Abstract
We have designed and developed an effective drug delivery system using biocompatible polymer of poly (ethylene glycol)-polyaspartic acid (mPEG-PAsp) for co-loading the chemotherapy drugs paclitaxel (PTX) and cisplatin (CP) in one nano-vehicle. This study aimed to improve the anti-cancer efficacy of combinations of chemotherapy drugs and reduce their side effects. mPEG-PAsp-(PTX/Pt) nano-micelles disperse well in aqueous solution and have a narrow size distribution (37.8 ± 3.2 nm) in dynamic light scattering (DLS). Drug release profiles found that CP released at pH 5.5 was significantly faster than that at pH 7.4. MPEG-PAsp-(PTX/Pt) nano-micelles displayed a significantly higher tumor inhibitory effect than mPEG-PAsp-PTX nano-micelles when the polymer concentrations reached 50 μg/mL. Our data indicated that polymer micelles of mPEG-PAsp loaded with the combined drug exert synergistic anti-tumor efficacy on SKOV3 ovarian cells via different action mechanisms. Results from our studies suggested that mPEG-PAsp-(PTX/Pt) nano-micelles are promising alternatives for carrying and improving the delivery of therapeutic drugs with different water solubilities.
Keywords: drug delivery, ovarian cancer, co-delivery nanosystem, paclitaxel, combination chemotherapy
Introduction
Ovarian cancer, which is one of the most common malignancies, is the leading cause of death among women worldwide as per the data provided by the Cancer Statistics Center of the American Cancer Society [1–4]. Initial debulking surgery followed by first-line platinum-based adjuvant chemotherapy is the routine management for approximately 80% of ovarian cancer patients. However, monotherapy through a single mechanism usually shows limited effect due to intrinsic or acquired drug resistance [5, 6]. Studies have shown that combination chemotherapy is effective in palliating related symptoms and extending life for patients with platinum-resistant ovarian cancer [7]. The drug combinations with different action mechanisms are widely used in clinical practice by killing cancer cells synergistically [6]. A combination of cisplatin (CP) along with paclitaxel (PTX) is currently the preferred treatment for ovarian cancer [8]. Unfortunately, existing disadvantages of delivery systems severely limit their clinical application such as off-target non-specific cytotoxicity and inevitably deleterious side effects [9]. Hence, novel drug delivery approaches are required for enhancing the anti-cancer effects and reducing the relative toxicity of combination chemotherapy in clinical management of ovarian cancer [10].
Nano-delivery systems have shown great potential over the past decade for effective drug delivery in a regulated manner to cancer cells ascribing to their modified surface, high stability and immense capacity [4]. Nano-particles (NPs) including lipid NPs, polymeric NPs, liposomes, dendrimers, oligomers and nanotubes have been frequently used for the delivery of chemotherapeutic drugs for treating cancer [11]. Among these NPs, polymeric micelles have emerged as one of the most promising drug delivery platforms with excellent biocompatibility [12]. Polymer micelles, composed of a hydrophobic inner core and a hydrophilic shell are particularly appealing because of several advantages. They improve the solubility of the hydrophobic drug in the body fluid, prolong the cycle time of the drugs and passively target tumor site by an enhanced permeability and retention (EPR) effect [4, 13]. They form into nano-micelles in aqueous solution in a self-assembled way [14]. In addition, they have the ability to control drug pharmacokinetics, improve the solubility of drugs and realize specific targeting by chemical modification while reducing the toxic side effects of normal organs. Therefore, the polymeric micelles have attracted considerable attention.
CP and PTX are widely used in combination chemotherapy for the treatment of gynecologic tumors such as cervical cancer and ovarian cancer [15, 16]. Compared to the single drug-loaded micelles, the superior anti-tumor activity of co-loaded PTX and CP nano-micelles was demonstrated in cisplatin-resistant human ovarian carcinoma A2780/CisR xenograft tumor [17]. However, the encapsulation and release of CP and PTX in a single polymer carrier remain challenging in biomaterials science and pharmaceutics. This is because CP has no functional groups that can be chemically conjugated with polymer [18]. Our objective is to develop a novel nano-micelles carrier that can overcome the hurdles mentioned above. Polyethylene glycol (PEG), which has good biocompatibility and is approved by FDA, has been widely used to as the hydrophilic segment of the polymer. A polymeric chain containing PEG prevents phagocytosis of the reticuloendothelial cell system and prolongs the blood circulation time that is beneficial for the treatment of cancer [19, 20]. Polyamino acids have received great attention in the field of biomedicine and materials science [21]. Among the polyamino acids, polyaspartic acid (PAsp) is characterized by biodegradability and low toxicity in the human body. Moreover, the small molecule drug can be attached to the side chain of polyamino acids by chemical bonding, which is particularly suitable for the drug carrier candidate.
In this paper, we chose mPEG-PAsp polymer as a delivery carrier of chemotherapy drugs, due to its biodegradable feature and good biocompatibility. PTX was covalently conjugated to mPEG-PAsp to form amphiphilic polymer (mPEG-PAsp-g-PTX). Hydrophobic PTX was encapsulated into the core of mPEG-PAsp-PTX nano-micelles by self-assembly in an aqueous solution. We then employed the carboxyl groups of mPEG-PAsp-PTX nano-micelles to chelate CP and achieve the goal of loading both hydrophilic and hydrophobic drugs in one nano-vehicle. The chemical structure of nano-particles was characterized by hydrogen nuclear magnetic spectrum (1HNMR) and dynamic light scattering (DLS). The cytotoxic efficacy of mPEG-PAsp-(PTX/Pt) nano-micelles was evaluated using SKOV3 cells in human ovarian cancer cells. We sought to take advantage of synergistic effects of chemotherapeutic combination to improve the anti-tumor efficacy of PTX and CP while minimizing systemic and cellular toxicity.
Materials and methods
Reagents
Polyethylene glycol monomethyl ether, p-toluenesulfonyl chloride and triphosgene were purchased from Sigma-Aldrich LLC. L-aspartic acid-β-benzyl ester, cisplatin and paclitaxel were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Dicyclohexylcarbodiimide (DCC) and dimethylaminopyridine (DMAP) were purchased from Sigma-Aldrich LCC.
Terminal amination modification of polyethylene glycol monomethylether (mPEG-NH2)
Polyethylene glycol monomethyl ether (10 g, Mw 5 kDa) were dissolved in 300 ml chloroform and 1 g triethylamine was added to it. The mixture was placed in an ice-bath. In addition, 10 ml of 1.9 g (10 mmol) p-toluenesulfonyl chloride was slowly dissolved in chloroform under an inert gas atmosphere. The mixing solution was stirred slowly and reacted for 5 d at room temperature. When the reaction solution was concentrated to 100 ml, diluted hydrochloric acid, saturated NaHCO3 solution and saturated NaCl solution were successively washed with water. Organic phases were obtained by separating and drying with anhydrous MgSO4. The organic phase was filtered and concentrated to 50 ml after precipitating in 500 ml diethyl ether. Sediments were collected and dried in a vacuum drying oven to obtain polyethylene glycol monomethyl ether tosylate (mPEG-Ts).
mPEG-Ts (10 g) were placed into a 500 ml round-bottom flask and inert gas was supplied to the system. In an ice-bath, 100 ml stronger ammonia water was added slowly through a dropping funnel and the mixture was stirred. The ice-bath was eliminated after finishing dropping and the mixture was stirred for 5 days at room temperature. At the end of the reaction, products were extracted three times with 100 ml chloroform and the organic phases were collected. Organic phases were successively washed with diluted hydrochloric acid, saturated NaHCO3 solution and saturated NaCl solution. Anhydrous MgSO4 was then added for drying and filtering. The filtrate was concentrated to 50 ml and precipitated in 500 ml cold ether. Sediments were collected and dried in a vacuum drying oven to get polyethylene glycol monomethyl ether-amino (mPEG-NH2).
Synthesis of L-aspartic acid-β-benzyl ester N-carboxylic acid anhydride (BLA-NCA)
L-aspartic acid-β-benzyl ester (5.00 g, 22.4 mmol) and triphosgene were put in a round-bottomed flask. 80 ml tetrahydrofuran (THF) was added to form a suspension. The mixture was heated to reflux and reacted for 4 h. Finally, the solution becomes clear. The solvent was eliminated by a rotary evaporator. THF and hexane were added for re-crystallization twice. BLA-NCA crystals were dried under vacuum conditions for 5 h so that they could be used later.
Synthesis of polyethylene glycol-poly (β-benzyl-L-aspartate) (mPEG-PBLA)
mPEG-NH2 (1 g) was put in a 100 ml reaction flask and dried under vacuum for 5 h. This was followed by the addition of 20 ml dry dichloromethane was added in to completely dissolve. Then, BLA-NCA was dissolved in 2 ml anhydrous dimethylformamide (DMF) and quickly added under inert gas shielding. The mixing solution was stirred and reacted for 3 days at room temperature. Later, the mixing solution was precipitated in 500 ml cold diethyl ether. Sediments were collected and dried in a vacuum drying oven to obtain mPEG-PBLA.
Synthesis of polyethylene glycol-polyaspartic acid (mPEG-PAsp)
mPEG-PBLA (440 mg) was placed in a round-bottom flask and 50 ml of methyl alcohol was dissolved by stirring. In addition, 440 mg NaOH was dissolved in 5 ml ultrapure water and dropped in slowly. The mixing solution was heated to 50°C and reacted for 48 h. One milliliter concentrated hydrochloric acid was then added in and stirred for 5 min. The solvent was eliminated through rotary evaporation. Subsequently, 10 ml of absolute ethyl alcohol was added to dissolve and filter the raw products. The filtrate was treated by rotary evaporation and the solvent was eliminated. Besides, 10 ml water was added to a dialysis bag with 1 kDa molecular cut off for 24 h dialysis desalination. Finally, the mixture was dried by freezing to obtain polyethylene glycol-polyaspartic acid (mPEG-PAsp) of the amphiphilic block copolymer.
Synthesis of polyethylene glycol-polyaspartic acid grafted paclitaxel (mPEG-PAsp-g-PTX)
mPEG-PAsp was put in a 100 ml reaction flask, dried under vacuum conditions and supplied with inert gas. Anhydrous DMF solution (5 ml) was added into the system. This was followed by the addition of 62 mg dicyclohexylcarbodiimide (DCC) by stirring for 30 min under room temperature. Dimethylaminopyridine (DMAP, 8 mg) and PTX (50 mg) were collected and then successively added into the reaction system. The mixing solution was heated to 60°C and reacted for 2 days. The solvent was eliminated through rotary evaporation and the mixing products were dissolved by chloroform and filtered. The filtrate was processed by a rotary evaporator and the solvent was eliminated to obtain mPEG-PAsp-g-PTX.
Preparation of mPEG-PAsp-PTX and mPEG-PAsp-(PTX/Pt) nano-micelles
mPEG-PAsp-g-PTX (3.7 mg) was dissolved into 400 ml THF. The ultrasonic power under room temperature was 130 W and the vibration amplitude was 65%. The solution was dropped into 5 ml ultrapure water slowly. The mixture solution was put in a dialysis bag (molecular weight cutoff, 1 kDa) after shaking well and dialyzed for 2 days in deionized water. Finally, the solution was concentrated to 3 ml by a rotary evaporator to obtain mPEG-PAsp-PTX nano-micelle solution.
CP (1 mg) was dissolved into 100 μL dimethyl sulfoxide (DMSO) and dropped into 1.5 ml mPEG-PAsp-PTX nano-micelle. The nano-micelle solution was placed at room temperature for 24 h and then was put in a dialysis bag (1 kDa molecular cut off) to dialysis for 2 days in deionized water. Finally, the solution was concentrated to 3 ml through a rotary evaporator to obtain mPEG-PAsp-(PTX/Pt) nano-micelles.
Cell lines and cell culture
Human ovarian cancer line SKOV3 was kindly provided by Affiliated Hospital of North Sichuan Medical College.
The cells were cultured in Dulbecco's modified eagle medium (DMEM) complete medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2.
Characterizations
The chemical structure of the synthesized copolymers was confirmed by the hydrogen nuclear magnetic spectrum (1HNMR), acquired by Bruker 400 M nuclear magnetic resonance spectrometer. Particle size and size distributions of mPEG-PAsp-PTX nano-micelles in aqueous solution were characterized through dynamic light scattering (DLS), measurement at 25°C using a Malvern Zetasizer Nano ZS90. GPC (Gel Permeation Chromatography, GPC) was performed to measure the molecular weight distribution of mPEG-PBLA, mPEG-PAsp and PEG-PAsp-g-PTX.
Cytotoxicity assay
The CCK-8 assay was performed to detect in vitro cell viability after treatment with CP and PTX. Briefly, SKOV3 cells were seeded into 96-well culture plate at the density of 1 × 105 cells per well in 200 μL DMEM medium with 5% fetal bovine serum (FBS) and maintained overnight in a humidified atmosphere of 5% CO2 at 37°C to allow cells to attach. The cells were then treated with various concentrations (12.5, 25, 37.5, 50 μg/mL) of mPEG-PAsp-PTX and mPEG-PAsp-PTX/Pt nano-micelles in the incubator. The untreated cells served as the blank control with the same density of cells. After 24 h incubation, 10 μl of CCK-8 solution was added to each well and the cell culture plate was reincubated at 37°C for another 30 min. The absorbance values were measured at 450 nm and cell viability was calculated. Cell viability of the blank control group was set at 100% and the relative cell viability was equal to optical density (OD) of the experimental group/OD of the control. This experiment was repeated three times by using at least five replicates of each concentration.
Drug release profiles
Fifteen milligrams mPEG-PAsp-(PTX/Pt) nano-micelle solution was freeze-dried and dissolved in 10 ml of phosphate buffer solution (PBS, 0.01 M). The solution was then placed into a dialysis bag (molecular weight cutoff of 3.5 kDa) and immersed in 45 ml of PBS, magnetic stirring at room temperature. One milliliter sample was withdrawn from the incubation medium at specified time intervals, and equal fresh PBS was immediately added into the incubation medium. Inductively coupled plasma mass spectrometry (ICP-MS) and UV-vis were used to measure the amount of PTX and CP released from the micelles, respectively. Drug release profiles were constructed by plotting the amount of drugs released over time.
Statistical analysis
All values were expressed as the mean ± standard error of at least three samples. Statistical analysis was performed using Student’s two-tailed test and P values less than 0.05 are considered statistically significant.
Results
Characterization of the block copolymer of mPEG-PAsp
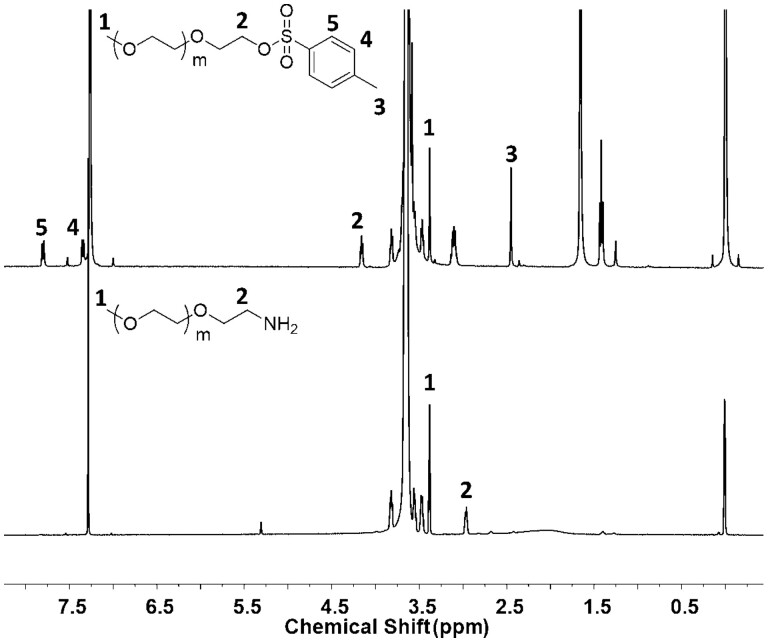
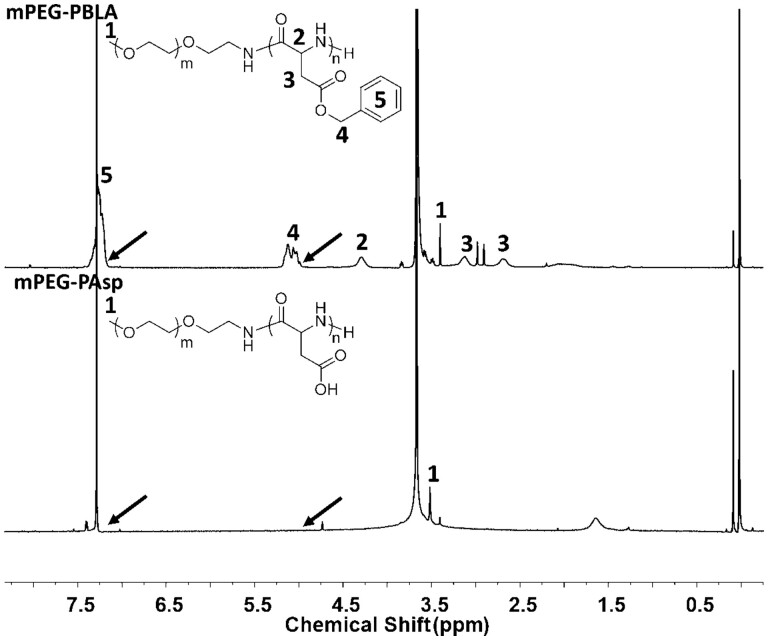
PEG-PBLA was synthesized via the ring-opening polymerization of β-benzyl-L-aspartate N-carboxy-anhydride (BLA-NCA) and then mPEG-PAsp was obtained by hydrolyzing PEG-PBLA in methyl alcohol. Commercial PEG-OH was used as the raw material in the experiment. Terminal hydroxy was modified into terminal amino PEG (mPEG-NH2) through a two-step reaction (Fig. 1).1HNMR of mPEG-Ts and mPEG-NH2 has been shown in Fig. 2. Characteristic peaks (2, 3, 4 and 5 peaks) of p-toluene sulfonate appeared in 1HNMR of mPEG-Ts and disappeared (3, 4 and 5 peaks) and shift (2 peaks) in 1HNMR of mPEG-NH2. These results confirmed the amination of PEG. Next, PEG-PBLA of the amphiphilic block copolymer was prepared by mPEG-NH2 triggering NCA ring-opening polymerization. The benzyl ester was then stripped off in alkaline conditions to obtain mPEG-PAsp. 1HNMR of the amphiphilic block copolymer of mPEG-PBLA and mPEG-PAsp has been shown in Fig. 3. The peaks of benzyl ester (4 and 5 peaks) disappeared after alkaline hydrolysis. Since the obtained PAsp does not have good solubility in weak-polarity solvents like chloroform, the 1HNMR mPEG-PAsp in CDCl3 is poor quality, and the peaks of polyaspartic acid did not appear. The average length of polyaspartic acid benzyl ester terminal can be calculated by the characteristic peak area integral of benzyl ester in 1HNMR of mPEG-PBLA, which was about 25 units and the average molecular weight of block polymer mPEG-PBLA was about 10.1 kDa. After alkaline hydrolysis, the average molecular weight of block copolymer mPEG-PAsp was about 7.8 KDa.
Figure 1.
The schematic formation of block copolymer mPEG-PAsp
Figure 2.
1H NMR Spectrum of mPEG-Ts (up) and mPEG-NH2 (down) in CDCl3
Figure 3.
1H NMR Spectrum of mPEG-PBLA (up) and mPEG-PAsp(down) in CDCl3
Characterization of mPEG-PAsp-g-PTX
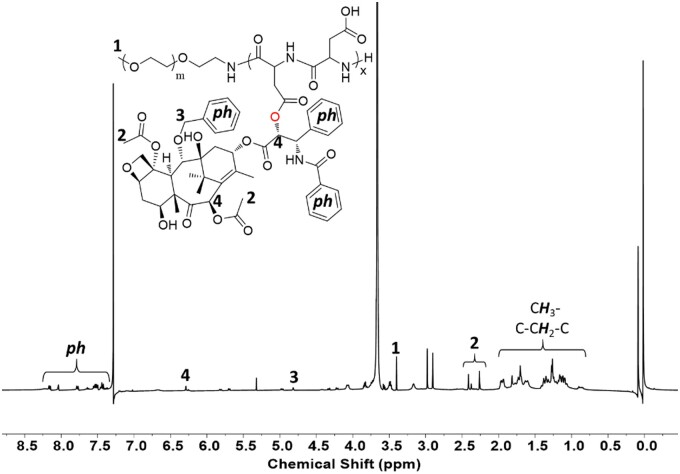
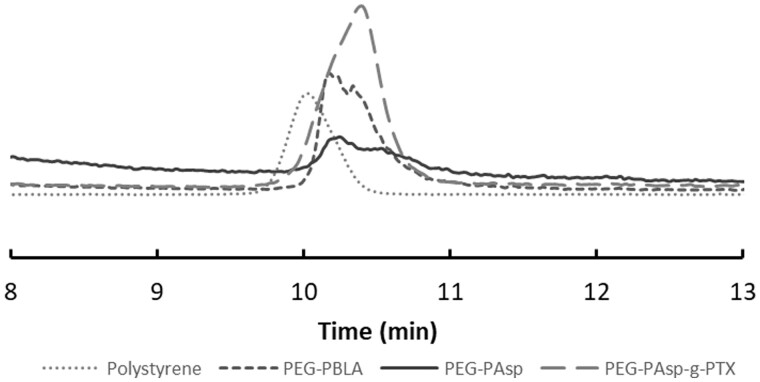
The block copolymer of mPEG-PAsp was grafted onto PTX via an esterification reaction. The obtained amphiphilic PEG-PAsp-g-PTX had good solubility in chloroform due to the weak-polarity of PTX. 1HNMR of mPEG-PAsp-g-PTX is shown in Fig. 4, where characteristic peaks of polyethylene glycol molecules and PTX molecules are observed. It is calculated based on the characteristic peak integral area with every polymer chain contains about 2.6 PTX ester, and the average molecular weight of graft block copolymer was about 10 kDa. GPC was performed to measure the molecular weight distribution of mPEG-PBLA, mPEG-PAsp and PEG-PAsp-g-PTX, the tetrahydrofuran as a mobile phase and polystyrene with molecular weight of 20 kDa as a standard sample. As shown in Fig. 5, three copolymers have molecular weights of less than 20 kDa, and the molecular weight distribution widens gradually in the sequence of mPEG-PBLA, mPEG-PAsp and PEG-PAsp-g-PTX.
Figure 4.
1H NMR Spectrum of graft block copolymer mPEG-PAsp-g-PTX in CDCl3
Figure 5.
GPC of mPEG-PBLA, mPEG-PAsp, mPEG-PAsp-g-PTX and polystyrene (standard sample with molecular weight of 20 kDa)
Characterization of mPEG-PAsp-PTX and mPEG-PAsp-(PTX/Pt) nano-micelles
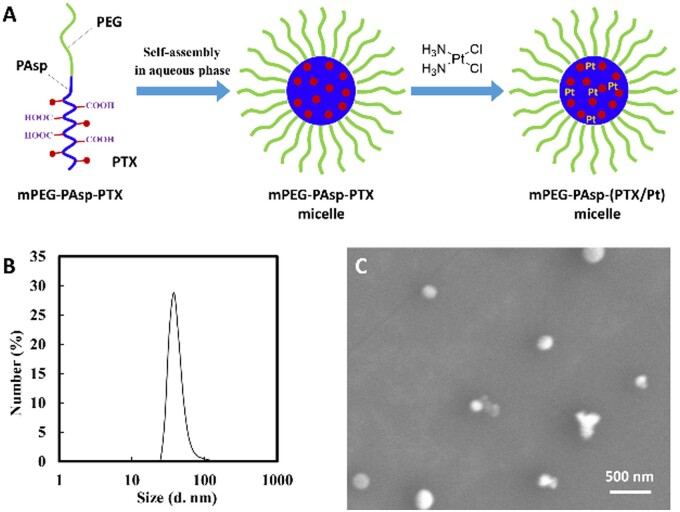
mPEG-PAsp-g-PTX is an amphiphilic polymer that can self-assemble into nano-micelles in an aqueous solution. The carboxyl-terminal of mPEG-PAsp-g-PTX further chelates CP to get mPEG-PAsp-(PTX/Pt) nano-micelles for achieving the goal of encapsulating two different water-soluble drugs into a single nanocarrier (Fig. 6A). MPEG-PAsp-(PTX/Pt) nano-micelles are good to disperse in aqueous solution and have a narrow size distribution (37.8 ± 3.2 nm) in DLS (Fig. 6B). Under scanning electronic microscopy (SEM), mPEG-PAsp-(PTX/Pt) nano-micelles appeared spherical with a diameter of around 100 nm (Fig. 6C).
Figure 6.
(A) Schematic illustration of mPEG-PAsp-PTX micelle formation and chelating cisplatin inside the micelle core by carboxyl; (B) DLS of mPEG-PAsp-(PTX/Pt) nano-micelles; (C) SEM of mPEG-PAsp-(PTX/Pt) nano-micelles
Drug release profiles
To evaluate the release behavior of the composite micelles, we carried out the PTX and CP release at room temperature in PBS solutions of pH 5.5 and pH 7.4. For each drug, the relative accumulative release weight percentage of the total drug payload in the sample was measured as a function of release time. As shown in Fig. 7, the platinum released at pH 5.5 was significantly faster than that at pH 7.4. To release 13% of the total CP took about 3 h at pH 5.5, while it took about 72 h at pH 7.4. The release of platinum from the nano-particles is because of the ligand exchange reaction of cisplatin from the carboxylates to the chloride ions in physiological saline, which was accelerated at lower pH. PTX showed a slower release rate from the composite micelles than platinum under the same condition, and displayed little difference at pH 5.5 and pH 7.4, implying that PTX can be released due to the slow hydrolytic reaction of the ester linkage in PBS.
Figure 7.
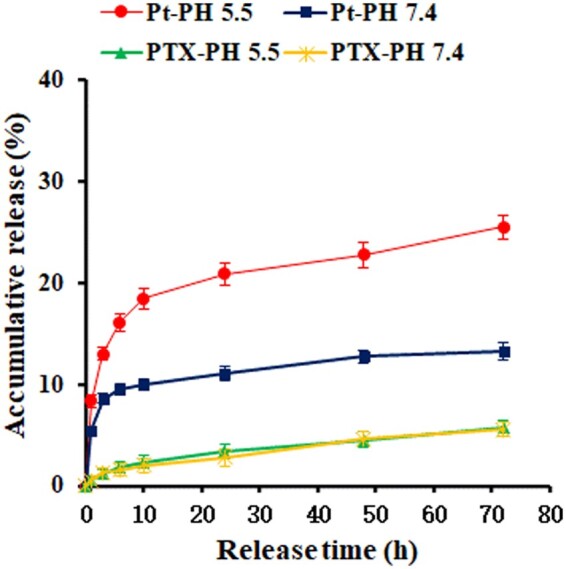
Drug release profiles of PTX and platinum from the nano-micelles in PBS solutions at pH 5.5 and pH 7.4
Cytotoxicity assay
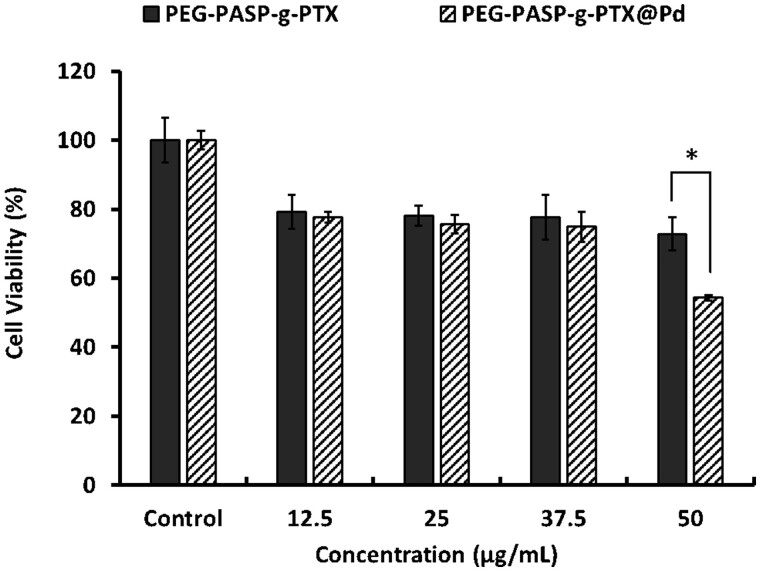
Cell activity of SKOV3 cells was evaluated by CCK-8 after incubation with different concentrations of nano-micelles for 24 h. Both mPEG-PAsp-(PTX/Pt) and mPEG-PAsp-PTX nano-micelles have a killing effect on cancer cells when polymer concentration ranged from 12.5 μg/mL to 50 μg/mL (Fig. 8). The anti-tumor activity of mPEG-PAsp-(PTX/Pt) nano-micelles was comparable to that of mPEG-PAsp-PTX nano-micelles when polymer concentration was below 37.5 μg/mL, which indicated that co-loaded combined drugs can be effectively released from the nano-micelles without losing their anti-cancer activity. Cell viability decreased from 72.8 ± 4.8% to 54.4 ± 0.8% when polymer concentration of mPEG-PAsp-(PTX/Pt) increased from 37.5 μg/mL to 50 μg/mL. Cell viability, on the other hand, did not exhibit significant differences between the different concentrations of mPEG-PAsp-PTX nano-micelles. Our data indicated that mPEG-PAsp-(PTX/Pt) nano-micelles displayed a significantly higher tumor inhibitory effect than that of mPEG-PAsp-g-PTX nano-micelles when polymer concentration reached 50 μg/mL (P < 0.05). The results indicated that higher cytotoxicity may be attributed to the synergistic effects derived from the combination of CP and PTX. Moreover, it can be observed that the anti-tumor activity of mPEG-PAsp-(PTX/Pt) nano-micelles increased significantly along with the dose increase. On the contrary, mPEG-PAsp-PTX nano-micelles did not exhibit a dose-dependent relationship, which may be attributed to the slow sustained-release of PTX from the nano-micelles as described in the drug release profiles.
Figure 8.
Cell viability of SKOV3 cells after incubation with different concentrations of nano-micelles for 24 h
Discussion
Currently, CP-based chemotherapy in combination with PTX is the standard care for patients with ovarian cancer. However, the traditional combination treatments, using CP and PTX showed limited treatment efficacy due to their distinct pharmacokinetics [22, 23]. PTX is poorly soluble in water and must be administered with a combination of dehydrated alcohol and Cremophor EL, which can lead to serious side effects including neurotoxicity, nephrotoxicity and hypersensitivity reactions [24]. Therefore, there is an urgent need for the development of an alternative mode of administration.
Nano-delivery systems have attracted increasing attention in recent years, especially for cancer therapies because they can improve the solubility of hydrophobic drugs and take advantage of the EPR effect for passive tumor targeting which helps to decrease the side effects of the drugs [25]. However, it is still very challenging to encapsulate two drugs with distinct physicochemical properties into a single nanocarrier.
In this study, we choose mPEG-PAsp that is a good biocompatible as the delivery carrier to encapsulate and deliver PTX and CP in a single nanoformulation. Terminal hydroxy was modified into terminal amino PEG (mPEG-NH2) via a two-step reaction. It could be seen that characteristic peaks of p-toluene sulfonate appeared in 1HNMR of mPEG-Ts and disappeared in 1HNMR of mPEG-NH2. These results indicated that the hydroxyl group successfully converted to an amino group, which is conducive to ring-opening polymerization of PEG-PBLA. The peak of benzyl ester disappeared after alkaline hydrolysis. This indicated the protection of carboxyl dibenzyl ester is successful. PTX covalently conjugated on mPEG-PAsp formed mPEG-PAsp-PTX micelles in an aqueous solution by self-assembly characteristics of the polymer. Every polymer chain contained 2.6 PTX molecules. The results are slightly lower than the value reported in the previous study. The main reason is the large molecular weight of PTX with a large steric hindrance [4, 26]. Free carboxyl groups of the mPEG-PAsp-PTX polymer micelles then chelated CP to achieve a co-delivery system of PTX and CP. GPC was used to qualitatively analyze the molecular weight and molecular weight distribution of mPEG-PBLA, mPEG-PAsp and PEG-PAsp-g-PTX. Three copolymers have molecular weights of less than 20 kDa, and the molecular weight distribution widens gradually with PTX modification. Unfortunately, exact molecular weights of copolymer were not obtained due to a lack of suitable standards samples.
The mPEG-PAsp-(PTX/Pt) nano-micelles have a size of 37.8 ± 3.2 nm in DLS, and show spherical under SEM. The fate of nano-materials after being introduced into the body has been proven to be size-dependent. Particles whose size was higher than 200 nm are often selectively accumulated in the liver and the spleen [20]. There is an easy uptake of the smaller particle size by tumor cells with abnormal blood vessels via endocytosis [27, 28]. The size of mPEG-PAsp-(PTX/Pt) nano-micelles falls into the optimal size range. Additionally, non-specific interaction between serum proteins and chemotherapy cargos was prevented by PEG providing the outer corona. The mPEG-PAsp-(PTX/Pt) nano-micelles are therefore expected to have a prolonged circulation time by EPR effect thus avoiding phagocytic and renal clearance. This helps to ensure the anti-tumor effect of chemotherapy regimens [29].
It was found that 13% release amount of the total takes about 3 h at pH 5.5 while it takes about 72 h at pH 7.4, as shown in the drug release profiles, indicating that CP is more susceptible to release at pH 5.5. The release characteristic of pH dependence is beneficial for the enhanced anti-cancer effect and the reducing toxic side effect. CP released at pH 5.5 is significantly faster than that at pH 7.4. While PTX showed a slow release rate from the composite micelles than CP under the same condition and displayed little difference at pH 5.5 and pH 7.4. The phenomenon may be because the hydrolytic reaction mPEG-PAsp-g-PTX is very slow in PBS, separating slowly from the nano-micelles. The reason was reported in previous reports [4, 30]. Once the nano-micelles enter the blood flow, nano-particles are effectively protected by biocompatible mPEG-PAsp from the attack of the reticuloendothelial system (RES). Subsequently, they may be internalized by the cancerous cells via endocytosis [30]. CP combined with sustained-release PTX exert their anti-tumor functions through their respective pathways, resulting in a synergistic anti-tumor effect.
In vitro cytotoxicity evaluation data revealed that mPEG-PAsp-(PTX/Pt) nano-micelles well delivered anti-tumor drugs to SKOV3 cells. Interestingly, when the polymer concentration reached 50 μg/mL, mPEG-PAsp-(PTX/Pt) nano-micelles exhibited a significantly higher tumor inhibitory effect than mPEG-PAsp-PTX nano-micelles after they were incubated with SKOV3 cells for 24 h. Once the mPEG-PAsp-(PTX/Pt) nano-micelles were taken up to the SKOV3 cells via endocytosis, they released PTX by the simple hydrolysis of the ester linkage formed by PTX with mPEG-PAsp [26]. The carboxyl group between the mPEG-PAsp polymer and CP is pH sensitive. The acidic environment inside tumor cells subsequently promoted the carboxyl group to break, which is beneficial for promoting the release of drugs and improving their efficacy [31]. The data confirmed mPEG-PAsp-(PTX/Pt) nano-micelles promoted the release of drugs and effectively suppressed cell viability by taking advantage of the synergistic effect of the two drugs. Previous studies have reported that CP not only inhibits DNA replication and transcription but also bonds and damages mitochondrial DNA, which in turn can lead to cell apoptosis [32–34]. PTX which is a microtubule-stabilizing agent not only inhibits the G2/M phase of the cell cycle by disrupting the normal tubule disassembles but also increases the expression of Bax and Bak which are two proapoptotic proteins. PTX enhances the apoptosis of CP-resistant tumor cells [35, 36]. Our results are comparable to the above papers. Therefore, PTX–CP combination therapy using a biocompatible mPEG-PAsp polymer as the delivery carrier shows great potential for the treatment of ovarian cancer in the future.
Conclusions
We have constructed a combination delivery nanosystem for hydrophilic cisplatin and hydrophobic paclitaxel using a biodegradable PEG-PAsp polymer as the carrier in one nano-vehicle. The novel delivery system was prepared to ensure that the size of particles was within the optimal range for effectively accumulating at the tumor site. The drug release profiles indicated that CP is more susceptible to release at pH 5.5 than at pH 7.4. In vitro cytotoxicity experiments confirmed that the dual drugs of mPEG-PAsp-(PTX/Pt) nano-micelles yield a synergistic anti-cancer effect against SKOV3 cells when the polymer concentration reached 50 μg/mL. These findings revealed that mPEG-PAsp-(PTX/Pt) nano-micelles have great potential as a co-delivery system with the increase of anti-tumor effects, the decrease of drug doses and the side effects. Therefore, this mPEG-PAsp polymer-based nanosystem not only provides an effective delivery strategy originating from the simultaneous combination of PTX and CP but also achieves the goal of delivering therapeutic drugs with different water solubilities.
It should be mentioned that only the in vitro experiments are focused on in this work. More adjustment and optimization for the drug loading efficacy by a range of feeding rates are needed to perform. The biosafety and anti-tumor activity of nano-micelles in vivo will be performed in our further research.
Funding
The work was supported by National Natural Science Foundation of China (81601490), the Key Project of Sichuan Education Department (16ZA0399), the Sichuan Science and Technology Program (2017JY0107, 2016JY0172) and the Leshan Science and Technology Bureau of China (17GZD041).
Conflict of interest statement. The authors declare that no conflict of interest.
References
- 1. Mignani S, Bryszewska M, Klajnert-Maculewicz B. et al. Advances in combination therapies based on nanoparticles for efficacious cancer treatment: an analytical report. Biomacromolecules 2015;16:1–27. [DOI] [PubMed] [Google Scholar]
- 2. Ai Z, Lu Y, Qiu S. et al. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett 2016;373:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4. Xiao H, Song H, Yang Q. et al. A prodrug strategy to deliver cisplatin(IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials 2012;33:6507–19. [DOI] [PubMed] [Google Scholar]
- 5. Gillet J-P, Gottesman MM.. Gottesman.Mechanisms of multidrug resistance in cancer. Methods Mol Biol 2010;596:47–76. [DOI] [PubMed] [Google Scholar]
- 6. Gatti L, Zuco V, Zaffaroni N. et al. Drug combinations with proteasome inhibitors in antitumor therapy. Curr Pharm Des 2013;19:4094–114. [DOI] [PubMed] [Google Scholar]
- 7. Woodcock J, Griffin JP, Behrman RE.. Development of novel combination therapies. N Engl J Med 2011;364:985–7. [DOI] [PubMed] [Google Scholar]
- 8. Zhang X-Z, Li X-J, Zhang H-Y. Valproic acid as a promising agent to combat Alzheimer's disease. Brain Res Bull 2010;81:3–6. [DOI] [PubMed] [Google Scholar]
- 9. O'Dwyer PW. Hypersensitivity reactions induced by etoposide. Cancer Treat Rep 1984;68:959–61. [PubMed] [Google Scholar]
- 10. Shao M, Zhu W, Lv X. et al. Encapsulation of chloroquine and doxorubicin by MPEG-PLA to enhance anticancer effects by lysosomes inhibition in ovarian cancer. Int J Nanomedicine 2018;13:8231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Zhang Zhu PT.. Ovarian carcinoma biological nanotherapy: comparison of the advantages and drawbacks of lipid, polymeric, and hybrid nanoparticles for cisplatin delivery. Biomed Pharmacother 2019;109:475–83. [DOI] [PubMed] [Google Scholar]
- 12. Mandal A, Bisht R, Rupenthal ID. et al. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release 2017;248:96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuzar Z, Kratochvil P.. Block and graft copolymer micelles in solution. Adv Colloid Interface Sci 1976;6:201–32. [Google Scholar]
- 14. Wang L, Shi C, Wright FA. et al. Multifunctional telodendrimer nanocarriers restore synergy of bortezomib and doxorubicin in ovarian cancer treatment. Cancer Res 2017;77:3293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rose PG, Blessing JA, Gershenson DM. et al. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. Am J Clin Oncol 1986;9:325. [DOI] [PubMed] [Google Scholar]
- 16. Moore KN, Herzog TJ, Lewin S. et al. A comparison of cisplatin/paclitaxel and carboplatin/paclitaxel in stage IVB, recurrent or persistent cervical cancer. Gynecol Oncol 2007;105:299–303. [DOI] [PubMed] [Google Scholar]
- 17. Wan X, Beaudoin JJ, Vinod N. et al. Co-delivery of paclitaxel and cisplatin in poly(2-oxazoline) polymeric micelles: implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 2019;192:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen W, Chen X, Luan J. et al. Sustained codelivery of cisplatin and paclitaxel via an injectable prodrug hydrogel for ovarian cancer treatment. ACS Appl Mater Interfaces 2017;9:40031–46. [DOI] [PubMed] [Google Scholar]
- 19. Domb AJ, Nudelman R.. Biodegradable polymers derived from natural fatty acids. J Polymer Sci A 2010;33:717–25. [Google Scholar]
- 20. Vu MT, Bach LG, Nguyen DC. et al. Modified carboxyl-terminated PAMAM dendrimers as great cytocompatible nano-based drug delivery system. Int J Mol Sci 2019;20:2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kricheldorf HR. Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides. Angew Chem Int Ed Engl 2006;45:5752–84. [DOI] [PubMed] [Google Scholar]
- 22. Zhang X, Liu Y, Kim YJ. et al. Co-delivery of carboplatin and paclitaxel via cross-linked multilamellar liposomes for ovarian cancer treatment. RSC Adv 2017;7:19685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohta T, Isobe M, Takahashi T. et al. The Akt and ERK activation by platinum-based chemotherapy in ovarian cancer is associated with favorable patient outcome. Anticancer Res 2009;29:4639–47. [PubMed] [Google Scholar]
- 24. He C, Tang Z, Tian H. et al. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv Drug Deliv Rev 2016;98:64–76. [DOI] [PubMed] [Google Scholar]
- 25. Ma P, Mumper RJ.. Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol 2013;4:1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X, Li Y, Chen X. et al. Synthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugate. Biomaterials 2005;26:2121–8. [DOI] [PubMed] [Google Scholar]
- 27. Luo J, Xiao K, Li Y. et al. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug Chem 2010;21:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee H, Fonge H, Hoang B. et al. The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Mol Pharmaceutics 2010;7:1195–208. [DOI] [PubMed] [Google Scholar]
- 29. Kataoka K, Harada A, Nagasaki Y.. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 2001;47:113–31. [DOI] [PubMed] [Google Scholar]
- 30. Li J, Li Z, Li M. et al. Synergistic effect and drug-resistance relief of paclitaxel and cisplatin caused by co-delivery using polymeric micelles. J Appl Polym Sci 2015;132:843–848. [Google Scholar]
- 31. Xu XY, Zhang XF, Wang XH. et al. Comparative study of paclitaxel physically encapsulated in and chemically conjugated with PEG-PLA. Polym Adv Technol 2009;20:843–8. [Google Scholar]
- 32. Davoodi P, Ng WC, Srinivasan MP. et al. Codelivery of anti-cancer agents via double-walled polymeric microparticles/injectable hydrogel: a promising approach for treatment of triple negative breast cancer. Biotechnol Bioeng 2017;114:2931–46. [DOI] [PubMed] [Google Scholar]
- 33. Mallick A, More P, Syed MM. et al. Nanoparticle-mediated mitochondrial damage induces apoptosis in cancer. ACS Appl Mater Interfaces 2016;8:13218–31. [DOI] [PubMed] [Google Scholar]
- 34. Romanini A, Tanganelli L, Carnino F. et al. First-line chemotherapy with epidoxorubicin, paclitaxel, and carboplatin for the treatment of advanced epithelial ovarian cancer patients. Gynecol Oncol 2003;89:354–9. [DOI] [PubMed] [Google Scholar]
- 35. Singla AK, Garg A, Aggarwal D.. Paclitaxel and its formulations. Int J Pharm 2002;235:179–92. [DOI] [PubMed] [Google Scholar]
- 36. Vanhoefer U, Harstrick A, Wilke H. et al. Schedule-dependent antagonism of paclitaxel and cisplatin in human gastric and ovarian carcinoma cell lines in vitro. Eur J Cancer 1995;31A:92–7. [DOI] [PubMed] [Google Scholar]