Abstract
Purpose:
The purpose of this study was to investigate the effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) versus sham stimulation on intracortical inhibition (ICI) and intracortical facilitation within the motor cortex. Such data are needed to better understand the presumed neurophysiologic effects of rTMS.
Methods:
The authors hypothesized that, compared with sham stimulation, 20 Hz rTMS will decrease ICI and increase intracortical facilitation in healthy volunteers. Using single-pulse and paired-pulse TMS, the authors evaluated prestimulation and poststimulation effects on motor cortex neurophysiology in neurologically healthy volunteers who received 2,000 stimuli of either 20 Hz rTMS (n = 11) or sham rTMS (n = 8). Primary outcomes were changes in ICI and intracortical facilitation and secondary outcomes were changes in motor threshold and motor evoked potential amplitude, and both were assessed using separate 2 × 2 (group × time) repeated-measures analysis of variance.
Results:
For ICI, there were main effects of time (P = 0.002) and group (P < 0.001) with a significant group-by-time interaction (P < 0.01). Intracortical inhibition decreased after rTMS, but was unchanged by sham rTMS. Intracortical facilitation results revealed a main effect of group (P = 0.02) and a significant group-by-time interaction (P = 0.048). Intracortical facilitation increased after rTMS and was slightly reduced after sham rTMS. The group-by-time interactions for motor threshold and motor evoked potential amplitude were not significant.
Conclusions:
High-frequency rTMS significantly influences the excitatory and inhibitory outputs of motor intracortical networks, specifically increasing intracortical facilitation and reducing ICI as compared with sham stimulation. Such changes were observed despite no significant changes in broader measures of motor cortex activation, that is, motor threshold and motor evoked potential amplitude.
Keywords: Transcranial magnetic stimulation, Brain, Neurostimulation, Intracortical facilitation, Intracortical inhibition
Repetitive transcranial magnetic stimulation (rTMS) provides a noninvasive means to stimulate the brain and to modulate its activity. Over the last decade, numerous protocols have been developed to use high-frequency rTMS (e.g., ≥ 5 Hz) as a clinical intervention for people with movement disorders arising from neurologic diseases such as stroke (Massie et al., 2013b), Parkinson disease (Khedr et al., 2006), and multiple sclerosis (Koch et al., 2008). In these disease states, high-frequency rTMS is applied to raise cortical excitability in support of improved motor activation. Indeed, some applications of rTMS have produced favorable behavioral results—implicating a therapeutic benefit of the technique (Emara et al., 2010; Khedr et al., 2009; Khedr et al., 2010; Massie et al., 2013b). Alternatively, other applications of rTMS have not produced the intended benefits to a clinically relevant degree (Ameli et al., 2009; Malcolm et al., 2007; Pomeroy et al., 2007; Theilig et al., 2011). Recent review articles on rTMS have implicated the need to better understand the underlying neurophysiologic mechanisms at play in therapeutic rTMS protocols and methods (Corti et al., 2012).
The most common neurophysiologic measures reported in the rTMS literature, resting motor threshold and motor evoked potential (MEP) size, are fairly broad measures of motor cortex excitability. Motor threshold, defined as the lowest magnetic stimulation intensity to produce an MEP in 50% of trials, has been described as a global measure of motor cortex excitability and perhaps is influenced by membrane excitability in postsynaptic neurons (Siebner and Rothwell, 2003). Similarly, MEP size is a broad measure believed to reflect the regional level of excitability in the corticospinal system (Pell et al., 2011). Although such measures provide some general insight into rTMS-induced changes, they do not implicate more specific mechanisms of action.
Measurement of inhibitory and facilitative intracortical activities yields a more direct measure of rTMS effects on the underlying neuronal network. For example, intracortical inhibition (ICI) and intracortical facilitation (ICF) may be assessed with paired-pulse transcranial magnetic stimulation (ppTMS). In the ppTMS paradigm, a suprathreshold test stimulus is preceded by a subthreshold conditioning stimulus (CS). The subthreshold CS intensity is thus too low to produce a corticospinal volley (Di Lazzaro et al., 1998), and therefore its effects are on cortical rather than subcortical or spinal levels. Depending on the interstimulus interval (ISI) between pulses, the CS will either inhibit or facilitate the normal MEP response. In ppTMS and compared with the unconditioned MEP, the conditioned MEP amplitude is reduced when the ISI is 1–4 milliseconds and increased when it is 5 to 30 milliseconds, respectively, reflecting ICI and ICF.
Although the capability to apply ppTMS to study the effects of rTMS has existed for some time, limitations exist in the current literature as follows: no sham condition, low number of rTMS stimuli (Fitzgerald et al., 2006a; Fitzgerald et al., 2006b; Peinemann et al., 2004), and few studies using higher frequency rTMS (e.g., 20 Hz) that are used in therapeutic applications (Fitzgerald et al., 2006a). The purpose of this study was, therefore, to investigate the effects of high-frequency rTMS versus sham stimulation on intracortical excitation and inhibition within the motor cortex. Such data are needed to better understand the presumed neurophysiologic effects of rTMS and must be established to validate the potential underlying neurologic mechanism targeted in therapeutic applications of rTMS. Our global hypothesis is that, compared with sham stimulation, 20 Hz rTMS will increase ICF and decrease ICI in healthy volunteers.
METHODS
Design
This study used a pretest and posttest design with an experimental group, which received rTMS. After recruiting and testing the experimental group, the investigators recognized the need to add a control group, which received sham stimulation. Pretest and posttest neurophysiologic measures were administered immediately before and after either rTMS or sham stimulation.
Subjects
Eleven neurologically intact right-handed individuals were recruited to participate in the rTMS group (6 women; mean age 25.6 ± 3.5 years). Subsequent to testing the rTMS group, we added a sham rTMS group (n = 8; 4 women; mean age 25.7 ± 3.0 years). All subjects met the following inclusion/exclusion criteria: no history of neurologic disease, seizures, epilepsy, or head injury leading to loss of consciousness within the last year; no implanted metal in the head or neck; no pacemaker or other implanted medical devices; not taking medications that lower the seizure threshold; not pregnant; not less than 18 years of age; and no evidence of epileptiform activity on a screening EEG. All study procedures were approved by the local institutional review board and all subjects provided written informed consent.
Experimental Procedures
Real rTMS
Subjects were comfortably seated in a dental chair, with the right forearm and hand supported. Bipolar surface electromyography electrodes were placed over the right first dorsal interosseous. Stimulation was delivered using the Magstim Rapid magnetic stimulator, through a 70-mm figure-of-eight–shaped coil (Magstim Ltd, Whitland, United Kingdom). The magnetic coil was placed over the left brain hemisphere, in contact with the scalp overlying the optimal area for eliciting MEPs in the contralateral first dorsal interosseous. The coil handle was oriented 45° from the midsagittal line to produce an induced current flow in the anteromedial direction, which is approximately perpendicular to the central sulcus (Laakso et al., 2014) and has been demonstrated as optimal for generating MEPs in intrinsic hand muscles (Laakso et al., 2014; Mills et al., 1992). Stimulus intensity was set at 90% of motor threshold for eliciting MEPs. We define motor threshold as the stimulator output that evokes an MEP of 50 μV in 5 of 10 trials (Chen et al., 1997). We approached motor threshold from below, that is, we started at low intensity (e.g., 25% output) and increased in 2% to 5% increments up to threshold. All subjects in the real rTMS group received 2,000 stimuli during rTMS, administered in the form of 50 trains of 40 stimuli. Stimulus rate was 20 Hz with a train duration of 2 seconds and an intertrain interval of 28 seconds. As an additional safety procedure, electromyography responses were observed between stimulation runs to monitor for post-TMS electromyography activity, as suggested by Chen et al. (1997).
Sham rTMS
All set-up procedures (e.g., subject positioning, electrode placement, coil orientation, etc) for subjects in the sham rTMS group were identical to the real rTMS group. Sham rTMS was administered using a specially designed Magstim figure-of-eight–shaped coil (Magstim Ltd). This coil generates a magnetic field, which is 95% attenuated, but produces noise and vibration comparable to those of a real magnetic coil (Mennemeier et al., 2009). Stimulus parameters and coil location for sham rTMS were identical to those for real rTMS.
TMS Testing Procedures
Before and immediately after real or sham rTMS, subjects underwent TMS testing trials to assess responses to single and paired-pulse stimulation to assess MEP amplitude, ICI, and ICF—the outcome measures of interest. Ten trials, each of single-pulse test stimulus, single-pulse CS, ICI, and ICF, were randomly ordered and successively administered (for a total of 40 trials each before rTMS and after rTMS). TMS was delivered with a figure-of-eight–shaped coil and 2 Magstim stimulators connected through a bistimulation module (Magstim Ltd). The coil was positioned over the optimal stimulating position to obtain MEPs, as determined during initial assessment of motor threshold and using the same coil orientation. For both ICI and ICF trials, the intensity of the CS was 90% of the resting motor threshold, and the test stimulus was 116% of the resting motor threshold (Chen et al., 1998). The ISIs were set at 2 milliseconds for ICI trials and 15 milliseconds for ICF trials. The intertrial interval was 6 seconds.
Statistical Analyses
Raw and statistical data analyses were performed by an investigator who was masked to group assignment. Prestimulation to poststimulation changes in motor threshold, single-pulse MEP amplitude, ICF, and ICI were assessed using separate 2 × 2 (group by time) analysis of variance with repeated measures on the second factor. Because considerable intersubject variability exists in the initial response to TMS (Hiscock et al., 2008; Pell et al., 2011), we anticipated that group differences may exist at pretest, that is, before rTMS delivery. Therefore, when between-groups pretest scores significantly differed on a given measure, we controlled for these differences by conducting a 2 × 2 (group by time) analysis of covariance with the pretest score entered as the covariate. IBM SPSS Statistics version 20 was used to run statistics.
RESULTS
All subjects in this study tolerated the procedures well with no serious adverse effects. One subject reported a mild headache at the end of rTMS, but this was related to neck muscle tension from holding his head still during the procedure.
There was no significant difference in the pretest motor thresholds between the groups (t = 0.28, P = 0.78). Motor threshold results revealed no main effects of time (F = 2.87, P = 0.11) or group (F = 0.03, P = 0.95), nor was there a significant group-by-time interaction (F = 1.68, P = 0.21). At pretest, MEP amplitudes during single-pulse TMS (i.e., at test stimulus intensity) were, on average, significantly larger for the rTMS group than the sham group (t = 2.51, P = 0.01). For single-pulse MEP amplitude, despite finding a main effect of group (F = 8.17, P = 0.005), there was no main effect of time (F = 0.40, P = 0.53) or significant interaction (F = 0.83, P = 0.36). When controlling for pretest single-pulse MEP amplitude (i.e., analysis of covariance results), we also found no significant group-by-time interaction (F = 1.98, P = 0.16).
For both rTMS and sham groups, the 2 milliseconds ISI produced inhibition consistent with previous work, (Roshan et al., 2003) although we found that pretest ICI MEP amplitudes were, on average, significantly larger for the rTMS group (t = 2.76, P = 0.01). Intracortical inhibition results revealed a main effect of time (F = 9.60, P = 0.002) and of group (F = 15.04, P < 0.001). We also found a significant group-by-time interaction for ICI (F = 7.90, P = 0.006). This group-by-time interaction remained significant when controlling for pretest differences between groups (F = 7.80, P = 0.006). Examination of group means demonstrated that ICI in the rTMS group decreased (represented by an increase in MEP amplitude from pretest to posttest) whereas the sham group experienced no change in pretest to posttest values (Fig. 1, left panel). During ICI trials, the rTMS group mean MEP amplitude significantly increased from a baseline of 0.97 mV (SE, ±0.18) to 1.70 mV (±0.43) post-rTMS (t = 3.24, P = 0.002), whereas the sham group mean MEP amplitude was essentially unchanged: 0.42 mV (±0.07) at baseline and 0.46 mV (±0.09) post-rTMS (t = 0.45, P = 0.65). Representative ICI MEPs are displayed in Figure 2. For both rTMS and sham groups, the 15 milliseconds ISI produced facilitation consistent with previous work (Chen et al., 1998), and there was no significant difference between groups at pretest (t = 1.41, P = 0.16). Intracortical facilitation results revealed a main effect of group (F = 5.79, P = 0.02) and a significant group-by-time interaction (F = 3.98, P = 0.048; Fig. 1, right panel). There was no main effect of time (F = 0.06, P = 0.80) for ICF. Examination of group means revealed that ICF MEP amplitude increased from a baseline of 3.2 mV (±0.35) to 3.6 mV (±0.39) post-rTMS in the rTMS group, and for the sham group, was 2.51 mV (±0.24) at baseline and 2.21 mV (±0.25) postsham stimulation (Table 1). Representative ICF MEPs are displayed in Figure 3.
FIG. 1.
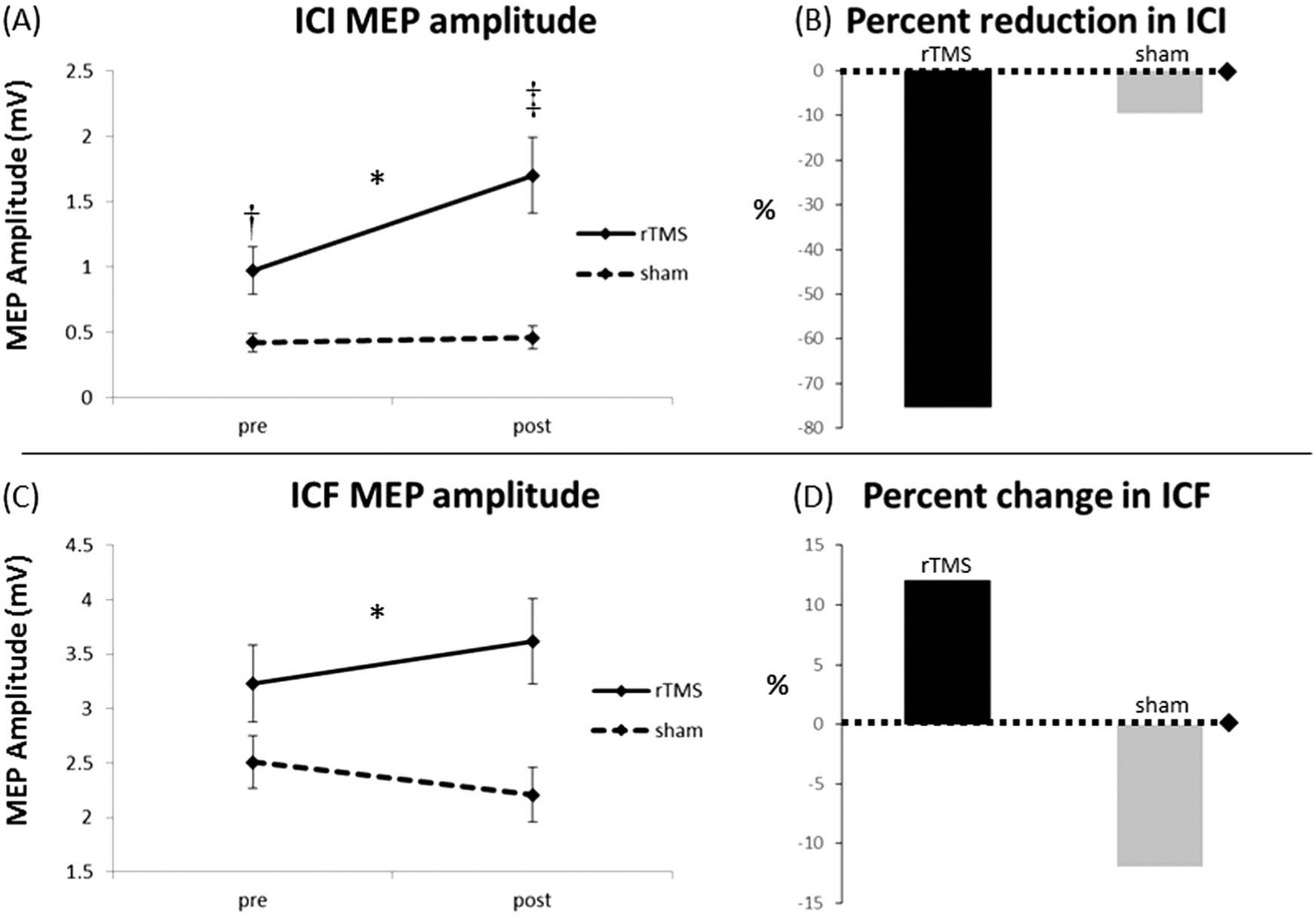
Intracortical inhibition (ICI) and intracortical facilitation (ICF) group results. After repetitive transcranial magnetic stimulation (rTMS), subjects displayed a reduction in ICI (represented as increase in MEP amplitude [A] and as a 75.3% reduction in ICI [B]) that was not observed in subjects who received sham stimulation. We found a significant group-by-time interaction effect with the rTMS group displaying a slight (12.1%) increase in ICF (C, D) and the sham group a slight decrease (12.0%) in ICF from pretesting to posttesting (right panel). *Significant group-by-time interaction effect (P < 0.05). ‡Significant within-group pretest to posttest change (P < 0.01). †Significant between-group difference at pretest (P = 0.01). MEP: motor evoked potential.
FIG. 2.
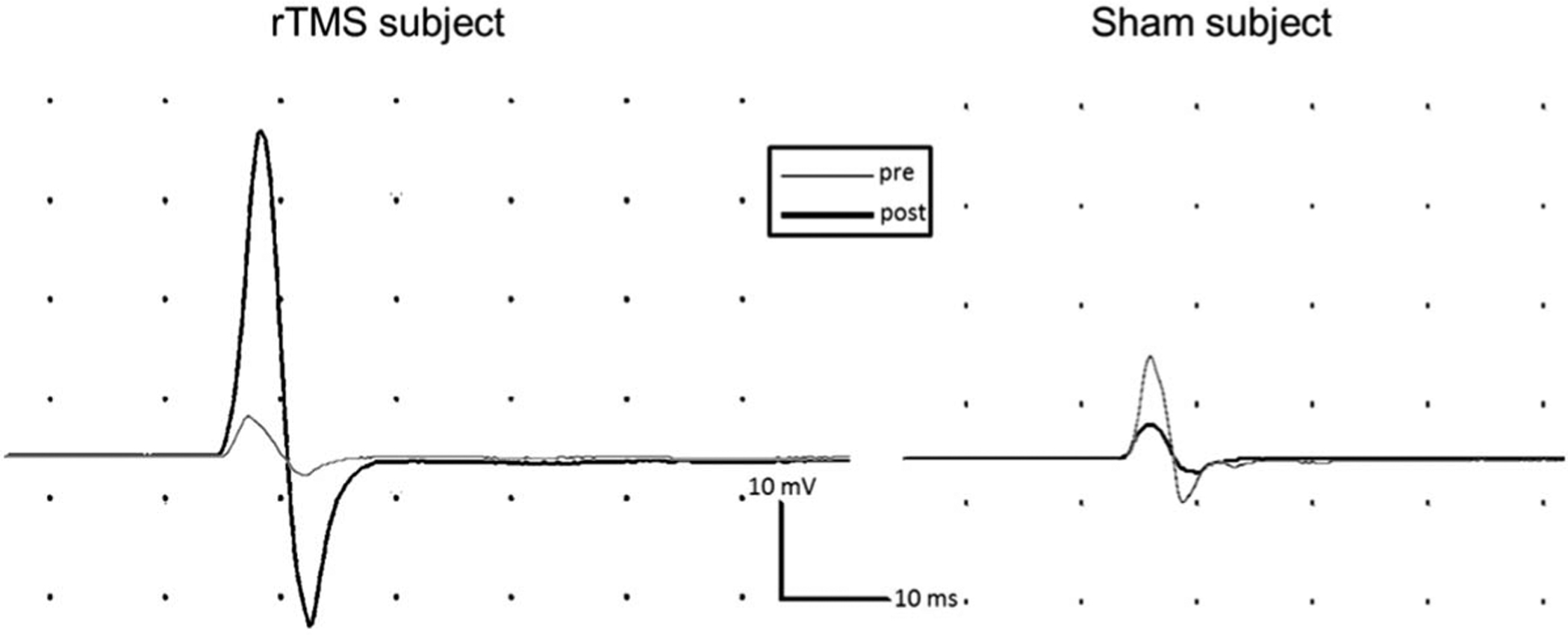
Representative intracortical inhibition (ICI) motor evoked potentials (MEPs) from a repetitive transcranial magnetic stimulation (rTMS) subject (left panel) and a sham subject (right panel). As displayed for a representative subject (left panel), subjects in the rTMS group displayed an increase in ICF MEP amplitude after rTMS—representing less inhibition, that is, reduced ICI. In contrast, the representative sham subject (right panel) demonstrated a slight decrease in ICI MEP amplitude after sham stimulation. ICF: intracortical facilitation.
TABLE 1.
Presession and Postsession Data by Group
| Measure | rTMS Group | Sham Group | Absolute Difference in Gain Scores Between Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | ||||||
| σ | (±SE) | σ | (±SE) | σ | (±SE) | σ | (±SE) | ||
| Motor threshold (% stimulator output) | 46.4 | (2.7) | 46.6 | (3.1) | 45.5 | (2.8) | 47.0 | (3.5) | 1.3 |
| Single-pulse MEP amplitude, mV | 2.08 | (0.26) | 2.32 | (0.33) | 1.28 | (0.12) | 1.24 | (0.13) | 0.2 |
| ICI, mV | 0.97 | (0.18) | 1.70 | (0.43) | 0.42 | (0.07) | 0.46 | (0.09) | 0.7* |
| ICF, mV | 3.23 | (0.35) | 3.62 | (0.39) | 2.51 | (0.24) | 2.21 | (0.25) | 0.1† |
Group-by-time interaction, P < 0.01.
Group-by-time interaction, P < 0.05.
ICI, intracortical inhibition; and ICF, intracortical facilitation; MEP, motor evoked potential; rTMS, repetitive transcranial magnetic stimulation.
FIG. 3.
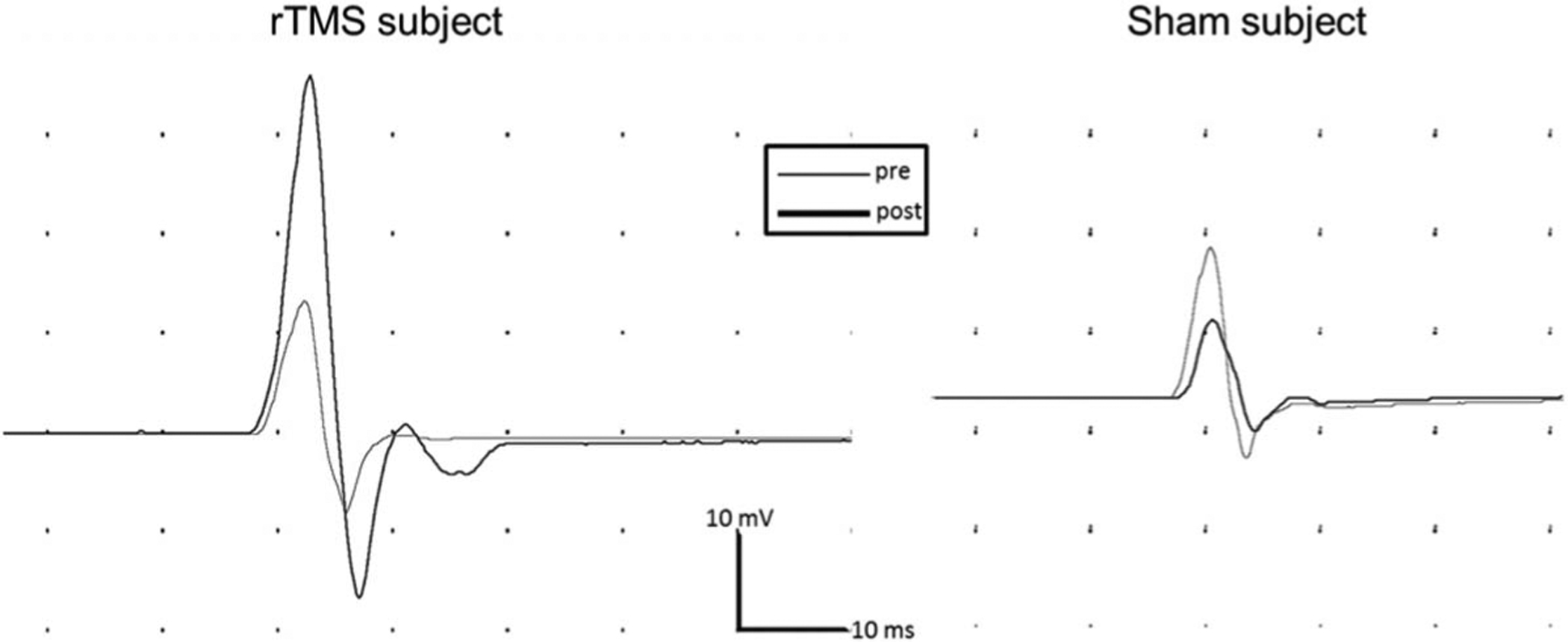
Representative intracortical facilitation (ICF) motor evoked potentials (MEPs) from a repetitive transcranial magnetic stimulation (rTMS) subject (left panel) and a sham subject (right panel). As displayed for a representative subject (left panel), subjects in the rTMS group displayed an increase in ICF MEP amplitude after rTMS. In contrast, the representative sham subject (right panel) demonstrated a slight decrease in ICF MEP amplitude after sham stimulation.
DISCUSSION
The results of this study demonstrate the effects of rTMS versus sham stimulation on neurophysiologic measures of intracortical excitability and inhibition within the motor cortex. We found that, compared with the sham group, those subjects receiving high-frequency rTMS experienced decreased ICI and increased ICF. Taken together, these two outcomes suggest a net increase in excitability, driven by a reduction in inhibition and an increase in excitation within the intracortical network that affects corticospinal activation. Interestingly, we found that commonly reported broad measures of excitability of the motor cortex and corticospinal system (i.e., motor threshold and single-pulse MEP size, respectively) were unaffected by high-frequency rTMS or sham stimulation. This finding differs from the finding of Jung et al. (2008) of increased MEP size post-rTMS, but is consistent with several other reports that show no change in these measures post-rTMS (Berardelli et al., 1999; Fierro et al., 2001; Fitzgerald et al., 2006b; Romeo et al., 2000). It may suggest that motor threshold and single-pulse MEP size are less-sensitive measures to assess motor system excitability after rTMS.
The method of rTMS administration and TMS testing must be examined when considering results of studies presenting neurophysiologic outcomes after rTMS. One key methodological variable relates to the “dosing” of rTMS. Our dosing parameters (2,000 stimuli) were based on our previous work that has used rTMS as a therapeutic intervention (Malcolm et al., 2007). With the intent of positively impacting recovery of motor function, such investigations tend to use relatively high doses of rTMS within a session. High-dose protocols are supported by the examination of the duration of rTMS effects related to dose of stimulation performed by Peinemann et al. (2004). These authors specifically showed that 1,800 stimuli produced a long-lasting increase in motor cortex excitability, an effect that was shorter-lasting after a medium dose (900 stimuli) and nonexistent at a low dose (150 stimuli). Our choice to use 2,000 stimuli evolved from the study of Peinemann et al and yielded similar results, that is, ICI decreased and ICF increased in both investigations. Several studies have used lower doses of rTMS (ranging from 5 to 1,600 stimuli), which have generally reduced ICI, but had no effect on ICF (Di Lazzaro et al., 2002; Huang and Rothwell, 2004; Pascual-Leone et al., 1998; Peinemann et al., 2000). One exception to this general outcome came from Wu et al. (2000), who used suprathreshold (rather than subthreshold used in this and other studies) low-dose rTMS (30 pulses) that decreased ICI and increased ICF. We did not specifically test varying doses of rTMS; however, based on findings of Peinemann et al, we submit that previous studies may have applied too few stimuli to produce a significant impact on especially ICF.
A second methodological variable that differs across studies is stimulus frequency. Most previous “fast” rTMS studies on ICF and ICI have used frequencies ranging from 5 to 10 Hz. We chose 20 Hz based on some of our studies (Malcolm et al., 2007) and other previous studies applying rTMS in clinical populations. For example, Corti et al. (2012) conducted a focused review on the use of rTMS to improve motor function after stroke and found that most clinical-efficacy studies use rTMS frequencies in the range of 10 to 20 Hz. We, therefore, selected a higher rTMS frequency such that our findings might inform future investigations, which use rTMS to alter intracortical neuroplastic mechanisms toward a therapeutic benefit. Furthermore, a possibility exists that higher frequency rTMS (i.e., 20 Hz) may have a more potent effect on ICI and ICF than moderately fast frequencies (i.e., 5–10 Hz). Certainly, we may only hypothesize that such a relationship exists and, therefore, a systematic investigation of the effects of various rTMS frequencies on ICI and ICF in nonclinical and clinical populations is warranted.
A third methodological issue often overlooked in previous studies is the lack of a sham rTMS condition. Clearly, actual effects of rTMS must be differentiated from the effects of subject accommodation to stimulation and shifts in subject arousal, which are tested with an effective placebo condition. Indeed, these are important variables to rule out, given initial evidence for the impact of attention-arousal shifts on motor cortex facilitation during rTMS (Conte et al., 2008; Milani et al., 2010). Our sham procedure used a specially designed sham coil to deliver the physical and auditory sensations of real rTMS, with the coil placed in contact with the subject’s head. In reviewing previous studies, which have collected pre-rTMS and post-rTMS ICI and ICF, only Jung et al. (2008) used a sham stimulation condition. These authors, however, used a real rTMS coil that was not placed in contact with the subject’s head, which may be a less than optimal method for assessing any placebo effects associated with the physical sensation of rTMS. We are confident in the strength of our sham condition, as no subject reported knowledge of condition assignment during the study.
The effects of high-frequency rTMS (≥ 5 Hz) have broadly been assumed to increase cortical excitability. A large majority of studies (for review, see [Fitzgerald et al., 2006a]) have used MEP amplitude and motor threshold as measures of excitatory modulation after rTMS. We consider motor threshold and MEP amplitude to be broad measures of motor cortex excitability, based on their presumed neurophysiologic bases. Motor threshold is believed to reflect the global excitability of cortical synapses involving excitatory inputs and corticospinal neurons, and also spinal cord level synapses between the corticospinal and alpha motor neurons (Talelli et al., 2006). Accordingly, motor threshold is influenced by the excitatory state of several elements in the motor nervous system. Similarly, MEP amplitude is believed to reflect the regional corticospinal system excitability. Changes in motor threshold (reduced) and MEP amplitude (increased) support the assumption that rTMS modulates cortical excitability. These broad measures, however, provide little insight into inhibitory and excitatory mechanisms on an intracortical level. In contrast, ICI and ICF reflect more specific cortical mechanisms that either inhibit or facilitate corticospinal activation of muscles of the hand: ICI is related to GABAB receptor-mediated inhibitory mechanisms (Sanger et al., 2001), whereas ICF is related to glutaminergic synaptic activity (Chen et al., 1998).
Our findings suggest that the generally accepted principle—high-frequency rTMS increases motor cortex excitability—occurs through action on both excitatory and inhibitory intracortical systems. In the case of excitatory interneurons, which directly or indirectly synapse on corticospinal neurons, high-frequency rTMS presumably raises their excitability closer to the firing threshold—thereby ultimately facilitating corticospinal firing to generate larger MEPs during ppTMS testing. Such action of rTMS tips the balance of excitation and inhibition toward excitation, so that the activity of inhibitory interneurons is less potent in modulating corticospinal activation. In our study, this is evidenced by reduced ICI as demonstrated by larger MEPs obtained during paired pulse ICI testing in the rTMS group than was seen in the sham control group.
The purpose of this study was to investigate the effects of high-frequency rTMS versus sham stimulation on intracortical excitation and inhibition within the motor cortex to provide further data about the presumed neurophysiologic effects of rTMS. Our results indicate that broader measures of motor cortex excitability, that is, motor threshold and single-pulse MEP amplitude, may lack sensitivity and may not capture rTMS-induced excitatory and inhibitory intracortical changes that influence the motor output of the brain. These findings are important to help validate the potential underlying neurophysiologic mechanisms targeted in therapeutic applications of rTMS. Future work is needed to ascertain the extent that varying rTMS intensities, frequencies, and doses influence intracortical mechanisms in especially clinical populations that might benefit from therapeutic brain stimulation.
Based on our findings in this study, our follow-up hypothesis is that modulation of ICI and ICF with rTMS may induce motor behavior changes independent of changes in motor threshold or MEP amplitude. Our rationale for this hypothesis is based on our view that the primary motor cortex is involved in more than just serving as the “final motor output” (e.g., it has a role in planning the execution of movements). Accordingly, changes in motor function may be apparent and paralleled by changes in ICI/ICF without seeing changes in motor threshold and MEP amplitude. This hypothesis was supported by findings in a study conducted in our laboratory (Massie et al., 2013a) in survivors of stroke, where changes in paretic hand force control were seen after rTMS to the contralateral motor cortex with significant changes in ICI/ICF but no change in motor threshold. Further research is needed to test this hypothesis, especially when multiple sessions of rTMS are delivered to each subject.
Acknowledgments
Supported by a grant from the Colorado State University College of Health and Human Sciences.
REFERENCES
- Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 2009;66:298–309. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Gilio F, et al. Effectsof repetitive cortical stimulation on the silent period evoked by magnetic stimulation. Exp Brain Res 1999;125:82–86. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105:415–421. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 1998;80:2870–2881. [DOI] [PubMed] [Google Scholar]
- Conte A, Belvisi D, Iezzi E, et al. Effects of attention on inhibitory and facilitatory phenomena elicited by paired-pulse transcranial magnetic stimulation in healthy subjects. Exp Brain Res 2008;186:393–399. [DOI] [PubMed] [Google Scholar]
- Corti M, Patten C, Triggs W. Repetitive transcranial magnetic stimulation of motor cortex after stroke: a focused review. Am J Phys Med Rehabil 2012;91:254–270. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, et al. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res 2002;147:108–113. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 1998;119:265–268. [DOI] [PubMed] [Google Scholar]
- Emara TH, Moustafa RR, Elnahas NM, et al. Repetitive transcranial magnetic stimulation at 1 Hz and 5 Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol 2010;17:1203–1209. [DOI] [PubMed] [Google Scholar]
- Fierro B, Piazza A, Brighina F, et al. Modulation of intracortical inhibition induced by low-and high-frequency repetitive transcranial magnetic stimulation. Exp Brain Res 2001;138:452–457. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 2006a;117:2584–2596. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Huntsman S, Gunewardene R, et al. A randomized trial of low-frequency right-prefrontal-cortex transcranial magnetic stimulation as augmentation in treatment-resistant major depression. Int J Neuropsychopharmacol 2006b;9:655–666. [DOI] [PubMed] [Google Scholar]
- Hiscock A, Miller S, Rothwell J, et al. Informing dose-finding studies of repetitive transcranial magnetic stimulation to enhance motor function: a qualitative systematic review. Neurorehabil Neural Repair 2008;22:228–249. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol 2004;115:1069–1075. [DOI] [PubMed] [Google Scholar]
- Jung SH, Shin JE, Jeong YS, et al. Changes in motor cortical excitability induced by high-frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clin Neurophysiol 2008;119:71–79. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Rothwell JC, Shawky OA, et al. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov Disord 2006;21:2201–2205. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Abdel-Fadeil MR, Farghali A, et al. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol 2009;16:1323–1330. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Etraby AE, Hemeda M, et al. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand 2010;121:30–37. [DOI] [PubMed] [Google Scholar]
- Koch G, Rossi S, Prosperetti C, et al. Improvement of hand dexterity following motor cortex rTMS in multiple sclerosis patients with cerebellar impairment. Mult Scler 2008;14:995–998. [DOI] [PubMed] [Google Scholar]
- Laakso I, Hirata A, Ugawa Y. Effects of coil orientation on the electric field induced by TMS over the hand motor area. Phys Med Biol 2014;59:203–218. [DOI] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy. Am J Phys Med Rehabil 2007;86:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie CL, Tracy BL, Malcolm MP. Functional repetitive transcranial magnetic stimulation increases motor cortex excitability in survivors of stroke. Clin Neurophysiol 2013a;124:371–378. [DOI] [PubMed] [Google Scholar]
- Massie CL, Tracy BL, Paxton RJ, et al. Repeated sessions of functional repetitive transcranial magnetic stimulation increases motor cortex excitability and motor control in survivors of stroke. NeuroRehabilitation 2013b;33:185–193. [DOI] [PubMed] [Google Scholar]
- Mennemeier MS, Triggs WJ, Chelette KC, et al. Sham transcranial magnetic stimulation using electrical stimulation of the scalp. Brain Stimul 2009;2:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani P, Piu P, Popa T, et al. Cortisol-induced effects on human cortical excitability. Brain Stimul 2010;3:131–139. [DOI] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol 1992;85:17–21. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 1998;15:333–343. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Mentschel C, et al. Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett 2000;296:21–24. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol 2004;115:1519–1526. [DOI] [PubMed] [Google Scholar]
- Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol 2011;93:59–98. [DOI] [PubMed] [Google Scholar]
- Pomeroy VM, Cloud G, Tallis RC, et al. Transcranial magnetic stimulation and muscle contraction to enhance stroke recovery: a randomized proof-of-principle and feasibility investigation. Neurorehabil Neural Repair 2007;21:509–517. [DOI] [PubMed] [Google Scholar]
- Romeo S, Gilio F, Pedace F, et al. Changes in the cortical silent period after repetitive magnetic stimulation of cortical motor areas. Exp Brain Res 2000;135:504–510. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res 2003;151:330–337. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol 2001;530:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 2003;148:1–16. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol 2006;117:1641–1659. [DOI] [PubMed] [Google Scholar]
- Theilig S, Podubecka J, Bosl K, et al. Functional neuromuscular stimulation to improve severe hand dysfunction after stroke: does inhibitory rTMS enhance therapeutic efficiency? Exp Neurol 2011;230:149–155. [DOI] [PubMed] [Google Scholar]
- Wu T, Sommer M, Tergau F, et al. Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett 2000;287:37–40. [DOI] [PubMed] [Google Scholar]


