Abstract
Purpose of review
Over 40% of patients with severe traumatic brain injury (TBI) show clinically significant neurological worsening within the acute admission period. This review addresses the importance of identifying the crashing TBI patient, the difficulties appreciating clinical neurological deterioration in the comatose patient and how neuromonitoring may provide continuous real-time ancillary information to detect physiologic worsening.
Recent findings
The latest editions of the Brain Trauma Foundation's Guidelines omitted management algorithms for adult patients with severe TBI. Subsequently, three consensus-based management algorithms were published using a Delphi method approach to provide a bridge between the evidence-based guidelines and integration of the individual treatment modalities at the bedside. These consensus statements highlight the serious situation of critical deterioration requiring emergent evaluation and guidance on sedation holds to obtain a neurological examination while balancing the potential risks of inducing a stress response.
Summary
One of the central tenets of neurocritical care is to detect the brain in trouble. The first and most fundamental neurological monitoring tool is the clinical exam. Ancillary neuromonitoring data may provide early physiologic biomarkers to help anticipate, prevent or halt secondary brain injury processes. Future research should seek to understand how data integration and visualization technologies may reduce the cognitive workload to improve timely detection of neurological deterioration.
Keywords: neurocritical care, neurological deterioration, neuromonitoring, secondary brain injury, traumatic brain injury
INTRODUCTION
The detection of secondary brain injury still remains one of the greatest clinical challenges in the management of severe traumatic brain injury (TBI). The development and severity of these secondary injury processes is a major determinant of outcome [1,2]. Consequently, one of the intensivist's primary responsibilities is to anticipate, prevent and halt secondary brain injury processes during the acute admission period, thereby supporting patients to reach their greatest recovery potential. This is achieved at the bedside through the early detection of clinical neurological deterioration, the maintenance of optimal systemic physiology and the prompt recognition of cerebrovascular pathophysiologic processes using an integrated neuromonitoring approach. The recently published fourth edition of the Brain Trauma Foundation (BTF) guidelines for treating severe TBI in adults consists of high-quality, evidence-based recommendations [3]. However, unlike prior editions [4,5], these guidelines no longer incorporate management algorithms for clinical use. Over the past year, two working groups published three consensus-based management algorithms incorporating expert clinical judgement in areas wherein current evidence is insufficient. Included in these consensus statements are recommendations to assist in recognizing, evaluating and treating neurological deterioration in patients with severe TBI [6▪▪,7▪▪,8]. This review aims to discuss the recent literature highlighting the importance of detecting the crashing TBI patient, the difficulties identifying neurological deterioration in the comatose patient and the use of neuromonitoring to detect pathophysiologic processes that may act as early biomarkers of neurological deterioration.
Box 1.

no caption available
NEUROLOGICAL DETERIORATION AFTER SEVERE TRAUMATIC BRAIN INJURY
Despite several decades of basic and clinical research, treatments to improve outcomes after TBI are limited. However, through these trials of neuroprotective therapies, we have gained considerable knowledge about the extent and timing of neurological deteriorations during the acute admission period and associated secondary brain injury processes [9–11]. Secondary brain injury develops over time, from hours to days, with activation of multiple tissue, cellular and molecular pathways (see Fig. 1). Over 40% of patients with severe TBI show significant neurological worsening within the acute admission period [10], warranting immediate medical management and consideration for surgical intervention. The majority of patients deteriorate within 72 h after injury, with a median time of 29 h [11]. In the International Selfotel Trial, the most common neurological deterioration detected was a change in pupillary reactivity (43%), followed by a decrease in the Glasgow Coma Scale (GCS) motor score of more than 1 (25%) [11]. Not unsurprisingly, patients suffering subsequent neurological deterioration have a significantly higher mortality rate and lower incidence of favourable outcomes than patients with no neurological worsening [9]. Increased intracranial volume accounts for the majority of identified reasons. Interestingly, only a small number of patients deteriorated due to cerebral ischemia and seizures (5 and 7%, respectively) in the International Selfotel Trial, and systemic complications or no definable cause accounted for a quarter of the identified reasons [9]. An understanding of neurological worsening is becoming increasingly important because prompt access to computed tomography (CT) scans within hospitals has resulted in rapid neuroimaging within minutes of admission to hospital before lesions have started to appear or evolve after the primary brain injury [12]. However, parenchymal lesions can expand over hours or days. In a cohort study of 352 patients with brain contusions to investigate the association between clinical and radiological deterioration, the volume of haemorrhage increased in 58% of patients from their first CT at the time of hospital admission to their follow up CTs [13].
FIGURE 1.
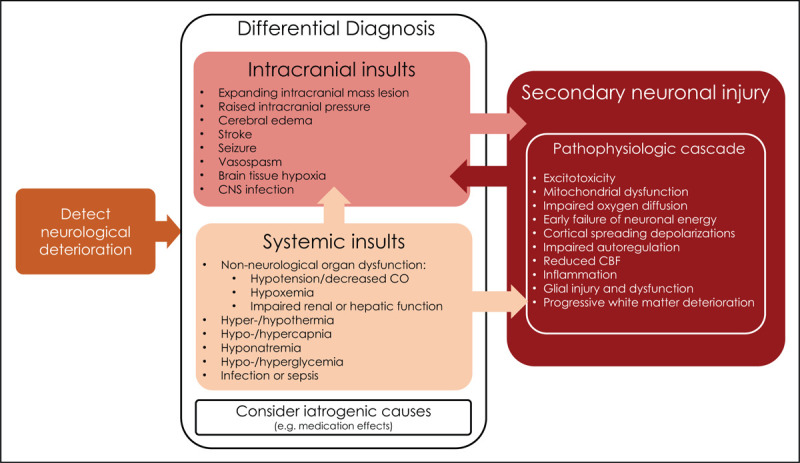
Neurological deterioration due to secondary insults on the brain [7▪▪,25,44,45]. Neurological deterioration may be due to intracranial, systemic or iatrogenic insults. Systemic and intracranial insults may drive secondary neuronal injury pathophysiologic processes occurring at a tissue, cellular and molecular level. The pathophysiology of secondary neuronal injury is complex and can involve several secondary pathological cascades that contribute to neuronal injury. Ongoing secondary neuronal injury processes may contribute to further intracranial events. Systemic insults may further add to the severity of the intracranial insults, for example hypoxemic event complicating ongoing cerebral oedema and aggravate the ongoing pathophysiologic cascade, for example systemic hypotension contributing to reduced CBF in the setting of impaired autoregulation. CBF, cerebral blood flow; CNS, central nervous system; CO, cardiac output.
DEFINING CLINICALLY SIGNIFICANT NEUROWORSENING
Deterioration of a patient's clinical status, or neuroworsening, was first defined as a potential intermediate-outcome variable for TBI trials [11]. The International Selfotel Trial defined neuroworsening as the occurrence of one or more of the following objective criteria: a spontaneous decrease in the Glasgow Coma Scale (GCS) motor score of at least 2 points (compared with the previous examination), a new loss of pupillary reactivity, interval development of pupillary asymmetry of at least 2 mm or deterioration in neurological status sufficient to warrant immediate medical or surgical intervention [11]. Neuroworsening was adapted as a clinical variable for the Benchmark Evidence from South American Trials: Treatment of Intracranial Pressure (BEST:TRIP) trial [14] and further refined by the Consensus REVised Imaging and Clinical Examination (CREVICE) Working Group for their ongoing work investigating the effectiveness of an Imaging and Clinical Examination (ICE) management protocol in resource-limited environments without intracranial pressure (ICP) monitoring [8]. The Seattle Severe Traumatic Brain Injury Consensus Conference (SIBICC) Working Group similarly adapted the definition using the clinical term ‘critical neuroworsening’ to promote recognition that this specific situation is a critical event requiring emergent evaluation and consideration of empiric therapy [6▪▪,7▪▪] (see Table 1).
Table 1.
| Criticala neuroworsening | Sedation hold needed | Continuously monitored |
| Spontaneous decrease in GCS motor score of ≥1 point (compared with the previous examination)b | Yes | No |
| New focal motor deficit | Yes | No |
| New decrease/loss of pupillary reactivity | No | No |
| New pupillary asymmetry (≥2 mmc) or bilateral mydriasis | No | No |
| Herniation syndrome/Cushing's triad | No | Yes (ICP, HR, BP, RR) |
BP, blood pressure; CREVICE, Consensus REVised Imaging and Clinical Examination; GCS, Glasgow Coma Scale; HR, heart rate; ICP, intracranial pressure; RR, respiratory rate; SIBICC, Seattle Severe Traumatic Brain Injury Consensus Conference; WG, working group.
The term ’Critical’ neuroworsening is used specifically by the SIBICC WG to promote its recognition as a critical event and guide expeditious evaluation and consideration of empiric therapy.
The ‘modified definition of neuroworsening’ now includes signs of the herniation syndrome and a lower threshold for GCS motor score (≥1 point).
Pupillary asymmetry quantification of ≥2 mm only utilized in the CREVICE protocol, the SIBICC WG does not quantify the difference in pupillary asymmetry.
DETECTING CLINICAL DETERIORATION WITH SEDATION INTERRUPTION
The first and most fundamental neurological monitoring tool is the repeated clinical examination, even in patients who are comatose or sedated [15]. The minimal requirement for the clinical examination includes an assessment of the level of consciousness, exclusion of new focal neurological deficits and measurement of pupillary size and reactivity to light. The feasibility of using the GCS tool in severe TBI has several potential limitations, confounders such as intoxication, hearing impairment, spinal cord injuries, hypotension, hypoxemia or administration of paralytics compromise assessment [16]. Furthermore, there are obstacles to the assessment of individual components of the GCS such as tracheal intubation precluding a verbal response and ocular trauma impeding eye opening. The motor response remains the main assessable component of the GCS, and fortunately, the prognostic value of the GCS is skewed towards the motor component, as studies have found this single category to be a strong predictor of outcome [17–19]. Sedation interruption, or the neurological wake-up test (NWT), is necessary in deeply sedated patients to evaluate for neurological deterioration, planning of neuroimaging and potential indications for surgical or medical interventions. However, the NWT can induce a stress reaction in severe TBI patients. Prior small single-centre cohort studies have found sedation holds in acutely brain-injured patients can cause transient rises in arterial blood pressure, heart rate, ICP and cerebral perfusion pressure (CPP), an increase in circulating stress hormones and differential results for brain tissue oxygen (PbtO2) and cerebral microdialysis (CMD) [20▪,21,22,23▪]. The clinical importance of these stress responses remains to be established, as little is known about the impact on patient outcome but should be carefully considered when deciding on the use and frequency of the NWT. The SIBICC Working Group recognized the balance between obtaining the most accurate neurological examination during a sedation hold and the potential hazards of temporarily halting sedation to perform these examinations [7▪▪]. Contraindications to the NWT reported in the literature have included uncontrolled intracranial hypertension, hyperthermia, status epilepticus, barbiturate treatment and acute respiratory distress syndrome (ARDS) [15]. The SIBICC Working Group was unsuccessful at gaining consensus on relative and absolute contraindications for sedation holds and therefore chose to construct decision-support matrices representing the most relevant clinical variables in differing intracranial hypertension scenarios [7▪▪]. The resulting heatmaps reflect the variability among expert clinicians in the perceived safety to perform a sedation hold in ICP-monitored severe TBI patients under differing conditions of pupillary status, GCS motor score, modified Marshall CT classification, duration of ‘controlled’ ICP with ongoing treatment, and degree of tiered therapy required to control any intracranial hypertension. Green, yellow and red indicate ‘safe to proceed’, ‘consider proceeding with caution’ and ‘do not proceed’, respectively, with transitional shades reflecting intermediate trends. Ultimately, it is up to the treating physician to consider the value of performing the NWT, weighing up the risks and benefits. The SIBICC Working Group recommend minimizing risks and enhancing the utility of sedation holds by coordinating the timing for all involved healthcare providers to be present (e.g. Intensivist, Neurosurgeon, ICU nurse) to maximize the safety and interpretation of the NWT [7▪▪]. Overall, sedation holds are not feasible in one-third of patients due to safety concerns and when the NWT is performed, over one-third of these trials are aborted due to critical increases in ICP and impending brain tissue hypoxia [20▪,24]. Little is known about the effectiveness of repeated NWTs to detect neurological deterioration and impact on outcomes; however, one small cohort study found that the NWT detected clinical neurological deterioration in one sedation hold out of a total of 54 trials performed [20▪]. However, given that 29–44% of patients with severe TBI will develop a clinically relevant neurological worsening during their ICU stay, the NWT may help to identify clinically important changes, arguing for repeated neurological examinations (See Table 2 for summary) [10,11,13]. The NWT may have a profound effect on patient management, with aggressive intervention in patients who show signs of progressive brainstem impairment, or reduced duration of ventilation in those recovering favourably.
Table 2.
Large studies reporting timing and features of neurological deterioration
| Study | Rate of neurological worsening | Time period for neuroworsening | Neuroworsening criteria | Radiological deterioration |
| Iaccarino et al.[13] | 32%∗ (111/352)(Clinical improvement in 6%, stable neurological function in 62%)∗29% of cohort had severe TBI, other patients had mild and moderate TBI | Clinical assessment: onset of neurological deterioration during the first 12 hours after traumaRadiological Assessment:- Injury to initial CT average 120 mins (IQR 63–98 mins)- 2nd CT average 9 hours after initial scan (IQR 154–312 mins)- 3rd CT average 38 hours after initial scan (IQR 12–14 hours) | - GCS decreased by >1 point- New pupillary abnormalities | On follow-up CT scans compared to admission CTPatients:58%- Evolution of hematoma (42% with >30% evolution) 46%- Increased edema volume30%- Onset/increase in basal cistern effacement 28%- Onset /increase of midline shift |
| Maas et al.[10] | 44% (375/846) | Within first 10 days | References Morris et al.[11] study for neuroworsening criteria | |
| Morris et al.[11] | 29% (117/409) | Median 29 h (range 3.3–447 h)a | Neuroworsening criteria (occurrence ≥ 1 of following):- Spontaneous decrease in GCS motor score ≥2 points (compared with previous exam)- New loss of pupillary reactivity- Interval development of pupillary asymmetry of ≥2 mm- Deterioration in neurologicalstatus sufficient to warrant immediate medical/surgical interventionEvents:a43%- Change in pupillary reactivity25%- Decrease ≥ 2 GCS motor score19%- Pupillary asymmetry >1 mm9%- Changes in ICP4% - Other (decrease GCS ≥ 2, new CT abnormalities, substantial change in systolic BP, systemic deterioration) |
Data from Juul et al. posthoc analysis of the International Selfotel Trial [9].
BP, blood pressure; CT computerized tomography; GCS, Glasgow Coma Scale; ICP, intracranial pressure; IQR, interquartile ratio; TBI, traumatic brain injury
DETECTING SECONDARY BRAIN INJURY WITH NEUROMONITORING
Due to the concerns of an increased stress response and the energy metabolic challenge to the injured brain, the use of the NWT has been questioned due to the increased access to neuromonitoring. However, the NWT remains the gold standard for clinical monitoring and should always be considered in TBI patients with stable baseline ICP and CPP readings. The overall aims of neuromonitoring are to identify neurophysiologic worsening that may indicate new or ongoing secondary processes, provide clear physiological data to guide and individualize therapy, improve pathophysiological understanding of cerebral disease in critical illness and assist with prognostication [25]. We will focus on the capability of neurophysiologic monitoring to identify neurological deterioration. Neuromonitoring can provide ancillary information when assessing which TBI patients can safely undergo the NWT. They also allow a physiologic examination, in place of a clinical examination, when it is either unsafe to perform the NWT in an unstable patient, the severity of the patient's illness obscures the clinical examination due to level of consciousness or medical interventions, such as an induced coma for intracranial hypertension, prohibit clinical examination. The combined use of multiple brain physiologic monitors, a platform often termed ‘multimodality neuromonitoring’ [26], can add additional information on brain tissue oxygenation, brain temperature and cerebral metabolism with the aim of providing a continuous, real-time evaluation of the brain's physiologic state to help prevent, detect and attenuate secondary brain injury [27]. Neuromonitoring devices can be divided into invasive and noninvasive (see Table 3 for an overview). Although the BTF guidelines for treating severe TBI advocate for threshold-based management treating ICP more than 22 mmHg due to the association with increased mortality [3], other emerging important neurophysiologic concepts to consider include the ICP intensity and duration or the ‘pressure-time burden’ [28▪], ICP trajectory [29] and individualized targets of ICP and CPP [30,31] are important physiologic concepts to consider when assessing brain function and predicting future neurological deterioration. Although clinical studies support the physiologic feasibility and biologic plausibility of monitoring and management based on the information from various cerebral physiologic monitors [25], data supporting this concept from randomized controlled trials are still required. The results of ongoing clinical trials to determine the effectiveness of multimodal neuromonitoring-targeted treatment (PbtO2 and ICP) versus ICP-directed therapy are eagerly awaited [32–34].
Table 3.
Commonly used neuromonitoring devices
| Device | Physiological parameter | Global vs. focal physiology | Interpretation/derived indices |
| Invasive neuromonitoring devices | |||
| ICP monitor (Intraparenchymal/ventricular catheters) | ICP | Global | Raised intracranial pressure reduces cerebral perfusionCPP, pressure-reactivity index, intracranial elastance |
| Parenchymal (PbtO2) | Brain tissue partialtension of oxygen | Focal | Oxygen diffusionBalance between oxygen supply and demand |
| Jugular venous oximetry (SjvO2) | Oxygen saturation of jugular haemoglobin | Global | Global cerebral oxygenation and extractionCerebral arterojugular difference in oxygen content |
| Cerebral microdialysis | Cerebral metabolism and biomarkers | Focal | Aerobic or anaerobic metabolism, brain injury severity and inflammation |
| Temperature monitoring (Intraparenchymal probe) | Brain temperature | Focal | Gradient between core and brain temperature |
| Intraparenchymal thermal diffusion flowmetry | Cerebral blood flow | Focal | Hypoperfusion or hyperperfusion |
| Noninvasive neuromonitoring devices | |||
| Electroencephalography | Cortical electrical activity | Global | Seizure activity, abnormal patterns |
| Optic nerve sheath ultrasonography | Optic nerve-sheathdiameter | Global | Elevated value is an indirect marker of raised ICP |
| Quantitative pupillometry | ICP | Global | Low NPi is associated with sustained elevations of ICP |
| Transcranial Doppler | Cerebral blood velocity | Focal | Indicative of regional cerebral ischemiaCritical closing pressure, cerebral arterial impedance |
| Near-infrared spectroscopy | Cerebrovascular oxygen saturation | Focal | Cerebral blood flow, cerebral autoregulation |
Adapted from Stochetti et al.[12].
DATA VISUALIZATION: INTEGRATING PHYSIOLOGICAL MONITORING TO HELP DETECT CRASHING PATIENTS
As neuromonitoring technology has advanced, the science of data integration and visualization has not kept pace. Tracking, quantifying and displaying dynamic neurophysiologic measures is crucial in the complex care of neurocritically ill patients, and needs to be put into the context of arterial blood pressure, temperature modulation, laboratory results, sedation levels and mechanical ventilator settings, as well as response to other therapeutic interventions. In today's neurocritical care environment, clinicians are required to assimilate these multiple streams of data in their heads in an attempt to understand the dynamic physiologic interactions between the injured brain and body and detect the brain in trouble. Clinicians are confronted with this high-dimensional data on a daily basis in the neurocritical care unit, with more than 200 data points to review for each patient during the morning ward round [35]. As humans, we find it significantly problematic to remember and simultaneously process data involving more than seven variables [36], and most clinicians are not able to judge the degree of relatedness between more than two variables [37,38]. Along with the introduction of multimodality neuromonitoring into the neurocritical care environment, the ability to acquire biomedical data has outstripped our ability to understand it, all of which greatly contributes to ‘information overload’ that can lead to missed opportunities to detect early physiologic signatures of neurological deterioration and preventable medical errors [27,39]. The fully automated ICU of the future where monitoring technology enables improvements in clinical care has been predicted since the 1970s, but the dream of complete physiologic monitoring captured by a single computer interface has yet to be realized [40,41]. Additional isolated monitoring devices present information on individual smaller displays that may or may not be integrated with the primary patient monitor. In this digital world, neurocritical care clinicians should be able to continually and rapidly evaluate the effect of treatments on the brain and be able to effortlessly track a patient's vital signs over minutes, hours and days. As fundamental as this may appear for comprehensive neurocritical care, visual plots of multiple waveform data streams that contains core neurophysiologic elements are not available at most institutions. However, there is evidence to suggest that even minor improvements in graphical user interfaces such as the presentation of simple line plots of trends or the addition of simple graphical indicators of trend direction could lead to clinically meaningful improvements in diagnostic accuracy and efficiency [42]. Furthermore, a recent systematic review and meta-analysis of 20 studies found that data integration and visualization technologies in critical care were associated with improvements in self-reported performance, mental and temporal demand, and effort compared with paper-based recording systems [43▪]. However, only 10% of data integration and visualization technology studies evaluated them in clinical settings. Unfortunately, there is a lack of robust evidence on how to integrate and display physiologic monitoring-derived information at the bedside to enhance time to detection of secondary brain injury and improve patient outcomes. The use of a systems design engineering approach to optimize the integration of high-resolution physiologic data will likely provide new insights into the complex neurophysiological relationships in critically ill patients with severe TBI, improve the time to detection of secondary brain injury processes and facilitate the translation of neuromonitoring-driven treatment paradigms.
CONCLUSION AND FUTURE RESEARCH DIRECTIONS
Critically ill patients with severe TBI are at risk of neuroworsening after the initial injury. Studies have shown that patients who subsequently deteriorate during their acute admission period have a much higher mortality and morbidity. The primary role of the intensivist and the critical care interdisciplinary team is the prompt recognition and treatment of any neurological deterioration. As a critical event, this may include empirical medical therapy until further imaging and assessment can be performed. The first monitor will always be the clinical examination, judged by whether it is appropriate to wake up the patient. Further studies are needed to understand whether the clinical information obtained by the NWT justify the risk of inducing a stress response and does this stress response result in subsequent worse longer-term outcomes. Future research should also seek to understand if the use of integrative neuromonitoring facilitates prompt recognition of earlier pathophysiologic deterioration and improves outcome, and whether novel data visualization techniques facilitate a better understanding of complex physiological relationships and improved care at the bedside.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
The authors have not disclosed any potential conflict of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Menon DK. Cerebral protection in severe brain injury: physiological determinants of outcome and their optimisation. Br Med Bull 1999; 55:226–258. [DOI] [PubMed] [Google Scholar]
- 2.Reilly P, et al. Patients with head injury who talk and die. Lancet 1975; 306:375–377. [DOI] [PubMed] [Google Scholar]
- 3.Carney N, et al. Guidelines for the management of severe traumatic brain injury, Fourth edition. Neurosurgery 2017; 80:6–15. [DOI] [PubMed] [Google Scholar]
- 4.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Critical pathway for the treatment of established intracranial hypertension. J Neurotrauma 2000; 17:537–538. [DOI] [PubMed] [Google Scholar]
- 5.Bullock R, et al. Guidelines for the management of severe head injury. Brain Trauma Foundation. Eur J Emerg Med 1996; 3:109–127. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Chesnut R, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 2020; 46:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a Delphi method-based consensus approach, to develop management protocols to fulfil the gap between published evidence and patient care. In this article, the group of authors developed three different treatment protocols for patients who have PbrO2 and ICP monitors. It represents a step forward to the integration of multimodal neuromonitoring into clinical practice.
- 7▪▪.Hawryluk GWJ, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 2019; 45:1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a Delphi method approach, 42 experts in TBI care developed a three-tier algorithm for treating elevated ICP. Other novel elements include outlines criteria for critical neuroworsening, guidance on the MAP challenge to measure static pressure autoregulation and two heat maps to guide ICP monitor removal and consideration of sedation holidays.
- 8.Chesnut RM, et al. Consensus-Based Management Protocol (CREVICE Protocol) for the treatment of severe traumatic brain injury based on imaging and clinical examination for use when intracranial pressure monitoring is not employed. J Neurotrauma 2020; 37:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juul N, et al. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. J Neurosurg 2000; 92:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Maas AIR, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol 2006; 5:38–45. [DOI] [PubMed] [Google Scholar]
- 11.Morris GF, et al. Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Executive Committee of the International Selfotel Trial. Neurosurgery 1998; 43:1369–1372. discussion 1372-1374. [PubMed] [Google Scholar]
- 12.Stocchetti N, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol 2017; 16:452–464. [DOI] [PubMed] [Google Scholar]
- 13.Iaccarino C, et al. Patients with brain contusions: predictors of outcome and relationship between radiological and clinical evolution. J Neurosurg 2014; 120:908–918. [DOI] [PubMed] [Google Scholar]
- 14.Chesnut RM, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 2012; 367:2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marklund N. The neurological wake-up test: a role in neurocritical care monitoring of traumatic brain injury patients? Front Neurol 2017; 8:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stocchetti N, et al. Inaccurate early assessment of neurological severity in head injury. J Neurotrauma 2004; 21:1131–1140. [DOI] [PubMed] [Google Scholar]
- 17.Murray GD, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007; 24:329–337. [DOI] [PubMed] [Google Scholar]
- 18.Marmarou A, et al. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed prehospital and on enrollment: an IMPACT analysis. J Neurotrauma 2007; 24:270–280. [DOI] [PubMed] [Google Scholar]
- 19.Healey C, et al. Improving the Glasgow Coma Scale score: motor score alone is a better predictor. J Trauma 2003; 54:671–678. discussion 678-680. [DOI] [PubMed] [Google Scholar]
- 20▪.Helbok R, et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care 2012; 16:R226. [DOI] [PMC free article] [PubMed] [Google Scholar]; A prospective observational study in a neuroscience ICU showed interruption of sedation revealed new relevant clinical information in only one trial and a large number of trials could not be performed or had to be stopped due to safety issues.
- 21.Skoglund K, et al. The neurological wake-up test increases stress hormone levels in patients with severe traumatic brain injury. Crit Care Med 2012; 40:216–222. [DOI] [PubMed] [Google Scholar]
- 22.Skoglund K, Enblad P, Marklund N. Effects of the neurological wake-up test on intracranial pressure and cerebral perfusion pressure in brain-injured patients. Neurocrit Care 2009; 11:135–142. [DOI] [PubMed] [Google Scholar]
- 23▪.Skoglund K, et al. The neurological wake-up test does not alter cerebral energy metabolism and oxygenation in patients with severe traumatic brain injury. Neurocrit Care 2014; 20:413–426. [DOI] [PubMed] [Google Scholar]; A single-centre cohort study showing the neurological wake up test induces stress response with an increase ICP and CPP levels, although it did not negatively alter focal neurochemistry or cerebral oxygenation in TBI patients.
- 24.Esnault P, et al. Early neurological wake-up test in intubated brain-injured patients: a long-term, single-centre experience. Aust Crit Care 2017; 30:273–278. [DOI] [PubMed] [Google Scholar]
- 25.Stocchetti N, et al. Clinical review: neuromonitoring: an update. Crit Care 2013; 17:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Roux P, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med 2014; 40:1189–1209. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt JM, De Georgia M. Multimodality monitoring: informatics, integration data display and analysis. Neurocrit Care 2014; 21: Suppl 2: S229–S238. [DOI] [PubMed] [Google Scholar]
- 28▪.Guiza F, et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med 2015; 41:1067–1076. [DOI] [PubMed] [Google Scholar]; This study presents the analysis of multicentre data from adults and paediatric population with TBI and ICP monitoring to assess the impact of duration and intensity of ICP insults on 6–month neurological outcomes. The authors showed how is the intracranial pressure time burden what is associated with worse outcomes. Also, that autoregulation impairment reduces the ability to tolerate intracranial pressure insults.
- 29.Jha RM, et al. Intracranial pressure trajectories: a novel approach to informing severe traumatic brain injury phenotypes. Crit Care Med 2018; 46:1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnelly J, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med 2017; 45:1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeiler FA, et al. Patient-specific ICP epidemiologic thresholds in adult traumatic brain injury: a CENTER-TBI Validation Study. J Neurosurg Anesthesiol 2021; 33:28–38. [DOI] [PubMed] [Google Scholar]
- 32. Lori Shutter, Ramon Diaz Arrastia, William Barsan, Sharon Yeatts. Brain Oxygen Optimization in Severe TBI, Phase 3 - Full Text View - ClinicalTrials.gov. ( https://clinicaltrials.gov/ct2/show/NCT03754114). [Accessed 22 February 2021] [Google Scholar]
- 33. Andrew Udy. BONANZA - ANZICS. 2021; Available from: https://www.anzics.com.au/current-active-endorsed-research/bonanza/. [Accessed 22 February 2021] [Google Scholar]
- 34.Payen JF, et al. Comparison of strategies for monitoring and treating patients at the early phase of severe traumatic brain injury: the multicentre randomised controlled OXY-TC trial study protocol. BMJ Open 2020; 10:e040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris A. Hall J, Wood L. Computer applications. Principles of critical care. New York: McGraw-Hill; 1992. 500–514. [Google Scholar]
- 36.Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev 1956; 63:81–97. [PubMed] [Google Scholar]
- 37.Imhoff M, et al. Dimension reduction for physiological variables using graphical modeling. AMIA Annu Symp Proc 2003. 313–317. [PMC free article] [PubMed] [Google Scholar]
- 38.Cambridge University Press, Jennings D, Amabile TM, Ross L. Kahneman D, Slovic P, Tversky A. Informal covariation assessment: data-based vs. theory-based judgments. Judgment under uncertainty: heuristics and biases 1982. 211–230. [Google Scholar]
- 39.Institute of Medicine Committee on Quality of Healthcare. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 40.Amaral AC, Rubenfeld GD. The future of critical care. Curr Opin Crit Care 2009; 15:308–313. [DOI] [PubMed] [Google Scholar]
- 41.Shubin H, et al. Monitoring the critically ill patient with the aid of a digital computer. Comput Biomed Res 1971; 4:460–473. [DOI] [PubMed] [Google Scholar]
- 42.Segall N, et al. Trend displays to support critical care: a systematic review. 2017 IEEE International Conference on Healthcare Informatics (ICHI), 2017: 305–313. [Google Scholar]
- 43▪.Lin YL, et al. Association of data integration technologies with intensive care clinician performance. JAMA Netw Open 2019; 2:e194392. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this systematic review and meta-analysis, the authors studied the published evidence on the association of user-centred DIVTs with intensive care clinician performance. Measures of the cognitive workload in this meta-analysis suggested that any DIVTs was an improvement over paper-based data in terms of consistency of the data and increase integration. On the contrary, the study also concluded that there is still work to do to enhance decision making based on the data shown by the DIVT.
- 44.Jones PA, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol 1994; 6:4–14. [PubMed] [Google Scholar]
- 45.Park E, Bell JD, Baker AJ. Traumatic brain injury: can the consequences be stopped? CMAJ 2008; 178:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


