Abstract
Introduction
Diabetic nephropathy (DN) develops in about 40% of patients with type 2 diabetes and remains the leading cause of end-stage renal disease. The mechanisms of DN remain to be elucidated. Oxidative stress is thought to be involved in the development of DN but antioxidant therapy has produced conflicting results. Therefore, we sought to define the role of antioxidant in retarding the development of DN in this study.
Research design and methods
We generated a new antioxidant/diabetes mouse model, LiasH/HLeprdb/db mice, by crossing db/db mice with LiasH/H mice, which have overexpressed Lias gene (~160%) compared with wild type, and also correspondingly increased endogenous antioxidant capacity. The new model was used to investigate whether predisposed increased endogenous antioxidant capacity was able to retard the development of DN. We systemically and dynamically examined main pathological alterations of DN and antioxidant biomarkers in blood and kidney mitochondria.
Results
LiasH/HLeprdb/db mice alleviated major pathological alterations in the early stage of DN, accompanied with significantly enhanced antioxidant defense. The model targets the main pathogenic factors by exerting multiple effects such as hypoglycemic, anti-inflammation, and antioxidant, especially protection of mitochondria.
Conclusion
The antioxidant animal model is not only very useful for elucidating the underlying mechanisms of DN but also brings insight into a new therapeutic strategy for clinical applications.
Keywords: antioxidant, kidney, diabetes complications, obesity
Significance of this study.
What is already known about this subject?
Oxidative stress is thought to be involved in the development of diabetic nephropathy (DN) but antioxidant therapy has produced conflicting results.
What are the new findings?
We generated a new antioxidant/diabetes mouse model by crossing db/db mice, a common type 2 diabetes mouse model, with LiasH/H mice, which have overexpressed Lias gene (~160%) compared with wild type, and also correspondingly increased endogenous antioxidant capacity.
The strengthened endogenous antioxidant capacity animal model significantly attenuated pathological changes of early stage in DN including microalbuminuria, mesangial matrix expansion, podocyte foot process effacement, and chronic inflammatory response, accompanied with increased antioxidant content in the blood and kidney mitochondria.
Strengthened antioxidant defense would be a valuable strategy for prevention and treatment of human DN.
How might these results change the focus of research or clinical practice?
Strengthened antioxidant defense would be a valuable strategy for prevention and treatment of human DN.
Introduction
Diabetes mellitus (DM) is the leading cause of cardiovascular and renal disease worldwide. In the last decades, the prevalence of type 2 diabetes mellitus (T2DM) has been increasing. Among microvascular complications of diabetes, DN develops in about 40% of patients with T2DM and T1DM and remains the leading cause of end-stage renal disease.1–3 The prevalence of diabetes including T1DM and T2DM is 11.6% in Chinese adults; this may represent up to 113.9 million adults.4 Among those with diabetes, the prevalence of chronic kidney disease is as high as 63.9%.4
The mechanisms of DN remain uncertain.5 Increased oxidative stress is proposed to be a major mechanism.1 Hyperglycemia is a major contributor to reactive oxygen species (ROS) production in mitochondria6 7 and subsequently oxidative stress.8 Accumulative evidence suggests that oxidative stress derived from impaired antioxidant defenses has been implicated in the pathogenesis of DN.9 However, antioxidant therapy has produced conflicting results.10 We postulate that impairment of antioxidant defense plays a critical role in the prevention and treatment of DN. The mouse models of antioxidant could be useful for a better understanding of the role of antioxidants in the onset and development of DN, although they usually exhibit modest kidney abnormalities and can only mimic the early stage of human DN.11
α-lipoic acid (LA) is a potent antioxidant, catalyzed by lipoic acid synthase (Lias) in the mitochondria. To test our hypothesis, we generated two mouse lines by modifying 3′ untranslated regions of Lias gene (Lias) to influence mRNA stability. These models exhibit different levels of gene expression (LiasLow/Low~10% and LiasHigh/High~160% of wild type) with corresponding LIAS expression, determined by real-time quantitative (qRT)-PCR and western blot. Our previous study showed that total plasma antioxidant capacity was significantly increased and lipid peroxidation induced by oxidative stress was exhibited in animal model with high expression of Lias gene.12 And the LiasH/H animals did not show any abnormal behaviors and performance in all generation in our long-term observations. By crossing LiasH/H with Leprdb/db mice, the most widely used model of type 2 diabetes,13 we generated the LiasH/HLeprdb/db mouse model. Our novel LiasH/HLeprdb/db mice mimic gain-of-function of antioxidant by creating a strengthened endogenous antioxidant microenvironment. They may amplify subtle changes that may not be apparent under basal conditions and will be helpful to identify LA targeted diagnostic biomarkers in mitochondria, and decipher the mechanisms of immune regulations during the development of the disease.
In this study, we sought to determine whether overexpression of Lias gene could attenuate oxidative stress and chronic inflammation in mice with DN. Our data demonstrated that LiasH/HLeprdb/db mice with increased endogenous antioxidant level showed alleviated major pathological alterations of T2DM at the early stage of DN.
Research design and methods
Animal models
Eight-week-old C57BL/J db/+ and db/db mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). Mice were fed ad libitum in a specific pathogen-free animal facility, LiasH/H mice on the same genetic background were mated with db/+ mice to finally obtain LiasH/HLeprdb/db. Male LiasH/HLeprdb/db mice will be used in our experiments since the lack of gender differences in the severity of renal injury in the Leprdb/db mouse model of DN.14 All animals were fed a normal chow produced according to NIH-07 standard and meet the hygienic standard of experimental animal compound feed (GB 14924.2) with 4% fat and 20.5% protein content (Puluteng, Shanghai, China). Water ad libitum. Animal experiments were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Xinxiang Medical University.
Measurement of blood metabolic variables
Blood glucose was monitored using OneTouch Verio Flex meter (Johnson-Johnson, New Brunswick, New Jersey, USA) after 5-hour fasting. Glycated hemoglobin (HbA1c) was measured from red cell lysates using high performance liquid chromatography (Bio-Rad, Richmond, California, USA). Serum levels of triglyceride (TG) and cholesterol (CHO) were determined using commercial assay kits (Jian-Cheng, Nan Jing, China). Levels of blood creatinine and blood urea nitrogen (BUN) were determined using commercial assay kits (Jian-Cheng, Nan Jing, China), and microalbuminuria was determined using ELISA kits (Ela science, Wuhan, China) following the manufacturer’s instruction.
Intraperitoneal glucose tolerance test (IPGTT)
All mice were fasted for 16 hours with free access to water before intraperitoneal injection of a glucose solution (0.5 mg/kg of body weight). Blood glucose was measured by the glucometer at designed timepoints after intraperitoneal injection of the glucose solution.
Measurement of urinary albumin excretion and creatinine concentration
Mice were singly placed in metabolic cages for 24 hours after acclimation and then food consumption, water intake, body weight, and urine output were recorded daily. Urinary albumin excretion per day was determined by mouse urinary albumin ELISA kit (Nanjing Bianzhen Biological Technology, China). Creatinine concentration in serum was measured by sarcosine oxidase method.
ELISA to assess oxidative stress and inflammation markers
Blood levels of total autoxidizing capability (T-AOC), reduced glutathione (GSH) and malondialdehyde (MDA) were measured using commercial kits purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China) following the manufacturer’s instructions. Mitochondria were isolated from kidney cortex. GSH concentration was determined using a chromogenic assay (Thermo, EnSpire 2300, USA). MDA concentration was assayed using a chromogenic assay. This assay measures free and protein-bound MDA without undue interference from the other lipid peroxidation products.15 Superoxide dismutase 2 (SOD2) activity was measured using the Beauchamp and Fridovich method.16 Levels of T-AOC were measured using commercial kits (Cell Biolabs, USA). The net absorbance values (at 490 nm) of antioxidants are compared with a known uric acid standard curve. Results are expressed as ‘μM copper reducing equivalents’ (μM CRE). The levels of serum interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α) and monocyte chemotactic protein-1 (MCP-1) in each group were analyzed by using ELISA kits (Dingguo Biotechnology, Beijing, China) according to the manufacturer’s instructions. The color generated was determined by measuring the optical density (OD) value at 450 nm with a spectrophotometric microtiter plate reader (Thermo, EnSpire 2300, USA). Levels of serum C-reactive protein (CRP) were determined using ELISA kits (Dingguo Biotechnology, Beijing, China).
Renal histopathology
The kidneys from mice were fixed in 4% paraformaldehyde at room temperature (RT) and then embedded in paraffin. Tissues were prepared as sections 3 µm in thickness and stained with periodic acid–Schiff (PAS) for examinations by light microscopy. The degree of glomerular injury was evaluated according to the degree of mesangial matrix expansion (MME)17 examined in 30 glomeruli per kidney with a ×100 objective oil immersion lens from each group of mice on PAS-stained kidney tissue sections. Sixteen slides of mouse kidney covering the whole spectrum of renal cortical were evaluated by an experienced animal pathologist. MME was examined in a blinded fashion and scored from 0 to 4 according to the ratio of glomerular expansion area/normal area: score 0, a normal glomerulus; score 1, increased mesangial matrix, 25% of glomerular tuft; score 2, MME of 25%–50% of glomerular tuft; score 3, MME of 50%–75%; and score 4, MME of >75% of the tuft.
Kidney mitochondrial dysfunction
Cytosolic and mitochondrial fractions were separated using a Mitochondria Isolation Kit (Beyotime, Shanghai, China) following the manufacturer’s instructions.
The mitochondrial SOD2 assay kit was used to determine the enzyme activities of SOD2 according to the manufacturer’s instructions (Beyotime Biotechnology, Shanghai, China). The ATP Colorimetric Assay Kit was used to measure the ATP content in the kidney cortex according to the manufacturer’s protocol (Jiancheng, Nan Jing, China).
Western blotting analysis
Total proteins were extracted from the kidneys. Homogenate (30 µg) was separated by 10% SDS-PAGE (Sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, New York, USA). Membranes were then incubated with the following primary antibodies: NF-κB p65 (1:1000, Cell Signaling, Boston, Massachusetts, USA), LIAS (1:1000, Proteintech, USA) and NRF2 antibodies (1:1000, Cell Signaling, Boston, Massachusetts, USA). Membranes were subsequently washed and incubated with horseradish peroxidase conjugated anti-rabbit secondary antibodies (1:3000, Earthox, San Francisco, California, USA). Immunoreactive bands were visualized on ECL Hyperfilm (GE Healthcare Bio-Sciences) using the Amersham ECL Prime Western Blotting Detection Reagent enhanced chemiluminescence (GE Healthcare Bio-Sciences, Pittsburgh, Pennsylvania, USA). All western blot analyses were performed at least three times. Membranes were incubated with rabbit polyclonal anti-GAPDH antibodies (1:5000, Proteintech, USA) or anti-COXIV to serve as controls for equal loading. The density of proteins in each band was determined using image J2X software and expressed as a relative value to the density of the corresponding band control protein immunoblot.
Transmission electron microscopic analysis
The right kidney cortical tissues were immediately fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 3 hours at RT. After, the specimens were postfixed with 1% osmium tetroxide. Ultrathin sections (70–80 nm thick) were made on an RMC Power Tome XL ultramicrotome (Shanghai Leica Instrument, Shanghai, China), stained with both uranyl acetate and lead citrate, and examined using Hitachi H-7500 transmission electron microscopy (Hitachi, Japan). Glomerular basement membrane (GBM) thickness was measured at 20 different sites of the GBM.
Statistical analysis
The data were expressed as mean±SE. Student’s t-test was used for the comparison between LiasH/HLeprdb/db and Lias+/+Leprdbdb/db mice or one-way analysis of variance for the two strains plus Lias+/+Leprdbdb/+ mice. Results were considered to be statistically significant when p value was less than 0.05.
Results
Alterations of metabolic parameters in LiasH/HLeprdb/db mice
To assess the impact of enhanced endogenous antioxidant activity derived from overexpressing Lias gene on metabolisms of the diabetic mice, we compared body weight and blood glucose levels between LiasH/HLeprdb/db and Lias+/+Leprdbdb/db mice. The body weights of male Lias+/+Leprdbdb/db mice were markedly heavier than that of Lias+/+Lepr db/+ (table 1). The body weights of male LiasH/HLeprdb/db mice at 7 months old was significantly heavier than their Lias+/+Leprdbdb/db littermates (table 1), likely due to metabolic regulation of LA since LA is a cofactor of several mitochondrial enzymes. We also found that the body weight in male LiasH/H mice was higher than that of male Lias+/+ mice.12 The body maybe regulated by metabolic homeostasis by storing body weight at the expense of excessive energy production in male LiasH/H mice.
Table 1.
Biological characteristics of three genotypes of mice at 7 months old
| Characteristics | Lias+/+Leprdb/+ | Lias+/+Leprdb/db | LiasH/HLeprdb/db | P1 | P2 |
| Body weight (g) | 31.54±1.05 | 65.56±2.03 | 74.27±1.71 | 0.001 | 0.001 |
| Right kidney weight (mg) | 244.26±8.04 | 242.58±7.61 | 248.85±13.90 | 0.909 | 0.669 |
| Chow intakes (g/day) | 10.11±0.09 | 16.03±0.72 | 13.98±0.18 | 0.001 | 0.093 |
| Water intakes (mL/day) | 14.60±0.84 | 13.33±0.05 | 11.15±0.11 | 0.035 | 0.001 |
| Urine output (mL/day) | 2.21±0.12 | 16.94±0.51 | 13.44±0.65 | 0.001 | 0.001 |
| TG (mM) | 0.75±0.030 | 1.35±0.10 | 1.16±0.11 | 0.001 | 0.542 |
| CHO (mM) | 2.37±0.21 | 6.06±0.62 | 9.35±0.77 | 0.001 | 0.015 |
All mice were male at 7 months of age. Data are represented as mean±SEM (n=10). P1 represents p value of Lias+/+Leprdbdb/db vs Lias+/+/Leprdb/+ mice and P2 stands for p value of LiasH/HLeprdb/db vs Lias+/+Leprdbdb/db mice.
CHO, cholesterol; TG, triglyceride.
We next determined blood glucose levels by measuring both glucose concentration and HbA1c levels at 7 months. Blood HbA1c concentrations in LiasH/HLeprdb/db mice were significantly lower than that of Lias+/+Leprdbdb/db mice (data not shown). HbA1c and fasting blood glucose levels at 7-month-old LiasH/HLeprdb/db mice were considerably lower than Lias+/+Leprdbdb/db mice (figure 1A and B), suggesting that increased endogenous antioxidant activity retarded the development of diabetes. To help diagnose type 2 diabetes, we also examine the mouse IPGTT at 7 months. The result showed that compared with LiasH/HLeprdb/db mice, Lias+/+Leprdbdb/db mice showed impaired glucose tolerance, as manifested by obviously increased glucose levels after glucose injection (p<0.05, all timepoints) (figure 1C).
Figure 1.
Effects of elevated endogenous antioxidant on glucose concentration, inflammation and renal dysfunction in 7-month-old Lias+/+Leprdbdb/db and in LiasH/HLeprdb/db mice. (A) Plasma glucose, (B) glycated hemoglobin (HbA1c) and (C) intraperitoneal glucose tolerance test (IPGTT) in mice that received glucose (0.5 g/kg body weight). Thereafter, the blood glucose was monitored at 10, 30, 60, 90, 120 and 180 min. The glucose level before glucose injection served as the baseline level for each mouse. (D) Urine microalbumin excretion (MAU) concentrations, and (E) serum creatinine and (F) blood urea nitrogen (BUN) levels. Urine albumin excretion per 24 hours on three urine collections. Lias+/+/Leprdb/+ mice as a non-obesity control. (G) Interleukin-1β (IL-1β), (H) tumor necrosis factor-α (TNF-α), (I) C-reactive protein (CRP) and (J) monocyte chemotactic protein-1 (MCP-1). Lias+/+/Leprdb/+ mice as a non-obesity control. Data are presented as the mean±SEM. # represents p value of Lias+/+Leprdbdb/db vs Lias+/+/Leprdb/+ mice and § stands for p value of LiasH/HLeprdb/db vs Lias+/+Leprdbdb/db mice: *p<0.05 and **p<0.01.
Reduction of systemic lipid content in LiasH/HLeprdb/db mice
Lipid metabolism disorder is associated with development of DN.18 Thus, concentrations of TG and total CHO in circulation were measured in the all animals. TG level at 7 months was markedly lower in LiasH/HLeprdb/db mice than in Lias+/+Leprdbdb/db mice (table 1). However, total CHO concentration at 7 months was not significantly different between these two groups (table 1). The results indicated that increased antioxidant activity resulted in hypolipidemia status, which may contribute to the renal protective effect.
Levels of plasma inflammatory mediators in LiasH/HLeprdb/db mice
In patients with T2DM, serum levels of proinflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-6 (IL-6) are elevated and have been widely used as a biomarker of T2DM.19 20 In addition, in patients with T2DM, elevated serum levels of CRP are closely associated with an increase in microalbuminuria excretion and renal dysfunction.21 To investigate potential anti-inflammatory role of enhanced endogenous antioxidant activity in the mouse model, we monitored serum concentrations of IL-1β, TNF-α, IL-6, CRP and MCP-1. The results showed that serum CRP, IL-1β and MCP-1 levels in LiasH/HLeprdb/db mice at 7 months of age were significantly lower than those of Lias+/+Leprdbdb/db mice (figure 1G–J), suggesting the inflammation was attenuated in LiasH/HLeprdb/db mice.
Upregulation of antioxidant activity ameliorated renal dysfunction
To investigate whether elevated endogenous antioxidant activity blocked the development of DN over time, urine albumin was examined before the mice were sacrificed. Our results showed that albuminuria was noticeably increased in Lias+/+Leprdbdb/db mice, which was significantly alleviated in LiasH/HLeprdb/db mice (figure 1D). The concentrations of serum creatinine and BUN in Leprdb/db mice during the early stage of diabetes did not differ significantly from those in non-diabetic mice of the same age (data not shown). However, after persistent hyperglycemia, the serum concentrations of creatinine were significantly elevated in Lias+/+Leprdbdb/db mice but this increase was partially offset in LiasH/HLeprdb/db mice (figure 1E). Serum BUN in LiasH/HLeprdb/db (for an unknown reason) and Lias+/+Leprdbdb/db mice significantly increased (figure 1F). The concomitant increases in BUN and creatinine suggested that the glomerular function was compromised. However, occurrence of hydration following persistent glycosuria likely contributed to the increased BUN in the diabetic animals.
Overexpression of Lias gene reduced oxidative stress in plasma and kidney mitochondria
To determine whether enhanced endogenous antioxidant activity systematically decreased oxidative stress, we measured plasma antioxidant content using a variety of biomarkers. We found that both T-AOC and reduced glutathione (GSH) in plasma significantly increased, while MDA in plasma significantly decreased in LiasH/HLeprdb/db mice (figure 2A–C). Mitochondrial dysfunction is a major source to produce ROS. Investigating mitochondrial redox status would gain a better understanding of DN mechanisms and prove our hypothesis that impairment of antioxidant defense plays a critical role in the prevention and treatment of DN. Thus, we examined entire antioxidant content T-AOC, antioxidant biomarker GSH and SOD2 activity and oxidative stress biomarker MDA in mitochondria isolated from kidney cortex. The results showed the highest T-AOC level in LiasH/HLeprdb/db mice (figure 2D). Our results also demonstrated that the level of GSH in Lias+/+Leprdbdb/db mice was suppressed by diabetes compared with non-diabetic Lias+/+Leprdbdb/+ mice (figure 2E). Increased endogenous antioxidant capacity in LiasH/HLeprdb/db mice elevated GSH level to close statistical significance (p=0.053) (figure 2E). SOD2 activity did not show any significant difference between LiasH/HLeprdb/db and Lias+/+Leprdbdb/db mice (figure 2F). But we found that MDA in the mitochondria significantly decreased in LiasH/HLeprdb/db mice compared with Lias+/+Leprdbdb/db mice (figure 2G). Furthermore, ATP yield in the kidney of LiasH/HLeprdb/db mice was lower than that in Lias+/+Leprdbdb/db mice (figure 2H). We concluded that decreased ATP production in LiasH/HLeprdb/db mice may serve as an adaptive mechanism to decrease ROS levels at the expense of ATP yield in states of excess substrate supply.
Figure 2.
Antioxidant content in serum and renal mitochondria in 7-month-old Lias+/+Leprdbdb/db and in LiasH/HLeprdb/db mice. Serum T-AOC (A), GSH (B) and MDA (C). Renal mitochondrial T-AOC (copper reducing equivalents) (D), GSH (E), SOD2 activity (F) and MDA (G). ATP production in renal mitochondria (H). Results represent the mean±SEM. *p<0.05 and **p<0.01. GSH, reduced glutathione; MDA, malondialdehyde; SOD2, superoxide dismutase 2; T-AOC, total autoxidizing capability.
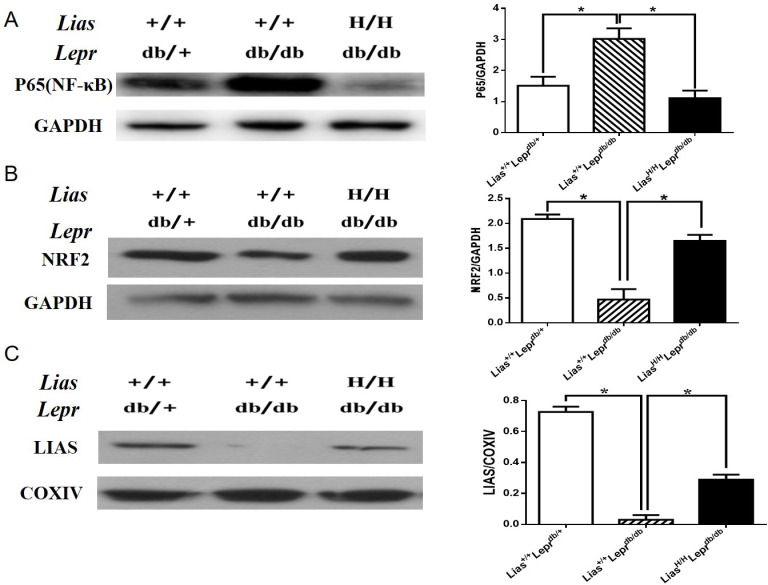
Because the NF-κB signal pathway plays a key role in orchestrating inflammatory response, we examined the effect of overexpression of Lias gene on the activation of NF-κB. Nuclear p65 expression was evidently increased in Lias+/+Leprdbdb/db mice (figure 3A) but significantly inhibited in LiasH/HLeprdb/db mice, indicating that enhanced endogenous antioxidant activity alleviated inflammatory response in diabetes.
Figure 3.
The expression of renal p65-NF-κB, NRF2 and LIAS proteins in kidneys of 7-month-old diabetic nephropathy mice. The protein levels in kidney lysates from Lias+/+Leprdbdb/+, Lias+/+Leprdbdb/db and LiasH/HLeprdb/db mice were measured using western blot. Representative image of kidney lysates immunoprobed with (A) anti-P65(NF-κB) antibody, (B) anti-NRF2 antibody and (C) anti–LIAS antibody. The values were normalized by GAPDH or cytochrome c oxidase (COXIV) expression. Bars represent the mean±SEM. *p<0.01.
Nrf2 is a transcription factor that plays a pivotal role in the endogenous antioxidant system.22 Our results showed that the protein level of Nrf2 was largely diminished in Lias+/+Leprdbdb/db mice compared with non-diabetic Lias+/+Leprdbdb/+ mice. However, the decline of Nrf2 level could be partially prevented in LiasH/HLeprdb/db mice (figure 3B). Since plasma and tissue LA cannot directly be measured due to a technical obstacle,23 changes of LA levels in kidney mitochondria were estimated by evaluating LIAS protein expression using western blot (figure 3C) and quantitatively assessed by densitometry. It was shown that levels of LIAS protein were markedly decreased in Lias+/+Leprdbdb/db mice, which could be partially restored in LiasH/HLeprdb/db mice.
Kidney histological alterations
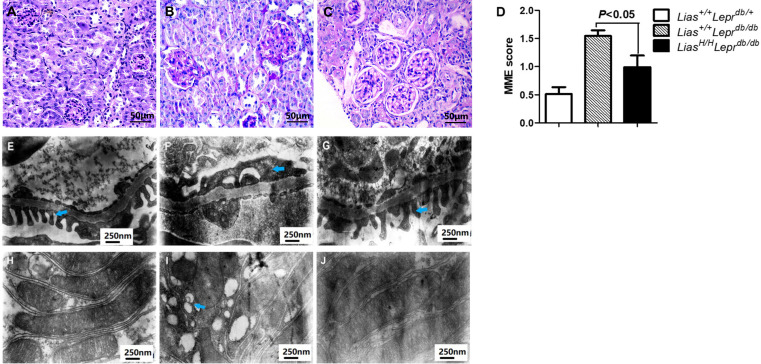
To investigate the potential role of increased Lias gene expression in diabetic glomerular injury, we examined MME, foot process effacement, proximal tubule mitochondria and GBM thickness. Notably, the extent of MME was significantly larger in Lias+/+Leprdbdb/db mice (figure 4B) than Lias+/+Leprdbdb/+ mice (figure 4A) but the expansion was attenuated in LiasH/HLeprdb/db mice (figure 4C). Semiquantitative analysis revealed significantly higher MME scores in Lias+/+Leprdbdb/db mice compared with LiasH/HLeprdb/db mice (figure 4D). Podocyte foot process effacement, assessed using transmission electron microscopy, were severer in Lias+/+Leprdbdb/db mice (figure 4F), compared with Lias+/+Leprdbdb/+ mice (figure 4E) but significantly improved in LiasH/HLeprdb/db mice (figure 4G). In addition, there were a few damaged mitochondria in proximal tubules of kidney of Lias+/+Leprdbdb/+ mice (figure 4H). However, more damaged mitochondria were observed in Lias+/+Leprdbdb/db mice (figure 4I) than in LiasH/HLeprdb/db mice (figure 4J), which suggested the involvement of mitochondria in the development of DN. GBM thickness were not significantly different among the three strains (data not shown).
Figure 4.
Representative images of mesangial matrix expansion (MME), ultrastructural alterations of the podocytes and proximal tubule mitochondrial damage in 7-month-old mouse kidneys. (A) Lias+/+Leprdbdb/+, (B) Lias+/+Leprdbdb/db and (C) LiasH/HLeprdb/db mice, the sections stained with periodic acid–Schiff (PAS). Original magnification ×400. (D) Quantitative analysis of mesangial expansion in kidney glomerulus from the three strains. Data are expressed as mean±SEM. Podocyte foot process effacement was significantly increased in Lias+/+Leprdbdb/db mice (F) compared to Lias+/+Leprdbdb/+ mice (E). The increase was significantly improved in LiasH/HLeprdb/db mice (G). Original magnification, ×30,000. A large numbers of damaged mitochondria (blue arrowheads) in kidney proximal tubules of Lias+/+Leprdbdb/db mice (I), and the damaged mitochondria were seldom observed in proximal tubules of LiasH/HLeprdb/db mice (J) and Lias+/+Leprdbdb/+ mice (H). Original magnification ×30,000.
Discussion
In this study, we found that enhanced endogenous antioxidant activity are associated: (1) significantly decreased blood glucose and HbA1c levels; (2) attenuated pathological changes of DN and improved renal function, as evidenced by improvements of serum creatinine, albuminuria, MME and podocyte foot process effacement; (3) decreased inflammatory response including the expression of proinflammatory cytokines/chemokines and NF-κB activity; and (4) reduced oxidative stress and strengthened antioxidant defense including increased NRF2 and Lias production. This change of endogenous antioxidant levels is within a physiological level. The concentration of the peak is higher than by 200% compared with the baseline when they gave LA (600 mg), which falls within pharmaceutical dose to healthy volunteers.24 Hence, we estimate that upregulation by ~60% of the Lias gene expression cannot reach that pharmaceutical level. Taken together, these results suggest that the new double mutant mouse model is a valuable tool to elucidate the antioxidant role in the development of DN derived from type 2 diabetes.
Mitochondria are a major source for oxidative stress and also a vulnerable target by oxidative stress. Thus, our research focused on potential mitochondrial protection by increased endogenous antioxidant capacity. Our results showed that total antioxidant content and GSH concentrations in cortex mitochondrial were higher in LiasH/HLeprdb/db mice. These findings are consistent with our previous observation that GSH content is significantly reduced if Lias gene expression level drops by 50%.25 GSH is the predominant intracellular non-protein thiol compound, and renal GSH levels are decreased during DN.26 Other investigators also showed that the effect of LA on reno-protection was associated with reduced oxidative stress and restoration of renal cortical levels of GSH.27 MDA is a peroxide formed by oxygen free radicals attacking unsaturated fatty acids in biomembrane, which can reflect the degree of lipid peroxidation in organism and the degree of damage of cells attacked by free radicals. Our results showed that MDA in both serum and mitochondria significantly decreased in LiasH/HLeprdb/db mice compared with Lias+/+Leprdbdb/db mice. In addition, the results from this study also revealed that mitochondrion damage in proximal tubules of LiasH/HLeprdb/db mice noticeably decreased compared with Lias+/+Leprdbdb/db mice, which confirmed our previous observation using different Lias mouse model.28 These results strongly suggest that damage of mitochondria impairment in proximal tubules play an important role in the development of DN. The scientific significance behind this interesting phenomenon is worth further study.
Accumulating evidence shows that the chronic low-grade inflammation is closely involved in the development of DN.29 Persistent hyperglycemic conditions are known to induce generation of not only oxidative stress but also proinflammatory cytokine in kidney cells.30 Inflammatory cytokines/chemokines acting as significant pathogenic mediators in DN are detected by preclinical studies and clinical trials.31 In recent years, many researchers have been convinced that inflammation, especially increased proinflammatory cytokines, plays a determinant role in the progression of DN.32 In this study, we demonstrated that elevated Lias gene expression limited the rise of serum CRP and inflammatory cytokines/chemokines, IL-1β and MCP-1 in LiasH/HLeprdb/db mice compared with Lias+/+Leprdbdb/db mice. The present study confirmed our previous observation that endogenous LA may have anti-inflammatory effects in a model of lipopolysaccharide (LPS)-induced inflammation in Lias+/-mice.33 Our new model also confirmed that other investigation that LA supplementation exerts anti-inflammatory action by decreasing the expression of inflammatory markers such as TNF-α and MCP-1 in kidney tissue.34
We further revealed that overexpression of Lias gene also significantly increased Nrf2 and decreased NF-κB production in kidney cortex of LiasH/HLeprdb/db mice. These results are consistent with another investigation that one of the mechanisms by which LA decreases oxidative stress is through activation of Nrf2 that leads to an increment in antioxidant defense.35 Nrf2 is a master regulator of antioxidant responses and thus maintain cellular redox homeostasis.36 Thus, our results indicate that suppression of oxidative stress by overexpression of Lias may go through manipulating endogenous antioxidant defense system. It was also shown that NF-κB was upregulated in Lias+/+Leprdbdb/db mice but suppressed in LiasH/HLeprdb/db mice, suggesting attenuation of inflammation by overexpression of Lias gene may be associated with inhibition of NF-κB activation. NF-κB is one of the most well-defined transcription factors that are highly activated in renal tissues of patients with human DN.37 Under hyperglycemic environment, NF-κB is activated and induces multiple key proinflammatory genes.38 Ying and coworkers have reported that the pretreatment of human umbilical vein endothelial cells with LA, but not with other tested antioxidants such as tiron, apocynin, and tempol, inhibits TNF-α-induced NF-κB activation.39
In conclusion, the pathogenesis of DN is complex involving numerous factors. LiasH/HLeprdb/db mouse model can resolve main factors by exerting multiple pharmacological effects such as hypoglycemic, anti-inflammation, and antioxidant, especially protection of mitochondria, all of which contributed to attenuation of DN. Thus, this antioxidant animal model is not only very useful for elucidating the underlying mechanisms of DN but also brings insight into a new therapeutic strategy for clinical applications.
Acknowledgments
The authors thank Ms Mingjing Gao and Mr Xudong Li for participation in this study, especially for mouse colony maintenance.
Footnotes
Contributors: Conceptualization: GX and XY. Methodology: YZ, TY, and CX. Validation: GX, SY and WW. Data curation: YZ, TY, MC, QG and GX. Writing—original draft preparation: YZ and GX. Writing—review and editing: XY and WW. Supervision: GX and SY. Project administration: WW and SY. Manuscript submission: XY. All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by the National Natural Science Foundation of China (81703183 to GX and 81773399 to YZ) and Scientific and Technological Research Project of Henan Provincial Science and Technology Department (182102310078 to GX).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Xinxiang Medical University (protocol number XYLL-2017086, approved March 3, 2017).
References
- 1.Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015;1:15018. 10.1038/nrdp.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Packham DK, Alves TP, Dwyer JP, et al. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: results from the DIAMETRIC (diabetes mellitus treatment for renal insufficiency consortium) database. Am J Kidney Dis 2012;59:75–83. 10.1053/j.ajkd.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJL, de Zeeuw D. The kidney in type 2 diabetes therapy. Rev Diabet Stud 2011;8:392–402. 10.1900/RDS.2011.8.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. 10.1001/jama.2013.168118 [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee N, Barma S, Konwar N, et al. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur J Pharmacol 2016;791:8–24. 10.1016/j.ejphar.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 6.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes 2015;64:663–72. 10.2337/db14-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi H, Casalena G, Shi S, et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes 2017;66:763–78. 10.2337/db16-0695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowotny K, Jung T, Höhn A, et al. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015;5:194–222. 10.3390/biom5010194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha JC, Banal C, Chow BSM, et al. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal 2016;25:657–84. 10.1089/ars.2016.6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavafi M. Complexity of diabetic nephropathy pathogenesis and design of investigations. J Renal Inj Prev 2013;2:59–62. 10.12861/jrip.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azushima K, Gurley SB, Coffman TM. Modelling diabetic nephropathy in mice. Nat Rev Nephrol 2018;14:48–56. 10.1038/nrneph.2017.142 [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Hiller S, Simington S, et al. Influence of different levels of lipoic acid synthase gene expression on diabetic nephropathy. PLoS One 2016;11:e0163208. 10.1371/journal.pone.0163208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauer SM, Lane P, Zhu D, et al. Renal structure and function in insulin-dependent diabetes mellitus in man. J Hypertens Suppl 1992;10:S17–20. 10.1097/00004872-199204001-00004 [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Li W, Yazdizadeh Shotorbani P, et al. Comparison of diabetic nephropathy between male and female eNOS-/-db/db mice. Am J Physiol Renal Physiol 2019;316:F889–97. 10.1152/ajprenal.00023.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 1990;9:515–40. 10.1016/0891-5849(90)90131-2 [DOI] [PubMed] [Google Scholar]
- 16.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 1971;44:276–87. 10.1016/0003-2697(71)90370-8 [DOI] [PubMed] [Google Scholar]
- 17.Wagner M, Alam A, Zimmermann J, et al. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol 2011;6:1573–9. 10.2215/CJN.00380111 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Ma KL, Liu J, et al. Dysregulation of low-density lipoprotein receptor contributes to podocyte injuries in diabetic nephropathy. Am J Physiol Endocrinol Metab 2015;308:E1140–8. 10.1152/ajpendo.00591.2014 [DOI] [PubMed] [Google Scholar]
- 19.Pradhan AD, Manson JE, Rifai N, et al. C-Reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. 10.1001/jama.286.3.327 [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2013;36:166–75. 10.2337/dc12-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Yatsuya H, Tamakoshi K, et al. Positive association between high-sensitivity C-reactive protein and incidence of type 2 diabetes mellitus in Japanese workers: 6-year follow-up. Diabetes Metab Res Rev 2013;29:398–405. 10.1002/dmrr.2406 [DOI] [PubMed] [Google Scholar]
- 22.Huang K, Chen C, Hao J, et al. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating SIRT1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol Cell Endocrinol 2015;399:178–89. 10.1016/j.mce.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 23.Carlson DA, Smith AR, Fischer SJ, et al. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev 2007;12:343–51. [PubMed] [Google Scholar]
- 24.Chen J, Jiang W, Cai J, et al. Quantification of lipoic acid in plasma by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2005;824:249–57. 10.1016/j.jchromb.2005.07.038 [DOI] [PubMed] [Google Scholar]
- 25.Yi X, Xu L, Kim K, et al. Genetic reduction of lipoic acid synthase expression modestly increases atherosclerosis in male, but not in female, apolipoprotein E-deficient mice. Atherosclerosis 2010;211:424–30. 10.1016/j.atherosclerosis.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winiarska K, Drozak J, Wegrzynowicz M, et al. Diabetes-induced changes in glucose synthesis, intracellular glutathione status and hydroxyl free radical generation in rabbit kidney-cortex tubules. Mol Cell Biochem 2004;261:91–8. 10.1023/B:MCBI.0000028742.83086.43 [DOI] [PubMed] [Google Scholar]
- 27.Melhem MF, Craven PA, Liachenko J, et al. Alpha-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol 2002;13:108–16. 10.1681/ASN.V131108 [DOI] [PubMed] [Google Scholar]
- 28.Yi X, Xu L, Hiller S, et al. Reduced expression of lipoic acid synthase accelerates diabetic nephropathy. J Am Soc Nephrol 2012;23:103–11. 10.1681/ASN.2011010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pérez-Morales RE, Del Pino MD, Valdivielso JM, et al. Inflammation in diabetic kidney disease. Nephron 2019;143:12–16. 10.1159/000493278 [DOI] [PubMed] [Google Scholar]
- 30.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 2002;288:2579–88. 10.1001/jama.288.20.2579 [DOI] [PubMed] [Google Scholar]
- 31.Wu C-C, Sytwu H-K, Lu K-C, et al. Role of T cells in type 2 diabetic nephropathy. Exp Diabetes Res 2011;2011:514738. 10.1155/2011/514738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci 2013;124:139–52. 10.1042/CS20120198 [DOI] [PubMed] [Google Scholar]
- 33.Yi X, Kim K, Yuan W, et al. Mice with heterozygous deficiency of lipoic acid synthase have an increased sensitivity to lipopolysaccharide-induced tissue injury. J Leukoc Biol 2009;85:146–53. 10.1189/jlb.0308161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang KP, Kim DH, Jung YJ, et al. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant 2009;24:3012–20. 10.1093/ndt/gfp242 [DOI] [PubMed] [Google Scholar]
- 35.Sena CM, Cipriano MA, Botelho MF, et al. Lipoic acid prevents high-fat diet-induced hepatic steatosis in Goto kakizaki rats by reducing oxidative stress through Nrf2 activation. Int J Mol Sci 2018;19:2706. 10.3390/ijms19092706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao B, Doan A, Hybertson BM. The clinical potential of influencing Nrf2 signaling in degenerative and immunological disorders. Clin Pharmacol 2014;6:19–34. 10.2147/CPAA.S35078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tak PP, Firestein GS. Nf-kappaB: a key role in inflammatory diseases. J Clin Invest 2001;107:7–11. 10.1172/JCI11830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez AP, Sharma K. Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev Mol Med 2009;11:e13. 10.1017/S1462399409001057 [DOI] [PubMed] [Google Scholar]
- 39.Ying Z, Kampfrath T, Sun Q, et al. Evidence that α-Lipoic acid inhibits NF-κB activation independent of its antioxidant function. Inflamm Res 2011;60:219–25. 10.1007/s00011-010-0256-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in a public, open access repository. Data are available on reasonable request.