Abstract
The worldwide spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the most severe public health crisis since the 1918 Spanish influenza pandemic. After the introduction of public health interventions aimed at reducing the number of COVID-19 cases, many countries across the world obtained success at containing the fast spread of SARS-CoV-2 during the first wave of the pandemic. However, the SARS-CoV-2 has resurged in many countries causing a even more devastating second wave. Brazil is one the most affected countries and currently is facing one of the worst public health crises in its history. Here, we discuss the unprecedented challenges faced by the Brazilian public health system in the midst of the second wave of the COVID-19 pandemic, particularly regarding the collapse of the Brazilian health system and the emergence of new variants of concern (VOCs). Finally, we suggest some insights using a one health approach that will help the country to face and overcome the current COVID-19 crisis.
Keywords: COVID-19, Coronavirus, Public health, SARS-CoV-2, One health, Variants
Highlights
-
•
Brazil currently is facing one of the worst public health crises in its history.
-
•
SARS-CoV-2 has resurged in Brazil causing a even more devastating second wave.
-
•
The uncontrolled transmission of SARS-CoV-2 have made Brazil an ideal breeding ground for the genesis and propagation of new variants.
-
•
Constant genomic surveillance should be implemented on a large scale in Brazil in order to establish policies to control the spread of SARS-CoV-2.
-
•
The current COVID-19 crisis in Brazil requires the integration and implementation of government interventions and stronger national coordination led by the ministry of health.
The COVID-19 pandemic is the most devastating public health crisis seen in the 21st century. The sustained human-to-human transmission, high virulence and lack of antivirals or vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) prompted health officials to establish stringent control measures to halt the spread of the disease. A range of public health measures including education, social distancing, movement restriction, isolation and quarantine policies, requirement to wear protective gear in public associated with health education and strengthening public health systems contributed to reduce the morbidity and mortality of COVID-19 during the first wave of the pandemic in many countries [1]. However, the number of SARS-CoV-2 infections has increased again in many countries causing a second and even third wave, particularly due to a relaxation of public health interventions associated with a lack of herd immunity in the population [1,2]. In many countries, the second wave has been more devastating than the first. This has been the case of Brazil, a tropical country that has become one of the epicenters during the COVID-19 pandemic course in the world. As of 27 June 2021, Brazil has >18.4 million laboratory-confirmed cases and > 513,474 deaths, the second highest death toll in the world from COVID-19 behind the United States [3]. The number of cases is probably underestimated since Brazil doses 246,306 tests per million inhabitants as opposed to 1,499,247 per million done by the USA, for instance (https://www.worldometers.info/coronavirus/). Moreover, Brazil has been facing many challenges for COVID-19 diagnosis, particularly regarding the lack of supplies and the limited number of laboratories for SARS-CoV-2 diagnosis [4,5].
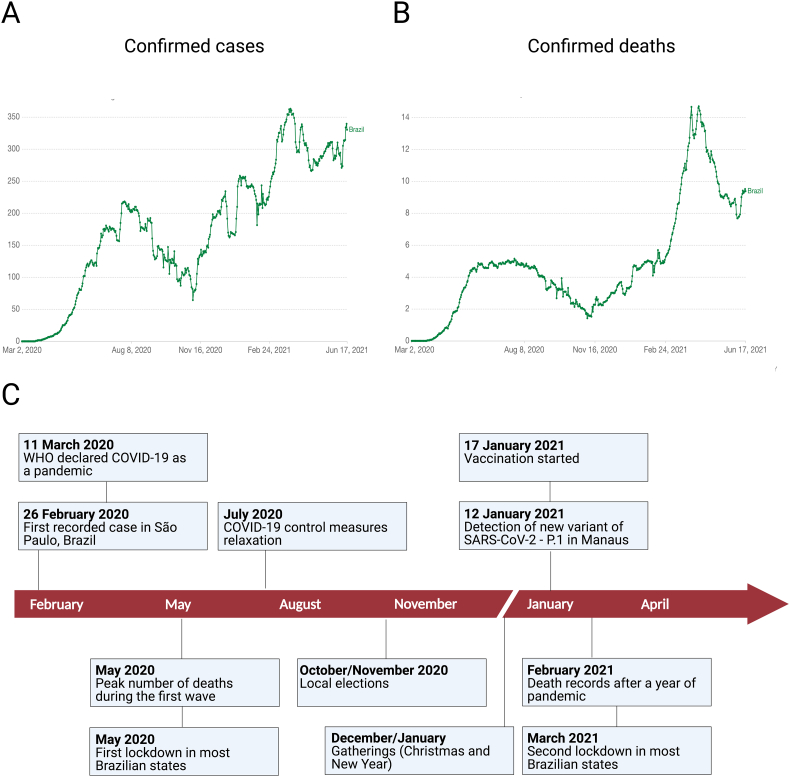
Brazil reported the first COVID-19 case in Latin America on 26 February 2020, which was diagnosed in a traveler returning to São Paulo after a work trip from Lombardy, Northern Italy (Fig. 1) [6]. Since then, the number of COVID-19 cases in Brazil has increased progressively and the country has the third highest number of COVID-19 cases in the world after the USA and India, comprising over 55% of the total number of reported COVID-19 cases in Latin America [3,7,8]. During the first wave, the number of cases and deaths increased steadily until reaching its peak by the end of May 2020 and then started to decline from September to early November 2020 (Fig. 1). Brazil has failed to implement a thorough national response plan to control and to prevent the rapid spread of SARS-CoV-2. By 9 January 2021, the country has been ranked the worst among 98 countries in the pandemic response by the Lowy Institute, an independent and non-partisan international policy research institution based in Australia [9]. Since the beginning of the pandemic, Brazil had four Health Ministers, including an army general between May 2020 and March 2021. The first two ministers were fired because they backed up science-based approaches and WHO recommendations to control the disease, including social distancing and mask wearing. The federal government has downplayed the impact of pandemic, discouraged the use of face masks, ignored quarantine and lockdown measures and advocated for the use of chloroquine and other unproven drugs for COVID-19 treatment, despite multiple clinical trials showing their ineffectiveness. Despite the lack of a robust federal response, most state governors and mayors imposed quarantines and social distancing measures to prevent the spread of the virus, which contributed to reduce the incidence and deaths due to COVID-19 [2,10].
Fig. 1.
Overview of the COVID-19 pandemic in Brazil. Fig. 1A shows the number of COVID-19 laboratory confirmed cases in Brazil as of June 18, 2021. Fig. 1B shows the number of COVID-19 deaths in Brazil as of June 18, 2021. Fig. 1C shows the timeline of the key events of the COVID-19 pandemic in Brazil. The first confirmed case was reported on 26 February 2020 in São Paulo, Brazil. Over the course of the following 16 months, more than 18.4 million cases and 513,000 deaths have been confirmed in Brazil. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization. Source: Johns Hopkins University CSSE COVID-19 Data (https://ourworldindata.org/). Created with Biorender.com
However, a second COVID-19 wave began in November 2020, which coincided with the local elections in all Brazilian cities that took place in October and November. The elections were characterized by political rallies, mass gatherings, distribution of political cards, and relaxation of public health measures. The resurgence of COVID-19 was most notably seen first in Manaus, a large city in the middle of the Amazon Forest in Northern Brazil, where the massive number of hospitalizations and oxygen shortage collapsed the city's health-care system resulting in a large number of deaths [2,11,12]. The crisis spread across the country, adding extra pressure and overload to the Brazilian Universal Healthcare Program (SUS), which offers universal access to the Brazilian population.
In March 2021, most Brazilian states reached 90% ICU occupancy rate, which represented the most severe health and hospital collapse in the country's history. Cases of patients being treated in hospital hallways, dying while awaiting vacancy in ICUs, or even sent away with no treatment multiplied throughout the country. Worryingly, patients admitted to hospitals as a suspected COVID-19 case are being mixed up with confirmed COVID-19 patients without results of a rapid test and many of them are contracting nosocomial SARS-CoV-2 and dying. In addition, the lethality in Brazilians ICUs is higher than the rest of the world. A retrospective study with 250,000 hospitalized COVID-19 patients in Brazil found that 80% of patients who needed invasive ventilation died, which is higher than the world average (~ 50%) [13].
What factors have led Brazil to this unprecedented crisis? Several non-mutually exclusive pathways may have contributed to explain current epidemiological scenario [2]. First, the relaxation of COVID-19 control protocols and massive agglomeration during the local elections in the end of 2020, followed by Christmas and New Year holidays and then the carnival holiday in February 2021. Second, the availability of the COVID-19 vaccine, albeit timid, in January 2021 may have created a false sense of security in the population and negatively interfered with people's compliance with COVID-19 preventive and control measures. These favorable conditions for SARS-CoV-2 transmission led to higher infection rates, especially in the more exposed young population [14]. Third, the actions of federal government have been widely known for its scientific denialism, disregard for social distancing protocols and dissemination of fake news against the COVID-19 vaccines, notably the Chinese-origin Coronavac vaccine. Fake news about Coronavac vaccine included that the vaccine had no scientific proof and raised equivocal questions about its origin, in spite of the publication of the vaccine's clinical trial results demonstrating its safety, tolerability, and immunogenicity [15,16]. Moreover, President Bolsonaro has refused to buy Pfizer's vaccine at half the price paid by other countries. He even said that the Pfizer's vaccine could cause unknown harms and that he would not be responsible if vaccinated people turn into alligators post-inoculation. Early vaccination could have avoided the death of million Brazilians and would have saved billions in losses caused by the economic shutdown and hospitalization of sick people.
The presence of a large naïve population, low testing rates, poor population adherence to COVID-19 prevention measures and the uncontrolled transmission of SARS-CoV-2 have made Brazil an ideal breeding ground for the genesis and propagation of new variants. Viral replication in immunocompromised individuals, the presence of long shedders and infection of SARS-CoV-2 in different hosts might also have contributed to the genesis of new variants.
Due to rapid spread of the SARS-CoV-2 in Brazil, a network of public health laboratories was mobilized to investigate and track the emergence of SARS-CoV-2 mutations and new variants. Despite the presence of national laboratories of excellence for genomic sequencing, the surveillance of SARS-CoV-2 genomes has been slow. Currently, 19,440 whole genomes of SARS-CoV-2 from Brazil have been sequenced and deposited at GISAID as of 27 June 2021, which represents the fifteenth country with the highest number of SARS-CoV-2 genomes deposits (https://www.gisaid.org/). Six variants of concern have emerged since the beginning of the pandemic and rapidly spread across the world: alpha [B.1.1.7] in the United Kingdom; beta [B.1.351] in South Africa; and gamma [P.1] in Brazil; epsilon [B.1.427] in the USA; epsilon [B.1.429] in the USA; delta [B.1.617.2] in India (Table 1) [[17], [18], [19], [20], [21]]. Four of these six variants have been detected in Brazil [12,18,[22], [23]]. The gamma variant, first identified in Manaus in early January 2021, has 35 nucleotide mutations (17 amino acid changes) and has spread throughout Brazil and outside [24,25]. Recent findings suggested that SARS-CoV-2 gamma variant infections are associated with higher viral loads (~ 10-fold) in infected patients, when compared with the non-gamma infections [12]. Using a dynamical model, a recent study has demonstrated that the gamma variant is more transmissible (estimated to be 1.7 to 2.4) when compared to non-gamma lineages, which may have accelerated the rapid propagation around the world [18]. In the same context, a recent study reported three cases of SARS-CoV-2 reinfection have been associated with the gamma variant [26], suggesting that the gamma variant may escape the neutralization generated by previous immune responses.
Table 1.
Features of the emerging SARS-CoV-2 variants around the world.
| Official name | B.1.1.7 | B.1.351 | P.1 | B.1.427 | B.1.429 | B.1.617.2 |
| WHO label | Alpha | Beta | Gamma | Epsilon | Epsilon | Delta |
| Other names used | N501YV1, 20I/501YV1, VOC 202012/1 |
N501YV2, 20H501YV2, VOC 202012/2 |
N501YV3, 20J/501YV3, VOC 202012/3 |
20C/S:452R | CAL.20C, 20C/S:452R | 20A/S:478 K |
| Country where first reported | United Kingdom | South Africa | Brazil | USA | USA | India |
| Spike mutations | 69del, 70del, 144del, E484K, S494P*, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H, K1191N | D80A, D215G, 241del, 242del, 243del, K417N, E484K, N501Y, D614G, A701V | L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I | L452R, D614G | S13I, W152C, L452R, D614G | T19R, (G142D), 156del, 157del, R158G, L452R, T478K, D614G, P681R, D950N |
| Transmissibility | ~50% increased transmissiona | ~50% increased transmissiong | Estimated to be 1.7 to 2.4i | ~20% increased transmissionj | ~20% increased transmissionj | Increased transmissibilityk |
| Lethality | Potential increased severity based on hospitalizations and mortality ratesb | Not lead to an increase in the mortality rateh | Not yet established | Not yet established | Not yet established | Not yet established |
| Immune escape from vaccine-induced immunity | Decrease by 2.0× (BNT162b2/Pfizer); Decrease by 1.8× (mRNA-1273/Moderna); Decrease by 1.8× (NVX-CoV2373/Novavax)c |
Decrease by ≤86× (AZD1222/Astrazeneca); Decrease by ≤8.6× (mRNA-1273/Moderna); Decrease by ≤6.5× (BNT162b2/Pfizer); Decrease by 1.6× (BBIBP-CorV/Sinopharm)c |
Decrease by 6.7× (BNT162b2/Pfizer); Decrease by 4.5× (mRNA-1273/Moderna)c |
Decrease by 2.0-fold in neutralizing titers from vaccine recipientsj | Decrease by 2.0-fold in neutralizing titers from vaccine recipientsj | Potential reduction in neutralization by post-vaccination serumj |
| Immune escape from natural-induced immunity | Minimal impact on neutralization by EUA therapeutic monoclonal antibodies and convalescent serumd, e | Moderate impact on neutralization by EUA therapeutic monoclonal antibodies and convalescent serumd, e | Moderate impact on neutralization by EUA therapeutic monoclonal antibodies and convalescent serumd, f | Reduced neutralization by convalescent serumj | Reduced neutralization by convalescent serumj | Impact on neutralization by EUA therapeutic monoclonal antibodiesd |
| Countries reportedl | 147 | 95 | 61 | – | – | 78 |
More recently, studies have pointed that COVID-19 infection with gamma variant could be associated for increasing the risk of hospitalization and ICU admission [27]. Interestingly, a yet to be reviewed study demonstrated that gamma variant is able to infect and replicate to high titers in the lungs of mice [28]. However, in vitro and in vivo studies are needed to better understand the effects and impact of specific mutations on viral transmissibility and disease severity of gamma variant and other SARS-CoV-2 lineages in animals and humans [29,30].
With regard to escape from natural-induced immunity and vaccine-induced immunity against gamma variant, Souza and colleagues demonstrated that the convalescent COVID-19 plasma had a 6-fold lower neutralization capacity against the gamma variant when compared to the B-lineage, suggesting a moderate impact on neutralization [31]. Regarding the response vaccine-induced immunity against gamma variant, the findings showed that the neutralizing activity for the gamma variant among vaccinated individuals was lower by a factor of 4.5 for the mRNA-1273 and 6.7 for the BNT162b2 vaccine [25]. The gamma variant can also evade FDA-approved therapeutic antibodies and antibodies induced by infection or vaccination as determined by in vitro studies [32]. Whether this ability to escape neutralizing antibodies is translated into lower vaccine efficacy is still a question to be addressed. In addition to the gamma variant, the scientific community should be alert to the emergence of new SARS-CoV-2 variants in Brazil.
1. Final considerations and public health perspectives
There are important lessons to be learned from the current COVID-19 pandemic in Brazil: (i) the profile of COVID-19 epidemiological progression in Brazil was largely influenced by the introduction of lockdown measures implemented by during the first wave of the pandemic and the subsequent easing of these measures, which may have directly influenced of the resurgence of the second wave. (ii) implementation of government interventions and stronger national coordination led by the ministry of health should be in place; (iii) constant genomic surveillance should be implemented on a large scale in Brazil in order to identify new variants and establish policies and countermeasures to control the spread of SARS-CoV-2; (iv) establishment of strong partnerships between the national public health sector, regulatory agencies and private sector to large-scale production of vaccines available should institutionalized to overcome the COVID-19 pandemic. A national campaign for COVID-19 education and prevention based on scientifically recommended approaches is key to control the pandemic until heard immunity is reached to a level able to halt the rate of SARS-CoV-2 transmission.
On the other hand, the concept of one health is still poor applied during the COVID-19 pandemic in Brazil. The major challenges to implement a one health approach in Brazil include absence of a legal governmental institution to implement the measures, poor organizational structure to support a one health approach application, poor coordination among different governmental sectors, and poor data sharing mechanism across governmental sectors. In this context, we invite health authorities, scientists, physicians, veterinarians and environmentalists to unify their efforts to address these gaps and prioritize this public health problem, where a one heath approach that will help the country to face and overcome the current COVID-19 crisis.
Authorship contribution statement
All authors contributed equally to this paper with the design of the manuscript, literature review and analysis, critical revision, editing, and approval of the final version.
Funding
The work in Dr. Pena's lab is funded by the Fiocruz Inova Program, IDRC-Canada and the Foundation for Science and Technology of Pernambuco - FACEPE, Brazil (grant number APQ-0560-2.12/19). S.J.R.d.S is supported by a PhD fellowship sponsored by the Foundation for Science and Technology of Pernambuco (FACEPE), reference number IBPG-1321-2.12/18. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
All authors declare no potential conflicts of interest.
Contributor Information
Severino Jefferson Ribeiro da Silva, Email: jeffersonbiotecviro@gmail.com.
Lindomar Pena, Email: lindomar.pena@fiocruz.br.
References
- 1.Okell C.L., Verity R., Watson O.J., Mishra S., Walker P., Whittaker C., Katzourakis A. Have deaths from COVID-19 in Europe plateaued due to herd immunity? Lancet. 2020;395(10241):e110–e111. doi: 10.1016/S0140-6736(20)31357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabino E.C., Buss L.F., Carvalho M.P.S., Prete C.A., Crispim M.A.E., Fraiji N.A., Pereira R.H.M. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva S.J.R.D., Magalhães J.J.F., Pena L. Simultaneous circulation of DENV, CHIKV, ZIKV and SARS-CoV-2 in Brazil: an inconvenient truth. One Health. 2021;12 doi: 10.1016/j.onehlt.2020.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews Q., Silva S.J.R.D., Norouzi M., Pena L.J., Pardee K. Adaptive, diverse and de-centralized diagnostics are key to the future of outbreak response. BMC Biol. 2020;18(1):153. doi: 10.1186/s12915-020-00891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PAHO Brasil confirma primeiro caso de infecção pelo novo coronavírus. https://www.paho.org/bra/index.php?option=com_content&view=article&id=6113:brasil-confirma-primeiro-caso-de-infeccao-pelo-novo-coronavirus&Itemid=812-:~:text=26%20de%20fevereiro%20de%202020,21%20de%20fevereiro%20deste%20ano, 2020 [Accessed 16 April 2021]
- 7.de Souza W.M., Buss L.F., Candido D.D.S., Carrera J.P., Li S., Zarebski A.E., Pereira R.H.M., Prete C.A. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat. Hum. Behav. 2020;4(8):856–865. doi: 10.1038/s41562-020-0928-4. [DOI] [PubMed] [Google Scholar]
- 8.Candido D.S., Claro I.M., de Jesus J.G., de Souza W.M., Moreira F.R.R., Dellicour S., Mellan T.A. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369(6508):1255–1260. doi: 10.1126/science.abd2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowy Institute Covid Performance Index. 2021. https://interactives.lowyinstitute.org/features/covid-performance/-rankings [Accessed 16 April 2021]
- 10.Ministério da Saúde Boletim epidemiológico especial - Doença pelo coronavírus COVID-19, 1-85. 2021. https://www.gov.br/saude/pt-br/media/pdf/2021/marco/05/boletim_epidemiologico_covid_52_final2.pdf
- 11.Buss L.F., Prete C.A., Abrahim C.M.M., Mendrone A., Salomon T., de Almeida-Neto C., França R.F.O. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371(6526):288–292. doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naveca F.G., Nascimento V., de Souza V.C., Corado A.L., Nascimento F., Silva G., Costa A. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat. Med. 2021;13 doi: 10.1038/s41591-021-01378-7. [DOI] [PubMed] [Google Scholar]
- 13.Ranzani O.T., Bastos L.S.L., Gelli J.G.M., Marchesi J.F., Baião F., Hamacher S., Bozza F.A. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir. Med. 2021;9(4):407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor L. Covid-19: Brazil's spiralling crisis is increasingly affecting young people. BMJ. 2021;373:n879. doi: 10.1136/bmj.n879. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z., Hu Y., Xu M., Chen Z., Yang W., Jiang Z., Li M. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21(6):803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 18.Faria N.R., Mellan T.A., Whittaker C., Claro I.M., Candido D.D.S., Mishra S., Crispim M.A.E. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;272(6544):815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rambaut A., Loman N., Pybus O., Barclay W., Barrett J., Carabelli A., Connor T. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. Virological. 2020 https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 [Google Scholar]
- 20.Zhang W., Davis B.D., Chen S.S., Sincuir Martinez J.M., Plummer J.T., Vail E. Emergence of a novel SARS-CoV-2 variant in Southern California. JAMA. 2021;325(13):1324–1326. doi: 10.1001/jama.2021.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh J., Rahman S.A., Ehtesham N.Z., Hira S., Hasnain S.E. SARS-CoV-2 variants of concern are emerging in India. Nat. Med. 2021 doi: 10.1038/s41591-021-01397-4. [DOI] [PubMed] [Google Scholar]
- 22.Reuters, Brazil Detects First Case of South African Variant, Grave Shortage Looms as Death Toll Soars. 2021. https://www.reuters.com/article/us-health-coronavirus-brazil-variant-idUSKBN2BU2KA [Accessed 16 April 2021]
- 23.Reuters, Brazil Identifies Indian Coronavirus Variant in Traveler through São Paulo, Rio Airports. 2021. https://www.reuters.com/business/healthcare-pharmaceuticals/brazil-identifies-coronavirus-strain-india-traveler-through-sao-paulo-rio-2021-05-26/ [Accessed 23 June 2021]
- 24.Tosta S., Giovanetti M., Nardy V.B., Silva L.R.D.O.D., Gómez M.K.A., Lima J.G., Cardoso C.W. 2021. Early Genomic Detection of SARS-CoV-2 P.1 Variant in Northeast Brazil. medRxiv. [DOI] [Google Scholar]
- 25.Abdool Karim S.S., de Oliveira T. New SARS-CoV-2 variants - clinical, public health, and vaccine implications. N. Engl. J. Med. 2021;384(19):1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naveca F., Costa C., Nascimento V., Souza V., Corado A., Nascimento F., Costa A. SARS-CoV-2 reinfection by the new Variant of Concern (VOC) P.1 in Amazonas, Brazil. Virological. 2021 https://virological.org/t/sars-cov-2-reinfection-by-the-new-variant-of-concern-voc-p-1-in-amazonas-brazil/596 [Google Scholar]
- 27.Funk T., Pharris A., Spiteri G., Bundle N., Melidou A., Carr M., Gonzalez G. Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Euro Surveill. 2021;26(16) doi: 10.2807/1560-7917.ES.2021.26.16.2100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montagutelli X., Prot M., Levillayer L., Salazar E.B., Jouvion G., Conquet L., Donati F. The B1.351 and P.1 variants extend SARS-CoV-2 host range to mice. bioRxiv. 2021 doi: 10.1101/2021.03.18.436013v1. [DOI] [Google Scholar]
- 29.Silva S.J.R.D., Mendes R.P.G., Silva C.T.A.D., Lorusso A., Kohl A., Pena L. Insights into SARS-CoV-2, the coronavirus underlying COVID-19: recent genomic data and the development of reverse genetics systems. J. Gen. Virol. 2020;101(10):1021–1024. doi: 10.1099/jgv.0.001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva S.J.R.D., Silva C.T.A.D., Mendes R.P.G., Pena L. Role of nonstructural proteins in the pathogenesis of SARS-CoV-2. J. Med. Virol. 2020;92(9):1427–1429. doi: 10.1002/jmv.25858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Souza W.M., Amorim M.R., Sesti-Costa R., Coimbra L.D., Toledo-Teixeira D.A., Parise P.L., Barbosa P.P. Levels of SARS-CoV-2 Lineage P.1 Neutralization by Antibodies Elicited after Natural Infection and Vaccination. 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3793486 SSRN. [DOI] [PMC free article] [PubMed]
- 32.Hoffmann M., Arora P., Groß R., Seidel S., Hörnich B.F., Hahn A.S., Krüger N. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384–2393. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies N.G., Jarvis C.I., Ed.munds W.J., Jewell N.P., Diaz-Ordaz K., Keogh R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA . FDA; 2021. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab.https://www.fda.gov/media/145802/download [Accessed 23 June 2021] [Google Scholar]
- 36.Wang P., Nair M., Liu L., Iketani S., Luo Y., Guo Y., Wang M. Antibody resistance of SARS-CoV-2 variants B.I.I.351 and B.I.I.7. bioRxiv. 2021 doi: 10.1101/2021.01.25.428137v3. [DOI] [PubMed] [Google Scholar]
- 37.Wang P., Wang M., Yu J., Cerutti G., Nair M.S., Huang Y., Kwong P.D. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. bioRxiv. 2021 doi: 10.1101/2021.03.01.433466v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson C., Russell T., Davies N., Kucharski A.A.J. Estimates of severity and transmissibility of novel SARS-CoV-2 variant 501Y.V2 in South Africa. CMMID Repos. 2021 https://cmmid.github.io/topics/covid19/sa-novel-variant.html [Google Scholar]
- 39.Deng X., Garcia-Knight M.A., Khalid M.M., Wang V.S.C., Morris M.K., Sotomayor-González A., Glasner D.R. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant California a L452R spike protein mutation. medRxiv. 2021 doi: 10.1101/2021.03.07.21252647v1. [DOI] [Google Scholar]
- 40.Allen H., Vusirikala A., Flannagan J., Twohig K.A., Zaidi A. Increased Household Transmission of COVID-19 Cases Associated with SARS-CoV-2 Variant of Concern B.1.617.2: A National Casecontrol Study. 2021. https://khub.net/documents/135939561/405676950/Increased+Household+Transmission+of+COVID-19+Cases+-+national+case+study.pdf/7f7764fb-ecb0-da31-77b3-b1a8ef7be9aa [Accessed 23 June 2021]