Abstract
Oxidative stress is an important factor in the etiology of several chronic diseases that include cardiovascular disease (CVD), Type 2 diabetes (T2D), and rheumatoid arthritis (RA). Oxidative stress can lead to inflammation, and this can contribute to these chronic diseases. Reducing inflammation and oxidative stress may, therefore, be useful in the prevention and treatment of these conditions. One of the treatment options for chronic diseases is the use of traditional medicine and herbs, such as Nigella sativa. This is one of the herbs that have recently been assessed for its ability to reduce inflammation and oxidative stress. We have reviewed the reported effects of N. sativa on risk factors of chronic diseases (CVD, DM, and RA) with emphasis on molecular and cellular mechanisms in controlling inflammation and oxidative stress. Various mechanisms have been proposed to contribute to the beneficial properties of N. sativa, including a reduction of lipid peroxidation via its antioxidant properties; agonist of peroxisome proliferator-activated receptor gamma in adipose tissue; activation of AMP-activated protein kinase, increased antioxidants, inhibition of nuclear factor-kappa B pathway; increased in interleukin-10 expression, CD4+ T-cell percentage, T regulatory cell percentage (CD4+ CD25+ T-cell) in peripheral blood, and CD4+/CD8+ ratio, but to prove this claim, it is necessary to conduct experimental and well-designed clinical trial studies with a larger sample size on the effects of N. sativa on these chronic diseases.
Keywords: Chronic disease, inflammation, Nigella sativa, oxidative stress
INTRODUCTION
Chronic diseases may be defined as a long-term illness with long treatment period that cannot currently be prevented by vaccines, or fully cured by medication and also causes physical changes in the body and reduces its performance.[1] According to the Centers for Disease Control (CDC) definition, cardiovascular disease (CVD), Type 2 diabetes (T2D), and rheumatoid arthritis (RA) are common chronic diseases globally.[1,2] CVD is associated with dysfunction of the heart and blood vessels and comprises disorders such as coronary heart disease and hypertension.[3] It has been estimated that the prevalence of hypertension and CVD reaches about 30% of the world's population by the year 2025.[4,5,6] T2D is the most common type of diabetes, accounting for 90% of all diabetes cases.[7] The number of people with T2D in the world was estimated to be approximately 125 million in 1997 and is expected to reach 300 million by 2025, and on average, the prevalence among adults is 8.5%.[8,9] RA is an autoimmune chronic disease that affects the small joints of the human body and affects approximately 1% of the population and is usually associated with severe disability.[10] Based on the National Health Council (NHC) reports, the burden of the cost of chronic diseases annually in the United States is about $ 1.3 trillion for seven common chronic diseases and now about 78% of total health care spending in the United States is spent on chronic diseases, and this trend is increasing.[11] It has been widely accepted that persistent oxidative stress leads to chronic inflammation and this inflammation can be effective in many chronic diseases such as CVD, T2D, and RA;[12] therefore, it is possible that reducing the level of inflammation and oxidative stress may ameliorate these diseases. One of the treatment options for chronic diseases is the use of traditional medicine and herbs, and it has been shown to be used in both prevention and treatment.[13] In general, the consumption of medicinal plants is increasing in different countries due to the evidence of the positive effects as well as the low side effects and availability of these plants.[14,15,16] Nigella sativa is one of the herbs that have recently been used to reduce inflammation and oxidative stress in various chronic diseases.[17]
N. sativa is also known as black seed, black cumin, habbatus sauda, and black caraway seed. It is cultivated in countries such as India, Pakistan, and some parts of Iran. The plant is a local herb in southern Europe, North Africa, and Asia.[18,19,20,21] N. sativa, as a 1-year-old plant, has flowers whose stem is covered with very delicate fluff and grows to a height of 20–30 cm; this plant's flowers are solitary and pale blue or white with 5 petals, the fruits are shaped like a large capsule containing 3–7 follicles, and the seeds in each follicle are black in color and have a bitter, spicy flavor[22] [Figure 1].
Figure 1.

Nigella sativa plant: Fruits, leaf, and flowers
N. sativa has been reported to contain more than 100 chemical compounds and has many reported therapeutic effects due to these compounds. N. sativa oil contains average, 35.6%–41.6% fat (constant oil), 22.7% protein, 32% carbohydrate, and 0.5%–1.6% essential fatty acid. The major fatty acid in this plant is linoleic acid (about 57.3% of fixed oil).[23,24] It also contains other compounds such as crude fiber, reducing sugars, mucilage, resin, alkaloids, sterols, tannins, flavonoids, saponins, minerals (such as Fe, zinc, Na, phosphorus, and calcium), and vitamins (C, B1, B3, B6, and B9 as water-soluble vitamins and A and E as a couple of fat-soluble vitamins).[24,25] The biological activity of N. sativa is attributed to the following proportions of forming components: volatile oils, such as thymoquinone (TQ) (30%–48%), P-cymene (7%–15%), carvacrol (6%–12%), 4-terpineol (2%–7%), trans-anethole (1%–4%), and sesquiterpene longifolene (1%–8%). A combination of four monoterpenes (d-limonene (carvone), carvone, pinene-α, and p-cymene), TQ, and its derivatives are the most pharmacologically active compounds.[24,26,27] TQ is not only a type of inhibitor for chemically reactive species containing oxygen such as superoxide and hydroxyl radicals but also inhibits leukotriene C4 and B4 production in humans and has anti-inflammatory effects.[27,28]
Recent studies have shown that N. sativa can be helpful and effective in the prevention and treatment of some chronic diseases, such as asthma, liver and kidney disease, influenza, and gastrointestinal problems.[21,29] Previous studies reported that N. sativa was used as antihypertensive, liver tonics, diuretics, antidiarrheal, appetite stimulant, analgesics, digestive, antibacterial, and in skin disorders,[30] and also, its effects on neurological and mental illness, cardiovascular disorders, cancer, diabetes, and inflammatory conditions have been shown.[31] Effects of an aqueous extract from N. sativa on glucose tolerance and body weight in rats were studied by Meddan et al.[32] In a similar study, N. sativa was reported to reduce the risk of CVD in diabetic patients.[33] Some studies have also shown the anti-inflammatory and analgesic effects of N. sativa in RA.[34]
Despite the effects of N. sativa on some chronic disease risk factors in previous studies, the exact mechanism of these effects has not been identified and the results of some studies have been inconsistent. Therefore, this study aims to review the possible molecular and cellular effects of N. sativa on inflammation, oxidative stress, and other related risk factors of chronic diseases (CVD, T2D, and RA).
SEARCH STRATEGY
PubMed-Medline, Google Scholar, Scopus, and Web of Science databases were searched to identify the relevant articles. The keywords including “nigella sativa,” OR “black seed,” OR “black cumin,” OR “habbatus sauda,” OR “black–caraway seed” in combination with “diabetes,” “insulin resistance,” “glucose,” “hemoglobin A1C,” “dyslipidemia,” “lipid profile,” “blood pressure,” “cardiovascular disease,” “heart disease,” “rheumatoid arthritis,” “atherosclerosis,” “oxidative stress,” “anti-oxidant,” “inflammation,” and “inflammatory markers” were used for an electronic search strategy. All the identified studies and review articles were reviewed. Subsequently, the eligible experimental and clinical trials were selected. These are papers reporting original studies on the mechanisms of action of N. sativa and almost all available animal and human studies. However, we mentioned that this study is a narrative review and not in a systematic framework.
EFFECTS OF NIGELLA SATIVA IN CARDIOVASCULAR RISK FACTORS
Laboratory animal studies
Three doses of hydroalcoholic extract of N. sativa (200, 400, and 600 mg/kg) were injected intraperitoneally in hypertensive rats showed a protective effect on blood pressure (BP) after 30 min of injection that was induced by TQ and its effects on angiotensin II.[35] In another study, improvements of cardiovascular risk factors including body weight, red blood cell and white blood cell, lipid profile (triglyceride [TG], low-density lipoprotein [LDL], and high-density lipoprotein [HDL]) and immune response (IgM and IgG), total antioxidant capacity, and malondialdehyde (MDA) after 8-week supplementation with N. sativa seed at doses of 300 and 600 g/kg in rabbits were proved.[36] This effect was dose dependent. The cardioprotective effects of N. sativa are summarized in Table 1.
Table 1.
Effects of Nigella sativa on cardiovascular, diabetes, and rheumatoid arthritis risk factors in experimental studies
| Author (year) | Country (reference number) | n | Type and dose of N.S administered | Duration | Outcome measures |
|---|---|---|---|---|---|
| Enayatfard et al. (2019)[35] | Iran | 21 hypertensive rats | 200, 400, and 600 mg/kg N.S | Once injection | Dose-dependent reduction in systolic blood pressure, mean arterial pressure, and heart rate |
| El-Gindy et al. (2019)[36] | Egypt | 54 growing V-line unsexed rabbits | 300 or 600 mg of N.S seed/kg | 8 weeks | Significantly improved body weight, ↑RBCs and WBCs ↑IgG and IgM immune responses, ↓ serum total lipids, TG, LDL, and MDA, and ↑HDL and total antioxidant capacity. |
| Ahmed and Hassanein (2013)[94] | Egypt | 45 Albino rats | N.S oil (4 ml/kg) orally | Once administration | ↓heart rate, ST-segment change, pro-inflammatory cytokines, oxidative stress, and cardiac tissue damage |
| Randhawa (2013)[95] | India | 16 Wistar albino rats | 0.2 ml/kg/day, intraperitoneally | 6 weeks | ↓blood pressure, oxidative injury, improved left ventricular function |
| Babaei Bonab and Tofighi (2019)[96] | Iran | 35 male Wistar rats with T2M | 400mg/kg/day | 8 weeks | Improvement in lipid profile (LDL, HDL, TC, and TG), FBG, HbA1c, and insulin resistance |
| Ahmad and Alkreathy (2018)[97] | Saudi Arabia | 48 male Albino Wistar rats | 2 ml/kg, p.o | 7 days | Improvement in lipid profile (TC, LDL, HDL, and TG) |
| Muneera et al. (2015)[98] | Pakistan | 30 Sprague Dawley rats | 1000mg/kg/day | 6 weeks | Improvement in the lipid profile of rats |
| Al-Hader et al. (1993)[99] | Jordan | Diabetic rabbits | 50 mg/kg volatile oil extract of N.S | 2, 4, and 6 h | Showed significant decreases in FBG levels |
| Meral et al. (2001)[44] | Turkey | 15 New Zealand male rabbits | 20 ml/kg aqueous extract of N.S | 2 months | ↑GSH and ceruloplasmin concentrations ↓MDA and glucose levels |
| El-Dakhakhny et al. (2002)[54] | Egypt | Diabetic rats | 0.4g/kg N.S oil | 2, 4, and 6 weeks | ↓blood glucose concentration |
| Kanter et al. (2003)[59] | Turkey | 46 male Wistar rats | 0.2 ml/kg/day volatile oil of N.S | 4 weeks | ↓GSH, glucose level, and serum NO ↑Insulin level |
| Fararh et al. (2004)[74] | Japan | Male Syrian hamsters | 400 mg/kg body weight/day of N.S oil | 4 weeks | ↓total glycated hemoglobin |
| Rchid et al. (2004)[61] | - | Rat pancreatic cells | 0.01, 0.1, 1, and 5 mg/ml whole, basic, and acidic subfractions of N.S | 30 min | A significant stimulatory effect on insulin release has been observed |
| Mansi et al. (2005)[55] | Jordan | Diabetic rats | 20 ml/kg/day aqueous extract of N.S | 15 days | ↑Insulin level and ↓Glucose level |
| Mansi (2006)[100] | Jordan | Diabetic rats | 20 ml/kg aqueous extract of N.S | 3 weeks | ↑Insulin level |
| Kaleem et al. (2006)[101] | India | Wister rats | 300 mg/kg/day ethanol extract of N.S | 4 weeks | ↑Catalase, SOD and insulin levels ↓Lipid peroxidation, GPX and glutathione ↓Body weight |
| Houcher et al. (2007)[56] | Algeria | Diabetic rats | 810 mg/kg/day 2.5 ml/kg/day methanol extract of N.S and N.S oil | 25 days | ↓Glucose level ↑TAC |
| Kanter (2008)[58] | Turkey | diabetic rats | N.S in a dose of 400 mg/kg body weight and TQ 50 mg/kg body weight once a day | 12 weeks | ↓Serum glucose |
| AL-Logmani (2009)[102] | Saudi Arabia | 40 diabetic male Wistar rats | N.S oil | 3 weeks | ↓Blood glucose |
| Meddah et al. (2009)[59] | Morocco | Rat jejunum |
In vitro: 0.1 pg/ml to 100 ng/ml In vivo: 2g/kg |
6 weeks |
In vivo: glucose tolerance and body weight improvement In vitro: inhibition of glucose absorption |
| Benhaddou-Andaloussi et al. (2010)[103] | Morocco | C2C12 skeletal muscle cells and 3T3-L1 adiposities | Ethanol extract of N.S | 18 h | ↑Glucose uptake in skeletal cells and adiposities |
| Fararh et al. (2010)[53] | Egypt | Diabetic rats | 50 mg/kg/day TQ | 20 days | ↓ Plasma glucose, TC, TG ↑ Insulin concentration |
| Abdelmeguid et al. (2010)[62] | Egypt | Diabetic rats | 2 mL⁄kg, i.p., 5% N. S Extract 0.2 mL⁄kg, i.p, N.S oil, or 3 mg⁄mL, i.p., TQ |
30 days | ↓ glucose and improve serum insulin levels |
| Salama (2011)[52] | Saudi Arabia | Diabetic rats | 500 mg/kg N.S oil | 4 weeks | ↓Glucose concentration ↑insulin, c-peptide, and TAC |
| Al-Logmani and Zari (2011)[104] | Saudi Arabia | Diabetic Wistar rats | Diet containing 5% N.S oil | 7 weeks |
N. sativa oil decreased blood glucose levels |
| Alimohammadi et al. (2013)[105] | Iran | Diabetic rats | 5, 10, and 20 mg/kg hydroalcoholic N.S extract | 32 days | 5 mg/kg: ↓FBS ↑Insulin secretion |
| Mohamed et al. (2015)[51] | Saudi Arabia | Nonalcoholic fatty liver in obese diabetic albino rats | 100mg/kg aqueous extract of N.S seed | 4 weeks | ↓FBS ↓TG, TC |
| Asaduzzaman et al. (2015)[106] | Bangladesh | Diabetic rats | 300 mg/kg body weight of ethanol extract of N.S | 28 days | ↓TG, TC, LDL, and FBG ↑HDL |
| Al-Trad et al. (2016)[107] | Jordan | Experimental diabetic rats | 50 mg/kg TQ and 2 mL/kg N.S oil | 10 weeks | N.S oil or TQ significantly reduced blood glucose level compared with that in untreated diabetic rats |
| Umar et al. (2012)[75] | India | Three groups of six Wistar albino rats each with collagen-induced arthritis | 5 mg/ kg TQ PO | 21 days | ↓ IL-1β, IL-6, TNF-α, IFN- γ, PGE2, articular elastase, myeloperoxidase, lipoxygenase, and NO ↑IL-10, SOD, GPX, and catalase |
| Vaillancourt et al. (2011)[76] | Canada | 24 female Lewis rats with lipopolysaccharide (LPS)-induced arthritis | 5 mg/ kg TQ PO | 7 days | ↓ LPS-induced IL-1β, TNF-α, MMP-13, Cox-2, and PGE2 |
| Tekeoglu et al. (2006)[108] | Turkey | Five groups of seven Wistar albino rats each with collagen-induced arthritis | 2.5 mg/kg and 5 mg/kg TQ PO | 7 days | ↓ TNF-α and IL-1β |
| Mohamed et al. (2003)[109] | Canada | 24 mice with inducing inflammation | 1 mg/kg TQ iv | 5 days and 12 days | Mice received TQ at day 12: Higher levels of GSH Significant reduction of symptoms of inflammation |
| Faisal et al. (2015)[110] | Pakistan | 32 rats with collagen-induced arthritis | 2mg/kg/day TQ by i.p injection | 15 days | ↓ in clinical score of inflammation and differentiation leucocyte counts |
| Zhong (2017) | China | 60 rabbit osteoarthritis model | 1mg/kg/day TQ by intra-articular injection | 9 weeks | ↓ in MMP-13 mRNA and cartilage lesions |
| Chen et al. (2010)[111] | China | 20 rabbit osteoarthritis model | 10 µmol/L TQ intra-articular injection |
4 weeks | Inhibited NF-kB p65 ↓ IL-1β Suppressed the MMP-1, MMP-3, and MMP-13 gene expression |
N.S=Nigella sativa; TQ=thymoquinone; i.p=intraperitoneal; RBC=Red blood cells; WBC=White blood cells; TG=Triglyceride; TC=Total cholesterol; LDL=Low-density lipoprotein; HDL=High-density lipoprotein; MDA=Malondialdehyde; IgG=Immunoglobulin G; IgM=Immunoglobulin M; FBG=Fasting blood glucose; HbA1C=Glycated hemoglobin; IL-1β=Interleukin-1 beta; IL-6=Interleukin-6; TNF-α=Tumor necrosis factor-alpha; IFN- γ=Interferon-gamma; PGE2=Prostaglandin E2; GSH=Glutathione; NO=Nitric oxide, GPX=Glutathione peroxidase; TAC=Total antioxidant capacity; IL-10=Interleukin-10; LPS=Lipopolysaccharide; MMP=Matrix metalloproteinase; Cox-2=Cyclooxygenase 2; NFkB= Nuclear factor-κB
Clinical studies
Clinical trials in human subjects showed similar results. Sabzghabaee et al.[37] have reported that 2 g/d N. sativa for 4 weeks in hyperlipidemic patients significantly reduced total cholesterol (TC), TG, and LDL compared to control group [Table 2]. Furthermore, 500 mg/d N. sativa in metabolic syndrome patients decreased systolic and diastolic BP and LDL-cholesterol (LDL-C) compared to control group.[38]
Table 2.
Effects of Nigella sativa on cardiovascular, diabetes, and rheumatoid arthritis risk factors in clinical trial studies
| Cardiovascular risk factors | ||||||
|---|---|---|---|---|---|---|
| Author (year) | Country (reference number) | Study design (sex) | Participant numbers | Type and dose of N.S administered | Duration (mean age of subjects) | Outcome measures |
| Darand et al. (2019)[112] | Iran | RCT (male/female) | 50 patients with NAFLD | 2 g/day | 12 weeks | ↓serum glucose and serum insulin ↑quantitative insulin sensitivity |
| Sabzghabaee et al. (2012)[37] | Iran | RCT (male/female) | 88 Hyperlipidemia patients | 2 g/d (capsule contained N.S crushed seeds) | 4 weeks | ↓TC, TG, and LDL, no significant difference in HDL, FBG |
| Dehkordi and Kamkhah (2008)[113] | Iran | RCT (male/female) | Patients with mild hypertension | Two test groups received 100 and 200 mg of N.S extract twice a day | 8 weeks | Significant dose-dependent decline in the levels of TC, TG, LDL, systolic and diastolic blood pressure |
| Najimi et al. (2013)[38] | India | Open labeled RCT (male/female) | 90 patients of metabolic syndrome | 500 mg capsule of N.S per day | 8 weeks | Significant improvement with reference to systolic and diastolic blood pressure and LDL-cholesterol, no significant difference in HDL, TG |
| Ibrahim et al. (2014)[114] | Malaysia | RCT (female) | 37 hyperlipidemic menopausal women | 500 mg capsule of N.S per day | 2 months | ↓ TC, LDL and TG, and increased HDL and FBG. No significant difference in diastolic and systolic blood pressure |
| Tasawar et al. (2011)[115] | Pakistan | RCT (male/female) | 80 patients with coronary artery diseases | 500 mg capsule of N.S per day | 6 months | ↓TC, LDL, VLDL, and TG ↑HDL-C |
| Bamosa et al. (1997)[116] | Saudi Arabia | CT (male) | 16 male adolescents | 2 capsules of 500 mg N.S twice daily | 2 weeks | ↓ glucose No significant difference for TG change |
| Farzaneh et al. (2014)[117] | Iran | RCT (female) | 20 sedentary overweight females | 3 capsules of 500 mg N.S daily | 8 weeks | ↓TC, LDL, TG, and body mass index, and ↑HDL-C |
| Najimi et al. (2008)[118] | India | CT (male/female) | 60 patients of metabolic syndrome | N.S oil 2.5 ml twice daily | 6 weeks | ↑TC, LDL, FBG No effect on TG, HDL, postprandial glucose, body weight, BMI, and waist circumference |
| Mahdavi et al. (2015)[119] | Iran | RCT (female) | 90 obese women | 3 g per day (1 g before each meal) N.S oil | 8 weeks | ↓VLD, TG, and BMI No effect on HDL, TC, and LDL |
| Rashidmayvan et al. (2019)[66] | Iran | RCT (male/female) | 44 patients with NAFLD | N.S oil | 8 weeks | ↓FBS, TG, TC, LDL, VLDL, AST and ALT, hs-CRP, IL-6, TNF-α ↑HDL-C N.S oil had no significant effect on serum levels of insulin, blood pressure, and GGT |
| Pelegrin et al. (2019)[69] | France | Pilot RCT (male) | 30 healthy male volunteers | N.S powder (1 g/day) | 4 weeks | ↓TC, LDL |
| Farhangi et al. (2018)[120] | Iran | RCT (male/female) | 40 patients with Hashimoto’s thyroiditis | 2 g N.S powder per day | 8 weeks | ↓ BMI, LDL, and TG ↑ HDL |
| Bhatti et al. (2016)[121] | Pakistan | CT (male/female) | 60 hyperlipidemic smokers | 1 g of N.S (kalonji) seed | 30 days | ↓TC, LDL, and TG, ↑ HDL |
| Diabetes risk factors | ||||||
| Bamosa et al. (2010)[64] | Saudi Arabia | RCT (male/female) | 94 patients with T2D | Capsules containing N.S (1, 2, and 3 g/day) | 12 weeks | ↓ FBS, 2hPG, and HbA1c |
| Kaatabi et al. (2012)[122] | Saudi Arabia | 94 diabetic patients | CT (F) | 1, 2, and 3 g/day of powdered N.S | 12 weeks | 1gr: ↑HDL-C 2gr: ↑ HDL; ↓TC, TG, and LDL-C; 3gr: ↑HDL; ↓ TC, TG, and LDL-C |
| Hosseini et al. (2013)[123] | Iran | RCT (male/female) | 70 patients with T2D | 5 ml/day N.S oil | 12 weeks | ↓FBS, 2hPG, BMI |
| Mirmiran et al. (2015)[68] | Iran | RCT (male/female) | 43 patients with T2D | 1000 mg extract of black seed oil | 8 weeks | ↓FBS, LDL, total cholesterol, and LDL/HDL |
| Heshmati et al. (2015)[124] | Iran | RCT (male/female) | 72 patients with | 3 g/day N.S oil or soft gel capsules | 12 weeks | ↓FBS, HbA1c, ↓LDL-C, and TG |
| Farhangi et al. (2018)[120] | Iran | RCT male/female) |
40 patients with Hashimoto’s thyroiditis | 2 g N.S powder per day | 8 weeks | ↓BMI ↑HDL ↓Serum concentrations of LDL and tri-TG |
| Rashidmayvan et al. (2019)[66] | Iran | RCT (male/female) |
44 patients with NAFLD | 1000 mg N.S oil per day | 8 weeks | ↓ FBS level ↓TG, TC, LDL, VLDL ↑ HDL |
| Pelegrin et al. (2019)[69] | France | Pilot RCT (male) | 30 healthy male volunteers | N.S powder (1 g/day) | 4-weeks | Ineffective on glucose-induced insulin secretion and insulin sensitivity |
| Rheumatoid arthritis risk factors | ||||||
| Hadi et al. (2016)[77] | Iran | RCT (female) | 45 patients with rheumatoid arthritis | two capsules 500 mg/day N.S oil | 8 weeks | ↓ MDA, NO ↑ Serum level of IL-10 |
| Kheirouri et al. (2016)[91] | Iran | RCT (female) | 45 patients with rheumatoid arthritis | two capsules 500 mg/day N.S oil | 8 weeks | ↓ hs-CRP level, cytotoxic T-cells (CD8+), DAS-28 score, and an improved number of swollen joints. ↓ Serum level of CD4+ T-cell percentage, T regulatory cell percentage (CD4+CD25+ T-cell) and CD4+/CD8+ ratio |
| Gheita and Kenawy (2012)[78] | Egypt | RCT (female) | 40 patients with rheumatoid arthritis | Two capsules 500 mg/day N.S oil | 4 weeks | ↓DAS-28, joint inflammation, and morning stiffness |
| Kooshki et al. (2016)[125] | Iran | RCT (male/female) | 40 elderly patients with knee osteoarthritis | Topical application of 3cc N.S oil on knee | 3 weeks | Pain reduction in the black seed group was greater than that of the acetaminophen group |
N.S=Nigella sativa; RCT=Randomized controlled trial; TG=Triglyceride; TC=Total cholesterol; LDL=Low-density lipoprotein; HDL=High-density lipoprotein; VLDL=Very-lowdensity lipoprotein; FBG=Fasting blood glucose; BMI=Body mass index; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; GGT=Gamma-glutamyltransferase; hs-CRP; high-sensitive C-reactive protein; IL-6=Interleukin-6; TNF-α=Tumor necrosis factor-α; T2D=Type 2 diabetes; FBS=Fasting blood sugar; 2hPG=2-h postprandially glucose; HbA1c=Glycosylated hemoglobin; NAFLD=Nonalcoholic fatty liver disease; MDA=Malondialdehyde; NO=Nitric oxide; DAS-28=Disease Activity Score-28
Effect on lipid profile
Improvements in serum lipid profile were investigated in human and animal studies. The meta-analysis on the effect of N. sativa on lipid profiles indicated that the levels of TC and LDL-C dropped significantly.[39,40] Sahebkar et al. showed significant reductions in serum TG.[40] Daryabeygi-Khotbehsara et al.[39] found that N. sativa seed oil reduced serum TG, while seed powder increased TG levels what may be a consequence for different preparation processes of crushed seeds and extracted oil in addition to chemical composition.[39] Another systematic review revealed that N. sativa supplementation might be effective in glycemic control, but its effects on anthropometric parameters and lipid profile were controversial.[41]
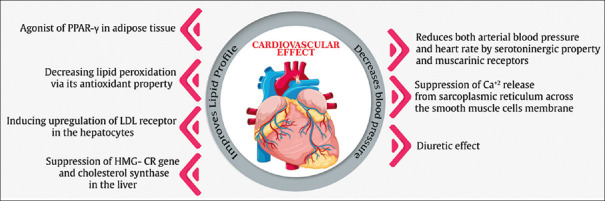
The effects of N. sativa on lipid profile may be related to its antioxidant properties,[42] and its agonist effects on peroxisome proliferator-activated receptor gamma (PPAR-γ),[43] reducing lipid peroxidation,[42] upregulation of the LDL receptor in the hepatocytes subsequently, cholesterol removal, and suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA R) gene and cholesterol synthase.[44]
Effect on blood pressure
N. sativa lowers BP sharply according to meta-analysis.[45] This may be connected to some factors such as diuretic effect, calcium channel-blocking properties, and cardiac depressant effect of N. sativa that are likely related to various components of N. sativa involved in this effect, including TQ, and fatty acids that contain substantial quantities of linoleic, oleic, and arachidonic acids, nigellicine, flavonoids, trans-anethole, p-cymene, α-pinene, limonene, carvone, and soluble fiber[41,46] volatile oils extracted from N. sativa and TQ may directly or indirectly reduce both BP and heart rate, by their serotoninergic and muscarinic properties.[47] N. sativa compounds are also reported to have endothelium-independent relaxation effects that may be due to suppression of Ca2+ release from the sarcoplasmic reticulum across the smooth muscle cell membrane and reduction of Ca2+ sensitivity and influx.[48] Furthermore, N. sativa may reduce BP by its diuretic effects and regulate the electrolytes and water content, and also control of blood volume; subsequently, cardiac output is reduced, an important regulator of BP.[49] Figure 2 shows the potential mechanisms of the effect of N. sativa on CVD.
Figure 2.
Potential mechanisms of the effect of N. sativa on cardiovascular disease. PPAR-γ = Peroxisome proliferator-activated receptor gamma; LDL receptor = Low-density lipoprotein receptor; HMG-CR = 3-Hydroxy-3-methylglutaryl-coenzyme A reductase
EFFECTS OF NIGELLA SATIVA IN TYPE 2 DIABETES
Laboratory animal studies
T2D mellitus is marked by hyperglycemia, dyslipidemia and increased oxidative markers that may be caused insulin resistance, and finally, overt diabetes.[50] Seeds of N. sativa have been used as a natural treatment for various diseases. Hypoglycemic, hypolipidemic, and antioxidant effects of N. sativa have been reported.[51,52,53] Numerous studies have been done in diabetic animal models. El-Dakhakhny et al. have shown that 0.4 g/kg N. sativa oil significantly diluted blood glucose concentration in diabetic rats after 6 weeks.[54] Insulin levels in diabetic rats treated with a dose of 20 ml/kg aqueous extract of N. sativa rose after 15 days.[55] Houcher et al. reported that intraperitoneally, a daily dosage of 810 mg/kg methanol extract and 2.5 ml/kg of N. sativa oil not only lowered glucose but also increased total antioxidant capacity levels in diabetic rats after treated for 25 days.[56] Kantar et al. showed that 50 mg/kg TQ administered orally grew both energy metabolism and insulin levels in diabetic rats after 20 days of insertion.[57,58,59] These studies also showed that a single daily dosage of 400 mg/kg and 50 mg/kg body weight N. sativa and TQ, respectively, administered orally in diabetic rats decreased serum glucose after the intervention.[58] Salama et al. in an animal study reported that oral 500 mg/kg N. sativa oil increased serum C-peptide, insulin, and total antioxidant capacity (TAC) concentration and decreased glucose level in a diabetic rat model.[52] Some studies assert that these antidiabetic properties of N. sativa are due to insulinotropic action,[60,61] hepatic gluconeogenesis inhibition, and its antioxidant effects.[52,56,62,63] Many studies have been done to confirm the properties of N. sativa on insulin sensitivity and release.[55] Several attempts have been worked on N. sativa, and they suggest that this plant improves insulin sensitivity by preventing the severity of oxidative stress. Insulin secretory effects of N. sativa have been probed on in vitro isolated rat pancreatic islets and saw that the secretion of insulin is increased in the presence of N. sativa. Table 1 summarizes the effects of N. sativa on diabetes in experimental studies.
Clinical studies
There have been several clinical studies that have evaluated the effects of oral N. sativa supplementation in different subject groups. Bamosa et al. in a clinical trial on 94 patients with diabetes showed that 2 g/day of N. sativa seed supplementation decreased fasting blood glucose (FBG), 2 h postprandially glucose, glycosylated hemoglobin (HbA1c), and insulin resistance after 12 weeks.[64] Hosseini et al. have reported a significant decrease in FBG, 2 h postprandial glucose, and HbA1c in 70 patients with T2D consuming (5 ml/day for 12 weeks) N. sativa oil.[65] The hypolipidemic and hypoglycemic effects of N. sativa oil (1000 mg/day for 8 weeks) were confirmed by Rashidmayvan et al. in a randomized, double-blind, placebo-controlled clinical trial performed in patients with nonalcoholic fatty liver.[66] Najmi et al. have been reported that the administration of N. sativa oil (2.5 mL twice a day for 6 weeks) to patients with metabolic syndrome significantly decreased FBG and LDL and increased HDL levels.[67] Hadi et al. in a clinical trial study investigated the effect 1000 mg/days extract of N. sativa oil supplementation on 43 patients with T2D. The results after 12-week supplementation of N. sativa oil have been shown a significant reduction in serum level of fasting blood sugar, HbA1c, LDL-C, and TG.[68] Table 2 summarizes clinical trials that evaluated the effects of N. sativa supplementation on healthy and patient subjects.
MECHANISMS OF HYPOGLYCEMIC EFFECT OF NIGELLA SATIVA
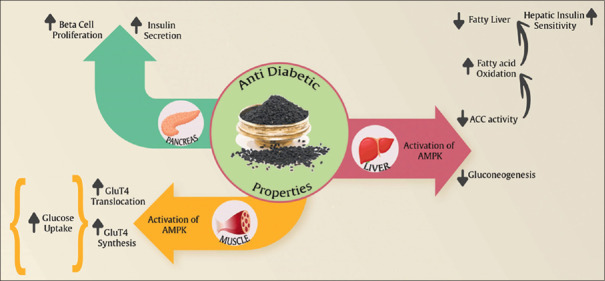
N. sativa promotes glucose homeostasis and improves the lipid profile in diabetic animals and humans with T2M through several routes. Mainly, N. sativa improve peripheral insulin sensitivity and circulating insulin.[52,53,69] It also enhances the activation of the AMP-activated protein kinase (AMPK) pathway in skeletal muscle and liver and to increased GLUT-4 in skeletal muscle.[70] Acetyl-CoA carboxylase (ACC) is a key component of the insulin-independent, metabolic sensing, and AMPK pathway. In fact, it has been reported that N. sativa stimulates ACC and the AMPK pathways in both hepatocyte cell lines and skeletal muscles in vitro.[70] Andaloussi et al. reported that in vivo N. sativa treatment can elevate the phosphorylation of ACC in skeletal muscle tissues and liver. The phosphorylation of ACC decreases its activity and shows a reduction of lipogenesis in the liver, whereas it increases fatty acid oxidation in skeletal muscle. These effects on lipid metabolism can justify the functionality of N. sativa to decrease plasma and tissue TG. Finally, it is known that the activation of the AMPK pathway can lead to growing synthesis of GLUT-4[71] [Figure 3]. Many researches suggested that the anti-hyperglycemia and anti-hyperlipidemia features of N. sativa are due to antioxidant components.[44,52,67,72,73] TQ and dithymoquinone are the main antioxidant components of N. sativa. Several studies have reported that N. sativa can enhance antioxidant enzymes and decrease lipid peroxidation.[73] When oxidative stress is reduced, this may help to regeneration of pancreatic beta-cells, increase number of islets, keep the integrity of pancreatic beta-cells, reduction in insulin resistance, increase insulin secretion and inhibition of advanced glycation end product.[59] N. sativa oil has been shown to decrease hepatic glucose production from gluconeogenic precursors (alanine, glycerol, and lactate) in STZ-induced diabetic hamster.[74] Furthermore, an in vitro study indicated direct inhibition of electrogenic intestinal absorption of glucose by N. sativa extract.[32]
Figure 3.
Potential mechanisms of the effect of N. sativa on diabetes. ACC = Acetyl-CoA carboxylase; AMPK = AMP-activated protein kinase
EFFECTS OF NIGELLA SATIVA IN RHEUMATOID ARTHRITIS
The anti-RA properties of N. sativa and its active ingredient TQ in an animal model were evaluated in experimentally induced arthritis. Findings of a study indicated that treatment with TQ orally (5 mg/kg once daily for 21 days) decreased levels of inflammatory cytokines (IL-1β, IL-6, tumor necrosis factor-α [TNF-α], interferon-gamma [IFN-γ], and prostaglandin E2 [PGE2]) and oxidative stress indices such as articular elastase, myeloperoxidase, lipoxygenase, nitric oxide (NO) significantly and increased levels of anti-inflammatory cytokine IL-10 and the activity of superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT).[75] The results of a study to elucidate the molecular mechanism of the protective effects of TQ showed that the administration of 5 mg/kg TQ orally for 7 days significantly reduced LPS-induced IL-1β, TNF-α, cyclooxygenase-2 (COX-2), metalloproteinase 13 (MMP-13), and PGE2.[76] Some of the studies reported on the effect of N. sativa on anti-RA are summarized in Table 1.
Clinical studies
Clinical trial studies in patients with RA have shown similar results. Hadi et al. and Kheirouri et al. indicated that two capsules of 500 mg/day N. sativa oil for 8 weeks in patients with RA is significantly reduced in MDA, NO, high-sensitivity C-reactive protein (hs-CRP) level, cytotoxic T-cells (CD8+), Disease Activity Score-28 (DAS-28), and a decreased number of swollen joints in comparison with baseline and placebo groups. Furthermore, N. sativa oil is significantly increased in serum level of IL-10, CD4+ T-cell percentage, T regulatory cell percentage (CD4+ CD25+ T-cell), and CD4+/CD8+ ratio. These findings suggest that N. sativa by shifting T-helper lymphocyte cells type 1 (Th1) to T-helper lymphocyte cells type 2 (Th2) can improve RA.[77] In another study, the administration of twice capsules 500 mg/day N. sativa oil for 4 weeks significantly reduced DAS-28, joint inflammation, and morning stiffness, which could be attributed to the effect of N. sativa on the immunomodulatory system.[78] Table 2 summarizes studies investigating the effects of N. sativa and its main active ingredient TQ on the anti-RA and anti-inflammatory in clinical studies.
A MECHANISM OF IMMUNO-MODULATORY PROPERTIES OF NIGELLA SATIVA
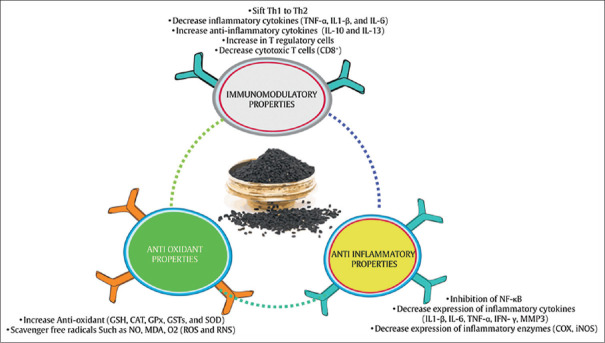
Several mechanisms for the anti-RA effects of N. sativa have been proposed. N. sativa with antioxidant, anti-inflammatory, and immunomodulatory properties can be effective.[77] Figure 4 shows the probable mechanisms of the effect of N. sativa on RA.
Figure 4.
Probable mechanisms of the effect of N. sativa on rheumatoid arthritis. NF-κB = Inhibition of the nuclear factor-kappa B; TNF-α = Tumor necrosis factor-alpha; IL-1β = Interleukin-1β; IL-6 = Interleukin-6; IFN-γ = Interferon-gamma; MMP-3 = Matrix metalloproteinase 3; COX = Cyclooxygenase; iNOS = Inducible nitric oxide synthase; GSH = Glutathione; CAT = Catalase; GPX = Glutathione peroxidase; GSTs = Glutathione S-transferases; SOD = Superoxide dismutase; NO = Nitric oxide; MDA = Malondialdehyde; ROS = Reactive oxygen species; RNS = Reactive nitrogen species; Th1 = Type 1 helper T-cells; Th2 = Type 2 helper T-cells; IL-10 = Interleukin-10; IL-13 = Interleukin-13
Antioxidant properties
According to the results of studies, reactive oxygen species (ROS), such as O2, OH, and reactive nitrogen species (RNS) such as NO, play an important role in the enhancement of inflammation and progression of RA.[75,79,80,81] Therefore, strengthening the antioxidant defense system is very important in reducing chronic diseases.[82] Numerous studies have shown the potentiation of the antioxidant system of N. sativa.[34] N. sativa exerts its antioxidant properties by direct and indirect antioxidant mechanisms and inhibits the expression of oxidative-producing enzymes (inducible NO synthase [iNOS]). Direct antioxidant activities of N. sativa may restore other antioxidants including glutathione (GSH), Vitamin E and Vitamin A, metal chelators, and scavenger free radicals (ROS and RNS). N. sativa also exerts its indirect antioxidant role by activating transcription factors involved in the expression of antioxidant enzymes including SOD, glutathione S-transferases (GSTs), GPX, and CAT.[83]
Anti-inflammatory properties
Inhibition of the nuclear factor-kappa B (NF-κB) pathway is a known mechanism marking anti-inflammatory effects of N. sativa.[84] NF-κB is a transcription factor that causes an exacerbation of inflammatory status. This transcription factor is present in the cytosol and has two subunits called P50 and P65. It also binds to the inhibitor of κB (IκBα) inhibitory protein. Factors such as ROS, TNFα, interleukin-1 beta (IL-1B), and bacterial lipopolysaccharides (LPSs) induce NF-κB activity.[85] These factors via activation of IκB kinase (IKK) results in the phosphorylation and destruction of IκBα which results in the activation of NF-κB and its transfer to the nucleus. NF-κB is located in a nucleus on a specific gene sequence that will eventually lead to increased expression of various types of inflammatory cytokines (IL-1β, IL-6, TNF-α, IFN-γ, and MMP3), COX-2, and iNOS.[86] These inflammatory cytokines are not only upregulated by NF-κB but also activate NF-κB leading to continuity of inflammatory status.[87] Therefore, N. sativa may probably rupture these interactions by NF-κB suppression and plays an important duty in its anti-inflammatory activity.[88] Inhibition of the NF-κB pathway by N. sativa can be done in several ways: (1) preventing NF-κB transfer from the cytosol to the nucleus, (2) blocking the NF-KB subunit P50 bonded to the promoter of genes expressing inflammatory factors, especially TNF-α, (3) inhibition of NF-κB p65 subunit nuclear expression, and (4) prevention of phosphorylation and degradation of I-KBα (binding of I-KBα to NF-κB causes inactivation of this transcription factor).[87]
Immunomodulatory properties
Studies suggest that N. sativa can regulate immune responses.[82] In RA, decreased expression of IL-10 produced by Th2 can be accountable for the dominance of T-helper 1 over T-helper 2 cells at sites of inflamed synovium and in the peripheral blood and reduce in Th2 may exacerbate the inflammatory process in RA. N. sativa probably shifts the immune response from Th1 that produces pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 to Th2 that results in anti-inflammatory cytokines such as IL-10 and IL-3. Studies have shown that an increase in IL-10 and IL-3 leads to an increase in T regulatory cell percentage (CD4+CD25+T-cell).[89,90,91] T regulatory cells reduce the abnormal proliferation of immune cells, such as cytotoxic T-cells (CD8+) and Th1 cells, which is responsible for the secretion of many inflammatory cytokines.[91,92,93] Therefore, N.sativa by regulation of T-lymphocytes leading to improve clinical symptoms of RA.[91]
CONCLUSION
This review article demonstrated that N. sativa could reduce oxidative stress and inflammation with various mechanisms have been proposed to contribute to the beneficial properties of N. sativa, including a reduction of lipid peroxidation via its antioxidant properties; agonist of PPAR-γ in adipose tissue; activation of AMPK, increased antioxidants inhibition of NF-κB pathway; increased in IL-10 expression, CD4+ T-cell percentage, T regulatory cell percentage in peripheral blood, and CD4+/CD8+ ratio. Therefore, N. sativa may be beneficial in chronic diseases (CVD, T2D, and RA) and can be used as an adjunct therapy. Furthermore, clinical studies have shown a positive effect of N. sativa on BP, FBG, and lipid profile, but to prove this claim, it is necessary to conduct experimental and well-designed clinical trial studies with a larger sample size on the effects of N. sativa on these chronic diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bernell S, Howard SW. Use your words carefully: What is a chronic disease? Front Public Health. 2016;4:159. doi: 10.3389/fpubh.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH, Mensah GA, Posner SF, Reed E, Simoes EJ, Engelgau MM, et al. When chronic conditions become acute: Prevention and control of chronic diseases and adverse health outcomes during natural disasters. Prev Chronic Dis. 2005;2 Spec no:A04. [PMC free article] [PubMed] [Google Scholar]
- 3.Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, et al. European Society of Cardiology: Cardiovascular disease statistics 2017. Eur Heart J. 2018;39:508–79. doi: 10.1093/eurheartj/ehx628. [DOI] [PubMed] [Google Scholar]
- 4.Pahlavani N, Jafari M, Sadeghi O, Rezaei M, Rasad H, Rahdar HA, et al. L-arginine supplementation and risk factors of cardiovascular diseases in healthy men: A double-blind randomized clinical trial. F1000Res. 2014;3:306. doi: 10.12688/f1000research.5877.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, et al. Obesity and prevalence of cardiovascular diseases and prognosis The obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–47. doi: 10.1016/j.pcad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Sabri M, Gheissari A, Mansourian M, Mohammadifard N, Sarrafzadegan N. Essential hypertension in children, a growing worldwide problem. J Res Med Sci. 2019;24:109. doi: 10.4103/jrms.JRMS_641_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, et al. Projection of diabetes burden through 2050: Impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24:1936–40. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pahlavani N, Roudi F, Zakerian M, Ferns GA, Navashenaq JG, Mashkouri A, et al. Possible molecular mechanisms of glucose-lowering activities of Momordica charantia (karela) in diabetes. J Cell Biochem. 2019;120:10921–9. doi: 10.1002/jcb.28483. [DOI] [PubMed] [Google Scholar]
- 10.Rudan I, Sidhu S, Papana A, Meng SJ, Xin-Wei Y, Wang W, et al. Prevalence of rheumatoid arthritis in low- and middle-income countries: A systematic review and analysis. J Glob Health. 2015;5:010409. doi: 10.7189/jogh.05.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: Can the U.S. health care workforce do the job? Health Aff (Millwood) 2009;28:64–74. doi: 10.1377/hlthaff.28.1.64. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules. 2015;20:9183–213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falci L, Shi Z, Greenlee H. Multiple chronic conditions and use of complementary and alternative medicine among us adults: Results from the 2012 National Health Interview Survey. Prev Chronic Dis. 2016;13:E61. doi: 10.5888/pcd13.150501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller KL, Liebowitz RS, Newby LK. Complementary and alternative medicine in cardiovascular disease: A review of biologically based approaches. Am Heart J. 2004;147:401–11. doi: 10.1016/j.ahj.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Wazaify M, Afifi FU, El-Khateeb M, Ajlouni K. Complementary and alternative medicine use among Jordanian patients with diabetes. Complement Ther Clin Pract. 2011;17:71–5. doi: 10.1016/j.ctcp.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Ganji-Arjenaki M, Rafieian-Kopaei M. Phytotherapies in inflammatory bowel disease. J Res Med Sci. 2019;24:42. doi: 10.4103/jrms.JRMS_590_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin J Nat Med. 2016;14:732–45. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- 18.Vafaee F, Hosseini M, Hassanzadeh Z, Edalatmanesh MA, Sadeghnia HR, Seghatoleslam M, et al. The effects of Nigella sativa hydro-alcoholic extract on memory and brain tissues oxidative damage after repeated seizures in rats. Iran J Pharm Res. 2015;14:547–57. [PMC free article] [PubMed] [Google Scholar]
- 19.Randhawa MA. Black seed, Nigella sativa, deserves more attention. J Ayub Med Coll Abbottabad. 2008;20:1–2. [PubMed] [Google Scholar]
- 20.Cho Ping N, Hashim NH, Hasan Adli DS. Effects of Nigella sativa (habbatus sauda) oil and nicotine chronic treatments on sperm parameters and testis histological features of rats. Evid Based Complement Alternat Med. 2014;2014:218293. doi: 10.1155/2014/218293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goreja W. Black Seed: Nature's Miracle Remedy. United States: Karger Publishers; 2003. [Google Scholar]
- 22.Gilani AU, Jabeen Q, Khan MA. A review of medicinal uses and pharmacological activities of Nigella sativa. Pak J Biol Sci. 2004;7:441–51. [Google Scholar]
- 23.Akhondian J, Parsa A, Rakhshande H. The effect of Nigella sativa L.(black cumin seed) on intractable pediatric seizures. Med Sci Monit. 2007;13:CR555–9. [PubMed] [Google Scholar]
- 24.Ramadan MF. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int J Food Sci Tech. 2007;42:1208–18. [Google Scholar]
- 25.Boskabady MH, Javan H, Sajady M, Rakhshandeh H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21:559–66. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 26.Khader M, Bresgen N, Eckl PM. In vitro toxicological properties of thymoquinone. Food Chem Toxicol. 2009;47:129–33. doi: 10.1016/j.fct.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity-the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 28.El Gazzar MA. Thymoquinone suppressses in vitro production of IL-5 and IL-13 by mast cells in response to lipopolysaccharide stimulation. Inflamm Res. 2007;56:345–51. doi: 10.1007/s00011-007-7051-0. [DOI] [PubMed] [Google Scholar]
- 29.Fallah Huseini H, Amini M, Mohtashami R, Ghamarchehre ME, Sadeqhi Z, Kianbakht S, et al. Blood pressure lowering effect of Nigella sativa L.seed oil in healthy volunteers: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2013;27:1849–53. doi: 10.1002/ptr.4944. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–52. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yimer EM, Tuem KB, Karim A, Ur-Rehman N, Anwar F. Nigella sativa L.(Black Cumin): A promising natural remedy for wide range of illnesses. Evid Based Complement Alternat Med. 2019;2019:1528635. doi: 10.1155/2019/1528635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121:419–24. doi: 10.1016/j.jep.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 33.Susilowati R, Ainuzzakki V, Nadif MR, Diana AR. The Efficacy of Nigella sativa L Extracts to Reduce Cardiovascular Disease Risk in Diabetic Dyslipidemia. Pune, India: AIP Conference Proceedings; 2019. [Google Scholar]
- 34.Mahboubi M, Mohammad Taghizadeh Kashani L, Mahboubi M. Nigella sativa fixed oil as alternative treatment in management of pain in arthritis rheumatoid. Phytomedicine. 2018;46:69–77. doi: 10.1016/j.phymed.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Enayatfard L, Mohebbati R, Niazmand S, Hosseini M, Shafei MN. The standardized extract of Nigella sativa and its major ingredient, thymoquinone, ameliorates angiotensin II-induced hypertension in rats. J Basic Clin Physiol Pharmacol. 2018;30:51–8. doi: 10.1515/jbcpp-2018-0074. [DOI] [PubMed] [Google Scholar]
- 36.El-Gindy Y, Zeweil H, Zahran S, El-Rahman MA, Eisa F. Hematologic, lipid profile, immunity, and antioxidant status of growing rabbits fed black seed as natural antioxidants. Trop Anim Health Prod. 2020;52:999–1004. doi: 10.1007/s11250-019-02091-x. [DOI] [PubMed] [Google Scholar]
- 37.Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: A randomized, placebo controlled clinical trial. Med Arch. 2012;66:198–200. doi: 10.5455/medarh.2012.66.198-200. [DOI] [PubMed] [Google Scholar]
- 38.Najmi A, Nasiruddin M, Khan R, Haque SF. Indigenous herbal product Nigella sativa proved effective as an antihypertensive in metabolic syndrome. Asian J Pharm Clin Res. 2013;6:61–4. [Google Scholar]
- 39.Daryabeygi-Khotbehsara R, Golzarand M, Ghaffari MP, Djafarian K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: A systematic review and meta-analysis. Complement Ther Med. 2017;35:6–13. doi: 10.1016/j.ctim.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Sahebkar A, Beccuti G, Simental-Mendía LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016;106:37–50. doi: 10.1016/j.phrs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Mohtashami A, Entezari MH. Effects of Nigella sativa supplementation on blood parameters and anthropometric indices in adults: A systematic review on clinical trials. J Res Med Sci. 2016;21:3. doi: 10.4103/1735-1995.175154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol. 2006;44:745–8. [PubMed] [Google Scholar]
- 43.Benhaddou-Andaloussi A, Martineau LC, Vallerand D, Haddad Y, Afshar A, Settaf A, et al. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab. 2010;12:148–57. doi: 10.1111/j.1463-1326.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 44.Meral I, Yener Z, Kahraman T, Mert N. Effect of Nigella sativa on glucose concentration, lipid peroxidation, anti-oxidant defence system and liver damage in experimentally-induced diabetic rabbits. J Vet Med Ser A. 2001;48:593–9. doi: 10.1046/j.1439-0442.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- 45.Sahebkar A, Soranna D, Liu X, Thomopoulos C, Simental-Mendia LE, Derosa G, et al. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J Hypertens. 2016;34:2127–35. doi: 10.1097/HJH.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 46.Doménech M, Roman P, Lapetra J, García de la Corte FJ, Sala-Vila A, de la Torre R, et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension. 2014;64:69–76. doi: 10.1161/HYPERTENSIONAHA.113.03353. [DOI] [PubMed] [Google Scholar]
- 47.el Tahir KE, Ashour MM, al-Harbi MM. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: Elucidation of the mechanism of action. Gen Pharmacol. 1993;24:1123–31. doi: 10.1016/0306-3623(93)90359-6. [DOI] [PubMed] [Google Scholar]
- 48.Peixoto-Neves D, Silva-Alves KS, Gomes MD, Lima FC, Lahlou S, Magalhães PJ, et al. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam Clin Pharmacol. 2010;24:341–50. doi: 10.1111/j.1472-8206.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 49.Zaoui A, Cherrah Y, Lacaille-Dubois MA, Settaf A, Amarouch H, Hassar M. [Diuretic and hypotensive effects of Nigella sativa in the spontaneously hypertensive rat] Therapie. 2000;55:379–82. [PubMed] [Google Scholar]
- 50.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–80. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohamed WS, Mostafa AM, Mohamed KM, Serwah AH. Effects of fenugreek, Nigella, and termis seeds in nonalcoholic fatty liver in obese diabetic albino rats. Arab J Gastroenterol. 2015;16:1–9. doi: 10.1016/j.ajg.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Salama RH. Hypoglycemic effect of lipoic acid, carnitine and Nigella sativa in diabetic rat model. Int J Health Sci (Qassim) 2011;5:126–34. [PMC free article] [PubMed] [Google Scholar]
- 53.Fararh KM, Ibrahim AK, Elsonosy YA. Thymoquinone enhances the activities of enzymes related to energy metabolism in peripheral leukocytes of diabetic rats. Res Vet Sci. 2010;88:400–4. doi: 10.1016/j.rvsc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 54.El-Dakhakhny M, Mady N, Lembert N, Ammon HP. The hypoglycemic effect of Nigella sativa oil is mediated by extrapancreatic actions. Planta Med. 2002;68:465–6. doi: 10.1055/s-2002-32084. [DOI] [PubMed] [Google Scholar]
- 55.Mansi KM. Effects of oral administration of water extract of Nigella sativa on serum concentrations of insulin and testosterone in alloxan-induced diabetic rats. Pak J Biol Sci. 2005;8:1152–6. [Google Scholar]
- 56.Houcher Z, Boudiaf K, Benboubetra M, Houcher BJ. Effects of methanolic extract and commercial oil of Nigella sativa L.on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines. 2007;18:8–18. [Google Scholar]
- 57.Kanter M, Akpolat M, Aktas C. Protective effects of the volatile oil of Nigella sativa seeds on beta-cell damage in streptozotocin-induced diabetic rats: A light and electron microscopic study. J Mol Histol. 2009;40:379–85. doi: 10.1007/s10735-009-9251-0. [DOI] [PubMed] [Google Scholar]
- 58.Kanter M. Effects of Nigella sativa and its major constituent, thymoquinone on sciatic nerves in experimental diabetic neuropathy. Neurochem Res. 2008;33:87–96. doi: 10.1007/s11064-007-9419-5. [DOI] [PubMed] [Google Scholar]
- 59.Kanter M, Meral I, Yener Z, Ozbek H, Demir H. Partial regeneration/proliferation of the beta-cells in the islets of langerhans by Nigella sativa L.in streptozotocin-induced diabetic rats. Tohoku J Exp Med. 2003;201:213–9. doi: 10.1620/tjem.201.213. [DOI] [PubMed] [Google Scholar]
- 60.Fararh KM, Ibrahim AK, Elsonosy YA. Thymoquinone enhances the activities of enzymes related to energy metabolism in peripheral leukocytes of diabetic rats. Res Vet Sci. 2010;88:400–4. doi: 10.1016/j.rvsc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Rchid H, Chevassus H, Nmila R, Guiral C, Petit P, Chokaïri M, et al. Nigella sativa seed extracts enhance glucose-induced insulin release from rat-isolated Langerhans islets. Fundam Clin Pharmacol. 2004;18:525–9. doi: 10.1111/j.1472-8206.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 62.Abdelmeguid NE, Fakhoury R, Kamal SM, Al Wafai RJ. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β-cells of streptozotocin-induced diabetic rats. J Diabetes. 2010;2:256–66. doi: 10.1111/j.1753-0407.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 63.Alimohammadi S, Hobbenaghi R, Javanbakht J, Kheradmand D, Mortezaee R, Tavakoli M, et al. Protective and antidiabetic effects of extract from Nigella sativa on blood glucose concentrations against streptozotocin (STZ)-induced diabetic in rats: an experimental study with histopathological evaluation. Diagn Pathol. 2013;8(1):1–7. doi: 10.1186/1746-1596-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54:344–54. [PubMed] [Google Scholar]
- 65.Hosseini M, Mirkarimi S, Amini M, Mohtashami R, Kianbakht S, Fallah HH. Effects of Nigella sativa L.seed oil in type II diabetic Patients: A randomized, double-blind, placebo-controlled clinical trial. J Med Plants. 2013:93–99. [Google Scholar]
- 66.Rashidmayvan M, Mohammadshahi M, Seyedian SS, Haghighizadeh MH. The effect of Nigella sativa oil on serum levels of inflammatory markers, liver enzymes, lipid profile, insulin and fasting blood sugar in patients with non-alcoholic fatty liver. J Diabetes Metab Disord. 2019;18:1–7. doi: 10.1007/s40200-019-00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Najmi A, Haque S, Naseeruddin M, Khan RJ. Effect of Nigella sativa oil on various clinical and biochemical parameters of metabolic syndrome. Int J Diabetes Dev Ctries. 2008;16:85–7. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirmiran P, Hosseinpour-Niazi S, Hedayati M, Azizi FJ, Metabolism Effect of Nigella sativa oil extract on lipid profiles in type 2 diabetic patients: A randomized, double blind, placebo-controlled clinical trial. IJEM. 2015;16:411–8. [Google Scholar]
- 69.Pelegrin S, Galtier F, Chalançon A, Gagnol JP, Barbanel AM, Pélissier Y, et al. Effects of Nigella sativa seeds (black cumin) on insulin secretion and lipid profile: A pilot study in healthy volunteers. Br J Clin Pharmacol. 2019;85:1607–11. doi: 10.1111/bcp.13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ali B, Louis MC, Diane V, Yara H, Pierre HS. Antidiabetic effects of Nigella sativa are mediated by activation of insulin and AMPK pathways, and by mitochondrial uncoupling. Can J Diabetes. 2008;32:333. [Google Scholar]
- 71.Habegger KM, Hoffman NJ, Ridenour CM, Brozinick JT, Elmendorf JS. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology. 2012;153:2130–41. doi: 10.1210/en.2011-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- 73.Hamdy NM, Taha RA. Effects of Nigella sativa oil and thymoquinone on oxidative stress and neuropathy in streptozotocin-induced diabetic rats. Pharmacology. 2009;84:127–34. doi: 10.1159/000234466. [DOI] [PubMed] [Google Scholar]
- 74.Fararh KM, Atoji Y, Shimizu Y, Shiina T, Nikami H, Takewaki T. Mechanisms of the hypoglycaemic and immunopotentiating effects of Nigella sativa L.oil in streptozotocin-induced diabetic hamsters. Res Vet Sci. 2004;77:123–9. doi: 10.1016/j.rvsc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Umar S, Zargan J, Umar K, Ahmad S, Katiyar CK, Khan HA. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem Biol Interact. 2012;197:40–6. doi: 10.1016/j.cbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Vaillancourt F, Silva P, Shi Q, Fahmi H, Fernandes JC, Benderdour M. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J Cell Biochem. 2011;112:107–17. doi: 10.1002/jcb.22884. [DOI] [PubMed] [Google Scholar]
- 77.Hadi V, Kheirouri S, Alizadeh M, Khabbazi A, Hosseini H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed. 2016;6:34–43. [PMC free article] [PubMed] [Google Scholar]
- 78.Gheita TA, Kenawy SA. Effectiveness of Nigella sativa oil in the management of rheumatoid arthritis patients: A placebo controlled study. Phytother Res. 2012;26:1246–8. doi: 10.1002/ptr.3679. [DOI] [PubMed] [Google Scholar]
- 79.Ebru U, Burak U, Yusuf S, Reyhan B, Arif K, Faruk TH, et al. Cardioprotective effects of Nigella sativa oil on cyclosporine A-induced cardiotoxicity in rats. Basic Clin Pharm Toxicol. 2008;103:574–80. doi: 10.1111/j.1742-7843.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- 80.El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N, et al. Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol. 2002;2:1603–11. doi: 10.1016/s1567-5769(02)00139-x. [DOI] [PubMed] [Google Scholar]
- 81.Sayed-Ahmed MM, Aleisa AM, Al-Rejaie SS, Al-Yahya AA, Al-Shabanah OA, Hafez MM, et al. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid Med Cell Longev. 2010;3:254–61. doi: 10.4161/oxim.3.4.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L.seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Amin B, Hosseinzadeh H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Med. 2016;82:8–16. doi: 10.1055/s-0035-1557838. [DOI] [PubMed] [Google Scholar]
- 84.Wilkins R, Tucci M, Benghuzzi H. Role of plant-derived antioxidants on NF-kb expression in LPS-stimulated macrophages-biomed 2011. Biomed Sci Instrum. 2011;47:222–7. [PubMed] [Google Scholar]
- 85.El Gazzar MA, El Mezayen R, Nicolls MR, Dreskin SC. Thymoquinone attenuates proinflammatory responses in lipopolysaccharide-activated mast cells by modulating NF-kappaB nuclear transactivation. Biochim Biophys Acta. 2007;1770:556–64. doi: 10.1016/j.bbagen.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Hoy WE. C-reactive protein and the risk of developing type 2 diabetes in Aboriginal Australians. Diabetes Res Clin Pract. 2007;76:37–43. doi: 10.1016/j.diabres.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Ahn KS, Aggarwal BB. Transcription factor NF-κB: A sensor for smoke and stress signals. Ann New York Acad Sci. 2005;1056:218–33. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 88.Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem Pharmacol. 2012;83:443–51. doi: 10.1016/j.bcp.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 89.Haq A, Abdullatif M, Lobo PI, Khabar KS, Sheth KV, al-Sedairy ST. Nigella sativa: Effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activity. Immunopharmacology. 1995;30:147–55. doi: 10.1016/0162-3109(95)00016-m. [DOI] [PubMed] [Google Scholar]
- 90.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+) CD25(+) T cells. J Autoimmun. 2001;16:115–23. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 91.Kheirouri S, Hadi V, Alizadeh M. Immunomodulatory effect of Nigella sativa oil on T lymphocytes in patients with rheumatoid arthritis. Immunol Invest. 2016;45:271–83. doi: 10.3109/08820139.2016.1153649. [DOI] [PubMed] [Google Scholar]
- 92.Yogesha SD, Khapli SM, Srivastava RK, Mangashetti LS, Pote ST, Mishra GC, et al. IL-3 inhibits TNF-alpha-induced bone resorption and prevents inflammatory arthritis. J Immunol. 2009;182:361–70. doi: 10.4049/jimmunol.182.1.361. [DOI] [PubMed] [Google Scholar]
- 93.Longhi MS, Ma Y, Mitry RR, Bogdanos DP, Heneghan M, Cheeseman P, et al. Effect of CD4+CD25+regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun. 2005;25:63–71. doi: 10.1016/j.jaut.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed MA, Hassanein KM. Cardio protective effects of Nigella sativa oil on lead induced cardio toxicity: Anti inflammatory and antioxidant mechanism. J Physiol Pathophysiol. 2013;4:72–80. [Google Scholar]
- 95.Taşar N, Şehirli Ö, Yiğiner Ö, Süleymanoğlu S, Yüksel M, Yeğen B, et al. Protective effects of Nigella sativa against hypertension-induced oxidative stress and cardiovascular dysfunction in rats. Marmara Pharm J. 2012;16:141–9. [Google Scholar]
- 96.Babaei Bonab S, Tofighi A. Effect of 8 weeks aerobic training and nigella supplement on insulin resistance, lipid profile and plasma level of hba1c in type 2 diabetic rats. J Zanjan Univ Med Sci Health Serv. 2019;27:20–9. [Google Scholar]
- 97.Ahmad A, Alkreathy HM. Comparative biochemical and histopathological studies on the efficacy of metformin and Nigella sativa oil against thioacetamide-induced acute hepatorenal damage in rats. Biomed Res. 2018;29:3106–16. [Google Scholar]
- 98.Muneera KE, Majeed A, Naveed AK. Comparative evaluation of Nigella sativa (Kalonji) and simvastatin for the treatment of hyperlipidemia and in the induction of hepatotoxicity. Pak J Pharm Sci. 2015;28:493–8. [PubMed] [Google Scholar]
- 99.Al-Hader A, Aqel M, Hasan ZJ. Hypoglycemic effects of the volatile oil of Nigella sativa seeds. Int J Pharm. 1993;31:96–100. [Google Scholar]
- 100.Mansi KS. Effects of oral administration of water extract of Nigella sativa on the hypothalamus pituitary adrenal axis in experimental diabetes. Int J Pharmacol. 2006;2:104–9. [Google Scholar]
- 101.Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol. 2006;44:745–8. [PubMed] [Google Scholar]
- 102.Al-Logmani AS. Effects of Nigella sativa L. and Cinnamomum zeylanicum Blume oils on some physiological parameters in streptozotocin-induced diabetic rats. Boletín latinoamericano y del caribe de plantas medicinales y aromática. 2009;8:86–96. [Google Scholar]
- 103.Benhaddou-Andaloussi A, Martineau LC, Vallerand D, Haddad Y, Afshar A, Settaf A, et al. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab. 2010;12:148–57. doi: 10.1111/j.1463-1326.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 104.Al-Logmani A, Zari TJ. Long-term effects of Nigella sativa L.oil on some physiological parameters in normal and streptozotocin-induced diabetic rats. J Diabetes Mellitus. 2011;1:46. [Google Scholar]
- 105.Alimohammadi S, Hobbenaghi R, Javanbakht J, Kheradmand D, Mortezaee R, Tavakoli M, et al. Protective and antidiabetic effects of extract from Nigella sativa on blood glucose concentrations against streptozotocin (STZ)-induced diabetic in rats: An experimental study with histopathological evaluation. Diagn Pathol. 2013;8:137. doi: 10.1186/1746-1596-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Asaduzzaman M, Nahar L, Hasan M, Khatun A, Tamanna Z, Huda N, et al. Hypoglycemic and hypolipidemic potential of Nigella sativa L.seed extract in streptozotocin (STZ)-induced diabetic rats. J Plant Biochem Physiol. 2015;3:158. [Google Scholar]
- 107.Al-Trad B, Al-Batayneh K, El-Metwally S, Alhazimi A, Ginawi I, Alaraj M, et al. Nigella sativa oil and thymoquinone ameliorate albuminuria and renal extracellular matrix accumulation in the experimental diabetic rats. Eur Rev Med Pharmacol Sci. 2016;20:2680–8. [PubMed] [Google Scholar]
- 108.Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21:895–7. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- 109.Mohamed A, Shoker A, Bendjelloul F, Mare A, Alzrigh M, Benghuzzi H, et al. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; an oxidative stress inhibitor. Biomed Sci Instrum. 2003;39:440–5. [PubMed] [Google Scholar]
- 110.Faisal R, Chiragh S, Popalzai AJ, Khalil Ur Rehman. Anti inflammatory effect of thymoquinone in comparison with methotrexate on pristane induced arthritis in rats. J Pak Med Assoc. 2015;65:519–25. [PubMed] [Google Scholar]
- 111.Chen WP, Tang JL, Bao JP, Wu LD. Thymoquinone inhibits matrix metalloproteinase expression in rabbit chondrocytes and cartilage in experimental osteoarthritis. Exp Biol Med (Maywood) 2010;235:1425–31. doi: 10.1258/ebm.2010.010174. [DOI] [PubMed] [Google Scholar]
- 112.Darand M, Darabi Z, Yari Z, Hedayati M, Shahrbaf MA, Khoncheh A, et al. The effects of black seed supplementation on cardiovascular risk factors in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019;33:2369–77. doi: 10.1002/ptr.6424. [DOI] [PubMed] [Google Scholar]
- 113.Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008;22:447–52. doi: 10.1111/j.1472-8206.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 114.Ibrahim RM, Hamdan NS, Mahmud R, Imam MU, Saini SM, Rashid SN, et al. A randomised controlled trial on hypolipidemic effects of Nigella sativa seeds powder in menopausal women. J Transl Med. 2014;12:82. doi: 10.1186/1479-5876-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tasawar Z, Siraj Z, Ahmad N, Lashari MH. The effects of Nigella sativa (Kalonji) on lipid profile in patients with stable coronary artery disease in Multan, Pakistan. Pak J Nutr. 2011;10:162–7. [Google Scholar]
- 116.Bamosa AO, Ali BA, Sowayan S. Effect of oral ingestion Nigella sativa seeds on some blood parameters. Saudi Pharm J. 1997;5:126–9. [Google Scholar]
- 117.Farzaneh E, Nia FR, Mehrtash M, Mirmoeini FS, Jalilvand M. The effects of 8-week Nigella sativa supplementation and aerobic training on lipid profile and VO2 max in sedentary overweight females. Int J Prev Med. 2014;5:210–6. [PMC free article] [PubMed] [Google Scholar]
- 118.Najmi A, Haque S, Naseeruddin M, Khan R. Effect of Nigella sativa oil on various clinical and biochemical parameters of metabolic syndrome. Int J Diabetes Dev Ctries. 2008;16:85–7. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mahdavi R, Namazi N, Alizadeh M, Farajnia S. Effects of Nigella sativa oil with a low-calorie diet on cardiometabolic risk factors in obese women: A randomized controlled clinical trial. Food Funct. 2015;6:2041–8. doi: 10.1039/c5fo00316d. [DOI] [PubMed] [Google Scholar]
- 120.Farhangi MA, Dehghan P, Tajmiri S. Powdered black cumin seeds strongly improves serum lipids, atherogenic index of plasma and modulates anthropometric features in patients with Hashimoto's thyroiditis. Lipids Health Dis. 2018;17:59. doi: 10.1186/s12944-018-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bhatti I, Inayat S, Uzair B, Menaa F, Bakhsh S, Khan H, et al. Effects of Nigella sativa (Kalonji) and honey on lipid profile of hyper lipidemic smokers. Ind J Pharmaceut Educ Res. 2016;50:376–84. [Google Scholar]
- 122.Kaatabi H, Bamosa AO, Lebda FM, Al Elq AH, Al-Sultan AI. Favorable impact of Nigella sativa seeds on lipid profile in type 2 diabetic patients. J Family Community Med. 2012;19:155–61. doi: 10.4103/2230-8229.102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hosseini M, Mirkarimi S, Amini M, Mohtashami R, Kianbakht S, Fallah Huseini HJ. Effects of Nigella sativa L.seed oil in Type II diabetic Patients: A randomized, double-blind, placebo-controlled clinical trial. J Med Plants. 2013;3:93–9. [Google Scholar]
- 124.Heshmati J, Namazi N, Memarzadeh MR, Taghizadeh M, Kolahdooz FJ. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Food Res Int. 2015;70:87–93. [Google Scholar]
- 125.Kooshki A, Forouzan R, Rakhshani MH, Mohammadi M. Effect of topical application of Nigella sativa oil and oral acetaminophen on pain in elderly with knee osteoarthritis: A crossover clinical trial. Electron Physician. 2016;8:3193–7. doi: 10.19082/3193. [DOI] [PMC free article] [PubMed] [Google Scholar]