Abstract
The success of immune-checkpoint blockade and chimeric antigen receptor (CAR) T cell therapies has established the remarkable capacity of the immune system to fight cancer. Over the past several years, it has become clear that immune cell responses to cancer are critically dependent upon metabolic programs that are specific to both immune cell type and function. Metabolic features of cancer cells and the tumor microenvironment impose constraints on immune cell metabolism that can favor immunosuppressive phenotypes and block antitumor responses. Advances in both preclinical and clinical studies have demonstrated that metabolic interventions can dramatically enhance the efficacy of immune-based therapies for cancer. As such, understanding the metabolic requirements of immune cells in the tumor microenvironment, as well as the limitations imposed therein, can have significant benefits for informing both current practice and future research in cancer immunotherapy.
Introduction
In order to support the tremendous growth that characterizes tumors, cancer cells engage unique metabolic programs (1). These programs not only serve to facilitate unbridled proliferation but also facilitate the ability of cancer to evade immune responses. The specialized metabolic programs used by cancer create a hypoxic, acidic, nutrient-depleted tumor microenvironment (TME), which presents a prodigious hurdle for effective antitumor immunity. The TME is well suited for immune cells that suppress effector function and thus promote tumor immune evasion. As such, therapeutically targeting metabolic pathways affords the unique opportunity to not only inhibit tumor growth, but also alter the TME in order to enhance the efficacy of immunotherapy. As our understanding of immune metabolism has increased, it has become apparent that targeting metabolism can also have the added bonus of directly enhancing antitumor immune responses. There are several in-depth reviews delineating the metabolic pathways of both cancer and anticancer immune cells (2–5). In this review, at the crossroads of immunometabolism and immunotherapy, we seek to bring to light, for the cancer immunotherapist, the multiple different facets whereby targeting metabolism can potentially enhance the efficacy of cancer immunotherapy.
Targeting Tumor Growth
The extraordinary potential of endogenous antitumor immune responses to treat cancer has been revealed by checkpoint blockade in a number of different tumor types (6). That is, by simply inhibiting an inhibitory pathway (blocking the negative signal delivered by PD-1 to T cells), a patient’s own tumor-specific T cells can eliminate their cancer. However, although remarkable, this single-agent therapy is only effective in a limited number of patients, in part consequent to the fact that tumor growth continues to outpace the rate of the immune response. Thus, it stands to reason that slowing down tumor growth and decreasing the tumor mass could enhance the efficacy of immunotherapy.
To this end, targeting tumor metabolism represents a powerful means to inhibit tumor growth. Cancer cells reprogram their metabolism to promote anabolic pathways and growth (7–10). In order to rapidly proliferate, cancer cells require proteins for growth, lipids for creating new membranes, and nucleic acids to support transcription and translation. For somatic cells, mitochondrial oxidation of nutrients, including glucose, amino acids, and fatty acids through the tricarboxylic acid (TCA) cycle, is used as an efficient means of generating ATP. However, because of their anabolic state, cancer cells reprogram their metabolism by upregulating the lactate-forming glycolysis called Warburg physiology (11, 12). This refers to using glycolysis to generate ATP even in the presence of oxygen, which allows for more rapid metabolism of glucose and regeneration of NAD+. Glycolytic intermediates enter other essential pathways, such as the pentose phosphate pathway, the one-carbon pathway, and the hexosamine biosynthesis pathway, all of which support high levels of cellular growth and proliferation. These pathways are readily inhibited by therapeutic agents. Studies also demonstrate that some cancers may use alternative fuels for energy generation, including lactate and branch-chained amino acids (13–15). Interestingly, many traditional chemotherapies such as methotrexate, 6-mercap-topurine (6-MP), and 5-fluorouracil (5-FU) are in fact metabolic inhibitors (16). Along these lines, the combination of carboplatin and pemetrexed, along with anti–PD-1, has demonstrated efficacy for the treatment of lung cancer (17). Although the efficacy in these trials is typically presented as a function of combination chemotherapy and immunotherapy, it is instructive to understand that pemetrexed is fundamentally a metabolic inhibitor that suppresses folate metabolism, as well as purine and pyrimidine synthesis.
Altering the TME
Targeting metabolism to directly inhibit cancer cell growth and proliferation is a straightforward approach to enhance the efficacy of immunotherapy. However, as mentioned above, tumor metabolism also profoundly influences the TME. The high metabolic activity of cancer cells, in addition to a disorganized, dysfunctional vasculature, can drive hypoxia and nutrient depletion in the TME, leading to competition for oxygen and nutrients between cells within the TME, including cancer and immune cells (18–20). For example, robust glucose uptake and glycolysis in cancer cells is associated with enhanced infiltration of immune-suppressive myeloid-derived suppressor cells (MDSC) and decreased antitumor immune responses among tumor-infiltrating lymphocytes (TIL; refs. 18–22). Targeting cancer cell glycolysis has been shown to preserve antitumor T-cell function and improve response to checkpoint immunotherapy (23). The Cancer Genome Atlas data demonstrate decreased immune responses in tumors with high expression of hexokinase 2, the rate-limiting enzyme of glycolysis (20). Amino acids may also be the subject of metabolic competition between cancer and immune cells. A report demonstrates that high methionine uptake in cancer cells can lead to epigenetic reprogramming of antitumor T cells and impaired antitumor function (24). Similarly, it has been reported that ovarian cancers can dampen T-cell glycolysis and effector function through micro-RNA-mediated suppression of the methyltransferase, EZH2 (25). Another study reports that TME hypoxia can induce T-cell exhaustion, specifically through dysregulated mitochondrial dynamics in the context of T-cell receptor (TCR) and PD-1 signaling (26). In addition to depriving immune cells of necessary nutrients, tumor metabolism also leads to the production of immunosuppressive metabolites, such as lactic acid (27), reactive oxygen species (ROS; ref. 28), kynurenine (29), polyamines (30–34), adenosine (35–40), and cholesterol (41), all of which suppress antitumor immunity. Thus, targeting tumor metabolism can enhance immunotherapy by creating a TME that is more hospitable to the antitumor immune response (Fig. 1, Immune suppression). To this end, trials are currently under way that seek to enhance immunotherapy by blocking the production of adenosine by the ectonucleotidase CD73, as well as blocking the adenosine receptor, A2aR. Likewise, in spite of initial disappointing trials, there still remains interest in preventing the depletion of tryptophan and the production of kynurenine by inhibiting IDO1. In this regard, a study has identified interleukin-4–induced-1 (IL4I1) as a critical activator of aryl hydrocarbon receptor (AHR) activity through IDO1-independent generation of indole metabolites and kynurenic acid (42). As such, this may explain clinical trial failures of IDO1-specific inhibitors for immunotherapy.
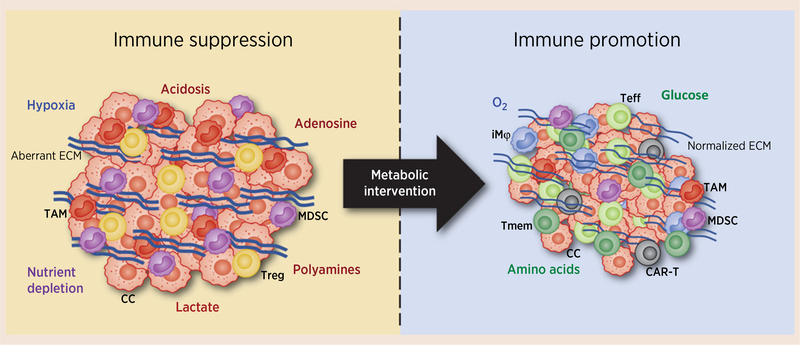
Figure 1.
Metabolic intervention has pleiotropic effects on tumor immunology. The metabolic programs of cancer cells function as immune checkpoints within the TME through a number of mechanisms, including depletion of oxygen and nutrients, generation of toxic metabolites (e.g., acid, adenosine, and lactate, polyamines), and the production of aberrant extracellular matrix (ECM; left, Immune suppression). These characteristics favor immune-suppressive phenotypes, including regulatory T cells, MDSCs, and tumor-associated macrophages, and suppress antitumor effector responses of T cells and natural killer cells. Targeted interventions designed to dismantle cancer cell metabolism can simultaneously suppress cancer cell growth, decrease the production of ECM components, limit nutrient deprivation, curtail generation of toxic metabolites, and reprogram the immune response, favoring T-cell persistence and inflammatory myeloid infiltration (right, Immune promotion). CAR-T, chimeric antigen receptor T cell; CC, cancer cell; iMϕ, inflammatory macrophage; TAM, tumor-associated macrophage; Teff, effector T cell; Tmem, long-lived memory T cell; Treg, regulatory T cell.
Another strategy is to alter the TME by directly inhibiting tumor metabolism. For example, inhibition of glutamine metabolism leads to a dramatic decrease in hypoxia, acidosis, and lactate production, as well as enhanced availability of nutrients in the TME for immune cells (43). Such an approach has the benefit of both inhibiting tumor growth and altering the TME. Interestingly, blocking glutamine metabolism also has the added benefit of leading to decreased kynurenine production by inhibiting the expression of IDO (44). It has also been demonstrated that T cell–generated IFNγ induces tumor cell lipid peroxidation and ferroptosis (45). This cytotoxic effect can be pharmacologically enhanced through inhibition of the glutamate–cystine antiporter system, leading to improved efficacy of checkpoint blockade. Finally, some tumors evade immune destruction by physically blocking infiltration of immune cells through the elaboration of extracellular matrix (ECM). This is particularly evident in pancreatic cancer (46). The production and elaboration of the ECM is metabolically demanding. Thus, targeting metabolism has the potential to overcome resistance to immunotherapy by making tumors accessible to immune cells. For example, a particular tumor may be classified as resistant to anti–PD-1. However, it is possible that anti–PD-1 is actually able to successfully unleash tumor-specific T cells, but these cells cannot infiltrate into the tumor. Thus, inhibition of the generation of ECM through metabolic therapy might readily convert such anti–PD-1–resistant tumors into susceptible tumors. Likewise, such a strategy mightserveto enhance the efficacy of adoptive cellular therapy (ACT).
Suppressive Myeloid Cells
Myeloid-derived cells make up a considerable proportion of the cells in the TME, contributing up to 40% of the mass of a tumor in some cancers (47). Many of these cells play important roles in promoting tumor immune evasion. In addition to expressing the immunosuppressive ligands PD-L1 and PD-L2, tumor-associated macrophages (TAM) also express immunosuppressive metabolic enzymes such as arginase-1, as well as IDO (30–33, 48, 49). Notably, the metabolism of TAMs is distinct and resembles the metabolic programming of M2 macrophages (50, 51). For example, similar to T-regulatory cells (Treg), M2 macrophages rely more on fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS) and are more reliant on glutamine metabolism than inflammatory M1 macrophages (50, 51). Studies have uncovered a distinct role for lipid uptake, accumulation, and oxidation, which are critical for TAM polarization and immunosuppressive activity (52). As such, blockade of lipid uptake or FAO suppresses the protumor activity of TAMs in mouse models. Similar to the M2-like immunosuppressive TAMs, tumor-associated immunosuppressive MDSCs also possess distinct metabolic programs. Blocking glycolysis or glutamine metabolism has been shown to inhibit the expansion and function of these suppressive cells in the TME (44, 53). Indeed, blocking glutamine metabolism with 6-Diazo-5-oxo-l-norleucine (DON) not only inhibited MDSC accumulation in tumors but also promoted the generation and function of inflammatory M1 macrophages. Thus, metabolic therapy has the potential to inhibit tumor growth, inhibit the generation and function of suppressive TAMs/MDSCs, and promote the accumulation of inflammatory M1 macrophages (Fig. 1, Immune promotion).
Enhancing Antitumor T Cells
The discussion thus far has emphasized the concept that targeting metabolism can inhibit tumor growth AND also positively influence the antitumor response by conditioning the TME and inhibiting the generation and function of suppressive cells. Effector CD4+ and CD8+ T cells are critical executors of the antitumor immune response. Upon activation, in the context of costimulation, these cells reprogram their metabolism to support their own prodigious growth and anabolic function (54). Early studies highlight the similarities in metabolic programming between activated, proliferating T cells and cancer cells (55–60), whereby activated T cells increase glycolysis even in the presence of oxygen (known as Warburg physiology). However, it is important to note that increased TCA cycle metabolism and OXPHOS are also instrumental (55–60). The similarity between tumor metabolism and activated T-cell metabolism raises the concern that targeting tumor metabolism might in fact inhibit antitumor T cells. As it turns out, this is not necessarily the case, as work has demonstrated that targeting tumor metabolism can simultaneously enhance antitumor T cells (43, 44). Indeed, in spite of their metabolic similarities, it has become clear that it is possible to differentially target cancer growth and the anticancer immune response. Although many cancer cells can be rigid in their metabolic programs, T cells can be plastic. It has been shown that acetate metabolism can overcome glucose restriction in CD8+ T-effector cells (61). Similarly, although blockade of glutamine metabolism can inhibit tumor growth, T cells can overcome this blockade by acquiring carbon via acetate metabolism (43).
Nonetheless, in spite of these alternative pathways, similar to cancer cells, targeting T-cell metabolism can inhibit proliferation and clonal expansion. Triple combination therapy using the glycolysis inhibitor 2-deoxyglucose (2-DG), glutamine inhibitor DON, and mitochondrial inhibitor metformin can inhibit activated T-cell proliferation and cytokine production (62), which has been shown to be an effective approach to preventing allograph rejection. Despite these dramatic suppressive effects through the simultaneous blockade of three independent metabolic pathways, targeted inhibition of any single pathway may actually enhance critical attributes, such as effector response upon rechallenge and resistance to activation-induced cell death. Blocking glycolysis with the inhibitor 2-DG will mitigate clonal expansion. However, it has been shown that 2-DG can also condition T cells to become more robust long-lived memory cells (63). To this end, inhibiting mTOR or AKT signaling, both of which will inhibit tumor growth, has been used to enhance the robustness of the antitumor T-cell response (63–65).
Along these lines, tumor immune evasion can take the form of chronic, nonproductive antigen-specific activation. CD8+ T cells in the TME can adopt a state of functional exhaustion, wherein they are poorly proliferative and unable to generate sufficient cytotoxicity against target cancer cells. Interestingly, exhausted T cells can also be defined by their (dysregulated) metabolism. Exhausted T cells can be characterized not just by the upregulation of PD-1 and loss of cytokine production, but also by mTOR signaling in the absence of productive glycolytic function and anabolic processes. To this end, PD-1 signaling inhibits the expression of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), which in turn leads to diminished mitochondrial function and less oxidative capacity compared with normal effector T cells (66, 67). Targeting metabolism can in part reverse this phenotype and restore function.
An important subtype of T cells, called regulatory T cells (Treg), relies on distinct metabolic programs and plays a critical role in dampening antitumor immune responses. This suppressive subset is defined by the FoxP3 transcription factor, which reprograms metabolism toward mitochondrial respiration (OXPHOS) through MYC suppression (68). Unlike antitumor effector T cells, immunosuppressive Tregs adapt to metabolic challenges within the TME, resisting lactate-induced suppression of function and proliferation. Interestingly, work has demonstrated that targeting CD36-mediated lipid metabolism in intratumoral Tregs can disrupt their ability to function in lactate-enriched environments and improve antitumor immune responses (69).
Enhancing ACT
Currently, checkpoint blockade and ACT in the form of chimeric antigen receptor (CAR) T cells represent the two stalwarts of clinically approved cancer immunotherapy. CAR-T cells are approved to treat a number of hematologic malignancies and have shown remarkable efficacy in patients with leukemia and lymphoma with extensive cancer burden. However, the progress of this approach has been stymied by two hurdles. First, it is clear that even after initial responses, lack of persistence of the adoptively transferred cells is a major mechanism of relapse (70). Second, in spite of the successes in hematologic malignancies, CAR-T cell therapy has yet to induce impressive, durable responses in solid tumors (71). Both of these problems have the potential to be overcome with metabolic therapy.
By design, CAR-T cells are generated through ex vivo activation and expansion. This process lends itself to metabolic intervention. For example, expanding T cells in the presence of an inhibitor of glycolysis can promote memory cell generation leading to enhanced persistence and function when the cells are adoptively transferred into tumor-bearing mice (63). In a similar fashion, inhibiting AKT signaling during ex vivo processing can promote the generation of T cells with transcriptional and metabolic profiles associated with enhanced memory (72). Likewise, the inclusion of increased arginine or potassium in the culture media can promote the enhanced generation of long-lived memory cells (73, 74). Other strategies, including inhibition of lactate dehydrogenase (LDH), the critical enzyme in aerobic glycolysis, T-cell sorting based on low mitochondrial membrane potential, and limiting ROS metabolism in T cells, can also enhance the generation of long-lived or stem-like antitumor T cells and enhance adoptive immunotherapy regimens (75–77).
The fact that CAR-T cells are genetically altered creates the opportunity for metabolic reprogramming by genetic means. First, it has been noted that the 4–1BB signaling domain is superior to the CD28 signaling domain in promoting the expansion of central memory T (Tcm) cells with increased mitochondrial biogenesis and oxidative metabolism (78–83). In this case, the design of the CAR itself can reprogram the cells metabolically. However, strategies to genetically engineer T cells further have also been used. For example, overexpression of phosphoenolpyruvate carboxykinase 1 (PCK1), which converts oxaloacetate (OAA) into phosphoenolpyruvate (PEP), has been shown to enhance the efficacy of adoptively transferred T cells (20). Likewise, it has been demonstrated that forced overexpression of PGC1a in donor T cells can promote mitochondrial fitness and prevent exhaustion of adoptively transferred cells (66). Alternatively, pharmacologically promoting mitochondrial fusion and inhibiting mitochondrial fission can lead to superior control of adoptively transferred T cells by enhancing memory generation with increased mitochondrial mass, OXPHOS, and spare respiratory capacity (SRC; ref. 84).
Although ex vivo metabolic interventions are appealing, treating patients either before or during ACT can be an effective strategy as well. The efficacy of even the most potent adoptively transferred cells may be readily thwarted by lack of access to the tumor. Likewise, the potency of antitumor T cells can be mitigated upon tumor infiltration by a hostile TME. To this end, the addition of metabolic therapy to CAR-T therapy might facilitate the success of this modality for solid tumors. Indeed, metabolic therapy can facilitate inhibiting tumor growth and condition the TME to make it more hospitable to antitumor immune responses during the period of cell processing. Treating patients with metabolic therapy post ACT has the potential to enhance the robustness of the adoptively transferred cells. As previously discussed, the inhibition of a number of metabolic targets, such as glutamine metabolism, glycolysis, mTOR, and AKT, not only serves to mitigate tumor growth but also promotes long-lived T-cell memory (63, 72, 73, 85). Thus, for example, the continued treatment with an inhibitor of glutamine metabolism, even after ACT, has the potential to both keep tumor growth in check and promote long-lived memory in the adoptively transferred cells (85).
Targeting Metabolism to Enhance the Depth and Breadth of Immunotherapy
Undoubtedly, the success of checkpoint blockade and CAR-T therapy has revolutionized the treatment of cancer. Patients with significant tumor burdens can now be cured by endogenous (checkpoint blockade) and exogenous (ACT) antitumor immune responses. However, although the successes have been impressive, it is clear that the task ahead is to build on these successes to enhance the depth of immunotherapy in patients with tumors deemed sensitive and the breadth of immunotherapy to tumors that have not, as of yet, demonstrated robust responses to immunotherapy. The addition of metabolic therapy to both target tumor metabolism and regulate immune metabolism has the potential to accelerate these goals (Fig. 1). Indeed, we view the addition of pemetrexed to immunotherapy for non–small cell lung cancer (NSCLC) as just the beginning. Specifically, targeting metabolism has the potential to enhance the efficacy of immunotherapy in NSCLC, melanoma, renal cell carcinoma, and other cancers for which checkpoint blockade has already been approved. Likewise, for cancers, such as prostate cancer, breast cancer, pancreatic cancer, and others, where immunotherapy has yet to show significant efficacy, targeting metabolism has the potential to both alter the TME and increase immune infiltration, converting these resistant tumors to susceptible ones. For these same reasons, metabolic therapy has the potential to facilitate the expansion of CAR-T therapy to solid tumors, as well as improve the overall efficacy of this approach, by enhancing the persistence of adoptively transferred cells.
Supplementary Material
Acknowledgments
This work was supported by the NIH (R01CA226765 and R01CA229451) and The Bloomberg–Kimmel Institute for Cancer Immunotherapy.
Authors’ Disclosures
R.D. Leone reports grants from NIH during the conduct of the study, other from Corvus (previous support for materials and other financial support to study A2aR inhibitor CPI-444) outside the submitted work, and a patent for Methods for Cancer and Immunotherapy Using Glutamine Analogues, including DON, licensed to Dracen Pharmaceuticals. J.D. Powell reports grants, personal fees, and other from Dracen Pharmaceuticals (scientific founder, consulting, equity), grants, personal fees, and other from Corvus (past sponsored research agreement with institution, consulting fees, and limited amount of equity), grants from AstraZeneca (to institution), Bristol-Myers Squibb (to institution), and Bluebird (past sponsored research agreement), and personal fees fromSitryx (scientific founder and equity) during the conduct of the study, as well as a patent for PCT/US16/44829 pending to Dracen Pharmaceuticals.
References
- 1.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv 2016;2:e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer 2020;20:516–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan D, Sanin DE, Pearce EJ, Pearce EL. Metabolic interventions in the immune response to cancer. Nat Rev Immunol 2019;19:324–35. [DOI] [PubMed] [Google Scholar]
- 4.Andrejeva G, Rathmell JC. Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab 2017;26: 49–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra L, Bonetti L, Brenner D. Metabolic modulation of immunity: a new concept in cancer immunotherapy. Cell Rep 2020;32:107848. [DOI] [PubMed] [Google Scholar]
- 6.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, DeBerardinis RJ. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab 2019;30:434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 2010;107:8788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma EH, Verway MJ, Johnson RM, Roy DG, Steadman M, Hayes S, et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8(+) T cells. Immunity 2019;51:856–70. [DOI] [PubMed] [Google Scholar]
- 10.Chen PH, Cai L, Huffman K, Yang C, Kim J, Faubert B, et al. Metabolic diversity in human non-small cell lung cancer cells. Mol Cell 2019;76:838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warburg O, Gawehn K, Geissler AW. [Metabolism of leukocytes]. Z Naturforsch B 1958;13B:515–6. [PubMed] [Google Scholar]
- 12.Bakker A. Einige Übereinstimmungen im Stoffwechsel der Carcinomzellen und Exsudatleukocyten. Klin Wochenschr 1927;6:252–4. [Google Scholar]
- 13.Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 2014;159:1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017;551:115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, et al. Lactate metabolism in human lung tumors. Cell 2017;171:358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 2013;13:572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaer DA, Geeganage S, Amaladas N, Lu ZH, Rasmussen ER, Sonyi A, et al. The folate pathway inhibitor pemetrexed pleiotropically enhances effects of cancer immunotherapy. Clin Cancer Res 2019;25:7175–88. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015;162:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukey MJ, Katt WP, Cerione RA. Targeting amino acid metabolism for cancer therapy. Drug Discov Today 2017;22:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 2015;162:1217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C, et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab 2018;27:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu Ke, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab 2018;28:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renner K, Bruss C, Schnell A, Koehl G, Becker HM, Fante M, et al. Restricting glycolysis preserves T cell effector functions and augments checkpoint therapy. Cell Rep 2019;29:135–50. [DOI] [PubMed] [Google Scholar]
- 24.Bian Y, Li W, Kremer DM, Sajjakulnukit P, Li S, Crespo J, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 2020;585: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao E, Maj T, Kryczek I, Li W, Wu Ke, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol 2016;17:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu YR, Imrichova H, Wang H, Chao T, Xiao Z, Gao M, et al. Disturbed mitochondrial dynamics in CD8(+) TILs reinforce T cell exhaustion. Nat Immunol 2020;21:1540–51. [DOI] [PubMed] [Google Scholar]
- 27.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab 2016;24:657–71. [DOI] [PubMed] [Google Scholar]
- 28.Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer 2019;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin Cancer Res 2019;25:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shearer JD, Richards JR, Mills CD, Caldwell MD. Differential regulation of macrophage arginine metabolism: a proposed role in wound healing. Am J Physiol 1997;272:E181–90. [DOI] [PubMed] [Google Scholar]
- 31.Ye C, Geng Z, Dominguez D, Chen S, Fan J, Qin L, et al. Targeting ornithine decarboxylase by a-difluoromethylornithine inhibits tumor growth by impairing myeloid-derived suppressor cells. J Immunol 2016;196:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills CD, Shearer J, Evans R, Caldwell MD. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol 1992;149:2709–14. [PubMed] [Google Scholar]
- 33.Hayes CS, Shicora AC, Keough MP, Snook AE, Burns MR, Gilmour SK. Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol Res 2014;2:274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gökmen SS, Aygit AC, Ayhan MS, Yorulmaz F, Gülen S. Significance of arginase and ornithine inmalignant tumors of the human skin. J Lab Clin Med 2001;137: 340–4. [DOI] [PubMed] [Google Scholar]
- 35.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 2013;36:921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J 2015;13: 265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer 2018;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol Immunother 2012;61:917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006;103:13132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leone RD, Sun IM, Oh MH, Sun IH, Wen J, Englert J, et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother 2018;67:1271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab 2019;30:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadik A, Somarribas Patterson LF, Öztürk S, Mohapatra SR, Panitz V, Secker PF, et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 2020;182:1252–70. [DOI] [PubMed] [Google Scholar]
- 43.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019;366:1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh MH, Sun IH, Zhao L, Leone RD, Sun IM, Xu W, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest 2020;130:3865–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019; 569:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karamitopoulou E. Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features. Br J Cancer 2019;121:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najac C, Chaumeil MM, Kohanbash G, Guglielmetti C, Gordon JW, Okada H, et al. Detection of inflammatory cell function using 13C magnetic resonance spectroscopy of hyperpolarized [6–13C]-arginine. Sci Rep 2016;6:31397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zajac E, Schweighofer B, Kupriyanova TA, Juncker-Jensen A, Minder P, Quigley JP, et al. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 2013;122:4054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, Shen Z, Wang Z, Wang X, Zhang H, Qin J, et al. Increased expression of IDO associates with poor postoperative clinical outcome of patients with gastric adenocarcinoma. Sci Rep 2016;6:21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 2006;4:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jha AK, Huang SC-C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015;42:419–30. [DOI] [PubMed] [Google Scholar]
- 52.Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res 2020;80:1438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jian SL, Chen WW, Su YC, Su YW, Chuang TH, Hsu SC, et al. Glycoly regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis. Cell Death Dis 2017;8:e2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel CH, Leone RD, Horton MR, Powell JD. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat Rev Drug Discov 2019;18: 669–88. [DOI] [PubMed] [Google Scholar]
- 55.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011;35:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med 2011;3:67ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang CH, Curtis JD, Maggi LB, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013;153:1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013;38:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002; 16:769–77. [DOI] [PubMed] [Google Scholar]
- 60.Menk AV, Scharping NE, Moreci RS, Zeng X, Guy C, Salvatore S, et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep 2018;22:1509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu J, Villa M, Sanin DE, Buck MD, O’Sullivan D, Ching R, et al. Acetate promotes T cell effector function during glucose restriction. Cell Rep 2019;27: 2063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee CF, Lo YC, Cheng CH, Furtmüller GJ, Oh B, Andrade-Oliveira V, et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep 2015; 13:760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 2013;123:4479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao Bo, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009;30:832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature 2009;460:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 2016;45:374–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 2016;45:358–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Angelin A, Gil-de-Gomez L,Dahiya S,Jiao J,Guo L, Levine MH,et al.Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab 2017;25:1282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol 2020;21:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghorashian S, Kramer AM, Onuoha S, Wright G, Bartram J, Richardson R, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med 2019;25:1408–14. [DOI] [PubMed] [Google Scholar]
- 71.Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol 2019;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res 2015;75:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019;363:eaau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 2016;167:829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hermans D, Gautam S, García-Cañaveras JC, Gromer D, Mitra S, Spolski R, et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8(+) T cell stemness and antitumor immunity. Proc Natl Acad Sci U S A 2020;117:6047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, Crompton JG, et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab 2016;23:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pilipow K, Scamardella E, Puccio S, Gautam S, De Paoli F, Mazza EMC, et al. Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI insight 2018;3:e122299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015;28:415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 2009;17:1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676–84. [DOI] [PubMed] [Google Scholar]
- 81.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol 2004;172:104–13. [DOI] [PubMed] [Google Scholar]
- 82.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 2016;44: 380–90. [DOI] [PubMed] [Google Scholar]
- 84.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 2016;166:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nabe S, Yamada T, Suzuki J, Toriyama K, Yasuoka T, Kuwahara M, et al. Reinforce the antitumor activity of CD8(+) T cells via glutamine restriction. Cancer Sci 2018;109:3737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.