Abstract
We believe the switch to a digital pathology (DP) workflow is imminent and it is essential to understand the economic implications of conversion. Many aspects of the adoption of DP will be disruptive and have a direct financial impact, both in short term costs, such as investment in equipment and personnel, and long term revenue potential, such as improved productivity and novel tests. The focus of this whitepaper is to educate pathologists, laboratorians and other stakeholders about the business and monetary considerations of converting to a digital pathology workflow. The components of a DP business plan will be thoroughly summarized, and guidance will be provided on how to build a case for adoption and implementation as well as a roadmap for transitioning from an analog to a digital pathology workflow in various laboratory settings. It is important to clarify that this publication is not intended to list prices although some financials will be mentioned as examples. The authors encourage readers who are evaluating conversion to a DP workflow to use this paper as a foundational guide for conducting a thorough and complete assessment while incorporating in current market pricing. Contributors to this paper analyzed peer-reviewed literature and data collected from various institutions, some of which are mentioned. Digital pathology will change the way we practice through facilitating patient access to expert pathology services and enabling image analysis tools and assays to aid in diagnosis, prognosis, risk stratification and therapeutic selection. Together, they will result in the delivery of valuable information from which to make better decisions and improve the health of patients.
Keywords: Business case, clinical laboratory, digital pathology, implementation, patient care
INTRODUCTION
A few definitions may be confusing and thus used interchangeably: Whole slide image (WSI), digital pathology (DP), DP system (DPS), DP workflow, telepathology, and computational pathology.
At its core, DP is based on capturing a high-resolution digital replica of a conventional glass slide, called a WSI. The WSI is acquired by scanning a glass slide with a whole slide scanner. The high-resolution digital WSI may be viewed on a computer, shared or stored. The DPS refers to the end-to-end components that result in the generation and viewing of digital WSI. It consists of the scanner, software, and the image viewer.[1,2,3] The DP workflow encompasses a broad set of processes implemented in the pathology laboratory of which the DPS is a component.[2] DP workflows enable computational pathology, where the WSI is translated to data and applied mathematical models for a more precise interpretation and to enable research and discovery. Computational pathology relies on image analysis software to extract data. The pixels in the WSI are translated to numeric values to be used for higher-level computational modeling. In recent years, more sophisticated image analysis software that uses machine learning approaches enabling artificial intelligence (AI) for a dynamic solution have been showing great promise in applications from computer assistive diagnosis to the discovery of novel biomarkers.[1,2,4] DP is thus a necessary step to adopt computational pathology. Telepathology is the remote transmission of pathology images for primary diagnosis, consultation, research, or education.
The reader is advised to review previous white papers from the Digital Pathology Association for an in-depth review of WSI[3] image analysis[1] and Computational Pathology.[5]
CURRENT STATUS
DP has been in use for many years in industrial and academic research. Image analysis techniques like quantification, measurements, and classification have been employed for decades.[1]
The last few years have seen increased interest in a DP workflow in the clinical areas, especially since the approval of whole slide imaging for primary diagnosis by regulatory agencies. In Europe, for example, many DPS have had Conformité Européene- In-Vitro Diagnostics (CE-IVD) marking/approval for several years which has allowed for the exponential growth of digital technology in countries like The Netherlands[6,7] Sweden[8,9] England[10,11,12,13,14,15] and Spain[16] all of which have reported major advances including primary diagnosis and 100% transition to a digital workflow. In North America, Canada has been making strides since the beginning of the decade of the 2010s;[17,18,19] and more recently in the United States with the approval by the Food and Drug Administration (FDA) of two WSI systems at the time of this paper.[20,21,22]
The thought that now the transition is happening in America creates both enthusiasm and anxiety in the diagnostic pathology community; the dilemma is should we run toward the change, invest early, and get ready versus wait until the technology is widely adopted and perhaps more affordable? This is particularly important for laboratories in community hospitals: Do they have a real value proposition? Are they going to see a short-term return on investment (ROI)? Or is this just future planning? Laboratories in academic institutions may not be much better off since they also must decide and create a plan that would make fiscal sense and satisfy their financial leadership. If the “watch and wait” approach is the answer, how long should laboratories wait? Many of the factors to consider before making this decision are financial, not only in the amount of dollars to be spent on new equipment and new Information Technology (IT) but also the time and effort needed to transition from analog to DP.
COSTS OF IMPLEMENTING DIGITAL PATHOLOGY
Many variables can affect costs
the volume of slides, the number of workstations to review digital slides, and the amount of time to retain the images are just a few of the drivers that cause DP initiative costs to range from tens of thousands of dollars to multimillion-dollar investments. There are required costs associated with the minimum requirements to start a digital deployment as well as optional costs, which are investments that can wait until after the initial deployment.
Required costs and minimum requirements
Imaging hardware
Whole slide scanning hardware varies considerably in cost, and the selection of a scanner depends on the laboratory workflow and volume as well as the anticipated use cases which may include any combination of research, archival only, education, conferences, telepathology, frozen sections, primary diagnosis including recuts, special stains, immunohistochemistry (IHC) and other ancillary testing.[3]
At present, scanners range in capacity from 1 slide to over 1000 slides. Scanning time, typically expressed as the number of seconds per 15 mm × 15 mm scanning area, can vary by scanner by a factor of 2 or more, and therefore, may also become an important consideration depending on the intended use case. In addition to throughput, additional considerations such as reliability, ease of use, image resolution/magnification supported, slide size format, and the ability to integrate into the software platform of choice must also be evaluated. Additional features may also be important to some users such as the continuous loading or ability to interrupt a scanning session to add new slides (and optionally, to prioritize them), optical factors such as the objective or illumination source, and the flexibility to add fluorescence or multispectral capabilities. An evaluation of cost relies first on determining the necessary features to meet workflow requirements. Currently, a single high-capacity (>100 slides) whole slide scanner ranges from $100,000 to $400,000. One of the most important distinguishing features that explain the price disparity is the software platform that comes with the scanner. Some scanners are accompanied only by minimal software, while others are bundled with elaborate software platforms to host and organize large image sets and to manage many of the requirements of a digital workflow. The needs for software will be determined by the intended use cases. Another factor that can influence pricing is whether the scanner includes a dedicated workstation for viewing and analyzing slides. Based on regulatory clearance, some manufacturers provide only the scanner, while others provide the complete pixel pipeline, which encompasses the scanner, viewing software, workstation, and display. However, nearly all scanners are either accompanied by viewing software or generate files that can be viewed by free software.
Despite the evolution of whole slide scanners toward greater capacity and automation, smaller capacity whole slide scanners and robotic microscopes (with scanning capabilities) have recently re-emerged in the market to meet more targeted applications. For example, small table-top scanners have found a niche by offering a fully portable solution to users who wish to bring this technology to multiple locations. Many of the smaller scanners use alternate technologies to set themselves apart from high-capacity scanners, such as hybrid real-time robotic devices with specialization for frozen sections or live viewing applications for telepathology. Furthermore, some can be considerably lower in cost than their high-capacity counterparts, which can provide a cost-effective alternative for departments who wish only to do occasional slide scanning (e.g., to support occasional presentations or publications).
Additional hardware may aid the integration of whole slide imaging into the laboratory workflow. For displays, it has been demonstrated that a modern 3-4-megapixel (MP) display is adequate; however, for primary diagnosis, medical-grade displays which provide higher resolutions (4-8 MP), contrast ratios, luminance, and color stability are preferred. In addition, most medical-grade display screens have embedded sensors that guarantee image consistency over a lifetime of 5 + years, Quality Assurance/Quality Control (QA/QC) software that is remotely accessible and technology that automatically senses the ambient lighting conditions of your workspace. As of today, there are no universal guidelines for displays in DP; however, when determining the required investment into displays, these are some minimum specifications to consider: 24” to 27” screen size, 3MP resolution, brightness/luminance of 350 cd/m2, contrast ratio of luminance from the brightest color to the darkest color of 1000:1, screen refresh rate of 60 Hz and QA/QC software with color calibration management to ensure daily consistency of color. A wide range of displays meet and/or exceed these specifications; therefore, the price range for medical-grade displays is also wide, ranging from $5,500 to $14,500. Again, based on regulatory clearance, certain devices may be bundled for purchase altogether.
The computing hardware and software needs of department users should be evaluated as part of an integration strategy, and where possible, coordinated with your IT department. Barcoding is essential for a clinical deployment of whole slide imaging. The adoption of barcoding is a critical safety improvement and eliminates manual data entry to connect slides with clinical information in the Laboratory Information Systems (LIS). Most scanners capture and interpret slide bar codes, which with integration can automatically associate scanned slides with the case in the LIS, Electronic Medical Record (EMR) or Electronic Health Record. This is an important feature for clinical laboratories as a “mix up” in patient cases may result in a potentially detrimental outcome for the patient and could have severe consequences for the laboratory. Also, if the bar code contains information that can link the slide to a specific stain, image analysis can be readily deployed in a semi-automated fashion. Using barcoding, specimen tracking systems have the potential to accommodate the slide scanning process into the workflow.
Software
In addition to the scanner and other necessary hardware, specific software is used to manage, view and manipulate the digital images. DP software may be included with the scanner hardware or sold separately by the scanner manufacturer. Software may also be acquired from third-party vendors and be compatible with several different slide scanner manufacturers. The image management software allows one to interact with the images efficiently, only accessing the part of the image in view. This allows for quick navigation of the WSI without having to physically transfer the entire file to one's workstation. Other common functions include the ability to rotate and zoom in/out of the image, perform measurements across the image as well as annotation on the images. Each organization needs to understand their unique requirements and evaluate the different software solutions in the same way a scanner is evaluated.
The integration of image management systems with an EMR or LIS system is important to gain overall operational efficiency. Understanding management software integration with the laboratory's unique EMR/LIS environment is critical to making the best decision.
Information technology infrastructure
When implementing DP, one of the largest costs will be the IT infrastructure that is necessary to move and store the images. If enterprise infrastructure is not in place, high-volume scanning will require an investment to grow the existing infrastructure. The size of the infrastructure needed will reside with the size of WSIs.
The size of the file in WSI is determined by:
Size of the scan area-typically 15 mm × 20 mm
Resolution- 5×, 10×, 20×, 40 × or higher (Note: ×40 is recommended for primary diagnosis)
Post-scan compression - certain image formats allow for compression.
Other factors (Z-planes, larger scan areas, etc.) also contribute to file size, but for the purposes of this section, assume a 15 mm × 20 mm scan area at 40x magnification with the resolution of. 25 microns per pixel and a Joint Photographic Experts Group 2000 compression algorithm with a compression ratio of 30:1. This file size will be approximately 15 gigabytes (GB) uncompressed, but with compression, it is reduced to 500 megabytes per slide. Based on these numbers and a given intended use case, an organization can calculate their storage and infrastructure needs per year. Each organization will need to consider the total volume of slides that would be expected to be scanned over time and the time the images will be kept, following College of American Pathologists (CAP) recommendations and the cumulative volume will drive the consideration of the different technology components needed to support DP in your organization.
An IT infrastructure to support DP may contain the following components: [Figure 1 and Table 1]. A possible scenario for using this layered storage for primary diagnosis with DP is described. At the beginning, the scanner in the laboratory, as it scans the glass slides and converts those into a digital image, will transfer the images to a pathology application or server. The application server will store the image on a high-performance storage system (Tier 1). This storage needs to keep up with the ingress of images from one or more scanners. It is often implemented with many SAS disks or Solid-State Storage. The application will read the bar code from the image and any metadata. The application will store the required information in a database.
Figure 1.
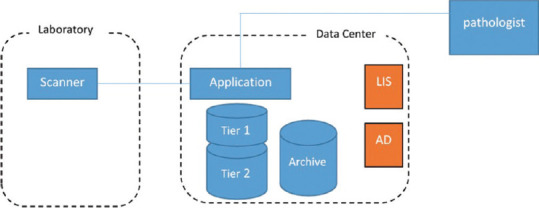
Information Technology infrastructure components
Table 1.
Storage comparison
| Tier | Type of storage | Purpose |
|---|---|---|
| Tier 1 | SSD/flash drives | Highest performance |
| Tier 2 | 15 k rpm hard drives | Balance of performance/capacity |
| Archive Tier 3 | 7.2 k/10 k rpm hard drives | High capacity/low performance |
SSD: Solid state drive
Pathologists performing primary diagnosis will use a workstation with a good quality medical grade display to connect to the pathology application.[23] The pathology application will display the cases, associated tissue images and metadata, and support the pathologist in diagnosing the tissue. After an initial period of frequent access when the pathologist is accessing all data, the final diagnosis will be complete, the case will be signed out and a few days later, after additional testing or consultations are completed, the access to the image will be less frequent. At this time, the images can be stored on a less expensive storage system (Tier 2). The image will still be online and viewable without delay. This type of storage is typically implemented with RAID sets of very large capacity disks. Cases that need to be kept for possible future reference (or for legal reasons) but are not likely to be accessed in the near future can be stored on an archive storage system (Tier 3). This can be implemented on less expensive storage systems such as tapes or cloud backup. If an archived case needs to be viewed, it first must be restored from Tier 3 (Archive) to Tier 1 or Tier 2 storage. This will be automated in the pathology application for the pathologist. Depending on the archiving technology used, the retrieval time can vary from minutes to hours/days.
By default, a backup of the pathology application server should be created to be used to recover from a potential disaster with the server. This can be done inexpensively by using tapes. Pricing of the IT system will vary greatly depending on how many months of online data storage is needed, does the server hardware need to be fully redundant and highly available, is a full backup of all image data needed. For example, a simple nonredundant system with 3 months of online data storage without an archive for a lab that produces 250,000 slides per year will cost around $90K. Whereas a fully redundant system including full image backup can cost much more. Your DP vendor should be able to advise you on the best configuration and pricing for your specific situation.
Labor
The adoption of DP requires some technical labor. DP technical staff may be titled differently at various institutions, but in this white paper, the term “scanning technician” will be used. The scanning technician has the role of loading the slides into the DP scanner and performing QA checks on the scanning process. Depending on the DP vendor, this may mean cleaning slides before loading them into cassettes, loading cassettes, performing focus point adjustment, troubleshooting any issues with the scanning process, or unloading the scanner. Recently, the DP Association (DPA), along with the National Society for Histotechnology, has created an online program to become certified in DP. This certification is recommended for technical staff who might be involved in the DP scanning process. Although the ratio of scan tech to scanners is variable across different institutions, this ratio should be between 0.3 full-time equivalents (FTE) to 1 FTE per scanner. The volume of slides to be scanned and the expected throughput will influence an institution's choice about the proper ratio of staffing. Although dedicated staff are likely necessary for retrospective scanning, upfront scanning may be performed by some existing personnel within the laboratory.
Besides the scan tech role, it is also important to assign an IT staff member to the application. A DPS will generally have at least two components-hardware and software. Both components will need support from IT staff.
Administrative/project management support is also critical during the implementation phase, as there are numerous meetings to attend and tasks that need to be performed. Although this may be an additional cost, good management that results in on-time or early implementation and avoiding costly mistakes will more than pay for itself.
Once the DPS is deployed, ongoing maintenance of the system should include periodic meetings with the different stakeholders. These meetings should assess the adoption and the process of the DPS.
Facility consideration
Depending on the method for deployment of the DPS, significant facility considerations may be necessary. Although most scanners take up the space of standard computers, there may be weight requirements depending on the manufacturer. Most scanners are also paired with a desktop computer, so space is necessary for a monitor and a workstation. The scanners also require dedicated network lines to upload images to a central storage repository. There are variable recommendations from DP scanner vendors about the need to have anti-vibration surfaces for the scanners to be located. The need for anti-vibration considerations may depend on the vendor as well as the size of the scanner itself.
Optional costs: Image analysis software and artificial intelligence
The Basic Science Image Analysis programs are not specifically designed for digital pathologists and therefore, tend not to be intuitive and easy to learn and use.
Slide scanner-based software is designed to be plug-and-play so that it is easier to use, but because they are built for automation and reproducibility, there is limited ability to create customized algorithms.
DP-inspired software works with images from any scan format and allows a higher level of customization and features data extraction compared to slide-scanner based software. The workflow makes it easy for anyone with minimal knowledge of pathology and image analysis.
Algorithm-based software typically requires a higher level of knowledge in image analysis. This software has virtually limitless capabilities in designing customized analysis approaches.
For a purchase license to own model, most image analysis software suites have an initial base cost of about $20K. The cost could be significantly higher (up to $80-;100K) if more features are added. Also, if additional licenses are added, this cost would be approximately $5-10K for each. After the initial cost and 1st year of use, most software typically has one of two maintenance models: Either an annual maintenance fee that includes full support and updates costing typically $5-10K per license, or a required annual maintenance fee to use the software. The latter will include the full support and updates and the cost is like the first model.
Other costs to consider are servers if network access is required to use the software. This will allow many users to share licenses. However, server computers can be expensive, especially if a graphic processing unit is required which could cost around $20-40K.
Also, vendors may develop new features such as deep learning, which will require additional purchase and most likely add cost to annual maintenance. Some vendors offer deep learning modules.
Local or cloud storage for images and data processed using deep learning technologies may also add to the cost that will include institutional IT support.
In a subscription model, the software may be budgeted at $1000-1500/month, which allows unsupervised use of the algorithm with customer service support. There is typically a one-time setup/configuration/training fee. Additional development that is customized will cost additional fees to be negotiated upfront [Table 2].
Table 2.
Common types of image analysis software (courtesy of Joseph Johnson, Moffitt Cancer Center)
| Image format compatibility | Technical knowledge level | Customization level | Features | |
|---|---|---|---|---|
| Basic science image analysis | Most image formats | Moderate | High | Variety of measurement tools |
| Access to image processing tools | ||||
| Some automation | ||||
| Slide scanner based | Limited image formats | Low | Lowmoderate | Direct access to images |
| Access to common algorithms | ||||
| US IVD for HER2/ER available | ||||
| Pattern recognition | ||||
| Batch processing | ||||
| Designed for digital pathology | ||||
| Digital pathology inspired | Most image formats | Moderate | Moderate | Workflow based |
| Easily adjustableparameters | ||||
| Batch processing | ||||
| Pattern recognition | ||||
| Access more feature data | ||||
| Designed for digital pathology | ||||
| Algorithm based | Most image formats | High | High | Fully customizable |
| Unique algorithms | ||||
| Even more feature data | ||||
| Batch processing |
IVD: In vitro diagnostics, ER: Estrogen receptor, HER2: Human epidermal growth factor receptor 2
VALUE PROPOSITION AND MARKET DRIVERS
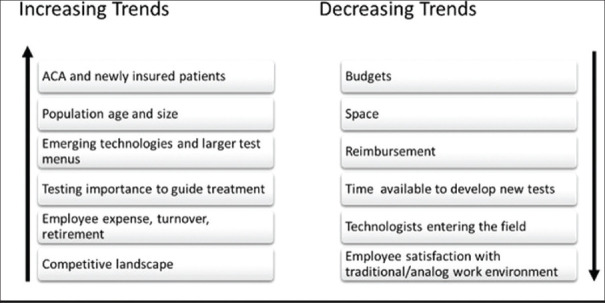
The pathology laboratory industry is seeing notable trends in recent years. An increase in newly insured patients and the growth ofan aging population result in greater demand for diagnostic laboratories than ever before. A growing number of commercial targeted therapies, an increase in available technologies and biomarkers analyzed, and an increasingly competitive landscape are also having an impact. This is creating an increased pathologist demand with projected pathologist shortages and increases in pathology specimen volume each year.[5,24,25,26,27] At the same time, we witness a decrease in reimbursements, budgets, space, and time to allocate for test development. Today's diagnostic laboratory must be more productive, nimble and innovative than ever before to provide responsible patient care and remain competitive [Figure 2].
Figure 2.
Digital imaging added value proposition (Courtesy of Orly Ardon, Memorial Sloan Kettering)
DP applications may help laboratories improve productivity, remain competitive, and adjust to some of the market trends by increasing capacity, driving down costs and improving patient care. All result in increased efficiency and an ability to respond to the price pressures of the competitive diagnostic testing market, contain healthcare expenses and address the foreseen pathologist shortage. In addition, adopting DP capabilities can lead to additional revenue-generating opportunities for laboratories that include research, drug discovery, companion diagnostics, and clinical trials.
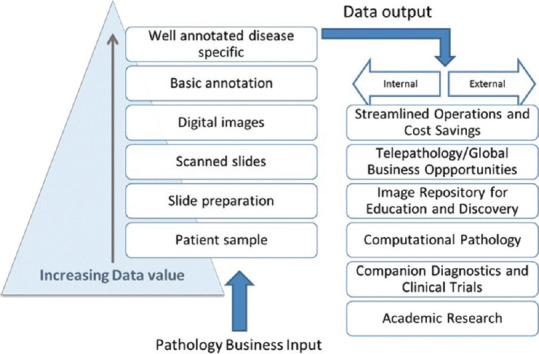
The basic requirement for developing these capabilities requires the digitization of a glass slide, with more added value for the data obtained in each step of the digital workflow and investment, as illustrated in Figure 1. Well annotated, archived slides would be a key requirement for additional revenue-generating business and educational opportunities for diagnostic laboratories and academic centers.
The main economic and patient drivers are increased capacity and efficiency and the creation of new opportunities for revenue generation. Streamlining clinical operations is crucial for reducing costs, improving turnaround time, diagnostic testing quality, and offering a full test menu [Figure 3].
Figure 3.
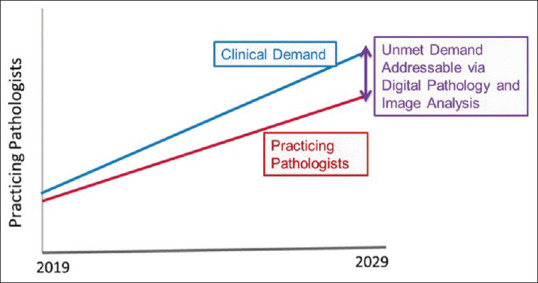
Projected shortage of practicing pathologists in the next 5 years Digital Pathology and Image Analysis Can Help Alleviate Pathologist Shortage (Courtesy of Orly Ardon, Memorial Sloan Kettering)
Pathologists are not only facing pressures related to shorter turn-around times and increasing reporting complexity, they are also faced with a looming workforce shortage. In recent decades, fewer pathologists are entering the field, while >75% of full-time pathologists are 45 years or older, making pathology one of the oldest specialties in the United States.[27] A key component of this is a proportional decrease in the number of medical students choosing pathology. According to the Accreditation Council for Graduate Medical Education, from 2007 to 2017, the number of pathology residency programs has decreased from 150 to 142. Although the number of residents has remained relatively stable (2312 in 2007-2008 and 2334 in 2016-2017), this is in contrast to a 19% increase in residents across all specialties.[24,25]
One model predicts a decrease in the number of U.S. pathologist FTE from 5.7/100,000 population to 3.7 per 100,000 population by 2030.[28] This is due to both a relative decrease in the number of new pathologists entering the workforce and a “retirement cliff” starting in 2015, peaking in 2021 and continuing until at least 2030. The CAP predictive model estimates that there would need to be an 8.1% increase in residency training positions per year from now through 2030 to keep up with the increased demand.[28] DPSs may delay the need to add new pathologists by increasing the efficiency of currently active pathologists. Robboy et al. applied a conservative estimate of a 5% increase in efficiency from the use of a DPS and an estimated annual case volume per pathologist of 3000 to estimate that a DPS would allow a pathologist to see 150 more cases per year. Based on this estimate, for a pathology practice with 10 pathologists seeing 30,000 cases and a 3% increase in cases per year, a DPS would allow the practice to delay adding another pathologist for >18 months. If the gain in efficiency is closer to 13% as described in one study[29] the effect of a DPS on decreased workforce expansion would be even greater. Another avenue to slow workforce expansion is to optimize the use of part-time and semi-retired pathologists. The flexibility of a DPS allows part-time workers to maximize the hours they can work, while potentially enticing pathologists who might otherwise retire to take advantage of remote working and continue to contribute on a part-time basis.[11] Finally, attracting the most-talented pathologists may be easier when pathologists can choose where they live and work.
The anticipated shortage of pathologists may be alleviated in part by DP workflows and image analysis tools [Figure 4]. Image analysis software providing aid with the more mundane pathology tasks like counting cells or mitosis will further address the shortage by increasing pathologist efficiency and productivity. Pathologists would be able to focus on the more engaging part of their job like challenging, unusual and rare cases, communicating and discussing findings, and educating the clinical teams.
Figure 4.
Current trends in diagnostic laboratories. The case for improved productivity (Courtesy of Orly Ardon, Memorial Sloan Kettering)
Younger generations of pathologists are becoming more technology savvy than previous generations.[30] Young pathologists may choose to accept positions in DP-enabled laboratories over traditional analog laboratories, which will further increase competition and drive up hiring expenses. Today's laboratories must consider their capabilities to hire and retain talent in the future when making decisions about adopting DP.
RETURN ON INVESTMENT
Considering the costs associated with implementing DP, it is important to evaluate opportunities to maximize ROI. This can be viewed in terms of both cost reduction and revenue opportunities.
Cost reduction
Cost reduction can be split into direct and indirect components. Direct cost reduction applies to areas of a pathology practice that have a quantifiable component, including shipping costs, overhead costs, and workforce efficiency. Indirect cost reduction includes areas more difficult to quantify but still likely to provide a ROI, such as improved communication between pathologists, streamlined access to diagnostic information, and improved pathologist recruitment.
Slide handling and archiving
Slide handling and archiving efficiencies are achieved with a DP workflow. Several steps in the current anatomical pathology sign-out workflow are manual. These include sorting and matching glass slides to corresponding accessioned cases in the LIS, followed by manual delivery to pathologists. If pathologists are not on-site, glass slides must be shipped. Shipping costs might include transferring of glass slides from the histology lab to the pathologists, transferring of slides to an off-site storage facility, sending slides out for consultation and costs associated with tracking slides. If pathologists are spread across multiple sites, this could involve various types of transport (from a regional lab to each site, between sites, etc.), each requiring packing and sorting, coordination of shipping and receiving, and extensive efforts to track the slides. With a DPS, the images can be sent directly from the scanning location (ideally located near the histology lab) to pathologists locally, throughout a region or globally, providing considerable savings on the costs associated with shipping and tracking. In addition, there is no risk of lost or broken slides, which traditionally would require recuts to be made at the expense of the laboratory. These tasks are not only time-and labor-intensive but also error-prone and costly (e.g., expense of using slide trays and courier services).[31] Besides the expense of handling the circulation of physical slides among pathologists, if the slides are not stored, they are returned to ordering clinicians, as is often the case in consultations. In contrast, digital images can be retained for tumor boards and educational purposes long after slides were shipped back; this may need an agreement between organizations.[32]
In the US, laboratories are required by Clinical Laboratory Improvement Amendments (CLIA) to store slides on-site or in long-term, climate-controlled slide storage, which is usually off-site and costly but necessary to protect the integrity of stored slides over many years.
The cost of short- and long-term storage is further divided between the slide storage and slide retrieval cost and may result in hundreds of dollars per case. In addition, laboratories are liable for lost or damaged glass slides and must account for the possibility of legal costs associated if loss or damage occurs. Digital WSIs ensure that the quality of the image does not deteriorate with time as a result of extreme temperature, light, and other environmental factors. Laboratories also have the advantage of lower-cost unlimited retrieval from the digital archive.
Today, US laboratories must store slides and Formalin-Fixed Paraffin-Embedded blocks per CLIA requirements; however, digitization eliminates the logistics and cost of retrieval. As more laboratories adopt digital workflows, Current CLIA requirements may change to accept digitized WSIs to replace physical slides.
Overhead costs
If pathologists are working remotely, there is no need for individual offices, thereby saving on real estate and facilities costs. In addition, once a practice is 100% digital, there would be a limited need to purchase and maintain microscopes.
As a result of having a DPS, imaging core facilities have been established at many academic institutions to serve as one central resource.[29,33,34] A central core laboratory that can receive specimens, process and scan slides and manage the distribution and storage of digital images is a more efficient business case for clinical operations. Cost reductions in laboratory instrumentation and infrastructure, slide storage facilities, and the hiring of additional technicians in redundant positions are realized. A central processing site will also result in consistent slide quality, streamlined workflows, and timely delivery of patient results.[29] Digital workflows may also allow the adjustment of demand and capacity by dividing workloads among multiple pathologists who may be in different facilities, so-called workload balancing. This flexibility can reduce the cost of hiring additional pathologists on location, which is especially advantageous when case volume cannot be accurately forecasted or when it is variable. The value of a remote pathology infrastructure can be further realized by including full-or part-time experts for remote consultations in rare or difficult cases (telepathology).[35,36,37]
Efficiency and turn around time
Another benefit of DP is the potential for time savings in the form of faster per case reads. Recent publications support the hypothesis that DP workflows are more efficient and therefore, a cost-saving measure. In 2014, Ho et al. reported a projected $17.73 million in savings for the first 5 years following implementation of DP for primary diagnostic use in a large academic medical center-based health care organization.[29] Another recent study by Vodovnik found time savings in digital WSI reads in comparison to analog glass slide reads.[38] They reported 400 consecutive cases (histopathology, nongynecological cytology and fine-needle aspiration cytology) in 1841 min (vs. 1956 min). He concluded that the diagnostic time to read a DP case may be up to 10% shorter than a traditional glass slide microscopy case. In addition, future improvements in efficiency may be realized through hardware and software advancements as well as a digitally trained workforce.[39]
Guo et al. evaluated the challenges and advantages of digital solutions for pathology sign-out in the United States and concluded that the integration of LIS with DPS will be instrumental to streamline workflow, reduce human error and improve the sign-out experience.[31] It is only a matter of time before multiple vendor solutions that offer fully digital workflows to enable effortless standardized diagnoses are available. Time savings may also be realized through eliminating the need to switch from microscope to computer screen and switch glass slides on the microscope. This is particularly helpful when viewing special stains and immunohistochemical stains. Most DPS's allow synchronous viewing of multiple slides, such as a routine hematoxylin and eosin (H and E) slide and multiple immunohistochemical stains, including the option to “link” slides so that the same area can be viewed on each slide.[11]
In a first-of-its-kind study of time savings in a large regional laboratory in the Netherlands, the authors reported a time savings of 19 h in an assessment of the impact of DP on logistical laboratory tasks.[7] In Spain, after introducing a DP workflow, Granada University pathologists reported a 21% annual increase in productivity.[16] Pathologists have felt increased pressure in recent years from efforts to minimize routine turn-around time, but also from increasingly complex reporting requirements and increasing caseloads.[40] In the UK, for example, the number of surgical pathology requests has increased on average 4.5% per year, without a concurrent increase in the pathology workforce.[41] DPS's have the potential to increase efficiency, helping to offset some of these increased costs. Previously mentioned is an estimated 13% increase in efficiency using a DPS, based on changes in time spent waiting for cases to arrive, organizing slides, searching for cases and matching cases to paperwork.[29] Future studies should be planned to evaluate time and cost savings in state-of-the-art DP laboratories.
Screening, digital annotations, and image analysis
Before the dissemination of cases for pathologist review, digital slides can undergo interactive screening, which in cytopathology has been shown to increase productivity and increase job satisfaction among cytopathologists and cytotechnologists by reducing the need for surface area screening by cytotechnologists.[42] The development and implementation of algorithms to prescreen surgical pathology slides will reduce the time needed for routine case analysis.
During their review of slides, pathologists often annotate directly on the slides indicating the presence or absence of tumor in lymph nodes, measurements of tumor from margins, or other areas of interest. With digital images, annotations can be applied in layers that can be hidden or shown as needed. Areas can also be flagged or bookmarked for instantaneous return for review. Measurements done on digital images have been shown to be more accurate and reproducible.[43] Image analysis of digital slides offers standardization of inter-batch stain variability.[44] Quantification of markers including Estrogen Receptor, Progesterone Receptor, Human Epidermal Growth Factor Receptor 2, (ER, PR, and Her2/Neu) for breast cancer cases on digital images has been shown to be better than manual quantification.[45,46] Mitoses count by image analysis on digital slides can reduce inter-observer variability.[47] 3-D recreation and virtual sectioning will allow recreation of specimen orientation that would otherwise be impossible.[48]
With digital slides, review cases can be automatically added to the review pathologist queue and are immediately available.[49]
Indirect cost reduction
The indirect component of cost reductions encompasses less quantifiable areas of the pathology practice and include pathologist safety and flexibility as DP allowed remote sign out during the COVID-19 pandemic, especially for aging or high-risk pathologist, as well as for those that needed to be quarantined but remain in good health. Furthrmore, another cost reduction is achieved by improved communication between pathologists and streamlined access to diagnostic material, both of which overlap with workforce efficiencies already discussed.
A CAP survey showed that 6.6% of cases were reviewed prior to sign-out in the laboratories surveyed.[50] In the UK, the Royal College of Pathologists recommends double reporting for cases of dysplasia in inflammatory bowel disease and Barrett's esophagus.[51] This increasing trend toward internal review and double reporting requires extra pathologist time in terms of identifying cases for review, marking areas of interest, and finding time to review slides with a colleague. Using a DPS integrated with the Anatomic Pathology (AP) LIS, pathologists can easily annotate an image, mark a case for review and send a message to a colleague to review the case. If desired, the slides can be reviewed synchronously, with the ability to transfer control between users as needed. Alternatively, a slide may be flagged for review by a colleague later, allowing each pathologist to make optimal use of his or her time. This results in increased efficiency for pathologists, producing cost savings as previously described.
REVENUE OPPORTUNITIES
The sources of revenue can be classified as traditional sources, which are the same sources utilized by analog pathology but enhanced by the new technology and “de novo sources” which are new streams of revenue that present themselves after the adoption of a DPS.
Traditional enhanced sources
This includes an increased number of incoming cases as well as an increased number of incoming consults, both through an expanded geography utilizing centralization and telepathology. Multiple kinds of consults may be augmented by DP. Digitization provides the flexibility to see the “same slide at the same time” with collaborators around the globe. Most notable, case-share consulting provides DP adopters an ability to more easily create new revenue streams. Case sharing is mutually beneficial for both the senders and receivers of cases. By outsourcing digitally, sign-out groups realize opportunities for expert second opinions from key sub-specialists, eliminate case backlogs and/or free up staff from performing quality assessments of diagnoses to outside groups or services. At the same time, case sharing can also be a lucrative mechanism to drive new revenue for pathology laboratories who insource cases. Performing secondary consults is a revenue opportunity for traditional glass-based diagnoses. However, with digitization, new and greater revenue opportunities arise.
This new insourced case consult revenue can be regional, domestic, or international. Regional digital consulting allows groups to enhance their local competitive footprint without opening new specimen collection facilities, histology laboratories, or placing pathologists in areas where case volume does not warrant full-time coverage. Therefore, case sharing in a region can provide new revenue while also minimizing additional expense.
With domestic digital consultations, the footprint expands more significantly to every other state. These cases may be secondary consults or primary diagnoses and may be solicited individually or as a contract with a specific institution or institutions. State board certifications may still apply to serving primary diagnosis patients from other states. Guidelines recommend checking your local laws.
International consultations are a promising model for digital consultations. Groups have been established specifically for this purpose. However, large laboratories, including academic medical centers offer DP consultations as a global service initiative. Bringing international cases for digital consult diagnosis has multiple revenue-generating benefits. Not only can the individual pathologist, department or institution gain revenue from the consultation itself, but when patients then travel to the site of the diagnosis for their therapy, pull-through revenue is realized from a patient who would have been outside the catchment area of the digital case sharing organization.
Organizations expanding globally may realize additional revenue streams, including the training of technical and professional groups, global branding, increasing market share, all resulting in overall institutional growth.[33,35]
Pathology laboratories who have adopted digital imaging today are building repository capabilities, for example, Memorial Sloan Kettering (MSK) Cancer Center has digitized 1,000,000 slides with an aggressive goal to create 100,000 digital images per month. Four million slides in MSK's slide archives will be scanned under this goal.[52]
Educational use databases with WSIs have the benefits of viewing images on demand and the ability to mirror real-life scenarios. In addition, networking and collaborative study for students is promoted, which is of importance for those individuals in rural or remote locations.[33,53]
NEW REVENUE OPPORTUNITIES
These include opportunities that did not exist or were not readily available before having a DPS, including WSI-based image analysis, computational pathology, precision medicine, drug development, and clinical trials as well as big data.
Image analysis, computational pathology and precision medicine
Pathology laboratories have infrastructure that includes pathologists, specimens, and outcomes that can generate big data for the creation of computer-assisted diagnostics tools. These resources allow the development of WSI analysis-based decision support tools. Such tools allow pathologists to perform tasks that were previously too difficult or impossible to perform manually.[1,5,54]
Computer-aided image analysis can uncover sub-visual features from morphology (e.g., texture, shape, architecture) that would otherwise be overlooked by a pathologist.[55,56]
In addition to aiding pathologists in manual disease grading, these identifications are also critical for subsequent automated feature analysis. Multiple teams developed algorithms to identify different features such as nuclei, lymphocytes, and mitoses.
Some histologic biomarkers can be correlated with disease progression independent of existing clinical and pathologic features.[3,33,34,54,56] Digital image analysis can improve the assessment of predictive biomarkers in tissue sections and overcome challenges associated with subjective analyses.[1,57]
One of the major goals of modern medicine is to increase patient specificity so that the right treatment is administered to the right patient at the right time with the right dose.
The research in high-throughput morphological analysis of histologic structures, automated grading of tumors and automated selection of desired regions of interest leads to machine learning-based classification of disease presence, grading, and outcome prediction for both basic and translational research. These are useful tools which can help stratify patients and enable the clustering of more homogeneous cohorts in precision assays studies.[58]
In 2013, 80% (60 out of 69) of candidate drugs and companies involved in personalized medicine research and development were focused on oncology.[59] There is a broad need for predictive and prognostic assays to distinguish aggressive from less-aggressive phenotypes of cancer to identify optimal therapies in individual patients and guide clinical trials. Most prognostic tests are based on gene expression assays. Recent studies have shown extensive genetic heterogeneity among cancer cells between tumors and even within the same tumor.[56,60] In fact, molecular signatures for both good and bad prognoses can be found in the same tumor. In reality, tumor morphology, as observed on a standard H and E slide, reflects the sum of all temporal genetic and epigenetic changes and alterations in tumor cells, thereby providing incredible utility for predicting tumor biology, clinical behavior, and treatment response.[56] Therefore, combining phenotypic data with molecular data can result in powerful diagnostic as well as therapeutic tools.[58] The integration of image and omics data to understand the spatial heterogeneity of tumor microenvironments is essential to help guide treatment and avoid unnecessary treatment expenses.[56,60]
Future personalized cancer treatments will require more than just matching a patient's tumor genomics with that of a central library due in part to intratumoral heterogeneity.
Using automated image analysis techniques may generate more precise data regarding intratumoral heterogeneity, which can be measured and used to define both prognosis and optimal therapeutic strategies.
As laboratory testing plays an increasing role in the era of personalized medicine, the role of the pathologist increases and can include image-guided tumor removal, in vivo microscopy, oversight of an increased number of imaging tests that may even overlap with radiology, and more input into a patient's clinical management.
A more critical need exists for consolidated interpretive reporting.[19] The final interpretation and reporting of a given sample will not only include microscopy data that is routinely included in a pathology report but also new ancillary techniques, including data generated from flow cytometry, molecular genetics, and cytogenetics. The depth of knowledge in all these technologies suggests a critical role for pathologists in the implementation of precision medicine.[19,58]
An additional advantage of expanded pathology reports is the patient-centric potential. Patients will be able to see and understand their diagnosis using a meaningful visual context. This could aid the clinical conversation that would not be possible otherwise and build trust and transparency between patient and clinician.
Additional revenue opportunities with pharmaceutical and biotech companies directly related to precision medicine might also be possible. Pathology laboratories may use a DP infrastructure to increase their revenue from partnerships and services in trials and drug development.
Drug development and clinical trials
The drug development pipeline is a complex discipline that relies on data obtained from genomics, proteomics, and metabolomics studies. It also requires new, rapidly advancing tools and techniques that will continue to be increasingly employed in the development process. In 2016, the cost of advancing a single drug from concept to market, including post-FDA approval Phase IV expenses, was estimated to exceed US $1 billion.[61]
The need for multiple data sources suggests that drug development is a huge market opportunity for DP. The pharmaceutical industry research and development ROI depends on innovative processing and the harnessing of information from multiple patients.[62,63,64] Heterogeneity is a crucial factor contributing to failure at the clinical trial phase. Genotypic and phenotypic data could potentially offer new ways to stratify patients and enable the clustering of more homogeneous cohorts.[63] The development of a therapeutic with a companion diagnostic therefore requires patient stratification to identify target patients who are more likely to benefit from therapy. This targeted therapy segment is attractive as it can reduce the time and drug development costs.
According to a research group, the total global clinical trials market is expected to reach $65.2 billion by 2025.[65] The key drivers impacting the growth are globalization of clinical trials, development of new personalized medicine treatments, augmenting evolution in technology, and boosting demand for clinical research organizations to conduct clinical trials. According to the authors of this report, the oncology segment is anticipated to witness the fastest growth. They estimate >$38 billion is currently being spent by the healthcare industry toward preclinical and clinical development of oncology therapy products.[65]
Today, DP is transforming clinical trials by introducing new standards of practice. Most recently, DP-based clinical trials have been completed in nephrology.[66,67,68]
Compared with conventional light microscopy, DP offers several advantages for clinical trials. These include providing a permanent dataset allowing full transparency for regulatory agencies, standard multiple users scoring protocols with reduced travel expenses and personnel time, targeted adjudication, and analytic reproducibility.[66,67]
According to a GenomeWeb study, the FDA approved 25 molecularly targeted indications in 2018.[69] These include 10 new molecular entities and 15 expanded indications of previously approved products in comparison to 19 approvals in 2017. Based on the growth in these FDA approvals, the companion diagnostic sector is also expected to grow.[69]
A 2018 estimate suggested approximately 90 Phase III, diagnostic-dependent drug trials were completed in the US, EU, and Asia this year compared to only 22 in 2017. The number of diagnostic-dependent drug trials is expected to double by the end of 2020. A number of these personalized medicine, diagnostic-dependent treatments will require histopathological data and digitized images for development, documentation, and regulatory filings.[70] As described in the personalized medicine section in this paper, laboratories with whole slide imaging capabilities could greatly benefit from participating in this testing segment.
For biotechnology and pharmaceutical companies, there is significant value in DP for both preclinical and clinical programs, with DP offering potential cost-savings. Pathology laboratories may be able to capitalize on a pharmaceutical services approach where the DP laboratory offers staining, scanning, image hosting, and image analysis services to pharmaceutical groups, driving significant revenue for the laboratory and saving expenses for the pharmaceutical company. However, pharmaceutical companies may be reluctant to outsource these services due to concerns over intellectual property issues. Laboratories should explore this idea further and understand what securities and capabilities need to be in place to meet the needs and address the concerns of pharmaceutical companies.
The importance of a quantitative, biomarker-driven, and overall “digital” pathology approach cannot be overstated to improve the success of the drug development workflow.
BIG DATA
While implementing DP workflows can include significant productivity improvements, another component of the ROI analysis should include the potential value of the data unlocked when slides are digitized.
The medical data insights “trifecta” includes medical image data, medical record data, and genomic data. The derived insights from these three data types is of growing interest to numerous entities, including pharmaceutical and In Vitro Diagnosis (IVD) manufacturers focused on discovering new disease biomarkers, treatment pathways, and other various discoveries. Hospitals and hospital organizations are establishing collaborative networks to share and analyze large data sets as they look toward the improved and optimized delivery of services based on data.
Extracting insights from GB of information on each patient/case and terabytes on patient populations is a considerable endeavor. Recent innovations in “big data” management tools and sophisticated machine learning algorithms facilitate the enormous analysis effort, which includes clustering, regression, classification, and anomaly detection, helping perform processes in a fraction of the time previously possible. Trained data scientists utilize the latest supervised and unsupervised deep learning techniques employing parallel processing methods associated with neural network architectures.
In the search for larger and more specific data sets, de-identified data is being procured from the institutional cohorts as well as individual independent laboratories partnered with data aggregators and data brokers. Data aggregators are using increasingly sophisticated platforms to provide contributors insights to the collective data and an opportunity to participate in revenue sharing models by connecting independent labs to a carefully cultivated network of data acquirers. Although there is some controversy currently about who has the rights to benefit from all this collected data, regulations should follow suit and specify the limitations. When it comes to collecting data at the order of magnitude desired by data scientists, independent laboratories are challenged approaching it alone. Participation through a data aggregator takes advantage of the network effect of established partnerships with a trusted vendor leveraging that vendor's know-how, platform and network, permitting even the smallest laboratory an opportunity to treat their DP data as a core asset and an investment opportunity for the long term.
ROAD MAP: BUILDING A PLAN AND DEVELOPING A BUSINESS PROPOSAL
Laboratories interested in exploring an investment in DP in any form need to start by building a thorough and clear plan for a DP strategy as well as a roadmap. Start by taking a full inventory of the departments and areas of the business needed and/or impacted at the beginning of the strategy development process. This will help streamline the entire project and increase the ease of implementation.
Phase I. Win senior leader support
Before and during the planning stage, senior leadership support is critical and needs to be included in strategy development. Senior leaders must agree and align that the time is right to explore a DP workflow and support activities to gather all the data necessary to make an informed decision.
Phase II. Establish a steering committee/task force
Once senior support has been established, the next step is to identify department stakeholders who should be included in the discovery, planning, and implementation phases.[6,29]
The following stakeholders should have representation from the start to form a task force or steering committee with the understanding that in smaller organizations various roles may correspond to a single person.
Pathologists
A pathologist champion should be identified. He/she will provide feedback and insight about how the change to a DP workflow will impact patient care and the daily workload, should make recommendations about the best plan to initiate a transition and the short-and long-term implementation. The pathologist champion will take the position of influencer within the group, helping to bridge the gap if there is resistance from other members of the department. See change management below.
Laboratory operations
A laboratory operations representative who is knowledgeable in all areas of operations that may be affected by the change should be included. This member should help design a control mechanism to avoid anticipated failures in the workflow and solutions to address failures should they occur.
Information technology
The IT department will play a large role in the entire process. A representative or representatives will evaluate what technology will be required upfront, for implementation and finally, to support the day-to-day functionality of a full DP platform. He/she needs to be able to address security, connectivity, systems, data warehouse and storage.
Compliance/legal
As the industry evolves and DP is more widely accepted and adopted, so will the associated laws and regulations. It is important to include a representative from legal and compliance to ensure that the plans created are in alignment with internal processes and adhere to existing laws and regulations. The representative should be proactive in understanding that DP technology is relatively novel, and as such the laws and regulations may not be complete, created, or passed. Working closely with regulatory agencies, staying informed, and when possible, influencing and educating authorities within the agencies will be the responsibility of the compliance and legal departments.
Managed care/market access
A representative or representatives from managed care and government payer contracting, collectively “market access,” should be invited to participate in the steering committee. Laboratories must be ready to advocate for new Current Procedural Terminology (CPT) codes to assure reimbursement as new use cases are created that do not have an equivalent in the current terminology. The representative from market access must be fully aware of the planned changes, able to add input to the lab plan, and ultimately work with the other members to develop a strategy for reimbursement and a dedicated CPT code. Laboratories moving toward DP might consider working with trade organizations, such as the DPA, to develop an industry strategy. Although laboratories, even academic ones, compete for pathologists, staff, and referrals, it makes sense to work together for CPT codes and policy change for the benefit of all as well as the benefit of patients.
Business development and strategy
Representative (s) from business development and strategy will provide input regarding industry trends and ways to use DP to generate revenue. Their expertise may be put to good use to facilitate the broad strategy, make necessary interdepartmental connections, and establish relationships with organizations outside the laboratory that may be critical to both a successful implementation plan and revenue generating opportunities. Private practice, commercial laboratories and academic institutions can benefit from the skills and experience of individuals from business development.
Finance
For many obvious and not so obvious reasons, the representative (s) from finance should be included from the beginning. Their support and cooperation may be gained if they are able to learn about the technology and the opportunities and efficiencies that will be created from the start. They will contribute valuable feedback regarding potential financial repercussions. Their support will also be critical to gaining leadership's support, and they will be able to guide, make recommendations and in some instances, approve funding for the project.
Project management
Including project management early is beneficial to the success of the program. An assigned project manager who is engaged early on will understand the program at inception, allowing her or him to draw on their knowledge during implementation. Even in small organizations, to have one person coordinate and manage all efforts will be paramount.
Phase III. Define purpose, objectives and goals
Once the DP steering committee has been identified, the first order of business will be to outline the purpose, objectives and goals of the DP program. Regardless of how comprehensive the program will be (as defined by the purpose), its objectives should be straight forward. Once an objective is defined, create goals and tasks required to complete that goal. Outline both short-term and long-term goals. Assign timelines to each task and goal to ensure that the project moves forward in a timely manner and to enforce upfront and clear communication channels with all members of the steering committee and laboratory leadership.
Phase IV. Develop short-term goals
What can be started on immediately? A few examples may include:
Attend conferences and have meetings with vendors
Conferences are a great way to start gathering helpful information from those who have already transitioned to DP as well as identify vendors and resources. Several conferences are held each year that detail how labs have implemented DP and what they learned along the way. (For example, the DPA Visions Conference held annually in October/November is a great resource for information gathering.)
Meet with scanner vendors and software vendors to get an idea of what is available. It will be important to understand the variability in scanners and software programs. Software and LIS compatibility is another critical piece of information to know upfront.
Educate internal stakeholders and influencers
While many individuals are very excited about emerging DP technology, there are others who do not yet see the value in it. Their opinions may be as varied as the reasons for those opinions, and it is helpful to understand their motivations. It is important to understand and address concerns very early on if a DP solution is to be successful. See Change Management below.
Phase V. Develop long-term goals
Consider what the “end game” looks like. Does it include 100% transition from glass slides to digital? Partial transition, such as certain case types or consults only? Research cases only? Immunohistochemical stains only? Retrospective cases? Prospective implementation? Once long-term goals are defined, decide what short-term goals and tasks are needed to achieve them. What equipment and personnel may be needed for the long-term strategy? What processes will have to be completed?
Phase VI. Assess objective, goals and task feasibility
After the potential impacts of a DP workflow implementation project have been considered, it will be up to the committee to determine if the plan is feasible. Objectives and goals may need to be revised several times until a plan is in place that matches what is realistic and practical for the organization.
Minimum requirements for any laboratory to get started include hardware, software, software integration, facilities and labor.
CHANGE MANAGEMENT WITHIN THE ORGANIZATION FOR LONG-TERM SUCCESS
In order to have long-term success, change management should be a priority from the beginning. Change starts by seeing an opportunity to do something in a better or more effective way. Being a champion of change is not easy and taking this on sometimes means going against the deeply embedded norms established within a group of individuals. A recent Harvard Business Review article on engaging health care providers in organizational change found that “when staff view innovations and changes as clashing with longstanding patient care values, they are less likely to adopt new behaviors and practices.”[71] Acknowledging there are obstacles or barriers to adoption is the first step to discerning how they can be overcome. Barriers can be seen as tangible (budget, resources, facilities, compliance, etc.,) and intangible (culture, understanding, fears, etc.,). Distinguishing them from the change at hand enables one to plan for and mitigate adoption risk with a clear understanding of what will be necessary to drive that adoption. For example, if the change is viewed as misaligned with the currently-held core values, then appropriate steps must be taken to align the change with these core values, typically this means building a better understanding of what the change (or future state) looks like and relating that back to those values. Taking the time upfront to listen to concerns and questions from others in the organization (or impacted stakeholder groups) will discern the barriers to be addressed and ultimately speed the transition and drive faster to integrate with their values.
After listening and assessing the current state of perceptions, concerns, potential barriers and awareness, an adoption strategy can be crafted with an action-oriented change plan aligned and integrated with the business case and implementation plan. Adoption should not be an after-thought. The thinking must be embedded in all planning and day-to-day actions to avoid the risk of negatively impacting adoption with the actions taken. Champions must consider how to build buy-in, break down barriers, and drive awareness and motivation for the change within the context of the business case.
How to overcome resistance? Resistance can take many forms and can arise from many different root causes such as a feeling of loss because change is perceived as taking things away including the pride of knowing how things are done. If change is viewed as taking something away, that void must be filled or at a minimum recognized and acknowledged with the future promise of regaining what will be lost. Consider addressing loss through the Kubler-Ross Stages of Grief model[72] or William Bridges' Transition Model[73] which theorizes that “the starting point for dealing with transition is not the outcome but the endings that people have in leaving the old situation behind.” Other causes of resistance that should be proactively addressed include a poor understanding of how to perform in the new process, lack of trust in the new process or technology or in the people driving the change. If any of these occur and hinder the new process, the change will be viewed as unsuccessful and people will revert or find workarounds that inhibit realizing the intended value of the change. Ensure that all impacts from the change (people, process, technology, facilities, performance, etc.,) are sufficiently addressed.
Preparing the business proposal
The approach to a business proposal to implement a DP workflow will likely differ depending upon the laboratory and the nature of their services whether predominantly academic, research or commercial. A stand-alone commercial US AP laboratory may need to look further out to realize returns in commercializing DP while a research laboratory focused on pharmaceutical opportunities may have a stronger case in the near term. An academic setting may have different goals that can be realized through digitizing their pathology process.
Outlining an approach specific to the audience is a crucial element of preparing the business proposal. The task force or steering committee's input will be critical to proposal development and acceptance.
While gathering the data to be submitted with the proposal, scanner, software, analysis algorithm software and other hardware vendors can provide support for capital and operating expenses depending upon ownership models. When possible, include multiple solutions in the proposal.
Quantifying the revenue-generating opportunities of going digital presents more of a challenge. It is best to make reasonable revenue assumptions at different intervals/milestones. Assumptions used to forecast an impact to top-line revenue should be clearly expressed. An honest iterative approach to implementing and scaling up over a period will ensure realistic expectations are set providing the timeline for a laboratory and the market to mature.
Market maturity is key as one looks at the impact of algorithmic detection, staging and clinical decision support functions as they relate to DP. Over time, adoption, support and reimbursement will grow and become established for these new tools and services.
Driving support for digitization may start with socializing the latest published benefits of applying algorithms to DP to the influencers in the organization. One example would be Google's use of their (LYmph Node Assistant) LYNA algorithm resulting in significant increases in specificity and sensitivity in identifying and outlining regions likely to contain tumors while also demonstrating the potential to reduce slide review time by roughly 47%.[74] Dialogue that opens up as a result of sharing articles with influencers can set the stage for a deeper dive into DP for the organization. Maintain a file of articles and abstracts that get to the point quickly.
Develop a narrative and provide data that highlights the insights gained by observing the network effect many other industries/technological advances have experienced over time. In such examples, organizations unable or unwilling to adopt tools miss collaboration opportunities, positive valuation effects and data asset building. For the laboratory, passing on DP may translate to missed collaboration opportunities with reference laboratories, payers and their clients. Various commercial laboratories may miss the opportunity to positively impact the valuation of the enterprise by establishing digital capabilities that are certain to open up markets, allow for collaboration, provide access to algorithmic support tools (some with potential revenue implications), as well as make the laboratory's most valuable asset (data) available to interested parties and prospective alliances and payers. Numerous examples of acquisitions made over the last few years were accomplished with the core asset purchased being the data. Calling these items out and presenting supporting financials relevant to your organization or citing examples of the financial implications to others can go a long way in making a strong body of support in the final proposal.
Finally, establish a regular cadence to meet with the decision makers who can provide the go/no-go decision in moving forward. This will ensure funding allocations and executive support when the time comes for a final decision.
REGULATION AND REIMBURSEMENT
Regulatory
Definitions of what constitutes a medical device/in vitro diagnostic device may differ, but generally they are considered any hardware or software or general material (i.e., reagents) that are used alone or in combination and are intended by the manufacturer to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment, or alleviation of disease. From this perspective diagnostic tests by way of DP platforms are considered medical devices.[75,76,77,78,79]
Through a strictly regulatory lens there are two main types of diagnostic tests, excluding tests under investigation as part of clinical trials: lab developed tests (LDT) and In Vitro Diagnosis tests (IVDs). Generally, health authorities, such as the FDA, regulate device manufacturers who market IVDs, but they have thus far not implemented regulations for clinical laboratories that employ LDTs [Table 3]. The FDA has contemplated the regulation of LDTs which has been a controversial debate over past several years.[79] In the U.S. the Center for Medicare and Medicaid Services (CMS) is the governing body that operationalizes the guidelines and testing standards that were established by the United States Congress through the CLIA of the Public Health Service Acts. CLIA guidelines are designed for all laboratory testing regardless of whether a laboratory employs an LDT or FDA-cleared or-approved IVD. Therefore, both tests are subject to CLIA guidelines. In the U.S., the FDA and CMS have overlapping responsibility in regulating the tests themselves; however, they differ with respect to what organizational entity over which they have authority[Table 3].
Table 3.
Differences in regulatory governance of diagnostic tests
| Government agency via regulatory authority | CMS via CLIA program | FDA via code of regulations (21 CFR) |
|---|---|---|
| Has regulatory authority over… | Clinical laboratories | Medical device manufacturers |
| Intent of regulations | Ensure accurate test results are performed and delivered | Ensure safety and efficacy of marketed devices and tests distributed to laboratories |
| Scope of analytical validity | Single laboratory where test is performed | All laboratories that use the device/test as intended in device labeling |
| Regulates IVD test | Yes – through laboratory oversight | Yes – through device manufacturer oversight |
| Regulates LDTs | Yes – through laboratory oversight | No – FDA has exercised ‘enforcement discretion’* thus far, which may change in future |
*Enforcement discretion is a form of regulation which allows the FDA to formally choose not to enforce the regulations. The FDA may decide to reverse this decision and regulate LDT in the future. CFR: Code of Federal Regulations, CMS: Centers for Medicare and Medicaid Services, CLIA: Clinical Laboratory Improvement Amendments, FDA: Food and Drug Administration, IVD: In vitro diagnostic, LDTs: Laboratory developed tests
When considering the use of DP, laboratories may choose devices that are sold by manufacturers and approved by health authorities for the intended use (i.e., IVD labeled) or laboratories may employ devices that are only intended to be used in their specific laboratory and validated as an LDT. If a laboratory chooses to employ an IVD labeled device for purposes outside the specific intended use for which it is labeled by the manufacturer, the device is then treated as an LDT. Regardless of the device type, all new devices/tests should be validated in the laboratory for its intended purpose. There are various sources to obtain more specifics on validation for DP.[77]
For devices labeled as an IVD (either as a Conformité Européene [CE] mark in Europe, FDA in the United States, etc.) validations can follow the manufacturer's instructions and the user can have reasonable assurances that the device will perform according to its specifications. This is because such devices were necessarily developed and manufactured under strict medical device regulations for its specific intended use and have been demonstrated to be safe and effective. In contrast, if a laboratory decides to employ equipment or software that was not developed under medical device regulations or they decide to use an IVD labeled device for “nonintended use purposes,” they should consider the device an LDT as indicated above and employ robust “fit for purpose” validations (analytical and clinical) as required by CLIA. Proper validation is essential given that the device has not been required to pass specific standards prior to marketing.
Reimbursement
To date, there are no new reimbursement models that directly support the implementation of DP technology. CPT code 88361 was introduced in the U.S. to cover costs associated with the morphometric quantitation using “computer-assisted technology” of breast IHC markers ER, PR, Her2. The narrow indication (i. e., breast IHC) is a major challenge when considering the use of this code for substantial reimbursement related to the implementation and broad use of DP devices in the clinical laboratory. Laboratories should consult reimbursement specialists in the context of their own use cases and volumes to determine if there are any revenue advantages that can be gained with the current payment models for their given geographic location. In most instances, laboratories will likely bill for their services in the same manner whether they use digital technology or microscopes.
It is reasonable to ask whether new or additional reimbursement may be implemented for DP, given its inherent value to the laboratory and wider healthcare delivery model. Inside and outside the U.S., the path to establish additional payment for any novel diagnostic technology is long and complex. For the device manufacturers, many factors need to be considered when presenting a case for payment to payers including technical assessments, regulatory approvals, clinical guidelines, patient and provider advocacy and cost/benefit analysis.[80] Of these, cost/benefit, changes to established clinical guidelines, and patient advocacy could be the most challenging bar for a technology that has yet to demonstrate significant value with respect to patient outcomes (i.e., DP). Regardless, the lack of standardization, transparency and guidance has been a major challenge for reimbursement in general even for those technologies that can demonstrate strong value.
In many countries, innovative technologies require a formal health technology assessment (HTA) in order to be considered for reimbursement, although this is not requirement in the U.S. for positive payment decisions. In the context of HTA, two main types of values are taken into consideration: “core elements” and “wider elements.”[81] Core elements of value include health benefits to the patient such as improved prognosis and/or survival and improved quality of life. Wider elements of value include a long list of nonhealth related aspects including reduced costs, convenience, improved productivity and others. By its very definition, it is likely that demonstrating 'core value' is a critical aspect of gaining reimbursement. The lack of emphasis on noncore value aspects, relative to core value, is a major challenge for technologies that can have great benefits on cost and efficiency but have not yet shown value with respect to health outcomes. Therefore, finding value in the form of additional revenue for the use of DP soon is unlikely to come from direct payment for the use of DP technologies. Nonprofit organizations such as the DPA will need to research and influence reimbursement strategies as the industry adopts this technology in the future. In the meantime, estimating a return on the investment should focus on cost savings and other revenue streams associated with the overall delivery of pathology services that are discussed in this paper.
CONCLUSIONS
Pathology laboratories, commercial, academic and community, will benefit from exploring and engaging in an internal discussion to implement a DP workflow. Change is imminent, and it will be highly beneficial for pathology and laboratory medicine as well as the patients and all those involved in their care. Laboratories starting the discussion now will be poised to make the best decision, one that is fiscally responsible, proactive and innovative, rather than waiting until forced into a DP workflow. It is the best approach to have the time to design an individual path toward transition, one that has clearly defined requirements, objectives, goals and a roadmap.
DP will definitively change the way pathology is practiced, especially when image analysis tools and assays are able to aid in diagnosis, prognosis, risk stratification and therapy selection. By more comprehensive pathology reports being generated for treating physicians, the pathologist will be able to educate and directly assist in the decision-making process. Providing guidance by monitoring responses to therapy and communicating those results to the physician, the pathologist's role will be transformed from a behind-the-scenes collaborator into a dynamic, engaged participant in the clinical team.
Financial support and sponsorship
Nil.
Conflicts of interest
Mark Lloyd is an employee of Inspirata, Inc. Gerard Slootweg is an employee of Philips. Bryan Van Meter is an employee of Barco, Inc.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2021/12/1/17/313004
REFERENCES
- 1.Aeffner F, Zarella MD, Buchbinder N, Bui MM, Goodman MR, Hartman DJ, et al. Introduction to digital image analysis in whole-slide imaging: A white paper from the digital pathology association. J Pathol Infor. 2019;10:9–9. doi: 10.4103/jpi.jpi_82_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna MG. Digital pathology. In: Narayan R, editor. Encyclopedia of Biomedical Engineering. Vol. 2. 2018. pp. 524–32. Elsevier. [Google Scholar]
- 3.Zarella MD, Bowman D, Aeffner F, Farahani N, Xthona A, Absar SF, et al. A practical guide to whole slide imaging: A white paper from the digital pathology association. Arch Pathol Lab Med. 2019;143:222–34. doi: 10.5858/arpa.2018-0343-RA. [DOI] [PubMed] [Google Scholar]
- 4.Tizhoosh HR, Pantanowitz L. Artificial intelligence and digital pathology: Challenges and opportunities. J Pathol Inform. 2018;9:38. doi: 10.4103/jpi.jpi_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abels E, Pantanowitz L, Aeffner F, Zarella MD, van der Laak J, Bui MM, et al. Computational pathology definitions, best practices, and recommendations for regulatory guidance: A white paper from the Digital Pathology Association. J Pathol. 2019;249:286–94. doi: 10.1002/path.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baidoshvili A. How to go digital in pathology. In: The Pathologist. London: Texere Publishing Limited; 2016. [Google Scholar]
- 7.Baidoshvili A, Bucur A, van Leeuwen J, van der Laak J, Kluin P, van Diest PJ. Evaluating the benefits of digital pathology implementation: Time savings in laboratory logistics. Histopathology. 2018;73:784–94. doi: 10.1111/his.13691. [DOI] [PubMed] [Google Scholar]
- 8.Thorstenson S, Molin J, Lundström C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: Digital pathology experiences 2006-2013. J Pathol Inform. 2014;5:14. doi: 10.4103/2153-3539.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asa SL, Bodén AC, Treanor D, Jarkman S, Lundström C, Pantanowitz L. 2020 vision of digital pathology in action. J Pathol Inform. 2019;10:27. doi: 10.4103/jpi.jpi_31_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams BJ, Bottoms D, Clark D, Treanor D. Future-proofing pathology part 2: Building a business case for digital pathology. J Clin Pathol. 2019;72:198–205. doi: 10.1136/jclinpath-2017-204926. [DOI] [PubMed] [Google Scholar]
- 11.Williams BJ, Bottoms D, Treanor D. Future-proofing pathology: The case for clinical adoption of digital pathology. J Clin Pathol. 2017;70:1010–8. doi: 10.1136/jclinpath-2017-204644. [DOI] [PubMed] [Google Scholar]
- 12.Williams BJ, Hanby A, Millican-Slater R, Nijhawan A, Verghese E, Treanor D. Digital pathology for the primary diagnosis of breast histopathological specimens: An innovative validation and concordance study on digital pathology validation and training. Histopathology. 2018;72:662–71. doi: 10.1111/his.13403. [DOI] [PubMed] [Google Scholar]
- 13.Williams BJ, Jayewardene D, Treanor D. Digital immunohistochemistry implementation, training and validation: Experience and technical notes from a large clinical laboratory. J Clin Pathol. 2019;72:373–8. doi: 10.1136/jclinpath-2018-205628. [DOI] [PubMed] [Google Scholar]
- 14.Steiner DF, MacDonald R, Liu Y, Truszkowski P, Hipp JD, Gammage C. Impact of Deep Learning Assistance on the Histopathologic Review of Lymph Nodes for Metastatic Breast Cancer. Am J Surg Pathol. 2018;42:1636–46. doi: 10.1097/PAS.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingdom RCoPU. Digital Pathology. Royal College of Pathologists. [[Last accessed on 2019 Jul 10]]. Available from:http://www.rcpath.org/discover-pathology/publicaffairs/ digital-pathology.html .
- 16.Retamero JA, Aneiros-Fernandez J, Del Moral RG. Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch Pathol Lab Med. 2020;144:221–8. doi: 10.5858/arpa.2018-0541-OA. [DOI] [PubMed] [Google Scholar]
- 17.Evans AJ, Salama ME, Henricks WH, Pantanowitz L. Implementation of whole slide imaging for clinical purposes: Issues to consider from the perspective of early adopters. Arch Pathol Lab Med. 2017;141:944–59. doi: 10.5858/arpa.2016-0074-OA. [DOI] [PubMed] [Google Scholar]
- 18.Tetu B, Evans A. Canadian licensure for the use of digital pathology for routine diagnoses: one more step toward a new era of pathology practice without borders. Arch Pathol Lab Med. 2014;138:302–4. doi: 10.5858/arpa.2013-0289-ED. [DOI] [PubMed] [Google Scholar]
- 19.Volynskaya Z, Chow H, Evans A, Wolff A, Lagmay-Traya C, Asa SL. Integrated pathology informatics enables high-quality personalized and precision medicine: Digital pathology and beyond. Arch Pathol Lab Med. 2018;142:369–82. doi: 10.5858/arpa.2017-0139-OA. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, Feldman MD, Abels E, Ashfaq R, Beltaifa S, Cacciabeve NG, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: A multicenter blinded randomized noninferiority study of 1992 cases (Pivotal Study) Am J Surg Pathol. 2018;42:39–52. doi: 10.1097/PAS.0000000000000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans AJ, Bauer TW, Bui MM, Cornish TC, Duncan H, Glassy EF, et al. US Food and drug administration approval of whole slide imaging for primary diagnosis: A key milestone is reached and new questions are raised. Arch Pathol Lab Med. 2018;142:1383–7. doi: 10.5858/arpa.2017-0496-CP. [DOI] [PubMed] [Google Scholar]
- 22.Hu YF FDA issuance of substancial equivalence determination for the Aperio AT2 DX system. Administration UfaD. 2019. [Google Scholar]
- 23.Zarella MD, Feldscher A. laboratory computer performance in a digital pathology environment: Outcomes from a single institution. J Pathol Inform. 2018;9:44. doi: 10.4103/jpi.jpi_47_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accreditation Council for Graduate Medical Education Data Resource Book Academic Year 2016-2017. 2017. [[Last accessed on 2019 Apr 15]]. Available from: http://www.acgme.org/About-Us/Publications-and-Resources/Graduate-Medical-Education-Data-Resource-Book .
- 25.Accreditation Council for Graduate Medical Education Data Resource Book Academic Year 2007-2008. 2008. [[Last accessed on 2019 Apr 15]]. Available from: http://www. acgme.org/About-Us/Publications-and-Resources/Graduate-Medical- Education-Data-Resource-Book .
- 26.Ellison EC, Pawlik TM, Way DP, Satiani B, Williams TE. Ten-year reassessment of the shortage of general surgeons: Increases in graduation numbers of general surgery residents are insufficient to meet the future demand for general surgeons. Surgery. 2018;164:726–32. doi: 10.1016/j.surg.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Petriceks AH, Salmi D. Trends in pathology graduate medical education programs and positions, 2001 to 2017. Acad Pathol. 2018 Mar;28(5) doi: 10.1177/2374289518765457. 2374289518765457. doi: 10.1177/2374289518765457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robboy SJ, Weintraub S, Horvath AE, Jensen BW, Alexander CB, Fody EP, et al. Pathologist workforce in the United States: I. Development of a predictive model to examine factors influencing supply. Arch Pathol Lab Med. 2013 Dec;137(12):1723–32. doi: 10.5858/arpa.2013-0200-OA. doi: 10.5858/arpa.2013-0200-OA. Epub 2013 Jun 5. PMID: 23738764. [DOI] [PubMed] [Google Scholar]
- 29.Ho J, Ahlers SM, Stratman C, Aridor O, Pantanowitz L, Fine JL, et al. Can digital pathology result in cost savings?A financial projection for digital pathology implementation at a large integrated health care organization. J Pathol Inform. 2014;5:33. doi: 10.4103/2153-3539.139714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdullah B. Generational challenges to radiology education and practice. Biomed Imaging Interv J. 2009;5:e31. doi: 10.2349/biij.5.4.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo H, Birsa J, Farahani N, Hartman DJ, Piccoli A, O'Leary M, et al. Digital pathology and anatomic pathology laboratory information system integration to support digital pathology sign-out. J Pathol Inform. 2016;7:23. doi: 10.4103/2153-3539.181767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ZW, Kohan J, Perkins SL, Hussong JW, Salama ME. Web-based oil immersion whole slide imaging increases efficiency and clinical team satisfaction in hematopathology tumor board. J Pathol Inform. 2014;5:41. doi: 10.4103/2153-3539.143336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aeffner F, Blanchard TW, Keel MK, Williams BH. Whole-slide imaging: The future is here. Vet Pathol. 2018;55:488–9. doi: 10.1177/0300985818771146. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton PW, Bankhead P, Wang Y, Hutchinson R, Kieran D, McArt DG, et al. Digital pathology and image analysis in tissue biomarker research. Methods. 2014;70:59–73. doi: 10.1016/j.ymeth.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Farris AB, Cohen C, Rogers TE, Smith GH. Whole slide imaging for analytical anatomic pathology and telepathology: Practical applications today, promises, and perils. Arch Pathol Lab Med. 2017;141:542–50. doi: 10.5858/arpa.2016-0265-SA. [DOI] [PubMed] [Google Scholar]
- 36.Griffin J, Treanor D. Digital pathology in clinical use: Where are we now and what is holding us back? Histopathology. 2017;70:134–45. doi: 10.1111/his.12993. [DOI] [PubMed] [Google Scholar]
- 37.Romero Lauro G, Cable W, Lesniak A, Tseytlin E, McHugh J, Parwani A, et al. Digital pathology consultations-a new era in digital imaging, challenges and practical applications. J Digit Imaging. 2013;26:668–77. doi: 10.1007/s10278-013-9572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vodovnik A. Diagnostic time in digital pathology: A comparative study on 400 cases. J Pathol Inform. 2016;7:4. doi: 10.4103/2153-3539.175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.House JC, Henderson-Jackson EB, Johnson JO, Lloyd MC, Dhillon J, Ahmad N, et al. Diagnostic digital cytopathology: Are we ready yet? J Pathol Inform. 2013;4:28. doi: 10.4103/2153-3539.120727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Independent Cancer Taskforce U. Cancer Research UK: Independent Cancer Taskforce U; 2015. Achieving World Class Cancer Outcomes. A Strategy for England 2015-2020. [Google Scholar]
- 41.UK CR. Testing Times to Come? An Evaluation of Pathologoy Capacity Across the UK. 2016 Cancer Research UK; 2016. [Google Scholar]
- 42.Pantanowitz L, Hornish M, Goulart RA. The impact of digital imaging in the field of cytopathology. Cytojournal. 2009;6:6. doi: 10.4103/1742-6413.48606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Urrego PA, Cronin AM, Al-Ahmadie HA, Gopalan A, Tickoo SK, Reuter VE, et al. Interobserver and intraobserver reproducibility in digital and routine microscopic assessment of prostate needle biopsies. Hum Pathol. 2011;42:68–74. doi: 10.1016/j.humpath.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Van Eycke YR, Allard J, Salmon I, Debeir O, Decaestecker C. Image processing in digital pathology: an opportunity to solve inter-batch variability of immunohistochemical staining. Sci Rep. 2017 Feb 21;7:42964. doi: 10.1038/srep42964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dennis J, Parsa R, Chau D, Koduru P, Peng Y, Fang Y, et al. Quantification of human epidermal growth factor receptor 2 immunohistochemistry using the Ventana Image Analysis System: Correlation with gene amplification by fluorescence in situ hybridization: The importance of instrument validation for achieving high (> 95%) concordance rate. Am J Surg Pathol. 2015;39:624–31. doi: 10.1097/PAS.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 46.Helin HO, Tuominen VJ, Ylinen O, Helin HJ, Isola J. Free digital image analysis software helps to resolve equivocal scores in HER2 immunohistochemistry. Virchows Arch. 2016;468:191–8. doi: 10.1007/s00428-015-1868-7. [DOI] [PubMed] [Google Scholar]
- 47.Veta M, van Diest PJ, Jiwa M, Al-Janabi S, Pluim JP. Mitosis counting in breast cancer: Object-level interobserver agreement and comparison to an automatic method. PLoS One. 2016;11:e0161286. doi: 10.1371/journal.pone.0161286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen I, Lucas M, Savci-Heijink CD, Meijer SL, Marquering HA, de Bruin DM, et al. Histopathology: ditch the slides, because digital and 3D are on show. World J Urol. 2018;36:549–55. doi: 10.1007/s00345-018-2202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owens SR, Dhir R, Yousem SA, Kelly SM, Piccoli A, Wiehagen L, et al. The development and testing of a laboratory information system-driven tool for pre-sign-out quality assurance of random surgical pathology reports. Am J Clin Pathol. 2010;133:836–41. doi: 10.1309/AJCPLN9DU9LNXSXA. [DOI] [PubMed] [Google Scholar]
- 50.Nakhleh RE, Bekeris LG, Souers RJ, Meier FA, Tworek JA. Surgical pathology case reviews before sign-out: A College of American Pathologists Q-Probes study of 45 laboratories. Arch Pathol Lab Med. 2010;134:740–3. doi: 10.5858/134.5.740. [DOI] [PubMed] [Google Scholar]
- 51.Pathologists TRCo. Tissue Pathways for Gastrointestinal and Pancreaticobiliary Pathology. London, England, UK: The Royal College of Pathologists; 2016. p. G085. [Google Scholar]
- 52.Newitt V. Digital Pathology: A 1st Anniversary Report Card. CAP Today. 2018 Apr [Google Scholar]
- 53.Van Es SL. Digital pathology: Semper ad meliora. Pathology. 2019;51:1–0. doi: 10.1016/j.pathol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Aeffner F, Adissu HA, Boyle MC, Cardiff RD, Hagendorn E, Hoenerhoff MJ, et al. Digital microscopy, image analysis, and virtual slide repository. ILAR J. 2018;59:66–79. doi: 10.1093/ilar/ily007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farahani N, Monteith CE. The coming paradigm shift: A transition from manual to automated microscopy. J Pathol Inform. 2016;7:35. doi: 10.4103/2153-3539.189698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhargava R, Madabhushi A. Emerging themes in image informatics and molecular analysis for digital pathology. Annu Rev Biomed Eng. 2016;18:387–412. doi: 10.1146/annurev-bioeng-112415-114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saeed-Vafa D, Magliocco AM. Practical applications of digital pathology. Cancer Control. 2015;22:137–41. doi: 10.1177/107327481502200203. [DOI] [PubMed] [Google Scholar]
- 58.Ching T, Himmelstein DS, Beaulieu-Jones BK, Kalinin AA, Do BT, Way GP, et al. Opportunities and obstacles for deep learning in biology and medicine. J R Soc Interface. 2018 Apr;15(141):20170387. doi: 10.1098/rsif.2017.0387. doi: 10.1098/rsif.2017.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milne CP, Cohen JP, Chakravarthy R. Market watch: Where is personalized medicine in industry heading? Nat Rev Drug Discov. 2015;14:812–3. doi: 10.1038/nrd4759. [DOI] [PubMed] [Google Scholar]
- 60.Lloyd KL, Cree IA, Savage RS. Prediction of resistance to chemotherapy in ovarian cancer: A systematic review. BMC Cancer. 2015;15:117. doi: 10.1186/s12885-015-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matthews H, Hanison J, Nirmalan N. “Omics”-Informed Drug and Biomarker Discovery: Opportunities, Challenges and Future Perspectives? Review Proteomes. 2016 Sep 12;4(3):28. doi: 10.3390/proteomes4030028. doi: 10.3390/proteomes4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matthews G, Williams N. Post-Brexit crystal ball gazing: The future for phase 1 clinical trials in the UK. Med Leg J. 2018;86:63–71. doi: 10.1177/0025817218767265. [DOI] [PubMed] [Google Scholar]
- 63.Madabhushi A, Lee G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med Image Anal. 2016;33:170–5. doi: 10.1016/j.media.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mroz P, Parwani AV, Kulesza P. Central pathology review for phase III clinical trials: The enabling effect of virtual microscopy. Arch Pathol Lab Med. 2013;137:492–5. doi: 10.5858/arpa.2012-0093-RA. [DOI] [PubMed] [Google Scholar]
- 65.Research GV. Clinical Trials Market Size, Share & Trends Analysis Report By Phase (I/II/III/IV), By Study Design (Interventional, Expanded Access), By Indication (Oncology, Diabetes, Obesity), And Segment Forecasts, 2019-2026. Gran View Research. 2019 Feb [Google Scholar]
- 66.Barisoni L, Gimpel C, Kain R, Laurinavicius A, Bueno G, Zeng C, et al. Digital pathology imaging as a novel platform for standardization and globalization of quantitative nephropathology. Clin Kidney J. 2017;10:176–87. doi: 10.1093/ckj/sfw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barisoni L, Hodgin JB. Digital pathology in nephrology clinical trials, research, and pathology practice. Curr Opin Nephrol Hypertens. 2017;26:450–9. doi: 10.1097/MNH.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barisoni L, Nast CC, Jennette JC, Hodgin JB, Herzenberg AM, Lemley KV, et al. Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE) Clin J Am Soc Nephrol. 2013;8:1449–59. doi: 10.2215/CJN.08370812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.T R. Personalized Medicine 2018: More Drugs, Greater NGS Adoption, Growing Appreciation of Dx Value. Genomeweb. 2018. [[Last accessed on 2019 Jul 15]]. Available from: http://www.genoweb.com/molecular-diagnostics/personalizedmedicine-2018-more-drugs-greater-ngs-adoption-growing# .
- 70.Andrews G, Williams AD. Internet psychotherapy and the future of personalized treatment. Depress Anxiety. 2014;31:912–5. doi: 10.1002/da.22302. [DOI] [PubMed] [Google Scholar]
- 71.Brett JF, Luciano MM. 3 Steps for Engaging Health Care Providers in Organizational Change. Harvard Business Review. 2018 18 October. [Google Scholar]
- 72.Kübler-Ross E, Kessler D. On Grief and Grieving: Finding the Meaning of Grief Through the Five Stages of loss. Toronto: Scribner; 2005. [Google Scholar]
- 73.Bridges W, Bridges SM. anaging Transitions: Making the Most of Change. Reading, MA: Addison-Wesley; 1991. [Google Scholar]
- 74.Steiner DF, MacDonald R, Liu Y, Truszkowski P, Hipp JD, Gammage C. Impact of Deep Learning Assistance on the Histopathologic Review of Lymph Nodes for Metastatic Breast Cancer. Am J Surg Pathol. 2018;42:1636–46. doi: 10.1097/PAS.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Contis L, et al. Validating whole slide imaging for diagnostic purposes in pathology: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137:1710–22. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hipp J. Digital Pathology Resource Guide. College of American Pathologists. 2017 [Google Scholar]
- 77.Abels E, Pantanowitz L. Current state of the regulatory trajectory for whole slide imaging devices in the USA. J Pathol Inform. 2017;8:23. doi: 10.4103/jpi.jpi_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Code of Federal Regulations Title 21. U.S. Food and Drug Administration. In: Services HaH Food and Drug Administration. https://www.fda.gov/medical-devices/medical-device-databases/codefederal-regulations-title-21-food-and-drugs. [Google Scholar]
- 79.Gatter K. FDA oversight of laboratory-developed tests: Where are we now? Arch Pathol Lab Med. 2017;141:746–8. doi: 10.5858/arpa.2017-0053-ED. [DOI] [PubMed] [Google Scholar]
- 80.Gustavsen GP, Pothier K Health Advances, the Reinbursement Landscape for Novel Diagnostics. Current Limitations, Real-World Impact and Proposed Solutions. 2010 [Google Scholar]
- 81.Henshall C, Schuller T HTAi Policy Forum. Health technology assessment, value-based decision making, and innovation. Int J Technol Assess Health Care. 2013;29:353–9. doi: 10.1017/S0266462313000378. [DOI] [PubMed] [Google Scholar]