Abstract
Background:
Despite routine use of echocardiographic parameters to evaluate the severity of cardiac amyloidosis (CA), this methodology has not been well validated. We developed a histopathologic schema for quantifying CA burden and evaluated its relationship with clinical outcomes. Additionally, echocardiographic parameters were tested as potential noninvasive indices of CA burden.
Methods:
We retrospectively studied 59 patients with CA (17 light chain, 42 transthyretin) who underwent endomyocardial biopsies. Light microscopy with staining was used to categorize CA burden as mild-to-moderate (<50%) or high (≥50%). Kaplan-Meier survival analysis was performed for the two groups. In 34 patients with good-quality echocardiograms, we measured left ventricular volumes, ejection fraction (EF), interventricular septal thickness (IVSt), posterior wall thickness (PWt), LV mass, lateral e′-velocity, and global longitudinal strain (GLS). These parameters were compared between the two groups.
Results:
Thirty-five patients had mild-to-moderate and 24 severe amyloid burden. Kaplan-Meier curves demonstrated a trend toward worse mortality with high CA burden, which was more common and associated with higher mortality specifically in transthyretin-type patients. Echocardiography-derived IVSt, PWt, and LV mass were directly related to CA burden, while LV EF, e’-velocity, and GLS magnitude were inversely related to CA burden.
Conclusions:
Our findings provided a signal that CA burden is a clinically important entity with potentially valuable prognostic information. Echocardiographic parameters of LV anatomy and function correlate with histopathologic burden of CA, which is inversely related to survival. Further studies are needed to determine whether these parameters could be used as imaging biomarkers of treatment-related changes in CA burden.
Keywords: cardiac amyloidosis, echocardiography, endomyocardial biopsy, longitudinal strain
1 |. INTRODUCTION
Cardiac amyloidosis (CA) is an uncommon cause of cardiomyopathy characterized by deposition in the heart of insoluble fibrils made of low-molecular-weight protein subunits, with two main types being AL (immunoglobulin light chain) and ATTR (transthyretin). Once thought as a uniformly fatal disease with few therapeutic options, the landscape of cardiac amyloidosis is changing, with emergence of novel classes of therapies beyond organ transplantation for both AL and ATTR subtypes.1,2 Nonetheless, the diagnosis of CA remains challenging and is frequently delayed or missed, often as a result of its nonspecific clinical and imaging features that overlap with cardiomyopathy of other etiologies. While endomyocardial biopsy (EMB) is traditionally considered the gold standard for diagnosis, few studies have explored its prognostic potential, and the published histopathologic methods to quantify CA burden are complex, time-consuming, and expensive.3 A simpler histopathologic method is needed to more readily quantify amyloid burden and help to establish initial prognosis and develop an optimal treatment plan. In addition, there is a need for imaging-based biomarkers to trend the burden of CA in order to monitor treatment response.
In this study, we aimed to develop a simple and inexpensive histopathologic schema to quantify the burden of CA in patients undergoing EMB and validated it against clinical outcomes, including hospital admission rate, arrhythmia, and survival. Our secondary goal was to study the relationship between amyloid burden determined using this histopathologic schema and standard echocardiographic parameters of cardiac anatomy and function, as potential imaging-based, noninvasive biomarkers of CA burden.
2 |. METHODS
2.1 |. Population and study design
This was a single-center, retrospective study of 59 patients with CA confirmed by EMB and no valvular abnormalities. Microscopic review of EMB specimens with quantitative analysis of the percentage of amyloid burden was performed to categorize each patient into one of two groups: mild-to-moderate (0%–49%) and high (≥50%) CA burden. In a subset of 32 patients, amyloid subtyping was performed by mass spectroscopy to determine the etiology of the disease. Review of electronic health record was used to obtain outcomes data including heart failure-related hospital admissions, cardiac arrhythmia, and deaths. Kaplan-Meier survival analysis was performed for the two groups. A subgroup of 34 patients who had echocardiograms available were used to identify indices of cardiac anatomy and function that may be related to CA burden and thus potentially used as noninvasive biomarkers of this clinically important entity. The study was approved by the Institutional Review Board (IRB) at the University of Chicago.
2.2 |. Analysis of EMB specimens
In each case, the EMB tissue sample was entirely contained in a single tissue block. Three hematoxylin and eosin (H&E)-stained slides comprising ten histologic levels from each biopsy sample were reviewed, and amyloid burden, that is, the fraction of amyloid deposition in the sample, was quantitated to the nearest 5% interval by two pathologists (DBC & ANH) working together at a multiheaded light microscope. In addition, a single Congo red-stained tissue section was reviewed to verify the presence of amyloid deposition and confirm the amyloid burden percentage obtained by H&E analysis. In cases where the H&E- and Congo red-stained slides showed disparate burdens, the amyloid burden from the Congo red-stained slide was used. Subtyping of amyloid was performed using liquid chromatography tandem mass spectrometry on peptides extracted from Congo red-positive areas of the paraffin-embedded EMB samples. Each case was characterized as either AL- or ATTR-type.
2.3 |. Two-dimensional echocardiography
Two-dimensional transthoracic echocardiography (2D TTE) was performed on a commercial system (Phillips Healthcare, Andover, MA, USA) using standardized scanning protocol. Standard views were recorded including parasternal short-axis, apical two-, three-and four-chamber views, as well as mitral and tissue Doppler imaging. Measurements were taken using QLAB 10 software (Phillips Healthcare). Endocardial boundaries were traced to obtain left ventricular end-systolic and end-diastolic volumes (LVESV, LVEDV) in the apical two- and four-chamber views, and ejection fraction (EF) was calculated using biplane method of disks. Interventricular septal thickness (IVSt), posterior wall thickness (PWt), LV mass, and lateral e′-velocity were measured according to the guidelines of the American Society of Echocardiography (ASE).4 LV longitudinal strain was measured in the apical two-, three-, and four-chamber views using speckle-tracking and then averaged to obtain GLS, while segments where tissue tracking was deemed inaccurate were excluded from analysis.
2.4 |. Statistical analysis
Categorical variables were expressed as percentages and compared between the two CA burden groups using the chi-square statistics. Continuous variables were expressed as mean ± SD and unpaired Student’s t-tests were used to test the significance of the differences between the two groups. A P-value of <0.05 was considered statistically significant. Kaplan-Meier survival analysis was performed to assess inter-group differences in survival, and log-rank test was used to assess their significance.
3 |. RESULTS
Of the 59 patients analyzed, 24 (41%) had high burden of CA, while the remaining 35 patients (59%) had low-to-moderate CA burden. Demographic data and clinical characteristics of the patient cohort are shown in Table 1, showing no statistically significant differences in patient characteristics such as age, gender, and comorbidities between the two groups.
TABLE 1.
Patient demographics and clinical characteristics of the two groups of study patients defined by the burden of cardiac amyloid
| Cardiac amyloid burden | P-value | ||
|---|---|---|---|
| Low-to-moderate (0%-49%) | High (50%-100%) | ||
| Age (years) | 69 ± 10 | 72 ± 9 | 0.21 |
| Female | 10/35 (29%) | 5/24 (21%) | 0.60 |
| Diabetes mellitus | 7/19 (37%) | 4/16 (25%) | 0.59 |
| Hypertension | 14/21 (67%) | 14/16 (88%) | 0.59 |
| Hyperlipidemia | 11/19 (58%) | 8/16 (50%) | 0.80 |
| Peripheral arterial disease | 2/19 (11%) | 0 (0%) | 0.20 |
| Prior stroke | 3/11 (16%) | 3/16 (19%) | 0.85 |
Fractions represent the number of each finding out of the number of patients in whom the corresponding data were available for analysis.
Figures 1, 2 show examples of histopathologic and echocardiographic images with the corresponding measurements obtained in two patients: one with a low burden of CA and the other with a high CA burden. It is easy to visually appreciate the extensive areas of amyloid deposits (lighter red colors) in the histopathology slide in Figure 2, compared to patchy, well-delineated deposits interspersed within normal myocardial tissue (darker red color) in Figure 1. Correspondingly, the echocardiographic images of the patient with low CA burden show mild LV hypertrophy and mild diastolic dysfunction, while those of the patient with high CA burden show a severe LV hypertrophy and higher grade of diastolic dysfunction.
FIGURE 1.
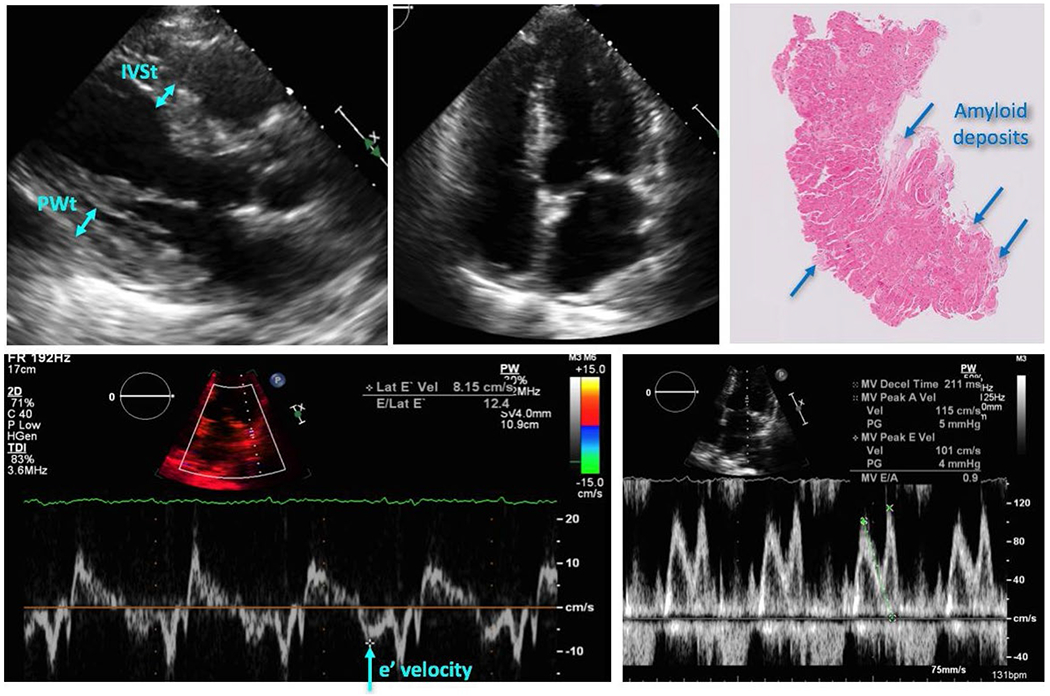
Example of echocardiographic images with the corresponding measurements and histopathology of the tissue sample, obtained in a patient with a low burden of cardiac amyloidosis (see text for details)
FIGURE 2.
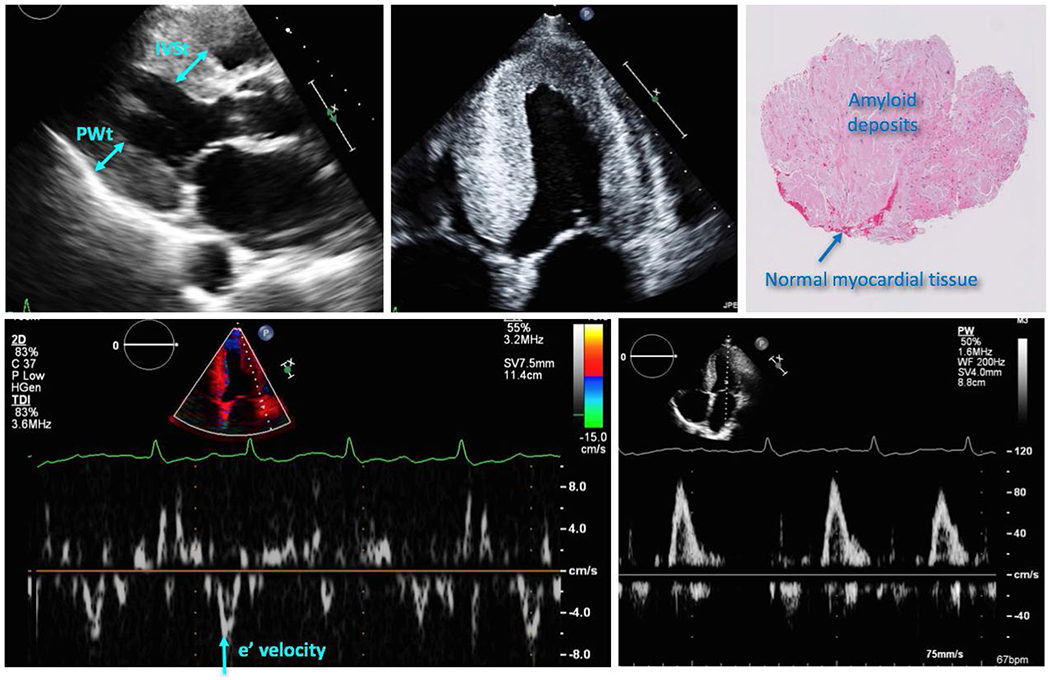
Example of echocardiographic images with the corresponding measurements and histopathology of the tissue sample, obtained in a patient with a high burden of cardiac amyloidosis (see text for details)
Table 2 lists the adverse cardiac events in the two groups. Interestingly, there was no statistically significant inter-group difference in the frequency of heart failure-related emergency department visits or hospital admission rates, occurrence of atrial or ventricular arrhythmia or heart block. However, survival in days from EMB showed a trend toward longer survival in patients with lower CA burden (681 ± 534 days) vs high CA burden (444 ± 405 days, P < 0.088). Kaplan-Meier curve also revealed divergence in survival between the two groups (Figure 3), albeit not reaching statistical significance.
TABLE 2.
Major adverse cardiac events in the two groups of study patients defined by the burden of cardiac amyloid
| Cardiac amyloid burden | P-value | ||
|---|---|---|---|
| Low-to-moderate (0%-49%) | High (50%-100%) | ||
| Emergency department visits for HF | 19/20 (95%) | 15/15 (100%) | 0.92 |
| Hospital admissions for HF | 18/19 (95%) | 15/15 (100%) | 0.91 |
| Atrial arrhythmias | 13/18 (72%) | 8/13 (62%) | 0.78 |
| Ventricular arrhythmias | 2/18 (11%) | 4/13 (31%) | 0.27 |
| Heart block | 8/17 (47%) | 5/14 (36%) | 0.68 |
| Death or transplantation at 1 year | 8/28 (29%) | 13/20 (65%) | 0.12 |
Fractions represent the number of each finding out of the number of patients in whom the corresponding data were available for analysis.
FIGURE 3.
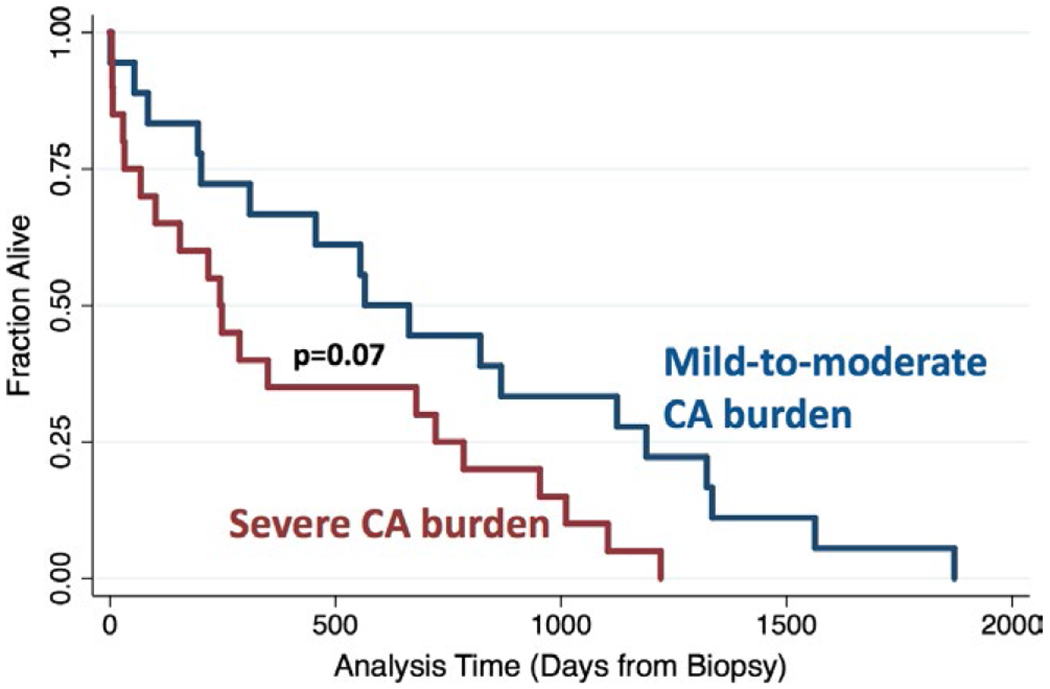
Kaplan-Meier survival curves of the two cardiac amyloidosis burden groups
Table 3 shows the breakdown of the amyloidosis subtyping for the 32 patients: 17 patients were classified as having low-to-moderate CA burden and the remaining 15 as high burden. Interestingly, 14/15 patients with high CA burden were ATTR-type. In contrast, all but one AL-type patients examined had mild-to-moderate CA burden per histopathology, while the majority (14/22) of ATTR-type patients were classified as having high CA burden. Of note, the difference in the prevalence of high CA burden between the two subtypes was statistically significant.
TABLE 3.
The distribution of amyloidosis subtypes in the two groups of study patients defined by the burden of cardiac amyloid
| Amyloid type | Cardiac amyloid burden | P-value | |
|---|---|---|---|
| Low-to-moderate (0%-49%) (N = 17) | High (50%-100%) (N = 15) | ||
| AL (N = 10) | 9 | 1 | 0.03 |
| ATTR (N = 22) | 8 | 14 | 0.29 |
| P-value | 0.84 | 0.004 | |
AL = light chain; TTR = transthyretin. Data shown only for patients in whom survival data were available for analysis (in parentheses).
In a subgroup of 24 patients who had both amyloidosis subtyping and outcomes data (1-year death or heart transplantation) available, 13 patients were classified as low-to-moderate and 11 as severe CA burden. Of these, the numbers of patients who reached the above endpoint was 3/13 and 5/11 (P = 0.41), respectively. Interestingly, when divided by amyloidosis type (Table 4), with ATTR-type, none of the 6 patients (0%) with low-to-moderate CA burden died or underwent heart transplantation, while of the 11 patients with high CA burden, 5 had reached this endpoint (45%). In contrast, with AL-type, 3/7 patients (43%) with low-to-moderate CA burden reached the endpoint, while there were no patients with high CA burden and outcomes data, so that there are no endpoints to report (0/0). Of note, within the patients with low-to-moderate CA burden, none of the ATTR-type reached the endpoint, while nearly half of the patients with AL-type did.
TABLE 4.
Death or heart transplantation within 1 year in the two groups of patients defined by the burden of cardiac amyloid, separated by amyloidosis type
| Amyloid type | Cardiac amyloid burden | P-value | |
|---|---|---|---|
| Low-to-moderate (0%-49%) (N = 13) | High (50%-100%) (N = 11) | ||
| AL (N = 7) | 3/7 | 0/0 | N/A |
| ATTR (N = 17) | 0/6 | 5/11 | 0.13 |
| P-value | 0.13 | N/A | |
Data shown only for patients in whom survival data were available for analysis (in parentheses).
Of the subgroup of 34 patients who had echocardiograms available, 13 had a high burden of CA, while the other 21 had a low-to-moderate CA burden. The majority of the echocardiographic parameters showed significant differences between these two subgroups (Table 5). Specifically, the group with high CA burden had higher values of total LV mass (341 ± 75 vs 289 ± 92 g), IVSt (1.89 ± 0.3 vs 1.47 ± 0.3 cm), and PWt (1.89 ± 0.3 vs 1.45 ± 0.2 cm), corresponding to more severe LV hypertrophy. At the same time, parameters of LV functional indices were lower in the high CA burden group, including EF (30 ± 11% vs 53 ± 15%), lateral e′-velocity (4.4 ± 1.4 vs 5.8 ± 1.8 cm/s), and GLS magnitude (−6.4 ± 3.7 vs −10.7 ± 4.9%) (Figure 4), reflecting more severe systolic and diastolic dysfunction.
TABLE 5.
Echocardiographic measurements obtained in the two groups of study patients defined by the burden of cardiac amyloid
| Echocardiographic parameters | Cardiac amyloid burden | P-value | |
|---|---|---|---|
| Low-to-moderate (0%-49%) (N = 21) | High (50%-100%) (N = 13) | ||
| LV ESV | 58 ± 21 | 90 ± 49 | 0.03 |
| LV EDV | 124 ± 24 | 125 ± 56 | 0.95 |
| LV EF (%) | 53 ± 15 | 30 ± 11 | 0.0003 |
| GLS (%) | −10.7 ± 4.9 | −6.4 ± 3.7 | 0.03 |
| IVSt (cm) | 1.47 ± 0.3 | 1.89 ± 0.3 | 0.003 |
| PWt (cm) | 1.45 ± 0.2 | 1.89 ± 0.3 | 0.001 |
| LV mass (g) | 289 ± 92 | 341 ± 75 | 0.02 |
| Lateral e′ (cm/s) | 5.8 ± 1.8 | 4.4 ± 1.4 | 0.05 |
FIGURE 4.
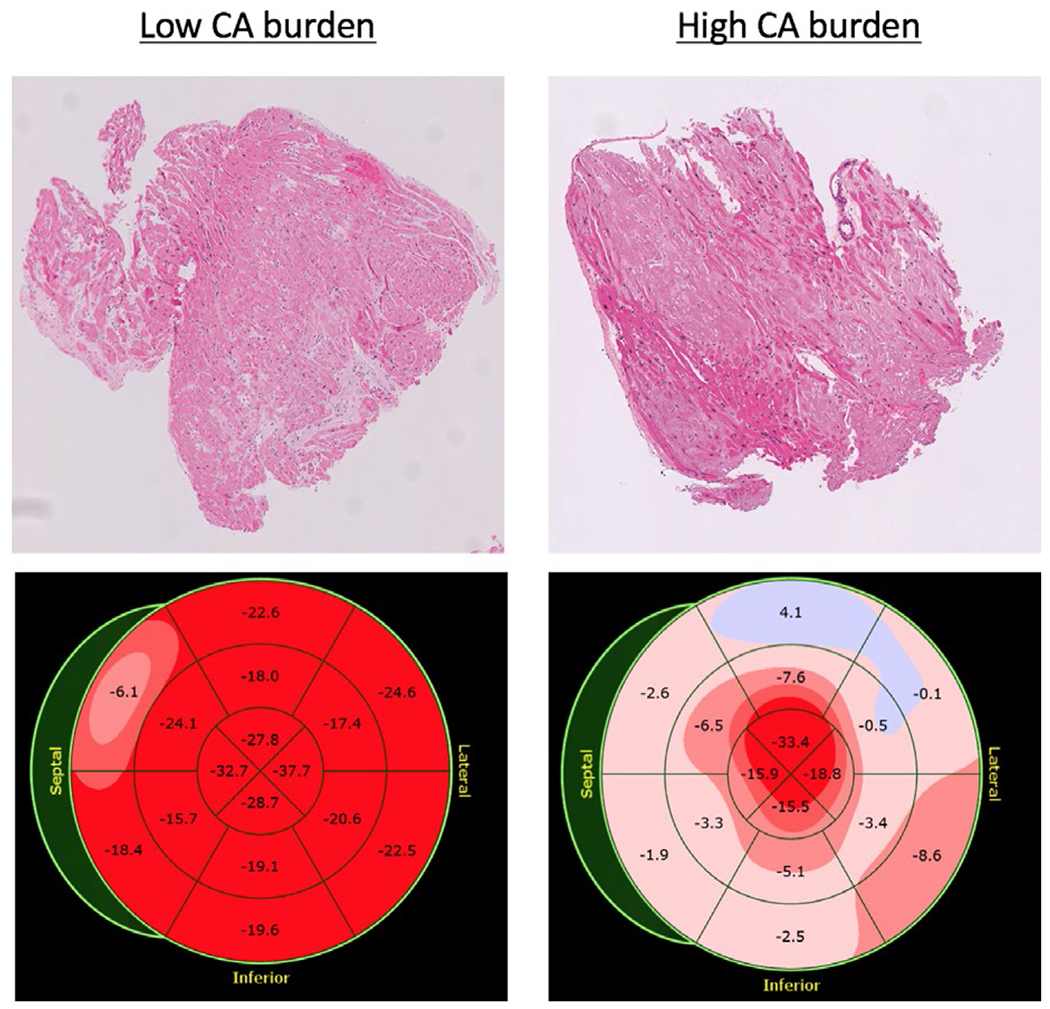
Examples of histopathology of the tissue samples (top) and bulls’ eye representation of global longitudinal strain (bottom) of a patient with mild (left) and high (right) cardiac amyloidosis burden
4 |. DISCUSSION
Cardiac amyloidosis is an often underdiagnosed condition due to its vague clinical presentation that overlaps with heart failure of other etiologies. Traditional 2D echocardiography is easy to obtain and noninvasive but may lack adequate sensitivity in this context.5 Histopathologic analysis of endomyocardial samples is traditionally considered the gold standard in diagnosis but is performed infrequently due to the need for invasive biopsy and risk of procedural complications. Few published reports exist regarding the prognostic value of EMB in cardiac amyloidosis or their correlation to standard echocardiographic findings. Pelikka et al reported that among a cohort of 30 patients with EMB-confirmed cardiac amyloidosis, mean survival was 5 months, which did not correlate with the extent of interstitial amyloidosis. Furthermore, only 68% had echocardiograms consistent with amyloidosis, while the remaining 32% were abnormal but nonspecific.6 Using a French cohort of 79 patients with a clinical diagnosis of cardiac amyloidosis, Ternacle et al reported good correlation between apical longitudinal strain and the rate of major adverse events. Interestingly, in the three patients who underwent heart transplantation, the longitudinal strain of the diseased native hearts correlated with macroscopic amyloid burden postexplantation.7
In this study, we demonstrated that beyond providing definitive disease diagnoses, histopathologic analysis of endomyocardial biopsies allows for quantification of amyloid burden at the tissue level, which correlated with echocardiographic parameters of cardiac function. Such histological knowledge may also carry prognostic value, as evidenced by a signal toward higher mortality in the group with high CA burden. Together, these findings pose the intriguing premise that in patients with clinical diagnoses of cardiac amyloidosis, the degree of echocardiographic abnormality may help to identify those with probable high CA burden and worse clinical outcome, as well as to track changes in amyloid burden in response to therapy. The latter possibility is starting to take on ever more practical significance in light of several therapeutic agents that demonstrated clinical efficacy in multiple ongoing clinical trials of ATTR amyloid cardiomyopathy and neuropathy. With an emerging armamentarium of disease-modifying therapies ranging from RNA/oligonucleotide-inhibition of transthyretin synthesis8,9 to small molecule stabilizers of transthyretin tetramer,10 traditional 2D echocardiography may well be re-tooled as a cost-effective imaging meter to monitor treatment response.
Subtyping using mass spectrometry showed that all samples but one of AL-type patients had low-to-moderate amyloid burden, while a majority of samples of TTR amyloidosis had high amyloid burden. This may simply reflect earlier clinical presentation in systemic AL amyloidosis (eg, constitutional B symptoms and other organ involvement) and availability of alternative diagnostic tools (eg, serum light chain ratio, bone marrow aspirates) for AL-subtype. Additionally, despite the fact that the AL-type patients had only low-to-moderate burden of CA, their mortality was considerably higher than the ATTR-type patients with the same burden category. This likely reflects the well-known prognostic differences between the two types of amyloidosis.11 It is possible that in our study, the divergence in survival curves between high- and low-amyloid burden groups was blunted in part because of a higher representation of ATTR patients in the high-burden group. Our data show that both the type and the burden of amyloid may carry prognostic information, and that CA burden may be particularly important the ATTR-type patients.
4.1 |. Limitations
We recognize several important limitations to the study. First, given the study is retrospective, not all echocardiographic data were available for analysis in all patients. To address this issue and avoid potential misinterpretation, we presented the actual numbers of patients in whom data were available as denominators in Tables 1 and 2. Secondly, the study was underpowered to detect difference in major clinical outcomes owing to the small sample size, though it still provided a strong signal that CA burden may be a clinically important entity and also captured significant differences in echocardiographic parameters of LV anatomy and function between the two groups of CA burden. Moreover, amyloidosis subtyping was not performed in every patient, thus limiting our ability to differentiate our findings according to the specific etiology. However, our data reflected interesting and potentially hypothesis generating differences between these two types of disease. Furthermore, our results do not provide a clear answer to whether echocardiographic analysis can aid in the diagnosis of patients with low-to-moderate CA burden. This is because this important question was not included in the study goals, since we could not have answered it within the constraints of this relatively small retrospective study and given the fact that we only included patients who had a high enough clinical suspicion of CA to warrant a referral for and ultimately have the diagnosis confirmed by EMB. Finally, many patients did not undergo imaging using newer generation of modalities such as cardiac MRI and technetium-labeled bone scintigraphy, which have improved the diagnostic specificity compared to echocardiography, and in some studies, been reported to exhibit prognostic value.12–14 The use of such advanced imaging technologies may yield stronger, more specific imaging correlations to the amyloid burden obtained from EMB.
5 |. CONCLUSIONS
We described a simple, quantitative histopathologic method to stratify burden of CA, which was shown to be associated with 2D echocardiographic parameters of cardiac dysfunction and may have prognostic value in predicting survival. Further studies using larger patient samples are needed to determine whether these associations are strong enough to allow imaging-based, noninvasive evaluation of CA burden in individual patients both at the time of diagnosis and over the course of treatment.
DISCLOSURES
AN was supported by a T32 Cardiovascular Sciences Training Grant (5T32HL7381). There are no conflicts of interest among the authors.
REFERENCES
- 1.Joseph NS, Kaufman JL. Novel approaches for the management of AL amyloidosis. Curr Hematol Malig Rep. 2018;13(3):212–219. [DOI] [PubMed] [Google Scholar]
- 2.Dahm CN, Cornell RF, Lenihan DJ. Advances in treatment of cardiac amyloid. Curr Treat Options Cardiovasc Med. 2018;20(5):37. [DOI] [PubMed] [Google Scholar]
- 3.Kristen AV, Brokbals E, Aus dem Siepen F, et al. . Cardiac amyloid load: a prognostic and predictive biomarker in patients with light-chain amyloidosis. J Am Coll Cardiol, 2016;68(1):13–24. [DOI] [PubMed] [Google Scholar]
- 4.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. e14. [DOI] [PubMed] [Google Scholar]
- 5.Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840–1849. [DOI] [PubMed] [Google Scholar]
- 6.Pellikka PA, Holmes DR Jr, Edwards WD, Nishimura RA, Tajik AJ, Kyle RA. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch Intern Med. 1988;148(3):662–666. [PubMed] [Google Scholar]
- 7.Ternacle J, Bodez D, Guellich A, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging. 2016;9(2):126–138. [DOI] [PubMed] [Google Scholar]
- 8.Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. [DOI] [PubMed] [Google Scholar]
- 9.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22–31. [DOI] [PubMed] [Google Scholar]
- 10.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. [DOI] [PubMed] [Google Scholar]
- 11.Dubrey SW, Cha K, Skinner M, LaValley M, Falk RH. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart. 1997;78(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana M, Pica S, Reant P, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castano A, Haq M, Narotsky DL, et al. Multicenter study of planar technetium 99 m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1(8):880–889. [DOI] [PubMed] [Google Scholar]
- 14.Gillmore JD, Maurer MS, Falk RH et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. [DOI] [PubMed] [Google Scholar]


