Abstract
Introduction
Current non-invasive treatments for erectile dysfunction (ED) include oral medications, intracavernosal injections, and vacuum-assisted devices. Though these therapies work well for many, a subset of patients have contraindications or are unsatisfied with these options. Restorative therapies for ED are a new frontier of treatments focused on regenerating diseased tissue and providing a potential “cure” for ED.
Aim
The aim of this position statement is to examine existing clinical trial data for restorative therapies and identify elements that require further research before widespread adoption.
Methods
A literature review was performed to identify all clinical trials performed with regenerative therapy for ED. This includes treatments such as stem cell therapy (SCT), platelet rich plasma (PRP), and restorative related technologies like low-intensity shockwave therapy (LiSWT).
Main Outcome Measures
Most clinical trials in restorative therapies were assessed for safety, feasibility, or efficacy. This included recording adverse events, changes in sexual function and erectile function questionnaires, and diagnostics measures.
Results
To date there is an absence of robust clinical data supporting the efficacy of restorative therapies regarding ED, though technologies such as LiSWT have established relative safety.
Conclusions
Restorative therapies are a promising technology that represents a new frontier of treatment geared towards reversing disease pathology rather than just treating symptoms. However, current published clinical studies are limited. Future work needs to be adequately powered, multi-center, randomized, sham/placebo-controlled trials in well-characterized patient populations to ensure safety and demonstrate efficacy. Until these studies are done, restorative therapies should be reserved for clinical trials and not offered in routine clinical practice. Liu JL, Chu KY, Gabrielson AT, et al. Restorative Therapies for Erectile Dysfunction: Position Statement From the Sexual Medicine Society of North America (SMSNA). J Sex Med 2021;9:100343
Key Words: Erectile dysfunction, Restorative therapies, Low intensity shock wave therapy, Stem cell therapy, Stromal vascular fraction, Platelet rich plasma
Abbreviations: ED, Erectile dysfunction; LiSWT, Low-intensity shock wave therapy; SCT, stem cell therapy; SVF, Stromal vascular fraction; PRP, Platelet-rich plasma; PDE5i, phosphodiesterase type 5 inhibitor; IIEF, International Index of Erectile Function; EHS, Erection Hardness Score; SHIM, Sexual Health Inventory for Men
INTRODUCTION
Erectile dysfunction (ED) refers to the inability to achieve or maintain an erection sufficient for satisfactory sexual performance and has significant negative impact on both men and their partners.1 Recent estimates suggest the overall prevalence of ED in North America to be between 22 and 58%.2 This number is expected to grow in tandem with the aging population.3 Several studies show ED is strongly associated with older age and increasingly common comorbidities such as hypertension, cardiovascular disease, and diabetes.2,4
Initial treatments include couples therapy and oral pharmacologic agents, namely phosphodiesterase type-5 (PDE5) inhibitors, as well as local pharmacotherapies to the penis like intra-urethral suppositories and intracavernosal injections.5,6 Though these treatments demonstrate good efficacy for men with mild to moderate ED there remains a cohort who either cannot tolerate these medications, have direct contraindications or represent a hard-to-treat ED population.6 These include men with post-prostatectomy ED, diabetes mellitus, and men with severe ED related to peripheral vascular disease and smoking.7,8 For the medication refractory patients, surgical treatment involves the placement of a penile prosthesis. Penile implants have high patient satisfaction, but this surgery is not without risks and potential complications.6,9 Sexual medicine providers recognize the importance of restoring spontaneous physiologic erections. In fact, most men and their partners report spontaneous erections preferable to pharmacologic and surgical approaches to ED.1,10 Therefore, the field has actively sought novel approaches that reverse organ dysfunction and restore neurovascular function of the penile vasculature.
Restorative therapies are based on the concept of repairing or replacing diseased tissue by stimulating endogenous regenerative capabilities. These treatments provide a promising alternative to the current management paradigms and represent a transition from modalities that only address disease symptoms to interventions aimed at restoring structure and function of erectile tissue.11 Restorative therapies include treatments such as stem cell therapy (SCT) or platelet rich plasma (PRP) and technologies based on regenerative principles, such as low-intensity shock wave therapy (LiSWT)12 which stimulate endogenous stem cell mobilization to diseased tissue. Many of these erectogenic treatments have been studied pre-clinically; however, there are limitations in the translation of these findings to humans (due to both study design and species to species variability) that require clinical trials. To this end, randomized controlled trials with appropriately powered placebo arms are severely lacking, thus limiting the widespread acceptance of these treatments.13 The aim of this position statement is to review the clinical studies that have been conducted utilizing restorative therapies and provide context regarding the next steps needed before restorative therapies can be considered for broad worldwide use in sexual medicine clinical practices. In doing so, we as the Sexual Medicine Society of North America (SMSNA) will provide an evidence-based position statement on restorative therapies
LOW-INTENSITY SHOCK WAVE THERAPY (LISWT)
Shockwave therapy has been utilized by urologists since the 1980s for the non-invasive fragmentation of kidney stones in the form of extracorporeal shockwave lithotripsy (ESWL).14 In recent years, there has been rapid investigation for its use in restorative therapy applications such as wound healing or bone fractures.15 Within the realm of sexual medicine there has been tremendous interest for LiSWT in the treatment of ED with a handful of preclinical studies followed by several clinical trials and meta-analyses already published.15, 16, 17, 18, 19
There are currently 3 types of LiSWT generators available on the market; electrohydraulic, electromagnetic, and piezoelectric.20 Though they differ in the energy source generating the shockwave, the mechanistic actions of the 3 are similar in producing acoustic waves that transfer energy to tissue leading to direct microscopic mechanical stress.15,17 Based on several preclinical studies shockwaves appear to improve erectile function through neo-angiogenesis, recruitment of progenitor cells and resident stem cells, improvement of microcirculation, vasodilation with subsequent increase in nitric oxide, decrease in fibrosis, and nerve regeneration.15,21, 22, 23, 24 Though these findings are encouraging and suggest a regenerative nature to LiSWT, there exists several limitations with these studies. The first limitation is the heterogeneity of shockwave generator and treatment protocols (dosing, frequency, and location) used, which make comparison of studies difficult. Additionally, the ED that was acutely reproduced in these animal studies was immediately treated with LiSWT, as opposed to the more chronic and complex disease state seen in real-life clinical situations.16
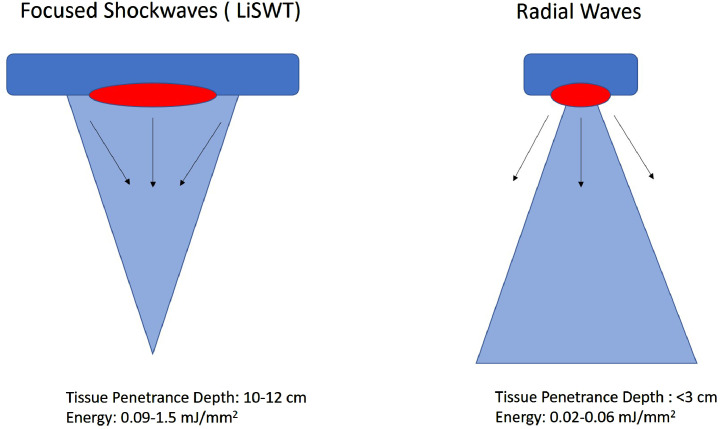
It is important to point out that radial wave therapy is often marketed for ED, but the clinical research is limited and for patients or providers, the technology is not equivalent to LiSWT. Radial wave generators produce dispersive waves away from the probe tip (Figure 1). Consequently, these waves have low tissue penetrance (less than 3 cm) and force of impact (0.02–0.06 mJ/mm2).25 LiSWT, on the other hand, focuses pressure waves in a shorter time frame (<10 nanoseconds) to target focal points at various tissue depth (10–12 cm) and at much higher energy (0.09–1.5 mJ/mm2).20,25 This stark difference in depth of tissue penetrance and energy accounts for the regenerative effects LiSWT on the tissue level. Therefore, radial wave therapy cannot be compared to LiSWT26 for management of ED as its disparate shock wave technology.
Figure 1.
LiSWT mechanism of action compared to radial wave therapy.
Nonetheless, promising preclinical data has led to thirteen published clinical trials studying the use of LiSWT for ED (Table 1). The first major study was a randomized control trial (RCT) performed by Vardi et al.27 Sixty patients, all PDE5 inhibitor responders, were recruited and split into LiSWT (n = 40) and sham (n = 20) groups. Patients were treated with 12 sessions of 300 shocks (Electrohydraulic, Omnispec ED1000, Medispec Ltd., Yehud, Israel) at an energy density of 0.09 mJ/mm2 and a frequency of 120 shocks/min at 5 treatment locations on the penis. Efficacy was evaluated by change in International Index of Erectile Function erectile function (IIEF-EF) scores, of which the Li-SWT group had improvement of 6.7 points compared to 3 points in the sham group (P= .03). Additionally, 19 of 28 men with baseline Erection Hardness Score (EHS < 2) were able to achieve penetrative erections (EHS > 3) upon completion of treatment compared to no improvement in the sham group. Though this initial study was promising, efficacy was only assessed 1 month after the final treatment, demonstrating only a short-term benefit. Kalyvianakis et al also studied 46 PDE5i responder patients utilizing the same shockwave generator with 2 treatment protocols (1 or 2 sessions for 6 weeks, each treatment consisted 5,000 shockwaves at 6 locations at an energy density of 0.05 mJ/mm2 and frequency of 8Hz), and observed IIEF-EF improvement at the longer interval of 6 months in the LiSWT group.28 The group found that LiSWT improved with both regimens and minimally clinical important difference (MCID) was achieved in 62% of the 1 session cohort and 71% in the 2 session cohort. Furthermore, the group added a second phase for patients who completed the first set of treatments, including 6 additional sessions with 2 regimens. Ultimately, they found a positive correlation with total number of sessions and MCID and IIEF-EF score. This study not only provided longer follow up than prior reports, but also suggested that there may be benefit to retreatment dosing. Looking at PDE5i non-responders Kitrey et al performed a RCT of 58 patients with 37 randomized to 12 sessions of 1,500 shocks of 0.09 mj/mm2 at 120 shocks/min with the same shockwave generator.29 They found 40.5% of the LiSWT group achieved MCID in IIEF-EF vs none in sham patients (P= .001), they also found that 54.1% of LiSWT patients had an EHS of 3, while no patients in the sham group attained this level (P< .0001).29 This study was the first to include double-blinding and sham control, however, the total number of patients and follow up was again limited. Two other studies also reported positive treatment efficacy with different types of LiSWT (Electromagnetic, Duolith SD1, Storz, Tagerwilen, Switzerland) and (Electropnematic, Swiss Dolorcast Smart, Electro Medical Systems, Switzerland) with different treatment protocols and in small patient cohorts.30,31 Likewise, some clinical trials did not observe improvements in their overall study population, but did note positive efficacy in subgroup analysis by stratification into ED severity.32,33 To date only 1 published trial showed no treatment efficacy through IIEF-EF or EHS score improvement in comparison to sham.21 Treatment in that trial consisted of two 5-week periods of weekly sessions, separated by a 4-week break (Piezoelectric, FBL10, Richard-Wolf GmBH - 600 shockwaves at energy density of 0.09 mJ/mm2 and frequency of 5Hz in 3 locations). A recent non-randomized study in a large (n = 425) cohort of patients with vasculogenic ED treated with LiSWT for 6 weeks found that at 30 months, 168 patients (39.5%) who responded to LiSWT still reported satisfactory erectile function with SHIM scores of 22-25 without using PDE5i (69 mild, 151 mild to moderate ED). The authors also observed that all 98 severe ED patients did not respond to LiSWT. Though the study design is missing randomization and blinding, the long follow up and volume of patients, stratified by severity of ED, is worth noting.34
Table 2.
Clinical trials with stem cell therapy and stromal vascular fraction
| Intervention | Authors | Year | Patients | Study design | Stem cell type | Findings: |
|---|---|---|---|---|---|---|
| SCT | Bahk et al | 2010 | n = 7 | non-randomized, single-blind study | Umbilical Cord Blood-derived | Diabetic ED patients treated single injection of cavernosal stem cells. 6/7 patients regained morning erections by 3 months, 2/7 regained rigidity enough for penetrance with aid of PDE5i. |
| Levy et al | 2015 | n = 8 | non-randomized, open-label | Placnetal matrix-derived | Heterogenous ED patients treated with single cavernosal injection of stem cells. 3/8 patients achieved erections at 3 months, and PSV increased from 50.7 cm/s to 73.9 cm/s at 6 months. | |
| Yiou et al | 2016 | n = 12 | non-randomized, pilot | Bone marrow mesenchymal | Post-prostatectomy patients in 4 groups, each group treated with a different dosage of cavernosal injected stem cells. No serious side effects. Significant improvement in IIEF-15 and EHS obersved at 6 months. Higher dosage of stem cells showed signficant improvement in spontaneous erections | |
| Yiou et al | 2017 | n = 6 | non-randomized, pilot | Bone marrow mesenchymal | Longer update on prior study and included 6 additional post-prostatectomy patients who saw similar findings in improvements of IIEF-15 and EHS. No prostate cancer recurrence from first study after following for 62.1 ± 11.7 months | |
| Al Demour et al | 2018 | n=4 | non-randomized, open-label | Bone marrow mesenchymal | Diabetic ED patients treated with 2 consecutive cavernosal injections. No reported adverse effects. Significant improvement in IIEF-15 and EHS. | |
| Schweizer et al | 2019 | n=7 | non-randomized, pilot | Bone marrow mesenchymal | Prostatectomy patients treated with single IV infuction of stem cells in 2 groups based on dosing several days prior to prostatectomy. No dose limiting toxicity was observed. No stem cells were detected in all subjects and no stem cells were noted in prostate specimen. At 2 years post prostatecotmy, there was no cancer recurrrence. Function studies showed significnat improvement of sexual function over the course of the study, | |
| SVF | Haahr et al | 2016 | n=17 | non-randomized, open label | Autologous adipose-derived | Post-prostatectomy patients were treated with single cavernosal injection. No major adverse events, minor events relatd to liposuction and ecchymosis from injection. 8/17 patients recovered erectile function to perform sexual intercourse. In post-hoc stratification, of the continent prostatectomy men 8/11 recovered erectile function, no incontinent man regained erectile function |
| Haahr et al | 2018 | n = 21 | non-randomized, open-label | Autologous adipose-derived | Post-prostatectomy patients were treated with single cavernosal injection. No major adverse events, 8 minor events related to liposuction. 8/15 of patients in continent group report erectile function enough for penetrance at 12 months. IIEF-5 unchanged at 1 month post treatment, but significantly increased at 6-7 months post tretment and sustained at 12 months. | |
| Khera et al | 2019 | n = 30 | randomized, with delayed cross over | Autologous adipose-derived | Heterogenous ED patients were treated with SVF injections. Importantly, patients abstained from erectogenic medications for 6 months following injections. Minor adverse events noted include pain and swelling at site of injection or liposuction. In treated patinets IIEF-EF scores improved at least 2-4 points from baseline starting at 3 months sustained through 9 months. Control experience no benefits at 6 months of treatment. |
Table 3.
Clinical trials of platelet-rich plasma
| Intervention | Authors | Year | Patients | Study design | Findings |
|---|---|---|---|---|---|
| PRP | Epifanova et al | 2017 | n = 75 (30 activated PRP, 30 activated PRP + PDE5i, 15 non-activated PRP) | randomized control | Heterogenous ED patients received weekly cavernosal injections for 3 weeks. Improvements were seen in IIEF-5, SEP, patient satisfaction, and penile duplex ultrasound parameters. |
| Matz et al | 2018 | n = 5 (4 with ED, 1 with ED + PD) | case series | Organic ED and one with Peyronie's and ED were reated with cavernosal injections, mean receipt of 2.1 injections. Improvement in IIEF-5 score by 4.1 points over 15.5 month follow-up |
Table 1.
Clinical trials of LiSWT
| Intervention | Authors | Year | Patients | Study design | Device | Treatment | Findings |
|---|---|---|---|---|---|---|---|
| LiSWT | Vardi et al | 2012 | n = 60 (40 LiSWT, 20 sham) | randomized, double-blind, sham control | Electrohydraulic, Omnispec ED1000, Medispec Ltd., Yehud, Israel | energy density of 0.09 mJ/mm2 and a frequency of 120 shocks/min; 12 week period (2 treatments/wk) | PDE5i responding patients treated with 12 sessions of 300 shocks. LiSWT had 6.7 point vs 3 point improvement in sham for IIEF-EF scores. 19/28 improved to an EHS > 3 in Li-SWT compared to sham. |
| Yee et al | 2014 | n = 58 (30 LiSWT, 20 sham) | randomized, double-blind, placebo control | Electrohydraulic, Omnispec ED1000, Medispec Ltd., Yehud, Israel | energy density of 0.09 mJ/mm2 and a frequency of 120 shocks/min; 300 shocks each at distal, mid, proximal penile shaft, left and right crura; 12 week period (2 treatments/wk) | Subgroup analyses revealed IIEF-EF improvement in LiSWT patients with baseline severe ED compared to sham. No differences in overall comparison. | |
| Srini et al | 2015 | n = 77 (60 LiSWT, 17 sham) | randomized, sham group | Electrohydraulic, Omnispec ED1000, Medispec Ltd., Yehud, Israel | energy density of 0.09 mJ/mm2 and a frequency of 120 shocks/min; 300 shocks each at distal, mid, proximal penile shaft, left and right crura; 12-week period (2 treatments/wk) | Subgroup analyses revealed IIEF-EF improvement in LiSWT patients of at least 7 points in moderate and severe ED patients compared to sham. 83% LiSWT had EHS ≥ 3 compared to regressed EHS in sham. | |
| Olsen et al | 2015 | n = 105 (51 LiSWT, 51 sham) | randomized, sham group | Electromagnetic, Duolith SD1, Storz, Tagerwilen, Switzerland | energy density of 0.15 mJ/mm2; 500 impulses at distal, centre, proximal part of corpora cavernosum (bilaterally); 5 week period (1 treatment/wk) | 29/51 Li-SWT improved to EHS ≥ 3 compared to 5/51 sham. No observable differences in IIEF-EF score improvements between the 2 groups. | |
| Kitrey et al | 2016 | n = 55 (37 LiSWT, 18 sham) | randomized, sham group | Electrohydraulic, Omnispec ED1000, Medispec Ltd., Yehud, Israel | energy density of 0.09 mj/mm2 at a frequency of 120 shocks/min; 3 week period (2 treatments/wk) | Patients treated with 12 sessions of 1,500 shocks. LiSWT, 40.5% achieved MCID in IIEF-EF improvement vs none in sham. 54.1% of Li-SWT had EHS ≥ 3 vs none in sham. | |
| Fojecki et al | 2017 | n = 118 (58 LiSWT, 60 sham) | randomized, sham group | Piezoelectric, FBL10, Richard-Wolf GmbH, Knitlingen, Germany | energy density of 0.09 mJ/mm2, 5Hz; 600 shocks to corpora cavernosa; 10-week period | No observed difference between IIEF-EF and EHS scores between LiSWT and sham groups. | |
| Fojecki et al | 2018 | n = 126 (43 Linear LiSWT 5 weekly sessions, 52 10 weekly sessions) | randomized | Piezoelectric, FBL10, Richard-Wolf GmbH, Knitlingen, Germany | energy density of 0.09 mJ/mm2, 5Hz; 600 shocks to corpora cavernosa; 10 week period | No noted differences between 2 cycles of linear LiSWT vs one cycle in ED outcomes. | |
| Kalvianakis et al | 2018 | n = 46 (30 LiSWT, 16 sham) | randomized, sham group | Electrohydraulic, Omnispec ED1000, Medispec Ltd., Yehud, Israel | energy density of 0.05 mJ/mm2 and frequency of 8Hz; 12 week period (2 treatments/wk) | PDE5i responding patients treated with either protocol (1 or 2 sessions for 6 weeks with 5000 shocks). LiSWT had 75% achieved MCID in IIEF-EF compared to only 25% in sham. | |
| Kalvianakis et al | 2018 | n = 42 (21 LiSWT 1 theapy/week, 21 LiSWT 2 therapies/week | randomized | Electromagnetic, Aries 2, Dornier MedTech GmbH, Wessling, Germany | energy density of 0.05 mJ/mm2, 8Hz; 1,000 shockwaves each to the left and right shaft, 1,000 shockwaves each to the 2 crura, and 500 shockwaves each to the left and right penile hilum; 6 week period | Two shockwave therapies per week resulted in better IIEF-EF outcomes. | |
| Yamaacake et al | 2019 | n = 20 (10 LiSWT, 10 sham) | randomized, sham group | Electropneumatic, Swiss Dolorclast Smart, Electro Medical Systems, Swizerland | energy density of 0.09 mJ/mm2 and a total of 2,000 shocks per session (throughout penile shaft); 3 weeks (2 treatments/wk) | Study population was kidney transplant recipients. Li-SWT, 70% had IIEF-EF score improvements of at least 5 compared to only 10% having that same interval improvement in sham. | |
| Kalvianakis et al | 2020 | n = 97 (4 LiSWT groups of various sessions/week and energy flux density (EFD)) | randomized | Electromagnetic, Aries 2, Dornier MedTech GmbH, Wessling, Germany | Group A: energy density of 0.05 mJ/mm2, 8 Hz, 2 sessions/wk, 12 sessions; Group B: energy density of 0.05 mJ/mm2, 8 Hz, 3 sessions/wk, 12 sessions; Group C: energy density of 0.096 mJ/mm2, 5 Hz, 2 sessions/wk, 12 sessions; Group D: energy density of 0.096 mJ/mm2, 5 Hz, 3 sessions/wk, 12 sessions | No noted differences between study groups of sessions/week and EFD in achieveing MCID in IIEF-EF scores. | |
| Ramasamy et al | 2020 | n = 80 (40 LiSWT 3600 shocks/week, 40 LiSWT 3600 shocks/2 weeks | randomized | Electromagnetic, MoreNova, DirexGroup, Israel | energy density of 0.09 mJ/mm2, 1Hz; Group A: 720 shocks on M,T,W,Th, F; Group B: 600 shocks on M, W, F (2 weeks) | Overall improvement of IIEF-EF and EHS scores between the 2 groups. Frequency of shockwave sessions did not affect outcomes. | |
| Adeldaeim et al | 2020 | n = 425 (all received 6 sessions over 6 weeks) | Non-randomized | PiezoWave2, Richard-Wolf GmbH, Knitlingen, Germany | first 500 shocks at energy density of 0.16 mJ/mm2 elevated to 0.20 mJ/mm for the rest of 6000 total shocks. Shocks distributed to 5 separate site with 1200 shocks each. | Largest study with signficant follow up (30 months) found age, DM, hypertension, smoking, obesity, hyperlipidemia all affected success of LiSWT and of those who had success from treatment, a majority (76.3%) had durability of resutls at follow up without need for PDE5i |
To better identify the ideal LiSWT treatment, there have been a few studies that have compared various treatment protocols. Ramasamy et al performed a RCT comparing patients receiving 3,600 shocks over 1 week (n = 40) vs 2 weeks (n = 40) (Electromagnetic, MoreNova, DirexGroup, Israel, energy density of 0.09 mJ/mm2 and frequency of 1Hz).35 Both groups observed improved IIEF-EF and EHS scores, but there was no discernible difference in outcomes between the 2 groups. Kalyvianakis et al also compared session frequency and energy density (EFD) by randomizing 97 patients into 4 treatment groups: (2 sessions/week, EFD 0.05 mJ/mm2), (3 sessions/week, EFD 0.05 mJ/mm2), (2 sessions/week, EFD 0.10 mJ/mm2), and (3 sessions/week, EFD 0.10 mJ/mm2).36 They found no significant differences between session frequency and EFD in MCID in IIEF- EF between groups, though there was a non-statistical significant trend towards EFD 0.10 mJ/mm2 having better efficacy. Fojecki et al also compared 2 cycles of linear LiSWT vs 1 cycle of linear LiSWT (same device settings as their prior publication21) and found no differences in IIEF-erectile function or EHS outcomes between the 2 treatment groups.18 Therefore, studies to date have not identified any clear statistical difference in treatment protocols.
Collectively, the cumulative results from the LiSWT clinical trials suggest a promising degree of efficacy and are encouraging for this technology. Importantly, across all trials, there were no documented adverse events with various LiSWT treatment protocols. Thus, at bare minimum LiSWT, within the parameters of studies performed, is safe. However, the shockwave generator types and protocols (energy settings, dosing, frequency of use, probe locations, and duration of therapy) were inconsistent between studies and consequently difficult to compare. Looking at the studies, most patient cohorts are small and heterogeneous, further complicating any type of comparison. Even in studies where patients have similar sources of ED (ie, prostatectomy) underlying comorbidities such as diabetes, age, and vascular disease makes randomization and patient grouping challenging.
A major limitation to most LiSWT studies is the lack of randomization to a sham control cohort. A number of studies have performed randomization and provided sham controls (ie, identical treatment protocols without shocks) and blinding both patients and providers, whenever possible. Blinding providers is especially important since end-point analysis often included functional surveys like EHS, IIEF, and SHIM. Therefore, clinical trials exploring the types of shockwave, utilization of sham control cohorts, and comparing patient populations would be very helpful in further identifying ideal treatment candidates and true ability to use this restorative therapy for management of ED. Likewise, more studies with variable protocols are needed to find the ideal dosing for maximal effect. Similarly, given the broad spectrum of treatment protocols future clinical trials should attempt to provide some standardization in both device settings (measured in total energy of treatment or EFD) as well as duration of treatment (6 months, 1 year, or greater). This would allow for larger multi-center studies and enable comparison between different trials. In particular, an area of interest is the durability of treatment response should be examined, since some studies with retreatment phases suggest that maintenance dosing after initial treatment may improve overall efficacy. Finally, the basic science behind the mechanisms of LiSWT must continue to be explored. The current hypothesis consists of a combination of circulation improvement, stem cell recruitment and activation, immune regulation, fibrosis reduction, and nerve repair.31 Though the actual mechanism may be a complex interplay of these components, additional work may identify more precise targets allowing for enhanced restorative effect.
STEM CELL THERAPY/ STROMAL VASCULAR FRACTION
SCT is an exciting application of restorative medicine with a theoretical promise of disease “cure.”37 Stem cells are unspecialized, undifferentiated cells found in both embryonic (ie, placental/umbilical) and adult (ie, mesenchymal: bone marrow, adipose) tissue.38 Given their precursory nature, these cells harbor self-renewal potential and the ability to differentiate into other types of cells. Recently, studies have shown that these cells exhibit regenerative effects by releasing growth factors, cytokines, and chemokines; upregulating pathways to reduce inflammation, inhibit apoptosis, improve wound healing, and drive angiogenesis and neuritogenesis.39 The paracrine effects of SCT have led to widespread application in several fields, including orthopedics, cardiology, and neurology.40,41
Within the clinical scope of ED, both mesenchymal and embryonic stem cells have been of particular interest.12,42,43 Embryonic stem cells are isolated from both the placental and umbilical cord blood. Although these cells can be considered as childbirth waste products, their origins raise ethical concerns and they possess an element of tumorigenic (teratoma) potential.44 Mesenchymal cells, derived from bone marrow and adipose tissue, among other sources, are more readily available and have minimal ethical considerations.41,45 Additionally, some researchers are exploring stromal vascular fraction (SVF), the heterogeneous mixture from which adipose mesenchymal progenitor cells are derived, including stem cell populations and endothelial cells.46 SVF is isolated via centrifugation from liposuction aspirate after the adipose tissue is digested by enzymes and includes not only adipose derived stem cells (ADSC), but also preadipocytes, lymphocytes, smooth muscle cells, and endothelial progenitor cells. By including the milieu along with the stem cells, there is synergistic activity that drives cellular adhesion, tissue remodeling, angiogenesis, and cell differentiation.46 These attributes provide stem cells with an ideal regenerative microenvironment.
Though preclinical data for SCT and SVF are promising and have led to important discoveries about the mechanisms of erectile tissue regeneration, clinical trial data in humans is limited47,48 (Table 1). There have been several small studies using similar protocols and patient populations. Two studies, in particular, looked at embryonically-derived stem cells. The earlier, done by Bahk et al looked at 7 diabetic patients with ED who failed medical therapy.49 Patients were treated with umbilical cord blood-derived stem cells (total of 1.5 × 107 cells) injected into both corpora cavernosa and compared against 3 control patients treated with saline. By 3 months, 6 out of the 7 patients had regained morning erections and when paired with PDE5 inhibitors before coitus, 2 out of the 7 patients achieved erections sufficient for penetration. Levy et al looked at 8 patients with organic ED for at least 6 months and those with baseline IIEF scores of 21 or higher were treated with placental matrix derived stem cells injected into the corpora cavernosal.50 Looking at penile Doppler ultrasonography the authors found significantly improved systolic velocity (PSV) between 6 weeks and 3 months follow up (25.5–56.6 cm/s to 32.5–66.7 cm/s, P < .5) and at 6 months follow up (PSV 50.7–73.9 cm/s P < .01). Of the 8 patients, 3 were able to achieve erection without pharmacologic assistance and the only adverse effect found was local ecchymosis. Al Demour et al looked at 4 diabetic patients with medication refractory ED.51 Patients were treated with 2 rounds of injections of bone marrow derived stem cells. Efficacy was assessed using the IIEF-15 and EHS for 12 months, tolerability of the injections immediately and at 24 hours, and safety for 2 years. Overall, the procedure was well tolerated, and no significant adverse events were reported. Three of the patients reported significant improvement from baseline IIEF-15 score (P = .4), and all patients reported significant increases in EHS (P = .2). Although these studies provide promising early results, they are limited by sample size and study design (blinding). Though the Al Demour et al study did look at 2 years of safety, the other studies did not follow patients beyond 11 months and none of the 3 studies followed efficacy beyond their initial study parameters.
Yiou et al focused on patients with ED post radical prostatectomy (RP) with the INtra-cavernous STem-cell INjection (INSTIN) clinical trial.52,53 In the first study they enrolled twelve patients with localized prostate cancer post RP with ED refractory to maximal medical treatment. This was a phase 1 dose escalating trial that included 4 groups treated with 4 doses of bone marrow-derived stem cells (2 × 107, 2 × 108, 1 × 109, 2 × 109 cells). The primary end point of the study was tolerance/safety, but the authors also assessed IIEF-15, EHS and color duplex Doppler penile US. The authors found no adverse events and all patients tolerated the injections well. At 6 months they found significant improvements in the intercourse satisfaction (6.8 ± 3.6 vs 3.9 ± 2.5, P = .4) and erectile function (17.4 ± 8.9 vs 7.3 ± 4.5, P = .006) domains of the IIEF-15 and EHS (2.6 ± 1.1 vs 1.3 ± 0.8, P = .008) in the total population. Based on the first study the authors added 6 additional patients with 1 × 109 dosing and followed the first twelve patients for updates on their clinical parameters. In the 6 new patients, the authors saw similar findings in improvements for IIEF-5 domains and EHS compared the with earlier study. It should be noted that these studies were powered only for safety, and there were no adverse events noted. Importantly, the group reported a decline in the improved erectile function over time, suggesting a role for repeat injections. Likewise, following the original twelve patients out to 61.1 ± 11.7 months, the authors found no prostate cancer recurrence. This is critical given the growth potential of SCT, leading to reservations about using them safely in cancer patients. Schweizer et al looked at cancer safety of SCT in 7 patients with clinical stage T1c prostate cancer and baseline PSA <10 ng/mL.54 Three patients received a dose of 1 × 106 stem cells per kilogram (maximum of 1 × 108 cells) IV infusion 4 days prior to planned RP. The other 4 patients received 2 × 106 stem cells per kilogram (maximum of 2 × 108 cells) IV infusion. Two were dosed 4 days prior to planned RP and the other two 6 days prior to RP. There were no delays in surgical treatment and all patients had undetectable PSA (<0.1 ng/mL) at 30 days postoperatively. Using human leukocyte antigen (HLA)-A locus the authors found low quantities of stem cell DNA within the prostate specimen. Using this approach, the authors did not detect any stem cell accumulation in the prostate tissue. Likewise, at 2 years post prostatectomy, the authors found no cancer recurrence. Though the study focused on cancer safety the authors did look at functional outcomes and noted expected post-surgical declines in urinary incontinence and ED using the Expanded Prostate Cancer Composite (EPIC) questionnaire. However, they did observe significant improvement in the EPIC sexual function score over the course of the study. Therefore, this study suggests both feasibility and relative safety of SCT in cancer patients.
To date there have only been 2 clinical studies published looking at SVF. Haahr et al looked at SVF injections in 21 men suffering from post RP ED refractory to medical therapy.55,56 Patients underwent liposuction under general anesthesia with isolation of adipose derived regenerative cells or SVF. Then the men received intracavernosal injection of the isolated SVF within 2 hours of harvest and were followed for 1 year for safety and tolerance with secondary analysis for improvement of erectile function. Injections were well tolerated and only minor events with liposuction (8 events) were noted. No serious adverse events were observed. Of the fifteen continent men, 8 reported erectile function satisfactory for penetration. However, the men who remained incontinent following RP did not regain erectile function. In another prospective 2-center trial for SVF. Thirty patients were randomized to either SVF vs control in a 2:1 fashion.13 Control patients were allowed to cross over to SVF treatment after 9 months or exit the study. Men with ED (IIEF< 26) greater than 6 months from RP, diabetes, and or vascular disease were all included in the study. The primary end point was restored erectile function, as determined by IIEF scores at 6 months; in addition, safety was followed in terms of adverse events for 36 months. Though the trial has not been published to date, early results reveal that 21 patients reported a total of 58 adverse events. The most common being bruising or pain at the site of SVF injection or liposuction within the first 48 hours. No serious adverse events have been reported to date. IIEF-5 scores demonstrated an improvement of at least 2–4 points from baseline beginning at 3 months and sustained at 9 months, no control patients experienced benefits at 6 months after treatment.
Despite the promising results of these early studies, it is important to recognize that these trials involve small cohorts, are open label, and utilize end points which demonstrate relative safety rather than efficacy. When taken together, the published studies include no more than 70 patients with variable inclusion and exclusion criteria. Most studies did look at adverse events as the primary end point, and, beyond minor complaints at the site of injection immediately after the procedure, no study noted any significant adverse event. This was also the case for the 2 studies that specifically looked at SCT in the setting of prostate cancer.53,54 These findings suggest that SCT and SVF are likely safe and well tolerated by patients and feasible in the post RP population. No patient in either study had evidence of PSA biochemical recurrence, with 1 study following patients up to 2 years. The results from the studies looking at efficacy must be cautiously and carefully analyzed. In ED research, the placebo effect can play a serious role in outcomes, and in the post RP population, a subgroup of patients may experience spontaneous recovery, independent of an intervention.57 The nocebo effect may complicate results, though most patients who elected to undergo invasive SCT therapy may overestimate the therapeutic benefit. Likewise, there exists significant heterogeneity among the studies including study design (blinding), stem cell source and quantity, and treatment regimen. In particular, for SVF additional characterization and quantification of the amount of stem cell content is critical to assess efficacy and compare trials. This diversity of reported elements makes comparison between studies difficult and reflects our incomplete knowledge of SCT/SVF biology. Questions remain regarding which stem cell sources are the most efficacious, including cell types under investigation like urine-derived stem cells. Likewise, discoveries in SVF have led to increased basic research in stem-cell-cultured media (secretome) begging the question of whether it is the stem cells or the associated milieu that drives regenerative processes.58 Therefore, in order for SCT/SVF to move forward we need clinical studies with translation science that look at mechanisms of action and underlying biology. To overcome the significant bias that plaques ED research, clinical studies moving forward need to be larger, placebo-controlled, double-blinded, and randomized trials.
PLATELET-RICH PLASMA (PRP)
The regenerative potential of platelet-rich plasma (PRP), autologous blood plasma with supraphysiologic concentrations of activated platelets, was first described in the 1980s within the field of maxillofacial surgery.59 Since that time, PRP has been utilized in a myriad of fields such as orthopedics, cardiology, dermatology, ophthalmology, and more recently, urology.60, 61, 62, 63, 64 However, despite its widespread adoption, PRP's biological underpinnings remain poorly understood. Limited preclinical data demonstrates that PRP is comprised of a rich milieu of growth factors (platelet-derived growth factor, insulin-like growth factor, vascular endothelial growth factor, insulin-like growth factor, epidermal growth factor, fibroblast growth factor) and activated platelets which work together to facilitate mitogenesis and neo-angiogenesis, thereby reconstituting diseased tissues.65 Components within PRP have also been shown to act as a scaffold for healing tissues.66 PRP can be prepared by sequential centrifugation of whole blood with removal of red blood cells and platelet-poor plasma, followed by addition of a platelet activating factor such as thrombin or 10% calcium chloride.67 Like SCT, PRP represents an attractive option for the treatment of ED because it can be selectively injected into the target organ to facilitate localized tissue healing and minimize systemic and non-target side effects.
To-date, only 3 preclinical manuscripts have evaluated mechanisms of action behind PRP.68, 69, 70 In these studies, PRP was shown to: (i) improve maximal intracavernosal pressures following exogenous nerve stimulation of the cavernous nerve at various time points post treatment (1 and 3 months), (ii) enhance myelination of cavernous nerve axons, and (iii) reduce expression of pro-fibrotic signaling molecules (TGF-beta 1) within the corporal bodies when compared to vehicle (saline only) or sham.68, 69, 70 However, these studies are severely limited by small sample sizes (n = 24, respectively), heterogeneous methods of isolating PRP and stimulating the cavernous nerves, and poor standardization of PRP concentrations. These studies have acknowledged that the clinical effects they demonstrate may be highly variable based on PRP preparation and administration parameters. Overall, very few basic science studies have attempted to characterize PRP's effect on erectile function recovery.
Despite early enthusiasm for PRP as a restorative treatment for ED, the available evidence to support its use in the clinical setting of ED is lacking with only 2 clinical trials performed to date (Table 1). The largest clinical study evaluating the efficacy of PRP in ED was performed in 75 patients with heterogeneous severities of ED (IIEF range 0–17).71 Patients were randomized to receive either activated PRP (addition of calcium chloride to promote α–granule degranulation, n = 30), activated PRP plus oral PDE-5 inhibitor (n = 30), or non-activated PRP (without calcium chloride, n = 15). Autologous PRP was obtained by 2-step centrifugation of 72 mL of autologous blood for maximum platelet concentrations up to 2,400 k/µL and administered in 4 mL injections to each corpus cavernosum. The procedure was performed weekly for 3 weeks. Primary outcomes included IIEF-5 score, SEP score, and penile duplex Doppler measurements with administration of prostaglandin E1 at 28 days, 90 days, and 180 days post-treatment. The authors report significant improvements in IIEF-5 and SEP scores, patient satisfaction, and penile duplex ultrasound vascular parameters. However, there are major limitations to this data including questionable statistical methods (ie, no description of baseline or net change in any of the primary or secondary end points), no description of the type, dosage or duration of PDE-5 inhibitor that was co-administered, and no placebo or control group as comparator. Furthermore, the authors suggest that PRP contains the amount of growth factors necessary for therapeutic effect; however, they do not measure growth factor concentrations, nor describe how differences in growth factor concentrations may modulate recovery of erectile function. The authors also conclude that PRP is both safe and cost-effective; however, they provide no adverse event data or cost analysis to support these claims.
The other published clinical study on PRP in ED was performed by Matz et al in which the authors treated 4 patients with organic ED and 1 patient with concomitant ED and PD with intracavernosal injection of platelet-rich fibrin matrix (PRFM).72 The authors noted objective improvements in IIEF-5 scores by 4.1 points over the 15.5 months follow-up period. The therapy was overall well tolerated with most patients experiencing only mild pain and localized bruising, without major adverse events. Nonetheless, this study is limited by small sample size and lack of placebo, as acknowledged by the authors.
Currently, no double-blinded prospective randomized controlled clinical trials has provided enough evidence to support the widespread use of PRP for treatment of ED, though some are currently in process. Preclinical studies delving into the mechanisms of action and pathways are critically important and randomized controlled trials of larger cohorts are needed before any claims about efficacy or safety can be made.
REGULATORY APPROVAL
To date, there is no Food and Drug Administration (FDA) regulatory approval for any restorative therapies for the treatment of ED. However, components of restorative therapies have been approved, such as the LiSWT devices themselves (albeit for treatment of alternative disease states), or clinical separators/centrifuges used in the preparation of PRP. Therefore, any use of these restorative therapies is considered off-label. Some restorative therapies are already being employed in practice as they circumvent traditional regulatory pathways. PRP, for instance, falls under HCT/P361 exemption, which states that tissue that is minimally manipulated once removed from a patient (ie, centrifugation only) may be autologously implanted into that same patient in a process that is exempt from FDA approval.73 One regulatory challenge of SCT and SVF is the fact that many isolates involve heterogeneous cell sources and formulations. As such, each component would need to be evaluated individually as a drug or device before approval could be granted.
ASPECTS FOR FUTURE STUDIES
There are several limitations that need to be addressed prior to widespread acceptance of restorative therapies. A key element is standardizing measurable validated outcome instruments which would quantify therapy efficacy as well as allow for comparison between studies. For LiSWT future studies need to address efficacy across different devices, dosing regimens, and the duration of treatments. SCT/SVF research will require a combination of advances in translational science as well as larger, double blind, RCT powered for both safety and efficacy. Finally, PRP simply does not have enough clinical evidence to support any current application. Therefore, additional well-designed clinical trials with PRP would be very important towards advancing restorative medicine in urology.
Important limitations to these future studies include costs and patient recruitment. With regards to funding, it is critical that patients are enrolled in studies at minimal additional cost to avoid financial incentives and reduce selection bias. Additionally, elements of study design, such as, double blinding, are critical to minimize provider bias, especially in studies with industrial funding. Patient recruitment is also a limiting factor, as highlighted by the small patient cohorts in current published literature. Methods to improve recruitment include opening up study design (ie, cross over with appropriate delayed interval) and multi-institutional collaborations. Though multi-institutional studies introduce variability, they increase study numbers and broaden patient selection. Considering that the current state of clinical trials in restorative therapies is to determine efficacy, appropriately powered studies by increased patient volume and eliminating bias through study design are critical to advancing this technology.
SMSNA POSITION STATEMENT ON RESTORATIVE THERAPIES
The SMSNA does not advocate for restorative therapies to be offered or used in routine clinical practice. However, the SMSNA strongly supports the development of novel erectogenic therapies, given that many men with ED either fail currently available treatments or find them unpalatable. Restorative therapies are an exciting avenue for this work, as they utilize regenerative medicine technologies to re-establish organ function. The emergence of restorative therapies such as low intensity shock wave therapy, stem cells therapies (including SVF) and platelet rich plasma therapy represents a new frontier of investigative therapies for ED treatment. At the moment, however the cumulative body of clinical trials for restorative therapies (Table 1) is largely incomplete, and many questions remain unanswered. The society, however, recognizes the need for adequately powered, multicenter, randomized, sham/placebo-controlled trials in well-characterized patient populations to ensure that efficacy and safety are demonstrated for any novel ED therapy.69 The society agrees with the regulatory agency pathway of approval including safety and efficacy studies to achieve our goals in diverse patient populations. Without FDA approval, the use of any novel therapy is considered off-label. To date, there is an absence of robust clinical trial data supporting restorative therapies’ efficacy in humans, although relative safety has been established for SCT/SVF and LiSWT. Furthermore, the precise treatment parameters for LiSWT such as: energy settings, dosing, frequency of use, and duration of therapy among others remains to be fully elucidated. Cell source allowing optimization of these evolving SCT and SVF therapies remain, as yet, undefined. Unlike conventional pharmacologic therapies which generally have a primary, well-defined target, the mechanism of action of restorative therapies is likely to be complex, involving a number of pathways inherent to the regenerative potential of the host. The SMSNA both advocates for and supports the application of high-quality research, both pre-clinical and clinical, aimed at better understanding the mechanisms involved, the magnitude and durability of benefit and the long-term safety of restorative therapies. Thus, given the current lack of regulatory agency approval for any restorative (regenerative) therapies for the treatment of ED and until such time as approval is granted, SMSNA believes that the use of shock waves or stem cells/SVF are investigational and platelet rich plasma is experimental and should only be conducted under research protocols in compliance with Institutional Review Board approval at little or no cost to the patient. Specifically, the SMSNA does not feel that it is appropriate or ethical for providers to advertise or otherwise make implicit or explicit claims of efficacy for these therapies pending further data. Similarly, patients considering such therapies should be fully informed as to the lack of data demonstrating clinically relevant efficacy and consented regarding the potential benefits and risks. In summary, at the current time, the SMSNA does not advocative for restorative therapies to be offered or used in routine clinical practice.
STATEMENT OF AUTHORSHIP
James L. Liu: Conceptualization and Writing-Original draft; Kevin Y. Chu: Conceptualization and Writing-Original draft; Andrew T. Gabrielson: Conceptualization and Writing-Original draft; Run Wang: Writing- Review & Editing; Landon Trost: Writing- Review & Editing; Gregory Broderick: Writing- Review & Editing; Kelvin Davies: Writing- Review & Editing; Gerald Brock: Writing- Review & Editing; John Mulhall: Writing- Review & Editing; Ranjith Ramasamy: Conceptualization and Writing-Original draft; Writing- Review & Editing, Supervision; Trinity J. Bivalacqua: Conceptualization and Writing-Original draft, Writing- Review & Editing, Supervision.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: None.
REFERENCES
- 1.Fisher WA, Rosen RC, Eardley I. Sexual experience of female partners of men with erectile dysfunction: The female experience of men's attitudes to life events and sexuality (females) study. J Sex Med. 2005;2:675–684. doi: 10.1111/j.1743-6109.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 2.Kessler A, Sollie S, Challacombe B. The global prevalence of erectile dysfunction: A review. BJU Int. 2019;124:587–599. doi: 10.1111/bju.14813. [DOI] [PubMed] [Google Scholar]
- 3.Aytaç IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Lee DM, Forti G. Age-related changes in general and sexual health in middle-aged and older men: Results from the European male ageing study (EMAS) J Sex Med. 2010;7:1362–1380. doi: 10.1111/j.1743-6109.2009.01601.x. [DOI] [PubMed] [Google Scholar]
- 5.Irwin GM. Erectile dysfunction. Prim Care. 2019;46:249–255. doi: 10.1016/j.pop.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AL, Nehra A, Breau RH. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Lee M, Sharifi R. Non-invasive management options for erectile dysfunction when a phosphodiesterase type 5 inhibitor fails. Drugs and Aging. 2018;35:175–187. doi: 10.1007/s40266-018-0528-4. [DOI] [PubMed] [Google Scholar]
- 9.Castiglione F, Ralph DJ, Muneer A. Surgical techniques for managing post-prostatectomy erectile dysfunction. Curr Urol Rep. 2017;18:90–99. doi: 10.1007/s11934-017-0735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burri A, Porst H. Results from an online survey investigating ED patients’ insights and treatment expectations. Int J Impot Res. 2015;27:191–196. doi: 10.1038/ijir.2015.14. [DOI] [PubMed] [Google Scholar]
- 11.Campbell JD, Milenkovic U, Albersen M. What is the future of erectile dysfunction therapy? Curr Sex Heal Reports. 2018;10:169–176. [Google Scholar]
- 12.Campbell JD, Milenkovic U, Usta MF. The good, bad, and the ugly of regenerative therapies for erectile dysfunction. Transl Androl Urol. 2020;9(Suppl 2):S252–S261. doi: 10.21037/tau.2019.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera M, Bivalacqua T, Goldstein I. An update on regenerative medicine clinical trials in erectile dysfunction: Have we made any progress? Eur Urol Focus. 2019;5:536–538. doi: 10.1016/j.euf.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Eisenmenger W. The mechanisms of stone fragmentation in ESWL. Ultrasound Med Biol. 2001;27:683–693. doi: 10.1016/s0301-5629(01)00345-3. [DOI] [PubMed] [Google Scholar]
- 15.Fode M, Hatzichristodoulou G, Serefoglu EC. Low-intensity shockwave therapy for erectile dysfunction: Is the evidence strong enough? Nat Rev Urol. 2017;14:593–606. doi: 10.1038/nrurol.2017.119. [DOI] [PubMed] [Google Scholar]
- 16.Sokolakis I, Dimitriadis F, Teo P. The basic science behind low-intensity extracorporeal shockwave therapy for erectile dysfunction: A systematic scoping review of pre-clinical studies. J Sex Med. 2019;16:168–194. doi: 10.1016/j.jsxm.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Sokolakis I, Hatzichristodoulou G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: A systematic review and meta-analysis of randomised controlled trials. Int J Impot Res. 2019;31:177–194. doi: 10.1038/s41443-019-0117-z. [DOI] [PubMed] [Google Scholar]
- 18.Fojecki GL, Tiessen S, Osther PJS. Effect of linear low-intensity extracorporeal shockwave therapy for erectile dysfunction—12-month follow-up of a randomized, double-blinded, sham-controlled study. Sex Med. 2018;6:1–7. doi: 10.1016/j.esxm.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong L, Chang D, Zhang X. Effect of low-intensity extracorporeal shock wave on the treatment of erectile dysfunction: a systematic review and meta-analysis. Am J Mens Health. 2019;13:1–14. doi: 10.1177/1557988319846749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz JE, Clavijo RI, Rizk P. The basic physics of waves, soundwaves, and shockwaves for erectile dysfunction. Sex Med Rev. 2020;8:100–105. doi: 10.1016/j.sxmr.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fojecki GL, Tiessen S, Osther PJS. Effect of low-energy linear shockwave therapy on erectile dysfunction—A double-blinded, sham-controlled, randomized clinical trial. J Sex Med. 2017;14:106–112. doi: 10.1016/j.jsxm.2016.11.307. [DOI] [PubMed] [Google Scholar]
- 22.Gotte G, Amelio E, Russo S. Short-time non-enzymatic nitric oxide synthesis from l-arginine and hydrogen peroxide induced by shock waves treatment. FEBS Lett. 2002;520:153–155. doi: 10.1016/s0014-5793(02)02807-7. [DOI] [PubMed] [Google Scholar]
- 23.Bongrazio M, Silva-Azevedo L DA, Bergmann EC. Shear stress modulates the expression of thrombospondin-1 and CD36 in endothelial cells in vitro and during shear stress-induced angiogenesis in vivo. Int J Immunopathol Pharmacol. 2006;19 205873920601900. [PubMed] [Google Scholar]
- 24.Behr-Roussel D, Giuliano F. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. Transl Androl Urol. 2016;5:977–979. doi: 10.21037/tau.2016.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Notarnicola A, Tamma R, Moretti L. Effects of radial shock waves therapy on osteoblasts activities. Musculoskelet Surg. 2012;96:183–189. doi: 10.1007/s12306-012-0213-4. [DOI] [PubMed] [Google Scholar]
- 26.Wu SS, Ericson KJ, Shoskes DA. Retrospective comparison of focused shockwave therapy and radial wave therapy for men with erectile dysfunction. Transl Androl Urol. 2020;9:2122–2128. doi: 10.21037/tau-20-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vardi Y, Appel B, Kilchevsky A. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol. 2012;187:1769–1775. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 28.Kalyvianakis D, Memmos E, Mykoniatis I. Low-intensity shockwave therapy for erectile dysfunction: A randomized clinical trial comparing 2 treatment protocols and the impact of repeating treatment. J Sex Med. 2018;15:334–345. doi: 10.1016/j.jsxm.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Kitrey ND, Gruenwald I, Appel B. Penile low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: A double-blind, sham controlled study. J Urol. 2016;195:1550–1555. doi: 10.1016/j.juro.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 30.Olsen AB, Persiani M, Boie S. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol. 2015;49:329–333. doi: 10.3109/21681805.2014.984326. [DOI] [PubMed] [Google Scholar]
- 31.Yamaçake KGR, Carneiro F, Cury J. Low-intensity shockwave therapy for erectile dysfunction in kidney transplant recipients. A prospective, randomized, double blinded, sham-controlled study with evaluation by penile Doppler ultrasonography. Int J Impot Res. 2019;31:195–203. doi: 10.1038/s41443-018-0062-2. [DOI] [PubMed] [Google Scholar]
- 32.Yee CH, Chan ESY, Hou SSM. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol. 2014;21:1041–1045. doi: 10.1111/iju.12506. [DOI] [PubMed] [Google Scholar]
- 33.Srini VS, Reddy RK, Shultz T, et al. Low intensity extracorporeal shockwave therapy for erectile dysfunction: A study in an Indian population. Vol 22.; 2015. [PubMed]
- 34.Adeldaeim HM, Abouyoussif T, Gebaly O El. Prognostic indicators for successful low-intensity extracorporeal shock wave therapy treatment of erectile dysfunction. Urology. 2021;149:133–139. doi: 10.1016/j.urology.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Patel P, Katz J, Lokeshwar SD. Phase II randomized, clinical trial evaluating 2 schedules of low-intensity shockwave therapy for the treatment of erectile dysfunction. Sex Med. 2020;8:214–222. doi: 10.1016/j.esxm.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalyvianakis D, Mykoniatis I, Memmos E. Low-intensity shockwave therapy (LiST) for erectile dysfunction: A randomized clinical trial assessing the impact of energy flux density (EFD) and frequency of sessions. Int J Impot Res. 2020;32:329–337. doi: 10.1038/s41443-019-0185-0. [DOI] [PubMed] [Google Scholar]
- 37.Hanson-Divers C, Jackson SE, Lue TF. Health outcomes variables important to patients in the treatment of erectile dysfunction. J Urol. 1998;159:1541–1547. doi: 10.1097/00005392-199805000-00037. [DOI] [PubMed] [Google Scholar]
- 38.Zakrzewski W, Dobrzyński M, Szymonowicz M. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akpancar S, Tatar O, Turgut H. The current perspectives of stem cell therapy in orthopedic surgery. Arch Trauma Res. 2016;5:37976. doi: 10.5812/atr.37976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakim L, Van Der Aa F, Albersen M. Emerging tools for erectile dysfunction: A role for regenerative medicine. Nat Rev Urol. 2012;9:520–536. doi: 10.1038/nrurol.2012.143. [DOI] [PubMed] [Google Scholar]
- 43.Matz EL, Terlecki R, Zhang Y. Stem cell therapy for erectile dysfunction. Sex Med Rev. 2019;7:321–328. doi: 10.1016/j.sxmr.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira MS. Placental-derived stem cells: Culture, differentiation and challenges. World J Stem Cells. 2015;7:769. doi: 10.4252/wjsc.v7.i4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells state-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264–e277. doi: 10.18295/squmj.2018.18.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haney NM, Gabrielson A, Kohn TP. The use of stromal vascular fraction in the treatment of male sexual dysfunction: A review of preclinical and clinical studies. Sex Med Rev. 2019;7:313–320. doi: 10.1016/j.sxmr.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Gur S, Abdel-Mageed AB, Sikka SC. Advances in stem cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2018;18:1137–1150. doi: 10.1080/14712598.2018.1534955. [DOI] [PubMed] [Google Scholar]
- 48.Lokeshwar SD, Patel P, Shah SM. A systematic review of human trials using stem cell therapy for erectile dysfunction. Sex Med Rev. 2020;8:122–130. doi: 10.1016/j.sxmr.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Bahk JY, Jung JH, Han H. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: Preliminary report of 7 cases. Exp Clin Transplant. 2010;8:150–160. [PubMed] [Google Scholar]
- 50.Levy JA, Marchand M, Iorio L. Determining the feasibility of managing erectile dysfunction in humans with placental-derived stem cells. J Am Osteopath Assoc. 2016;116:e1–e5. doi: 10.7556/jaoa.2016.007. [DOI] [PubMed] [Google Scholar]
- 51.Al Demour S, Jafar H, Adwan S. Safety and potential therapeutic effect of two intracavernous autologous bone marrow derived mesenchymal stem cells injections in diabetic patients with erectile dysfunction: An open label phase I clinical trial. Urol Int. 2018;101:358–365. doi: 10.1159/000492120. [DOI] [PubMed] [Google Scholar]
- 52.Yiou R, Hamidou L, Birebent B. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: An open dose-escalation pilot study. Eur Urol. 2016;69:988–991. doi: 10.1016/j.eururo.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 53.Yiou R, Hamidou L, Birebent B. Intracavernous injections of bone marrow mononucleated cells for postradical prostatectomy erectile dysfunction: Final results of the INSTIN clinical trial. Eur Urol Focus. 2017;3:643–645. doi: 10.1016/j.euf.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Schweizer MT, Wang H, Bivalacqua TJ. A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived mesenchymal stem cells in men with localized prostate cancer. Stem Cells Transl Med. 2019;8:441–449. doi: 10.1002/sctm.18-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haahr MK, Jensen CH, Toyserkani NM. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: An open-label phase I clinical trial. EBioMedicine. 2016;5:204–210. doi: 10.1016/j.ebiom.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haahr MK, Harken Jensen C, Toyserkani NM. A 12-month follow-up after a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: An open-label phase I clinical trial. Urology. 2018;121:203.e6–203.e13. doi: 10.1016/j.urology.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 57.De Araujo AC, Da Silva FG, Salvi F. The management of erectile dysfunction with placebo only: Does it work? J Sex Med. 2009;6:3440–3448. doi: 10.1111/j.1743-6109.2009.01496.x. [DOI] [PubMed] [Google Scholar]
- 58.Jayaraman P, Nathan P, Vasanthan P. Stem cells conditioned medium: A new approach to skin wound healing management. Cell Biol Int. 2013;37:1122–1128. doi: 10.1002/cbin.10138. [DOI] [PubMed] [Google Scholar]
- 59.Alves R, Grimalt R. A review of platelet-rich plasma: History, biology, mechanism of action, and classification. Ski Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anitua E, Muruzabal F, de la Fuente M. Plasma rich in growth factors for the treatment of ocular surface diseases. Curr Eye Res. 2016;41:875–882. doi: 10.3109/02713683.2015.1104362. [DOI] [PubMed] [Google Scholar]
- 61.Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis. Am J Sports Med. 2015;44:884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 62.Foster TE, Puskas BL, Mandelbaum BR. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 63.Patel AN, Selzman CH, Kumpati GS. Evaluation of autologous platelet rich plasma for cardiac surgery: Outcome analysis of 2000 patients. J Cardiothorac Surg. 2016;11:62. doi: 10.1186/s13019-016-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taschieri S, Lolato A, Ofer M. Immediate post-extraction implants with or without pure platelet-rich plasma: A 5-year follow-up study. Oral Maxillofac Surg. 2017;21:147–157. doi: 10.1007/s10006-017-0609-2. [DOI] [PubMed] [Google Scholar]
- 65.Epifanova MV, Gvasalia BR, Durashov MA. Platelet-rich plasma therapy for male sexual dysfunction: Myth or reality? Sex Med Rev. 2020;8:106–113. doi: 10.1016/j.sxmr.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 66.El-Sharkawy H, Kantarci A, Deady J. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 67.Kushida S, Kakudo N, Morimoto N. Platelet and growth factor concentrations in activated platelet-rich plasma: A comparison of seven commercial separation systems. J Artif Organs. 2014;17:186–192. doi: 10.1007/s10047-014-0761-5. [DOI] [PubMed] [Google Scholar]
- 68.Ding XG, Li SW, Zheng XM. The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian J Androl. 2009;11:215–221. doi: 10.1038/aja.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu CC, Wu YN, Ho HO. The neuroprotective effect of platelet-rich plasma on erectile function in bilateral cavernous nerve injury rat model. J Sex Med. 2012;9:2838–2848. doi: 10.1111/j.1743-6109.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- 70.Wu YN, Wu CC, Sheu MT. Optimization of platelet-rich plasma and its effects on the recovery of erectile function after bilateral cavernous nerve injury in a rat model. J Tissue Eng Regen Med. 2016;10:E294–E304. doi: 10.1002/term.1806. [DOI] [PubMed] [Google Scholar]
- 71.Epifanova M V, Chalyi ME, Krasnov AO. Investigation of mechanisms of action of growth factors of autologous platelet-rich plasma used to treat erectile dysfunction. Urologiia. 2017;4:46–48. [PubMed] [Google Scholar]
- 72.Matz EL, Pearlman AM, Terlecki RP. Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol. 2018;59(1):61–65. doi: 10.4111/icu.2018.59.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones IA, Togashi RC, Thomas Vangsness C. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11(4):558–565. doi: 10.1007/s12178-018-9514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]