Abstract
Nerve guide conduits (NGCs), as alternatives to nerve autografts and allografts, have been widely explored as an advanced tool for the treatment of peripheral nerve injury. However, the repairing efficiency of NGCs still needs significant improvements. Functional NGCs that provide a more favorable microenvironment for promoting axonal elongation and myelination are of great importance. In recent years, 3D printing technologies have been widely applied in the fabrication of customized and complex constructs, exhibiting great potential for tissue engineering applications, especially for the construction of functional NGCs. In this review, we introduce the 3D printing technologies for manufacturing functional NGCs, including inkjet printing, extrusion printing, stereolithography-based printing and indirect printing. Further, we summarize the current methods and strategies for constructing functional NGCs, such as designing special conduit architectures, using appropriate materials and co-printing with different biological cues. Finally, the challenges and prospects for construction of functional NGCs are also presented.
Keywords: Nerve guide conduits, Functionalization, Peripheral nerve repair, 3D printing
Background
Peripheral nerve injury (PNI) is one of the most common neuropathies, mainly caused by trauma, accident and disease [1,2]. PNI often impairs the motor and sensory abilities of patients and results in paralysis and disability, which may significantly reduce the quality of a patient’s life [3]. The self-repairing ability of peripheral nerves is extremely limited and treating PNI remains challenges in regenerative medicine [4]. Currently, autografts are regarded as the gold standard to repair PNI. However, there are also some disadvantages of autografts, such as the need for multiple surgeries, neuroma formation, lack of nerve donors and donor-site morbidity [5–7]. Hence, exploring alternative therapy is of vital significance. Nerve guide conduits (NGCs), as exogenous alternatives to bridge the injured nerve stumps, can build a beneficial microenvironment for promoting nerve recovery by complex mechanisms [8]. So far, some single-channel NGCs, such as Neuromaix®, NeuraGen® and Reaxon Plus® [9,10], have been approved by the Food and Drug Administration (FDA) for PNI treatment. However, the curative effect of normal NGCs in the therapy of long-gap PNI is not very satisfactory and still needs much improvement.
Functional NGCs can provide a favorable microenvironment, which is useful when treating PNI and beneficial to the functional recovery of the injured nerve [11–13]. To develop functional NGCs, some physico-chemical cues and biological cues are introduced to promote axonal elongation and myelin sheath formation. On the one hand, the customized structures of NGCs can provide biomimetic physical support to facilitate nerve regeneration. On the other hand, the material composition of NGCs and the corresponding physical and chemical properties may affect cellular behaviors and further influence the efficiency of nerve recovery. For the biological cues, some biological elements such as cells, neurotrophic factors and drugs are also integrated with NGCs to enhance the repair efficiency.
Recently, the newly emerging 3D printing which can construct complex structures rapidly has been applied to fabricate many meaningful products, such as personalized vascular topologies, cardiac patches and hearts [14–19], exhibiting great potential applications in tissue engineering. 3D printing can not only fabricate flexible NGCs with sophisticated structures, but also combine the scaffolds with cells and growth factors to mimic the extracellular matrix [20]. Moreover, 3D printing is a highly flexible processing technique with high precision [21,22] and thus is superior for the construction of functional NGCs for PNI repair.
Herein, we introduce the current technologies for 3D printing NGCs based on different principles and summarize the current methods and strategies of preparing functional NGCs, including designing conduit structures and materials, and co-printing with different biological cues (Fig. 1). Finally, the challenges and prospects for the construction of functional NGCs are discussed.
Fig. 1.
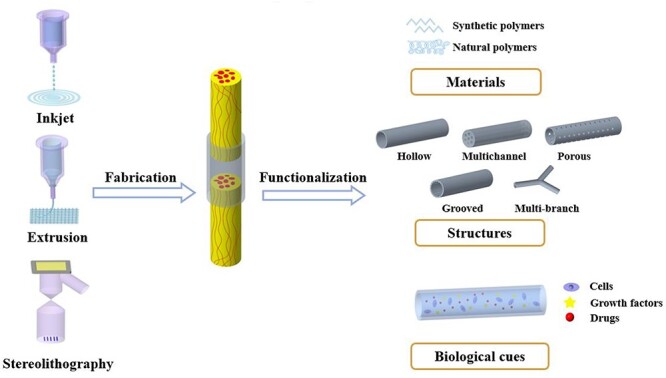
Schematic diagram of 3D printing technologies for NGCs fabrication and the strategies for construction of functionalNGCs
Review
3D printing technologies for NGCs
Different from the traditional fabrication methods of NGCs, such as solvent casting, freeze drying and electrospinning [23,24], 3D printing technology can fabricate NGCs with personalized characteristics to imitate the natural structure of nerves. NGCs can be created layer-by-layer by rapid prototyping machines, which is assisted by computer-aided design (CAD) technology by collecting and digitizing the complex microarchitectural information of native tissue from the images acquired by computed tomography (CT) or magnetic resonance imaging (MRI) [25–28]. In general, extrusion, inkjet and stereolithography printing are the most widely used 3D printing methods to fabricate NGCs. Besides, indirect 3D printing is another way to provide different structures for NGCs by printing different molds. The characteristics of different 3D printing technologies for NGCs are summarized in Table 1.
Table 1.
Different kinds of 3D printing technologies forNGCs
| Materials | Advantages | Disadvantages | Reference |
|---|---|---|---|
| PLA/PCL, collagen | 1. High speed 2. Low cost |
1. Low mechanical strength 2. Bioprinting limited. Unable to control the directionality and size of droplets precisely |
[29,31,32,50] |
| PLLA, PLGA, GelMA, alginate dialdehyde etc. | 1. Low cost 2. Multiple materials mixed printing 3. Higher cell viability |
1. Lower resolution than inkjet 2. Low speed 3. Nozzle clogging |
[35,37–39] |
| Photopolymerizable monomer like GelMA, PEGDA, silk-GMA, glycerol sebacate methacrylate and composites. | 1. Higher resolution than extrusion 2. High fabrication speed 3. Personalization |
1. Expensive 2. Photocurable resin limited 3. Possible cytotoxicity of residual photoinitiator and uncured resin |
[41,44,51–54] |
| GelMA, alginate methacrylate etc. | 1. Convenience 2. Low cost |
1. Microstructures limited 2. Personalisation |
[46–49,55] |
Inkjet 3D printing
Inkjet printing is also known as drop-on-demand printing. The bioink drop can be artificially controlled and delivered to predefined locations by thermal or piezoelectric forces while printing. The resolution of inkjet printing can range from 1.0 to 500 μm depending on the bioink and sizes of nozzles [29,30]. A cylindrical NGC composed of polylactic acid (PLA) and poly(ε-caprolactone) (PCL) was constructed by a piezoelectric inkjet system with a resolution of 1.0 μm [31]. Another study has shown that neuronal and Schwan cells can keep normal cell proliferation and viability when printed with a 60 μm single-nozzle piezoelectric inkjet device, indicating that this technique can be utilized for bioprinting application [32].
Extrusion 3D printing
Different from the drop-by-drop of inkjet printing, extrusion printers fabricate a 3D scaffold line-by-line with a movable nozzle driven by pneumatic or mechanical dispensing systems, with a resolution ranging from 100 to 500 μm [33,34]. Johnson et al. developed a custom 3D printing system based on microextrusion and structured light scanning (SLS) technique, and 3D-printed silicone bifurcated NGCs, which significantly enhanced the functional recovery of a 10 mm-defect of a rat sciatic nerve bifurcation [35]. In another study, uniaxial and multichannel conductive NGCs were pressure-extruded with a minimal 100 μm tip. These conduits with excellent physical and mechanical performances could be sutured with a 2 cm nerve defect from the dorsal branches of the ulnar nerve in an unfixed human cadaver by complex surgical process [36]. Besides, through bioprinting technology, different HUVECs and Schwann cell-laden scaffolds, such as gelatin and sodium alginate composite scaffolds, have been developed to promote the recovery of injured peripheral nerves, [37–39].
Stereolithography-based 3D printing
Utilizing laser beam scanning or image projection modeling, stereolithography-based 3D printing deposits cell-laden bioink in a reservoir with precise control of deposition. In general, the resolution is ~2.5–200 μm [33,40]. Nowadays, some optimized stereolithography 3D printers have been applied to construct NGCs, such as a laser-based microstereolithography (mSL) printer with a resolution of 50 μm and a projection stereolithography (PSLA) system with a resolution of 15 μm [41,42]. Particularly, digital light processing (DLP) 3D printing is developed by applying digital micromirror devices (DMDs) as a dynamic pattern generator. In DLP 3D printing an entire layer of material is simultaneously polymerized upon light exposure, which offers excellent image stability and fidelity. The theoretical resolution of a DLP printer can reach 2.5 μm [43]. The Chen group has fabricated NGCs in different complexity and size by DLP printing, such as an anatomically appropriately sized human facial NGC and special NGCs with four linear 4 mm microchannels [44]. Our group has also fabricated hydrogel NGCs rapidly by a continuous DLP 3D printing process [45]. In general, due to the special photocuring characteristics of printed biomaterials, the availability of proper bioinks for stereolithography-based 3D printing is limited.
Indirect 3D printing
Comparing the above three methods, with indirect 3D printing it is much easier to fabricate a mold that provides some simple structures for NGCs. We 3D-printed molds with a ‘lock and key’ structure to form cryopolymerized GelMA NGCs in different shapes [46], such as multichannel and bifurcating. With the same method, we also constructed a porous conduit with shape-memory property, and found that the conduits could facilitate the functional recovery of the transected model after neurorrhaphy [47]. Other researchers also utilized sacrificial molding to construct structures, such as poloxamer 407 and gelatin [48,49]. Indirect 3D printing is convenient and low-cost for manufacturing. However, it is difficult to fabricate complex personalized NGCs with high resolution.
Strategies for 3D printing functional conduits
Structure design
Structural cues of NGCs play an important role in physically supporting and connecting the injured nerves in peripheral nerve repair. The design of conduit structures can be divided into biomimic structures and personally customized structures, to make the reproduction of complex nerve features possible.
Biomimic structures
In general, the biomimic structure of NGCs can be classified as hollow [56], multi-channel [57–59] or porous [60]. Hollow conduits with the simplest structure are the most widely used in peripheral nerve repair, some of which have been approved in clinic. However, they are not favorable for directional alignment of axons and exchange of nutrients and growth factors. It has been demonstrated that multichannel conduits are superior for axonal regeneration as they control axonal dispersion and decrease mismatch [61], especially 4-channel conduits [44,62]. However, it has also been reported that internal lumen diameter, materials and the physical properties of multi-lumen conduits can also influence axon regeneration [52]. In addition, porous NGCs could facilitate nutrient exchange which may be beneficial for nerve repair. Multi-pore design is not easy for 3D printing NGCs, but it could be achieved with the help of other approaches such as salt leaching and solvent casting.
Furthermore, some topographical cues of biomimic structures have also been introduced into the structural design of NGCs. It has been reported that the longitudinal groove topography of the biomaterial surface could promote the directional alignment and migration of Schwann cells as well as the neurite orientation and elongation of PC12 cells in vitro [63–67]. Behbehani et al. provided a potential in vitro model to explore different dimensions of grooved conduits and evaluate the performances of different cells [68]. Meanwhile, NGCs with different sizes of grooved structures on the inner surface have also been applied for nerve repair in vivo [69]. The smaller ones, such as nanogrooves [70] and 3 μm stripe micropatterns on the inner wall of NGCs [71], were demonstrated to effectively facilitate sciatic nerve regeneration. Most of these grooved conduits were prepared by electrospinning. For 3D printing fabrication, NGCs with microgroove structure on the inner wall were manufactured by laser-based mSL with a size resolution of 50 μm, which could support the growth, differentiation and orientation of dorsal root ganglions (DRG) in vitro and facilitate nerve repair across a 3 mm defect [41]. The results demonstrated that 3D printed customized grooved conduits had great potential for functional nerve repair. However, the 3D printing technologies for customized grooved conduits should be further improved.
Personally customized structures
To improve the recovery efficacy of injured nerves with complex morphological geometry, the anatomical structures and different functions of nerves need to be valued, like bifurcated sciatic nerve and trifacial nerve. In a study, researchers fabricated ‘Y’ shaped NGCs to match inherent tissue anatomies by microextrusion printing. Through customizing the geometries of the scaffolds and boosting function with biomimetic microgrooves and bioactive gradients, they found that the 3D printed functional NGCs could accelerate the regeneration of injured nerves by multiple nerve pathways [35]. The 3D printed Y-tube with a loop by interposing with an autograft could effectively prevent the formation of neuroma, demonstrating great potential for surgical treatment of traumatic neuromas. Using advanced DLP 3D printing, Zhu et al. fabricated a life-size biomimetic multi-branched NGC (5.5 cm in length) featuring different branches of a complex branched human facial nerve, showing great potential for the design of personally structured NGCs [44].
Scaffold material characteristics
The materials of NGCs are also critical for nerve repair, and the ideal is that they should closely mimic the native environment in order to achieve the best possible induction of nerve regeneration [72]. In general, the material for 3D printing conduits can be subcategorized into natural and synthetic. The natural polymers for conduits mainly include collagen, chitosan, gelatin, silk fibroin and hyaluronic acid, while the most commonly used synthetic ones are PCL [73], PLA [74], polyglycolic acid (PGA) and poly (lactic-co-glycolic acid) (PLGA) [75]. This section summarizes recent research into the characteristics of NGCs scaffold material, including biocompatibility, mechanical properties and conductivity.
Biocompatibility
Biocompatibility is a basic requirement of NGCs. Namely, when the conduit is implanted in vivo, it should provide a matrix to promote cell adhesion, differentiation and growth, but not cause toxic reactions, such as hemolysis, coagulation or immune response. Generally, matrix materials usually offer bioactive properties, which can actively regulate cell behaviors and functions, including adhesion, proliferation, differentiation and migration. In particular, the cell-binding domain arginyl-glycyl-aspartic acid (RGD) peptide motif of gelatin and collagen is of great importance. Tao et al. fabricated NGCs with GelMA, which showed good compatibility with HUVECs and Schwann cells, and further enhanced the effect for nerve repair by incorporating drug-loaded nanoparticles [45]. Ning et al. used RGD chemically modified alginate with HA and fibrinogen as hydrogel precursors. Bioprinted Schwann cells and DRG neurons showed satisfactory cell ability and morphology [76]. In another study, substrates functionalized with I3QGK peptide nanofibers enhanced PC12 cells attachment, proliferation and differentiation, and showed potential for nerve regeneration [77]. In fact, the degradation rate of the biomaterial should closely match the regeneration rate of nerve to avoid injury caused by the second retrieval surgery. Most degradable biomaterials can be biodegraded by hydrolase and the products should be at least not harmful for the organism; such materials include some synthetic materials and most natural materials, like PCL, PLGA and collagen. Gong et al. sutured rat sciatic nerves with GelMA nerve conduits which were functionalized with engelbreth-holm-swarm (EHS) hydrogel. The conduits slowly degraded and completely disappeared after 16 weeks, and exhibited favorable performance to promote the regeneration of injured sciatic nerve [55]. In a study, Singh et al. 3D-printed poly (glycerol sebacate methacrylate) NGCs to repair a 3 mm gap of Thy-1-YFP-H mice, and this synthetic material could be slowly degraded by lipase [54].
Mechanical properties
Mechanical properties are essential characteristics for surgical operation with NGCs. Furthermore, the substrate stiffness of NGCs has been proved to have a significant influence on neuronal cells, including cell survival, spreading, adhesion, migration, neurotrophic function and average neurite length [78–81]. A study showed that rat Schwann cell precursor line (SpL201) cells presented better performance of adhesion and proliferation on stiffer substrates (in the range 1–100 MPa), while PC12 cells preferred softer ones. This might be due to mechano-transduction mechanisms involving integrins, focal adhesions and actin–myosin-associated signal pathways [78,82,83]. However, for more details, Wu et al. reported that 10 wt% GelMA hydrogels exhibited a Young’s modulus of 34.9 KPa. This modulus was optimal for nerve regeneration of PC12 cells, including outgrowth characteristics and morphology [84]. Otherwise, with an elastic modulus of 7.45 kPa, the polyacrylamide gel substrate represented the most suitable physical support for nerve repair to regulate the biological neurotrophic behavior and function of Schwann cells [85]. The differences were caused by different substrate material compositions, cell types and specific microenvironment for cell survival. The effects of hydrogel stiffness gradient fractions on neural cell orientation and behavior have also been explored in recent research [86,87].
Conductivity
In addition, electrical stimulation in particular has been demonstrated to control directional migration of neuronal and glial cells, neurite extension and differentiation of stem cells, providing insights into nerve regeneration. Recently, some conductive polymers, such as PPy and PAA, have been blended with other degradable polymers (PCL) to fabricate conductive NGCs by electrohydrodynamic jet 3D printing. These conductive conduits exhibited great performance in neuronal cell proliferation and stem cell differentiation [73,88–90]. In addition, carbon-based conductive materials, such as black phosphorus [91], carbon nanotubes and graphene [92,93] can also be doped to matrix material. Scaini et al. demonstrated that single-layer graphene deposited on electrically insulating substrates can alter neuronal excitability by changing membrane-associated functions [93]. And Park et al. prepared hybrid materials by dispersing reduced graphene oxide (rGO) in GelMA hydrogel and found that rGO/GelMA with good conductivity and mechanical properties could promote the neurite outgrowth of PC12 cells. Further, the rGO/GelMA-based NGCs could improve nerve recovery in electrophysiology, gastrocnemius muscle weight and the sciatic nerve function index [94]. Apart from conductive biomaterials, piezoelectric materials which provide in situ artificial electrical stimulation, have also been explored for peripheral nerve regeneration, such as zinc oxide [95] and polyvinylidene fluoride [96]. However, neurotoxicity should be highlighted for all of these conductive materials, as how to minimize toxicity or explore non-toxic conductive materials is very important [97].
To construct a favorable microenvironment for maximal PNI recovery, it is necessary to consider all of the matrix materials’ biocompatibility, biodegradability, conductivity and mechanical properties for NGCs. At the same time, the matrix materials must be printable to meet the need of different 3D printers, including viscosity, gelation methods and rheological properties. Currently, the characteristics of most existing materials are still not ideal. Thus, a combination of two or three kinds of materials which have special functions that can synergistically functionalize the NGCs may be an effective strategy.
Functional biological cues
Another potential strategy for efficient nerve recovery is to functionalize the customized NGCs with biological cues including cells, exogenous bioactive factors and drugs (Table 2). These functional biological cues could improve neural signals around or through the injured region in some cases and provide a more permissive environment for nerve regeneration.
Table 2.
Biological cues in conduits for nerve repair
| Additive | Type | Material | Gap size | Research time | Application | Ref. |
|---|---|---|---|---|---|---|
| Schwann cell | Cell | Silk–gold nanocomposite | 10 mm | 18 months | Enhancing myelination of the regenerated nerves | [100] |
| Neural crest stem cell | Cell | Polycaprolactone nanofiber | 15 mm | 6/12 weeks | Promoting regeneration across large nerve gaps | [104] |
| MSC | Cell | EPO-loaded Chitosan | 5 mm | 4/8 weeks | Accelerating nerve healing process | [103] |
| ASC | Cell | Cryopolymerized gelatin methacryloyl |
10 mm | 4/8/16 weeks | Providing a conducive environment for nerve recovery | [46] |
| OEC | Cell | Collagen–chitosan | 15 mm | 12 weeks | Promoting axonal regeneration and functional recovery |
[101] |
| CNTF/bFGF | Factor | Collagen | 35 mm in minipig | 6 months | Repairing 35 mm gap in minipigs | [112] |
| NGF | Factor | PCLA/silk fibroin | 15 mm | 12 weeks | Significant improvements in promoting and guiding neurite outgrowth | [113] |
| GDNF | Factor | GDNF/PCL | 5 cm | 1 year | Increasing nerve conduction velocity and average area occupied by individual Schwann cells | [114] |
| Platelet | Cytoplasm | GelMA/PEGDA | 10 mm | 12 weeks | Promoting hydrogel conduits in peripheral nerve repair | [56] |
| Melatonin | Drug | PCL | 15 mm | 12 weeks | Improving recovery efficiency by inhibiting oxidative stress and inflammation | [117] |
| XMU-MP-1 | Drug | GelMA | 10 mm | 3 months | Promoting the proliferation, migration and neurotrophic factors secretion of Schwann cells | [45] |
| PSA/HNK-1 | Drug | Polyethylene | 5 mm | 8/15 weeks | Increasing the ratio of myelinated axons/improvements in myelination | [120] |
| RGFP966 | Drug | GelMA | 10 mm | 3 months | Efficiently bridging a 10-mm gap of rat sciatic nerve by promoting remyelination of axons | [118] |
Live cells
Cell-based therapy has great application potential in PNI repair. Different types of cells that are beneficial to injured nerve regeneration, including glial cells and stem cells, have been integrated into NCGs in recent decades [98]. Schwann cells, a native glial population that can form myelin sheath and secrete neurotrophic factors, are neuroprotective and have been widely applied in PNI repair [99]. In a study, the nanocomposite-based NGCs loaded with the rat Schwann cell line could considerably improve axonal myelination and nerve regeneration, as shown by analysis of regenerated nerve [100]. In another study, native glial olfactory ensheathing cells (OECs) were seeded in perfluorotributylamine-loaded collagen–chitosan NGCs. The results showed this strategy could increase oxygen supply and promote axon regeneration [101]. Recently, Wu et al. 3D bioprinted a composite scaffold with hybrid bioinks of gelatin, sodium alginate and Schwann cells. They found that the 3D scaffold significantly enhanced cell adhesion and upregulated the level of related neurotrophic factors compared to 2D culture, which showed promising application of 3D bioprinted NGCs with cells [37].
In addition, different kinds of stem cells such as mesenchymal stem cells (MSCs), adipose-derived stem cells (ADSCs), neural stem cells (NSCs) and induced pluripotent stem cells (iPSCs) have been encapsulated into NGCs for PNI repair [102]. Zhang et al. combined MSCs with erythropoietin-loaded chitosan nerve conduits and found this strategy could accelerate nerve repair and morphological recovery in vivo [103]. Hu et al. seeded ADSCs on the cryoGelMA conduit and demonstrated that the mRNA levels of cells such as brain-derived neurotrophic factors (BDNF) were significantly up-regulated [46]. Besides, other stem cells, like bone marrow-derived stem cells (BMSC), skin derived stem cells (SDSC) and amniotic fluid mesenchymal stem cells (AFMSC), have also exhibited great potential in repair of PNI [104–107]. Qian et al. showed that the differentiation of BMSC into Schwann-like cells was facilitated by PCL NGCs that were functionalized with Au nanoparticles and polydopamine (PDA) [60].
Growth factors
Growth factors as another biological cue are important for functional nerve repair. Various kinds of growth factors have been incorporated into NGCs to improve the recovery efficacy, including nerve growth factors (NGF), glial cell line-derived neurotrophic factor (GDNF), ciliary neurotrophic factors (CNTF), BDNF, fibroblast growth factors (FGF) etc. [108–110]. These factors in conduits can be released to regulate the function of transplanted cells and host cells through different signaling pathways [111]. Specifically, they can improve the survival, proliferation and migration of transplanted cells, promote the infiltration of host cells, enhance the neuronal differentiation of stem cells and improve the neural regenerative capacities of Schwann cells [98]. This may provide a more beneficial biological microenvironment for nerve repair. Cui et al. reported that the collagen NGCs containing CNTF and bFGF could promote PNI repair across a 35 mm distance in minipig models [112]. The concentration gradients or distribution of the growth factors could affect the repair efficacy. In a study, NGCs functionalized with concentration gradients of NGF exhibited superior outcomes of 15 mm-long-gap nerve regeneration than those of NGCs with uniform NGF [113]. In addition, long-term sustained-release of the growth factors from conduits may construct a temporal-beneficial microenvironment for the recovery of injured nerves. Fadia et al. fabricated PCL NGCs with GDNF microspheres that provided sustained release of GDNF in a 5 cm nerve defect of rhesus macaques for >50 days [114]. Interestingly, in our recent study, we 3D-printed functional NGCs by incorporating bioinks with live platelets isolated from whole blood. This kind of conduit could slow down the activation of the live platelets and provide sustained release of multiple growth factors, which significantly improved nerve recovery in vivo [56].
Drugs
Currently, several pharmaceuticals have been approved by the FDA for PNI therapy through local injection or systemic administration, but effective results have not been achieved. Alternatively, drug-loaded NGCs can provide sustained release of a drug locally and reduce systemic side-effects; they typically stimulate nerve regeneration and myelination by acting on targets, activating signaling pathways, and promoting the growth and proliferation of the cells involved [115]. Melatonin can reduce oxidative stress and inhibit inflammation after nerve injury thus promoting the recovery of peripheral nerves [116]. 3D printed melatonin/PCL NGCs exhibited better biocompatibility than PCL NGCs by accelerating the proliferation of Schwann cells and upregulating neural expression [116,117]. Recently, some small molecules have been reported to be functional in accelerating nerve regeneration and recovery. Tao et al. functionalized 3D-printed NGCs with XMU-MP-1, and found that this small molecule could promote the functional recovery of injured nerves through inhibiting the Hippo pathway [45]. Xu et al. 3D-printed NGCs incorporating RGFP966-loaded nanoparticles, and demonstrated that RGFP966 could efficiently repair the injured nerve by activating the PI3K-AKT-ERK signaling pathway to enhance the remyelination of Schwann cells [118]. It has also been reported that incorporating drugs within the luminal filler of the conduits can also promote nerve regeneration after injury [55,119,120].
3D printing technology is a convenient method that can be used to fabricate different morphological NGCs that could influence neural cell proliferation and migration. Additionally, the microstructures of NGCs can also affect the controlled and sustained release of growth factors and drugs to offer specific cues to the regenerating tissues [121]. Integrating cells, growth factors and drugs into biomaterials to prepare functional NGCs may be a potential strategy to build a more ideal microenvironment for functional recovery of injured nerves. However, the problems of cell viability, stability of growth factors and controlled release of additives that greatly affect the efficiency of nerve regeneration should be considered.
Conclusions
The ideal functional NGCs require physico-chemical cues and biological cues to synergistically construct a biomimetic microenvironment and maintain physiological balance for nerve repair [122]. The emerging 3D printing technology with design flexibility, personalized customization and controllable manufacturing precision is an advanced method to achieve the functionalization of NGCs. Currently, remarkable advances have been made in the fabrication and functionalization of 3D printed NGCs and their corresponding applications in peripheral nerve repair and regeneration [123]. However, there are still some challenges for the future. Firstly, to precisely fabricate customized functional NGCs, the printing system should be optimized, especially with regard to precision and speed. Secondly, the bioinks for NGCs are limited. The characteristics of printable materials for conduits, such as biodegradation, biocompatibility and mechanical properties, need to be further improved. Using a combination of different biomaterials to fabricate suitable NGCs could be a choice for multifunctional conduits. Thirdly, aiming for clinical translation of neural tissue engineering, fabricating human-scale constructs for long-gap injuries and big animal models for PNI still remain a great challenge. What is more, exploring and clarifying the mechanisms of the repair of injured nerves is of great significance and could offer direction on how to design new strategies for NGCs. Generally, the gradual advancement in biomaterials and 3D printing techniques provide promise for the fabrication of human-scale customized constructs for long-gap PNI with ideal therapeutic effect.
Abbreviations
ADSC: Adipose-derived stem cells; BDNF: Brain-derived neurotrophic factors; BMSC: Bone marrow-derived stem cells; CNTF: Ciliary neurotrophic factors; DLP: Digital light processing; GDNF: Glial cell line-derived neurotrophic factor; MSC: Mesenchymal stem cells; mSL: Microstereolithography; NGC: Nerve guide conduits; NGF: Nerve growth factors; PLA: Polylactic acid; PCL: Poly(ε-caprolactone); PLGA: Poly (lactic-co-glycolic acid); PNI: Peripheral nerve injury; rGO: Reduced graphene oxide
Conflict of interest
None declared.
References
- 1. Rigoni M, Negro S. Signals orchestrating peripheral nerve repair. Cell 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carvalho CR, Silva-Correia J, Oliveira JM, Reis RL. Nanotechnology in peripheral nerve repair and reconstruction. Adv Drug Deliv Rev 2019;148:308–43. [DOI] [PubMed] [Google Scholar]
- 3. Mukhatyar V, Karumbaiah L, Yeh J, Bellamkonda R. Tissue Engineering Strategies Designed to Realize the Endogenous Regenerative Potential of Peripheral Nerves. Advanced Materials: NA-NA, 2009. [Google Scholar]
- 4. Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA et al. A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron 2016;89:956–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am 2013;95:2144–51. [DOI] [PubMed] [Google Scholar]
- 6. Raza C, Riaz HA, Anjum R, Shakeel NA. Repair strategies for injured peripheral nerve: review. Life Sci 2020;243. [DOI] [PubMed] [Google Scholar]
- 7. Deumens R, Bozkurt A, Meek MF, Marcus MA, Joosten EA, Weis J et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol 2010;92:245–76. [DOI] [PubMed] [Google Scholar]
- 8. Yin J, Zhang DM, Xiang Y, Wei P, Yang ZY, Wang ZH et al. The influence of cross-sectional morphology on the compressive resistance of polymeric nerve conduits. Polymer 2018;148:93–100. [Google Scholar]
- 9. Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 2012;43:553–72. [DOI] [PubMed] [Google Scholar]
- 10. Kornfeld T, Vogt PM, Radtke C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med Wochenschr 2019;169:240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bellamkonda RV. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials 2006;27:3515–8. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Qu W, Li D, Shi K, Li R, Han Y et al. Functional polymer-based nerve guide conduits to promote peripheral nerve regeneration. Adv Mater Interfaces 2020;7. [Google Scholar]
- 13. Manoukian OS, Baker JT, Rudraiah S, Arul MR, Vella AT, Domb AJ et al. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J Control Release 2020;317:78–95. [DOI] [PubMed] [Google Scholar]
- 14. Ma X, Liu J, Zhu W, Tang M, Lawrence N, Yu C et al. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev 2018;132:235–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–85. [DOI] [PubMed] [Google Scholar]
- 16. Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 2016;34:312–9. [DOI] [PubMed] [Google Scholar]
- 17. Suo HR, Li L, Zhang CX, Yin J, Xu KD, Liu JY et al. Glucosamine-grafted methacrylated gelatin hydrogels as potential biomaterials for cartilage repair. Journal of Biomedical Materials Research Part B-Applied Biomaterials 2020;108:990–9. [DOI] [PubMed] [Google Scholar]
- 18. Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ et al. BIOMEDICINE multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019;364: 458-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and Perfusable cardiac patches and hearts. Adv Sci 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maiti B, Diaz Diaz D. 3D printed polymeric hydrogels for nerve regeneration. Polymers 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ford S, Despeisse M. Additive manufacturing and sustainability: an exploratory study of the advantages and challenges. J Clean Prod 2016;137:1573–87. [Google Scholar]
- 22. Wang X, Jiang M, Zhou ZW, Gou JH, Hui D. 3D printing of polymer matrix composites: a review and prospective. Composites Part B-Engineering 2017;110:442–58. [Google Scholar]
- 23. Sarker M, Naghieh S, McInnes AD, Schreyer DJ, Chen X. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol J 2018;13. [DOI] [PubMed] [Google Scholar]
- 24. Xue JJ, Wu T, Dai YQ, Xia YN. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 2019;119:5298–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling L, Yang Y. Exploration on creative product customization design based on 3D printing technology research. Appl Mech Mater 2015;709:509–12. [Google Scholar]
- 26. Ghosh U, Ning S, Wang Y, Kong YL. Addressing unmet clinical needs with 3D printing technologies. Adv Healthc Mater 2018;7. [DOI] [PubMed] [Google Scholar]
- 27. Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010;5:335–41. [DOI] [PubMed] [Google Scholar]
- 28. Felix HM, Rosenbush KA, Lannen AM, Pooley RA, Siegel JL, Brown BL et al. Creation of an optic nerve sheath diameter ultrasound model for NeuroICU education. Mil Med Res 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gu Q, Zhu H, Li J, Li X, Hao J, Wallace GG et al. Three-dimensional bioprinting speeds up smart regenerative medicine. Natl Sci Rev 2016;3:331–44. [Google Scholar]
- 30. Yu X, Zhang T, Li Y. 3D printing and bioprinting nerve conduits for neural tissue engineering. Polymers 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radulescu D, Dhar S, Young CM, Taylor DW, Trost H-J, Hayes DJ et al. Tissue engineering scaffolds for nerve regeneration manufactured by ink-jet technology. Mater Sci Eng C 2007;27:534–9. [Google Scholar]
- 32. Tse C, Whiteley R, Yu T, Stringer J, MacNeil S, Haycock JW et al. Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication 2016;8. [DOI] [PubMed] [Google Scholar]
- 33. Cui H, Nowicki M, Fisher JP, Zhang LG. 3D bioprinting for organ regeneration. Adv Healthc Mater 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang R, Wang Y, Yao B, Hu T, Li Z, Liu Y et al. Redirecting differentiation of mammary progenitor cells by 3D bioprinted sweat gland microenvironment. Burns & Trauma 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL et al. 3D printed anatomical nerve regeneration pathways. Adv Funct Mater 2015;25:6205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jakus AE, Secor EB, Rutz AL, Jordan SW, Hersam MC, Shah RN. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015;9:4636–48. [DOI] [PubMed] [Google Scholar]
- 37. Wu Z, Li Q, Xie S, Shan X, Cai Z. In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatin-sodium alginate/rat Schwann-cell scaffold. Korean J Couns Psychother 2020;109:110530–0. [DOI] [PubMed] [Google Scholar]
- 38. Guo G, Zhu Y, Wu Z, Ji H, Lu X, Zhou Y et al. Circulating monocytes accelerate acute liver failure by IL-6 secretion in monkey. J Cell Mol Med 2018;22:4056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soltan N, Ning L, Mohabatpour F, Papagerakis P, Printability CX. Cell viability in bioprinting alginate Dialdehyde-Gelatin scaffolds. ACS Biomater Sci Eng 2019;5:2976–87. [DOI] [PubMed] [Google Scholar]
- 40. Ligon SC, Liska R, Stampfl J, Gurr M, Muelhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev 2017;117:10212–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pateman CJ, Harding AJ, Glen A, Taylor CS, Christmas CR, Robinson PP et al. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 2015;49:77–89. [DOI] [PubMed] [Google Scholar]
- 42. Singh A, Asikainen S, Teotia AK, Shiekh PA, Huotilainen E, Qayoom I et al. Biomimetic Photocurable three-dimensional printed nerve guidance channels with aligned Cryomatrix lumen for peripheral nerve regeneration. ACS Appl Mater Interfaces 2018;10:43327–42. [DOI] [PubMed] [Google Scholar]
- 43. Lee SJ, Esworthy T, Stake S, Miao S, Zuo YY, Harris BT et al. Advances in 3D bioprinting for neural tissue engineering. Advanced Biosystems 2018;2. [Google Scholar]
- 44. Zhu W, Tringale KR, Woller SA, You S, Johnson S, Shen H et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater Today 2018;21:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tao J, Zhang J, Du T, Xu X, Deng X, Chen S et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater 2019;90:49–59. [DOI] [PubMed] [Google Scholar]
- 46. Hu Y, Wu Y, Gou Z, Tao J, Zhang J, Liu Q et al. 3D-engineering of Cellularized conduits for peripheral nerve regeneration. Sci Rep 2016;6:32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tao J, Hu Y, Wang S, Zhang J, Liu X, Gou Z et al. A 3D-engineered porous conduit for peripheral nerve repair. Sci Rep 2017;7:46038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueller M, Becher J, Schnabelrauch M, Zenobi-Wong M. Printing Thermoresponsive reverse Molds for the creation of patterned two-component hydrogels for 3D cell culture. Jove-Journal of Visualized Experiments 2013;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Naghieh S, Sarker MD, Abelseth E, Chen X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J Mech Behav Biomed Mater 2019;93:183–93. [DOI] [PubMed] [Google Scholar]
- 50. Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB et al. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006;27:3580–8. [DOI] [PubMed] [Google Scholar]
- 51. Zhang AP, Qu X, Soman P, Hribar KC, Lee JW, Chen S et al. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv Mater 2012;24:4266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Evangelista MS, Perez M, Salibian AA, Hassan JM, Darcy S, Paydar KZ et al. Single-lumen and multi-lumen poly(ethylene glycol) nerve conduits fabricated by Stereolithography for peripheral nerve regeneration in vivo. J Reconstr Microsurg 2015;31:327–35. [DOI] [PubMed] [Google Scholar]
- 53. Lee S-J, Nowicki M, Harris B, Zhang LG. Fabrication of a highly aligned neural scaffold via a table top Stereolithography 3D printing and electrospinning. Tissue Eng Part A 2017;23: 491-+. [DOI] [PubMed] [Google Scholar]
- 54. Singh D, Harding AJ, Albadawi E, Boissonade FM, Haycock JW, Claeyssens F. Additive manufactured biodegradable poly (glycerol sebacate methacrylate) nerve guidance conduits. Acta Biomater 2018;78:48–63. [DOI] [PubMed] [Google Scholar]
- 55. Gong H, Fei H, Xu Q, Gou M, Chen HH. 3D-engineered GelMA conduit filled with ECM promotes regeneration of peripheral nerve. J Biomed Mater Res A 2020;108:805–13. [DOI] [PubMed] [Google Scholar]
- 56. Tao J, Liu H, Wu W, Zhang J, Liu S, Zhang J et al. 3D-printed nerve conduits with live platelets for effective peripheral nerve repair. Adv Funct Mater 2020. [Google Scholar]
- 57. Wang J, Xiong H, Zhu T, Liu Y, Pan H, Fan C et al. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano 2020. [DOI] [PubMed] [Google Scholar]
- 58. Sun X, Bai Y, Zhai H, Liu S, Zhang C, Xu Y et al. Devising micro/nano-architectures in multi-channel nerve conduits towards a pro-regenerative matrix for the repair of spinal cord injury. Acta Biomater 2019;86:194–206. [DOI] [PubMed] [Google Scholar]
- 59. Diez-Ahedo R, Mendibil X, Carmen Marquez-Posadas M, Quintana I, Gonzalez F, Javier Rodriguez F et al. UV-casting on Methacrylated PCL for the production of a peripheral nerve implant containing an Array of porous aligned microchannels. Polymers 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qian Y, Song J, Zheng W, Zhao X, Ouyang Y, Yuan W et al. 3D manufacture of gold nanocomposite channels facilitates neural differentiation and regeneration. Adv Funct Mater 2018;28. [Google Scholar]
- 61. Yao L, de Ruiter GC, Wang H, Knight AM, Spinner RJ, Yaszemski MJ et al. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 2010;31:5789–97. [DOI] [PubMed] [Google Scholar]
- 62. Arcaute K, Mann BK, Wicker RB. Fabrication of off-the-shelf multilumen poly(ethylene glycol) nerve guidance conduits using Stereolithography. Tissue Engineering Part C-Methods 2011;17:27–38. [DOI] [PubMed] [Google Scholar]
- 63. He J, Sun C, Gu Z, Yang Y, Gu M, Xue C et al. Morphology, migration, and transcriptome analysis of Schwann cell culture on butterfly wings with different surface architectures. ACS Nano 2018;12:9660–8. [DOI] [PubMed] [Google Scholar]
- 64. Jiang T, Zhao J, Yu S, Mao Z, Gao C, Zhu Y et al. Untangling the response of bone tumor cells and bone forming cells to matrix stiffness and adhesion ligand density by means of hydrogels. Biomaterials 2019;188:130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang D, Xu S, Wu S, Gao C. Micropatterned poly (d,l-lactide-co-caprolactone) films entrapped with gelatin for promoting the alignment and directional migration of Schwann cells. J Mater Chem B 2018;6:1226–37. [DOI] [PubMed] [Google Scholar]
- 66. Zhang D, Wu S, Feng J, Duan Y, Xing D, Gao C. Micropatterned biodegradable polyesters clicked with CQAASIKVAV promote cell alignment, directional migration, and neurite outgrowth. Acta Biomater 2018;74:143–55. [DOI] [PubMed] [Google Scholar]
- 67. Zhang DM, Suo HR, Qian J, Yin J, Fu JZ, Huang Y. Physical understanding of axonal growth patterns on grooved substrates: groove ridge crossing versus longitudinal alignment. Bio-Design and Manufacturing 2020;3:348–60. [Google Scholar]
- 68. Behbehani M, Glen A, Taylor CS, Schuhmacher A, Claeyssens F, Haycock JW. Pre-clinical evaluation of advanced nerve guide conduits using a novel 3D in vitro testing model. International Journal of Bioprinting 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang ZH, Wu YB, Xiang Y, Kruth MB, Wei P, Dai GL et al. Efficacy of large groove texture on rat sciatic nerve RegenerationIn VivoUsing Polyacrylonitrile nerve conduits. Ann Biomed Eng 2020. [DOI] [PubMed] [Google Scholar]
- 70. Huang C, Ouyang Y, Niu H, He N, Ke Q, Jin X et al. Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal Nanogrooves on a Fiber surface. ACS Appl Mater Interfaces 2015;7:7189–96. [DOI] [PubMed] [Google Scholar]
- 71. Zhang D, Yao Y, Duan Y, Yu X, Shi H, Nakkala JR et al. Surface-anchored graphene oxide Nanosheets on cell-scale micropatterned poly (d,l-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl Mater Interfaces 2020;12:7915–30. [DOI] [PubMed] [Google Scholar]
- 72. Jiang L-B, Su D-H, Ding S-L, Zhang Q-C, Li Z-F, Chen F-C et al. Salt-assisted toughening of protein hydrogel with controlled degradation for bone regeneration. Adv Funct Mater 2019;29. [Google Scholar]
- 73. Vijayavenkataraman S, Kannan S, Cao T, Fuh JYH, Sriram G, Lu WF. 3D-printed PCL/PPy conductive scaffolds as three-dimensional porous nerve guide conduits (NGCs) for peripheral nerve injury repair. Front Bioeng Biotechnol 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sun B, Zhou Z, Li D, Wu T, Zheng H, Liu J et al. Polypyrrole-coated poly (l-lactic acid-co-epsilon-caprolactone)/silk fibroin nanofibrous nerve guidance conduit induced nerve regeneration in rat. Materials Science & Engineering C-Materials for Biological Applications 2019;94:190–9. [DOI] [PubMed] [Google Scholar]
- 75. Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods. Acta Biomater 2020;106:54–69. [DOI] [PubMed] [Google Scholar]
- 76. Ning L, Zhu N, Mohabatpour F, Sarker MD, Schreyer DJ, Chen X. Bioprinting Schwann cell-laden scaffolds from low-viscosity hydrogel compositions. J Mater Chem B 2019;7:4538–51. [Google Scholar]
- 77. Sun W, Zhang Y, Gregory DA, Jimenez-Franco A, Tomeh MA, Lv S et al. Patterning the neuronal cells via inkjet printing of self-assembled peptides on silk scaffolds. Progress in Natural Science-Materials International 2020;30:686–96. [Google Scholar]
- 78. Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 2009;10:21–33. [DOI] [PubMed] [Google Scholar]
- 79. Lv HW, Wang HP, Zhang ZJ, Yang W, Liu WB, Li YL et al. Biomaterial stiffness determines stem cell fate. Life Sci 2017;178:42–8. [DOI] [PubMed] [Google Scholar]
- 80. Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M et al. Role of YAP/TAZ in mechanotransduction. Nature 2011;474:179–U212. [DOI] [PubMed] [Google Scholar]
- 81. Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 2016;15: 326-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cai L, Zhang L, Dong JY, Wang SF. Photocured biodegradable polymer substrates of varying stiffness and microgroove dimensions for promoting nerve cell guidance and differentiation. Langmuir 2012;28:12557–68. [DOI] [PubMed] [Google Scholar]
- 83. Wang SF, Kempen DHR, de Ruiter GCW, Cai L, Spinner RJ, Windebank AJ et al. Molecularly engineered biodegradable polymer networks with a wide range of stiffness for bone and peripheral nerve regeneration. Adv Funct Mater 2015;25:2715–24. [Google Scholar]
- 84. Wu YB, Xiang Y, Fang JH, Li XK, Lin ZW, Dai GL et al. The influence of the stiffness of GelMA substrate on the outgrowth of PC12 cells. Biosci Rep 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gu Y, Ji Y, Zhao Y, Liu Y, Ding F, Gu X et al. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012;33:6672–81. [DOI] [PubMed] [Google Scholar]
- 86. Kayal C, Moeendarbary E, Shipley RJ, Phillips JB. Mechanical response of neural cells to physiologically relevant stiffness gradients. Adv Healthc Mater 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lavrentieva A, Fleischhammer T, Enders A, Pirmahboub H, Bahnemann J, Pepelanova I. Fabrication of stiffness gradients of GelMA hydrogels using a 3D printed micromixer. Macromol Biosci 2020;20:e2000107. [DOI] [PubMed] [Google Scholar]
- 88. Vijayavenkataraman S, Zhang S, Thaharah S, Sriram G, Lu WF, Fuh JYH. Electrohydrodynamic jet 3D printed nerve guide conduits (NGCs) for peripheral nerve injury repair. Polymers 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vijayavenkataraman S, Thaharah S, Zhang S, Lu WF, Fuh JYH. 3D-printed PCL/rGO conductive scaffolds for peripheral nerve injury repair. Artif Organs 2019;43:515–23. [DOI] [PubMed] [Google Scholar]
- 90. Vijayavenkataraman S, Thaharah S, Zhang S, Lu WF, Fuh JYH. Electrohydrodynamic jet 3D-printed PCL/PAA conductive scaffolds with tunable biodegradability as nerve guide conduits (NGCs) for peripheral nerve injury repair. Materials & Design 2019;162:171–84. [Google Scholar]
- 91. Qian Y, Yuan W-E, Cheng Y, Yang Y, Qu X, Fan C. Concentrically integrative bioassembly of a three-dimensional black phosphorus Nanoscaffold for restoring neurogenesis. Angiogenesis, and Immune Homeostasis Nano Letters 2019;19:8990–9001. [DOI] [PubMed] [Google Scholar]
- 92. Qian Y, Zhao X, Han Q, Chen W, Li H, Yuan W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat Commun 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pampaloni NP, Lottner M, Giugliano M, Matruglio A, D'Amico F, Prato M et al. Single-layer graphene modulates neuronal communication and augments membrane ion currents. Nat Nanotechnol 2018;13: 755-+. [DOI] [PubMed] [Google Scholar]
- 94. Park J, Jeon J, Kim B, Lee MS, Park S, Lim J et al. Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration. Adv Funct Mater 2020;30. [Google Scholar]
- 95. Qian Y, Cheng Y, Song J, Xu Y, Yuan W-E, Fan C et al. Mechano-informed biomimetic polymer scaffolds by incorporating self-powered zinc oxide Nanogenerators enhance motor recovery and neural function. Small 2020;16. [DOI] [PubMed] [Google Scholar]
- 96. Cheng Y, Xu Y, Qian Y, Chen X, Ouyang Y, Yuan W-E. 3D structured self-powered PVDF/PCL scaffolds for peripheral nerve regeneration. Nano Energy 2020;69. [Google Scholar]
- 97. Fei J, Wen X, Lin X, Saijilafu WW, Ren O et al. Biocompatibility and neurotoxicity of magnesium alloys potentially used for neural repairs. Materials Science & Engineering C-Materials for Biological Applications 2017;78:1155–63. [DOI] [PubMed] [Google Scholar]
- 98. Wang ZZ, Sakiyama-Elbert SE. Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp Neurol 2019;319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cerqueira SR, Lee YS, Cornelison RC, Mertz MW, Wachs RA, Schmidt CE et al. Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials 2018;177:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Das S, Sharma M, Saharia D, Sarma KK, Sarma MG, Borthakur BB et al. In vivo studies of silk based gold nano-composite conduits for functional peripheral nerve regeneration. Biomaterials 2015;62:66–75. [DOI] [PubMed] [Google Scholar]
- 101. Zhu S, Ge J, Wang Y, Qi F, Ma T, Wang M et al. A synthetic oxygen carrier-olfactory ensheathing cell composition system for the promotion of sciatic nerve regeneration. Biomaterials 2014;35:1450–61. [DOI] [PubMed] [Google Scholar]
- 102. Yi S, Zhang Y, Gu X, Huang L, Zhang K, Qian T et al. Application of stem cells in peripheral nerve regeneration. Burns & Trauma 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang W, Zhang L, Liu J, Zhang L, Zhang J, Tang P. Repairing sciatic nerve injury with an EPO-loaded nerve conduit and sandwiched-in strategy of transplanting mesenchymal stem cells. Biomaterials 2017;142:90–100. [DOI] [PubMed] [Google Scholar]
- 104. Du J, Zhen G, Chen H, Zhang S, Qing L, Yang X et al. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials 2018;181:347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hu N, Wu H, Xue C, Gong Y, Wu J, Xiao Z et al. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials 2013;34:100–11. [DOI] [PubMed] [Google Scholar]
- 106. Marchesi C, Pluderi M, Colleoni F, Belicchi M, Meregalli M, Farini A et al. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia 2007;55:425–38. [DOI] [PubMed] [Google Scholar]
- 107. Pan HC, Cheng FC, Chen CJ, Lai SZ, Lee CW, Yang DY et al. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J Clin Neurosci 2007;14:1089–98. [DOI] [PubMed] [Google Scholar]
- 108. Hu Z, An K, Wang J, Xu X, Chen Z, Hu J et al. Preparation and drug release behavior of tussah silk fibroin composite membrane. Fibers and Polymers 2020;21:252–61. [Google Scholar]
- 109. Chen R, Zhou L, Yang H, Zheng H, Zhou Y, Hu Z et al. Degradation behavior and immunological detection of silk fibroin exposure to enzymes. Anal Sci 2019;35:1243–9. [DOI] [PubMed] [Google Scholar]
- 110. Kokai LE, Ghaznavi AM, Marra KG. Incorporation of double-walled microspheres into polymer nerve guides for the sustained delivery of glial cell line-derived neurotrophic factor. Biomaterials 2010;31:2313–22. [DOI] [PubMed] [Google Scholar]
- 111. Nagappan PG, Chen H, Wang D-Y. Neuroregeneration and plasticity: a review of the physiological mechanisms for achieving functional recovery postinjury. Mil Med Res 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cui Y, Lu C, Meng D, Xiao Z, Hou X, Ding W et al. Collagen scaffolds modified with CNTF and bFGF promote facial nerve regeneration in minipigs. Biomaterials 2014;35:7819–27. [DOI] [PubMed] [Google Scholar]
- 113. Tang S, Zhu J, Xu Y, Xiang AP, Jiang MH, Quan D. The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials 2013;34:7086–96. [DOI] [PubMed] [Google Scholar]
- 114. Fadia NB, Bliley JM, DiBernardo GA, Crammond DJ, Schilling BK, Sivak WN et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci Transl Med 2020;12. [DOI] [PubMed] [Google Scholar]
- 115. Bota O, Fodor L. The influence of drugs on peripheral nerve regeneration. Drug Metab Rev 2019;51:266–92. [DOI] [PubMed] [Google Scholar]
- 116. Chang HM, Liu CH, Hsu WM, Chen LY, Wang HP, Wu TH et al. Proliferative effects of melatonin on Schwann cells: implication for nerve regeneration following peripheral nerve injury. J Pineal Res 2014;56:322–32. [DOI] [PubMed] [Google Scholar]
- 117. Qian Y, Han Q, Zhao X, Song J, Cheng Y, Fang Z et al. 3D melatonin nerve scaffold reduces oxidative stress and inflammation and increases autophagy in peripheral nerve regeneration. J Pineal Res 2018;65:e12516. [DOI] [PubMed] [Google Scholar]
- 118. Xu X, Tao J, Wang S, Yang L, Zhang J, Zhang J et al. 3D printing of nerve conduits with nanoparticle-encapsulated RGFP966. Appl Mater Today 2019;16:247–56. [Google Scholar]
- 119. Pace LA, Plate JF, Smith TL, Van Dyke ME. The effect of human hair keratin hydrogel on early cellular response to sciatic nerve injury in a rat model. Biomaterials 2013;34:5907–14. [DOI] [PubMed] [Google Scholar]
- 120. Masand SN, Chen J, Perron IJ, Hammerling BC, Loers G, Schachner M et al. The effect of glycomimetic functionalized collagen on peripheral nerve repair. Biomaterials 2012;33:8353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang JM, Chen YW, Huang YL, Wu WB, Deng XM, Liu HF et al. A 3D-printed self-adhesive bandage with drug release for peripheral nerve repair. Adv Sci 2020; n/a(n/a):2002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yan Z, Qian Y. Rectify the injury-induced microenvironment imbalance in peripheral nerve repair. Adv Mater Let 2020;11:20101562. [Google Scholar]
- 123. Joung D, Lavoie NS, Guo S-Z, Park SH, Parr AM, McAlpine MC. 3D printed neural regeneration devices. Adv Funct Mater 2020;30. [DOI] [PMC free article] [PubMed] [Google Scholar]


