. All the participants were performed with single-embryo transfer.
RNA isolation and quality control
The culture media were collected from embryos at cleavage and blastocyst stages during in vitro fertilization cycles. For ddPCR, we extracted RNA directly from each single sample. For sequencing and RT-qPCR, in order to get enough RNA, we mixed 15 samples of embryo culture media for each group, cleavage stage with successful pregnancy, cleavage stage with failed pregnancy, blastocyst stage with successful pregnancy, and blastocyst stage with failed pregnancy. The culture media were centrifuged at 1500g for 10 min to remove the granulosa cells and dead sperm. Before RNA extraction, all samples were spiked with C. elegans-miR-39 (QIAGEN, Hilden, Germany) to act as an endogenous control. Then the total RNA was extracted using the miRNeasy Serum/Plasma Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. The concentration and purity of RNA samples were determined by NanoDrop ND-1000 spectrophotometer (Thermo Fisher, Wilmington, DE, USA) and 2100 Bioanalyzer Instruments (Agilent, Santa Clara, CA, USA).
High-throughput miRNA sequencing
We performed the high-throughput miRNA sequencing on the culture media of embryos at cleavage and blastocyst stages with different pregnancy outcomes. The miRNA sequencing library was constructed using the TruSeq® miRNA Sample Prep Kit v2 (Illumina, San Diego, CA, USA). Briefly, the total RNA of each sample was used to prepare the miRNA sequencing library as the following steps: 3′-adaptor ligation, 5′-adaptor ligation, cDNA synthesis, PCR amplification, and gel purification for 145–160 bp PCR-amplified fragments. After quantification with Qubit (Thermo Fisher, Waltham, MA, USA), the libraries were captured on cBOT (Illumina, San Diego, CA, USA) to be amplified in situ as clusters, and finally, they were sequenced on the HiSeq™ 2500 System (Illumina, San Diego, CA, USA) as per the manufacturer’s instructions.
After sequencing, the adaptor sequences were trimmed and the quality-filtered reads were harvested as clean reads. The clean reads were clustered to unique sequences and mapped to databases of human genome, RFam, RepBase, mRNA database, miRBase using bowtie software, allowing up to one mismatch. Based on miRNA biogenesis model, we used miRDeep2 software to predict novel miRNAs [33]. Then, the clean reads of each sample were aligned to merged miRNA databases (known miRNAs from miRBase plus the newly predicted miRNAs) to calculate the miRNA expression levels. The numbers of mapped tags were defined as the raw expression levels of that miRNAs. To correct for the difference in tag counts between samples, the tag counts were scaled to TPM (the copy number of transcripts per million) based on the total number of tags aligned [34]. Identification of differentially expressed miRNAs was performed with the DESeq software. Fold change and p value were calculated to identify the differentially expressed miRNAs in culture media collected from embryos with different pregnancy outcomes. The miRNAs, matched p ≤ 0. 05 and log2 (fold change) ≥ 1 (upregulated) or <−1 (downregulated), were considered differentially expressed miRNAs.
RT-qPCR validation
The RT-qPCR assay was subsequently performed to further confirm the sequencing results with mixed samples from two centers (Wuhan Tongji Reproductive Medicine Hospital and Wuhan Union Hospital) and 15 samples were mixed for each group. Reverse transcription for mixed samples was performed with the miScript II RT Kit (QIAGEN, Hilden, Germany). qPCR was performed on the LightCycler® 96 SW 1.1 real-time PCR detection system (Roche, Mannheim, Germany) with the miScript SYBR® Green PCR Kit (QIAGEN, Hilden, Germany). The forward primers used were listed in supplemental Table 1. The universal reverse primer was provided by the PCR kit. The PCR reactions were run in triplicate and C. elegans-miR-39 mimic (QIAGEN, Hilden, Germany) was used as endogenous control to normalize the relative expression levels of the miRNAs. Blank samples, namely water and culture media never exposed to embryo culture were run in parallel and showed no amplification for any of the tested miRNAs.
ddPCR validation
In order to verify the results of mixed samples, we performed ddPCR to detect the expression of target miRNAs at a single sample level (100 cleavage samples in total, 50 with successful pregnancy and 50 with failed pregnancy). The cDNA template for ddPCR was prepared using TaqMan™ Advanced miRNA cDNA Synthesis Kit (Thermo Fisher, Pleasanton, CA, USA). The ddPCR was carried out with the QX200™ Droplet Digital™ PCR system (Bio-Rad, Hercules, CA, USA). The reaction mixture consisted of 10 of 2ddPCR supermix for probes (Bio-Rad, Hercules, CA, USA), 1 l of TaqMan® Advanced miRNA Assay (Thermo Fisher, Pleasanton, CA, USA), and 5 l of diluted cDNA in a final volume of 20 l. The mixture was dispensed into a separate well of a disposable DG8 cartridge (Bio-Rad, Hercules, CA, USA). Then, 70 l of droplet generation oil (Bio-Rad, Hercules, CA, USA) was added into each of the corresponding oil well before the cartridge was loaded into the droplet generator (Bio-Rad, Hercules, CA, USA). After the mixture was partitioned into monodisperse water-in-oil emulsion, the droplets were transferred to a 96-well PCR plate (Eppendorf, Hamburg, Germany), heat sealed with foil, and amplified on a C1000 thermal cycler (Bio-Rad, Hercules, CA, USA). Following amplification, the plate was placed onto the droplet reader (Bio-Rad, Hercules, CA, USA). The ddPCR data were analyzed with QuantaSoft analysis software (Bio-Rad, Hercules, CA, USA). The C. elegans-miR-39 mimic (QIAGEN, Hilden, Germany) was used as endogenous control to normalize the relative expression levels of the miRNAs.
Target prediction and pathway analyses of miRNAs
Target genes of the differentially expressed miRNAs were predicted using the miRanda algorithms (http://www.microrna.org/). The distribution of these miRNA-target genes on human genome and their interactions were identified by Circos (http://circos.ca/) [35]. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using DAVID (https://david.ncifcrf.gov/).
Statistical analyses
Continuous variables are presented as mean ± standard deviation (SD) for normally distributed data, or as median and interquartile range. Normally distributed data were compared using the Student’s t-test and non-normally distributed data using the Mann-Whitney U test. A p value less than 0.05 was considered to be statistically significant. Receiver operating characteristic (ROC) analyses were performed for differentially expressed miRNAs. All analyses were performed using Graph Pad Prism v5.0 (GraphPad Software, La Jolla, CA, USA).
Results
Characteristics of study population
Embryo culture media were collected from cultured embryos of infertile patients who underwent routine IVF or ICSI at reproductive medicine center. The characteristics and treatments of the patients were described in Table 1 (samples used for miRNA Sequencing). According to the morphologic criteria [36], [37], [38], only embryos with good quality were included for both the successful pregnancy group and the failed pregnancy group. For cleavage embryos, the blastomeres were in equal size and no cytoplasmic fragments were observed, while, the blastocysts included were fully expanded with a clear structure of the inner cell mass and trophectoderm (Fig. 1A).
Table 1.
Characteristics and treatments of study population involved in miRNA Sequencing.
| Variable | Successful pregnancy |
Failed pregnancy |
|||
|---|---|---|---|---|---|
| Cleavage (n = 15) |
Blastocyst (n = 15) |
Cleavage (n = 15) |
Blastocyst (n = 15) |
||
| Age, years(±SD) | 31.2 ± 5.2 | 29.3 ± 2.7 | 32.2 ± 7.5 | 30.2 ± 3.8 | |
| Diagnosis | Primary infertility | 13 | 10 | 9 | 11 |
| Secondary infertility | 2 | 5 | 6 | 4 | |
| Duration of infertility, years | 1–5 | 10 | 10 | 12 | 11 |
| 6–10 | 4 | 5 | 2 | 4 | |
| >10 | 1 | 0 | 1 | 0 | |
| Sperm source | AIH | 7 | 5 | 10 | 4 |
| AID | 6 | 10 | 4 | 11 | |
| TESA | 2 | 0 | 1 | 0 | |
| ART method | IVF | 7 | 12 | 7 | 11 |
| ICSI | 8 | 3 | 8 | 4 | |
Notes: AID, artificial insemination by donor; AIH, artificial insemination by husband; TESA, testicular sperm aspiration; ART, assisted reproductive technology; IVF, in-vitro fertilization; ICSI, intracytoplasmic sperm injection; SD, standard deviation.
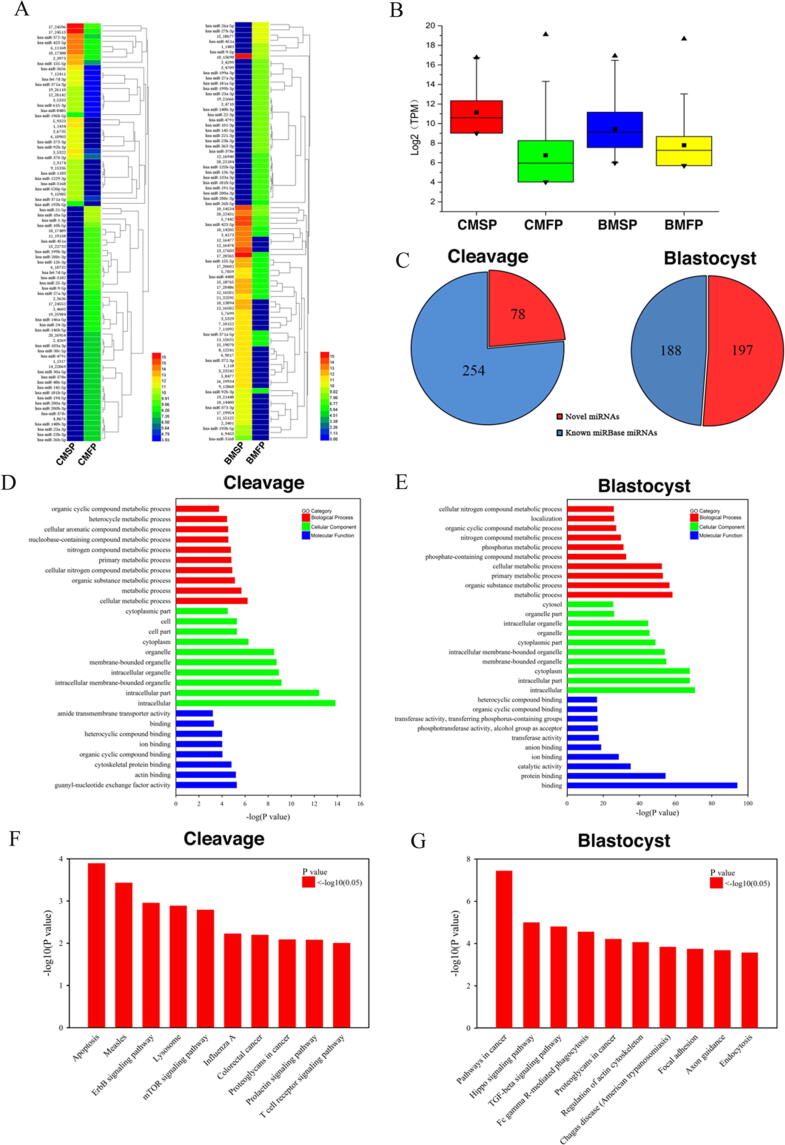
Fig. 1.
Detection of miRNAs by high-throughput sequencing in embryo culture media collected at different stages of preimplantation development. A. Representative pictures of the embryos at two different stages of preimplantation development (cleavage and blastocyst stages) with different pregnancy outcomes. B. Comparison of the miRNA profiles between the cleavage and blastocyst embryo culture media samples. The Venn diagram shows that 148 miRNAs detected in cleavage stage culture media were also present in blastocyst culture media. C. The distribution of detected miRNAs in human genome. D. The classification of detected small RNAs. CMSP: culture media of cleavage embryo with successful pregnancy. CMFP: culture media of cleavage embryo with failed pregnancy. BMSP: culture media of blastocyst with successful pregnancy. BMFP: culture media of blastocyst with failed pregnancy.
MiRNA profiling in culture media of human embryos
With high-throughput miRNA sequencing, we detected the miRNA profiles of human embryo culture media at different developmental stages. Following the quality control filter, 332 miRNAs were detected in cleavage embryo culture media, and 385 miRNAs were detected in blastocyst culture media. In total, 148 miRNAs were common in embryo culture media collected from both of the two developmental stages (Fig. 1B). These miRNAs were mapped to the human genome and most of them were distributed in the intron regions (Fig. 1C). As shown in Fig. 1D, miRNAs only accounted for a tiny proportion of all the small RNAs species (Fig. 1D). Among all the detected miRNAs, the numbers of novel predicted miRNAs for cleavage and blastocyst stages were 78 and 197, respectively (Fig. 2C).
Fig. 2.
Comparison analysis of differentially expressed miRNAs in human embryo culture media with different reproductive outcomes. A. Unsupervised hierarchical cluster analysis of the differentially expressed miRNAs in culture media of embryos with different pregnancy outcomes. The expression intensity of each miRNA in each sample varies from high (red) to low (blue). B. Box plot built on log2 (transcript per million) of miRNAs detected in culture media from embryos with successful and failed pregnancy. C. The novel miRNAs predicted in embryo culture media at cleavage and blastocyst stages. D, E. Gene ontology (GO) analysis of the differentially expressed miRNAs at cleavage and blastocyst stages, respectively. It contains 3 parts: biological process, cellular component, and molecular function. F, G. The enriched KEGG signaling pathways associated with the differentially expressed miRNAs at cleavage and blastocyst stages, respectively. The top 10 pathways were listed.
Identification of differentially expressed miRNAs in culture media collected from embryos with different pregnancy outcomes
Next, we compared the miRNA profiles of embryo culture media with different pregnancy outcomes at similar developmental stages (cleavage stage or blastocyst stage). Unsupervised hierarchical clustering of the differentially expressed miRNAs indicated a good separation of the samples with different pregnancy outcomes, suggesting that the culture media of embryos with successful pregnancy exhibit unique miRNA expression signatures as compared to those with failed pregnancy (Fig. 2A). Furthermore, the total expression level of miRNAs detected in the culture media of embryos with successful pregnancy was higher than that of embryos with failed pregnancy, no matter at cleavage or blastocyst stage (Fig. 2B).
Bioinformatics analysis of differentially expressed miRNAs and their target genes
The differentially expressed miRNAs detected in the human embryo culture media are predicted to regulate genes involved in multiple biological processes and pathways. GO analysis showed that the genes were enriched in a variety of biological functions, including cellular metabolic processes, cellular components and biomolecular binding (Fig. 2D, E). The KEGG signaling pathways associated with these differentially expressed miRNAs in embryo culture media were also analyzed (Fig. 2F, G). For cleavage embryos, the results pointed out that these miRNAs may have a role in apoptosis, ErbB, and mTOR signaling pathways (Fig. 2F). For blastocyst, they were mainly involved in Hippo, TGF-beta, and Fc gamma R-mediated phagocytosis signaling pathways (Fig. 2G). All of these pathways have been reported to be related to the regulation of embryo implantation and development. We also implemented a genome mapping by Circos to identify unequivocal interactions between these differentially expressed miRNAs and their targets (Supplemental Fig. 1).
Validation of selected miRNAs in culture media collected from embryos with different pregnancy outcomes
We mapped the differentially expressed miRNAs to the miRNAs enriched in signaling pathways described above using the following algorithms: miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/), miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php), miRPathDB (https://mpd.bioinf.uni-sb.de/). The miRNAs with high expression levels and high fold changes between two groups in the high-throughput sequencing analyses were included for validation. In total, 18 miRNAs (hsa-miR-372-3p, hsa-miR-373-3p, hsa-miR-155-5p, hsa-miR-423-5p, hsa-miR-92b-3p, hsa-miR-3168, hsa-miR-193-5p, hsa-miR-371a-5p, hsa-miR-4488, hsa-miR-21-5p, hsa-miR-26b-5p, hsa-miR-10a-5p, hsa-miR-27a-3p, hsa-miR-451a, hsa-miR-200b-3p, hsa-miR-200c-3p, hsa-miR-26a-5p, hsa-miR-27b-3p) were selected for RT-qPCR and further analysis (Fig. 3A, B). The significantly different expression levels of these 18 miRNAs between two groups were identified by the high-throughput miRNA sequencing (Fig. 3C). For the mixed samples collected from Wuhan Tongji Reproductive Medicine Hospital, the RT-qPCR results revealed that the cleavage and blastocyst culture media both highlighted the increased expression of hsa-miR-372-3p and hsa-miR-373-3p, and the decreased expression of hsa-miR-451a, hsa-miR-200c-3p, hsa-miR-27a-3p, and hsa-miR-26b-5p for embryos with successful pregnancy. In addition, the downregulation of hsa-miR-21-5p and hsa-miR-10a-5p in culture media was specific for cleavage embryos with successful pregnancy; whereas blastocysts with successful pregnancy presented the reduced expression of hsa-miR-26a-5p and hsa-miR-27b-3p in the culture media, as compared to culture media from embryos with failed pregnancy (Fig. 3D, E). We also confirmed the consistent expression of these miRNAs in the samples collected from Wuhan Union Hospital (Fig. 3F, G). RT-qPCR results of the other selected miRNAs presented no significance or they were not detected. Furthermore, a regulatory network was built to further elucidate the target genes of the validated miRNAs (Supplemental Fig. 2). As expected, these miRNAs play regulatory roles in the expression of important genes like large tumor suppressor homolog 2 (LATS2), mechanistic target of rapamycin kinase (MTOR), phosphatase and tensin homolog (PTEN), MYC proto-oncogene (MYC), and B-cell lymphoma 2 (BCL2), etc.
Fig. 3.
Identification and validation of selected miRNAs. A, B. The Venn diagram shows the selection of miRNAs for RT-qPCR verification. C. Heatmap of the high- throughput sequencing results for the 18 selected miRNAs detected in culture media of embryos at cleavage and blastocyst stages with different pregnancy outcomes. Range of colors (red to blue) shows the range of expression values (high to low). D-G. Fold change of significantly upregulated (red) and downregulated (green) miRNAs in culture media of embryos with successful pregnancy compared to embryos with failed pregnancy. D, F. RT-qPCR confirmed the different expression pattern of 8 miRNAs in the cleavage embryo culture media collected from Wuhan Tongji Reproductive Medicine Hospital (D) and Wuhan Union Hospital (F). E, G. RT-qPCR confirmed the different expression pattern of 8 miRNAs in the blastocyst culture media collected from Wuhan Tongji Reproductive Medicine Hospital (E) and Wuhan Union Hospital (G). RT-qPCR data indicated relative expression of the samples with successful pregnancy with respect to the samples with failed pregnancy. Error bars indicate mean ± SD of three replicate experiments.
ddPCR analysis of the screened miRNAs at single embryo level
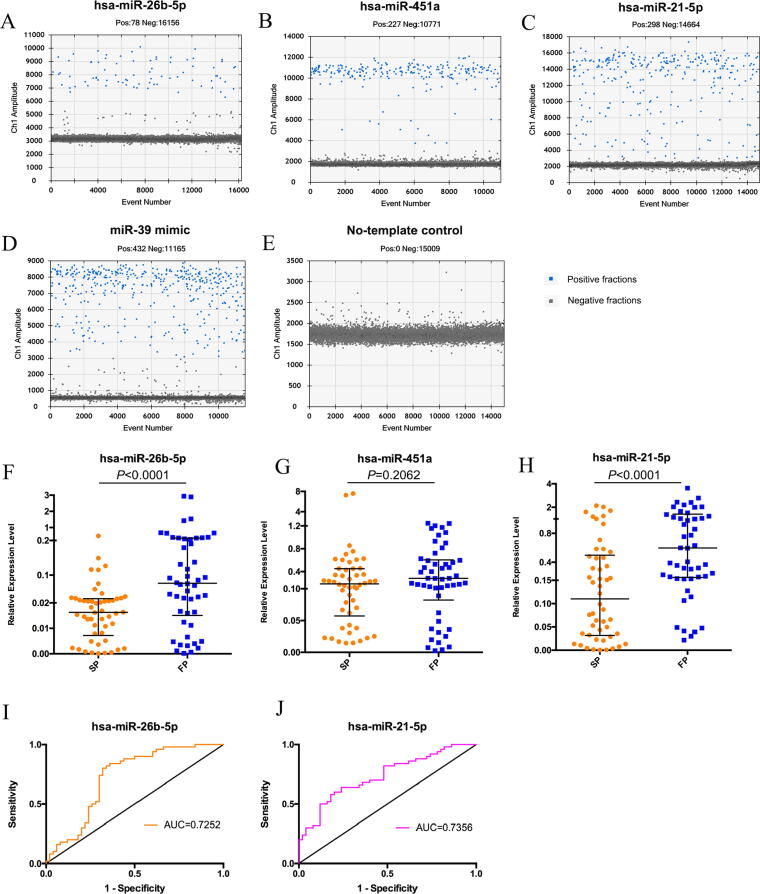
In order to explore the predictive potential of the above selected miRNAs, we performed ddPCR to detect their expression at a single embryo level, and only hsa-miR-26b-5p, hsa-miR-451a and hsa-miR-21-5p could be stably detected in the culture medium of one single embryo at cleavage stage (Fig. 4A-E). With QuantaSoft analysis, we validated the downregulation of hsa-miR-26b-5p and hsa-miR-21-5p in the culture media at a single embryo level (Fig. 4F, H), but no significant difference was detected for hsa-miR-451a at single embryo level (Fig. 4G). Furthermore, we conducted ROC analyses for hsa-miR-26b-5p and hsa-miR-21-5p. The area under the curve (AUC) to discriminate embryos with successful pregnancy from those with failed pregnancy was 0.7252 (95% confidence interval (CI): 0.6217–0.8287) for hsa-miR-26b-5p and 0.7356 (95% CI: 0.6386–0.8326) for hsa-miR-21-5p, suggesting the predictive potential of these two miRNAs for pregnancy outcomes during IVF/ICSI-ET cycles (Fig. 4I, J).
Fig. 4.
Validation of differentially expressed miRNAs at single embryo level. A-E. ddPCR results for target miRNAs. (A) hsa-miR-26b-5p. (B) hsa-miR-451a. (C) hsa-miR-21-5p. (D) C. elegans-miR-39 mimic. (E) No-template control. F-H. Median and interquartile range of hsa-miR-26b-5p (F), hsa-miR-451a (G) and hsa-miR-21-5p (H) levels detected by ddPCR in 50 samples for each group. The corresponding significance values were calculated via a Mann-Whitney U test. SP: Successful pregnancy. FP: Failed pregnancy. I, J. ROC analysis of hsa-miR-26b-5p (I) and hsa-miR-21-5p (J) levels for prediction of reproductive outcomes in human embryo culture media.
Discussion
In the present study, we have characterized the miRNA profiles in culture media of human embryos during IVF/ICSI-ET cycles by high-throughput sequencing. The embryo culture media showed distinct miRNA profiles between the successful pregnancy group and the failed pregnancy group both at cleavage and blastocyst stages, thereby suggesting that miRNAs secreted by human embryos might be correlated with pregnancy outcomes. Thus, miRNAs in human embryo culture media could be developed as predictive biomarkers for reproductive outcomes of IVF/ICSI-ET cycles.
In previous studies using miRNA array method, miRNAs could be reliably detected and analyzed from spent blastocyst culture media [16], [25], [39]. However, fresh cleavage-stage embryos are also widely used in clinical practice [40], [41]. To experienced embryologists, the selection of the morphologically best embryos on day 3 (cleavage stage) presents only 23% predictive value for day 5 blastocyst formation in vitro, suggesting that day 3 morphology may be an inaccurate predictor of an embryo’s implantation capability [42], [43], [44]. Therefore, more evaluation criteria are necessary for cleavage-stage embryo selection before blastocyst stage. Here, we have detected stable miRNA expression in culture media collected from embryos both at cleavage and blastocyst stages. Moreover, the comparison analyses indicated that miRNAs were differentially expressed between samples with successful pregnancy and failed pregnancy, which might be useful in embryo evaluation.
Bioinformatics analysis of these different miRNA profiles identified several target genes, enriched in biological processes and pathways. It is interesting that miR-372-3p and miR-373-3p were predicted to modulate the expression of LATS2, a serine-threonine kinase acting as a cell cycle inhibitor [45], [46]. Our results showed that miR-372-3p and miR-373-3p were upregulated in the culture media of embryos with successful pregnancy, which would repress their target gene, the cell cycle inhibitor LATS2, promoting the cell cycle progression in early embryo development. In addition, LATS2 also participates in the regulation of Hippo-YAP signaling, which is consistent with the results of KEGG pathway analysis for blastocyst stage [47]. Furthermore, miR-21-5p, miR-451a and miR-200c-3p targeted at the apoptosis-related gene, BCL2, which regulates cell death process [48]. The reduced levels of these miRNAs could activate the expression of BCL2, leading to the inhibition of apoptosis during preimplantation embryo development. We also observed that apoptosis was the most significantly enriched pathway for the differentially expressed miRNAs of samples at cleavage stage. Additionally, MYC was the predicted target of miR-373-3p, miR-21-5p, miR-451a and miR-26a-5p; meanwhile, PETN was predicted to be the target of four down-regulated miRNAs, miR-26a-5p, miR-21-5p, miR-10a-5p and miR-26b-5p. Both MYC and PETN genes are involved in numerous biological functions, like the regulation of cell cycle, proliferation, growth and differentiation [49], [50]. After all, the exact roles of these miRNAs in human embryo development have yet to be determined by further research.
In most reproductive clinics, a commonly used approach for embryo selection is to grade embryos based on their morphological features and embryo scores are still accepted as the best predictor of pregnancy from IVF [51], [52], [53]. However, the evaluation of morphology might be insufficient to identify the best suited embryos for transfer to increase the pregnancy rate per cycle [7], [42]. Thus, the exploration for additional biomarkers to morphologic criteria will contribute to better selection of the embryos, which has been one of the most important challenges in the development of ART. Recently, several non-invasive biomarkers for embryonic selection have been reported to be related to embryo quality, like mRNA fragments and secreted proteins as well as metabolomic profiles in culture media [6], [54], [55], [56], [57], [58], [59], [60]. But these biomarkers may not be reliably detectable and stable for a long time. Notably, PGS helps to select the best embryo for transfer and improve the chance of achieving a successful pregnancy, whereas it is invasive and expensive [61]. By contrast, miRNAs are stable, consistently expressed, easily detected, and involved in multiple physiological and pathological processes [23], [25], [62], [63], [64]. In order to get enough amount of RNA, we used mixed samples for high-throughput sequencing and RT-qPCR, and the results were not specific for embryos or patients. Validation using more sensitive and efficient detection methods for miRNA profiles in single embryo culture medium is more conducive for clinical application. Thus, we performed ddPCR validation at single-embryo level, and the results revealed that hsa-miR-26b-5p and hsa-miR-21-5p detected in culture media could serve as potential biomarkers for reproductive outcomes. Nevertheless, our study was retrospective and we did not investigate the underlying mechanisms that might explain the reduced expression of hsa-miR-26b-5p and hsa-miR-21-5p in culture media of human embryos with successful pregnancy. Additional prospective and mechanism research are required before the miRNA biomarkers can be confidently used in clinical settings.
Conclusions
In conclusion, our results indicate that miRNAs in human embryo culture media may serve as novel and non-invasive biomarkers for embryo selection during IVF/ICSI-ET cycles. Combined with morphological evaluation, it would be helpful to increase the success rates and to reduce the high multiple pregnancy rates of ART. More clinical trials are needed to determine the sensitivity and specificity of miRNA biomarkers for embryo selection, and more basic research is also necessary to develop better detection methods of miRNAs with low input.
Availability of data and materials
All relevant data are available from the authors upon reasonable request. The miRNA-seq data were deposited in the NCBI Gene Expression Omnibus (NCBI GEO: GSE142824).
Compliance with Ethics Requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgments
Acknowledgements
We thank Dr. Yanmei Luo and Dr. Yan Luo from Bio-Rad for their technical support in ddPCR.
Funding
This work was supported by the National Natural Science Foundation of China (81901484) and the Health commission of Hubei Province scientific research project (WJ2019H263).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.01.003.
Contributor Information
Ling Zhang, Email: zhling312@163.com.
Wenpei Xiang, Email: wpxiang2010@gmail.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Inhorn M.C., Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 2.Boivin J., Bunting L., Collins J.A., Nygren K.G. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 3.Zegers-Hochschild F., Adamson G.D., de Mouzon J. The international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod. 2009;24(11):2683–2687. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 4.Chen A.A., Tan L., Suraj V., Reijo Pera R., Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99(4):1035–1043. doi: 10.1016/j.fertnstert.2013.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasiri N., Eftekhari-Yazdi P. An overview of the available methods for morphological scoring of pre-implantation embryos in in vitro fertilization. Cell J. 2015;16(4):392–405. doi: 10.22074/cellj.2015.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodgaard T., Heegaard P.M., Callesen H. Non-invasive assessment of in-vitro embryo quality to improve transfer success. Reprod Biomed Online. 2015;31(5):585–592. doi: 10.1016/j.rbmo.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Fragouli E., Alfarawati S., Daphnis D.D. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Hum Reprod. 2010;26(2):480–490. doi: 10.1093/humrep/deq344. [DOI] [PubMed] [Google Scholar]
- 8.Lu L., Lv B., Huang K., Xue Z., Zhu X., Fan G. Recent advances in preimplantation genetic diagnosis and screening. J Assist Reprod Genet. 2016;33(9):1129–1134. doi: 10.1007/s10815-016-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeesch J.R., Voet T., Devriendt K. Prenatal and pre-implantation genetic diagnosis. Nat Rev Genet. 2016;17(10):643–656. doi: 10.1038/nrg.2016.97. [DOI] [PubMed] [Google Scholar]
- 10.Xu J., Fang R., Chen L., Chen D., Xiao J.P., Yang W. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci USA. 2016;113(42):11907–11912. doi: 10.1073/pnas.1613294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 12.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Weber J.A., Baxter D.H., Zhang S. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Zhang S., Weber J., Baxter D., Galas D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38(20):7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbluth E.M., Shelton D.N., Wells L.M., Sparks A.E., Van Voorhis B.J. Human embryos secrete microRNAs into culture media–a potential biomarker for implantation. Fertil Steril. 2014;101(5):1493–1500. doi: 10.1016/j.fertnstert.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 17.Kropp J., Salih S.M., Khatib H. Expression of microRNAs in bovine and human pre-implantation embryo culture media. Front Genet. 2014;5:91. doi: 10.3389/fgene.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuman C., Van Sinderen M., Gantier M.P. Human Blastocyst Secreted microRNA Regulate Endometrial Epithelial Cell Adhesion. EBioMedicine. 2015;2(10):1528–1535. doi: 10.1016/j.ebiom.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent L.C. MicroRNAs in embryonic stem cells and early embryonic development. J Cell Mol Med. 2008;12(6a):2181–2188. doi: 10.1111/j.1582-4934.2008.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 21.Kropp J., Khatib H. Characterization of microRNA in bovine in vitro culture media associated with embryo quality and development. J Dairy Sci. 2015;98(9):6552–6563. doi: 10.3168/jds.2015-9510. [DOI] [PubMed] [Google Scholar]
- 22.Lin X., Beckers E., Mc Cafferty S. Bovine Embryo-Secreted microRNA-30c Is a Potential Non-invasive Biomarker for Hampered Preimplantation Developmental Competence. Front Genet. 2019;10:315. doi: 10.3389/fgene.2019.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X., Ba Y., Ma L. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell P.S., Parkin R.K., Kroh E.M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capalbo A., Ubaldi F.M., Cimadomo D. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 2016;105(1):225–235. doi: 10.1016/j.fertnstert.2015.09.014. e221-223. [DOI] [PubMed] [Google Scholar]
- 26.Hindson C.M., Chevillet J.R., Briggs H.A. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10(10):1003–1005. doi: 10.1038/nmeth.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinheiro L.B., Coleman V.A., Hindson C.M. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem. 2012;84(2):1003–1011. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson S., Follo M., Haenel D. Droplet digital PCR as a novel detection method for quantifying microRNAs in acute myocardial infarction. Int J Cardiol. 2018;257:247–254. doi: 10.1016/j.ijcard.2017.10.111. [DOI] [PubMed] [Google Scholar]
- 29.Campomenosi P., Gini E., Noonan D.M. A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. 2016;16(1):60. doi: 10.1186/s12896-016-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hindson B.J., Ness K.D., Masquelier D.A. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whale A.S., Huggett J.F., Cowen S. Comparison of microfluidic digital PCR and conventional quantitative PCR for measuring copy number variation. Nucleic Acids Res. 2012;40(11) doi: 10.1093/nar/gks203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao S., Lin H., Chen S. Sensitive detection of Porcine circovirus-2 by droplet digital polymerase chain reaction. J Vet Diagn Invest. 2015;27(6):784–788. doi: 10.1177/1040638715608358. [DOI] [PubMed] [Google Scholar]
- 33.Friedlander M.R., Chen W., Adamidi C. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol. 2008;26(4):407–415. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- 34.Vaz C., Ahmad H.M., Sharma P. Analysis of microRNA transcriptome by deep sequencing of small RNA libraries of peripheral blood. BMC Genomics. 2010;11:288. doi: 10.1186/1471-2164-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krzywinski M., Schein J., Birol I. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niakan K.K., Han J., Pedersen R.A., Simon C., Pera R.A. Human pre-implantation embryo development. Development. 2012;139(5):829–841. doi: 10.1242/dev.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutting R., Morroll D., Roberts S.A., Pickering S., Rutherford A. Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb) 2008;11(3):131–146. doi: 10.1080/14647270802302629. [DOI] [PubMed] [Google Scholar]
- 38.The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26(6):1270–83. [DOI] [PubMed]
- 39.Cimadomo D., Rienzi L., Giancani A. Definition and validation of a custom protocol to detect miRNAs in the spent media after blastocyst culture: searching for biomarkers of implantation. Hum Reprod. 2019;34(9):1746–1761. doi: 10.1093/humrep/dez119. [DOI] [PubMed] [Google Scholar]
- 40.Rhenman A., Berglund L., Brodin T. Which set of embryo variables is most predictive for live birth? A prospective study in 6252 single embryo transfers to construct an embryo score for the ranking and selection of embryos. Hum Reprod. 2015;30(1):28–36. doi: 10.1093/humrep/deu295. [DOI] [PubMed] [Google Scholar]
- 41.van Loendersloot L., van Wely M., van der Veen F., Bossuyt P., Repping S. Selection of embryos for transfer in IVF: ranking embryos based on their implantation potential using morphological scoring. Reprod Biomed Online. 2014;29(2):222–230. doi: 10.1016/j.rbmo.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Milki A.A., Hinckley M.D., Gebhardt J., Dasig D., Westphal L.M., Behr B. Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril. 2002;77(6):1191–1195. doi: 10.1016/s0015-0282(02)03104-7. [DOI] [PubMed] [Google Scholar]
- 43.Dennis S.J., Thomas M.A., Williams D.B., Robins J.C. Embryo morphology score on day 3 is predictive of implantation and live birth rates. J Assist Reprod Genet. 2006;23(4):171–175. doi: 10.1007/s10815-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rijnders P.M., Jansen C.A. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13(1O):2869–2873. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- 45.Voorhoeve P.M., le Sage C., Schrier M. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124(6):1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 46.Lee K.H., Goan Y.G., Hsiao M. MicroRNA-373 (miR-373) post-transcriptionally regulates large tumor suppressor, homolog 2 (LATS2) and stimulates proliferation in human esophageal cancer. Exp Cell Res. 2009;315(15):2529–2538. doi: 10.1016/j.yexcr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Hoa L., Kulaberoglu Y., Gundogdu R. The characterisation of LATS2 kinase regulation in Hippo-YAP signalling. Cell Signal. 2016;28(5):488–497. doi: 10.1016/j.cellsig.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Cory S., Adams J.M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 49.Bretones G., Delgado M.D., Leon J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849(5):506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins B.D., Hodakoski C., Barrows D., Mense S.M., Parsons R.E. PTEN function: the long and the short of it. Trends Biochem Sci. 2014;39(4):183–190. doi: 10.1016/j.tibs.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian Y.L., Ye Y.H., Xu C.M., Jin F., Huang H.F. Accuracy of a combined score of zygote and embryo morphology for selecting the best embryos for IVF. J Zhejiang Univ Sci B. 2008;9(8):649–655. doi: 10.1631/jzus.B0820003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisch J.D., Sher G., Adamowicz M., Keskintepe L. The graduated embryo score predicts the outcome of assisted reproductive technologies better than a single day 3 evaluation and achieves results associated with blastocyst transfer from day 3 embryo transfer. Fertil Steril. 2003;80(6):1352–1358. doi: 10.1016/j.fertnstert.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Ziebe S., Petersen K., Lindenberg S., Andersen A.G., Gabrielsen A., Andersen A.N. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12(7):1545–1549. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 54.Seli E., Sakkas D., Scott R., Kwok S.C., Rosendahl S.M., Burns D.H. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88(5):1350–1357. doi: 10.1016/j.fertnstert.2007.07.1390. [DOI] [PubMed] [Google Scholar]
- 55.Mains L.M., Christenson L., Yang B., Sparks A.E., Mathur S., Van Voorhis B.J. Identification of apolipoprotein A1 in the human embryonic secretome. Fertil Steril. 2011;96(2):422–427. doi: 10.1016/j.fertnstert.2011.05.049. e422. [DOI] [PubMed] [Google Scholar]
- 56.Hardarson T., Ahlström A., Rogberg L. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2011;27(1):89–96. doi: 10.1093/humrep/der373. [DOI] [PubMed] [Google Scholar]
- 57.Vergouw C.G., Kieslinger D.C., Kostelijk E.H. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012;27(8):2304–2311. doi: 10.1093/humrep/des175. [DOI] [PubMed] [Google Scholar]
- 58.Stigliani S., Anserini P., Venturini P., Scaruffi P. Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Hum Reprod. 2013;28(10):2652–2660. doi: 10.1093/humrep/det314. [DOI] [PubMed] [Google Scholar]
- 59.Stigliani S., Persico L., Lagazio C., Anserini P., Venturini P., Scaruffi P. Mitochondrial DNA in Day 3 embryo culture medium is a novel, non-invasive biomarker of blastocyst potential and implantation outcome. Mol Hum Reprod. 2014;20(12):1238–1246. doi: 10.1093/molehr/gau086. [DOI] [PubMed] [Google Scholar]
- 60.Kropp J., Khatib H. mRNA fragments in in vitro culture media are associated with bovine preimplantation embryonic development. Front Genet. 2015;6 doi: 10.3389/fgene.2015.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zamora S., Clavero A., Gonzalvo M.C. PGS-FISH in reproductive medicine and perspective directions for improvement: a systematic review. J Assist Reprod Genet. 2011;28(8):747–757. doi: 10.1007/s10815-011-9578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mraz M., Malinova K., Mayer J., Pospisilova S. MicroRNA isolation and stability in stored RNA samples. Biochem Biophys Res Commun. 2009;390(1):1–4. doi: 10.1016/j.bbrc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 63.Ma R., Jiang T., Kang X. Circulating microRNAs in cancer: origin, function and application. J Exp Clin Cancer Res. 2012;31(1):38. doi: 10.1186/1756-9966-31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomankova T., Petrek M., Kriegova E. Involvement of microRNAs in physiological and pathological processes in the lung. Respir Res. 2010;11(1):159. doi: 10.1186/1465-9921-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the authors upon reasonable request. The miRNA-seq data were deposited in the NCBI Gene Expression Omnibus (NCBI GEO: GSE142824).