Abstract
A fraction of the OA patient population is affected by post-traumatic osteoarthritis (PTOA) following acute joint injuries. Stopping or reversing the progression of PTOA following joint injury could improve long-term functional outcomes, reduced disability, and medical costs. To more effectively treat articular cartilage injury, we have developed a novel cell-based therapy that involves the pre-targeting of apoptotic chondrocytes and the delivery of healthy, metabolically active chondrocytes using click chemistry. Specifically, a pre-targeting agent was prepared via conjugating apoptotic binding peptide (ApoPep-1) and trans-cyclooctene (TCO) onto polyethylene glycol (PEG) polymer carrier. The pre-targeting agent would be introduced to injured areas of articular cartilage, leading to the accumulation of TCO groups on the injured areas from actively binding to apoptotic chondrocytes. Subsequently, methyltetrazine (Tz)-bearing chondrocytes would be immobilized on the surface of TCO-coated injured cartilage via Tz-TCO click chemistry reaction. Using an ex vivo human cartilage explant PTOA model, the effectiveness of this new approach was evaluated. Our studies show that this novel approach (Tz-TCO click chemistry) significantly enhanced the immobilization of healthy and metabolically active chondrocytes to the areas of apoptotic chondrocytes. Histological analyses demonstrated that this treatment regimen would significantly reduce the area of cartilage degeneration and enhance ECM regeneration. The results support that Tz-TCO click chemistry-mediated cell delivery approach has great potential in clinical applications for targeting and treatment of cartilage injury.
Keywords: chondrocyte, cartilage regeneration, cell therapy, click chemistry
Introduction
Post-traumatic arthritis typically occurs following joint trauma and commonly leads to the long-term affliction, osteoarthritis (OA). This type of OA is referred to as post-traumatic OA (PTOA) and has been estimated to represent 10% of all patients with OA. OA left unchecked can progress to the complete loss of the joint’s articular surface with resultant bone on bone articulation, subchondral sclerosis and cyst formation, and osteophyte formation. Acute joint injuries, intraarticular fractures, ligament and chondral injuries, and meniscal tears are common causes that lead to PTOA [1]. In the United States, the number of patients who suffer from PTOA of the knee, hip or ankle nearly reaches six million, accounting for about 3 billion dollars in annual societal expenditures [2]. Approximately 50% of joint trauma patients develop OA [3]. PTOA is a more common complication in young adults and these injuries may endure for years asymptomatically and worsen over time. Studies on ligament and meniscus injuries have shown that acute molecular changes can take place in the joint decades prior to detecting structural changes [4]. Definite changes are present in late stage (Grade 3 and 4) OA, where treatment often necessitates joint replacement surgery to restore motion and reduce pain. However, young adults are not ideal candidates for joint replacement or fusion treatments, as this invasive surgery is associated with considerable surgical and systemic risks, costs, and these artificial joints have a limited life span. Additionally, higher energy injuries to the surface of articular cartilage reflect a higher risk for rapid onset of PTOA [5]. Young adults with joint injuries that are at higher risk of developing OA should be addressed in the prevention of PTOA [6]. Therefore, there is a need to develop such a therapeutic strategy, to prevent the ultimate development of PTOA and to potentially reverse articular cartilage degeneration from further destruction.
The most significant effect in joint trauma is damage to the articular cartilage, as the degenerative process following injury is largely immutable. The tissue has an extremely limited self-repair capacity due to its intrinsic characteristics of chondrocyte scarcity within the tissues and vascular deficiency [7]. Articular cartilage repair is a challenge, and currently, there are no approved treatments to target post-traumatic arthritis and no available means to prevent OA. Standard non-operative treatment for post-traumatic arthritis generally involves the use of nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy to strengthen the muscles surrounding the joint, weight reduction, and intra-articular injections with corticosteroids and hyaluronic preparations. Despite reducing inflammation and the associated pain, these methods are aimed at sustaining joint comfort and function and do not cure arthritis [8–10]. Surgical intervention is considered if the arthritis progresses and the previous measures are no longer effective. Surgical treatment for post-traumatic arthritis typically includes debridement, reconstruction, or replacement of the damaged joint surfaces.
Cell-based therapies have been emerging in the field of articular cartilage tissue engineering/regeneration in recent years. Autologous chondrocyte transplantation (ACT) as a cell-based therapy for OA involves harvesting and expanding chondrocytes from non-articular areas of the affected joint and then re-implantation of these chondrocytes back into the articular cartilage defects [11]. Although, studies have found this treatment to relieve patient’s symptoms in the short-term. Significant long-term cartilage regeneration was found only in patients with minimal or surface-level articular defects. Even though this treatment is able to repair small defects, it fails in generating new hyaline cartilage [12]. Additionally, complications including donor site morbidity and fibrocartilage formation were reported [13, 14]. Furthermore, this bolus administration of expanded chondrocytes lacks specificity and the ability of these chondrocytes to anchor to injured and degenerative sites. Although in some cases this treatment regimen has been shown to improve function and reduce pain when the defects are advanced and well defined, this cell-based treatment has not been used to treat or prevent post-traumatic cartilage degradation, which is the objective of this investigation [3, 15].
To achieve our objectives, we developed a novel strategy to deliver healthy chondrocytes directly to areas of injured cartilage via a two-step process (Scheme 1B): (i) apoptotic cell targeting and (ii) chondrocyte cell delivery and immobilization at the injured site(s). For the apoptotic targeting, a nano-probe was designed to target the apoptotic chondrocytes, since chondrocyte apoptosis is a well-recognized hallmark of early stage OA [16]. Cell death is established as a notable mechanism in OA joint pathology development and has become a focal point in post-traumatic cartilage injury research [17]. Furthermore, in the immediate response of cartilage to mechanical trauma, chondrocyte death occurs and apoptosis markers elevate which can begin the cartilaginous degradative process toward PTOA [18, 19]. Previous studies have found that there are more apoptotic cells presented on the injured cartilage compared with the normal tissue [20–22]. Equally important, increasing evidences show that there is a positive correlation between apoptotic cells and cartilage matrix depletion [23, 24]. Here, ApoPep-1, a six amino acid peptide (CQRPPR) was used as a ligand to target apoptotic chondrocytes. Earlier studies have shown that the ApoPep-1 probe can identify and bind apoptotic chondrocytes [25]. For chondrocyte delivery, we used copper-free click chemistry strategy to engineer chondrocytes [26]. This strategy has recently been employed for cell engineering, cell transplantation and imaging reports/drug delivery in the diagnosis and treatment of cancers [27]. Finally, to simulate the osteoarthritic environment, the feasibility of this approach was evaluated using an ex vivo induced human cartilage injury model [28]. Overall results show that the proposed click chemistry strategy possesses good translational potential with high specificity, fast conjugation rate, and biocompatibility.
Scheme 1.
Two-step approach for pre-targeting apoptotic chondrocytes and subsequent Tz-chondrocyte delivery. (A) Tz-engineering of chondrocytes and (B) their targeted delivery to injured cartilage surface
Materials and methods
Apoptotic cell-binding peptide (CQRPPR) (United BioSystems Inc., Herndon, VA), heterobifunctional maleimide polyethylene glycol amine (Mw : 3.5k, Mal-PEG-NH2) (JenKem Technol, Plano, TX), tetraacetylated N-azidoacetyl-d-mannosamine (Ac4ManNAz), methyltetrazine DBCO (DBCO-Tz) and TCO-NHS ester (Click Chemistry Tools, Scottsdale, AZ), sulfo-cyanine7 N-hydroxysuccinimide ester (Lumiprobe Corp., Hallandale Beach, FL), Vybrant™ DiD cell-labeling solution (Thermo Fisher, Waltham, MA), and all other chemicals (Sigma-Aldrich Corporation, St Louis, MO).
Synthesis of pre-targeting agent (CQRPPR-PEG-TCO)
Mal-PEG-NH2 (15.7 mM) and TCO-NHS ester (25.1 mM) were dissolved in 2 ml of dimethyl sulfoxide, to which 4.5 μl of triethylamine was added. After stirring at room temperature overnight, the intermediate product (MAL-PEG-TCO) was purified by dialysis against DI water and then collected by lyophilization. The intermediate yield was determined to be 72.7%. Next, 80 mg of MAL-PEG-TCO and 35 mg of peptide (CQRPPR) were dissolved in 5 ml of phosphate-buffered saline (PBS, pH 7.2), and the conjugation reaction proceeded at room temperature for 24 h. Following dialysis against DI water, the pre-targeting agent was freeze-dried for 1H-NMR characterization and further use. Meanwhile, using sulfo-cyanine5 NHS ester (Lumiprobe Co., Hunt Valley, MD), some apoptosis-binding optical probe (CQRPPR-PEG-Cy5) was prepared as described in a recent publication [25].
Isolation and Tz-engineering of human chondrocytes
Chondrocytes were isolated from arthritic human cartilage explants isolated from patients during joint replacement surgery as previously published [29]. To produce Tz-bearing chondrocyte surfaces, the isolated chondrocytes were incubated with DMEM complete medium supplemented with 50 μM Ac4ManNAz in 37.0°C for 3 days as previously described [30], then washed with PBS once, and cultured in DMEM complete medium supplemented with DBCO-Tz with different concentrations for 45 min (Scheme 1A). The introduction of the Azide (N3) group and Tz group to the cell surface was confirmed using fluorescence imaging with AFDye 488 DBCO and Cy5-TCO respectively (Click Chemistry Tools). The effect of DBCO-Tz dose (0−100 μM) on chondrocyte viability was evaluated by MTT assay. The proliferative activity of Tz-engineered chondrocytes was also compared with unlabeled chondrocytes [25].
In vitro Tz-chondrocyte stability and TCO interactions
In vitro availability of Tz presented on chondrocyte membranes
The availability of Tz-groups on chondrocyte surfaces was studied over 7 days using fluorescence measurements as previously described [31]. Tz-bearing chondrocytes and unlabeled chondrocytes (as control) were seeded in a 96-well plate (2 × 104 cells/well). The cells were incubated with 50 μl of AFDye 488 TCO (50 μM TCO in DMEM) in 37.0°C for 1 h and then washed 3× with PBS. At each sampling time (0, 1, 3, 5 and 7 days), the fluorescence intensity of each well was acquired using a microplate reader (SpectraMax Gemini XPS, Molecular Devices). The excitation wavelength and emission wavelength used for the TCO dye was 490 and 540 nm, respectively. The availability of cell surface bound Tz was determined by dividing the fluorescent intensity at specific time points with respect to Day 0 and calculating the percentage of Tz-groups available on the cell surface over time.
In vitro testing of Tz-chondrocyte interaction with TCO-coated surface
The targeted delivery efficacy of Tz-chondrocytes was tested in vitro using TCO-coated wells. For that, a 24-well plate was first coated with 200 μl of 100 μg/ml TCO-conjugated albumin or unconjugated albumin (as control). Three million Tz-chondrocytes were incubated with 6 μl of Vybrant™ DiD cell-labeling solution (Thermo Fisher Scientific, Inc., Waltham, MA) in 100 μl serum free (SF)-DMEM in 37.0°C for 30 min. After the incubation, the cells were washed with PBS and resuspended in SF-DMEM. The Tz-chondrocytes or chondrocytes (as control) (1 × 105 cells/well) were added to each well of the TCO-coated well-plate and incubated in 37.0°C for 5, 15 and 30 min to provide sufficient time for Tz-TCO reactions since Tz-TCO click chemistry is known to have short reaction times (in minutes) and very fast reaction rates [26, 32–34]. After the incubation, the cells were washed 3× with PBS and were lysed with 200 μl/well of 1% Triton-X-100 in PBS. The lysate was transferred to a 96-well plate (black wall/clear bottom) for fluorescence measurements as described earlier [25]. The numbers of adherent chondrocytes under different treatment conditions were estimated based on the fluorescence intensities.
In vitro assessment of pre-targeting agent (ApoPep-1-PEG-TCO) functionality
Apoptosis was induced in chondrocytes by a sodium nitroprusside (SNP) treatment as previously described [25]. The apoptotic cells were then used to assess the functionality of the pre-targeting agent. Briefly, apoptotic cells were incubated with react with pre-targeting agent (ApoPep-1-PEG-TCO) (0.05 mg/ml in SF-DMEM) at 37.0°C for 30 min. The incubated cells were washed and then incubated with Tz-dye (0.05 mg/ml in SF-DMEM) at 37.0°C for 30 min. After washing with SF-DMEM 3×, the cell-associated fluorescent intensity was then measured using a fluorescence plate reader (SpectraMax Gemini XPS, Molecular Devices). The effect of pre-targeting agent dose (0 − 500 µg/ml) on chondrocyte viability was evaluated with Alamar Blue assay.
Ex vivo evaluation of pre-targeted cell delivery to injured cartilage in PTOA model
Ex vivo study to assess the therapeutic efficacy of pre-targeting cell delivery
Arthritic cartilage tissues isolated during total joint replacement surgery obtained per our institution’s approved IRB agreement without patients’ identity were used in this investigation. The efficacy of our approach to deliver chondrocytes to injured cartilage and then to promote tissue regeneration was evaluated using an ex vivo explant PTOA model. For that, areas of minimal cartilage damage were selected for the following experiments. A mechanical force was applied onto cartilage tissue using a modified procedure to induce injury [28]. After mechanically injuring the tissue for 3 h in DMEM media, Cy5-labeled pre-targeting agents were added onto the cartilage tissue. After incubating with the pre-targeting agent for 30 min, the tissue was washed with PBS to remove any unbound agent, then imaged to assess the tissue targeting efficacy of pre-targeting agent. Next, Tz-labeled human chondrocytes (1000 and 3000 cells/μl) were added onto the cartilage tissues. Similarly, after incubating for 30 min the tissue was washed with PBS to remove any unbound cells. The tissue was then imaged to assess the cell delivery efficacy of Tz-chondrocytes. Some of the tissues were then incubated at 37.0°C for 1 week. An in-house imaging system developed previously in our laboratory was used in the imaging study [35]. The imaging system consists of a digital CCD camera (ORCA®-R2, Hamamatsu) capable of detecting fluorescence illuminated by an LED ring light source (RL200, Smart Vision Lights). For the measurements, the cartilage was placed under the mounted light source (with adjustable height) for the duration of the imaging experiments (Excitation 670 nm, Emission 700 nm). The images were then analyzed using PortableView, an in-house image processing software developed with the portable imager. Three regions of interest (ROIs) from the same tissues were quantified to produce the mean fluorescence intensity.
Histology and immunohistochemistry
At the end of the 1-week culture, the tissue was then embedded in OCT and cryosectioned for histological evaluation. Hematoxylin and Eosin (H&E) staining was used for morphological assessment, collagen type II (Santa Cruz Biotechnology, USA) and collagen type I alpha 1 (Santa Cruz Biotechnology) staining was used for evaluation of collagen, and Safranin-O staining was used for GAG quantification as previously described [36]. Diaminobenzidine (DAB, Sigma, USA) chromogen was used for development of color. Photomicrographs were obtained using a light microscope and images were quantified for GAG and DAB (collagens) intensity using ImageJ software. Briefly, three ROIs from the same region (100 µm2) on the surface of the cartilage were selected for the quantification.
Ex vivo time course study of pre-targeting agent and cell delivery to injured cartilage
Real time imaging studies were carried out to assess the binding of both pre-targeting agents to apoptotic sites, and Tz-chondrocytes to the pre-targeting agents in a simulated clinical environment. For that, Cy5-labeled pre-targeting agents were added onto the cartilage tissue submerged in DMEM (bolus administration) then imaged under visible light and fluorescent light using the portable imager (Ex. 670 nm, Em. 700 nm) at 0 (before addition of probe), 20, 40 and 60 min as previously described [35]. Next, Vybrant™ DiD-stained Tz-chondrocytes (60 × 103 cells in 60 μl) were added onto the cartilage explants in the same manner and then imaged (Ex. 670 nm, Em. 700) at 0, 20, 40 and 60 min. ROIs were selected to quantitatively analyze the accumulation of the pre-targeted agents and cells.
Statistical analysis
All statistical analyses were performed using GraphPad Prism® software (version 9). Student’s t-test was used to perform the statistical analysis for in vitro experimental results. Paired t-tests were used to analyze ex vivo imaging data. Comparisons when P ≤ 0.05 were designated as statistically significant. Results were presented as mean ± standard deviation (SD).
Results
Isolation and Tz-engineering of human chondrocytes
In vitro labeling of chondrocytes with Tz
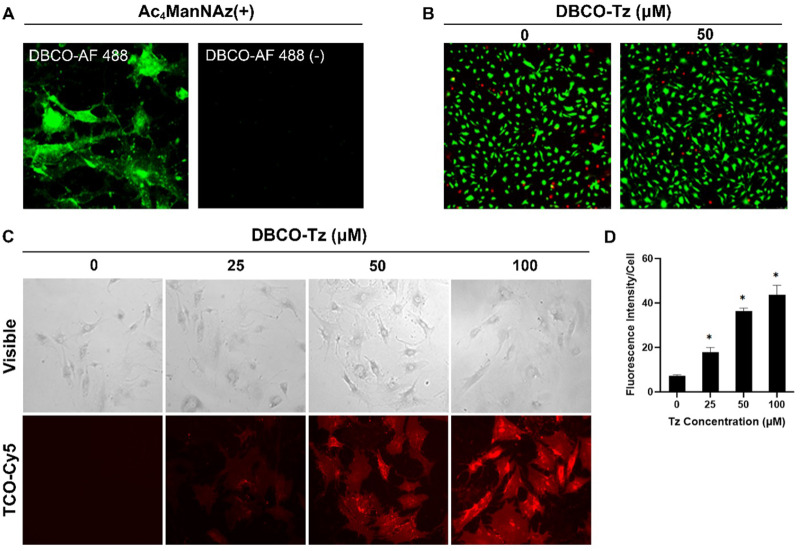
A two-step procedure (Scheme 1A) was used to introduce Tz onto the surface of human chondrocytes. First, the chondrocytes were incubated with Ac4ManNAz (50 μM) to label the cell surface with Azide (N3) groups via a metabolic glycoengineering method as reported before [30]. Second, via reaction between N3 and DBCO, Tz-expressing chondrocytes (Tz-DBCO-N3-chondrocytes) were produced. To verify this process, we first examined the incorporation of N3 onto the Ac4ManNAz-treated chondrocytes by staining the cells with AF488 Dye-conjugated DBCO-Tz which could specifically react with N3 via copper-free click chemistry (Fig. 1A). The live/dead staining showed that N3 conjugation has no significant influence on cell viability, since the viability of the N3-labeled chondrocytes reacted with DBCO-Tz has no significant difference compared with the unlabeled chondrocytes (Fig. 1B). Subsequently, the conjugation efficiency of DBCO-Tz to N3 conjugated cells was then determined by incubating the N3-labeled chondrocytes with different concentrations of DBCO-Tz (0−100 μM). As expected, the chondrocyte-associated fluorescent signal intensity increases with the increase in DBCO-Tz concentration (Fig. 1C and D). These results indicate that Tz-chondrocytes can be produced via the two-step process and the amount of Tz on the chondrocytes is dose-dependent on DBCO-Tz.
Figure 1.
Analysis of the viability and DBCO reactivity of N3 conjugated human chondrocytes. (A) Fluorescence images of N3 conjugated human chondrocytes reacted with or without incubation with AF488 dye-conjugated DBCO (50 μM). (B) Live (green)/dead (red) staining of chondrocytes with or without DBCO-Tz (0 − 50 μM) labeled chondrocytes. (C) Cy5 fluorescence images of DBCO-Tz (0 − 100 μM) treated chondrocytes incubated with TCO-Cy5. (D) Cy5 fluorescence intensities of DBCO-Tz (0 − 100 μM) treated chondrocytes (n = 4, Student’s t-test, *P < 0.05 versus 0 μM). Results presented as mean ± SD.
Cytotoxicity and function of Tz-chondrocytes
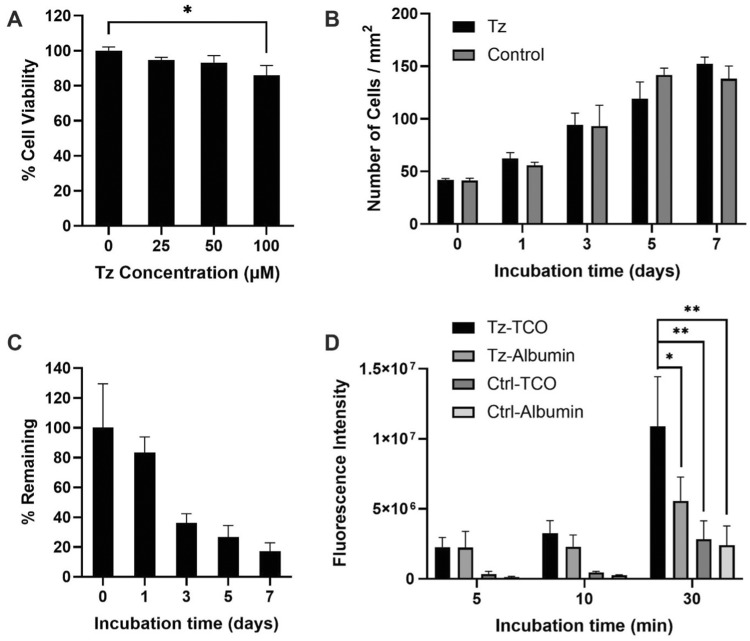
The cytotoxicity of DBCO-Tz to human chondrocytes was determined via MTS assay (Fig. 2A). In this experiment, we found that the treatment of chondrocytes with DBCO-Tz exerted no apparent toxicity up to 50 μM. Significant cell toxicity was evident with 100 μM DBCO-Tz which demonstrated ∼85% cell viability compared with 0 μM controls. Based on these results, 50 μM DBCO-Tz was determined as the optimal concentration for cell labeling. Next, proliferative potential of the Tz-conjugated chondrocytes was studied over the course of 7 days (Fig. 2B). Interestingly, we find that Tz-conjugation would not affect the cell proliferation for up to 7 days. The cell proliferation was not conducted beyond 7 days since that would require subculturing the cells.
Figure 2.
Cytotoxicity and functional analysis of Tz-chondrocytes. (A) Cell viability of DBCO-Tz (0 − 100 μM) labeled chondrocytes (n = 3, Student’s t-test, *P < 0.05). (B) Proliferation of DBCO-Tz (50 μM) labeled chondrocytes from Day 0 to 7. (C) Stability test of DBCO-Tz (50 μM) labeled chondrocytes from Day 0 to 7. (D) In vitro time-dependent targeting analysis of Tz-chondrocytes (n = 4, Student’s t-test, *P < 0.05, **P < 0.01). Results presented as mean ± SD.
In vitro Tz-chondrocyte availability and TCO interactions
Availability of Tz functional group on chondrocytes
The availability of Tz (50 μM) on cell surface was studied over time by reacting with fluorescent labeled TCO at different time points: 0, 1, 3, 5 and 7 days (Fig. 2C). For availability calculations, the background was subtracted (unlabeled chondrocytes) and the Tz-associated fluorescence was normalized to Day 0 (100%). We found that the Tz availability decreases by ∼20% every 24 h following Tz-engineering of chondrocytes (∼1% reduction in 1 h). Approximately 80% and 40% of Tz was available (with Day 0 being 100%) to react with TCO after 1 and 3 days, respectively. These results indicated that Tz-chondrocytes can maintain surface Tz functional groups to react with TCO within 3 days from Tz-engineering.
Reactivity of Tz-chondrocytes with TCO
Next, we assessed the targeted delivery capability of the Tz-chondrocytes to TCO-coated surfaces in vitro. For that, DiD labeled Tz-conjugated chondrocytes were incubated in wells coated with TCO-conjugated albumin (Tz-cell/TCO-albumin) for 5, 10 and 30 min. Here, Tz-cell/Albumin (Tz-cells were incubated in the wells coated with albumin), Cell/TCO-albumin (Cells were incubated in the wells coated with TCO-conjugated albumin) and Cell/Albumin (Cells were incubated in the wells coated with albumin) were used as the control groups. While bovine serum albumin was used as a surface blocking agent, it may lead to cell adhesion due to non-specific binding [37]. Hence, the inclusion of the albumin groups in the experiment serves as controls for non-specific binding. After washing the wells 3× with PBS, the numbers of adherent cells were estimated based on cell-associated fluorescent intensity measurement (Fig. 2D). As expected, the results show that Tz-chondrocytes have high affinity to TCO-coated surfaces, and the number of Tz-chondrocytes adherent onto TCO-surfaces increased with time (Fig. 2D). When incubated for 30 min, Tz-cell/TCO-albumin group showed more than a 4-fold higher signal than both Cell/TCO-albumin and Cell/Albumin groups, indicating that the majority (∼75%) of Tz-chondrocyte accumulation on TCO-albumin coated wells is via specific Tz-TCO reactions. Interestingly, we found that Tz-chondrocytes also have high affinity for albumin coated surfaces. The Tz-cell/TCO-albumin group showed 2-fold higher fluorescence compared with the Tz-cell/Albumin group. Although the cause for such non-specific binding has yet to be determined, it is possible that the hydrophobicity of Tz groups on the cell surface provided enhanced interactions with the adsorbed albumin. Our results also show that both Cell/TCO-albumin and Cell/Albumin groups are found to have slightly increased in the number of adherent chondrocytes with time (Fig. 2D).
In vitro characterization of pre-targeting agent (ApoPep-1-PEG-TCO)
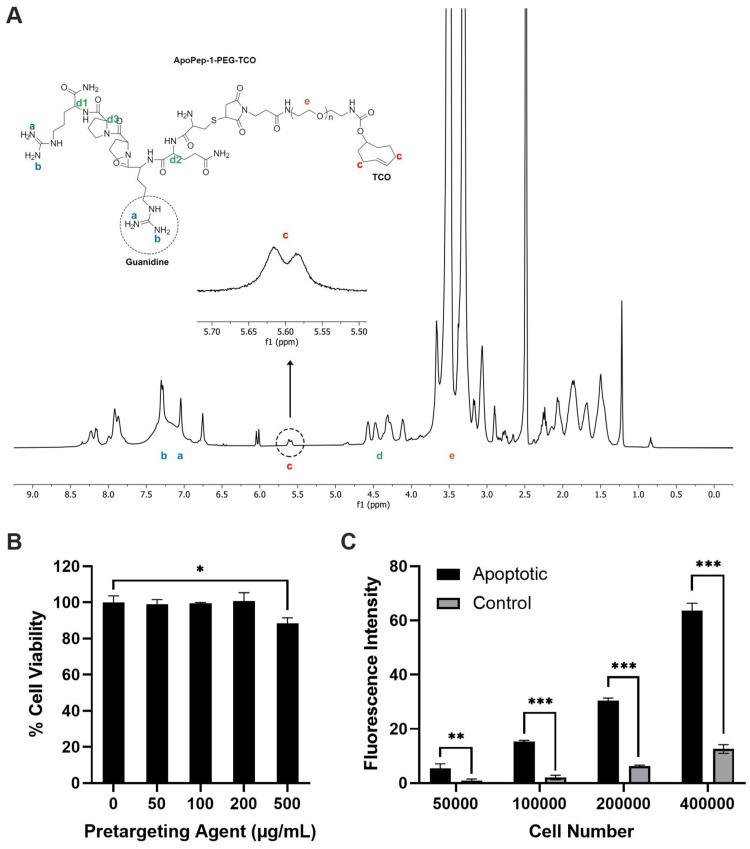
Next, injured cartilage pre-targeting agent was developed and evaluated for its ability to target apoptotic cells on injured cartilage. The pre-targeting agent (CQRPPR-PEG-TCO) was first prepared and 1H-NMR measurement (Fig. 3A) showed identifying peaks for the peptide, PEG and TCO, suggesting the successful preparation of the agent. The chemical shifts at 7.0 − 7.5 ppm were assigned to the Guanidine groups, and the multiple peaks between 4.25 and 4.7 ppm were assigned to the tertiary hydrogens next to the amide bonds. Also, the multiple peaks between 5.5 and 5.7 ppm (highlighted in the figure) correspond to the protons of CH2 next to the alkene (C=C), which is the characteristic structure of TCO. The highest peak at 3.5 ppm belongs to the PEG structure.
Figure 3.
Characterization of pre-targeting agents. (A) ¹H-NMR spectrum of the pre-targeting agent. (B) Chondrocyte viability when exposed to different concentrations of pre-targeting agent (0–500 µg/ml) (n = 3, Student’s t-test, *P < 0.05). (C) In vitro pre-targeting of apoptotic chondrocytes (n = 3, Student’s t-test, **P < 0.0001). Results presented as mean ± SD.
The cytotoxicity of the pre-targeting agent was determined using Alamar Blue assay (Fig. 3B). The results show that the pre-targeting agent was no apparent cell toxicity up to 200 µg/ml of the pre-targeting agent (>97%). Significant cell toxicity (∼85%) was observed with 500 µg/ml pre-targeting agent (P < 0.05). Next, the ability of the pre-targeting agent to detect osteoarthritic cartilage was first assessed using apoptotic cells (Fig. 3C). By incubating the pre-targeting agents with variously treated cells, we find that fluorescence intensities of apoptotic cells were significantly higher than healthy cells (P < 0.001) and up to 7-fold higher than healthy cells.
Visualization of pre-targeted cell delivery to injured human cartilage
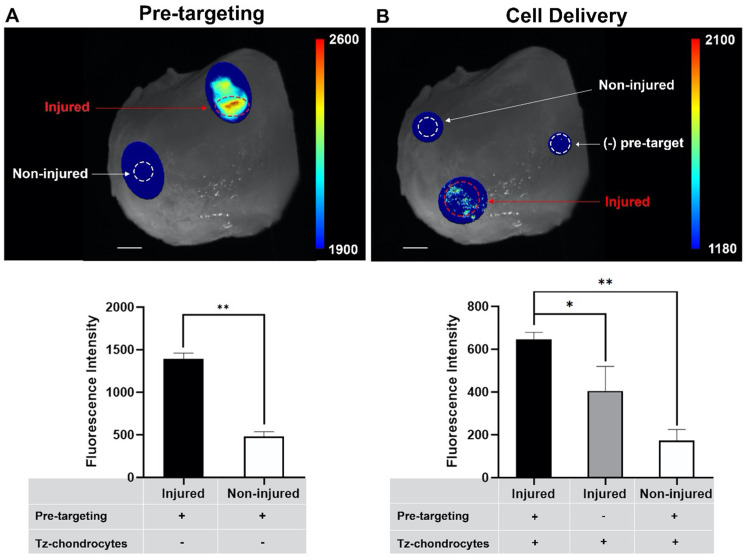
An ex vivo study was carried out to assess the efficacy of the pre-targeting approach using mechanically injured freshly isolated human cartilage tissue. Briefly, we first explored if the pre-targeting agent could accumulate at the mechanically injured cartilage tissue through targeting to the apoptotic cells. This is a very critical step to express TCO groups on the damaged tissue, which allows for accumulation of the Tz-chondrocytes via TCO-Tz click chemistry reaction. For that, the pre-targeting agent conjugated with TCO dye was applied to mechanically injured cartilage explants, and then ex vivo images were captured using an in-house imaging system (Fig. 4A). We found that the damaged tissues emitted a strong fluorescence signal while the signal was hardly detectable on the healthy tissues. Quantification analysis further indicated that the damaged tissue had ∼3-fold higher fluorescence intensity compared with the control (non-injured) tissue in 20 min (Fig. 4A). These results suggest that the pre-targeting agent was able to accumulate on the injured cartilage areas via targeting of the apoptotic chondrocytes induced by the mechanical force. Next, we explored whether delivered Tz-chondrocytes would accumulate at the pre-targeted apoptotic sites via Tz-TCO click chemistry reaction. For that, pre-targeting agent was administered to mechanically injured tissue for 30 min. Then, Tz-chondrocytes labeled with Vybrant™ DiD-staining were introduced and ex vivo images were taken using our imaging system. Similarly, ex vivo imaging (Fig. 4B) showed that our approach triggered more chondrocyte accumulation at the area of the injured tissue than the control groups: 2-fold higher than injured tissue without pre-targeting treatment, and 4-fold higher than non-injured tissue (Fig. 4B). These results suggest that our pre-targeting agent was able to facilitate the Tz-chondrocyte delivery via click chemistry approach indicated by the accumulation of Tz-chondrocytes at the areas where the cartilage had been injured.
Figure 4.
Ex vivo assessment of pre-targeting agent and cell delivery efficacy. (A) Fluorescent images of pre-targeting agent binding to apoptotic sites before and after wash and quantitative analysis of fluorescent images obtained from the probe binding tests (after washing) (nROI = 4, paired t-test, **P < 0.05, **P < 0.01). (B) Fluorescent images of Tz-chondrocyte delivery before and after wash and quantitative analysis of Tz-chondrocyte (60 × 103 cells/60 µl) delivery fluorescent images (after washing) (nROI = 4, paired t-test, *P < 0.05, **P < 0.01). Results presented as mean ± SD. Scale bar = 5 mm.
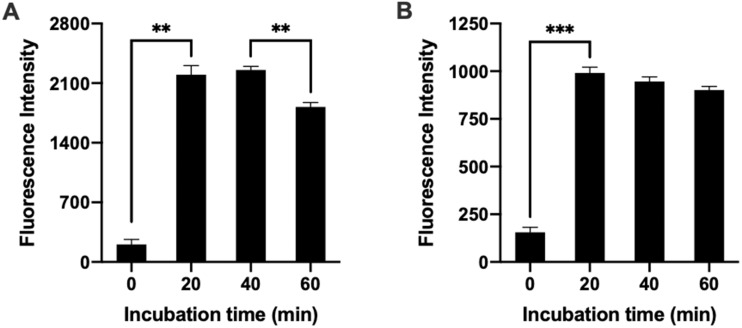
To better simulate the clinical environment and optimize the treatment condition, the pre-targeting approach was investigated using freshly isolated osteoarthritic cartilage tissue ex vivo. For that, we tested the two-step targeted delivery method using real time imaging over 2 h to explore the binding of both the pre-targeting agent and Tz-chondrocytes when administered sequentially without washing to simulate a clinical setting. For each step, the pre-targeting agent and Tz-chondrocytes were administered to the cartilage tissue submerged in media (0 min) and imaged after 20, 40 and 60 min (Fig. 5A). As expected, the pre-targeting agents were found to have high affinity for injured areas of the explants’ areas within 20 min. The amounts of pre-targeting agents on the injured areas showed about ∼15% decrease in intensity from 40 to 60 min with statistical significance. The decrease in intensity may be caused by the reduction of the non-specific binding between pre-targeting agents and the tissue. Later, even when suspended in media, the pre-targeting agent and Tz-chondrocytes were able to accumulate and reach a plateau within 20 min of administration (Fig. 5B). There was a slight decrease of Tz-chondrocytes over 60 min, however, the differences between all three time points are not statistically significant.
Figure 5.
Ex vivo imaging of pre-targeting agent and Tz-chondrocyte delivery time-dependent study. Pre-targeting agent was introduced to tissue in media and imaged at 20-, 40- and 60-min post. Subsequently, Tz-chondrocytes were administered to the media and imaged at 20-, 40- and 60-min post. (A) Quantification of pre-targeting agent on injured cartilage at different time points (nROI = 3, paired t-test: **P < 0.001). (B) Quantification of Tz-chondrocytes at different time points following 60 min incubation with pre-targeting agent (nROI = 3, paired t-test: ***P < 0.001). Results presented as mean ± SD.
Ex vivo evaluation of the efficacy of pre-targeting approach to improve the cartilage regeneration in situ
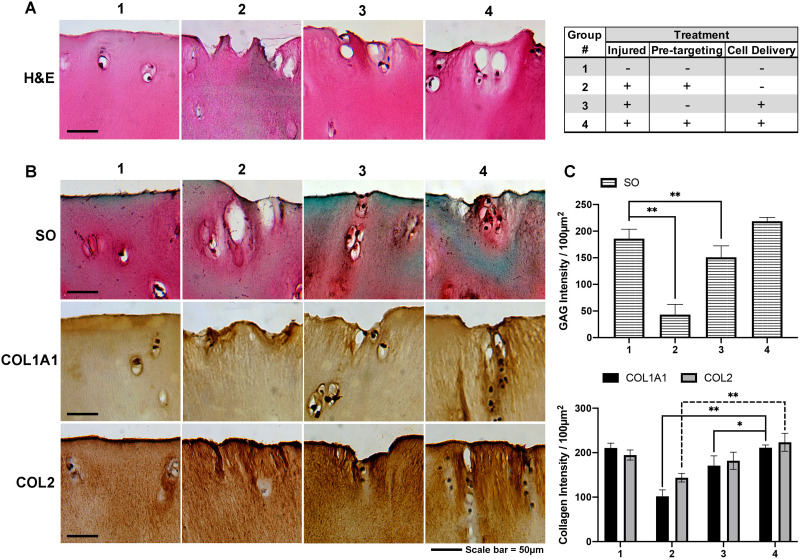
By targeting the delivery of chondrocytes to the injured cartilage sites, further studies were performed to determine whether the pre-targeting approach would promote the regeneration of cartilage tissue in situ. For that, injured cartilage tissue (as described above) was treated with pre-targeting agents (30 min) and chondrocytes (30 min), and then cultured for 1 week in DMEM supplemented with 10% FBS. Representative samples of control (non-injured), injured without pre-targeting, injured without cell delivery, and injured with both pre-targeting and cell delivery tissue then underwent histological analyses, including H&E, Safranin-O, collagen type I alpha 1 and collagen type II. The histological results showed that the pre-targeting cell delivery approach was able to deliver chondrocytes to the sites of injured cartilage (Fig. 6A) and that the areas where the delivered chondrocytes accumulated expressed greater amounts of GAG (Safranin-O), collagen type 1 (Col 1A1) and collagen type II (Col II) than those without delivered chondrocytes (Fig. 6B). Quantification analysis for the pre-targeting cell delivery group showed that compared with the non-treated group, there was an ∼80, 80 and ∼60% increase in the formation of GAG, type I and type II collagen, respectively (Fig. 6C). The GAG, type I and type II collagen intensities for the group that received the pre-targeting treatment was not statistically significant compared with the control (non-injured) groups. Compared with the control (non-injured) group, the non-targeting treatment group shows ∼45, 24 and 25% increase in the formation of GAG, type I and type II collagen, respectively (Fig. 6C). Similarly, the percent increase of formation of GAG and collagens using the pre-targeting approach was higher (∼40 − 50%) than that of the non-targeting group (Fig. 6C). These results show that our pre-targeting approach to treat injured cartilage has a greater effectiveness compared with the conventional non-targeting approach.
Figure 6.
Histological evaluation of osteoarthritic cartilage tissue following different treatment for 1 week. Treatment groups included (1) non-injured; (2) injured without both pre-targeting and Tz-chondrocyte delivery; (3) injured without pre-targeting with Tz-chondrocyte delivery and (4) injured with both pre-targeting and Tz-chondrocyte delivery. (A) Delivery of chondrocytes to injured sites (H&E). (B) Safranin-O (so) staining, collagen type I α1 (COL1A1) and collagen type II (COL2) staining of Tz-chondrocyte delivery to human cartilage tissue treated with apoptotic targeting ligand (after 7 days). (C) Quantification of GAG, COL1A1 and COL2 intensity per 100 µm2 area (n = 3, Student’s t-test, *P < 0.01). Results presented as mean ± SD. Scale bar = 50 µm.
Discussion
Cartilage tissue has poor regenerative ability after injury and during degeneration due to its intrinsic avascular structure, the limited number of resident chondrocytes, and the structure of intact articular cartilage [7]. OA, also referred to as ‘wear and tear’ arthritis, commonly develops with aging and use, but may also develop following an acute joint injury. To restore the injured cartilage tissue, chondrocyte-based therapies have been extensively explored to promote chondrogenesis in the degenerative defects via transplantation of the metabolically active chondrocytes to the defect with or without the aid of scaffolds [38]. However, even with satisfactory outcomes in short term, long-term restoration of these cartilage defects with articular cartilage has not been generally achieved [39]. Failure to fully restore these injured areas may be because the structure of the scaffold does not exactly resemble that of cartilage, and the potential for chondrocytes to engraft onto the cartilage tissue is low for scaffold-free approaches. This investigation was designed to test a novel strategy to enhance the engraftment of normal chondrocytes onto areas of injured cartilage using a pre-targeting/Copper-free click chemistry conjugation system. To produce acutely injured cartilage, we used a PTOA model where cartilage tissue was mechanically injured to induce chondrocyte apoptosis. Pre-targeting ligand was first administered, which specifically targeted the cartilage defects and bound to apoptotic chondrocytes. Once the residual pre-targeting TCO ligand (non-targeting ligand) was cleared, complementary Tz-chondrocytes were delivered, and the Tz-TCO click chemistry reaction was shown to occur between the pre-targeting ligand and the Tz-chondrocytes leading to the enhanced engraftment of chondrocytes at the area of the cartilage defect and injury.
Several recent studies have employed similar strategies to improve drug or imaging probe’s accumulation in the disease site for the treatment and diagnosis of some cancers [40, 41]. Meanwhile, this pre-targeting method has also been extended to improve tissue regeneration via improvement of stem cell delivery. A recent investigation has showed that using this strategy can enhance the binding of endothelial progenitor cells to injured blood vessels, leading to effective heart healing [42]. In applying this strategy to selectively deliver healthy chondrocytes to degenerative and injured cartilage, we found chondrocytes were immobilized on top of the injured cartilage via TCO-Tz click chemistry reaction following bolus treatment with pre-targeting TCO ligand during both ex vivo imaging studies and histological analysis. Based on the observations, our approach may pave a path to treat the damaged cartilage via enhancing chondrocyte delivery for tissue regeneration.
To design an effective pre-targeting ligand, the accessible targeting biomarker must be recognized by the target cell. Chondrocyte death has been recognized to play a role in joint pathology, especially following joint injury [18]. Since a large number of apoptotic chondrocytes reside in early degenerative and acutely injured cartilage (but not in the cartilage) [20–22], and earlier evidence has shown a histone H1-binding peptide (CQRPPR) to effectively bind the histone H1 protein exposed on the apoptotic cell’s surface, several imaging probes based on the peptide have been explored to identify cartilage lesions via active targeting of apoptotic cells [41–43]. In this study, PEG polymer was used as a vehicle to prepare the pre-targeting ligand. The peptide was coupled to one end of the PEG to enhance accumulation of the pre-targeting ligand on the apoptotic chondrocytes, while the other end of the PEG was linked with TCO groups for chondrocyte capture. It is well documented that PEG has been commonly used for the development of drug delivery systems and imaging probes to lessen nonspecific binding and improve bioavailability [44]. Thus other carrier polymers and concentrations were not tested in this study and will be compared and optimized in future work. The in vitro experiments confirmed that the pre-targeting ligand could preferentially accumulate at the injured tissue by actively binding to the apoptotic cells, thus resulting in accumulation of the ligand at the damaged cartilage site. Furthermore, it has been noted that other than the six amino acid peptide several well-recognized apoptosis-binding ligands, including phosphatidylserine-binding Annexin V and apoptotic marker heat shock protein 60 (HSP60)-binding peptide (P17), may be employed to develop the apoptosis-targeting ligands [45, 46]. Besides apoptotic cells, collagen type II may serve as an alternative targeting biomarker since it exists in the deep zone of the cartilage consisting of 90–95% of the collagen network in cartilage, and is exposed to the synovial cavity after cartilage injury [47]. In fact, a collagen type II-binding peptide (WYRGRL) conjugated nanoparticle was investigated for targeting articular cartilage [48].
Copper-free click chemistry has been drawing an increasing attraction for modification of cellular membrane and cell delivery in recent years [32]. Indeed, copper-free click chemistry has the benefits of having high biocompatibility, and fast and selective chemical reactions in biological systems [32]. Our results confirmed that the chondrocyte surface is easily modified with Tz group. The Tz-chondrocytes show a slight reduction in proliferative potential compared with the control chondrocytes, however, the cell viability is not significantly compromised by the Tz conjugation. Similar results were observed by another group [30]. A substantial amount of Tz group is found present on the cell surface for up to 3 days which provides sufficient time for the Tz-TCO reaction and cell immobilization. Using an ex vivo injured cartilage tissue model, we found that our pre-targeting/click chemistry-directed approach could enhance the accumulation of the chondrocytes at the cartilage defect. Finally, immunohistochemical and histological analysis revealed that this cell delivery strategy can promote ECM regeneration for cartilage repair [49, 50]. Although the semi-quantitative approach based on histological staining is not the most accurate method for ECM measurements, ROIs-based quantitation has been used to compare collagen and GAG productions between different treatments in many published works and allows for the localization of ECM production at the site of cartilage injuries specifically [51–53]. More accurate approaches to GAG and collagens quantitation such as Dimethylmethylene Blue assay and enzyme-linked immunosorbent assay, respectively, will be explored in future works.
As a proof of concept, our studies show a promising outcome in the restoration of the injured cartilage tissue using the pre-targeting, click chemistry-directed chondrocyte delivery approach on degenerative and mechanically injured human cartilage tissue. To verify the long-term efficacy of this approach, further studies will need to be carried out using a validated articular cartilage animal model. In addition, to achieve even better treatment outcomes, investigations will be needed to determine whether the use of nanoparticles in place of the PEG polymer would serve as a better pre-targeting ligand. Using nanoparticles as deliver carriers offers two advantages over the use of PEG. First, because nanoparticles have a prolonged circulation time in systems, a greater number of nanoparticles will be able to accumulate on the targeted areas. and provide a greater number of reactive sites, leading to more chondrocyte accumulation [54]. Secondly, some bioactive agents, such as transforming growth factor beta and insulin like growth factor I, which have been shown to play an important role in maintaining chondrocyte phenotype during the expansion and transplantation and promoting chondrocyte ECM production, may be loaded onto the pre-targeting nanoparticles, theoretically leading to an improvement in the treatment of the injured tissue [55].
Conclusion
A novel approach has been developed to effectively target and deliver metabolically active chondrocytes to the site of injured cartilage. Our results show that the damaged cartilage can be repaired by the delivered chondrocytes and that this work may lay a foundation for the treatment of acutely injured cartilage that could stop or even reverse the degenerative process associated with PTOA.
Acknowledgements
We thank Martin Dakota Nicholas for assistance in conducting part of the tissue imaging work. This work was partially supported by UTA Research & Scholarship Excellence Gift. The authors acknowledge the financial support from the National Heart, Lung, and Blood Institute [NIH T32 HL134613 to C.C.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest statement. No potential conflict of interest was reported by the authors.
References
- 1. Kramer WC, Hendricks KJ, Wang J.. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med 2011;4:285–98. [PMC free article] [PubMed] [Google Scholar]
- 2. Brown TD, Johnston RC, Saltzman CL. et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 2006;20:739–44. [DOI] [PubMed] [Google Scholar]
- 3. Lotz MK, Kraus VB.. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther 2010;12:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Favero M, Ramonda R, Goldring MB. et al. Early knee osteoarthritis. RMD Open 2015;1:e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckwalter JA. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther 2003;33:578–88. [DOI] [PubMed] [Google Scholar]
- 6. Gelber AC, Hochberg MC, Mead LA. et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med 2000;133:321. [DOI] [PubMed] [Google Scholar]
- 7. Newman AP. Articular cartilage repair. Am J Sports Med 1998;26:309–24. [DOI] [PubMed] [Google Scholar]
- 8. Tao K, Rey-Rico A, Frisch J. et al. rAAV-mediated combined gene transfer and overexpression of TGF-β and SOX9 remodels human osteoarthritic articular cartilage. J Orthop Res 2016;34:2181–90. [DOI] [PubMed] [Google Scholar]
- 9. Bellavia D, Veronesi F, Carina V. et al. Gene therapy for chondral and osteochondral regeneration: is the future now? Cell Mol Life Sci 2018;75:649–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowman S, Awad ME, Hamrick MW. et al. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med 2018;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brittberg M, Lindahl A, Nilsson A. et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889–95. [DOI] [PubMed] [Google Scholar]
- 12. Nie X, Chuah YJ, Zhu W. et al. Decellularized tissue engineered hyaline cartilage graft for articular cartilage repair. Biomaterials 2020;235:119821. [DOI] [PubMed] [Google Scholar]
- 13. Brown WE, Potter HG, Marx RG. et al. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res 2004;422:214–23. [DOI] [PubMed] [Google Scholar]
- 14. Henderson I, Gui J, Lavigne P.. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy 2006;22:1318–24.e1. [DOI] [PubMed] [Google Scholar]
- 15. Ji X, Zhang H.. Current strategies for the treatment of early stage osteoarthritis. Front Mech Eng 2019;5:57. [Google Scholar]
- 16. Charlier E, Relic B, Deroyer C. et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. IJMS 2016;17:2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kühn K, D'Lima DD, Hashimoto S. et al. Cell death in cartilage. Osteoarthr Cartil 2004;12:1–16. [DOI] [PubMed] [Google Scholar]
- 18. D'Lima DD, Hashimoto S, Chen PC. et al. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthr Cartil 2001;9:712–9. [DOI] [PubMed] [Google Scholar]
- 19. Kim HT, Lo MY, Pillarisetty R.. Chondrocyte apoptosis following intraarticular fracture in humans. Osteoarthritis Cartil 2002;10:747–9. [DOI] [PubMed] [Google Scholar]
- 20. Thomas CM, Fuller CJ, Whittles CE. et al. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthr Cartil 2007;15:27–34. [DOI] [PubMed] [Google Scholar]
- 21. D’Lima DD, Hashimoto S, Chen PC. et al. Cartilage injury induces chondrocyte apoptosis. J Bone Joint Surg Ser A 2001;83:19–21. [DOI] [PubMed] [Google Scholar]
- 22. Chen CT, Burton-Wurster N, Borden C. et al. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res 2001;19:703–11. [DOI] [PubMed] [Google Scholar]
- 23. Hashimoto S, Ochs RL, Komiya S. et al. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum 1998;41:1632–8. [DOI] [PubMed] [Google Scholar]
- 24. Hwang HS, Kim HA.. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci 2015;16:26035–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang Y, Zhou J, Hakamivala A. et al. An optical probe for detecting chondrocyte apoptosis in response to mechanical injury. Sci Rep 2017;7:10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blackman ML, Royzen M, Fox JM.. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand diels−alder reactivity. J Am Chem Soc 2008;130:13518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takayama Y, Kusamori K, Nishikawa M.. Click chemistry as a tool for cell engineering and drug delivery. Molecules 2019;24:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis CG, Eisner E, McGlynn M. et al. Posttraumatic chondrocyte apoptosis in the murine xiphoid. Cartilage 2013;4:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naranda J, Gradišnik L, Gorenjak M. et al. Isolation and characterization of human articular chondrocytes from surgical waste after total knee arthroplasty (TKA). PeerJ 2017;5:e3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon HI, Yhee JY, Na JH. et al. Bioorthogonal copper free click chemistry for labeling and tracking of chondrocytes in vivo. Bioconjug Chem 2016;27:927–36. [DOI] [PubMed] [Google Scholar]
- 31. Li S, Cong W, Hakamivala A. et al. Hyaluronic acid-based optical probe for the diagnosis of human osteoarthritic cartilage. Nanotheranostics 2018;2:347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim E, Koo H.. Biomedical applications of copper-free click chemistry::in vitro, in vivo, and ex vivo. Chem Sci 2019;10:7835–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patterson DM, Nazarova LA, Prescher JA.. Finding the right (bioorthogonal) chemistry. ACS Chem Biol 2014;9:592–605. [DOI] [PubMed] [Google Scholar]
- 34. Devaraj NK, Thurber GM, Keliher EJ. et al. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc Natl Acad Sci U S A 2012;109:4762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dacy A, Haider N, Davis K. et al. Design and evaluation of an imager for assessing wound inflammatory responses and bioburden in a pig model. J Biomed Opt 2019;25:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hakamivala A, li S, Robinson K. et al. Recruitment of endogenous progenitor cells by erythropoietin loaded particles for in situ cartilage regeneration. Bioact Mater 2020;5:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koblinski JE, Wu M, Demeler B. et al. Matrix cell adhesion activation by non-adhesion proteins. J Cell Sci 2005;118:2965–2974. [DOI] [PubMed] [Google Scholar]
- 38. Lamplot JD, Schafer KA, Matava MJ.. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sport Med 2018;6:232596711876187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hapuarachchige S, Huang CT, Donnelly MC. et al. Cellular delivery of bioorthogonal pretargeting therapeutics in PSMA-positive prostate cancer. Mol Pharm 2020;17:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stéen EJL, Edem PE, Nørregaard K. et al. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018;179:209–45. [DOI] [PubMed] [Google Scholar]
- 41. Li Z, Shen D, Hu S. et al. Pretargeting and bioorthogonal click chemistry-mediated endogenous stem cell homing for heart repair. ACS Nano 2018;12:12193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang K, Purushotham S, Lee JY. et al. In vivo imaging of tumor apoptosis using histone H1-targeting peptide. J Control Release 2010;148:283–91. [DOI] [PubMed] [Google Scholar]
- 43. Che X, Chi L, Park CY. et al. A novel method to detect articular chondrocyte death during early stages of osteoarthritis using a non-invasive ApoPep-1 probe. Arthritis Res Ther 2015;17:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D'souza AA, Shegokar R.. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 2016;13:1257–75. [DOI] [PubMed] [Google Scholar]
- 45. Kolodgie FD, Petrov A, Virmani R. et al. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: A technique with potential for noninvasive imaging of vulnerable plaque. Circulation 2003;108:3134–9. [DOI] [PubMed] [Google Scholar]
- 46. Yang S, Meng J, Yang Y. et al. A HSP60-targeting peptide for cell apoptosis imaging. Oncogenesis 2016;5:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buckwalter JA, Mankin HJ, Grodzinsky AJ.. Articular cartilage and osteoarthritis. Instr Course Lect 2005;54:465–80. [PubMed] [Google Scholar]
- 48. Rothenfluh DA, Bermudez H, O'Neil CP. et al. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater 2008;7:248–54. [DOI] [PubMed] [Google Scholar]
- 49. Saha S, Kirkham J, Wood D. et al. Informing future cartilage repair strategies: a comparative study of three different human cell types for cartilage tissue engineering. Cell Tissue Res 2013;352:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee HJ, Seo Y, Kim HS. et al. Regulation of the viscoelastic properties of hyaluronate-alginate hybrid hydrogel as an injectable for chondrocyte delivery. ACS Omega 2020;5:15567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cao F, Qi J, Song H. et al. Tsmu solution improves rabbit osteochondral allograft preservation and transplantation outcome. Cell Tissue Bank 2018;19:549–58. [DOI] [PubMed] [Google Scholar]
- 52. Wang J, Zhang F, Tsang WP. et al. Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 2017;120:11–21. [DOI] [PubMed] [Google Scholar]
- 53. Hakamivala AShuxin liRobinson Ket al. . Recruitment of endogenous progenitor cells by erythropoietin loaded particles for in situ cartilage regeneration. Bioact Mater 2020;5:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morachis JM, Mahmoud EA, Almutairi A.. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol Rev 2012;64:505–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mobasheri A, Kalamegam G, Musumeci G. et al. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014;78:188–98. [DOI] [PubMed] [Google Scholar]