Abstract
Objective
There is no specific antiviral treatment available for coronavirus disease 2019 (COVID-19). Among the possible natural constituents is carrageenan, a polymer derived from marine algae that possesses a variety of antiviral properties. The purpose of this review was to summarize the evidence supporting carrageenan subtypes' antiviral activity against the emerging severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of COVID-19.
Methods
PubMed/MEDLINE and Google Scholar searches were conducted for publications using the terms ‘carrageenan’, ‘iota carrageenan’, ‘kappa carrageenan’, lambda-carrageenan’, 'coronavirus', ‘common cold’, 'rhinovirus', and ‘SARS-CoV-2′ search was also done in grey literature to increase our understanding. A search for the word “carrageenan” was also carried out. Most of the publications were discussed in narrative.
Results
Carrageenan has been shown to have potent antiviral activity against both coronaviruses (coronavirus NL63, SARS-CoV-2) and non-coronaviruses such as dengue virus, herpes simplex virus, cytomegalovirus, vaccinia virus, vesicular stomatitis virus, sindbis virus, human immunodeficiency virus, influenza virus, human papillomavirus, rabies virus, junin virus, tacaribe virus, African swine fever, bovine herpes virus, suid herpes virus, and rhinovirus. No in vivo study has been conducted using carrageenan as an anti-SARS-CoV-2 agent. The majority of the in vivo research was done on influenza, a respiratory virus that causes common cold together with coronavirus. Thus, various clinical trials were conducted to determine the transferability of these in vitro data to clinical effectiveness against SARS-CoV-2. When combined with oral ivermectin, nasally administered iota-carrageenan improved outcome in COVID-19 patients. It is still being tested in clinics for single-dose administration.
Conclusion
Though the carrageenan exhibited potent antiviral activity against SARS-CoV-2 and was used to treat COVID-19 under emergency protocol in conjunction with oral medications such as ivermectin, there is no solid evidence from clinical trials to support its efficacy. Thus, clinical trials are required to assess its efficacy for COVID-19 treatment prior to broad application.
Keywords: COVID-19, SARS-CoV-2, Nasal spray, Carrageenan, Iota-carrageenan, Clinical trial
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which first appeared in late December 2019 in China's Hubei Province, has had a negative impact on the majority of affected countries' national healthcare systems.1, 2, 3 SARS-CoV-2 infection is primarily a respiratory infection.4 , 5 However, numerous other organs may also be impacted,6, 7, 8 with numerous unknown consequences.9, 10, 11 Furthermore, the COVID-19 Global cases database indicated that over 136 million confirmed COVID-19 cases had been reported as of April 12, 2021, resulting in 2,949,409 deaths.12 The virus spreads quickly in through the world because of its high reproductive number R 0, which is estimated to be 3.25 ≤ R0 ≤ 3.4, which means that one infected person can spread the virus to up to three unvaccinated people.13 The SARS-CoV-2 virus is a member of the Coronaviridae family and the Betacoronavirus genus.14 , 15 SARS-CoV shares 79.6% of its sequence with the virus.16
Numerous COVID-19 drug candidates have been proposed, each with a distinct mechanism of action,14 , 17, 18, 19 including the use of convalescent plasma and interferon, as well as inhibitors of the interleukin 6 receptor, which have the potential to suppress the cytokine storm.14 , 20 , 21 Chloroquine and its hydroxy-form, both of which inhibit viral entry via endocytosis, endosomal acidification, and angiotensin converting enzyme 2 glycosylation, were used to treat COVID-19 14, 22, as well as ivermectin, which inhibits viral protein nuclear transport.18 , 23 Additionally, antiviral agents that inhibit proteases (e.g., remdesivir) and nucleotide or nucleoside analogs that inhibit viral RNA synthesis have been repurposed for the treatment of SARS-CoV-2 infection.14 , 17 Remdesivir has been approved by FDA.24 , 25 Corticosteroids, however, have also been shown to be effective against severe and potentially fatal COVID-19 infection and recently have been approved by the World Health Organization.26 , 27 Additional sources of drug candidates must be investigated, including natural sources such as carrageenan. Carrageenan is a naturally occurring component of marine algae28 , 29 and thus may be a viable alternative source of COVID-19 emergency treatment, as previous research has demonstrated that carrageenan is actively used to treat viruses that cause the common cold.30, 31, 32 Thus, the purpose of this review was to synthesize evidence from in vitro, in vivo, and clinical trials in order to provide comprehensive information about the possibility of treating COVID-19 patients with carrageenan derived from marine algae.
2. Methods
PubMed/MEDLINE and Google Scholar searches for articles were conducted using the title and abstract terms ‘carrageenan’, ‘iota carrageenan’, ‘kappa carrageenan’, lambda carrageenan’, 'coronavirus', and ‘SARS-CoV-2'. ClinicalTrials.gov and isrctn.com databases were also searched for recent clinical trials evaluating the efficacy of carrageenan for COVID-19. The publications till April 21, 2021 were then classified and analyzed according to their type of study: in vitro, in vivo, or clinical trials.
3. Results
3.1. Carrageenan: an overview
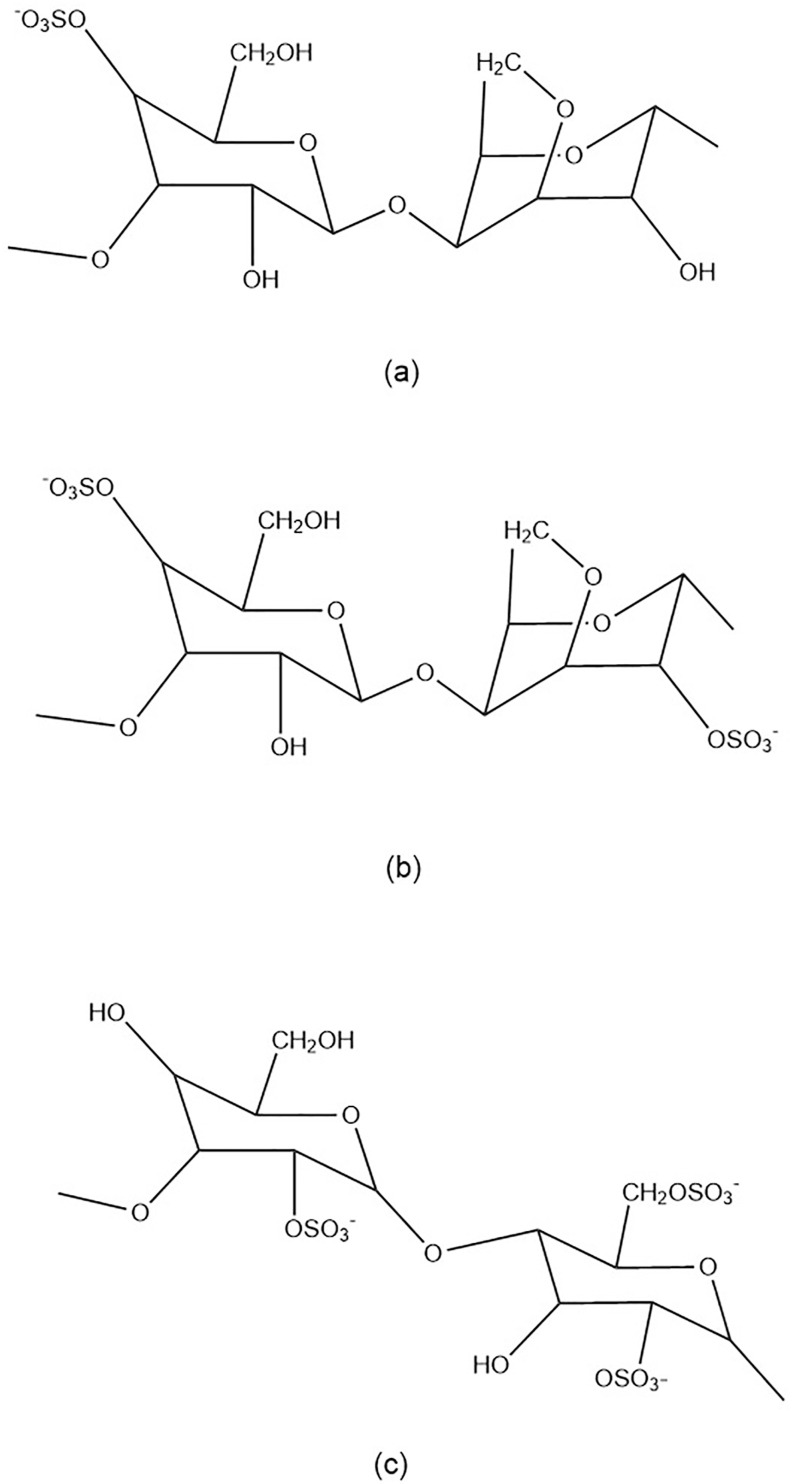
Carrageenan is a soluble sulphate galactose polymer derived from the marine alga Rhodophyceae. Its constituents are found in the algae's outer cell wall and intracellular matrix.33 The galactan backbone of the polymer is synthesized in the Golgi bodies of the cell, while the sulfation step occurs in the cell wall via a sulfotransferase pathway.33, 34, 35 Carrageenan bioactives are polydisperse and have a wide range of molecular weights. It is primarily found in the Gigartinaceae and Solieriaceae families,36 , 37 as well as the Furcellariaceae, Solieriaceae, Phyllophoraceae, and Hypneaceae families.33 , 38 Carrageenan is commonly used as an emulsifier, stabilizer, thickener, and gelling agent in topical products, cosmetics, and food preparations due to its emulsifying, stabilizing, thickening, and gelling properties.39, 40, 41 There are three major subtypes of carrageenan: kappa (Fig. 1 a), iota (Fig. 1b), and lambda (Fig. 1c), which differ in their location and number of sulphate moieties on the hexose scaffold skeleton and contain one, two, or three negatively charged sulphate ester groups per disaccharide repeating unit, respectively.42 The US Food and Drug Administration (21 CFR 172.620) has generally recognized its polymer as safe for topical application and consumption.43 Carrageenan, as a biomolecule, possesses a variety of biological properties, including,44 , 45 antioxidant,46 anti-bacteria,47 anticoagulant,48, 49, 50 and immunomodulator.51 , 52 Numerous reports indicate that carrageenan is also an antiviral, including against respiratory viruses.53, 54, 55
Fig. 1.
Representation of the stereo-chemically fundamental carrageenan repeating sequence. (a) Kappa-carrageenan consisted of 4-O-Sulfato-beta-d-galactopyranosyl unit and 3,6-Anhydro-alpha- d-galactopyranosyl unit, (b) iota-carrageenan consisted 4-O-Sulfato-beta-d-galactopyranosyl unit and 3,6-Anhydro-2-0-sulfato-alpha-d-galactopyranosyl unit, (c) lambda-carrageenan consisted of 2-O-Sulfato-beta-d-galactopyranosyl unit and 2,6-Di-0-sulfato-alpha-d-galactopyranosyl unit.
3.2. In vitro evidence
A study published in 1987 found that iota-carrageenan, a sulphated polysaccharide derived from marine red algae, effectively inhibited several viruses in cultured cells, including African swine fever (ASF), encephalomyocarditis virus (EMC), herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), semliki forest virus (SFV), and Vaccinia.53 Therefore, it was without effect against adenovirus type 5, measles, polio type 1 and vesicular stomatitis virus.53 A year later, a study using other types of polyanionic carrageenan including kappa and lambda also showed antivirus properties to HSV-1, HSV-2, cytomegalovirus (CMV), vaccinia, sindbis, and human immunodeficiency virus type 1 (HIV-1).56 The half-maximal inhibitory concentration (IC50), a concentration of a molecule required to inhibit virus-induced cytopathogenicity by 50%, varied between 0.3 and 12 μg/ml and 0.2–1.9 μg/ml, for kappa-and lambda-carrageenan, respectively (as shown in Table 1 ). In 1990, researchers discovered that kappa-carrageenan inhibited arenavirus replication in Vero cells in a potent and selective manner, including junin (IC50 = 0.3 g/ml) and tacaribe (IC50 = 0.2 g/ml), whereas its sulphated polysaccharide was not inhibitory to host cell proliferation even at a concentration of 200 g/ml57 The inhibition of the host cell could be achieved at a concentration of 3000 μg/ml.58
Table 1.
Carrageenan (kappa-, iota-, and lambda-) efficacy against some viruses in vitro, including SARS-CoV-2.
| Type | Virus | Group | Cell lines | IC50 or EC50 (μg/ml) | References |
|---|---|---|---|---|---|
| Kappa- | DENV-1 | RNA virus | Vero | EC50 = >50 | 61 |
| DENV-2 | RNA virus | Vero | EC50 = 1.8 | 61 | |
| DENV-3 | RNA virus | Vero | EC50 = 6.3 | 61 | |
| DENV-4 | RNA virus | Vero | EC50 = >50 | 61 | |
| HSV-1 | DNA virus | Vero | IC50 = 1.9 | 95 | |
| HSV-2 | DNA virus | Vero | IC50 = 1.6 | 95 | |
| HSV-1 | DNA virus | PRK | IC50 = 3.7 | 56 | |
| HSV-2 | DNA virus | PRK | IC50 = 2 | 56 | |
| CMV | DNA virus | HEL | IC50 = 2.8 | 56 | |
| Vaccinia | DNA virus | PRK | IC50 = 36 | 56 | |
| VSV | RNA virus | PRK | IC50 = 0.3 | 56 | |
| VSV | RNA virus | HeLa | IC50 = 7 | 56 | |
| VSV | RNA virus | Vero | IC50 = 7 | 56 | |
| Sindbis virus | RNA virus | Vero | IC50 = 7 | 56 | |
| HIV-1 | RNA virus | MT-4 | IC50 = 12 | 56 | |
| H1N1 | RNA virus | MDCK | IC50 = 32.1 | 69 | |
| HPV16 | DNA virus | HeLa | IC50 = 0.044 | 96 | |
| RABVs SAD-L16 | RNA virus | HEK-293T | IC50 = 15.89 | 97 | |
| RABVs SAD-L16 | RNA virus | NA | IC50 = 22.10 | 97 | |
| RABVs SAD-L16 | RNA virus | SK-N-SH | IC50 = 19.93 | 97 | |
| RABVs SAD-L16 | RNA virus | BSR | IC50 = 57.70 | 97 | |
| JV | RNA virus | Vero | IC50 = 0.3 | 57 | |
| TACV | RNA virus | Vero | IC50 = 0.2 | 57 | |
| ASF | DNA virus | Vero | EC50 = 150 | 58 | |
| H1N1 | RNA virus | MDCK | EC50 = 89.57 | 98 | |
| Iota- | DENV-1 | RNA virus | Vero | EC50 = 40.7 | 61 |
| DENV-2 | RNA virus | Vero | EC50 = 0.4 | 61,99 | |
| DENV-2 | RNA virus | C6/36 HT | EC50 = 7 | 99 | |
| DENV-2 | RNA virus | HepG2 | EC50 = 0.14 | 61 | |
| DENV-3 | RNA virus | Vero | EC50 = 4.1 | 61 | |
| DENV-3 | RNA virus | HepG2 | EC50 = 0.63 | 61 | |
| DENV-4 | RNA virus | Vero | EC50 = 8.2 | 61 | |
| HPV16 | DNA virus | HeLa | IC50 = 0.006 | 96 | |
| H1N1 | RNA virus | MDCK | IC50 = 0.39 | 71 | |
| H3N2 | RNA virus | MDCK | IC50 = 0.92 | 71 | |
| H5N1 | RNA virus | MDCK | IC50 = 10.14 | 71 | |
| H7N7 | RNA virus | MDCK | IC50 = 118.48 | 71 | |
| SSPL | RNA virus | ACE2-HEK293 cells | IC50 = 2.6 | 75 | |
| SARS-CoV-2 (western blot) | RNA virus | Vero | IC50 = 0.046 | 75 | |
| SARS-CoV-2 (PCR) | RNA virus | Vero | IC50 = 1.54 | 75 | |
| hCoV OC43 | RNA virus | Vero | IC50 = 0.33 | 75 | |
| Lambda- | DENV-1 | RNA virus | Vero | EC50 = >50 | 61 |
| DENV-2 | RNA virus | Vero | EC50 = 0.15 | 61 | |
| DENV-2 | RNA virus | HepG2 | EC50 = 0.22 | 61 | |
| DENV-3 | RNA virus | Vero | EC50 = 2 | 61 | |
| DENV-3 | RNA virus | HepG2 | EC50 = 0.14 | 61 | |
| DENV-4 | RNA virus | Vero | EC50 = 4.2 | 61 | |
| BoHV-1 | DNA virus | MDBK | IC50 = 0.52 | 100 | |
| SuHV-1 | DNA virus | MDBK | IC50 = 10.42 | 100 | |
| HSV-1 | DNA virus | PRK | IC50 = 1.6 | 56 | |
| HSV-2 | DNA virus | PRK | IC50 = 1.5 | 56 | |
| HSV-1 | DNA virus | Astrocytes | IC50 = 1.6 | 67 | |
| HSV-2 | DNA virus | Astrocytes | IC50 = 0.9 | 67 | |
| HSV-1 | DNA virus | Vero | IC50 = 0.4 | 67 | |
| HSV-2 | DNA virus | Vero | IC50 = 0.4 | 67 | |
| CMV | DNA virus | HEL | IC50 = 0.3 | 56 | |
| Vaccinia | DNA virus | PRK | IC50 = 16 | 56 | |
| VSV | RNA virus | PRK | IC50 = 0.2 | 56 | |
| VSV | RNA virus | HeLa | IC50 = 4 | 56 | |
| VSV | RNA virus | Vero | IC50 = 2 | 56 | |
| Sindbis virus | RNA virus | Vero | IC50 = 2 | 56 | |
| HIV-1 | RNA virus | MT-4 | IC50 = 1.9 | 56 | |
| H1N1 | RNA virus | MDCK | IC50 = 0.04 | 70 | |
| H3N2 | RNA virus | MDCK | IC50 = 0.3 | 54 | |
| Influenza B | RNA virus | MDCK | IC50 = 1.4 | 54 | |
| SARS-CoV-2 | RNA virus | Vero | EC50 = 0.9 | 54 | |
| HPV16 | DNA virus | HeLa | IC50 = 0.010 | 96 | |
| ASF | DNA virus | Vero | EC50 = 25 | 58 |
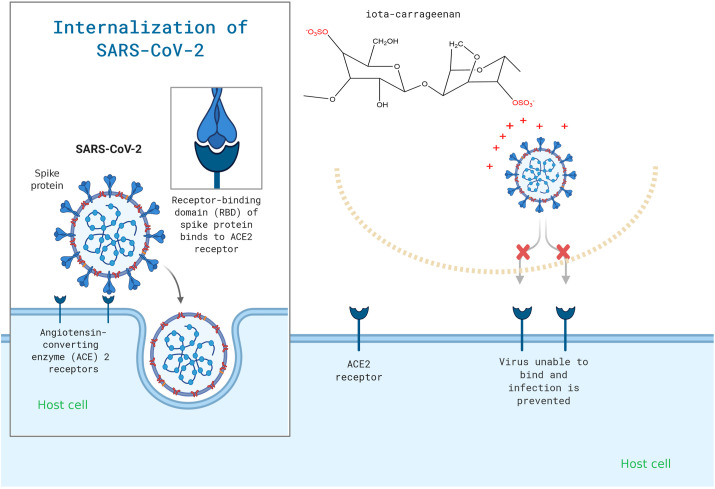
Carrageenan works by inhibiting enveloped RNA or DNA viruses, preventing them from attaching to or entering host cells.32 , 59, 60, 61 Like other sulphated polysaccharides including heparin, dextran sulphate, and pentosan polysulfate, carrageenan was also shown to inhibit the first step of the replication cycle of HIV-1 virus adsorption to CD4+ T-cell membrane.56 , 62 , 63 They specifically interact with the viral envelop of glycoprotein gp120 and inhibit the interaction of the virus with CD4. 64, 65, 66 Their negative charges shield off the positively charged amino acids at the viral envelop (Fig. 2 ). The natural kappa-carrageenan from red seaweed Gigartina skottsbergii showed anti HSV with the IC50 ranging from 0.9 to 1.6 and 0.4 μg/ml for mouse astrocytes and Vero cells, respectively.67 Its kappa-form is also reported to be strong and effective against human enterovirus 71 by preventing replication during viral adsorption.68
Fig. 2.
Carrageenan may be used to specifically target the viral attachment of SARS-CoV-2.
It became apparent that carrageenan has a broad spectrum of antiviral properties. Kappa-, iota-, and lambda-carrageenan are also reported to have shown antiviral activity through several viruses causing human respiratory disease which mostly belong to RNA viruses including influenza, rhinovirus and coronavirus (Table 1). A report showed that the derivative of kappa-carrageenan called kappa-carrageenan oligosaccharide (CO-1) with a molecular weight of 2000 Da was reported to be effective at inhibiting the replication of influenza A (H1N1) in MDCK cells with the half-maximal effective concentration (EC50). The effective concentration required to inhibit virus-induced cytopathogenicity by 50%, was 32.1 μg/ml.69 The inhibition of H1N1 was also shown in iota-carrageenan by inhibition of direct binding to its virus particles with an EC50 varied from 0.04 to 0.20 μg/ml.70 Its iota-form also inhibits H1N1, H3N2, H5N1 and H7N7 with IC50 of 0.39, 0.92, 10.14 and 118.48 μg/ml, respectively.71 In addition, lambda-carrageenan was effective in inhibiting both influenza A and B with EC50 varying between 0.3 and 1.4 μg/ml.54 The size of the molecule matters to the effectiveness of the inhibition. Carrageenan's low molecular weight has the potential to effectively inhibit virus particles.69 Varying chain lengths might be attributed to the water solubility properties and the abilities to internalize the host-cells.69 , 72 , 73 Thus, in 2008, a study reported that carrageenan showed antiviral properties against HRVs or human rhinoviruses in HeLa cells.32 Additionally, iota-carrageenan inhibits replication of HRV serotypes 1A, 8, 14, 16, 83, 84 on primary human nasal epithelial (HNep) cells.32 Iota-carrageenan also showed inhibitory properties against hCoV OC43, a member of beta-coronaviridae that is a frequent cause of respiratory illness,74 with IC50 of 0.33 μg/ml.75 A study published recently found that kappa-carrageenan inhibits emerging SARS-CoV-2 (MOI of 0.05) in Vero cells, with an EC50 value of 0.9 g/ml.54 SARS-CoV-2 entry process was inhibited Its kappa-form.54 Another study showed that iota-carrageenan neutralized SARS-CoV-2 Spike pseudotyped lentivirus (SSPL) at MOI of 0.1 in a dose-dependent manner with IC50 of 2.58 μg/ml.75 Additionally, Prieschl-Grassauer and her colleague compared the effectiveness of iota-, kappa-, and lambda using SSPL at 10 and 100 μg/ml. Thus, they found that iota-form effectively inhibited the virus at 10 μg/ml while kappa-and lambda-form were only active at 100 μg/ml.75 To get more deep-insight, SARS-CoV-2 isolated from a 61-year-old patient and was amplified in Vero B4 cells and its inhibitory activities were conducted using iota-carrageenan. The IC50 ranged from 0.046 to 1.54 g/ml.75 The activity of iota-form against SARS-CoV-2 was also studied using Vero E6 and showed less inhibition compared with Vero B4. 76 , 77 A study using two commercial pharmaceutical products, namely viruseptin nasal contained 1.2 mg/ml iota-and 0.4 mg/ml kappa-carrageenan (A) and viruseptin oral contained 1.2 mg/ml iota-form (B), showed inhibitory activity against SARS-CoV-2 at IC50 of 20 and 37 μg/ml, respectively.78 Without additives, in the pure form of carrageenan, A and B inhibited SARS-CoV-2 at IC50 of 21 and 33 μg/ml, respectively.78 Thus, the result is quite similar between products with additives and in pure form. Another research group investigated the inhibitory effect of iota-carrageenan on SARS-CoV-2 in Vero E6 and discovered a similar trend: at 600, 60, and 6 g/ml, with a xylitol concentration of 50 mg/ml, can indeed reduce over 4.25 log 10 copies/ml viruses compared to control.79
3.3. In vivo evidence
In 1993, an in vivo study was carried out using ICR mice (abbreviated from Institute of Cancer Research mice) that had been infected with cytomegalovirus and treated intraperitoneally with various doses of iota-carrageenan.80 Iota-carrageenan at 0.5 mg confers a greater protective effect on mice infected with murine cytomegalovirus via a host-mediated mechanism.80 In 1999, an in vivo study was conducted using HSV-2-infected female Swiss Webster mice with a single dose (10 mg/ml) of lambda- and iota-carrageenan.81 Post-exposure revealed that lambda-carrageenan provides significant protection (p < 0.05) against disease caused by intravaginal HSV-2 challenge.81 However, that is not the case for the iota-form.81 Therefore, a single dose of kappa-carrageenan protected 9 of 10 BALB/c mice infected with HSV-2 intravaginally.82 Another study showed that iota-carrageenan provides significant protection to BALB/cAnNCr mice-challenged HPV16 pseudovirus by either mechanical-or chemical disruption.83
In previous in vitro studies, all carrageenan subtypes showed antiviral activity against influenza virus (Table 1). Thus, several researchers investigated combined therapy of carrageenan with available influenza drugs on the market, including prodrug oseltamivir and zanamivir, an active drug. A study using the combination of iota-carrageenan with oseltamivir led to significantly enhanced survival of mice-infected H1N1 in comparison with the respective mono-therapies.70 The combination of kappa-and iota-carrageenan for the treatment of lethally H7N7 infected C57Bl/6 mice showed better therapeutic effect (p < 0.0004) compared to the use of iota-form alone (p < 0.1507).71 Moreover, the combination of kappa and iota-carrageenan with 3 mg/kg BW/day zanamivir used for treatment of H7N7 infected C57Bl/6 mice revealed synergistically elevated survival of mice in comparison of both mono-therapies.71 However, no reports on the in vivo study using kappa-, iota-, or lambda carrageenan have been published recently. Therefore, the evident from molecular docking showed that carrageenan exhibited promising inhibitory action by showing a binding energy score of-8.4 kcal/mol against SARS-CoV-2 Mpro at two crucial residues, namely Cys 145 and His 41 .84
3.4. Clinical evidence
The common cold is caused by a variety of respiratory viruses, including human rhinoviruses (HRV), coronaviruses, human enteroviruses (HEV), parainfluenza viruses, respiratory syncytial virus (RSV), and influenza viruses. On the other hand, HRVs are the most common cause of respiratory tract infection in all age groups.30 , 85 In 2008, under the European clinical trial registry number 2007-007577-23, double blind randomized clinical trials were conducted to evaluate the efficacy and safety of iota-carrageenan in treating patients with the common cold.86 Around 35 adult patients in the United Kingdom with early symptoms of the common cold received 0.12% iota-carrageenan in saline solution three times daily for up to four days, in comparison to a placebo. Its administration has been shown to decrease viral load in nasal lavages (p = 0.009) and symptoms of the common cold (p = 0.046). Additionally, several pro-inflammatory mediators such as IFN-2, IL-8, IL-10, IL-1, IP-10, GRO, G-CSF, Fractalkine, and FGF-2 are produced.86 Likewise, this study also used a large sample size of adult patients (n = 211) in Austria from 2010 to 2011 and was registered as ISRCTN80148028 87. The results indicated that administering iota-nasal carrageenan's spray significantly decreased the duration of symptoms (p = 0.037) and viral titers in nasal fluids (p = 0.024).87 Additional large-scale studies were conducted in the United Kingdom (NCT01944631)88 and Austria (NCT04533906), both of which show a similar trend to two previous studies (Table 2 ).
Table 2.
A list of clinical trials involving carrageenan for the common cold and SARS-CoV-2 infection that have been registered on ClinicalTrials.gov.
| Identifier number | Title | Intervention | Status | Phase | Location |
|---|---|---|---|---|---|
| NCT01944631 | Iota-carrageenan nasal spray in common cold | Four times daily for 4–10 days, nasal spray containing 1.20 g/l iota-carrageenan in saline | completed | 4 | UK |
| NCT04533906 | Study to Investigate if Sucking a Coldamaris Lozenge Elutes Sufficient Iota-carrageenan to Inactivate Usual Common Cold Viruses | Sucking iota-carrageenan containing lozenge | completed | NA | Austria |
| NCT04425850 | Usefulness of Topic Ivermectin and Carrageenan to Prevent Contagion of Covid 19 (IVERCAR) | Ivermectin nasal spray and Iota carrageenan nasal spray (used as buccal drops 5 times a day). Application to the nose and oral cavity on a topical basis. | completed | NA | Argentina |
| NCT04701710 | Prophylaxis Covid-19 in healthcare agents by intensive treatment with ivermectin and iota-carrageenan (Ivercar-Tuc) | Ivermectin 2 drops of 6 mg equals 12 mg every 7 days orally, and iota-carrageenan 6 sprays daily for 4 weeks | completed | 2 | Argentina |
| NCT04793984 | Efficacy and Safety Evaluation of Inhaleen Inhalation in Hospitalized COVID-19 Patients | Inhalation of Carragelose® (contains 1.2 mg/ml iota-carrageenan) three times daily | recruiting | – | Austria |
| NCT04681001 | Prophylactic treatment with carragelose nasal spray to prevent SARS-CoV-2, COVID-19, infections in health care workers | Nasal spray of Coldamaris pro. (contains 1.2 mg/ml iota-carrageenan) into nostrils and mouth | recruiting | – | Austria |
| NCT04590365 | Iota-carrageenan nasal spray COVID-19 prophylaxis for healthcare professionals (ICE-COVID) | Coldamaris plus nasal and throat spray (Iota-carrageenan 0.12% in 0.5% saline) | recruiting | – | UK |
Furthermore, a randomized double-blind trial involving 153 children was conducted between 2009 and 2010 under the registration number ISRCTN52519535 to determine the efficacy of 12% iota-carrageenan in saline solution.31 The report concluded that there is no statistically significant difference in total symptom score between the treated and placebo groups.31 As a result, between the treated and placebo groups, significant differences in clearance disease (p = 0.038), lower incidence of secondary infections by other respiratory viruses (p = 0.046), and viral load reduction (p = 0.026) were observed.31 Thus, these studies showed that treatment with iota-carrageenan nasal spray is safe and effective for children and adults experiencing early symptoms of the common cold.31
As iota-carrageenan inhibited SARS-CoV-2 in vitro with an IC50 of 1.54 g/ml (as shown in Table 1), this effect was mediated by the interaction of positively charged domains on the glycoprotein envelope involved in binding to protein glycans on the host cell surface (Fig. 2). SARS-CoV-2 S-protein is slightly more positively charged compared to S-spike protein of SARS-CoV due to the addition of four positively charged residues and the elimination of five negatively charged residues.89 Moreover, it was effective at treating common colds caused by both rhinoviruses and coronaviruses.85 , 87 Thus, the transferability of these in vitro data into clinical effectiveness for COVID-19 could be performed and evaluated.
Iota-carrageenan could be administered directly to patients infected with COVID-19 via nasal spray in conjunction with an oral antiviral such as ivermectin. Ivermectin was found to have significant antiviral activity against SARS-CoV-2 in three clinical trials: NCT04343092 (Effectiveness of ivermectin as adjunct therapy in COVID-19 management),90 NCT04422561 (Prophylactic ivermectin in COVID-19 contacts),91 , 92 and NCT04381884 (Ivermectin effect on SARS-CoV-2 replication in patients with COVID-19).92
In 2020, a clinical trial involving 229 participants was conducted at Eurnekian Public Hospital in Buenos Aires, Argentina under the registry number NCT04425850 (Table 2) to determine the efficacy of a topical treatment with iota carrageenan (nasal and buccal) and ivermectin (buccal). No adverse events were observed in the treated COVID-19 patients over a 30-day period.93 In 2021, a randomized controlled 1-1 clinical trial under registration NCT04701710 was conducted to prioritize health personnel infected with SARS-CoV-2 (n = 232) at Argentina's Public Healthcare Centre, using the same iota-carrageenan and ivermectin regimen as in the previous study.94 The result indicated that the number of subjects diagnosed with COVID-19 was significantly lower in the treated group, at 3.4%, than in the control group, at 21.4% (p = 0.0001).94 The treated group also had a lower prevalence of mild to moderate COVID-19 presentation compared to the control group.94 This intensive preventive treatment with iota-carrageenan and ivermectin was able to significantly reduce the number of infected health care workers.94 The study using iota-carrageenan in a single dose is still ongoing under the register numbers NCT04793984, NCT04681001, and NCT04590365. Therefore, the results of these ongoing clinical trials are required to provide conclusive evidence of iota-carrageenan's efficacy in patients with COVID-19. Additionally, there is a dearth of data on carrageenan, particularly regarding drug–drug, drug–gene, and drug–disease interactions. This information is critical in predicting potential adverse events that may occur during treatment.
4. Conclusion
Numerous carrageenan subtypes, including kappa-, iota-, and lambda-, inhibit SARS-CoV-2 infection in vitro by interfering with virus adsorption and internalization. The antiviral activity of iota-carrageenan as a single dose administered nasally is being studied in several clinical trials, whereas its co-administration with ivermectin was studied in two clinical trials and demonstrated improvement in outcome for COVID-19 patients. However, large-scale clinical trials should be conducted to demonstrate the efficacy of iota-carrageenan and its kappa-and lambda-subtypes in the treatment of COVID-19 patients.
Funding
The author wishes to express his gratitude to Program for Prioritas Nasional Ilmu Pengetahuan Kebumian, the Indonesian Institute of Sciences (MALSAI/5942. SDB.002).
Declaration of competing interest
None declared.
References
- 1.Chakraborty I., Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene C.J., Burleson S.L., Crosby J.C., Heimann M.A., Pigott D.C. Coronavirus disease 2019: international public health considerations. Journal of the American College of Emergency Physicians Open. 2020;1:70–77. doi: 10.1002/emp2.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nainu F., Abidin R.S., Bahar M.A., et al. SARS-CoV-2 reinfection and implications for vaccine development. Hum Vaccines Immunother. 2020;16:3061–3073. doi: 10.1080/21645515.2020.1830683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. J Am Med Assoc. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou Y.J., Okuda K., Edwards C.E., et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446. doi: 10.1016/j.cell.2020.05.042. e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav Med Infect Dis. 2020:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robba C., Battaglini D., Pelosi P., Rocco P.R. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expet Rev Respir Med. 2020;14:865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:1–8. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syahrul S., Maliga H.A., Ilmawan M., et al. Hemorrhagic and ischemic stroke in patients with coronavirus disease 2019: incidence, risk factors, and pathogenesis-a systematic review and meta-analysis. F1000Research. 2021:10. doi: 10.12688/f1000research.42308.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutiawati E., Fahriani M., Mamada S.S., et al. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms-a systematic review and meta-analysis. F1000Research. 2021:10. doi: 10.12688/f1000research.28393.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf F., Fahriani M., Mamada S.S., et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: a systematic review and meta-analysis. F1000Research. 2021;10:301. doi: 10.12688/f1000research.52216.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worldometers COVID-19 coronavirus pandemic. 2021. https://www.worldometers.info/coronavirus/2020
- 13.Shao N., Cheng J., Chen W. The reproductive number R0 of COVID-19 based on estimate of a statistical time delay dynamical system. MedRxiv. 2020 doi: 10.1101/2020.02.17.20023747. [DOI] [Google Scholar]
- 14.Frediansyah A., Tiwari R., Sharun K., Dhama K., Harapan H. Vol. 9. Clinical Epidemiology and Global Health; 2020. Antivirals for COVID-19: A Critical Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontanet A., Autran B., Lina B., Kieny M.P., Karim S.S.A., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet Infect Dis. 2021;397:952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frediansyah A., Nainu F., Dhama K., Mudatsir M., Harapan H. Vol. 9. Clinical Epidemiology and Global Health; 2020. Remdesivir and its Antiviral Activity against COVID-19: A Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mudatsir M., Yufika A., Nainu F., et al. Antiviral activity of ivermectin against SARS-CoV-2: an old-fashioned dog with a new trick—a literature review. Sci Pharm. 2020;88:36. [Google Scholar]
- 19.Kaur H., Shekhar N., Sharma S., Sarma P., Prakash A., Medhi B. Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes. Pharmacol Rep. 2021:1–14. doi: 10.1007/s43440-020-00195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. J Am Med Assoc. 2020;323:1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz P., Righy C., Gadelha M., et al. Effect of convalescent plasma in critically ill patients with COVID-19: an observational study. Front Med. 2021;8 doi: 10.3389/fmed.2021.630982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020;15:247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S.N., Atkinson S.C., Wang C., et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir Res. 2020;177:104760. doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- 24.Drożdżal S., Rosik J., Lechowicz K., et al. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updates. 2020:100719. doi: 10.1016/j.drup.2020.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirzada R.H., Haseeb M., Batool M., Kim M., Choi S. Remdesivir and ledipasvir among the FDA-approved antiviral drugs have potential to inhibit SARS-CoV-2 replication. Cells. 2021;10:1052. doi: 10.3390/cells10051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. “Solidarity” Clinical Trial for COVID-19 Treatments 2020.
- 27.Mishra G.P., Mulani J. Corticosteroids for COVID-19: the search for an optimum duration of therapy. The Lancet. Respiratory Medicine. 2021;9:e8. doi: 10.1016/S2213-2600(20)30530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khotimchenko M., Tiasto V., Kalitnik A., et al. Antitumor potential of carrageenans from marine red algae. Carbohydr Polym. 2020:116568. doi: 10.1016/j.carbpol.2020.116568. [DOI] [PubMed] [Google Scholar]
- 29.Kuznetsova T.A., Andryukov B.G., Besednova N.N., Zaporozhets T.S., Kalinin A.V. Marine algae polysaccharides as basis for wound dressings, drug delivery, and tissue engineering: a review. J Mar Sci Eng. 2020;8:481. [Google Scholar]
- 30.Mäkelä M.J., Puhakka T., Ruuskanen O., et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fazekas T., Eickhoff P., Pruckner N., et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Compl Alternative Med. 2012;12:1–8. doi: 10.1186/1472-6882-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J. 2008;5:1–13. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Therkelsen G.H. Elsevier; 1993. Carrageenan. Industrial Gums; pp. 145–180. [Google Scholar]
- 34.Wong K.F., Craigie J.S. Sulfohydrolase activity and carrageenan biosynthesis in Chondrus crispus (Rhodophyceae) Plant Physiol. 1978;61:663–666. doi: 10.1104/pp.61.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Jimenez P., Mantesa S.R., Robaina R.R. Expression of genes related to carrageenan synthesis during carposporogenesis of the red seaweed grateloupia imbricata. Mar Drugs. 2020;18:432. doi: 10.3390/md18090432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falshaw R., Furneaux R.H. Carrageenan from the tetrasporic stage of Gigartina decipiens (Gigartinaceae, rhodophyta) Carbohydr Res. 1994;252:171–182. doi: 10.1016/0008-6215(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 37.Freile-Pelegrín Y., Robledo D. Carrageenan of eucheuma isiforme (Solieriaceae, rhodophyta) from Nicaragua. J Appl Phycol. 2008;20:537–541. [Google Scholar]
- 38.Nussinovitch A. Springer; 1997. Carrageenans. Hydrocolloid Applications; pp. 40–62. [Google Scholar]
- 39.Zia K.M., Tabasum S., Nasif M., et al. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int J Biol Macromol. 2017;96:282–301. doi: 10.1016/j.ijbiomac.2016.11.095. [DOI] [PubMed] [Google Scholar]
- 40.Stanley N. Production, properties and uses of carrageenan. Production utilization of products from commercial seaweeds. FAO Fish Tech Pap. 1987;288:116–146. [Google Scholar]
- 41.Garrec D.A., Norton I.T. Kappa carrageenan fluid gel material properties. Part 2: Tribology. Food Hydrocolloids. 2013;33:160–167. [Google Scholar]
- 42.Di Rosa M. Biological properties of carrageenan. J Pharm Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 43.Weiner M.L. Food additive carrageenan: Part II: a critical review of carrageenan in vivo safety studies. Crit Rev Toxicol. 2014;44:244–269. doi: 10.3109/10408444.2013.861798. [DOI] [PubMed] [Google Scholar]
- 44.Luo M., Shao B., Nie W., et al. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci Rep. 2015;5:1–12. doi: 10.1038/srep11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emam H.E., Ahmed HBs. Antitumor/antiviral carbon quantum dots based on carrageenan and pullulan. Int J Biol Macromol. 2021;170:688–700. doi: 10.1016/j.ijbiomac.2020.12.151. [DOI] [PubMed] [Google Scholar]
- 46.Mani S.D., Govindan M., Muthamilarasan M., Nagarathnam R. A sulfated polysaccharide κ-carrageenan induced antioxidant defense and proteomic changes in chloroplast against leaf spot disease of tomato. J Appl Phycol. 2021:1–15. [Google Scholar]
- 47.Júnior E.H., Gonçalves A.G., Noseda M.D., Duarte M.E.R., Murakami F.S., Ducatti D.R. Semi-synthesis of N-alkyl-kappa-carrageenan derivatives and evaluation of their antibacterial activity. Carbohydr Res. 2021;499:108234. doi: 10.1016/j.carres.2021.108234. [DOI] [PubMed] [Google Scholar]
- 48.Carlucci M.J., Pujol C.A., Ciancia M., et al. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: correlation between structure and biological activity. Int J Biol Macromol. 1997;20:97–105. doi: 10.1016/s0141-8130(96)01145-2. [DOI] [PubMed] [Google Scholar]
- 49.de Araújo C.A., Noseda M.D., Cipriani T.R., Gonçalves A.G., Duarte M.E.R., Ducatti D.R. Selective sulfation of carrageenans and the influence of sulfate regiochemistry on anticoagulant properties. Carbohydr Polym. 2013;91:483–491. doi: 10.1016/j.carbpol.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 50.dos Santos-Fidencio G.C., Gonçalves A.G., Noseda M.D., Duarte M.E.R., Ducatti D.R. Effects of carboxyl group on the anticoagulant activity of oxidized carrageenans. Carbohydr Polym. 2019;214:286–293. doi: 10.1016/j.carbpol.2019.03.057. [DOI] [PubMed] [Google Scholar]
- 51.Yuan H., Song J., Li X., Li N., Dai J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Canc Lett. 2006;243:228–234. doi: 10.1016/j.canlet.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 52.Cicinskas E., Kalitnik A.A., Karetin Y.A., Ram M.S.G.M., Achary A., Kravchenko A.O. Immunomodulating properties of carrageenan from tichocarpus crinitus. Inflammation. 2020:1–10. doi: 10.1007/s10753-020-01216-x. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez M., Alarcón B., Carrasco L. Polysaccharides as antiviral agents: antiviral activity of carrageenan. Antimicrob Agents Chemother. 1987;31:1388–1393. doi: 10.1128/aac.31.9.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang Y., Shin H., Lee M.K., et al. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci Rep. 2021;11:1–12. doi: 10.1038/s41598-020-80896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pacheco-Quito E.-M., Ruiz-Caro R., Carrageenan Veiga M-D. Drug delivery systems and other biomedical applications. Mar Drugs. 2020;18:583. doi: 10.3390/md18110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baba M., Snoeck R., Pauwels R., de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrei G., De Clercq E. Inhibitory effect of selected antiviral compounds on arenavirus replication in vitro. Antivir Res. 1990;14:287–299. doi: 10.1016/0166-3542(90)90009-v. [DOI] [PubMed] [Google Scholar]
- 58.García-Villalón D., Gil-Fernández C. Antiviral activity of sulfated polysaccharides against African swine fever virus. Antivir Res. 1991;15:139–148. doi: 10.1016/0166-3542(91)90031-l. [DOI] [PubMed] [Google Scholar]
- 59.Baba M., Nakajima M., Schols D., Pauwels R., Balzarini J., De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antivir Res. 1988;9:335–343. doi: 10.1016/0166-3542(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 60.Guo C., Zhu Z., Yu P., et al. Inhibitory effect of iota-carrageenan on porcine reproductive and respiratory syndrome virus in vitro. Antivir Ther. 2019;24:261–270. doi: 10.3851/IMP3295. [DOI] [PubMed] [Google Scholar]
- 61.Talarico L.B., Damonte E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virol J. 2007;363:473–485. doi: 10.1016/j.virol.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 62.Nakashima H., Yoshida O., Baba M., De Clercq E., Yamamoto N. Anti-HIV activity of dextran sulphate as determined under different experimental conditions. Antivir Res. 1989;11:233–246. doi: 10.1016/0166-3542(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 63.Yutaro K., Osamu Y., Ryusuke N., et al. Inhibition of HIV-1 infectivity with curdlan sulfate in vitro. Biochem Pharmacol. 1990;39:793–797. doi: 10.1016/0006-2952(90)90161-d. [DOI] [PubMed] [Google Scholar]
- 64.Mitsuya H., Looney D.J., Kuno S., Ueno R., Wong-Staal F., Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Sci Total Environ. 1988;240:646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- 65.Sugawara I., Itoh W., Kimura S., Mori S., Shimada K. Further characterization of sulfated homopolysaccharides as anti-HIV agents. Experientia. 1989;45:996–998. doi: 10.1007/BF01953060. [DOI] [PubMed] [Google Scholar]
- 66.McClure M.O., Moore J.P., Blanc D.F., et al. Investigations into the mechanism by which sulfated polysaccharides inhibit HIV infection in vitro. AIDS Res Hum Retrovir. 1992;8:19–26. doi: 10.1089/aid.1992.8.19. [DOI] [PubMed] [Google Scholar]
- 67.Carlucci M., Scolaro L., Damonte E. Inhibitory action of natural carrageenans on Herpes simplex virus infection of mouse astrocytes. Chemotherapy. 1999;45:429–436. doi: 10.1159/000007236. [DOI] [PubMed] [Google Scholar]
- 68.Chiu Y.-H., Chan Y.-L., Tsai L.-W., Li T.-L., Wu C.-J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir Res. 2012;95:128–134. doi: 10.1016/j.antiviral.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Wang W., Zhang P., Hao C., Zhang X.-E., Cui Z.-Q., Guan H.-S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir Res. 2011;92:237–246. doi: 10.1016/j.antiviral.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Leibbrandt A., Meier C., König-Schuster M., et al. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PloS One. 2010;5 doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morokutti-Kurz M., König-Schuster M., Koller C., et al. The intranasal application of Zanamivir and carrageenan is synergistically active against influenza A virus in the murine model. PloS One. 2015;10 doi: 10.1371/journal.pone.0128794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji J., Wang L.C., Wu H., Luan H.M. Bio-function summary of marine oligosaccharides. Int J Biol. 2011;3(1):74–86. [Google Scholar]
- 73.Ning J., Zhang W., Yi Y., et al. Synthesis of β-(1→ 6)-branched β-(1→ 3) glucohexaose and its analogues containing an α-(1→ 3) linked bond with antitumor activity. 2003;11:2193–2203. doi: 10.1016/s0968-0896(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 74.McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. PNAS. 1967;57:933. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morokutti-Kurz M., Fröba M., Graf P., et al. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PloS One. 2021;16(2) doi: 10.1371/journal.pone.0237480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song S., Peng H., Wang Q., et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020;11(9):7415–7420. doi: 10.1039/D0FO02017F. [DOI] [PubMed] [Google Scholar]
- 77.Gasbarri M., V’kovski P., Torriani G., et al. Sars-cov-2 inhibition by sulfonated compounds. Microorganisms. 2020;8(12):1894. doi: 10.3390/microorganisms8121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schütz D., Conzelmann C., Fois G., et al. Carrageenan containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol. 2021;320(5):L750–L756. doi: 10.1152/ajplung.00552.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bansal S., Jonsson C.B., Taylor S.L., et al. bioRxiv; 2020. Iota-carrageenan and xylitol inhibit SARS-CoV-2 in cell culture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamasuna R., Eizuru Y., Shishime Y., Minamishima Y. Protective effect of carrageenan against murine cytomegalovirus infection in mice. Antiviral Chem Chemother. 1993;4:353–360. [Google Scholar]
- 81.Bourne K.Z., Bourne N., Reising S.F., Stanberry L.R. Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir Res. 1999;42:219–226. doi: 10.1016/s0166-3542(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 82.Carlucci M., Scolaro L., Noseda M., Cerezo A., Damonte E. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir Res. 2004;64:137–141. doi: 10.1016/j.antiviral.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Roberts J.N., Buck C.B., Thompson C.D., et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 84.Naidoo D., Roy A., Kar P., Mutanda T., Anandraj A., Dynamics Cyanobacterial Metabolites as Promising Drug Leads against the Mpro and PLpro of SARS-CoV-2: An in Silico Analysis. J Biomol Struct Dyn. 2020;18:1–13. doi: 10.1080/07391102.2020.1794972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eccles R., Meier C., Jawad M., Weinmüllner R., Grassauer A., Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res. 2010;11:1–10. doi: 10.1186/1465-9921-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ludwig M., Enzenhofer E., Schneider S., et al. Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir Res. 2013;14:1–11. doi: 10.1186/1465-9921-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eccles R., Winther B., Johnston S., Robinson P., Trampisch M., Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res. 2015;16:1–11. doi: 10.1186/s12931-015-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hassanzadeh K., Perez Pena H., Dragotto J., et al. Considerations around the SARS-CoV-2 Spike Protein with particular attention to COVID-19 brain infection and neurological symptoms. ACS Chem Neurosci. 2020;11:2361–2369. doi: 10.1021/acschemneuro.0c00373. [DOI] [PubMed] [Google Scholar]
- 90.Gorial F.I., Mashhadani S., Sayaly H.M., et al. Effectiveness of ivermectin as add-on therapy in COVID-19 management (pilot trial) medRxiv. 2020 doi: 10.1101/2020.02.17.20023747. [DOI] [Google Scholar]
- 91.Shoumann W.M., Hegazy A.A., Nafae R.M., et al. Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomized clinical trial. J Clin Diagn Res. 2021:15. [Google Scholar]
- 92.Formiga F.R., Leblanc R., de Souza Rebouças J., Farias L.P., de Oliveira R.N., Pena L. Ivermectin: an award-winning drug with expected antiviral activity against COVID-19. J Contr Release. 2021;329:758–761. doi: 10.1016/j.jconrel.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Héctor C., Roberto H., Psaltis A., Veronica C. Study of the efficacy and safety of topical ivermectin+ iota-carrageenan in the prophylaxis against COVID-19 in health personnel. Journal of Biomedical Research and Clinical Investigation. 2020;2 [Google Scholar]
- 94.Chahla R.E., Ruiz L.M., Ortega E.S., et al. A randomized trial-intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in healthcare agents. medRxiv. 2021 doi: 10.1101/2021.03.26.21254398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kolender A.A., Pujol C.A., Damonte E.B., Cerezo A.S., Matulewicz M. Sulfation of kappa-carrageenan and antiviral activity. Anales-Asociation Quimica Argentina. 1998;86:304–311. Fernandi Garcia Cambeiro. [Google Scholar]
- 96.Buck C.B., Thompson C.D., Roberts J.N., Müller M., Lowy D.R., Schiller J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS pathogen. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo Z., Tian D., Zhou M., et al. λ-carrageenan P32 is a potent inhibitor of rabies virus infection. PloS One. 2015;10 doi: 10.1371/journal.pone.0140586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shao Q., Guo Q., ping Xu W., Li Z., tong Zhao T. Specific inhibitory effect of κ-carrageenan polysaccharide on swine pandemic 2009 H1N1 influenza virus. PloS One. 2015;10 doi: 10.1371/journal.pone.0126577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Talarico L.B., Noseda M.D., Ducatti D.R., Duarte M.E., Damonte E.B. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J Gen Virol. 2011;92:1332–1342. doi: 10.1099/vir.0.028522-0. [DOI] [PubMed] [Google Scholar]
- 100.Diogo J.V., Novo S.G., González M.J., Ciancia M., Bratanich A.C. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res Vet Sci. 2015;98:142–144. doi: 10.1016/j.rvsc.2014.11.010. [DOI] [PubMed] [Google Scholar]