Abstract
Since the completion of the Human Genome Project, progress toward translating genomic research discoveries to address population health issues has been limited. Several meetings of social and behavioral scientists have outlined priority research areas where advancement of translational research could increase population health benefits of genomic discoveries. In this review, we track the pace of progress, study size and design, and focus of genomics translational research from 2012 to 2018 and its concordance with five social and behavioral science recommended priorities. We conducted a review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Guidelines for Scoping Reviews. Steps involved completing a search in five databases and a hand search of bibliographies of relevant literature. Our search (from 2012 to 2018) yielded 4,538 unique studies; 117 were included in the final analyses. Two coders extracted data including items from the PICOTS framework. Analysis included descriptive statistics to help identify trends in pace, study size and design, and translational priority area. Among the 117 studies included in our final sample, nearly half focused on genomics applications that have evidence to support translation or implementation into practice (Centers for Disease Control and Prevention Tier 1 applications). Common study designs were cross-sectional (40.2%) and qualitative (24.8%), with average sample sizes of 716 across all studies. Most often, studies addressed public understanding of genetics and genomics (33.3%), risk communication (29.1%), and intervention development and testing of interventions to promote behavior change (19.7%). The number of studies that address social and behavioral science priority areas is extremely limited and the pace of this research continues to lag behind basic science advances. Much of the research identified in this review is descriptive and related to public understanding, risk communication, and intervention development and testing of interventions to promote behavior change. The field has been slow to develop and evaluate public health-friendly interventions and test implementation approaches that could enable health benefits and equitable access to genomic discoveries. As the completion of the human genome approaches its 20th anniversary, full engagement of transdisciplinary efforts to address translation challenges will be required to close this gap.
Keywords: Translation, Genomics, Social and behavioral sciences
Social and behavioral sciences have been slow to develop and evaluate public health friendly interventions and test implementation approaches that could enable health benefits and equitable access to genomic discoveries. Full engagement of transdisciplinary efforts to address translation challenges will be required to close this gap.
INTRODUCTION
Since the completion of the Human Genome Project in 2003, substantial attention has been focused on pursuing genomic discoveries to improve the understanding of genetic mechanisms in disease development. Technological advances also have greatly reduced the cost of sequencing an individual’s genome, resulting in increasingly affordable and widely available information for researchers, clinicians, and the public [1, 2]. This excitement has been matched with significant financial investment and steep upward trends in publications related to basic genomics and biomedical research [3]. Nevertheless, considerably less progress has been made in translational research [4], defined as research that focuses on the evaluation and implementation of genomics discovery to improve the health of individuals and address population health disparities [5–7].
Challenges related to translating genomic discovery for clinical and public health impact requires a broad base of expertise [8]. Indeed, many have suggested that bringing knowledge and skills from the social and behavioral sciences to research teams is an essential aspect of fulfilling the translational agenda in genomics [9, 10]. As early as 2005, social and behavioral scientists began identifying research priorities to inform efforts to translate genomic discovery into population health benefits [11]. Other more recent initiatives have further increased discussions about the accessibility of genomics and elevated the importance of social and behavioral sciences in translational efforts. These include several reports from the Roundtable on Genomics and Precision Health, which was established by the National Academies of Medicine [7, 11–15].
Furthermore, in 2010, the National Human Genome Research Institute (NHGRI) advanced the conversation by publishing the proceedings of an interdisciplinary group of scientists who developed overarching recommendations for translational social and behavioral science research in genomics [14]. The group identified a need to improve genetic literacy, identify whether genomic information improves risk communication and adoption of health behaviors, and explore whether genomic discovery can elucidate new behavioral intervention targets. Other ideas generated for this social and behavioral science agenda have included developing interventions for health behavior change and studying the implementation of genomics applications [7, 11–14]. Since initial calls for engagement, social and behavioral investigators have made some progress in translating genetics applications for population health [9]. For example, social and behavioral researchers have investigated individuals’ preference to learn genomics information, finding that decision support is needed to help individuals make informed decisions about their genomics results [16]. Other efforts have included investigating the impact of family health history knowledge on cancer risk perceptions and uptake of cancer screening among family members [17, 18].
Given these initiatives, opportunities are great for social and behavioral scientists to engage in supporting genomics translation; however, to date, there have been no systematic efforts to consider the extent to which the recommendations for translation research priorities identified by social and behavioral scientists have been heeded. Thus, the objective of the current review is to conduct a scoping review of the literature to assess the pace of progress in this direction and provide descriptive information about studies (sample size and study design), as well as to assess the concordance of translational research with priorities outlined by behavioral and social sciences in the past decade.
METHODS
Developing search criteria (inclusion/exclusion criteria)
We identified social and behavioral science translational priority areas within the field of genomics. Using key articles and commentaries that have discussed research agendas related to genomics translation since 2005, we extracted priority areas suggested by previous consensus reports [7, 11–14]. A comprehensive list of potential research areas was identified. We then mapped the overlap between these areas and merged themes through rounds of discussion among coauthors. The final set of priority areas included: (i) public understanding of genetics and genomics (public included patients, providers, and the general public; also included the development of measures to assess public understanding), (ii) genetics and genomics risk communication (including comparisons of risk communication strategies), (iii) adequate reach of genomics-informed interventions (e.g., extent to which a genomics application has reached target audiences, consideration of disparities in access to genetics-informed applications), (iv) intervention development and testing of interventions to promote behavior change (e.g., testing the potential for genomics to increase the potency of health behavior interventions or assessing the impact of genomic information on health behaviors), and (v) new behavioral targets informed by genomics and genetics (e.g., identification of behavioral phenotypes that underpin behavioral adherence, which are likely to have common genetic underpinnings).
Conducting the search
Methods for this literature review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis for Scoping Reviews guidelines and was facilitated by a professional research librarian (S.P.). We completed literature searches in Medline/PubMed, Embase, PsychINFO, Web of Science, and the Public Health Genomics and Precision Health Knowledge Base (PHGKB) database from the Centers for Disease Control and Prevention (CDC). The search took place on October 4, 2018. We searched databases from January 2012 (since the PHGKB began tracking articles related to genomics translation) to October 2018. Additional modifications and updates were made on March 3, 2020. Keywords included concepts related to genetics, genomics, precision medicine, intervention, translation, and implementation (see Supplementary Table 1 for the full set of search criteria). We also hand-searched bibliographies of relevant literature and previous systematic reviews to identify studies that were not captured in the initial database searches. All titles were imported into EndNote version X8 and Covidence was used to organize and manage the review process [19, 20].
Study selection
Inclusion criteria for studies were: English language, published between 2012 and October 2018, full text provided, and a focus on one of the five social and behavioral sciences translational areas. All study designs were included. We excluded studies that were editorials, abstracts, or posters. Only one article was selected per study. We also excluded studies focused on family health history (FHH; n = 66) for two reasons: (i) FHH is not a translational product of genome discovery (though there have been refinements in FHH based on genomic advances) and (ii) at least four systematic reviews on the topic have been published since 2012 [17, 18, 21–23]. We also excluded newborn screening (n = 24) studies from our review as there have been several prior reviews and we chose to focus on studies of adults [24–27].
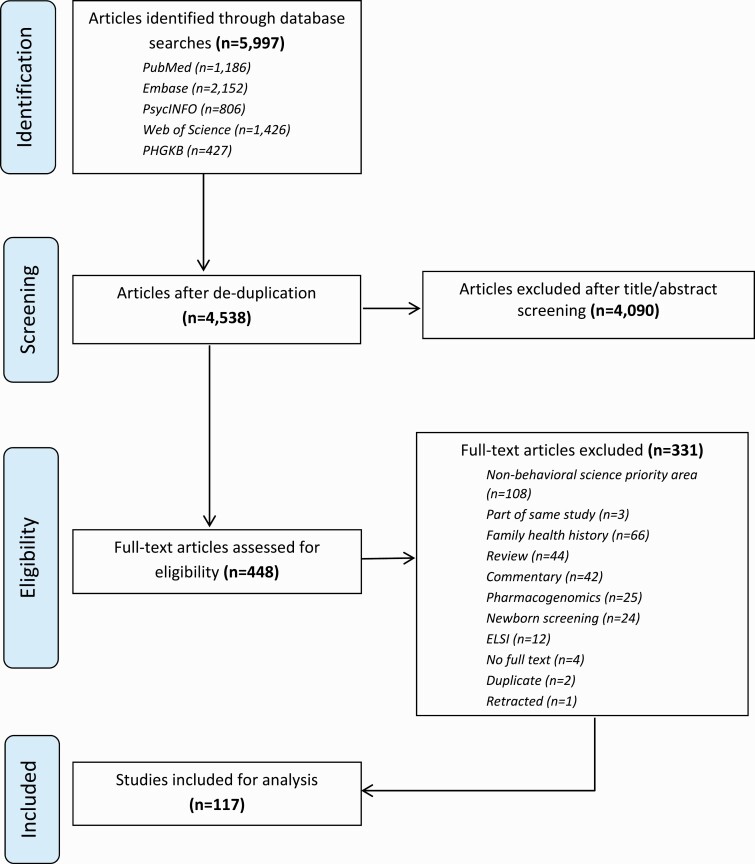
Our search resulted in 4,538 unique studies. Two reviewers (C.G.A. and S.P.) then conducted initial title and abstract screening to identify full studies that were assessed for eligibility. A total of 448 studies were assessed and 331 were removed during this process, resulting in 117 studies included in our final analysis (Fig. 1).
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis for Scoping Reviews flow diagram depicting the flow of information through phases of review process. Note: Articles that described the same study were removed at the full-text screening stage so that only one article per study was included in our final sample.
Data extraction
Data extraction took place using a Google Survey Form, where two authors (C.G.A. and S.P.) simultaneously extracted information from each of the 117 studies. We used elements of the PICOTS framework to extract information from each study: study aims or goals (I), sample size, study population, and participants (P), location (USA or international; S), study design (e.g., randomized control trial, cohort, case–control, cross-sectional, case report, and other), and outcomes (O). We also identified social and behavioral science priority areas (determined one priority area per study). If multiple social and behavioral science priority areas were possible (e.g., intervention development that focused on risk communication), the two primary coders (C.G.A. and S.P.) consulted with the study team to determine the primary priority area. The results of each study were also categorized into one of three tiers based on CDC’s public health genomics evidence tiers (Box 1). These applications included: Tier 1 (an evidence base that supports its implementation into practice), Tier 2 (an evidence base that is insufficient to support its use), or Tier 3 (an evidence base that is against or discourages its use). Other extraction elements included: funding sources (government, nongovernment, institutional, international, and none; see Supplementary Table 2 for all studies and details) [28].
Implications.
Practice: Practitioners can promote existing evidence-based translation efforts (e.g., Tier 1 genomics applications) in collaboration with social and behavioral researchers.
Policy: Policymakers can use social and behavioral approaches by aligning priorities with the translation continuum and five priority areas for translational genomics research.
Research: Social and behavioral scientists should engage in interdisciplinary research collaborations that consider the five priority areas for translational genomics research.
Box 1. Definitions of genomics evidence tiers.
Tier 1—Genomic applications that have a base of synthesized evidence that supports implementation in practice.
Tier 2—Genomic applications that have synthesized evidence that is insufficient to support their implementation in routine practice. Nevertheless, the evidence may be useful for informing selective use strategies (such as in clinical trials) through individual clinical, or public health policy, decision-making.
Tier 3—Applications either (i) have synthesized evidence that supports recommendations against or discourages use or (ii) no relevant synthesized evidence is available.
Data synthesis and analysis
After completing data extraction, we analyzed our results to identify trends in the pace of research (number of studies, CDC tiers, and funding mechanisms), descriptive information about study design and sample size, and description of translational priority areas, including the number of studies in each priority area and characteristics of these studies.
RESULTS
Pace of genomics translation research in behavioral and social sciences, tiers, and funding mechanisms
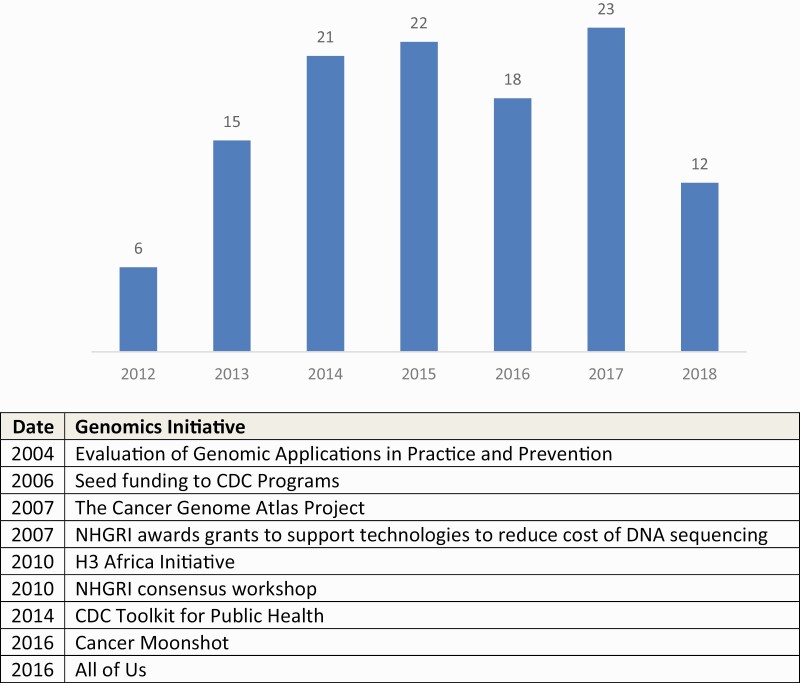
An average of 16.7 studies was published each year from 2012 to October 2018 (n = 117 studies). There was an upward trend in the number of studies during this time ranging from 6 (2012) to 23 (2017; Fig. 2).
Fig 2.
Annual trends in number of studies as they relate to key genomics initiatives. Key initiatives are included to highlight opportunities that may spark interest in genomics research among social and behavioral scientist. Note: 2018 includes studies through October 2018 search.
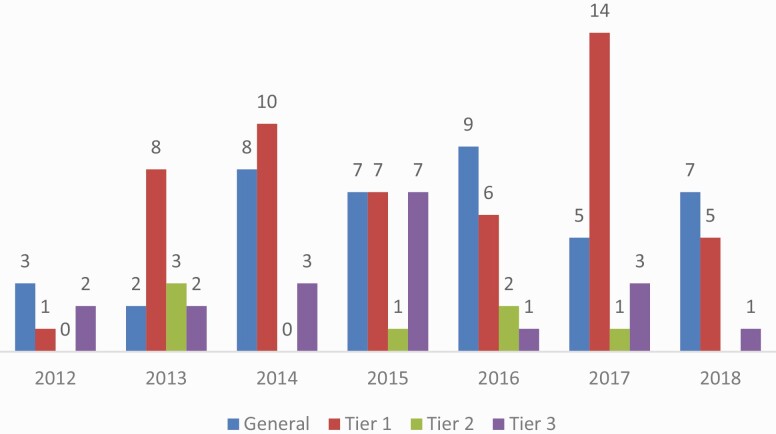
Nearly half of the studies (43.6%) presented evidence to support implementation into practice (Tier 1 applications; Table 1). Hereditary Breast and Ovarian Cancer (HBOC) accounted for 76% of Tier 1 applications. These studies largely involved assessing knowledge, beliefs, and understanding of HBOC-related issues (e.g., genetic testing, return of results, and sharing results with family members) among those tested with genes that predispose to HBOC. Fewer studies focused on genes related to colon cancer in Lynch syndrome (18%). These studies included models of education about Lynch syndrome and addressing clinic or organizational-level issues related to the implementation of Lynch syndrome programs. The number of studies about genetics in general (i.e., unrelated to a specific evidence-based genomics Tier or specific genetic condition or disease) also increased slightly over time, while studies addressing Tier 2 and Tier 3, tiers where evidence is insufficient or unavailable to support implementation, remained consistently low (Fig. 3).
Table 1.
Overview of sample
| N or mean | % or range SD | |
|---|---|---|
| Studies per yeara | 16.7 | |
| Sample size | 716.14 | 2,123.3 |
| Study design | ||
| Cross-sectional study | 47 | 40.17 |
| Qualitative | 29 | 24.79 |
| RCT | 21 | 17.95 |
| Cohort study | 8 | 6.84 |
| Mix methods | 8 | 6.84 |
| Case report | 3 | 2.56 |
| Case–control study | 1 | 0.85 |
| Priority area | ||
| Public understanding of genetics and genomics | 39 | 33.33 |
| Risk communication | 34 | 29.06 |
| Intervention development and testing | 23 | 19.66 |
| Adequate reach of intervention | 21 | 17.95 |
| New behavioral targets | 0 | 0 |
| Tiers | ||
| Tier 1 | 51 | 43.59 |
| General genetics | 40 | 34.19 |
| Tier 3 | 19 | 16.24 |
| Tier 2 | 7 | 5.98 |
| Located in USA | 82 | 70.09 |
| Funding type | ||
| Government | 57 | 48.72 |
| Not listed | 26 | 22.22 |
| International | 16 | 13.68 |
| Nongovernment | 15 | 12.82 |
| Institutional funding | 3 | 2.56 |
a2018 studies were only included through October 2018. Average number of studies per year was calculated based on seven full years, including all of 2018.
Fig 3.
Annual trends in number of studies based on evidence tiers. Note: 2018 includes studies through October 2018 search. “General” refers to articles that are unrelated to a specific evidence-based genomics tier-specific genetic condition or disease.
Nearly half of the studies (n = 57) were government funded, with the majority funded by the NHGRI (34.4%) and the National Cancer Institute (NCI; 31.2%). Studies funded by NHGRI included a wide range of topics, such as testing noninferiority trials to compare new strategies for the delivery of genetic test results [29–32] and disclosure of genetic information to family members [33–36]. Examples of NCI-funded studies included: sharing of cancer-genetic results with family members [37], assessing participant recruitment and retention [38, 39], and knowledge and beliefs about cancer genomics [40–42].
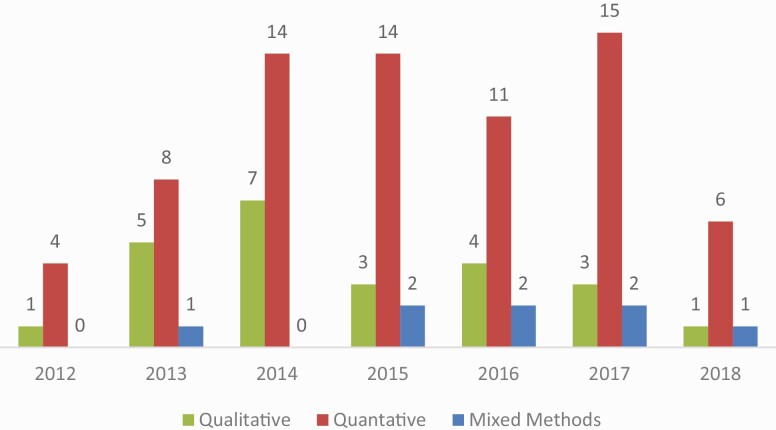
Study sample sizes and study designs
Of the 117 studies, most were descriptive cross-sectional surveys (40.2%; Fig. 4). These studies assessed: the public’s understanding of genetics and genomics (e.g., knowledge, beliefs, attitudes, and expectations) [41–51], provider’s understanding and motivations [52–58], and the impact on target audiences of receiving personalized genomic results [59].
Fig 4.
Annual trends in number of studies based on study design. Note: 2018 includes studies through October 2018 search.
Qualitative studies (24.8%) were less common across all years. Qualitative research topics were similar to those described in cross-sectional research; however, these studies included a diverse sample of key informants, including genetic counselors [60, 61], clinicians [62], lay individuals [63–82], and leadership from organizations of interest [83, 84].
Randomized trials (17.9%) involved the trials to compare different approaches to genetic counseling (e.g., web-based platform vs. standard care, examination of recruitment strategies for genetic counseling, and mode of delivery) [29, 30, 85–87] and assess the impact of genetic counseling on motivation and behaviors (e.g., for diabetes prevention behaviors, Alzheimer’s disease risk, and dietary intake) [88, 89, 90–92].
The average study sample size across all years and all studies was 716 (Standard Deviation 2,123). The average sample size increased from 510 participants in 2012 to 1,229 participants in 2017. Sample size was commonly determined by the data set being used for secondary data analysis (e.g., Health Information National Trends Survey, National Health and Nutrition Examination Survey, and National Health Interview Survey).
Engagement in translational priority areas
Of the five identified social and behavioral science priority areas, studies most commonly addressed public understanding of genetics and genomics (33.3%). Among studies that addressed public understanding, the majority were cross-sectional (64.1%) and focused on general genetics (51.3%; see Supplementary Table 3). This category had the largest average sample size (n = 1,353) of all focus areas. Public understanding studies largely were designed to assess the awareness and perceptions of genetics [43, 49, 51, 57, 66, 93] and demographic factors associated with understanding [42, 46, 47]. Others sought to characterize participation in genetics and genomics research [45, 53, 91] and assess the interpretation and comprehension of results [71]. Two studies focused on genetic literacy and psychometric properties of a genetic literacy tool [50, 92]. Finally, studies considered attitudes and knowledge of genetics among providers [52, 93], including the assessment of education and training needs [62, 94, 95].
The next most common focus area was risk communication (29.1%). Risk communication included both qualitative (29.4%) or cross-sectional (29.4%) methods. Risk communication studies were most likely to relate to Tier 1 applications (e.g., HBOC and Lynch syndrome; 58.8%). Studies commonly evaluated ways to improve risk communication through enhancing genetic counselor communication skills [60, 61], identifying the best format for delivering risk information (e.g., comparing genetic counselor to web platforms in delivery of risk information) [63, 80], and a better understanding of patient preferences for receiving risk information [96]. Numerous studies described risk communication patterns amongst affected individuals and their family members [33, 36, 37, 59, 66, 97–99], such as communication patterns that influenced the likelihood that family members would consider genetic testing [100]. Also, studies assessed the accuracy of recall of genetic risk among patients [101, 102] and ways to manage uncertainty about genetic test results [103, 104].
Behavior change intervention development and testing were the focus of 19.7% of studies. About half were randomized control trials (47.8%), mostly conducted in the USA (82.6%), and federally funded (73.9%). Most studies in this category were evaluating the provision of genetic information as the intervention. For example, studies provided personalized genetic information about Apolipoprotein E genes, diabetes, or single-nucleotide polymorphisms relating to common disease risk to assess whether disclosure of this information resulted in behavior changes, such as smoking cessation, cancer screening, and dietary intake [105, 106, 85, 87, 107, 108]. One study described an intervention to improve the uptake of genetic counseling and testing among high-risk breast cancer survivors [109] and another discussed the development of an educational intervention to improve the understanding of gene–environment interaction [65]. Interventions also included clinician decision support for familial hypercholesterolemia and to improve clinician’s confidence in incorporating genetics into practice [54].
Studies related to adequate reach (18%) were primarily qualitative (43%) and focused on Tier 1 applications (62%). Studies focusing on adequate reach had the smallest average sample size of all categories (sample size = 273). Often, studies in this category described facilitators and barriers to uptake of genetic counseling and testing among diverse populations. Most of these studies aimed to characterize reasons for underrepresentation of minority populations and how to better engage them in genetic counseling and testing [66, 69, 110, 111, 75, 112–114]. A few of the studies evaluated innovative recruitment strategies for hard to reach populations, including partnerships with advocacy and community-based organizations [81, 115]. Other studies evaluated informed consent processes, such as using different informed consent procedures to ensure understanding among participants [116, 74]. Lastly, studies characterized systems-level barriers to achieving adequate reach and implementation of genomics interventions [117, 82, 118]. Regarding the final social and behavioral priority area, we found no studies that were categorized as new behavioral intervention targets.
DISCUSSION
With this scoping review, we sought to describe translational research and its concordance with priorities outlined by social and behavioral scientists since 2012. The number of studies published marks slow advancement in research related to genomics discovery translation. On average, 16.7 studies were published each year as compared to approximately 97,000 basic science genomic studies published in the same time period. This slow pace of translational research in behavioral and social sciences has been noted in other fields. For example, a systematic review of grants in diabetes research identified 8% of the portfolio as focused on psychosocial or social science [119]. The authors of the review noted that 8% was very low, despite being substantially higher than social and behavioral science contributions to genomics translation. These results suggest the critical need to continue building evidence in the five key priority areas where social and behavioral researchers can be important interdisciplinary partners.
One of the challenges underpinning slow translation is the inability to galvanize social and behavioral scientists around research priorities for genomic translation. Previous systematic focus group discussions with social and behavioral scientist members of the Society of Behavioral Medicine (SBM) lend useful insights. For example, SBM participants indicated that they have been hesitant to engage in genomics translational research due to a lack of understanding of what is considered “translation ready.” [120] These participants also were largely unaware of evidence-based ratings on genomic applications. Additional concerns were related to social and behavioral scientists’ inability to find collaborators with genetics expertise willing to engage in translation research. Efforts to address these challenges, such as supportive collaborative networks to inform and engage social and behavioral scientists, could accelerate genomics translation research.
Drolet and Lorenzi describe a biomedical research translation (BRT) model that also could be useful for guiding social and behavioral scientists in considering translation research gaps [121]. The BRT continuum follows knowledge translation research from bench to human application with bridges and the potential for “chasms” on the way to clinical application, established clinical practice, and public health. The BRT holds that evidence needed to move scientific discoveries into practice and population health benefits differ across this translation continuum. Much of the social and behavioral translation research since 2012 has focused on the CDC’s Tier 1 genomic applications, that is, those with an evidence base to support clinical and public health deployment. These research needs present relatively late in the translation continuum.
Translation research related to Tier 1 applications have been focused on two priority research areas, public understanding and risk communication [7, 11–14]. These studies used descriptive methodologies—most commonly cross-sectional and qualitative methods—to identify differences in knowledge and beliefs about genomics, preferences for delivery of results, and provider-level facilitators and barriers to implementation. The studies also had considerable overlap in the types of research questions addressed. Drolet and Lorenzi’s framework suggests that fostering efficient translation to clinical practice and public health impact will require systematic research on implementation and adaptation. Thus, the limited research related to the implementation of established Tier 1 applications is surprising and represents an important chasm in the translation continuum [121].
Drolet and Lorenzi’s BRT model suggests that translation research related to Tier 2 (applications not ready for routine implementation but potentially useful in decision-making, n = 7 studies) and Tier 3 applications (applications that are unsuitable for implementation or have no evidence for implementation, n = 19 studies) would give priority to determining the safety and efficacy of these applications (i.e., clinical trials). Currently, behavioral and social scientists’ involvement in Tier 2 and Tier 3 applications largely consider public understanding (e.g., attitudes and awareness about genetic testing for certain diseases and direct to consumer testing) and risk communication about results. Going forward, accelerating potential translation will require research that examines promising applications with respect to whether they are efficacious compared to standard practice in reducing risk or improving health outcomes. Considering the specific research needs concordant with the translation continuum in the identified priority areas could reduce the likelihood of chasms occurring at multiple points in the genomic discovery translation continuum.
Lastly, we note that social and behavioral scientists’ contributions to basic science discovery are largely lacking. For example, we identified no progress in the priority area “new behavioral targets informed by genomics and genetics.” As previously suggested, research exploring the commonalities across behaviors and possibility of adherence phenotypes that may have genetic underpinnings is nascent [14]. Similarly, genetic discovery contributions to potential biomarkers of moderating and mediating mechanisms targeted by interventions and indicators of intervention benefit have not been explored [14]. Research in this area could produce more specific and sensitive information about how social and behavioral interventions operate and guide new approaches to tailoring.
Some large-scale and coordinated efforts to advance translational genomics research are currently underway, including the National Human Genome Research Institute’s Electronic Medical Records (eMRGE) network [122], Implementing Genomics in Practice (IGNITE) Network [123], National Institutes of Health (NIH) All of Us Research Program [124], and National Cancer Institute’s Cancer Moonshot [125] to accelerate cancer research. The CDC’s Office of Genomics and Precision Public Health also actively works to translate genetics to the public [126, 127]. Each of these initiatives offer great opportunities to accelerate the pace of social and behavioral scientists’ involvement in translational genomics research.
Limitations
There are limitations to consider in the current scoping review. First, we did not score studies to report the quality of evidence [128]. As the number of studies increases, future systematic reviews will be needed to characterize the rigor of studies (e.g., use of validated measures and whether studies are appropriately powered) and include more descriptive demographic information about the populations targeted by these studies. Second, we selected the year 2012 as the start of our review timeframe, which was the beginning of the PHGKB tracking system, and, 2 years after, a large NHGRI consensus report was released delineating priority areas for social and behavioral scientist in genomics [14]. The PHGKB was the main source of identifying studies, along with other databases, including PubMed, Embase, and Web of Science. We deemed 2012 the year to begin our search as it provided over 8 years since the initial calls for social and behavioral scientists to be involved with genomic translation (beginning in 2005) and over 10 years since the completion of the Human Genome Project. However, it is possible that our search missed relevant studies that were published prior to 2012. Third, we focused on published literature, likely missing some efforts that were reported only in gray literature (e.g., reports, nonpeer reviewed). Given our focus on research, this was an appropriate exclusion criterion; however, some reports or efforts that may have taken place (e.g., Tier 1 applications in state health departments) were likely missed. Furthermore, we excluded some common genetic translation applications, such as newborn screening and family health history, as prior reviews have focused on these applications; this eliminated 90 studies. Given our objective to generally characterize the field of ongoing research, our sampling unit was study and not manuscript. Only one article was included per study. For this reason, comparisons of the number of publications for discovery and translation are not entirely congruent. However, the inclusion of multiple manuscripts per study would not have greatly shifted the discovery–translation ratio. Finally, we restricted our search criteria to English only and may have missed additional translation research.
CONCLUSION
In the realm of biomedical research, the vast majority of genomic studies continue to focus on discovering genes and related mechanisms that impact disease. As these findings accumulate, it becomes increasingly important that emphasis is placed on building an evidence base to guide the translation of this knowledge to achieve population health impact. Our review suggests that this effort is moving slowly. Indeed, the pace of translational research in behavioral and social sciences raises the concern that genomic applications will not reach populations that could benefit from them, could exacerbate health disparities, and undermine the potential of precision public health and medicine [129]. Ongoing large-scale NIH-funded programs offer the chance to expand upon and accelerate research in five priority areas that have been suggested consistently to advance translation impact (public understanding, risk communication, adequate reach of interventions, intervention development and testing of interventions to promote behavior change, and new behavioral targets). Intensified effort is needed to anticipate and identify genomics translation chasms related to these five suggested areas where social and behavioral science contributions are critical.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding: None to report
Compliance with Ethical Standards
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Authors’ Contributions: All authors contributed to conceptualization and design of systematic review. C.G.A. and S.P. performed the review in consultation with M.J.K., L.C.B., and C.M.M. C.G.A. and C.M.M. oversaw writing of the manuscript. All authors contributed to editing and approving the final manuscript.
Ethical Approval: This article does not contain any studies with human participants performed by any of the authors. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: This study does not involve human participants and informed consent was, therefore, not required.
Supplementary Material
References
- 1. Caulfield T, Evans J, McGuire A, et al. Reflections on the cost of “low-cost” whole genome sequencing: Framing the health policy debate. PLoS Biol. 2013;11(11):e1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Human Genome Research Institute. The cost of sequencing a human genome. 2019; Available at https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Accessibility verified November 1, 2018.
- 3. Roberts MC, Clyne M, Kennedy AE, Chambers DA, Khoury MJ. The current state of funded NIH grants in implementation science in genomic medicine: A portfolio analysis. Genet Med. 2019;21(5):1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Best A, Hiatt RA, Norman CD. Knowledge integration: Conceptualizing communications in cancer control systems. Patient Educ Couns. 2008;71(3):319–327. [DOI] [PubMed] [Google Scholar]
- 5. Leavitt M. Medscape’s response to the institute of medicine report: Crossing the quality chasm: a new health system for the 21st century. MedGenMed. 2001;3(2):2. [PubMed] [Google Scholar]
- 6. Chambers DA. Commentary: Increasing the connectivity between implementation science and public health: advancing methodology, evidence integration, and sustainability. Annu Rev Public Health. 2018;39:1–4. [DOI] [PubMed] [Google Scholar]
- 7. Khoury MJ, Clauser SB, Freedman AN, et al. Population sciences, translational research, and the opportunities and challenges for genomics to reduce the burden of cancer in the 21st century. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaphingst KA, Ivanovich J, Lyons S, et al. Preferences for learning different types of genome sequencing results among young breast cancer patients: Role of psychological and clinical factors. Transl Behav Med. 2018;8(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turbitt E, Biesecker BB. A primer in genomics for social and behavioral investigators. Transl Behav Med. 2020;10(2):451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McBride CM. Translation research to apply genomics to improve health promotion: Is it worth the investment? Transl Behav Med. 2018;8(1):54–58. [DOI] [PubMed] [Google Scholar]
- 11. Wang C, Bowen DJ, Kardia SL. Research and practice opportunities at the intersection of health education, health behavior, and genomics. Health Educ Behav. 2005;32(5):686–701. [DOI] [PubMed] [Google Scholar]
- 12. McBride CM, Bryan AD, Bray MS, Swan GE, Green ED. Health behavior change: Can genomics improve behavioral adherence? Am J Public Health. 2012;102(3):401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McBride CM, Abrams LR, Koehly LM. Using a historical lens to envision the next generation of genomic translation research. Public Health Genomics. 2015;18(5):272–282. [DOI] [PubMed] [Google Scholar]
- 14. McBride CM, Bowen D, Brody LC, et al. Future health applications of genomics: Priorities for communication, behavioral, and social sciences research. Am J Prev Med. 2010;38(5):556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Academies Press. Roundtable on genomics and precision health: Reports and resources.2020; Available at https://www.nap.edu/initiative/roundtable-on-genomics-and-precision-health. Accessibility verified November 1, 2018.
- 16. Overby CL A Clinical Decision Support Model for Incorporating Pharmacogenomics Knowledge Into Electronic Health Records for Drug Therapy Individualization: A Microcosm of Personalized Medicine. Ann Arbor, MI: ProQuest Information & Learning; 2013. [Google Scholar]
- 17. Paalosalo-Harris K, Skirton H. Mixed method systematic review: The relationship between breast cancer risk perception and health-protective behaviour in women with family history of breast cancer. J Adv Nurs. 2017;73(4):760–774. [DOI] [PubMed] [Google Scholar]
- 18. Ait Ouakrim D, Boussioutas A, Lockett T, et al. Screening practices of unaffected people at familial risk of colorectal cancer. Cancer Prev Res (Phila). 2012;5(2):240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endnote, X8 E.2018; Available at https://endnote.com/. Accessibility verified November 1, 2018.
- 20. Covidence. 2020; Available at https://www.covidence.org/reviews/active. Accessibility verified November 1, 2018.
- 21. Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of genetic testing by relatives of lynch syndrome probands: A systematic review. Clin Gastroenterol Hepatol. 2013;11(9):1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quinn GP, Pal T, Murphy D, Vadaparampil ST, Kumar A. High-risk consumers’ perceptions of preimplantation genetic diagnosis for hereditary cancers: A systematic review and meta-analysis. Genet Med. 2012;14(2):191–200. [DOI] [PubMed] [Google Scholar]
- 23. Wilson BJ, Qureshi N, Santaguida P, et al. Systematic review: Family history in risk assessment for common diseases. Ann Intern Med. 2009;151(12):878–885. [DOI] [PubMed] [Google Scholar]
- 24. Goldsmith L, Jackson L, O’Connor A, Skirton H. Direct-to-consumer genomic testing: Systematic review of the literature on user perspectives. Eur J Hum Genet. 2012;20(8):811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanji A, Khoza-Shangase K, Moroe N. Newborn hearing screening protocols and their outcomes: A systematic review. Int J Pediatr Otorhinolaryngol. 2018;115:104–109. [DOI] [PubMed] [Google Scholar]
- 26. Kayton A. Newborn screening: A literature review. Neonatal Netw. 2007;26(2):85–95. [DOI] [PubMed] [Google Scholar]
- 27. Taylor-Phillips S, Stinton C, Ferrante di Ruffano L, Seedat F, Clarke A, Deeks JJ. Association between use of systematic reviews and national policy recommendations on screening newborn babies for rare diseases: Systematic review and meta-analysis. BMJ. 2018;361:k1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dotson WD, Douglas MP, Kolor K, et al. Prioritizing genomic applications for action by level of evidence: A horizon-scanning method. Clin Pharmacol Ther. 2014;95(4):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sweet K, Gordon ES, Sturm AC, et al. Design and implementation of a randomized controlled trial of genomic counseling for patients with chronic disease. J Pers Med. 2014;4(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sweet K, Sturm AC, Schmidlen T, et al. Outcomes of a randomized controlled trial of genomic counseling for patients receiving personalized and actionable complex disease reports. J Genet Couns. 2017;26(5):980–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biesecker BB, Klein W, Lewis KL, et al. How do research participants perceive “uncertainty” in genome sequencing? Genet Med. 2014;16(12):977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hooker GW, Peay H, Erby L, Bayless T, Biesecker BB, Roter DL. Genetic literacy and patient perceptions of IBD testing utility and disease control: A randomized vignette study of genetic testing. Inflamm Bowel Dis. 2014;20(5):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharff ME, DeMarco TA, Mays D, et al. Parenting through genetic uncertainty: Themes in the disclosure of breast cancer risk information to children. Genet Test Mol Biomarkers. 2012;16(5):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farkas Patenaude A, DeMarco TA, Peshkin BN, et al. Talking to children about maternal BRCA1/2 genetic test results: A qualitative study of parental perceptions and advice. J Genet Couns. 2013;22(3):303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamilton JG, Mays D, DeMarco T, Tercyak KP. Modeling the dyadic effects of parenting, stress, and coping on parent-child communication in families tested for hereditary breast-ovarian cancer risk. Fam Cancer. 2016;15(4):513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenberg M, Smith RA. Support seeking or familial obligation: An investigation of motives for disclosing genetic test results. Health Commun. 2016;31(6):668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chopra I, Kelly KM. Cancer risk information sharing: The experience of individuals receiving genetic counseling for BRCA1/2 mutations. J Health Commun. 2017;22(2):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ewing A, Thompson N, Ricks-Santi L. Strategies for enrollment of African Americans into cancer genetic studies. J Cancer Educ. 2015;30(1):108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Condit CM, Shen L, Edwards KL, Bowen DJ, Korngiebel DM, Johnson CO. Participants’ role expectations in genetics research and re-consent: Revising the theory and methods of mental models research relating to roles. J Health Commun. 2016;21(suppl 2):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glanz K, Volpicelli K, Kanetsky PA, et al. Melanoma genetic testing, counseling, and adherence to skin cancer prevention and detection behaviors. Cancer Epidemiol Biomarkers Prev. 2013;22(4):607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen AB, Oh A, Moser RP, Patrick H. Perceptions of the roles of behaviour and genetics in disease risk: Are they associated with behaviour change attempts. Psychol Health. 2015;30(3):336–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hurtado-de-Mendoza A, Jackson MC, Anderson L, Sheppard VB. The role of knowledge on genetic counseling and testing in black cancer survivors at increased risk of carrying a BRCA1/2 mutation. J Genet Couns. 2017;26(1):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waters EA, Muff J, Hamilton JG. Multifactorial beliefs about the role of genetics and behavior in common health conditions: Prevalence and associations with participant characteristics and engagement in health behaviors. Genet Med. 2014;16(12):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ready K, Haque IS, Srinivasan BS, Marshall JR. Knowledge and attitudes regarding expanded genetic carrier screening among women’s healthcare providers. Fertil Steril. 2012;97(2):407–413. [DOI] [PubMed] [Google Scholar]
- 45. McVeigh TP, Sweeney KJ, Kerin MJ, Gallagher DJ. A qualitative analysis of the attitudes of Irish patients towards participation in genetic-based research. Ir J Med Sci. 2016;185(4):825–831. [DOI] [PubMed] [Google Scholar]
- 46. Maio M, Carrion P, Yaremco E, Austin JC. Awareness of genetic counseling and perceptions of its purpose: A survey of the Canadian public. J Genet Couns. 2013;22(6):762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krakow M, Ratcliff CL, Hesse BW, Greenberg-Worisek AJ. Assessing genetic literacy awareness and knowledge gaps in the us population: Results from the health information national trends survey. Public Health Genomics. 2017;20(6):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kammin T, Fenton AK, Thirlaway K. A genetic lung cancer susceptibility test may have a positive effect on smoking cessation. J Genet Couns. 2015;24(3):522–531. [DOI] [PubMed] [Google Scholar]
- 49. Etchegary H, Green J, Parfrey P, Street C, Pullman D. Community engagement with genetics: Public perceptions and expectations about genetics research. Health Expect. 2015;18(5):1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abrams LR, McBride CM, Hooker GW, Cappella JN, Koehly LM. The many facets of genetic literacy: Assessing the scalability of multiple measures for broad use in survey research. Plos One. 2015;10(10):e0141532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bian J, Zhao Y, Salloum RG, et al. Using social media data to understand the impact of promotional information on laypeople’s discussions: A case study of lynch syndrome. J Med Internet Res. 2017;19(12):e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Teng I, Spigelman A. Attitudes and knowledge of medical practitioners to hereditary cancer clinics and cancer genetic testing. Fam Cancer. 2014;13(2):311–324. [DOI] [PubMed] [Google Scholar]
- 53. Tan YY, Spurdle AB, Obermair A. Knowledge, attitudes and referral patterns of lynch syndrome: A survey of clinicians in australia. J Pers Med. 2014;4(2):218–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reed EK, Johansen Taber KA, Ingram Nissen T, et al. What works in genomics education: Outcomes of an evidenced-based instructional model for community-based physicians. Genet Med. 2016;18(7):737–745. [DOI] [PubMed] [Google Scholar]
- 55. Hauser D, Obeng AO, Fei K, Ramos MA, Horowitz CR. Views of primary care providers on testing patients for genetic risks for common chronic diseases. Health Aff (Millwood). 2018;37(5):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hasnie AA, Kumbamu A, Safarova MS, Caraballo PJ, Kullo IJ. A clinical decision support tool for familial hypercholesterolemia based on physician input. Mayo Clin Proc Innov Qual Outcomes. 2018;2(2):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Evenson SA, Hoyme HE, Haugen-Rogers JE, Larson EA, Puumala SE. Patient and physician perceptions of genetic testing in primary care. S D Med. 2016;69(11):487–493. [PubMed] [Google Scholar]
- 58. Batais MA, Almigbal TH, Bin Abdulhak AA, Altaradi HB, AlHabib KF. Assessment of physicians’ awareness and knowledge of familial hypercholesterolemia in Saudi Arabia: Is there a gap? Plos One. 2017;12(8):e0183494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smit AK, Keogh LA, Newson AJ, et al. Does personalized melanoma genomic risk information trigger conversations about skin cancer prevention and skin examination with family, friends and health professionals? Br J Dermatol. 2017;177(3):779–790. [DOI] [PubMed] [Google Scholar]
- 60. Aasen T, Skolbekken JA. Preparing for and communicating uncertainty in cancer genetic counselling sessions in Norway: An interpretative phenomenological analysis. Health Risk Soc. 2014;16(4):370–389. [Google Scholar]
- 61. Cheng JKY, Guerra C, Pasick RJ, Schillinger D, Luce J, Joseph G. Cancer genetic counseling communication with low-income Chinese immigrants. J Community Genet. 2018;9(3):263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carroll JC, Makuwaza T, Manca DP, et al. Primary care providers’ experiences with and perceptions of personalized genomic medicine. Can Fam Physician. 2016;62(10):e626–e635. [PMC free article] [PubMed] [Google Scholar]
- 63. Smit AK, Keogh LA, Hersch J, et al. Public preferences for communicating personal genomic risk information: A focus group study. Health Expect. 2016;19(6):1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Adams I, Christopher J, Williams KP, Sheppard VB. What black women know and want to know about counseling and testing for BRCA1/2. J Cancer Educ. 2015;30(2):344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Allen CG, McBride CM, Engdawork K, Ayode D, Tadele G. Applying mental model methods to characterize understanding of gene-environment influences: The case of podoconiosis in Ethiopia. Crit Public Health. 2019;29(1):84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bruwer Z, Futter M, Ramesar R. Communicating cancer risk within an African context: Experiences, disclosure patterns and uptake rates following genetic testing for Lynch syndrome. Patient Educ Couns. 2013;92(1):53–60. [DOI] [PubMed] [Google Scholar]
- 67. Chen E, Miller GE. Socioeconomic status and health: Mediating and moderating factors. Annu Rev Clin Psychol. 2013;9:723–749. [DOI] [PubMed] [Google Scholar]
- 68. Dean M, Davidson LG. Previvors’ uncertainty management strategies for hereditary breast and ovarian cancer. Health Commun. 2018;33(2):122–130. [DOI] [PubMed] [Google Scholar]
- 69. Gleeson M, Meiser B, Barlow-Stewart K, et al. Communication and information needs of women diagnosed with ovarian cancer regarding treatment-focused genetic testing. Oncol Nurs Forum. 2013;40(3):275–283. [DOI] [PubMed] [Google Scholar]
- 70. Godino L, Jackson L, Turchetti D, Hennessy C, Skirton H. Decision making and experiences of young adults undergoing presymptomatic genetic testing for familial cancer: A longitudinal grounded theory study. Eur J Hum Genet. 2018;26(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA. “It’s not like judgment day”: Public understanding of and reactions to personalized genomic risk information. J Genet Couns. 2012;21(3):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nishigaki M, Tokunaga-Nakawatase Y, Nishida J, Kazuma K. The effect of genetic counseling for adult offspring of patients with type 2 diabetes on attitudes toward diabetes and its heredity: A randomized controlled trial. J Genet Couns. 2014;23(5):762–769. [DOI] [PubMed] [Google Scholar]
- 73. Sheppard VB, Graves KD, Christopher J, Hurtado-de-Mendoza A, Talley C, Williams KP. African American women’s limited knowledge and experiences with genetic counseling for hereditary breast cancer. J Genet Couns. 2014;23(3):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Skinner HG, Calancie L, Vu MB, et al. Using community-based participatory research principles to develop more understandable recruitment and informed consent documents in genomic research. Plos One. 2015;10(5):e0125466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sussner KM, Edwards T, Villagra C, et al. BRCA genetic counseling among at-risk Latinas in New York City: New beliefs shape new generation. J Genet Couns. 2015;24(1):134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sussner KM, Jandorf L, Thompson HS, Valdimarsdottir HB. Barriers and facilitators to BRCA genetic counseling among at-risk Latinas in New York City. Psychooncology. 2013;22(7):1594–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Underhill ML, Crotser CB. Seeking balance: Decision support needs of women without cancer and a deleterious BRCA1 or BRCA2 mutation. J Genet Couns. 2014;23(3):350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vadaparampil ST, Nam K, Malo T, et al. Development of a multimedia psychoeducational intervention to increase uptake of BRCA genetic counseling among high risk breast cancer survivors. Psycho-oncology. 2014;23:74–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Miller FA, Hayeems RZ, Bytautas JP, et al. Testing personalized medicine: Patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. Eur J Hum Genet. 2014;22(3):391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Biesecker BB, Lewis KL, Umstead KL, et al. Web platform vs in-person genetic counselor for return of carrier results from exome sequencing: A randomized clinical trial. JAMA Intern Med. 2018;178(3):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bressler T, Popp B. Orthodox Jewish thought leaders’ insights regarding BRCA mutations: A descriptive study. J Oncol Pract. 2017;13(4):e303–e309. [DOI] [PubMed] [Google Scholar]
- 82. Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16(3):238–245. [DOI] [PubMed] [Google Scholar]
- 83. Adam S, Birch PH, Coe RR, et al. Assessing an interactive online tool to support parents’ genomic testing decisions. J Genet Couns. 2018. [DOI] [PubMed] [Google Scholar]
- 84. Haga SB, Barry WT, Mills R, et al. Impact of delivery models on understanding genomic risk for type 2 diabetes. Public Health Genomics. 2014;17(2):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grant RW, O’Brien KE, Waxler JL, et al. Personalized genetic risk counseling to motivate diabetes prevention: A randomized trial. Diabetes Care. 2013;36(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mills R, Powell J, Barry W, Haga SB. Information-seeking and sharing behavior following genomic testing for diabetes risk. J Genet Couns. 2015;24(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ahn WK, Lebowitz MS. An experiment assessing effects of personalized feedback about genetic susceptibility to obesity on attitudes towards diet and exercise. Appetite. 2018;120:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Glanz K, Volpicelli K, Jepson C, Ming ME, Schuchter LM, Armstrong K. Effects of tailored risk communications for skin cancer prevention and detection: The PennSCAPE randomized trial. Cancer Epidemiol Biomarkers Prev. 2015;24(2):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nielsen DE, El-Sohemy A. A randomized trial of genetic information for personalized nutrition. Genes Nutr. 2012;7(4):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harding B, Egan R, Kannu P, MacKenzie JJ. Parents’ understanding of genetics and heritability. J Genet Couns. 2017;26(3):541–547. [DOI] [PubMed] [Google Scholar]
- 91. Schmidlen TJ, Scheinfeldt L, Zhaoyang R, et al. Genetic knowledge among participants in the coriell personalized medicine collaborative. J Genet Couns. 2016;25(2):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bouhnik AD, N’Diaye K, Evans DG, et al. Validation of a scale for assessing attitudes towards outcomes of genetic cancer testing among primary care providers and breast specialists. Plos One. 2017;12(6):e0178447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Birmingham WC, Agarwal N, Kohlmann W, et al. Patient and provider attitudes toward genomic testing for prostate cancer susceptibility: A mixed method study. BMC Health Serv Res. 2013;13:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen LS, Kim M. Needs assessment in genomic education: A survey of health educators in the United States. Health Promot Pract. 2014;15(4):592–598. [DOI] [PubMed] [Google Scholar]
- 95. Chen LS, Zhao S, Stelzig D, et al. Development and evaluation of a genomics training program for community health workers in Texas. Genet Med. 2018;20(9):1030–1037. [DOI] [PubMed] [Google Scholar]
- 96. Flores KG, Steffen LE, McLouth CJ, et al. Factors associated with interest in gene-panel testing and risk communication preferences in women from BRCA1/2 negative families. J Genet Couns. 2017;26(3):480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dilzell K, Kingham K, Ormond K, Ladabaum U. Evaluating the utilization of educational materials in communicating about Lynch syndrome to at-risk relatives. Fam Cancer. 2014;13(3):381–389. [DOI] [PubMed] [Google Scholar]
- 98. Katapodi MC, Northouse LL, Milliron KJ, Liu G, Merajver SD. Individual and family characteristics associated with BRCA1/2 genetic testing in high-risk families. Psychooncology. 2013;22(6):1336–1343. [DOI] [PubMed] [Google Scholar]
- 99. Patenaude AF, Tung N, Ryan PD, et al. Young adult daughters of BRCA1/2 positive mothers: What do they know about hereditary cancer and how much do they worry? Psychooncology. 2013;22(9):2024–2031. [DOI] [PubMed] [Google Scholar]
- 100. Anderson AE, Flores KG, Boonyasiriwat W, et al. Interest and informational preferences regarding genomic testing for modest increases in colorectal cancer risk. Public Health Genomics. 2014;17(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Besser AG, Sanderson SC, Roberts JS, et al. Factors affecting recall of different types of personal genetic information about Alzheimer’s disease risk: The REVEAL study. Public Health Genomics. 2015;18(2):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jacobs C, Dancyger C, Smith JA, Michie S. Accuracy of recall of information about a cancer-predisposing BRCA1/2 gene mutation among patients and relatives. Eur J Hum Genet. 2015;23(2):147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dean M. Celebrity Health Announcements and online health information seeking: An analysis of Angelina Jolie’s preventative health decision. Health Commun. 2016;31(6):752–761. [DOI] [PubMed] [Google Scholar]
- 104. Fisher CL, Roccotagliata T, Rising CJ, Kissane DW, Glogowski EA, Bylund CL. “I Don’t Want to Be an Ostrich”: Managing mothers’ uncertainty during BRCA1/2 genetic counseling. J Genet Couns. 2017;26(3):455–468. [DOI] [PubMed] [Google Scholar]
- 105. Christensen KD, Roberts JS, Whitehouse PJ, et al. ; REVEAL Study Group . Disclosing pleiotropic effects during genetic risk assessment for alzheimer disease: a randomized trial. Ann Intern Med. 2016;164(3):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: A randomized controlled trial. Plos One. 2014;9(11):e112665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vassy JL, O’Brien KE, Waxler JL, et al. Impact of literacy and numeracy on motivation for behavior change after diabetes genetic risk testing. Med Decis Making. 2012;32(4):606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Graves KD, Leventhal KG, Nusbaum R, et al. Behavioral and psychosocial responses to genomic testing for colorectal cancer risk. Genomics. 2013;102(2):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vadaparampil ST, Malo TL, Nam KM, Nelson A, de la Cruz CZ, Quinn GP. From observation to intervention: Development of a psychoeducational intervention to increase uptake of BRCA genetic counseling among high-risk breast cancer survivors. J Cancer Educ. 2014;29(4):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rajpal N, Muñoz J, Peshkin BN, Graves KD. Insights into BRCA1/2 genetic counseling from ethnically diverse Latina breast cancer survivors. J Genet Couns. 2017;26(6):1221–1237. [DOI] [PubMed] [Google Scholar]
- 111. Shaw J, Bulsara C, Cohen PA, et al. Investigating barriers to genetic counseling and germline mutation testing in women with suspected hereditary breast and ovarian cancer syndrome and Lynch syndrome. Patient Educ Couns. 2018;101(5):938–944. [DOI] [PubMed] [Google Scholar]
- 112. Fogel AL, Jaju PD, Li S, Halpern-Felsher B, Tang JY, Sarin KY. Factors influencing and modifying the decision to pursue genetic testing for skin cancer risk. J Am Acad Dermatol. 2017;76(5):829–835.e1. [DOI] [PubMed] [Google Scholar]
- 113. Jones T, Lockhart JS, Mendelsohn-Victor KE, et al. Use of cancer genetics services in African-American young breast cancer survivors. Am J Prev Med. 2016;51(4):427–436. [DOI] [PubMed] [Google Scholar]
- 114. Hafertepen L, Pastorino A, Morman N, et al. Barriers to genetic testing in newly diagnosed breast cancer patients: Do surgeons limit testing? Am J Surg. 2017;214(1):105–110. [DOI] [PubMed] [Google Scholar]
- 115. Burton-Chase AM, Parker WM, Hennig K, Sisson F, Bruzzone LL. The use of social media to recruit participants with rare conditions: Lynch syndrome as an example. JMIR Res Protoc. 2017;6(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Shelton AK, Freeman BD, Fish AF, Bachman JA, Richardson LI. A computer-based education intervention to enhance surrogates’ informed consent for genomics research. Am J Crit Care. 2015;24(2):148–155. [DOI] [PubMed] [Google Scholar]
- 117. Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: Experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genomics. 2017;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schneider JL, Davis J, Kauffman TL, et al. Stakeholder perspectives on implementing a universal Lynch syndrome screening program: A qualitative study of early barriers and facilitators. Genet Med. 2016;18(2):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jones A, Vallis M, Cooke D, Pouwer F. Review of research grant allocation to psychosocial studies in diabetes research. Diabet Med. 2016;33(12):1673–1676. [DOI] [PubMed] [Google Scholar]
- 120. McBride CM, Graves KD, Kaphingst KA, et al. Behavioral and social scientists’ reflections of genomics: A systematic evaluation within the Society of Behavioral Medicine. Available at: https://pubmed.ncbi.nlm.nih.gov/30950497/ [DOI] [PMC free article] [PubMed]
- 121. Drolet BC, Lorenzi NM. Translational research: Understanding the continuum from bench to bedside. Transl Res. 2011;157(1):1–5. [DOI] [PubMed] [Google Scholar]
- 122. National Human Genome Research Institute. Electronic Medical Records and Genomics (eMERGE) Network. 2020. Available at: https://emerge-network.org/ [Google Scholar]
- 123. National Human Genome Research Institute. Implementing genomics in practice (IGNITE) II: Pragmatic Clinical Trial Network. 2020; Available at https://www.genome.gov/Funded-Programs-Projects/Implementing-Genomics-in-Practice-IGNITE-2-Pragmatic-Clinical-Trials-Network. Accessibility verified November 1, 2018.
- 124. National Instututes of Health. All of Us Research Program. 2018; Available at https://allofus.nih.gov/. Accessibility verified November 1, 2018.
- 125. National Cancer Institute. Cancer Moonshot. 2020. Available at: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative. Accessibility verified November 1, 2018. [Google Scholar]
- 126. Green RF, Ari M, Kolor K, et al. Evaluating the role of public health in implementation of genomics-related recommendations: A case study of hereditary cancers using the CDC Science Impact Framework. Genet Med. 2019;21(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Khoury MJ, Bowen MS, Clyne M, et al. From public health genomics to precision public health: A 20-year journey. Genet Med. 2018;20(6):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Roberts MC, Mensah GA, Khoury MJ. Leveraging implementation science to address health disparities in genomic medicine: Examples from the field. Ethn Dis. 2019;29(suppl 1):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.