Abstract
Implantable cardioverter defibrillators (ICDs) have been a relative contraindication to cardiovascular magnetic resonance imaging. Although cardiovascular magnetic resonance provides valuable information regarding scar in patients with ventricular arrhythmias or cardiomyopathy, ICDs in these patients frequently cause artifacts hindering accurate interpretation of both cine and late gadolinium enhancement (LGE) images. We sought to quantify the frequency and severity of artifact on LGE images and assess whether a modified wideband LGE protocol could improve the diagnostic yield of scar identification in agreement with invasive electroanatomic mapping (EAM). Forty-nine patients with ICDs and ventricular tachycardia (VT) or cardiomyopathy underwent CMR (Philips 1.5T), including standard and wideband LGE imaging. A safety algorithm was followed throughout the protocol. Standard and wideband LGE short-axis images were graded using an artifact score on a per-slice basis. LGE on wideband images was compared with EAM in 27 of 49 patients who underwent VT ablation. There were no adverse patient- or device-related events. With standard LGE imaging, 84% of patients demonstrated some degree of hyperenhancement artifact, which persisted in 22% on wideband LGE but with much less extent. Wideband LGE imaging resulted in an increase from 48% to 94% diagnostic-quality slices, with a significant reduction in artifact score, and correlated with EAM in 21 of 27 patients (78%). In conclusion, assessment of standard LGE is markedly limited by artifact in patients with ICD. The use of wideband LGE significantly improves image quality and can accurately localize myocardial scar before VT ablation.
Although the use of cardiovascular magnetic resonance (CMR) in patients with non-MR conditional implantable cardioverter defibrillator (ICD) is primarily an off-label indication, there is a growing evidence to suggest that, with appropriate precautions, it can be performed safely.1,2 However, few studies focused on CMR in patients with ICDs,3,4 nor has the impact of ICDs on image quality been systematically evaluated. Previous studies aimed at scar assessment in patients with ICDs demonstrated considerable limitations regarding device-associated artifact, which may involve >50% of the myocardium.5,6 Specifically, hyperenhancement artifact related to ICD-induced frequency offset of susceptible myocardium may mimic late gadolinium enhancement (LGE) obscuring the ability to determine the extent of scar, or even falsely suggesting scar presence. Recent studies have tested the feasibility of a wideband (WB) protocol at 1.5 and 3T for standard and 3D LGE imaging, showing significantly reduced hyperenhancement artifact, thereby offering potential for accurate scar detection in patients with ICDs.7–9 However, WB LGE has not been widely implemented in clinical practice, and published literature to date reported WB imaging in association with a single vendor and remained largely relegated to research applications. It is important to further investigate the value of the WB protocol, including validation by independent sites and on different vendor platforms. We aimed to: (1) determine the baseline burden of artifact on standard LGE images; (2) test the feasibility and establish the safety of the WB protocol for scar assessment in patients with ICDs; (3) assess the reduction of artifact with WB LGE imaging; and (4) compare the agreement between standard and WB LGE assessment of scar localization with invasive electroanatomic mapping (EAM) as a reference.
Methods
We prospectively enrolled 49 patients with clinical ventricular tachycardia (VT) or heart failure and ICDs, referred for CMR assessment of myocardial scar (February 2016 to April 2018). Inclusion criteria were as follows: (1) age >18 years; (2) glomerular filtration rate >30 ml/min/1.73 m2 to permit administration of gadolinium-based contrast; (3) ability to tolerate CMR protocol. Exclusion criteria were ongoing unstable ventricular arrhythmia, gadolinium allergy, ICD implantation within 30 days, and abandoned and/or retained leads or suspected lead malfunction and/or fracture. The study was approved by the Institutional Review Board and each patient provided informed consent.
A general safety algorithm was followed for all patients before, during and after CMR (Figure 1). Specifically, pre- and post-CMR device interrogation was performed to ensure stability of ICD parameters and inhibit inappropriate tachyarrhythmia therapies during imaging. Patients were carefully monitored by an advanced cardiac life support certified nurse and a physician, with continuous electrocardiography and pulse oximetry, and blood pressure assessments every 5 minutes.
Figure 1.
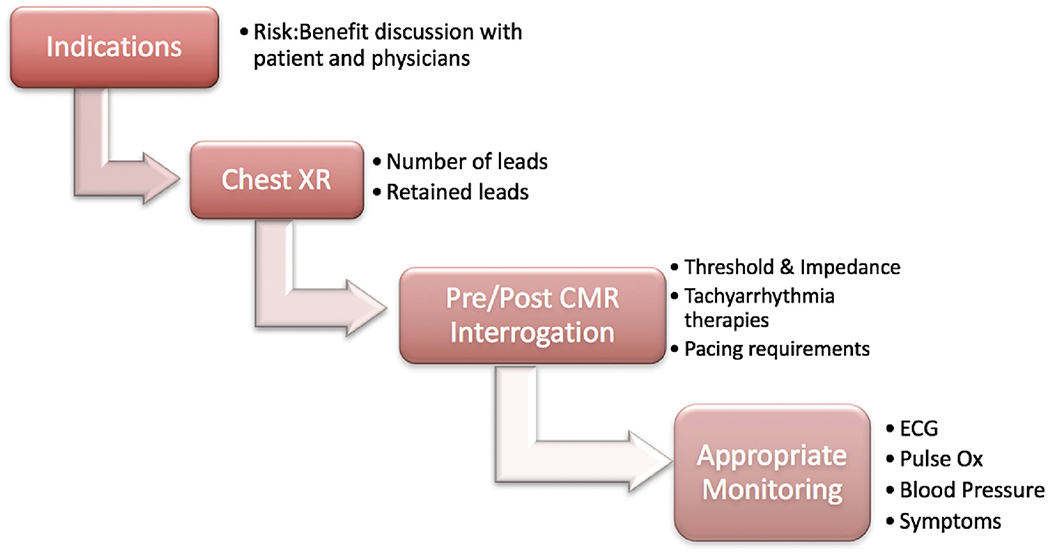
Patient safety algorithm. This demonstrates the clinical decision-making that determined patient eligibility for CMR with acceptable safety, and device and patient monitoring performed before, during and after the CMR exam. CMR = cardiovascular magnetic resonance.
In all patients, the presence of ICD-related artifacts on images obtained with and without WB was determined and graded for extent of the artifact and compared between these 2 acquisition modes. In a subgroup of 27 patients who were referred for VT ablation, scars identified on standard LGE and separately WB LGE images were compared against the reference of invasive EAM.
CMR was performed using a 1.5T scanner (Achieva, Philips) with a 5-channel surface coil. The study protocol initially involved supine position, but was subsequently modified to raise the arm above the head on the ipsilateral side of the ICD, in an effort to increase the distance between the pulse generator and the heart (Figure 2) to minimize imaging artifacts. Immediately following initial survey views, precontrast 4- and 2-chamber scout views were obtained at 3 frequency shifts: −1500, 0 and +1500 Hz (Figure 3). The frequency shift with minimal ICD-related artifact was identified. Next, a gadolinium-based contrast agent was administered (0.1 to 0.2 mmol/kg, depending on renal function), whereas retrospectively gated short-axis cine images were obtained spanning the left ventricle from base to apex (temporal resolution 25 to 40 ms). Ten minutes after contrast administration, standard and WB LGE images were acquired in the 2-, 3-, and 4-chamber views as well as short-axis stack. Standard LGE images were acquired using a T1-weighted gradient-echo pulse sequence with a phase-sensitive inversion recovery reconstruction (typical inversion time 200 to 300 ms, voxel size 2 × 2 × 10 mm, SENSE factor 1–2). WB LGE images were acquired with the optimal frequency shift. The WB protocol modified the standard inversion pulse bandwidth from 1.5 to 3.8 kHz, based on previously published work.9 Heart rate, electrocardiography, pulse oximetry, and blood pressure were monitored throughout the duration of the study.
Figure 2.
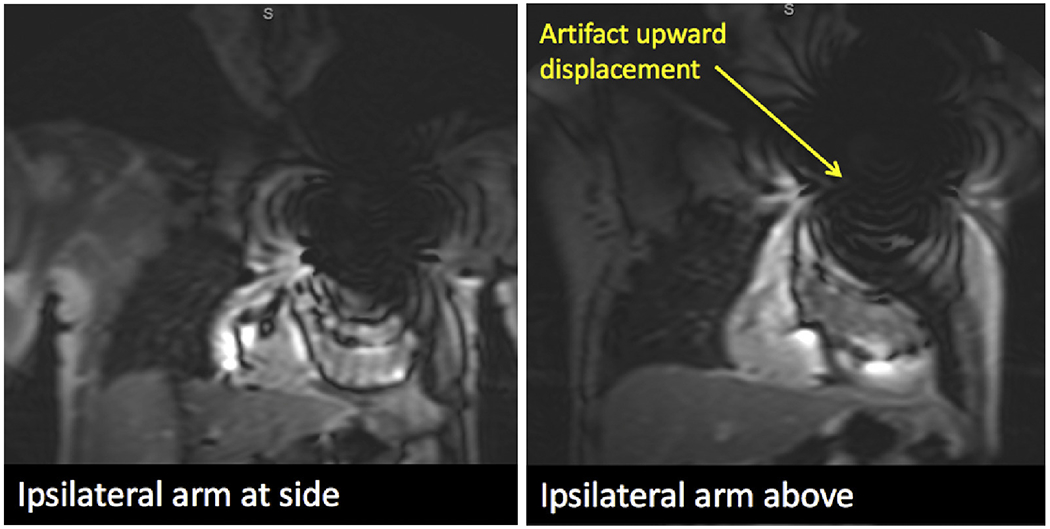
Optimal patient positioning. This example demonstrates a significant 4 cm displacement of ripple artifact from the ICD generator, due to a maneuver in which the ipsilateral arm of the ICD generator was raised above the head for the duration of the CMR exam. ICD = implantable cardioverter defibrillator.
Figure 3.
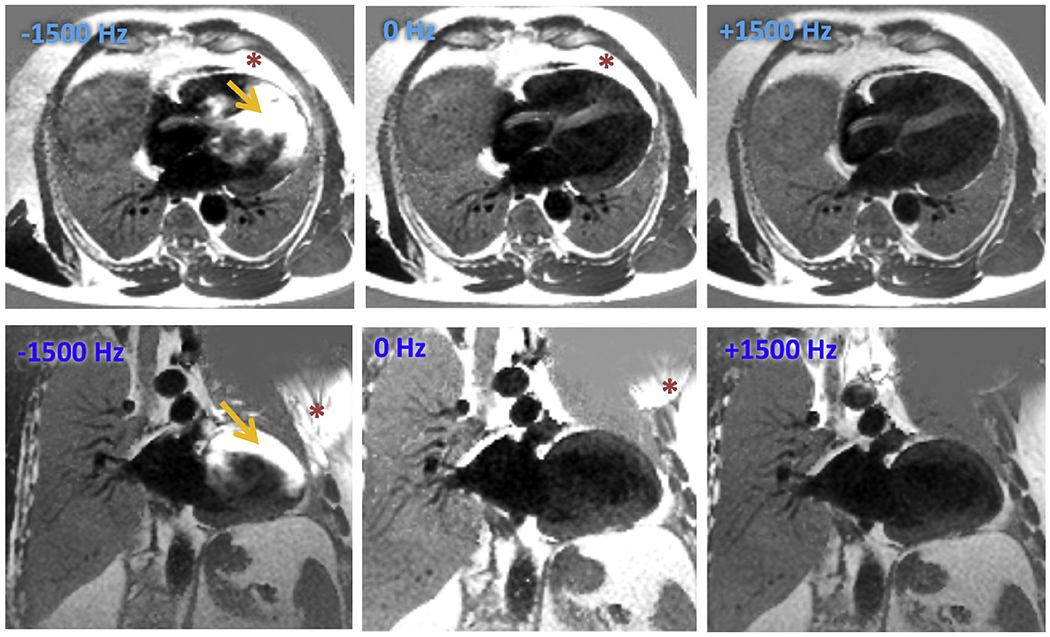
Selection of optimal frequency shift. 4-chamber view (top row) and 2-chamber (bottom row) views at three pre-selected frequency shifts (−1500 Hz, 0 Hz and +1500 Hz, respectively) were utilized before contrast administration. Optimal frequency shift was identified by the image with the least prominent hyperenhancement artifact. Asterisk (*) represents the artifact from the ICD generator itself or its effects on the extracardiac and orange arrows represents how this artifact affects the myocardium. In this example, artifact is least prominent at +1500 Hz and was selected as the appropriate frequency shift for the wide band late gadolinium enhancement images.
The presence of artifact was determined by a level 3 trained CMR expert per short-axis slice for both standard LGE and WB LGE images. Patients were given an artifact score (number of slices with artifact divided by the total number of short-axis slices).
Clinical data including demographics, ICD vendor and/or type and history of previous VT ablation were obtained from the medical records. For patients who proceeded to invasive VT ablation, EAM (assorted vendors) was performed to delineate low-voltage regions or fractionated signals suggestive of scar. These data were used as a reference for comparisons to CMR-based scar localization by both standard and WB LGE. Endocardial approach was the procedural default, unless CMR data suggested an epicardial or mid-myocardial focus or intraprocedural data demonstrated a failed endocardial approach.
Continuous variables are reported as means ± standard deviations. Significance of differences was determined using paired t Tests. Categorical variables were expressed as numbers or proportions and the differences were tested using chi-square statistics.
Results
The clinical data of the study patients are shown in Table 1. The overall prevalence of LGE was 71% (n = 35). Twenty-seven patients (55%) underwent VT ablation following their CMR exam. The average duration of the CMR examination, with addition of the WB protocol, was 54 ± 14 minutes, not significantly longer than standard clinical examinations without WB imaging in the control subjects: 50 ± 15 (p = 0.28). The majority of WB imaging was performed at a frequency offset of +1500 Hz. Although most of the patients had non-MR conditional devices, there were no adverse events. Pre- and post-CMR ICD parameters are shown in Table 2. There were no significant changes in parameters and no tachy-therapies or failure to pace during or immediately after CMR.
Table 1.
Clinical characteristics of patients and CMR findings
| Age (year) | 60 ± 11 |
| Men | 84% |
| Etiology of cardiomyopathy | |
| Ischemic | 22% |
| Nonischemic | 76% |
| Other | 2% |
| Side of implantable cardioverter defibrillators (left-sided) | 98% |
| Average number of leads | 2.1 ± 0.7 |
| Age of implantable cardioverter defibrillator (year) | 3.9 ± 3.4 |
| Distance from device generator to left ventricle (cm) | 9.1 ± 2.3 |
| Average duration of cardiac MRI including wideband (minutes) | 54 ± 14 |
| Late gadolinium enhancement present | 71% |
| Ventricular tachycardia ablation post cardiac MRI | 55% |
Table 2.
Device parameters by interrogation precardiac MRI and the difference between pre- and postcardiac MRI
| Precardiac MRI value | Postcardiac MRI minus precardiac MRI | |
|---|---|---|
| Sensing threshold (mV) | ||
| Right atrium | 3.5 ± 1.9 | −0.09 ± 1.2 |
| Right ventricle | 11.7 ± 5.4 | −0.08 ± 2.4 |
| Left ventricle | − | − |
| Pacing threshold (V) | ||
| Right atrium | 0.8 ± 0.3 | 0.04 ± 0.2 |
| Right ventricle | 0.9 ± 0.3 | 0.01 ± 0.2 |
| Left ventricle | 1 ± 0.4 | 0 ± 0.1 |
| Impedance (Ohm) | ||
| Right atrium | 501 ± 178 | 14 ± 80 |
| Right ventricle | 500± 171 | 11 ± 70 |
| Left ventricle | 607 ± 230 | −0.01 ± 0.07 |
| High-voltage lead impedance (Ohm) | ||
| Right ventricle | 60 ± 14 | 0.7 ± 7 |
On standard LGE images, hyperenhancement artifact of any severity, which could be mistaken for LGE, was encountered in the majority of patients with ICD (41 of 49 or 84%).
The most common location of artifact was throughout the anterior wall, with the apical segment most commonly involved followed by the mid and basal anterior segments. The basal anteroseptum and basal anterolateral segments were also commonly impacted. After applying the WB LGE protocol at manually selected frequency shifts, the majority of these cases (32 of 41 or 79%) demonstrated complete resolution of artifact. With WB LGE imaging, there was no evidence of LGE in 10 of 41 patients (24%) with baseline hyperenhancement artifact, thus clarifying the artefactual nature of this finding on standard LGE images.
In terms of diagnostic quality of the standard LGE images, 278 of 533 short-axis slices (52%) were deemed as nondiagnostic when the myocardium had any hyper-resonance or signal void artifact preventing complete visualization of the muscle. With WB LGE imaging, the number of nondiagnostic slices was considerably lower (32 of 537 or 6%, p < 0.01). In keeping with this effect of WB LGE imaging, the artifact score was significantly reduced (Table 3): only 11 of 49 patients demonstrated hyperenhancement artifact of any kind and, the artifact size was small relative to the heart size.
Table 3.
Comparison of standard LGE with WB LGE for diagnostic interpretation
| Standard late gadolinium enhancement | Sideband late gadolinium enhancement | |
|---|---|---|
| Diagnostic short-axis slices (n (%)) | 255 (48) | 505 (94)* |
| Artifact score | 0.51 ± 0.31 | 0.06 ± 0.13* |
p Value <0.05.
With standard LGE imaging, the presence and location of LGE was in agreement with at least one focus of scar on EAM in 10 of 27 cases. In contrast, with WB imaging, the agreement with EAM was noted in 21 of 27 cases, reflecting considerable improvement in scar localization. This included absence of LGE, which correlated with no EAM evidence of left ventricular scar in 2 patients (Figure 4). Two patients had right ventricular endocardial mapping alone, so agreement could not be assessed for left ventricular scar.
Figure 4.
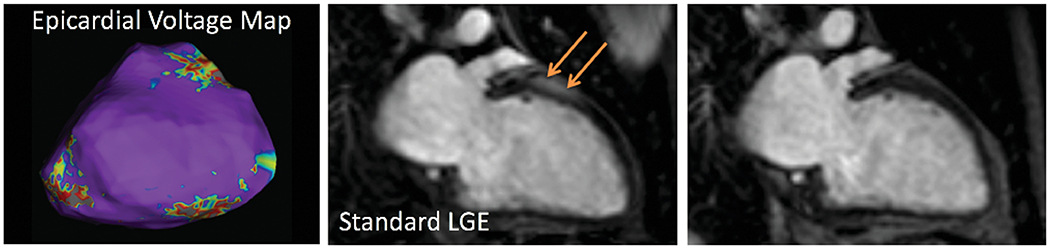
Example: Hyperenhancement artifact simulated anterior wall LGE (middle) but was completely resolved with WB imaging (right). This correlated with epicardial voltage mapping (left) showing normal voltage patterns and no electroanatomic evidence of scar (purple denotes normal myocardium, whereas scar area would be depicted by gray, which is absent in this image; see examples in Figures 5 and 6). LGE = late gadolinium enhancement; WB = wideband.
Discussion
We sought to assess how the presence of an ICD impacted the image quality and diagnostic utility of standard-LGE images by comparing the frequency of artifacts and their effects on scar identification. We additionally sought to determine to what extent the use of a WB-LGE protocol could improve the clinical utility of LGE-CMR imaging. Our study confirmed that the presence of an ICD results in standard LGE images that are frequently of nondiagnostic quality. The use of a WB-LGE imaging protocol was shown to nearly universally mitigate the artifact. Importantly, the presence and location of scar on WB-LGE images correlated with scar maps determined by EAM better than standard LGE images. Furthermore, by adhering to a safety algorithm to ensure appropriate patient selection and monitoring, we obtained diagnostic quality LGE images in all patients without any adverse events. This was particularly notable given the spectrum of device manufacturers, and the fact that most devices in our study were non-MR conditional.
The detection of scar in ischemic and nonischemic cardiomyopathies using CMR provides valuable prognostic information, and may help guide appropriate patient selection for device therapies and preprocedural planning for VT ablation.10–12 There is an increasing emphasis on the use of a substrate-guided approach to eradicate VT, in which the mechanism of ventricular dysrhythmia is often scar-mediated. Although VT ablation in patients with cardiomyopathy has not been shown to portend a mortality benefit to date, it offers substantial reductions in ICD shocks and VT burden, and therefore has important therapeutic benefits.13 Substrate mapping can effectively exploit scar imaging using techniques such as LGE-CMR to identify scar foci that can then be targeted during ablation.14 However, routine CMR imaging of patients with VT is often limited by the presence of ICDs. The advent of MR-conditional ICDs and pacemakers has alleviated concerns regarding the safety of CMR exams in these patients. Nevertheless, they remain a relatively recent addition to the market, thus many patients seen in everyday clinical practice may not have MR-conditional devices.15
As the number of patients with ICDs increases, the availability of accurate and safe CMR imaging techniques may prove increasingly useful.16 This is due to the unique ability of CMR to provide tissue characterization, with particular emphasis on its ability to detect LGE. Defining the presence of LGE by CMR is critical for risk stratification, and has been shown to be an independent predictor of sudden death or ventricular arrhythmias.17 Location of LGE correlates with ablation sites in patients with cardiomyopathy, and can therefore guide identification of arrhythmia substrate for invasive electrophysiology procedures, which are increasingly utilized for treatment of scar-mediated VT.10 However, due to safety concerns and ICD-induced artifacts that can make LGE assessment inaccurate, CMR-based scar assessment in patients with ICDs has not been well-studied.
The ferromagnetic components of an ICD generator and lead system have variable effects on the homogeneity of the magnetic field, which can distort cine images with ripple and signal void artifacts, and impair the uniformity of myocardial nulling that is integral to adequate assessment of myocardial fibrosis with LGE imaging. Previous studies have demonstrated up to a 2 to 6 kHz frequency shift in susceptible myocardial regions in the presence of an ICD.9 It is in these areas where hyperenhancement artifact appears, either mimicking or obscuring true foci of myocardial fibrosis. Recent studies9,18,19 have definitively shown the advantages of widening the bandwidth of the inversion pulse to 3.8 kHz from the standard 1 to 1.5 kHz used for LGE image sequences, which ameliorates the off-resonance effects of ICDs. This was successfully demonstrated utilizing a protocol in which WB LGE was obtained at 3 frequency offsets, resulting in 4 total acquisitions of LGE (standard, and 3 WB LGE datasets). This also required periodic prolongation of the selected inversion time to ensure adequate myocardial nulling.
With the help of these important developments in WB sequence, we sought to streamline our acquisition protocol. In our WB sequence, this entailed the use of a precontrast scout technique, which allows the determination of an offset frequency that is least likely to exhibit hyperenhancement artifact. We then performed WB LGE in one acquisition, utilizing the optimal and previously identified frequency shift. Abbreviating the duration of LGE imaging also minimized the need for adjustment of TI time. Therefore, a strategy of using the correctly identified frequency shift with a widened radiofrequency bandwidth inversion pulse can be harnessed to improve the diagnostic yield of LGE imaging, with a mean scan time comparable to many clinical CMR protocols. Furthermore, in our study, WB LGE use was expanded to include a magnetic resonance imaging (MRI) manufacturer different from all previous publications. In this regard, our study helps pave the way for a more widespread clinical use.
Interestingly, the subtype and location of the ICD may also play a role in optimizing LGE techniques. The majority of our patients had left-sided implants, for which we observed the most common frequency offset, resulting in minimal artifact at +1500 Hz. In one patient with a right-sided implant, there was minimal artifact on both standard and WB LGE images. In 6 patients with subcutaneous ICDs who were imaged during this study but not included in the analysis, the lateral wall was most affected by artifact, as opposed to the commonly affected anterior segments, and resolved best with a frequency offset of −1500 Hz. This suggests that the optimal frequency shift to minimize hyperenhancement depends on patient factors, including the laterality and type of device.
In our study, we were able to eliminate hyperenhancement artifact in 61% of patients, and when remained, the it was very small relative to the size of the ventricle. In one patient, hyperenhancement artifact simulated anterior wall LGE, but was completely resolved with WB imaging (Figure 4) and resulted in deferment of an invasive catheter ablation. In another patient, concomitant hyperenhancement artifact obscured the presence of true epicardial, inferolateral scar (Figure 5), that correlated with clinical VT and was the focus of catheter ablation. In a third patient, large transmural inferolateral and lateral wall infarct correlated with intraprocedural EAM (Figure 6).
Figure 5.
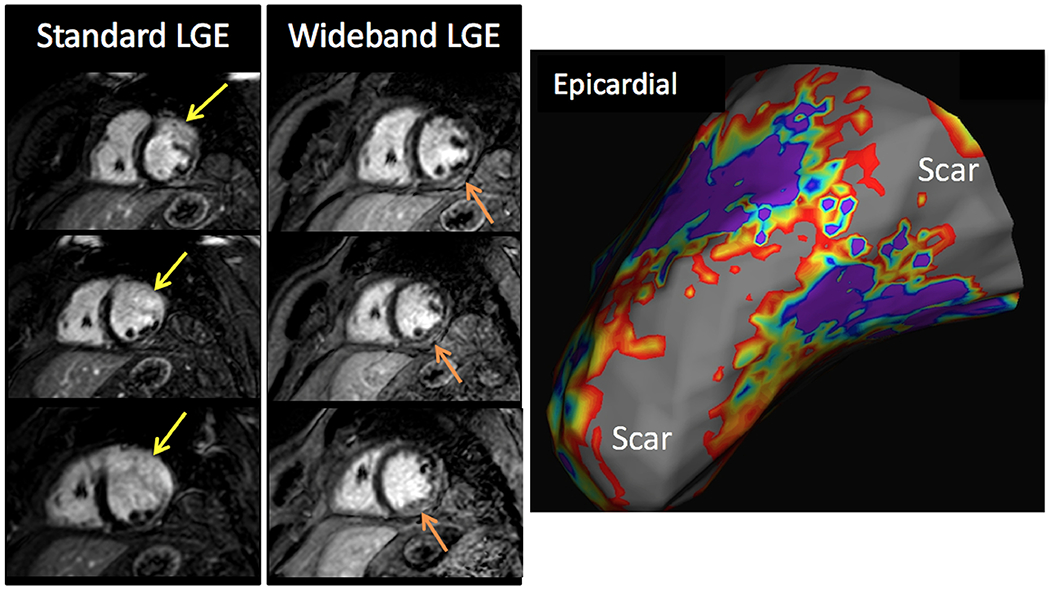
Example: The presence of concomitant hyperenhancement artifact (yellow arrows) can obscure true scar, as evidenced in this patient for whom there was underlying severe basal to mid lateral wall scar (orange arrows) made more evident by WB imaging. This correlated with clinical VT and focus of catheter ablation findings (right, gray region indicating scar in the epicardial/endocardial basal lateral walls). VT = ventricular tachycardia.
Figure 6.
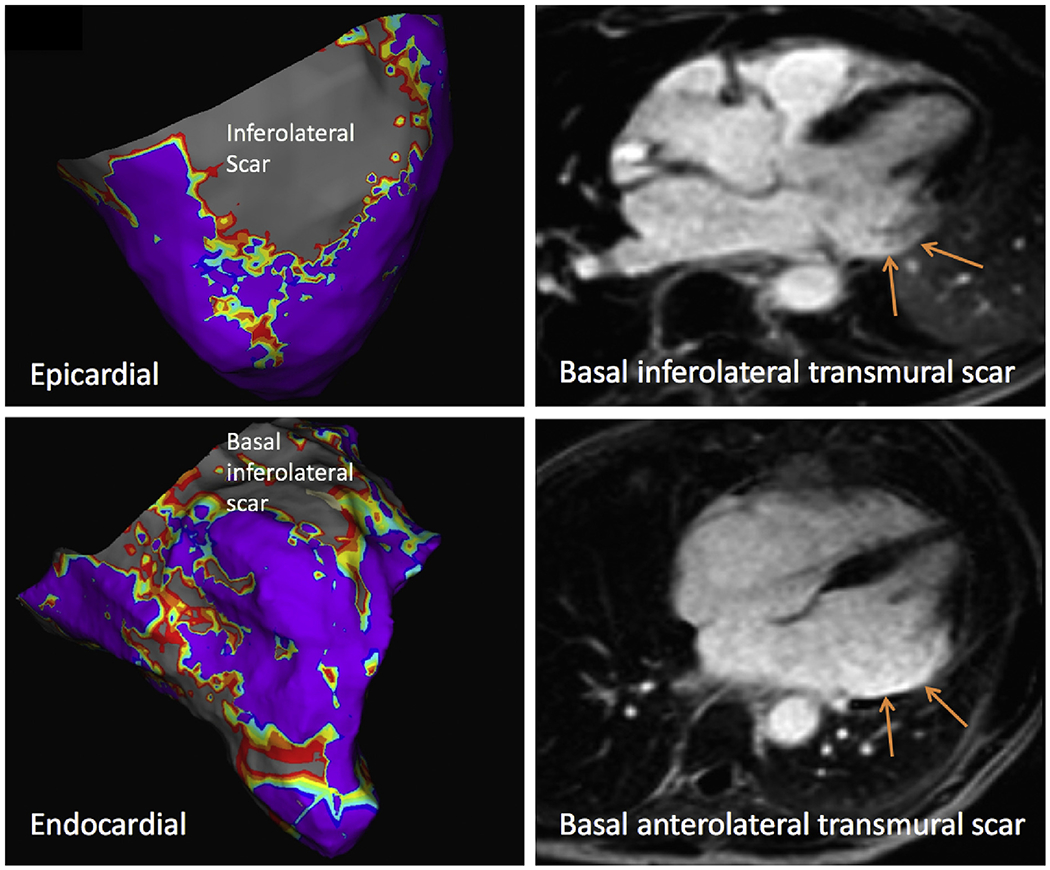
Example: Severe and transmural inferolateral and anterolateral wall infarct (right panels) was well-correlated with voltage mapping of scar (left, gray region indicating scar in the epicardial/endocardial basal lateral walls).
Our results also suggest a supplemental role for CMR scar assessment in patients with clinical VT considered for invasive ablation. In 78% of patients who underwent VT ablation, the EAM data correlated in part or fully with CMR-based scar localization. Although catheter ablation of VT comes with the inherent risks, it is increasingly recognized as an effective treatment for patients with recurrent VT in both ischemic and nonischemic cardiomyopathy, although a related mortality benefit is yet to be proven.20,21 With further studies of CMR-guided catheter ablation, it may be possible to identify CMR features associated with better or worse procedural outcomes. Our results provide support to its use as an adjunctive tool for procedural planning.
A growing body of literature, including recent data from the MagnaSafe registry,22,23 has challenged the perceived contraindication of performing nonthoracic MRI at 1.5T in patients with non-MR conditional devices.1,2 However, this study does not address the totality of MRI exams in patients with ICDs, as CMR was not evaluated. Furthermore, the majority of exams were performed in patients with pacemakers and not ICDs, and no subcutaneous ICDs were included.1,2 However, there are theoretical risks of alterations in device programming, as well as arrhythmia events, which necessitate thoughtful patient selection and close monitoring during CMR imaging. We do not advocate that CMR be routinely used for all patients with ICDs, but in the correct clinical context, it can reliably and safely provide important prognostic and diagnostic information through LGE assessment.
As safety data accrues, there is still an unmet need to define how additional CMR techniques, such as T1- and T2-weighted and phase-encoded velocity imaging, used for tissue characterization and the evaluation of valvular pathology, can be interpreted in the setting of ICD associated artifacts. There are efforts underway to develop T1-mapping sequences using WB pulse sequences, which show promise but they remain within the exploratory realm.24,25 There are many practical applications of CMR, which are susceptible to ICD related artifacts, but the impact on image quality and subsequent diagnostic interpretation is not well known and warrants further investigation.
One limitation of this methodology is that it may not be reliably applied to patients with subcutaneous ICDs because of the variant patterns in artifact that occur with imaging at different frequencies. The main limitation of our study is the small sample size, especially as it relates to the intertechnique comparisons in the subgroups of patients who underwent EAM. Furthermore, not every patient underwent endo- and epicardial voltage mapping, which was performed at the discretion of the physician performing the procedure. Finally, segmental correlation of scar and EAM findings would require robust coregistration tool, which was not available in this study; accordingly, our analysis was performed on a per patient basis.
In conclusion, the utilization of the WB LGE protocol substantially reduces hyperenhancement artifact in patients with ICDs, in whom assessment of underlying scar is needed. Using a protocol that is achievable in the same timeframe as many clinical CMR exams, these patients can be safely imaged with excellent diagnostic yield. In those who proceed to catheter ablation for VT, there is good agreement between scar location and extent by CMR and EAM. This is a promising application of CMR for the management of patients with cardiomyopathy and/or ventricular arrhythmias.
Funding:
Research support from Philips (ARP, KK, RML). AS was supported by funding from the NIH T32 Training Grant (#5T32HL7381).
References
- 1.Nazarian S, Hansford R, Roguin A, Goldsher D, Zviman MM, Lardo AC, Caffo BS, Frick KD, Kraut MA, Kamel IR, Calkins H, Berger RD, Bluemke DA, Halperin HR. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med 2011;155:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RW, Boyle NG, Frabizzio JV, Birgersdotter-Green U, Higgins SL, Lampert R, Machado CE, Martin ET, Rivard AL, Rubenstein JC, Schaerf RH, Schwartz JD, Shah DJ, Tomassoni GF, Tominaga GT, Tonkin AE, Uretsky S, Wolff SD. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med 2017;376:755–764. [DOI] [PubMed] [Google Scholar]
- 3.Naehle CP, Kreuz J, Strach K, Schwab JO, Pingel S, Luechinger R, Fimmers R, Schild H, Thomas D. Safety, feasibility, and diagnostic value of cardiac magnetic resonance imaging in patients with cardiac pacemakers and implantable cardioverters/defibrillators at 1.5 T. Am Heart J 2011;161:1096–1105. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki T, Hansford R, Zviman MM, Kolandaivelu A, Bluemke DA, Berger RD, Calkins H, Halperin HR, Nazarian S. Quantitative assessment of artifacts on cardiac magnetic resonance imaging of patients with pacemakers and implantable cardioverter-defibrillators. Circ Cardiovasc Imaging 2011;4:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwood L, Attili A, Luba F, Ibrahim EH, Parmar H, Stojanovska J, Gadoth-Goodman S, Fette C, Oral H, Bogun F. Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace 2017;19:812–817. [DOI] [PubMed] [Google Scholar]
- 6.Mesubi O, Ahmad G, Jeudy J, Jimenez A, Kuk R, Saliaris A, See V, Shorofsky S, Dickfeld T. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol 2014;37:1274–1283. [DOI] [PubMed] [Google Scholar]
- 7.Ranjan R, McGann CJ, Jeong EK, Hong K, Kholmovski EG, Blauer J, Wilson BD, Marrouche NF, Kim D. Wideband late gadolinium enhanced magnetic resonance imaging for imaging myocardial scar without image artefacts induced by implantable cardioverter-defibrillator: a feasibility study at 3 T. Europace 2015;17:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashid S, Rapacchi S, Shivkumar K, Plotnik A, Finn JP, Hu P. Modified wideband three-dimensional late gadolinium enhancement MRI for patients with implantable cardiac devices. Magn Reson Med 2016;75:572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JP, Hu P. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology 2014;270:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreu D, Ortiz-Perez JT, Boussy T, Fernandez-Armenta J, de Caralt TM, Perea RJ, Prat-Gonzalez S, Mont L, Brugada J, Berruezo A. Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the approach needed for ablation. Eur HeartJ 2014;35:1316–1326. [DOI] [PubMed] [Google Scholar]
- 11.Disertori M, Rigoni M, Pace N, Casolo G, Mase M, Gonzini L, Lucci D, Nollo G, Ravelli F. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging 2016;9:1046–1055. [DOI] [PubMed] [Google Scholar]
- 12.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JA, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation 2005;112:2821–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang JJ, Santangeli P, Callans DJ. Long-term Outcomes of ventricular tachycardia ablation in different types of structural heart disease. Arrhythm Electrophysiol Rev 2015;4:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickfeld T, Tian J, Ahmad G, Jimenez A, Turgeman A, Kuk R, Peters M, Saliaris A, Saba M, Shorofsky S, Jeudy J. MRI-guided ventricular tachycardia ablation: integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol 2011;4:172–184. [DOI] [PubMed] [Google Scholar]
- 15.Miller JD, Nazarian S, Halperin HR. Implantable electronic cardiac devices and compatibility with magnetic resonance imaging. J Am Coll Cardiol 2016;68:1590–1598. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch D, Greenspon AJ. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993-2006. Pacing Clin Electrophysiol 2010;33:705–711. [DOI] [PubMed] [Google Scholar]
- 17.Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA, Sramko M, Masci PG, Barison A, McKenna P, Mordi I, Haugaa KH, Leyva F, Rodriguez Capitan J, Satoh H, Nabeta T, Dallaglio PD, Campbell NG, Sabate X, Cequier A. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail 2017;5:28–38. [DOI] [PubMed] [Google Scholar]
- 18.Stevens SM, Tung R, Rashid S, Gima J, Cote S, Pavez G, Khan S, Ennis DB, Finn JP, Boyle N, Shivkumar K, Hu P. Device artifact reduction for magnetic resonance imaging of patients with implantable cardioverter-defibrillators and ventricular tachycardia: late gadolinium enhancement correlation with electroanatomic mapping. Heart Rhythm 2014;11:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do DH, Eyvazian V, Bayoneta AJ, Hu P, Finn JP, Bradfield JS, Shivkumar K, Boyle NG. Cardiac magnetic resonance imaging using wideband sequences in patients with nonconditional cardiac implanted electronic devices. Heart Rhythm 2018;15:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinov B, Fiedler L, Schonbauer R, Bollmann A, Rolf S, Piorkowski C, Hindricks G, Arya A. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014;129:728–736. [DOI] [PubMed] [Google Scholar]
- 21.Proietti R, Essebag V, Beardsall J, Hache P, Pantano A, Wulffhart Z, Juta R, Tsang B, Joza J, Nascimento T, Pegoraro V, Khaykin Y, Verma A. Substrate-guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long-term outcome. Europace 2015;17:461–467. [DOI] [PubMed] [Google Scholar]
- 22.Mason S, Osborn JS, Dhar R, Tonkin A, Ethington JD, Le V, Benuzillo J, Lappe DL, Knowlton KU, Bunch TJ, Anderson JL. Real world MRI experience with nonconditional and conditional cardiac rhythm devices after MagnaSafe. J Cardiovasc Electrophysiol 2017;28:1468–1474. [DOI] [PubMed] [Google Scholar]
- 23.Russo RJ. Determining the risks of clinically indicated nonthoracic magnetic resonance imaging at 1.5 T for patients with pacemakers and implantable cardioverter-defibrillators: rationale and design of the MagnaSafe Registry. Am Heart J 2013;165:266–272. [DOI] [PubMed] [Google Scholar]
- 24.Hong K, Jeong EK, Wall TS, Drakos SG, Kim D. Wideband arrhythmia-Insensitive-rapid (AIR) pulse sequence for cardiac T1 mapping without image artifacts induced by an implantable-cardioverter-defibrillator. Magn Reson Med 2015;74:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao J, Rashid S, Renella P, Nguyen KL, Hu P. Myocardial T1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magn Reson Med 2017;77:1495–1504. [DOI] [PubMed] [Google Scholar]


