In adults with COVID-19 (the disease caused by infection with severe acute respiratory syndrome coronavirus, SARS-CoV-2), the prevalence of acute neurologic symptoms (e.g., headaches, anosmia, seizure) and conditions (e.g., encephalopathy, stroke, delirium, encephalitis) ranges widely, from 4.4% to 100% of cases (1, 2). Neurologic manifestations in children younger than 18 years with COVID-19 is also relatively common. For example, in the United States, of nearly 3,700 cases, 17% had nonspecific neurologic conditions such as headache, fatigue, and myalgia, and 1% presented with encephalopathy, seizures, and meningeal signs (3). Worldwide, a report of nearly 1,400 pediatric patients described similar prevalence of headache (4%), anosmia (2%), seizures (0.7%), and cerebrovascular stroke (0.7%) (4).
The pathophysiology of acute and postacute neurologic manifestations of COVID-19 is likely multifactorial. Each of the following mechanistic pathways could interactively or independently cause disease: 1) direct viral invasion and replication in the CNS, 2) large vessel or microvascular insufficiency due to vasoconstriction and/or occlusion, 3) nonspecific effects of severe systemic COVID-19 illness or treatment, and 4) immune system dysregulation and autoimmunity.
VIRAL INVASION OF THE NERVOUS SYSTEM
Cellular invasion by the SARS-CoV-2 begins with binding of the viral spike protein to a transmembrane receptor, followed by viral membrane fusion with the cellular membrane after activation of the spike protein by cellular proteases.
SARS-CoV-2 binds to the angiotensin-converting enzyme (ACE) 2 receptor, a protein coexpressed with the protease transmembrane serine protease 2 (TMPRSS2) in endothelial cells throughout the body. ACE2 is particularly abundant in the small intestine, kidney, lungs, and heart (5). ACE2 is also present in human adult and fetal brain, with highest expression in the pons and medulla oblongata (6). In mice, brain ACE2 protein is higher in the early postnatal period than in the adult, whereas ACE2 activity is similar (7). ACE2 is also expressed in components of the cerebral vasculature and blood-brain barrier (BBB): that is, the endothelium, pericytes, and contractile cells (8–10). Purkinje cells, cortical layer V neurons, astrocytes, and micrglia also express ACE2 and TMPRSS2 (10). SARS-CoV-2 may bind instead, or also, to the neuronal adhesion molecule neuropilin (1 and undergo activation by Furin, an ubiquitous protease, to allow entry into the host cell (11). Neuropilin 1 is a glycoprotein essential for normal nervous and cardiovascular system formation and function in vertebrates. It is expressed in immune (i.e., macrophages, microglia) and nonimmune cells (i.e., endothelia, neurons) (12, 13). Neuropilin 1 has essential roles in axon guidance, dendrite formation, and cerebral vasculogenesis, among other processes. Indeed, mouse brain neuropilin 1 expression is two to three times higher in embryonic than adult tissues (14, 15). Of note, the developing brain expresses the main receptors and proteins considered necessary for SARS-CoV-2 invasion into neural cells. Therefore, direct viral invasion of the CNS or peripheral nervous system is biologically plausible across the age spectrum, but evidence supporting this mechanism as the sole or predominant pathophysiologic process in patients is scarce (16). Direct viral invasion of the CNS would require either viremia and BBB disruption or transsynaptic viral passage along cranial nerves V, VII, IX, and X, using nasopharyngeal, respiratory, and/or gastrointestinal tracts as entry points (16, 17). Figure 1A illustrates transsynaptic passage starting at the nasal neuroepithelium traveling via olfactory pathways to the brain stem and cortical areas. Viral entry via the respiratory system (Fig. 1B) leads to systemic inflammation and viremia, setting the stage for CNS invasion via BBB disruption (Figure 1, C and D).
Figure 1.
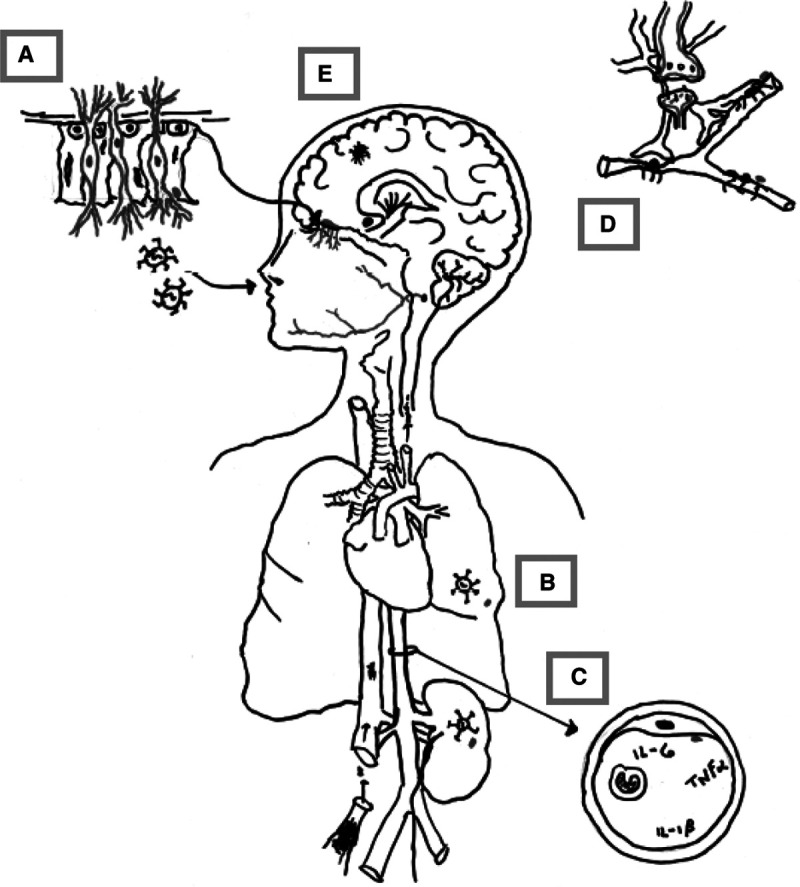
Schematic of hypothesized neuropathology of COVID-19 neurologic manifestations. A, Expanded view of the neuroepithelium (arrow) showing olfactory neurons, neural stem cells, and sustentacular cells. Olfactory neurons are bipolar, projecting axons that traverse the cribriform plate apically and the nasal cavity basally. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binding to angiotensin-converting enzyme (ACE) 2 on sustentacular cell membranes enveloping basal dendrites may allow viral invasion into olfactory neurons followed by transsynaptic spread via cranial nerves and olfactory pathways to enter the brainstem, basal ganglia, and cortex. B, Inhaled viral particles easily bind to ACE2 on respiratory epithelium to replicate and enter the bloodstream. C, Shows a cross-section of the vasculature with a cartoon of viremia, the “cytokine storm” and the ACE2-expressing vascular endothelium. SARS-CoV-2 binding to endothelial ACE2 enhances viremia and multiple organ involvement. D, Schematically shows the blood-brain barrier and ACE2 expressing pericytes. ACE2 loss decreases flow in the cerebral microcirculation, in part by pericyte action on cerebral vessels. Finally, E shows cerebral microinfarcts from vascular plugging and vasoconstriction. IL = interleukin, TNFα = tumor necrosis factor-α.
Transsynaptic entry into the brain via the nasal cavity is supported by data from human autopsy and biopsy tissues and by work using animal models (10, 18, 19). The nasal olfactory epithelium is a neuroepithelium containing neural stem cells, sustentacular (or supporting) cells and olfactory sensory neuronal dendrites in which ACE2 messenger RNA and protein are coexpressed with neuronal markers (19). Sustentacular cells express ACE2 messenger RNA and protein at levels similar to those found in the respiratory epithelium (18). Sustentacular cells wrap around the apical dendrites of olfactory sensory neurons, the bipolar neurons whose axons pierce the cribriform plate to synapse at the olfactory bulb. Whether or not sustentacular cells can transfer SARS-CoV-2 to these neurons, possibly via exosomes as noted in some herpesviruses, is unknown (10, 18). SARS-CoV-2 could thus travel transsynaptically from the olfactory bulb to the olfactory tubercule and cortex and/or to the brainstem and medulla (17, 19).
The likely importance of the olfactory route as a pathway to the CNS is supported by reports of MRI confirmed involvement of the olfactory cortex and brainstem in both adults and children (20, 21). However, reports of clinical encephalitis, meningitis, and intracranial ischemia/hemorrhage, in general, lack evidence of SARS-CoV-2 in the cerebrospinal fluid (CSF) (16, 22). Polymerase chain reaction SARS-CoV-2 positivity in brain slices has been noted (23, 24), but pathologic evidence of viral-specific injury in autopsy studies is lacking.
THE PROTHROMBOTIC STATE AND THE CNS (IMPAIRED LARGE VESSEL OR MICROVASCULAR BLOOD FLOW)
A feature that distinguishes neurologic disease associated with COVID-19 from that seen in most other respiratory viruses is the marked prothrombotic state and increased risk of stroke, particularly ischemic rather than hemorrhagic (16). Thrombotic and thromboembolic strokes have been reported in COVID-19 patients across the age spectrum, ranging from the elderly to those as young as 7 years (25, 26) depicted schematically in Figure 1E. SARS-CoV-2 infection is associated with cerebral large vessel and microcirculatory occlusion or insufficiency in young adults and children (25, 27, 28). Case reports of thrombotic and hemorrhagic stroke in children with acute COVID-19 are growing (4, 29–31).
Loss of ACE2 activity secondary to SARS-CoV-2 infection is likely to play an important role in the cerebral vascular insufficiency, endotheliopathy, and neuropsychiatric manifestations of COVID-19. Microcirculatory insufficiency and endotheliopathy in the CNS of COVID-19 patients (32) are supported by the predominance of hypoxic injury and/or microvascular plugging (Fig. 1E) in brain autopsies from adults who died from COVID-19 even in those without systemic hypoxia or respiratory failure (24, 26). As shown in Figure 2, SARS-CoV-2 bound to cell surface ACE2, followed by viral entry, depletes ACE2 (9). ACE2 loss has multiple effects. First, ACE2 normally counteracts angiotensin II, a potent vasoconstrictor, procoagulant, and inflammatory neuropeptide, via a number of pathways. ACE2 not only directly inactivates angiotensin II but it also produces angiotensin 1–7, an agonist at the Mas receptor (MasR) that counteracts the actions of angiotensin II by promoting anti-inflammatory, anticoagulant, vasodilatory, and antioxidant effects downstream of MasR. MasR agonists are protective against ischemia in vitro and in vivo (33). Hence, ACE2 depletion and subsequent renin-angiotensin system (RAS) disequilibrium could produce the endothelitis, inflammation, and prothrombotic state associated with COVID-19 (34, 35). ACE2 acts on numerous other substrates in the brain, including the endogenous opioid neuropeptides known as dynorphins. (36) ACE2 loss leading to unopposed bradykinin, neurotensin, and dynorphin levels could help explain increased vascular permeability, delirium, and high sedative requirements in COVID-19 patients (37). Preclinical studies show that the ACE2/angiotensin 1–7/MasR-axis affects cognition, anxiety, depression, and other mood disorders. In support of the role of ACE2, high circulating angiotensin II levels are correlated with disease severity in critically ill COVID-19 patients (38). In summary, ACE2 loss could account for vascular and nonvascular neurologic manifestations associated with COVID-19.
Figure 2.
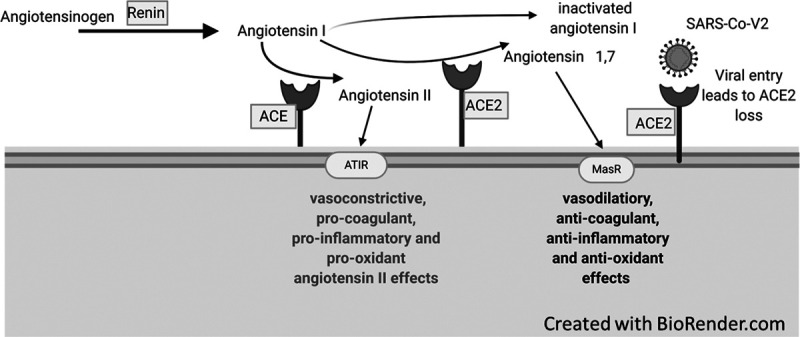
Angiotensin-converting enzyme (ACE) 2 and Mas receptor (MasR) pathways. Renin, produced in the kidney, acts on circulating angiotensinogen to produce angiotensin I. Angiotensin I is the physiologically inactive precursor of angiotensin II. The conversion of angiotensin I to angiotensin II is catalyzed by ACE, a type I integral membrane protein found primarily in the vascular endothelium of the lungs and kidneys. Angiotensin II exerts vasoconstrictive procoagulant, proinflammatory, and prooxidant effects via the angiotensin receptor (AT1R). Angiotensin II may instead be inactivated by the ACE2, a homologous type I integral membrane protein expressed in the vascular endothelium, lungs, kidney, adrenal cortex, arterioles, and brain. ACE2 also converts angiotensin I and angiotensin II into angiotensin 1–9 and angiotensin 1–7, respectively. Angiotensin 1–7 activates the MasR to promote anti-inflammatory, anticoagulant, vasodilatory, and antioxidant effects. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
SYSTEMIC FACTORS (CRITICAL ILLNESS AND THE CNS)
Seizures, delirium, and encephalopathy observed in many critically ill patients with COVID-19 are likely related in whole or in part to hepatic and/or renal failure, medications, hypoxia, and hypotension. The pooled estimates of seizure and encephalopathy frequency in children with severe COVID-19 are 3.1% and 12.6% of cases, respectively (3).
IMMUNE DYSREGULATION AND THE CNS
Immune dysregulation resulting in “cytokine storm” and macrophage activation, possibly related to inefficient innate immunity and impaired viral clearance, could produce neurologic manifestations from systemic effects and/or BBB breakdown (39). Acute or late neurologic manifestations of COVID-19 in adults and children may also be triggered by autoimmunity. Immune profiling suggests that autoantibodies in multiystem inflammatory syndrome in children (MIS-C) (the COVID-19–associated MIS-C) play a role in organ dysfunction including the brain (40, 41). Shared sequence similarity between SARS-CoV-2 and sialic acid residues on neural tissue as well as the postinfectious nature of MIS-C supports an autoimmune hypothesis in neurologic manifestations of COVID-19 in adults and children alike after resolution of the acute infection (22, 40, 42). Serum and CSF from a series of adult patients with severe COVID-19 infection contained high-affinity SARS-CoV-2–neutralizing antibodies that cross-react with mammalian self-antigens, including self-antigens found in the CNS (43). Postinfectious neurologic manifestations include Guillain-Barre syndrome and acute disseminated encephalomyelitis (3, 44, 45). In COVID-19, the presence of antiphospholipid antibodies in patients with severe thrombosis and the correlation between anti-interferon antibodies and severity of disease also support the possible role of autoimmunity (46, 47).
CONCLUDING REMARKS ABOUT THERAPIES
Knowledge about mechanisms of neurologic disease and immunologic response to SARS-CoV-2 is scarce, but rapidly growing. Experimental and clinical data suggest a major role for inflammation in the genesis of neurologic complications of COVID-19 in adults and children, with potential pathophysiologic involvement of disequilibrium in the RAS. This growing knowledge about potential mechanisms as outlined in this PCCM Concise Clinical Science review supports the currently used clinical interventions such as steroids in COVID-19 and steroids and IV immunoglobulin in MIS-C. Future potential immunologic interventions include blocking agents (against interleukin-1 and -6, for example) and autoreactive cell and autoantibody depletion with plasmapheresis and/or anti-CD20 monoclonal antibodies to ameliorate neurologic complications of COVID-19.
Other possible pharmacologic approaches include combatting RAS disequilibrium induced by ACE2 loss using MasR agonists. In this regard, a clinical trial (NCT04452435) of the MasR agonist C21 for treating nonneurologic complications of COVID-19 was recently completed (48). This group had previously demonstrated successful treatment of rodent stroke after nasal delivery of C21, suggesting the potential for future trials for treating or preventing neurologic complications of COVID-19 (49). There are also ongoing mechanistic studies of the cytokine storm in pediatric COVID-19, with or without MIS-C (NCT04538495; NCT04588363), which should yield highly valuable insights into systemic disease pathogenesis. However, studies using biosamples and imaging relevant to the CNS and peripheral nervous system are needed to gain further understanding of the mechanisms of neurologic disease in pediatric COVID-19.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 2020; 16:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharifian-Dorche M, Huot P, Osherov M, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020; 417:117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panda PK, Sharawat IK, Panda P, et al. Neurological complications of SARS-CoV-2 infection in children: A systematic review and meta-analysis. J Trop Pediatr. 2020; 66:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranabothu S, Onteddu S, Nalleballe K, et al. Spectrum of COVID-19 in children. Acta Paediatr. 2020; 109:1899–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020; 251:228–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukiw WJ, Pogue A, Hill JM. SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol. 2020; 57:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song R, Preston G, Yosypiv IV. Ontogeny of angiotensin-converting enzyme 2. Pediatr Res. 2012; 71:13–19 [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Jin H, Zhu Y, et al. Diverse functions and mechanisms of pericytes in ischemic stroke. Curr Neuropharmacol. 2017; 15:892–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson FA, Mihealsick RP, Wagener BM, et al. Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection. Am J Physiol Heart Circ Physiol. 2020; 319:H1059–H1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fodoulian L, Tuberosa J, Rossier D, et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience. 2020; 23:101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020; 370:856–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wild JR, Staton CA, Chapple K, et al. Neuropilins: Expression and roles in the epithelium. Int J Exp Pathol. 2012; 93:81–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S, Bag AK, Singh RK, et al. Multifaceted role of neuropilins in the immune system: Potential targets for immunotherapy. Front Immunol. 2017; 8:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neufeld G, Kessler O, Herzog Y. The interaction of neuropilin-1 and neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol. 2002; 515:81–90 [DOI] [PubMed] [Google Scholar]
- 15.Yue F, Cheng Y, Breschi A, et al. ; Mouse ENCODE Consortium. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014; 515:355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020; 183:16–27.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anoop UR, Verma K. Happy hypoxemia in COVID-19-A neural hypothesis. ACS Chem Neurosci. 2020; 11:1865–1867 [DOI] [PubMed] [Google Scholar]
- 18.Bilinska K, Jakubowska P, Von Bartheld CS, et al. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: Identification of cell types and trends with age. ACS Chem Neurosci. 2020; 11:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021; 24:168–175 [DOI] [PubMed] [Google Scholar]
- 20.Thu SS, Matin N, Levine SR. Olfactory gyrus intracerebral hemorrhage in a patient with COVID-19 infection. J Clin Neurosci. 2020; 79:275–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindan CE, Mankad K, Ram D, et al. ; ASPNR PECOBIG Collaborator Group. Neuroimaging manifestations in children with SARS-CoV-2 infection: A multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021; 5:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdel-Mannan O, Eyre M, Lobel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020; 77:1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020; 92:699–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med. 2020; 383:989–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez Amezcua JM, Jain R, Kleinman G, et al. COVID-19-induced neurovascular injury: A case series with emphasis on pathophysiological mechanisms. SN Compr Clin Med. 2020; 2:2109–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe. 2020; 1:e290–e299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crippa S, Kägi G, Graf L, et al. Stroke in a young adult with mild COVID-19 suggesting endotheliitis. New Microbes New Infect. 2020; 38:100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulko E, Overby P, Ali S, et al. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. AJNR Am J Neuroradiol. 2020; 41:2348–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appavu B, Deng D, Dowling MM, et al. Arteritis and large vessel occlusive strokes in children after COVID-19 infection. Pediatrics. 2021; 147:e2020023440. [DOI] [PubMed] [Google Scholar]
- 30.Mirzaee SMM, Gonçalves FG, Mohammadifard M, et al. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020; 297:E274–E275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beslow LA, Linds AB, Fox CK, et al. ; International Pediatric Stroke Study Group. Pediatric ischemic stroke: An infrequent complication of SARS-CoV-2. Ann Neurol. 2021; 89:657–665 [DOI] [PubMed] [Google Scholar]
- 32.Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, et al. Cerebrovascular disease in patients with COVID-19: Neuroimaging, histological and clinical description. Brain. 2020; 143:3089–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alenina N, Bader M. ACE2 in brain physiology and pathophysiology: Evidence from transgenic animal models. Neurochem Res. 2019; 44:1323–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miesbach W, Makris M. COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020; 26:1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra A, O’Farrell FM, Reynell C, et al. Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat Protoc. 2014; 9:323–336 [DOI] [PubMed] [Google Scholar]
- 36.Mehrabadi ME, Hemmati R, Tashakor A, et al. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomed Pharmacother. 2021; 137:111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarrahi A, Ahluwalia M, Khodadadi H, et al. Neurological consequences of COVID-19: What have we learned and where do we go from here? J Neuroinflammation. 2020; 17:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020; 63:364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020; 72:1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020; 183:968–981.e967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruber CN, Patel RS, Trachtman R, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell. 2020; 183:982–995.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrenfeld M: Covid-19 and autoimmunity. Autoimmun Rev. 2020; 19:102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreye J, Reincke SM, Prüss H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat Rev Immunol. 2020; 20:645–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalakas MC. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020; 7:e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Miranda Henriques-Souza AM, de Melo ACMG, de Aguiar Coelho Silva Madeiro B, et al. Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology. 2021; 63:141–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connell NT, Battinelli EM, Connors JM. Coagulopathy of COVID-19 and antiphospholipid antibodies. J Thromb Haemost. 2020; 18:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steckelings UM, Sumners C. Correcting the imbalanced protective RAS in COVID-19 with angiotensin AT2-receptor agonists. Clin Sci (Lond). 2020; 134:2987–3006 [DOI] [PubMed] [Google Scholar]
- 49.Bennion DM, Jones CH, Dang AN, et al. Protective effects of the angiotensin II AT2 receptor agonist compound 21 in ischemic stroke: A nose-to-brain delivery approach. Clin Sci (Lond). 2018; 132:581–593 [DOI] [PubMed] [Google Scholar]


