Prototheca species are unicellular, achlorophyllic algae that are ubiquitously distributed in nature [1]. Human protothecosis typically involves the cutaneous and subcutaneous tissues, and rarely causes systemic disease [2]. We report here a case of chronic meningitis due to Prototheca zopfii in an immunocompetent adolescent girl.
The patient is an Amish girl who was in her usual state of health until the age of 13 (in 2014) when she developed increasing fatigue. In the fall of 2015, she developed a shuffling gait and had trouble bending over. In 2016 she started to complain of headaches and had a computed tomographic scan of her head at an outside institution, which was reportedly unremarkable. Later in 2016 she developed pain in her lumbar spine and bilateral lower extremities. The pain appeared gradually, was worse in the popliteal region, and progressed to the point that she had difficulty extending her knees and ambulating despite physical therapy. Given continued symptoms, the patient traveled to Tijuana, Mexico, where she underwent brain imaging showing hydrocephalus, with subsequent placement of a ventriculoperitoneal (VP) shunt in 2016. The patient’s back and bilateral lower extremity pain improved; however, her ability to extend her knees and to ambulate did not. In 2017 she presented to the Clinic for Special Children where she underwent evaluation for failure to thrive and amenorrhea. Repeat brain magnetic resonance imaging (MRI) in May 2017 was remarkable for hydrocephalus and leptomeningeal enhancement over the dorsal and ventral corticomedullary junction and cerebellum. She was subsequently referred to the neurosurgery division at Nemours/Alfred I. duPont Hospital for Children (AIDHC) where aerobic cultures of cerebrospinal fluid (CSF) obtained from her VP shunt did not grow any bacteria. Later in 2017 she returned to Mexico for further evaluation and was treated for a “culture-negative infection” with 1 week of intravenous antibiotics.
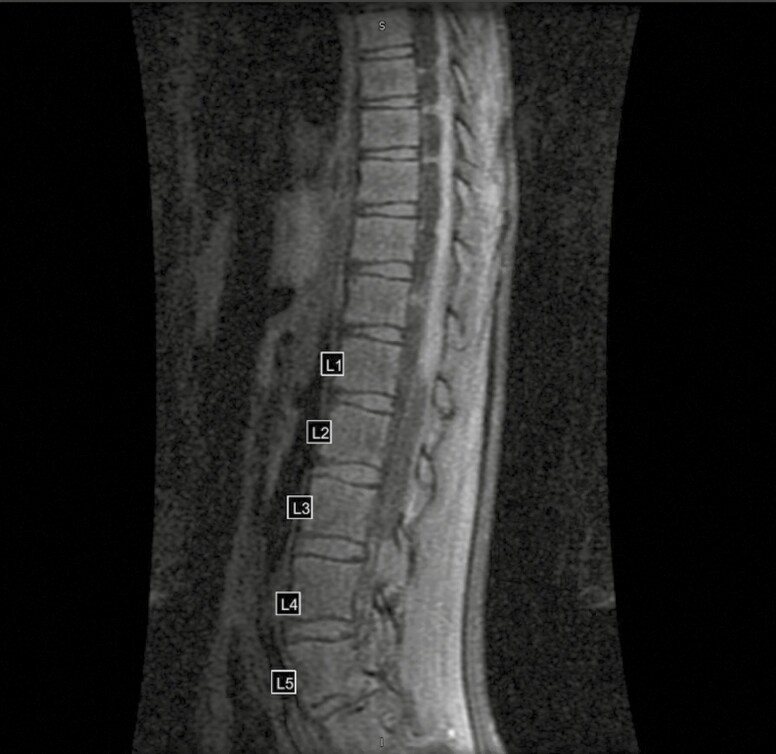
She returned to the neurosurgery clinic at AIDHC in 2018 with continued headache, lower back and bilateral leg pain, poor appetite, and weight loss. At this time her body mass index was 14 kg/m2, and her examination was notable for Tanner stage 2 breasts and Tanner stage 1 pubic hair, and inability to flex at the waist beyond 15 degrees when her knees were extended. The remainder of her examination, including assessment of cranial nerves, sensation, and strength, were within normal limits. The patient underwent MRI of her brain and spine. The imaging, displayed in Figure 1, showed a flattened and deformed spinal cord due to adjacent septations and loculations with enhancement along the surface of the spinal cord consistent with a chronic inflammatory process. Ventriculomegaly with septations across the lateral ventricles and diffuse meningeal enhancement over the bilateral cerebral hemispheres were also noted. Laboratory studies obtained at the time showed an elevated erythrocyte sedimentation rate to 39 mm/hour, with a normal white blood cell (WBC) count (11 200/µL but with a neutrophilic predominance (80.4%).
Figure 1.
T2 fast recovery fast spin echo magnetic resonance image of spine showing evidence of chronic meningitis.
The patient’s medical history was significant for failure to enter puberty, and her only past surgical history was placement of the previously described VP shunt. The patient was Amish and lived on a farm in rural Pennsylvania with her mother, father, and 8 siblings. Her family history was unremarkable, and immunizations were up to date. Notable exposures included travel to Mexico, swimming in freshwater ponds in Pennsylvania, and working on a farm, including the milking of cows.
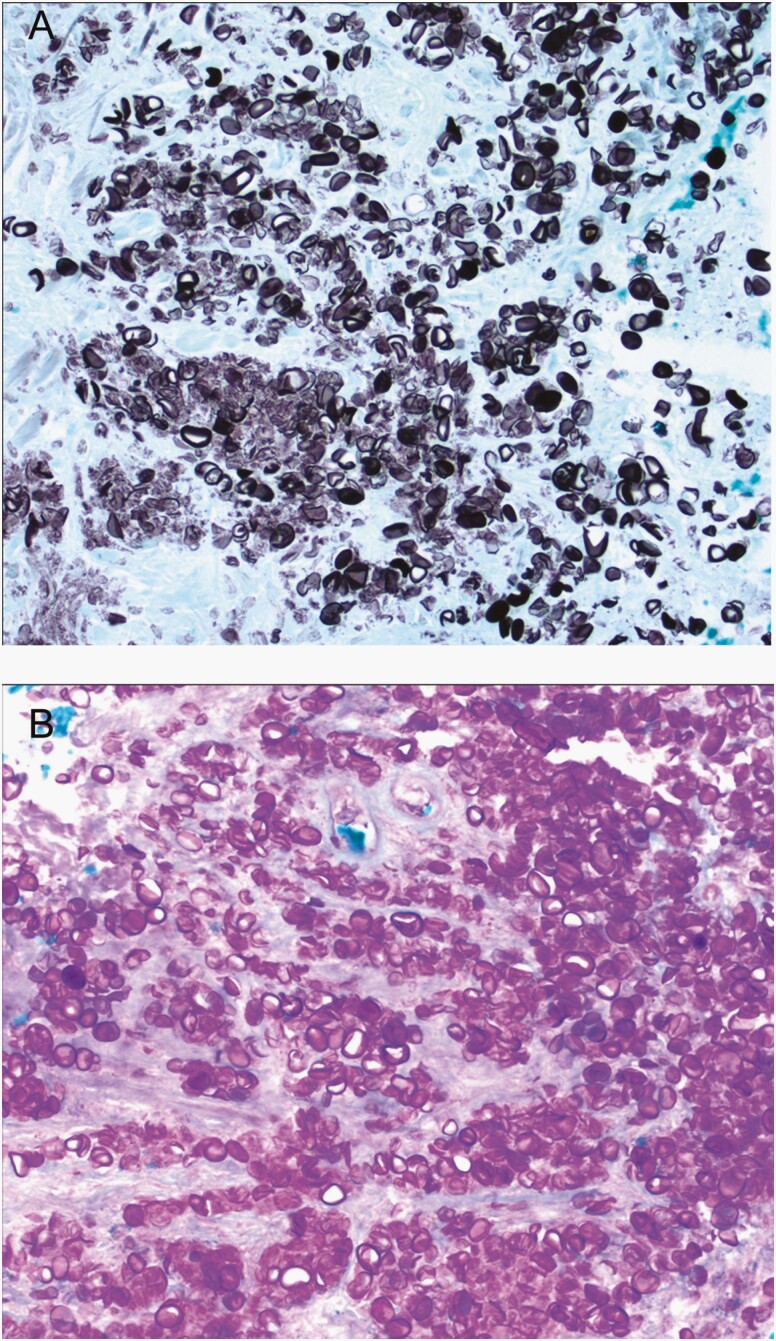
The patient subsequently underwent a lumbar puncture, which showed 560 WBCs and 3400 red blood cells/μL. A differential and glucose/protein were not performed. Gram stain was negative, as was a BioFire FilmArray Meningitis/Encephalitis panel. Aerobic, mycobacterial, and fungal cultures were negative. Serum testing for human immunodeficiency virus and tuberculosis were negative. Testing for cryptococcus antigen from CSF and serum, histoplasma antigen from urine and CSF, and coccidioides serology were all negative. A leptomeningeal biopsy was performed and a “chalky material” overlying the meninges was described by the neurosurgeon. The biopsy, shown in Figure 2, was notable for numerous infectious agents in areas of necrosis and in macrophages. The organisms stained strongly positive for Grocott methenamine silver and periodic acid-Schiff but negative for mucicarmine. No hyphae or budding yeast were noted. The patient was discharged on fluconazole for presumed fungal meningitis. Four weeks later, fungal cultures from the leptomeningeal tissue biopsy showed growth of a microorganism, and P zopfii was identified via D2 sequencing at the Mayo Clinic. Antifungal susceptibility was performed on the isolate showing the following minimum inhibitory concentrations: amphotericin B = 0.5 µg/mL, caspofungin > 8 µg/mL, fluconazole = 32 µg/mL, voriconazole = 0.125 µg/mL, ketoconazole = 1 µg/mL, itraconazole = 1 µg/mL, and 5-fluorocytosine > 64 µg/mL.
Figure 2.
Leptomeningeal biopsy showing numerous infectious elements stained with Grocott methenamine silver (A) and periodic acid-Schiff (B) stains.
The patient was treated with liposomal amphotericin B and treatment was complicated by hypokalemia, anemia, and intermittent acute kidney injury as demonstrated by elevated creatinine. She underwent gastrostomy tube placement, and had substantial improvement in her growth. She also required admission to the hospital on 2 separate occasions, once for a peripherally inserted central catheter malfunction and another time for methicillin-sensitive Staphylococcus aureus central line–associated bloodstream infection and central line–associated thrombosis. At the end of therapy, the patient exhibited reduced leg pain and improvement in her ability to participate in activities of daily living, though she still had limited range of motion in her hip flexors. Due to lack of insurance and family preference, a follow-up MRI and meningeal biopsy were not obtained. A whole exome sequence looking for immunodeficiency was performed at the National Institutes of Health and showed no variations associated with immunodeficiency.
Prototheca species are achlorophyllic algae that are a rare cause of disease in humans. Most cases of Prototheca infection occur as cutaneous disease or olecranon bursitis, but systemic disease, including meningitis, peritonitis, endocarditis, infections of the spleen, and bloodstream infection, has been reported. Infections have been reported in immunocompetent people, but the majority of systemic infections involve patients with AIDS, immunosuppression, or immune system defects [1, 2]. There are 5 species of Prototheca: P zopfii, P wickerhamii, P stagnora, P ulmea, and P blaschkeae, with the vast majority of infections being due to P wickerhamii. Meningitis due to Prototheca species has rarely been reported [3–5] but to our knowledge, this is the first case of meningitis due to P zopfii.
Prototheca species are found ubiquitously in the environment including in freshwater and saltwater as well as in animals such as cattle and deer. Cow’s milk has also been implicated as a source of Prototheca, and Prototheca species are a known cause of bovine mastitis [1]. The exposure that led to our patient’s infection is unknown, but we do note that she not only swam in freshwater ponds, but also had frequent exposure to soil on her family’s farm, and prior to her illness, she frequently milked cows. Many Prototheca infections are due to traumatic inoculation and it is possible that the patient’s infection was introduced during the insertion of her ventriculoperitoneal shunt or subsequent manipulation of the shunt. Although immunodeficiency is frequently seen in patients with disseminated protothecosis, whole exome sequencing did not identify a cause in our patient. Our patient was very malnourished, and it is possible that this led to a state of functional immunodeficiency, though the malnourished state may also have been secondary to this chronic infection.
There are no established breakpoints for antifungal agents for Prototheca species, or randomized trials to guide treatment length. Patients are frequently treated with amphotericin B or an azole antifungal, but response to antimicrobial therapy is variable [6]. Our patient showed clinical improvement in her pain and functioning after 12 months of treatment and given the adverse effects of this agent, we elected to stop treatment at this time. At the writing of this publication in 2020, the patient remains alive and able to perform activities of daily living. The differential for chronic meningitis is broad and includes fungal causes such as histoplasmosis and coccidioidomycosis, tuberculosis, malignancy, and sarcoidosis. As this case demonstrates, Prototheca zopfii should be considered as well.
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev 2007; 20:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thiele D, Bergmann A. Protothecosis in human medicine. Int J Hyg Environ Health 2002; 204:297–302. [DOI] [PubMed] [Google Scholar]
- 3. Zhang QQ, Zhu LP, Weng XH, et al. Meningitis due to Prototheca wickerhamii: rare case in China. Med Mycol 2007; 45:85–8. [DOI] [PubMed] [Google Scholar]
- 4. Takaki K, Okada K, Umeno M, et al. Chronic Prototheca meningitis. Scand J Infect Dis 1996; 28:321–3. [DOI] [PubMed] [Google Scholar]
- 5. Ahn A, Choe YJ, Chang J, et al. Chronic eosinophilic meningoencephalitis by Prototheca wickerhamii in an immunocompetent boy. Pediatr Infect Dis J 2017; 36:687–9. [DOI] [PubMed] [Google Scholar]
- 6. Leimann BC, Monteiro PC, Lazéra M, et al. Protothecosis. Med Mycol 2004; 42:95–106. [DOI] [PubMed] [Google Scholar]