Supplemental Digital Content is available in the text.
Keywords: children, coronavirus disease 2019, intensive care unit, multisystem inflammatory syndrome in children, outcomes, severe acute respiratory syndrome coronavirus 2
Abstract
OBJECTIVES:
To compare clinical characteristics and outcomes of children admitted to the PICU for severe acute respiratory syndrome coronavirus 2–related illness with or without multisystem inflammatory syndrome in children. The secondary objective was to identify explanatory factors associated with outcome of critical illness defined by a composite index of in-hospital mortality and organ system support requirement.
DESIGN:
Retrospective cohort study.
SETTING:
Thirty-eight PICUs within the Viral Infection and Respiratory Illness Universal Study registry from March 2020 to January 2021.
PATIENTS:
Children less than 18 years with severe acute respiratory syndrome coronavirus 2–related illness with or without multisystem inflammatory syndrome in children.
MEASUREMENTS AND MAIN RESULTS:
Of 394 patients, 171 (43.4%) had multisystem inflammatory syndrome in children. Children with multisystem inflammatory syndrome in children were more likely younger (2–12 yr vs adolescents; p < 0.01), Black (35.6% vs 21.9%; p < 0.01), present with fever/abdominal pain than cough/dyspnea (p < 0.01), and less likely to have comorbidities (33.3% vs 61.9%; p < 0.01) compared with those without multisystem inflammatory syndrome in children. Inflammatory marker levels, use of inotropes/vasopressors, corticosteroids, and anticoagulants were higher in multisystem inflammatory syndrome in children patients (p < 0.01). Overall mortality was 3.8% (15/394), with no difference in the two groups. Diagnosis of multisystem inflammatory syndrome in children was associated with longer duration of hospitalization as compared to nonmultisystem inflammatory syndrome in children (7.5 d[interquartile range, 5–11] vs 5.3 d [interquartile range, 3–11 d]; p < 0.01). Critical illness occurred in 164 patients (41.6%) and was more common in patients with multisystem inflammatory syndrome in children compared with those without (55.6% vs 30.9%; p < 0.01). Multivariable analysis failed to show an association between critical illness and age, race, sex, greater than or equal to three signs and symptoms, or greater than or equal to two comorbidities among the multisystem inflammatory syndrome in children cohort. Among nonmultisystem inflammatory syndrome in children patients, the presence of greater than or equal to two comorbidities was associated with greater odds of critical illness (odds ratio 2.95 [95% CI, 1.61–5.40]; p < 0.01).
CONCLUSIONS:
This study delineates significant clinically relevant differences in presentation, explanatory factors, and outcomes among children admitted to PICU with severe acute respiratory syndrome coronavirus 2–related illness stratified by multisystem inflammatory syndrome in children.
AT THE BEDSIDE
Although prior literature in this field describes the clinical course of children with SARS-CoV-2 disease, we have focused on patients with organ dysfunction and mortality in order to delineate findings for those with or without MIS-C.
This study provides further insight into explanatory factors associated with critical SARS-CoV-2 disease in children stratified by MIS-C, which may allow clinicians to better risk-stratify these patients.
Future research in pediatric SARS-CoV-2 disease—stratified by MIS-C and non–MIS-C disease—is needed in clarifying therapeutic and management decisions, addressing consequences of racial disparity, and better understanding the impact of obesity.
Since the early description of coronavirus disease 2019 (COVID-19) (1), many reports have been published on its characteristics and outcomes in both adults and children (2–5). As opposed to the severe, often fatal disease in the elderly population (6), studies examining pediatric patients have shown lower mortality (4, 5). However, there is a subset of children with COVID-19 who present with sepsis/shock-like features (7). On May 14, 2020, the Centers for Disease Control and Prevention (CDC) published a surveillance definition for this condition known as multisystem inflammatory syndrome in children (MIS-C) (8). Reports on the clinical characteristics, outcomes, and treatment of patients with MIS-C have now been published (9). Two studies have compared pediatric patients with and without MIS-C, including a study from United Kingdom (10), and more recently, the CDC (11), which have covered periods up to July and October 2020, respectively. However, none of the studies captured patient characteristics during the third wave in the United States (November 2020 to January 2021).
Prior studies have used ICU admission as the criterion for critical COVID-19 (4, 9). However, available resources may lead to significant heterogeneity in ICU admission criteria between centers. This issue has likely been confounded by hospital resource management planning whereby COVID-19 patients are cohorted, often to the ICU. Therefore, studies using ICU admission as the criterion to define critical illness may be limited in providing an accurate picture of critical COVID-19 in children (4). In this report, we have used the “Society of Critical Care Medicine (SCCM)” Virus Infection and Respiratory Illness Universal Study (VIRUS) registry (COVID-19 Registry [sccmcovid19.org]) (12, 13) to study a period of COVID-19 that is inclusive of the third wave of the disease in the United States. Our primary objective is to describe the PICU admissions stratified by the presence or absence of MIS-C. Our secondary objective is to identify explanatory factors associated with the critical illness stratified by MIS-C and non–MIS-C.
MATERIALS AND METHODS
We performed a retrospective review of SCCM’s VIRUS registry (NCT 04323787). The VIRUS study has been approved by the Mayo Clinic Institutional Review Board (IRB) (number 20-002610) and by the IRBs of all the participating centers. All patients (birth to < 18 yr) for whom data were entered into the VIRUS registry from March 2020 to January 2021 were included in the current report. Patients admitted to the PICU with a non–COVID-19 related reason for admission or readmission were excluded from the VIRUS registry. We also excluded the following PICU patients: those with missing essential demographic data (weight, sex) or incomplete outcome variables (hospital discharge status and hospital length of stay [LOS] ); those in whom MIS-C status (i.e., presence or absence) was unknown; and one patient with a diagnosis of trauma as the primary reason for admission. No further exclusions based on signs/symptoms or admission diagnosis were applied during analysis. There is the possibility of significant overlap between the patients included in the VIRUS registry and those already reported in the literature. Therefore, in keeping with current reporting recommendations (14), we have provided details of VIRUS centers from which similar data may have been reported elsewhere. Of note, we sent out a survey to VIRUS participating centers to delineate overlapping involvement in research projects, and we have also assessed the potential that the current report has patients that overlap with existing publications (SDC, http://links.lww.com/PCC/B784).
REPORT IN CONTEXT
Children suffer from two forms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease: multisystem inflammatory syndrome in children (MIS-C) and non–MIS-C. There is a paucity of literature comparing these entities in pediatric critical care.
The rapid development of knowledge related to SARS-CoV-2 disease in children has led to numerous studies across a range of disease severity. However, there has been limited focus on critical illness requiring organ support or resulting in death.
This report highlights differences between MIS-C and non–MIS-C SARS-CoV-2 disease related to their presentation, clinical characteristics, management, and outcomes among critically ill children and suggests future research areas as our understanding of these entities continue to evolve.
Measurements
Demographic, clinical characteristics, management, outcomes, and severity of illness data were extracted from the VIRUS Research Electronic Data Capture (REDCap) database (15), and patients were classified as either MIS-C or non–MIS-C cases. Adjudication of MIS-C was performed by individual sites based on CDC criteria (16) and not by the VIRUS research team. Data were reviewed for completeness and categorized. Age was stratified into discrete categories: neonate (≤ 28 d), infant (>28 d to < 2 yr), child (≥ 2 to < 12 yr), and adolescent (≥ 12 yr) (17). For children greater than or equal to 2 years, body mass index (BMI) percentiles were calculated based on the CDC growth variables using R package childsds (V0.7.6) (18). Percentiles were classified as underweight, normal, overweight, and obese using the CDC criteria (19). There were 26 categories of signs and symptoms, 41 pediatric comorbidities, and 36 categories of COVID-19–related complications in the VIRUS registry report. These were organized into organ system groups based on potential clinical relevance. For example, constitutional signs and symptoms included fever, chills, myalgia, malaise, night sweats, lymphadenopathy, rash, or arthralgia. Brain natriuretic peptide (BNP)/pro-BNP elevation was included as a discreet complication (yes/no) in the database and was included as such in the analysis. Patients with presenting signs or symptoms in greater than or equal to three organ systems and greater than or equal to two comorbidities were separately evaluated. Pediatric Risk of Mortality (PRISM) III score (20) was determined using the online calculator if a summative score was not already entered by the individual site (21). Patients with evidence of other respiratory viral infections were categorized as “viral coinfection.” Patients with concurrent blood, urine, or respiratory bacterial infections were combined into a category of “bacterial coinfection.” Medications administered and entered into the database on days 0, 1, 2, 3, 7, 14, or 21 were evaluated. Laboratory markers suggestive of inflammation (i.e., applicable lowest or highest values on day 0, 1, or 2), including total leukocyte count, platelet count, C-reactive protein, procalcitonin, ferritin, interleukin-6, serum albumin, and alanine transaminase, were extracted and evaluated.
Outcome Measures and Exposure of Interest
The outcome variables included hospital and ICU LOS (among survivors) and mortality at hospital discharge. The secondary outcome (critical illness) was a composite index of in-hospital mortality and organ support requirement, defined as a need for one or more of the following: 1) positive pressure ventilation (invasive or noninvasive); 2) vasoactive-inotropic support; 3) pulmonary vasodilator therapy (inhaled nitric oxide, epoprostenol); 4) extracorporeal life support; and/or 5) new renal replacement therapy. This classification was adapted from the National Institutes of Health (NIH) definition of critical COVID-19 (22) although with the utilization of more objective organ support variables. Patients admitted to the ICU but who did not require interventions mentioned above were categorized as having a moderate illness.
Statistical Analysis
Descriptive statistics were performed for continuous and categorical variables reported as median with interquartile range (IQR) and number with percentages, respectively. The nonparametric Wilcoxon rank-sum test was used for continuous variables and chi-square or Fisher exact test for categorical variables as appropriate. Multivariable logistic regression was performed to assess explanatory factors associated with critical COVID-19 illness compared with moderate illness (separately for MIS-C and non–MIS-C). Odds ratios (ORs) and 95% CIs (95% CI) are presented. We a priori identified potential confounders with various exposures and outcomes. Age (dichotomized as adolescent or nonadolescent), race (Black, White, and others), sex, greater than or equal to three signs/symptoms in different organ systems, and greater than or equal to two comorbidities were included in the model. As both PRISM III and MIS-C assess the severity of disease in their definitions, we reasoned that there was too much overlap with our primary outcome to include them in the models without introducing bias. Collinearity was assessed using the variance inflation factor (23). Missing data were assumed to be missing completely at random (MCAR), and a complete case analysis was performed (24). A p value of less than 0.05 was considered significant. Statistical analysis was performed using JMP Pro Version 14.2.0 and SAS V9.4 (SAS Institute, Cary, NC). This study’s reporting conforms to The Strengthening the Reporting of Observational Studies in Epidemiology statement (SDC, http://links.lww.com/PCC/B784).
RESULTS
A total of 1,134 patients less than 18 years were entered into the VIRUS registry from March 2020 to January 2021. Complete outcome data on patients meeting inclusion criteria were available for 884 patients, of which 406 were admitted to the ICU (45.9%). Twelve patients were excluded because their MIS-C status was unknown. Data were analyzed for 394 ICU patients from 38 centers (6 countries, 92.6% [365/394] in the United States). Out of this cohort, 171 (43.4%) were admitted with MIS-C and 223 (56.5%) without MIS-C (Fig. 1). Overall, 81.7% of patients had a positive polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Almost all patients without MIS-C had positive PCR (213/223; 95%). SARS-CoV-2 antibody information was available for 151 patients, most of them in the MIS-C (n = 126) group, with an 83.3% positivity rate (Table 1).
Figure 1.
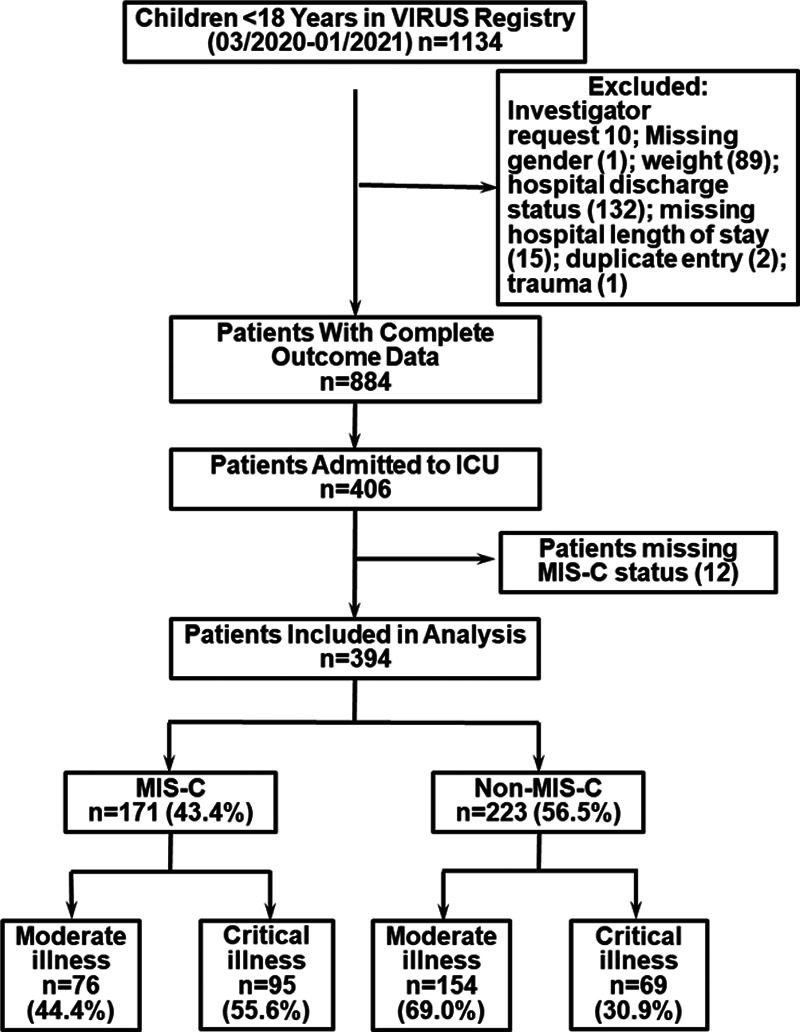
Patient recruitment consort diagram. MIS-C = multisystem inflammatory syndrome in children, VIRUS = Viral Infection and Respiratory Illness Universal Study.
TABLE 1.
Demographic Distribution
| Category | Subcategory | Total COVID Cohort (n = 394), n (%) | MIS-C (n = 171), n (%) | Non-MIS-C (n = 223), n (%) | p |
|---|---|---|---|---|---|
| Age (yr), median (interquartile range) | 10 (3.1–15.0) | 8.7 (5.0–13) | 10.8 (1.8–15) | 0.64 | |
| Age categoriesa | Neonate | 10 (2.5) | 1 (0.6) | 9 (4.0) | < 0.01 |
| Infant | 71 (18.0) | 23 (13.5) | 48 (21.5) | ||
| Child | 148 (7.6) | 85 (49.7) | 63 (28.2) | ||
| Adolescent | 165 (41.9) | 62 (36.3) | 103 (46.2) | ||
| Sex | Males | 218 (55.3) | 103 (60.2) | 115 (51.6) | 0.10 |
| Raceb | Black | 104 (26.4) | 57 (35.6) | 47 (21.9) | < 0.01 |
| White | 164 (41.6) | 70 (44.90) | 94 (43.7) | ||
| Other | 106 (26.9) | 32 (10.1) | 74 (34.4) | ||
| Ethnicityc | Hispanic | 111 (32.2) | 38 (27.3) | 73 (35.4) | 0.17 |
| Body mass index categoriesd | Underweight | 23 (7.4) | 8 (5.5) | 15 (9.4) | 0.44 |
| Normal | 111 (36.5) | 57 (39.3) | 54 (34.0) | ||
| Overweight | 36 (11.8) | 19 (13.1) | 17 (10.7) | ||
| Obese | 134 (44.1) | 61 (42.1) | 73 (45.9) | ||
| Countrye | % United States | 365 (92.6) | 169 (93.0) | 206 (92.4) | 0.85 |
| Symptomsf | Fever | 278 (70.6) | 154 (90.6) | 124 (55.6) | < 0.01 |
| Nausea/vomiting | 163 (41.4) | 99 (57.9) | 64 (28.7) | < 0.01 | |
| Cough | 128 (32.5) | 41 (24.0) | 87 (39.1) | < 0.01 | |
| Abdominal pain | 118 (29.9) | 80 (46.8) | 38 (17.0) | < 0.01 | |
| Dyspnea | 116 (29.4) | 31 (18.3) | 85 (38.1) | < 0.01 | |
| ≥ 3 signs or symptomsg | Yes | 229 (58.1) | 123 (71.9) | 106 (47.5) | < 0.01 |
| Comorbidities | Yes | 195 (49.5) | 57 (33.3) | 138 (61.9) | < 0.01 |
| ≥ 2 comorbidities | 110 (28.0) | 25 (14.6) | 85 (38.1) | < 0.01 | |
| Comorbidity categoriesh | Asthma | 58 (14.7) | 21 (12.3%) | 37 (16.6) | 0.32 |
| Seizures disorder | 41 (10.4) | 7 (4.1) | 34 (15.3) | < 0.01 | |
| Developmental delay | 33 (8.4) | 8 (4.7) | 25 (11.2) | 0.03 | |
| Diabetes | 19 (4.8) | 4 (2.3) | 15 (6.7) | 0.06 | |
| Chroniclung disease/bronchopulmonary dysplasia | 17 (4.3) | 0 (0) | 17 (7.6) | < 0.01 | |
| Viral coinfection | Yes | 25 (6.3) | 13 (7.65) | 12 (5.4) | 0.41 |
| Bacterial coinfectioni | Blood | 19 (4.8) | 9 (5.3) | 10 (4.5) | 0.55 |
| Urine | 33 (8.4) | 9 (5.3) | 24 (10.8) | < 0.01 | |
| Respiratory | 16 (4.0) | 7 (3.7) | 9 (4.0) | 0.67 | |
| Overall | 60 (15.1) | 23 (13.5) | 37 (16.6) | 0.40 | |
| Pediatric Risk of Mortality III score, median (interquartile range) | 3 (0–8) (n = 309) | 5 (1.8–9.3) (n = 159) | 2.0 (0–7) (n = 159) | < 0.01 | |
| COVID 2019 polymerase chain reaction | Positive | 322 (81.7) | 112 (64.7) | 213 (95.0) | < 0.01 |
| Severe acute respiratory syndrome coronavirus 2 immunoglobulin Gj | Positive | 115/151 (76.2) | 105/126 (83.3) | 10/25 (40) | < 0.01 |
| Critical illness k | Yes | 164 (41.6) | 95 (55.6) | 69 (30.9) | < 0.01 |
COVID = coronavirus disease, MIS-C = multisystem inflammatory syndrome in children.
aNeonate (≤ 28 d); infant (> 28 d to < 2 yr); child (≥ 2 to 12 yr); adolescent (≥ 12 yr).
bOther include Asian-American 10 (2.5%), East Asian 1 (0.2%), Mixed race 5 (1.2%), South Asian 25 (6.2%), South East Asian 1 (0.2%), West Asian 4 (1.0%), and others 60 (15.1%). Race listed as unknown in 20 patients (excluded from above analysis).
cEthnicity missing data on 49 patients (12.4%).
dBody mass index (BMI) categories only for ≥ 2 yr. Out of 313 patients ≥ 2 yr of age, BMI available for 304 patients (missing data on 9 [3.0%] patients).
eCroatia 2 (0.5%); India 12 (3.0%); Japan 1 (0.2%); Pakistan 12 (3.0%); Saudi Arabia 2 (0.5%).
f, hTop five sign symptoms and comorbidities. Comparative list of all signs and symptoms and comorbidities provided in SDC (http://links.lww.com/PCC/B784).
gGreater than or equal to three signs and symptoms in different organ system categories as presented in SDC (http://links.lww.com/PCC/B784).
iPercentage represents positive culture out of total patients. Blood, urine, and respiratory cultures performed on 285, 180, and 54 patients, respectively.
jMissing data regarding severe acute respiratory syndrome coronavirus 2 immunoglobulin G on 113 patients (2 [1.1%] MIS-C group and 111 [49.7%] non-MIS-C). Not done in 43 patients (25.4%) in MIS-C group and 87 patients (77.6%) in non-MIS-C group.
kCritical disease represents invasive respiratory support (ventilator, nitric oxide) or invasive hemodynamic support (inotropes/vasopressors/extracorporeal membrane oxygenation) or invasive renal support (new continuous renal replacement therapy/hemodialysis) or hospital mortality.
p values represent comparison between MIS-C and non-MIS-C group. ICU admission source: hospital emergency department 219 (59.7%), floor 61 (16.6%), and transfer 78 (21.3%). No significant difference in the admission source between MIS-C and non-MIS-C group.
Comparison of MIS-C and non-MIS-C Presentation
The median age of the cohort was 10 years (IQR, 3.1–15 yr). Although the two groups’ median age was similar, there were significant differences in the distribution of age categories between the MIS-C and non–MIS-C. Black patients comprised 26.4% of ICU admissions. There was a significant difference in racial distribution (p < 0.01) between the two groups, with a higher proportion of Black patients in the MIS-C group compared with the non–MIS-C group (35.6% vs 21.9%, respectively). Obesity was present in 44.1% of all ICU admissions (134/304) in children greater than or equal to 2 years, but there was no difference in the proportion of patients across BMI categories in those with and without MIS-C.
Fever was the most common presenting symptom occurring in 70.6% (278/394). Significant differences were noted in the distribution of presenting signs and symptoms between MIS-C and non–MIS-C. MIS-C patients had a higher prevalence of fever, abdominal pain, and nausea/vomiting. In comparison, non–MIS-C patients more often had cough and dyspnea. Almost 50% of all patients (195/394) had comorbidities. The prevalence of comorbidities was lower in MIS-C (33.3% [57/171]) vs non–MIS-C (61.9% [138/223]) (p < 0.01). Patients with MIS-C had higher laboratory evidence of inflammation in all eight markers analyzed (SDC, http://links.lww.com/PCC/B784). Demographics, presenting signs, comorbidities, and clinical characteristics are summarized in Table 1 (SDC, http://links.lww.com/PCC/B784).
Organ Support and Pharmacologic/Immunomodulatory Therapy
Invasive and noninvasive ventilation was used in 23.1% of patients (91/394) and 19.0% of patients (75/394), respectively. No difference was found in the proportion of patients with or without MIS-C requiring respiratory support, other than supplemental oxygen via face mask. Vasopressors/inotropes were used in a quarter of the cohort and more often in MIS-C versus non–MIS-C patients (42.6% [73/171] vs 9.9% [22/224]; p < 0.01). Corticosteroids were the most common medication, administered to 44.3% of patients (176/394). Corticosteroid use was more common in MIS-C versus non–MIS-C patients (67.3% vs 27.3%; p < 0.01). Therapeutic anticoagulation was used in 15.3% of patients, with more frequent use in MIS-C patients (22.8% [39/171] vs 9.9% [22/223]; p < 0.01) (Table 2).
TABLE 2.
ICU Management Among Pediatric Coronavirus Disease 2019 Patients
| Category | Subcategory | Total Coronavirus Disease Cohort (n = 394), n (%) | MIS-C (n = 171), n (%) | Non-MIS-C (n = 223), n (%) | p |
|---|---|---|---|---|---|
| Respiratory supporta | Noneb | 140 (35.2) | 65 (38.0) | 75 (33.6) | 0.40 |
| Face mask oxygenation | 35 (8.8) | 7 (4.1) | 28 (12.6) | < 0.01 | |
| Nasal cannula | 140 (35.2) | 57 (33.3) | 83 (37.2) | 0.45 | |
| High-flow nasal cannula | 107 (26.9) | 45 (26.3) | 61 (27.4) | 0.91 | |
| Noninvasive ventilation | 75 (19.0) | 35 (20.5) | 39 (17.5) | 0.52 | |
| Invasive ventilation | 91 (23.1) | 43 (25.2) | 45 (20.2) | 0.27 | |
| Nitric oxided | 15 (3.7) | 10 (5.9) | 5 (2.2) | 0.11 | |
| Other organ supporta | Vasopressors/inotropes | 95 (24.1) | 73 (42.6) | 22 (9.9) | < 0.01 |
| Continuous renal replacement therapy/hemodialysis | 1 (0.2) | 1 (0.6) | 0 (0) | 0.43 | |
| Extracorporeal life support | 11 (2.7) | 5 (2.9) | 4 (1.8) | 0.51 | |
| Neuromuscular blockade | 39 (9.8) | 17 (9.9) | 22 (9.9) | 1.00 | |
| Antiviral, Immunomodulatory and other medicationsc | Corticosteroids | 176 (44.3) | 115 (67.3) | 61 (27.3) | < 0.01 |
| Any antiviral | 82 (20.6) | 27 (15.8) | 55 (24.7) | 0.03 | |
| Remdesivire | 61 (15.3) | 20 (11.7) | 41 (18.4) | 0.09 | |
| Azithromycin | 35 (8.8) | 13 (7.6) | 22 (9.9) | 0.48 | |
| Hydroxychloroquine | 19 (4.7) | 5 (2.9) | 14 (6.3) | 0.17 | |
| Therapeutic anticoagulation | 61 (15.3) | 39 (22.8) | 22 (9.9) | < 0.01 | |
| Convalescent plasma | 4 (1.0) | 1 (0.6) | 3 (1.4) | 0.64 | |
| IV immunoglobulin | 83 (20.9) | 78 (45.6) | 5 (2.2) | < 0.01 | |
| Anakinra | 23 (5.7) | 21 (12.3) | 2 (0.9) | < 0.01 |
MIS-C = multisystem inflammatory syndrome in children.
aRespiratory and other organ support represent support provided at any time during the hospitalization.
bOf 140 patients who did not require any O2 support, one required continuous renal replacement therapy/hemodialysis, and 15 patients (10.7%) required inotropes or vasopressors.
cRepresent medications given on either of the day 0, 1, 2, 3, 7, 14, or 21 of hospitalization. Organ support and therapeutics not mutually exclusive.
dOr epoprostenol.
eRemdesivir only approved by U.S. Food and Drug Administration for use in patients 12 yr old or older.
Outcomes and Complications
In-hospital mortality was 3.8% (15/394), with no difference identified in MIS-C versus non–MIS-C patients. Median ICU and hospital LOS among cohort survivors were 3.8 days (IQR, 2.0–7.7 d) and 6.3 days (IQR, 3.8–11.0 d), respectively. Hospital LOS among survivors was significantly longer in the MIS-C group as compared to the non–MIS-C group (7.5 [IQR, 5.0–11.0] vs 5.3 d [3.0–11.0 d]; p < 0.01). The median duration of invasive ventilation among survivors was 4.9 days (IQR, 1.6–7.2 d), with no difference between MIS-C and non–MIS-C patients. COVID-19–related complications were reported in 66.7% (263/394). The proportion of complications by organ system and proportion of individual complications were higher in MIS-C patients. BNP/pro-BNP elevation occurred in 32.7% of MIS-C patients (56/171) compared with 3.1% of the non–MIS-C group (7/223) (Table 3) (SDC, http://links.lww.com/PCC/B784).
TABLE 3.
Outcomes and Complications
| S. No. | Category | Total Coronavirus Disease Cohort (n = 394) | MIS-C (n = 171) | Non-MIS-C (n = 223) | p |
|---|---|---|---|---|---|
| 1 | High-flow nasal cannula duration, median (IQR) (d)a | 2.2 (1.1–3.8) (n = 85) | 2.0 (1.0–2.8) (n = 37) | 3.0 (1.0–4.9) (n = 48) | 0.02 |
| 2 | Noninvasive ventilation duration, median (IQR) (d)a | 1.9 (0.6–4.2) (n = 69) | 1.9 (1.0–3.2) (n = 32) | 2.0 (0.3–5.1) (n = 37) | 1.00 |
| 3 | Invasive ventilator duration, median (IQR) (d)a | 4.9 (1.6–7.2) (n = 68) | 4.7 (2.0–6.3) (n = 30) | 5.1 (1.5–10.3) (n = 38) | 0.79 |
| 4 | ICU length of stay, median (IQR) (d)a | 3.8 (2.0–7.7) (n = 369) | 4.1 (2.6–7.0) (n = 158) | 3.5 (1.6–8.0) (n = 211) | 0.06 |
| 5 | Hospital length of stay, median (IQR) (d)a | 6.3 (3.8–11.0) (n = 379) | 7.5 (5.0–11.0) (n = 163) | 5.3 (3.0–11.0) (n = 216) | < 0.01 |
| 6 | Hospital mortality, n (%) | 15/394 (3.8) | 8/173 (4.7) | 7/224 (3.1) | 0.44 |
| 7 | Any complicationb, n (%) | 263 (66.7) | 129 (75.4) | 134 (60.9) | < 0.01 |
| c | BNP/pro-BNP elevation | 63 (16.0) | 56 (32.7) | 7 (3.1) | < 0.01 |
| Acute kidney injury | 62 (15.7) | 43 (25.1) | 19 (8.5) | < 0.01 | |
| Septic shock | 44 (11.2) | 35 (20.5) | 9 (4.0) | < 0.01 | |
| Myocarditis | 38 (9.6) | 32 (18.7) | 6 (2.7) | < 0.01 | |
| Anemia | 35 (8.9) | 23 (13.4) | 12 (5.4) | < 0.01 |
BNP = brain natriuretic peptide, IQR = interquartile range, MIS-C = multisystem inflammatory syndrome in children.
aSurvivors only.
bIncludes count of discrete options provided in SDC (http://links.lww.com/PCC/B784) and self-reported in the registry (as complication yes/no).
cFive most common complication. Complete list of all recorded complications and organ system categories provided in (SDC, http://links.lww.com/PCC/B784).
Missing HFNC duration in 16 (15.6%), noninvasive ventilator duration in 2 (2.8%), invasive ventilator duration in 6 (8.5%), and ICU length of stay in 10 (2.5%).
Explanatory Factors and Critical Illness
Critical illness occurred in 41.6% (164/394) and was more common among those with MIS-C (55.6% [95/171] vs 30.9% [69/223]; p < 0.01) (Table 1). Patients with critical illness in the MIS-C group were older (median age 10 [IQR, 6–13.4] vs 7 [IQR, 2–12.8], p < 0.01), with a higher proportion of patients greater than or equal to 2 years old, as compared to the moderate illness group. There was, however, no significant difference in the age distribution of patients with critical and moderate illness in the non–MIS-C group. A higher proportion of patients with critical non–MIS-C had greater than or equal to two comorbidities and bacterial coinfection (SDC, http://links.lww.com/PCC/B784). On multivariable analysis including a priori defined variables of age (adolescent vs child/infant), race, sex, greater than or equal to three signs/symptoms in different organ systems, and greater than or equal to two comorbidities, we failed to identify any associations with critical illness in MIS-C patients (Fig. 2A). However, in non–MIS-C patients, the presence of greater than or equal to two comorbidities was associated with greater OR of critical illness (adjusted OR, 2.95 [95% CI, 1.6–5.40], p < 0.01) (Fig. 2B).
Figure 2.
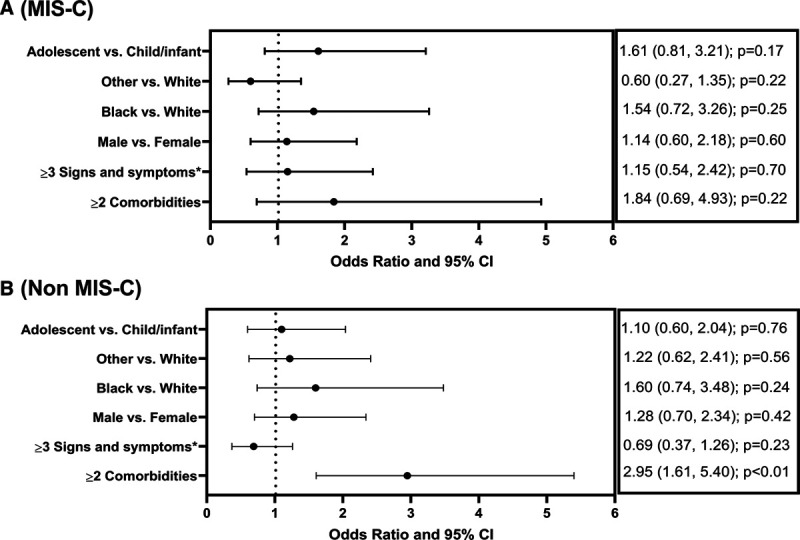
Multivariable logistic regression to assess independent explanatory factors associated with the critical illness among those with multisystem inflammatory syndrome in children (MIS-C) (A) and non-MIS-C (B) patients. Race category combined to three (Black, White, and Others). For logistic regression, patients with race listed as unknown (n = 20; 5% of the total cohort) were included in the “Other” category. Adolescent greater than or equal to 12 yr. *In different organ systems. Organ system categorization for signs and symptoms and a complete list of comorbidities listed in the SDC (http://links.lww.com/PCC/B784).
DISCUSSION
In this report from SCCM’s VIRUS registry (March 2020 to January 2021), we have provided details of 394 COVID-19–related PICU admissions from 38 hospitals. We have compared patient demographics, clinical course, management received, and outcomes between MIS-C and non–MIS-C patients. Additionally, we have stratified patients by critical versus moderate illness and identified unique patient characteristics that may relate to the risk-of-development of critical illness in the two presentations of pediatric COVID-19.
In this report, MIS-C and non–MIS-C pediatric patients exhibited significant differences in regard to demographics, symptoms, and clinical characteristics. Two other studies have compared patients with and without MIS-C. Swann et al (10) in the United Kingdom included 52 MIS-C and 404 non–MIS-C patients (< 19 yr) admitted to the hospital. In this study, MIS-C patients defined using the World Health Organization criteria, rather than the CDC criteria, were significantly older and required more respiratory support. In our study, we have not identified any difference in respiratory support needed, but a greater proportion of our patients in the MIS-C group were younger children. A more contemporary report by Feldstein et al (11) from the CDC-funded “Overcoming COVID-19” network describes a series of 1,116 patients (< 21 yr) admitted into hospital (not all to the ICU) in the United States up to October 2020. The findings in our report (e.g., age characteristics, presenting signs and symptoms, complications, and inflammatory markers) align with those identified in this CDC report (11). The proportion of ICU admissions in the CDC study appears significantly higher than the proportion we have observed in the VIRUS cohort (58.3% vs 45.9%, z score 5.4 and p < 0.05), but we have to acknowledge that the samples used in both studies are not independent (SDC, http://links.lww.com/PCC/B784, overlap details). That said, this apparent difference may reflect the exclusion of general hospital floor admissions for the non–MIS-C patients in the CDC registry after August 13, 2020.
We defined critical COVID-19 as the requirement of organ support (respiratory, cardiovascular, or renal) or death. Our critical illness subclassification is adapted from the NIH criteria for critical COVID-19 (22); however, we have used organ support variables to identify children with a critical illness. Dong et al (25) also classified children with acute organ dysfunction as critical COVID-19, whereas Cunningham et al (26) used a composite measure of mechanical ventilation or death to identify risk factors for severe disease in young adults. We classified and compared patients with and without critical illness separately for MIS-C and non–MIS-C patients. Although we identified age group differences in critical MIS-C on univariate comparison, multivariable analysis did not reveal any demographic or disease factors associated with greater odds of critical illness in MIS-C. This observation is similar to prior reports where MIS-C is reported in previously healthy children (8, 9). Our finding that in non–MIS-C cases, multiple comorbidities were associated with greater odds of critical illness has also been described in other studies (5, 10). A remarkably high proportion of patients in both MIS-C and non–MIS-C groups in our study were obese (44.1%). This proportion is more than twice the U.S. obesity rates for children 2–19 years old (17%) (27). Although the impact of obesity on adult COVID-19 has been studied (28), further research is needed to describe the predisposition of obese children to COVID-19.
The mortality rate (3.8%) and need for invasive ventilation (23.1%) we observed are comparable with other studies (4, 5, 10). However, in our cohort, there was less use of ventilatory support compared with the prior Virtual Pediatric Systems (VPS) report of 69% (29). The VPS population included 24% adults and counted ventilator use as a proportion of total days (rather than the percentage of total patients), which may explain the difference in findings (29).
In this report, we have also described the contemporary management of MIS-C and non–MIS-C groups of COVID-19 pediatric patients. The management described mostly aligns with the current recommendations (30). IV immunoglobulin (IVIG) is recommended as first-line therapy for MIS-C (30) but was used in only 45% of MIS-C patients in our cohort compared with 85% in another report (8). This finding is possibly due to heightened MIS-C recognition over time and anchoring bias (31), where patients with mild MIS-C may not require therapy such as IVIG. Some centers may also have prioritized data entry on certain days, and we may have missed capturing medication administration.
There are several limitations in our study. A complete case analysis such as ours assumes data are MCAR. If the MCAR assumption is not met, a complete case analysis can induce bias (32). A proportion of patients might still be hospitalized when data were extracted, contributing to missing outcome data and exclusion from analysis. Even though we have described a large cohort of patients, the sample size is still relatively small. To avoid overfitting the model, only five variables were included in the logistic regression. As such, there may be other explanatory factors associated with the outcome that could have been missed. Although unlikely, our critical illness definition may miss patients who did not require selected interventions. Even though patients with an incidental diagnosis of COVID-19 were explicitly excluded from the VIRUS registry, it is possible that some centers included borderline cases in the registry. Similarly, patient characterization into MIS-C and non–MIS-C groups was made by individual centers based on published CDC criteria, leaving the possibility for inappropriate classification. Further, we compared MIS-C and non–MIS-C as two distinct conditions although they may have overlapping presentations in some patients. Some patients may be entered into the registry before MIS-C was more formally recognized.
In conclusion, although both are epidemiologically linked with SARS-CoV-2, the two primary pediatric manifestations of COVID-19—MIS-C and non–MIS-C—have different presentations, demographic predispositions, explanatory factors, disease course, and management strategies. Overall, MIS-C is more often associated with a critical illness, higher proportion of complications, and more prolonged hospital LOS, than the non–MIS-C presentation of pediatric SARS-CoV-2 infection.
ACKNOWLEDGMENTS
We want to thank Ms. Mary Reidy, Ms. Colleen McNamara for their contributions to the Discovery Viral Infection and Respiratory Illness Universal Study: coronavirus disease 2019 Registry activities. We acknowledge Mr. Jeremy McGarvey, MS (senior statistician, OSF Healthcare Peoria) for assistance in calculating obesity percentiles, Dr. John Brinton, PhD (Department of Biostatistics and Informatics, Colorado School of Public Health, University of Colorado, Aurora, CO) for guidance in statistical analysis. We want to thank Dr(s) Aysun Tekin and Romil Singh for the manual calculation of Pediatric Risk of Mortality scores.
Supplementary Material
Footnotes
*See also p. 651.
The Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group served as collaborators and site investigators are listed in the Appendix (http://links.lww.com/PCC/B784).
Presented, in part, as a STAR research presentation at the Society of Critical Care Medicine’s 50th Annual National Congress 2021, virtual conference February 1-13, 2021.
Clinical Trial Registration: NCT04323787.
Drs. Tripathi and Gist contributed equally.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Supported, in part, by the Gordon and Betty Moore Foundation and Janssen Research and Development, LLC.
Dr. Bjornstad’s institution received funding from the International Society of Nephrology and Bioporto. Drs. Kashyap’s, Ms. Boman’s, Dr. Kumar’s institutions received funding from The Gordon and Betty Moore Foundation. Drs. Kashyap’s, Ms. Boman’s, and Dr. Kumar’s institutions received funding from Janssen and Janssen LLC. Dr. Kashyap receives funding from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute: R01HL 130881, UG3/UH3HL 141722; Gordon and Betty Moore Foundation Janssen Research and Development, LLC; and royalties from Ambient Clinical Analytics. Inc. Dr. Chiotos funded by Agency for Healthcare Research and Quality (K12-HS026393). Dr. Heneghan’s institution received funding from Society of Critical Care Medicine (SCCM). Dr Zimmerman’s institution received funding from the NIH, Immunexpress, Seattle, and he received funding from Elsevier Publishing Company. Dr. Kumar receives funding from the Gordon and Betty Moore Foundation, Centers for Disease Control and Prevention Foundation through the University of Washington, and Janssen Research and Development, LLC. Dr. Bhalala currently funded by NIH (site-principal investigator [PI] for Stress Hydrocortisone in Pediatric Septic Shock—R01HD096901), The Children’s Hospital of Philadelphia (site-PI for Pediatric Resuscitation Quality Collaborative), Voelcker Pilot Grant (PI for a project on prearrest electrocardiographic changes), The Children’s Hospital of San Antonio Endowed Chair Funds for ancillary projects related to SCCM Viral Infection and Respiratory Illness Universal Study (VIRUS) (coronavirus disease 2019) Registry and SCCM VIRUS electronic medical record automation pilot. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579:270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bialek S, Gierke R, Hughes M, et al. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR. 2020; 69:422–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Götzinger F, Santiago-García B, Noguera-Julián A, et al. ; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020; 4:653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020; 174:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the U.S. JAMA Intern Med. 2020; 180:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020; 395:1607–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR. 2020; 69:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020; 383:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swann OV, Holden KA, Turtle L, et al. ; ISARIC4C Investigators. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: Prospective multicentre observational cohort study. BMJ. 2020; 370:m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021; 325:1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkey AJ, Kumar VK, Harhay MO, et al. The viral infection and respiratory illness universal study (VIRUS): An international registry of coronavirus 2019-related critical illness. Crit Care Explor. 2020; 2:e0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walkey AJ, Sheldrick RC, Kashyap R, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: The society of critical care medicine discovery viral infection and respiratory illness universal study registry. Crit Care Med. 2020; 48:e1038–e1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauchner H, Golub RM, Zylke J. Editorial concern-possible reporting of the same patients with COVID-19 in different reports. JAMA. 2020; 323:1256. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J of Biomed Inform. 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control (CDC): Multisystem Inflammatory Syndrome (MIS-C), 2020. Available at: https://www.cdc.gov/mis-c/index.html. Accessed March 22, 2021
- 17.U.S Food & Drug Administration: Pediatric Exclusivity Study Age Group, 2014. Available at: https://www.fda.gov/drugs/data-standards-manual-monographs/pediatric-exclusivity-study-age-group. Accessed October 25, 2020
- 18.Vogel M: Childsds: Data and Methods Around Reference Values in Pediatrics, 2020. Available at: https://CRAN.R-project.org/package=childsds. Accessed October 25, 2020
- 19.Centers for Disease Control (CDC). Use and Interpretation of the WHO and CDC growth charts for children from birth to 20 years in the United States. In: Division of Nutrition PA, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, 2013. Available at: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/growthchart.pdf. Accessed March 22, 2021 [Google Scholar]
- 20.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988; 16:1110–1116 [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Pediatric Critical Care Research Network (CPCCRN): PRISM III Calculator, 2020. Available at: https://www.cpccrn.org/calculators/prismiiicalculator/. Assesed September 13, 2020
- 22.National Instuites of Health (NIH). Clinical Spectrum of SARS-CoV-2 Infection. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines 2020. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed February 12, 2021
- 23.Thompson CG, Kim RS, Aloe AM, et al. Extracting the variance inflation factor and other multicollinearity diagnostics from typical regression results. Basic Appl Soc Psych. 2017; 39:81–90. [Google Scholar]
- 24.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007; 4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020; 145:e20200702. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham JW, Vaduganathan M, Claggett BL, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020; 181:379–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016; 315:2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kass DA. COVID-19 and severe obesity: A big problem? Ann Intern Med. 2020; 173:840–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachdeva R, Rice TB, Reisner B, et al. The impact of coronavirus disease 2019 pandemic on U.S. and Canadian PICUs. Pediatr Crit Care Med. 2020; 21:e643–e650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson LA, Canna SW, Friedman KG, et al. american college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020; 72:1791–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss SL, Peters MJ, Agus MSD, et al. ; Children’s Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children Taskforce. Perspective of the surviving sepsis campaign on the management of pediatric sepsis in the era of coronavirus disease 2019. Pediatr Crit Care Med. 2020; 21:e1031–e1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001; 25:464–469 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


