Abstract
Background
Antithrombin 3 (AT3) is a physiological inhibitor of thrombin, and serum AT3 activity was found to decrease at the status of type 2 diabetes (T2D). T2D was presented with an increased risk of thrombotic complications at the background of impaired insulin sensitivity. The aim of this study was to investigate the relationship between insulin sensitivity indices and serum AT3 activity in patients with T2D.
Methods
We conducted a cross-sectional study in patients with T2D who consented to participate in the study at the Endocrinology Department of Affiliated 2 Hospital of Nantong University from January 2015 to June 2018. All patients received serum AT3 activity test and 75 g oral glucose tolerance test (OGTT). Basal and systemic insulin sensitivity were assessed by homeostasis model assessment of insulin resistance (HOMA-IR) and Matsuda index (ISIMatsuda), respectively, from the OGTT. And other relevant clinical data were also collected.
Results
Total of 1612 patients with T2D were enrolled in the study, with a mean age of 58.67 ± 13.09 years and a median diabetes duration of 6 years (interquartile range, 1–10 years). Across ascending quartiles of serum AT3 activity, HOMA-IR progressively decreased, while ISIMatsuda progressively increased (all P for trend < 0.001). Moreover, serum AT3 activity was negatively correlated with HOMA-IR (r = −0.189, P < 0.001) and positively correlated with ISIMatsuda (r = 0.221, P < 0.001). After adjusting for other metabolic risk factors, hemostatic parameters and glucose-lowering therapies by multivariate linear regression analysis, HOMA-IR (β = −0.185, t = −5.960, P < 0.001) and ISIMatsuda (β = 0.197, t = 6.632, P < 0.001) remained independently associated with the serum AT3 activity in patients with T2D, respectively.
Conclusions
Reduced basal and systemic insulin sensitivity are associated with decreased serum AT3 activity in patients with T2D.
Keywords: antithrombin 3, diabetes, insulin sensitivity, type 2 diabetes
Introduction
Individuals with type 2 diabetes (T2D) suffer from an increased risk of thrombotic complications such as myocardial infarction (MI), stroke, and venous thromboembolism (VTE) (1). Hyperglycemia, increased oxidative stress, and the formation of glycosylation end products (AGEs) collectively contribute to hypercoagulation in T2D (2).
Insulin resistance, also termed 'reduced insulin sensitivity', is of central importance in the pathogenesis and progression of T2D and contributes to many metabolic disorders, including hypercoagulation, hypo-fibrinolysis, dyslipidemia and hypertension (3). The hyperinsulinemic-euglycemic clamp technique is the gold standard for evaluating insulin resistance in humans but is costly for large sample clinical studies. An alternative method of evaluating insulin sensitivity has been derived from the oral glucose tolerance test (OGTT). Basal insulin resistance assessed by homeostasis model assessment of insulin resistance (HOMA-IR) and systemic insulin sensitivity assessed by Matsuda’s insulin sensitivity index (ISIMatsuda) can be easily derived from OGTT and are practical for application in large-scale clinical studies (4, 5). And previous clinical studies have demonstrated that these insulin resistance indices were independently associated with poor outcomes of cardiovascular disease (CVD) and acute ischemic stroke (6, 7).
Antithrombin 3 (AT3), one of the most important natural anticoagulants, plays a key role in controlling coagulation balance (8). Accumulating evidence has demonstrated that serum AT3 activity decreased in patients with T2D, stroke, and left atrial thrombus, when compared with normoglycemic subjects (9, 10, 11). AT3 activity deficiency may lead to vascular degenerative complications in T2D, such as diabetic retinopathy (12). The main mechanism may be that AT3 contains a link site for thrombin and heparin from which thrombin links with AT3 and then forms thrombin-AT3 complex, the inducer of thrombosis (13). Under the pathological condition of T2D, hyperglycemia promotes the product of AGEs; as a result, non-enzymatic glycation leads to structural modifications of AT3 and favors thrombosis (14). However, the incidence and progression of T2D are primarily attributed to impaired insulin sensitivity, and the relationship between serum AT3 activity and insulin sensitivity in T2D has not been fully elucidated. We hypothesize that reduced insulin sensitivity may play a vital role in the decreased AT3 activity in T2D.
Therefore, the present study we designed is to investigate the relationship between serum AT3 activity and insulin sensitivity indices, assessed by HOMA-IR and ISIMatsuda, in a large sample of patients with T2D.
Methods
Study design and participants
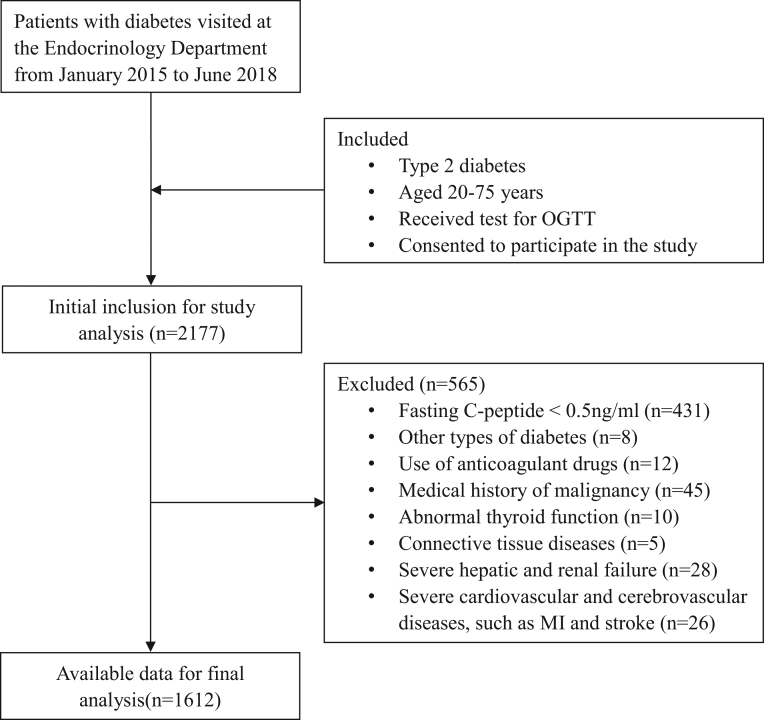
We conducted this cross-sectional study in patients with T2D who visited the Endocrinology Department of Affiliated 2 Hospital of Nantong University from January 2015 to June 2018. The study flowchart is shown in Fig. 1. The inclusion criteria were as follows: (i) diagnosis of T2D according to the American Diabetes Association (ADA) criteria in 2011; (ii) aged 20–75 years; (iii) received a test for OGTT; (iv) fasting C-peptide ≥ 0.5 ng/mL; and (v) consented to participate in the study. The exclusion criteria were as follows: (i) other types of diabetes; (ii) use of anticoagulant drugs; (iii) medical history of malignancy; (iv) abnormal thyroid function; (v) connective tissue diseases; (vi) severe hepatic and renal failure; and (vii) severe cardiovascular and cerebrovascular diseases, such as MI and stroke. Finally, a total of 1612 T2D patients with complete data were pooled for analysis.
Figure 1.
The study flowchart.
Basic data collection
The basic information of all participants was obtained, including age, sex, height, weight, blood pressure, diabetes duration, previous and present medical history, and history of glucose-lowering therapies. Glucose-lowering therapies included lifestyle alone, insulin treatments, insulin-secretagogues, pioglitazone, α-glucosidase inhibitors (AGIs), glucagon-like peptide-1 receptor agonists (GLP-1RAs) and dipeptidyl peptidase 4 inhibitors (DPP-4Is). The BMI was calculated as the ratio of the weight (kg) to the square of the height (m2). Hypertension was identified by systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure (DBP) ≥ 90 mmHg, a history of hypertension or current use of antihypertensive drugs.
Calculation of insulin sensitivity indices from OGTT
A 75 g OGTT was administered in the all recruited patients after an overnight fast of at least 12 h. Venous blood samples were drawn at fasting (0) and at 0.5, 1, 2, and 3 h after the glucose load for the measurement of serum glucose and insulin levels. Basal insulin resistance was assessed by HOMA-IR, which was defined as follows: HOMA-IR = (fasting glucose × fasting insulin)/22.5. Systemic insulin sensitivity was assessed by ISIMatsuda, which was calculated by the following formula: ISIMatsuda = 10,000/sqrt (fasting glucose × fasting insulin × mean glucose × mean insulin).
Laboratory examination
As biomarkers from the OGTT, serum insulin level (using the chemiluminescence method) was measured with an immunoassay system (DxI 800, Beckman Coulter), and serum glucose level (using the oxidase method) was measured with an automated biochemical instrument (Model 7600, Hitachi). Meanwhile, fasting venous blood samples were also collected from all patients for the measurement of other clinical biomarkers. Coagulation function indices, serum AT3 activity (using the chromogenic substrate assay), and prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen (Fg) (using the solidification method) were measured with an automated blood coagulation analyzer (CS-5100 system, Sysmex). Serum creatinine (Scr), uric acid (UA), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were measured with an automated biochemical analyzer (Model 7600; Hitachi). Glycosylated hemoglobin (HbA1c) was measured with an ion exchange-based HPLC method (D-10 system, Bio-Rad).
Statistical analysis
We used SPSS for Windows, standard version 19.0 (IBM Co.), to input and analyze the data. To analyze the association of serum AT3 activity with insulin sensitivity indices and other clinical variables, all patients were divided into four subgroups by quartiles of the AT3 activity levels (Table 1). Clinical variables are displayed as means ± s.d.s for normally distributed data, medians (25 and 75% interquartile ranges) for skewed data and frequencies (percentages) for categorical data. The insulin sensitivity indices in our study were skewed, a natural logarithm transformation (ln) was used in the further analysis, such as lnHOMA-IR and lnISIMatsuda.
Table 1.
Clinical characteristics of the participants according to serum AT3 activity quartiles.
| Variables | Total | Q1 | Q2 | Q3 | Q4 | P for trend |
|---|---|---|---|---|---|---|
| AT3 activity (%) (range) | 92.07 ± 16.92 (38.60–143.90) | 71.35 ± 6.41 (38.60–79.70) | 85.55 ± 3.34 (79.71–91.15) | 97.08 ± 3.60 (91.16–103.77) | 114.36 ± 9.13 (103.78–143.90) | <0.001 |
| n | 1612 | 405 | 401 | 403 | 403 | – |
| Age (years) | 58.67 ± 13.09 | 62.19 ± 13.28 | 58.08 ± 12.75 | 57.59 ± 13.26 | 56.81 ± 12.41 | <0.001 |
| Male, n (%) | 898 (55.7) | 226 (55.8) | 225 (56.1) | 217 (53.8) | 230 (57.1) | 0.826 |
| BMI (kg/m2) | 25.80 ± 3.52 | 25.75 ± 3.54 | 25.99 ± 3.42 | 26.02 ± 3.68 | 25.36 ± 3.39 | 0.076 |
| SBP (mmHg) | 136.8 ± 18.02 | 136.5 ± 17.9 | 136.4 ± 17.9 | 138.5 ± 18.28 | 135.7 ± 17.9 | 0.165 |
| DBP (mmHg) | 79.3 ± 10.9 | 78.2 ± 10.9 | 79.3 ± 11.6 | 80.64 ± 11.0 | 79.3 ± 9.9 | 0.016 |
| Diabetes duration (years) | 6 (1–10) | 6 (1–10) | 6 (2–10) | 5 (2–10) | 5 (1–10) | 0.108 |
| Glucose-lowering therapies | ||||||
| Lifestyle alone, n (%) | 132 (8.2) | 8 (2.0) | 16 (4.0) | 37 (9.2) | 71 (17.6) | <0.001 |
| Insulin treatments, n (%) | 869 (53.9) | 226 (55.8) | 227 (56.6) | 206 (51.1) | 210 (52.1) | 0.314 |
| Insulin-secretagogues, n (%) | 857 (53.2) | 227 (56.0) | 203 (50.6) | 204 (50.6) | 223 (55.3) | 0.240 |
| Metformin, n (%) | 933 (57.9) | 227 (56.0) | 209 (52.1) | 264 (65.5) | 233 (57.8) | 0.001 |
| Pioglitazone, n (%) | 594 (36.8) | 166 (41.0) | 156 (38.9) | 132 (32.8) | 140 (34.7) | 0.061 |
| AGIs, n (%) | 424 (26.3) | 131 (32.3) | 105 (26.2) | 102 (25.3) | 86 (21.3) | 0.005 |
| GLP-1RAs, n (%) | 54 (3.3) | 12 (3.0) | 17 (4.2) | 15 (3.7) | 10 (2.5) | 0.517 |
| DPP-4Is, n (%) | 456 (28.3) | 102 (25.2) | 101 (25.2) | 128 (31.8) | 125 (31.0) | 0.053 |
| Hypertension, n (%) | 823 (51.1) | 223 (55.1) | 211 (52.6) | 197 (48.9) | 192 (47.6) | 0.131 |
| TG (mmol/L) | 1.85 (1.20–2.96) | 1.65 (1.07–2.75) | 1.87 (1.27–2.84) | 2.02 (1.24–3.03) | 1.89 (1.22–3.18) | 0.008 |
| TC (mmol/L) | 4.51 ± 1.17 | 4.31 ± 0.96 | 4.42 ± 1.03 | 4.65 ± 1.42 | 4.67 ± 1.20 | <0.001 |
| HDL-C (mmol/L) | 1.03 ± 0.27 | 0.99 ± 0.25 | 0.99 ± 0.27 | 1.04 ± 0.27 | 1.09 ± 0.29 | <0.001 |
| LDL-C (mmol/L) | 2.56 ± 0.80 | 2.40 ± 0.71 | 2.49 ± 0.70 | 2.65 ± 0.78 | 2.69 ± 0.95 | <0.001 |
| Scr (umol/L) | 59.14 ± 22.14 | 61.48 ± 19.26 | 59.27 ± 25.43 | 56.21 ± 19.14 | 59.57 ± 23.80 | 0.010 |
| HbA1c (%) | 9.21 ± 2.08 | 9.09 ± 1.96 | 9.14 ± 2.14 | 9.25 ± 1.97 | 9.34 ± 2.25 | 0.330 |
| PT (s) | 11.83 ± 0.95 | 12.31 ± 1.06 | 11.87 ± 0.72 | 11.67 ± 0.84 | 11.46 ± 0.95 | <0.001 |
| APTT (s) | 30.01 ± 5.82 | 31.88 ± 6.49 | 30.38 ± 5.20 | 29.76 ± 5.67 | 28.02 ± 5.15 | <0.001 |
| Fg (g/L) | 2.62 ± 0.84 | 2.60 ± 0.94 | 2.59 ± 0.83 | 2.55 ± 0.78 | 2.74 ± 0.76 | 0.009 |
| lnHOMA-IR | 1.39 ± 0.88 | 1.60 ± 0.85 | 1.46 ± 0.88 | 1.36 ± 0.89 | 1.15 ± 0.84 | <0.001 |
| lnISIMatsuda | 4.05 ± 0.70 | 3.86 ± 0.67 | 3.99 ± 0.71 | 4.08 ± 0.69 | 4.28 ± 0.67 | <0.001 |
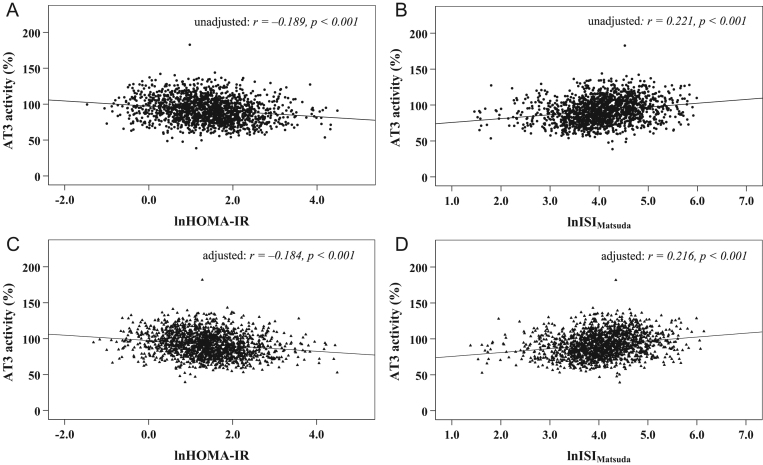
ANOVA followed by a post-test for linear trend was used to compare the trends in continuous data, and a linear-by-linear association chi-squared test was used to compare the trends in categorical data among the four subgroups based on serum AT3 activity quartiles. Moreover, Pearson’s test was conducted in the correlation of serum AT3 activity with lnHOMA-IR and lnISIMatsuda (Fig. 2A and B). Considering that the exogenous insulin injections may have an influence on insulin sensitivity, the partial correlation was used to adjust the effect of exogenous insulin on the correlation of AT3 activity with lnHOMA-IR and lnISIMatsuda (Fig. 2C and D). Furthermore, we used multivariable linear regression analyses to explore the independent effects of insulin sensitivity indices on serum AT3 activity (Tables 2 and 3). The initial model 0 was unadjusted; model 1 was adjusted for age, sex, diabetes duration, BMI, SBP, DBP, TC, TG, HDL-C, LDL-C, Scr, PT, APTT, Fg, HbA1c and glucose-lowering therapies. Statistical significance was identified when a value of P was less than 0.05.
Figure 2.
Scatter plot for the correlations between insulin sensitivity indices and AT3 activity (A and B: unadjusted; C and D: adjusted for insulin treatments).
Table 2.
Association of lnHOMA-IR with AT3 activity by multiple linear regression.
| Model | B (95% CI) | β | t | P value |
|---|---|---|---|---|
| Model 0 | −3.631 (−4.555 to −2.707) | −0.189 | −7.708 | <0.001 |
| Model 1 | −3.472 (−4.615 to −2.329) | −0.185 | −5.960 | <0.001 |
Model 0: unadjusted; Model 1: adjusted for age, sex, BMI, SBP, DBP, diabetes duration, TC, TG, HDL-C, LDL-C, Scr, PT, APTT, Fg, HbA1c and glucose-lowering therapies.
Table 3.
Association of lnISIMatsuda with AT3 activity by multiple linear regression.
| Model | B (95% CI) | β | t | P value |
|---|---|---|---|---|
| Model 0 | 5.349 (4.193 to 6.504) | 0.221 | 9.080 | <0.001 |
| Model 1 | 4.636 (3.264 to 6.007) | 0.197 | 6.632 | <0.001 |
Model 0: unadjusted; Model 1: adjusted for age, sex, BMI, SBP, DBP, diabetic duration, TC, TG, HDL-C, LDL-C, Scr, PT, APTT, Fg, HbA1c and glucose-lowering therapies.
Results
Clinical characteristics of the participants
Table 1 displays the characteristics of the 1612 recruited patients for the total and according to quartiles of serum AT3 activity. The normal reference of serum AT3 activity is between 70 and 140% in our laboratory, and 8.1% (n = 131) of recruited patients had a level of serum AT3 activity less than 70%. The average serum AT3 activity of total patients was 92.07 ± 16.92% and of the AT3 activity quartiles was 71.35 ± 6.41% (first quartile, Q1), 85.55 ± 3.34% (second quartile, Q2), 97.08 ± 3.60% (third quartile, Q3) and 114.36 ± 9.13% (fourth quartile, Q4), respectively. With the quartiles of the serum AT3 activity increasing, DBP, TG, TC, HDL-C, LDL-C, Fg and lnISIMatsuda levels significantly tended to increase, while age, Scr, PT, APTT and lnHOMA-IR levels significantly decreased (P for trend < 0.001). However, the male ratio, BMI, SBP, diabetes duration, prevalence of hypertension and HbA1c did not show any differences among the AT3 activity quartiles.
Correlations between serum AT3 activity and insulin sensitivity indices
Pearson’s correlation tests showed that serum AT3 activity significantly negatively correlated with lnHOMA-IR (r = −0.189, P < 0.001) (Fig. 2A) and positively correlated with lnISIMatsuda (r = 0.221, P < 0.001) (Fig. 2B). After adjusting for exogenous insulin injections by partial correlation analyses, serum AT3 activity still significantly negatively correlated with lnHOMA-IR (r = −0.184, P < 0.001) (Fig. 2C) and positively correlated with lnISIMatsuda (r = 0.216, P < 0.001) (Fig. 2D). Serum AT3 activity tended to decrease with the deterioration in insulin sensitivity.
Multiple linear regression analysis with serum AT3 activity as the dependent variable
Tables 2 and 3 show the degree of the influence of insulin sensitivity indices (lnHOMA-IR and lnISIMatsuda) on serum AT3 activity by multivariate linear regression analyses in all recruited patients with T2D. After adjusting for demographic parameters, lipids profile, Scr, HbA1c, coagulation function indices and glucose-lowering therapies by multivariate linear regression analyses, HOMA-IR (β = −0.185, t = −5.960, P < 0.001) (Table 2) and ISIMatsuda (β = 0.197, t = 6.632, P < 0.001) (Table 3) remained independently associated with the serum AT3 activity in patients with T2D, respectively.
Discussion
In the present study, we investigated the association of insulin sensitivity with the serum AT3 activity in a large Chinese population with T2D (n = 1612). The main findings of our study are shown as following: first, basal insulin resistance assessed by HOMA-IR and systemic insulin sensitivity assessed by ISIMatsuda were closely associated with serum AT3 activity in T2D patients; secondly, the associations of the two insulin sensitivity indices with serum AT3 activity were independent of glucose-lowering therapies and other metabolic risk factors. These findings imply that both impaired basal and systemic insulin sensitivity may contribute to decreased serum AT3 activity in patients with T2D.
Atherothrombotic events, such as myocardial infarction, ischemic stroke and cardiovascular death, are the result of platelet adhesion and activation on the ruptured atherosclerotic plaques. T2D is associated with a hypercoagulable state, and patients with T2D are at a high risk of atherothrombotic events (15, 16). Hyperglycemia, an extrinsic hallmark of T2D, together with dyslipidemia, obesity, aging, pregnancy, surgery and trauma may play a major role in the formation of a hypercoagulable state (17, 18, 19). Hyperglycemia was reported to be involved in the platelet activation, leukocyte aggregation, microparticles formation, endothelial dysfunction, impaired plaque remodeling, activation of coagulation and decreased fibrinolytic activity (20). Ephraim et al. (21) revealed that patients with T2D, who have a high risk of coagulation, were presented with shortened APTT and PT when compared with healthy controls. Moreover, these T2D patients were also characterized by the downregulation of serum AT3 activity, especially when they were accompanied by diabetic complications. In our present study, 8.1% of recruited patients had a level of serum AT3 activity less than 70%, whereas the prevalence of AT3 deficiency in healthy controls was 0.02 to 0.17% and in patients with VTE was 1.1% (22, 23). Under the circumstance of diabetes, chronic hyperglycemia may result in the excess formation of non-enzymatic glycosylation products and lead to structural modifications in various proteins (24, 25, 26). Serum AT3, the natural anticoagulant, is also prone to be non-enzymatically modified under the condition of hyperglycemia, which may facilitate the occurrence of atherothrombotic events (27). However, HbA1c did not show any differences among the quartiles of serum AT3 activity in our study. The possible reason might be that 91.8% of recruited patients of our study have received one or more glucose-lowering agents, which may attenuate the relationship between HbA1c levels and serum AT3 activity.
As a natural anticoagulant, the main physiological function of AT3 is to regulate coagulation via the inhibition of factor IIa (related to PT), IXa, Xa, XIa and XIIa (related to APTT) (28). In our study, with the quartiles of the serum AT3 activity increasing, PT and APTT levels significantly tended to decrease while Fg levels significantly increased. In addition, serum AT3 activity was found to be correlated with lipids profile (29). Our data showed that HDL-C, LDL-C, TG and TC tended to increase across ascending quartiles of serum AT3 activity. Moreover, patients with higher age showed a lower activity of AT3. A previous basic study has proved that aging may lead to decreased AT3 activity (30). Additionally, Addai-Mensah et al. (31) found that poorly managed T2D was associated with reduced levels and activity of AT3 compared to well-managed T2D.
The intrinsic pathogenesis of T2D is primarily attributed to impaired insulin sensitivity(32). With the background of insulin resistance, T2D patients were at a high risk for ischemic cardio-cerebrovascular events when compared to nondiabetic subjects, in which thrombosis played an important role (15, 33, 34, 35, 36, 37). And insulin resistance may promote the prothrombotic state and subsequent formation of thromboembolism in T2D (38). In our present study, we investigated the association of insulin sensitivity with the AT3 activity in patients with T2D, and we found that decreased AT3 activity was in relation to both the impaired basal and systemic insulin sensitivity. And decreased AT3 activity may lead to an increase in thrombin and thrombin-activatable fibrinolysis inhibitor (TFAI). The TFAI was also an indicator of insulin resistance in T2D and polycystic ovary syndrome (PCOS) (39, 40). Furthermore, serum AT3 activity was found to decrease acutely in obese patients with COVID-19 and poor prognosis who were often presented with severe insulin resistance (41), while serum AT3 activity increased after gastric bypass surgery in severely obese patients because of weight loss and improvement in insulin resistance (42). These changes may be at least partially explained by the finding of our present study that serum AT3 activity was closely connected to insulin sensitivity. However, Ragab et al. (43) indicated that there was no correlation between AT3 activity and HOMA-IR in patients with metabolic syndrome, which was different from this study. The possible explanation is that the AT3 activity may be affected by other factors such as blood lipids and blood pressure in patients with metabolic syndrome.
Several possible mechanisms may explain the link between decreased AT3 activity and insulin resistance in T2D. First, thrombin activity enhanced when serum AT3 activity reduced. In T2D db/db mice, the binding process of thrombin to its receptors stimulated the release of inflammatory cytokines such as monocyte chemoattractant protein-1 (MCP1) and interleukin-6 (IL6), which contributed to the insulin resistance (44). Secondly, under the condition of insulin resistance, the mitogen-activated protein kinase (MAPK) pathway of binding insulin is activated, which promotes the secretion of endothelin-1, a platelet activator, vasoconstrictor and mitogenic stimulus for vascular smooth muscle cells (45). As a result, the coagulation process enhanced while the antithrombin process weakened. Thirdly, under the circumstance of insulin resistance, the secretion of glucose-stimulated insulin increases, which induces tissue factor (TF) expression in monocytes leading to increased TF procoagulant activity and decreased antithrombin effect (46).
There are some limitations of this study. First, decreased AT3 activity could not directly represent the probability of adverse atherothrombotic events. Secondly, it was a cross-sectional observational study that could not definitively illustrate the causality of the association between reduced insulin sensitivity and decreased AT3 activity. Thirdly, insulin sensitivity should be assessed by the gold standard of hyperinsulinemic-euglycemic clamp technique, but the method was difficult to be applied in large epidemiological studies. HOMA-IR and ISIMatsuda are derivative indicators from OGTT and could be easily used in these studies. Finally, our study was performed in a Chinese population from the same hospital, and our findings may lack generalizability to other populations.
Conclusions
In conclusion, reduced basal and systemic insulin sensitivity are associated with decreased serum AT3 activity in patients with T2D. And our findings imply that therapies targeted to impaired insulin sensitivity may improve serum AT3 activity in patients with T2D.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The study was funded by the Social Development Projects of Nantong (MS12019019, HS2020005), the Medical Research Project of Nantong Health Commission (2020JCC010), and the Medical Research Project of Nantong University (2019HY002).
Ethical statement
All participants were required of written informed consents, as well, the study was approved by the Second Affiliated Hospital of Nantong University Research Ethics Committee.
Data availability
The current data are available to all interested researchers upon reasonable request. Requests for access to data should be made to the principal investigators of the study.
Author contribution statement
H W, X W and J S participated in the design of the study, data collection, analysis of the data, and drafting of the manuscript. H W and J C conceived of the study, participated in its design and revised the manuscript. H W and J S participated in the analysis of the data and revised the manuscript. H W, J C, D Z and X W participated in data collection. All authors read and approved the final manuscript.
References
- 1.Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB. Hyperglycemia: a prothrombotic factor? Journal of Thrombosis and Haemostasis 2010. 8 1663–1669. ( 10.1111/j.1538-7836.2010.03910.x) [DOI] [PubMed] [Google Scholar]
- 2.Domingueti CP, Dusse LM, Carvalho Md, De Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. Journal of Diabetes and Its Complications 2016. 30 738–745. ( 10.1016/j.jdiacomp.2015.12.018) [DOI] [PubMed] [Google Scholar]
- 3.Reusch JE.Current concepts in insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome. American Journal of Cardiology 2002. 90 19G–26G. ( 10.1016/s0002-9149(0202555-9) [DOI] [PubMed] [Google Scholar]
- 4.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000. 23 57–63 . ( 10.2337/diacare.23.1.57) [DOI] [PubMed] [Google Scholar]
- 5.Matsuda M, Defronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999. 22 1462–1470. ( 10.2337/diacare.22.9.1462) [DOI] [PubMed] [Google Scholar]
- 6.Ago T, Matsuo R, Hata J, Wakisaka Y, Kuroda J, Kitazono T, Kamouchi M. & Fukuoka Stroke Registry. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology 2018. 90 e1470–e1477. ( 10.1212/WNL.0000000000005358) [DOI] [PubMed] [Google Scholar]
- 7.Gotoh S, Doi Y, Hata J, Ninomiya T, Mukai N, Fukuhara M, Kamouchi M, Kitazono T, Kiyohara Y. Insulin resistance and the development of cardiovascular disease in a Japanese community: the Hisayama study. Journal of Atherosclerosis and Thrombosis 2012. 19 977–985. ( 10.5551/jat.13698) [DOI] [PubMed] [Google Scholar]
- 8.Mammen EF.Antithrombin: its physiological importance and role in DIC. Seminars in Thrombosis and Hemostasis 1998. 24 19–25. ( 10.1055/s-2007-995819) [DOI] [PubMed] [Google Scholar]
- 9.Barillari G, Fabbro E, Pasca S, Bigotto E. Coagulation and oxidative stress plasmatic levels in a type 2 diabetes population. Blood Coagulation and Fibrinolysis 2009. 20 290–296. ( 10.1097/MBC.0b013e328329e49b) [DOI] [PubMed] [Google Scholar]
- 10.Meng R, Li ZY, Ji X, Ding Y, Meng S, Wang X. Antithrombin III associated with fibrinogen predicts the risk of cerebral ischemic stroke. Clinical Neurology and Neurosurgery 2011. 113 380–386. ( 10.1016/j.clineuro.2010.12.016) [DOI] [PubMed] [Google Scholar]
- 11.Choi SW, Kim BB, Choi DH, Park G, Shin BC, Song H, Kim D, Kim DM. Stroke or left atrial thrombus prediction using antithrombin III and mean platelet volume in patients with nonvalvular atrial fibrillation. Clinical Cardiology 2017. 40 1013–1019. ( 10.1002/clc.22759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monnier L, Follea G, Mirouze J. Antithrombin III deficiency in diabetes mellitus: influence on vascular degenerative complications. Hormone and Metabolic Research 1978. 10 470–473. ( 10.1055/s-0028-1093373) [DOI] [PubMed] [Google Scholar]
- 13.Stehling F, Weber R, Ozcelik A, Brocker M, Volbracht L, Diener HC, Busch E. Acute changes of coagulation and fibrinolysis parameters after experimental thromboembolic stroke and thrombolytic therapy. Neuroscience Letters 2008. 441 39–43. ( 10.1016/j.neulet.2008.05.102) [DOI] [PubMed] [Google Scholar]
- 14.Ceriello A, Quatraro A, Marchi E, Barbanti M, Dello Russo P, Lefebvre P, Giugliano D. The role of hyperglycaemia-induced alterations of antithrombin III and factor X activation in the thrombin hyperactivity of diabetes mellitus. Diabetic Medicine 1990. 7 343–348. ( 10.1111/j.1464-5491.1990.tb01402.x) [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New England Journal of Medicine 1998. 339 229–234. ( 10.1056/NEJM199807233390404) [DOI] [PubMed] [Google Scholar]
- 16.Nazimek-Siewniak B, Moczulski D, Grzeszczak W. Risk of macrovascular and microvascular complications in type 2 diabetes: results of longitudinal study design. Journal of Diabetes and Its Complications 2002. 16 271–276. ( 10.1016/s1056-8727(0100184-2) [DOI] [PubMed] [Google Scholar]
- 17.Faber DR, De Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obesity Reviews 2009. 10 554–563. ( 10.1111/j.1467-789X.2009.00593.x) [DOI] [PubMed] [Google Scholar]
- 18.Gorar S, Alioglu B, Ademoglu E, Uyar S, Bekdemir H, Candan Z, Saglam B, Koc G, Culha C, Aral Y. Is there a tendency for thrombosis in gestational diabetes mellitus? Journal of Laboratory Physicians 2016. 8 101–105. ( 10.4103/0974-2727.180790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romano M, Guagnano MT, Pacini G, Vigneri S, Falco A, Marinopiccoli M, Manigrasso MR, Basili S, Davi G. Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. Journal of Clinical Endocrinology and Metabolism 2003. 88 5321–5326. ( 10.1210/jc.2003-030508) [DOI] [PubMed] [Google Scholar]
- 20.Morel O, Jesel L, Abbas M, Morel N. Prothrombotic changes in diabetes mellitus. Seminars in Thrombosis and Hemostasis 2013. 39 477–488. ( 10.1055/s-0033-1343888) [DOI] [PubMed] [Google Scholar]
- 21.Ephraim RK, Awuku YA, Adu P, Ampomah LT, Adoba P, Panford S, Ninnoni JP, Agbodzakey H. High risk of coagulopathy among type-2 diabetes mellitus clients at a municipal hospital in Ghana. Ghana Medical Journal 2017. 51 101–107. ( 10.4314/gmj.v51i3.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tait RC, Walker ID, Perry DJ, Islam SI, Daly ME, Mccall F, Conkie JA, Carrell RW. Prevalence of antithrombin deficiency in the healthy population. British Journal of Haematology 1994. 87 106–112. ( 10.1111/j.1365-2141.1994.tb04878.x) [DOI] [PubMed] [Google Scholar]
- 23.De Stefano V, Finazzi G, Mannucci PM. Inherited thrombophilia: pathogenesis, clinical syndromes, and management. Blood 1996. 87 3531–3544. ( 10.1182/blood.V87.9.3531.bloodjournal8793531) [DOI] [PubMed] [Google Scholar]
- 24.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006. 114 597–605. ( 10.1161/CIRCULATIONAHA.106.621854) [DOI] [PubMed] [Google Scholar]
- 25.Jasmin JF, Mercier I, Hnasko R, Cheung MW, Tanowitz HB, Dupuis J, Lisanti MP. Lung remodeling and pulmonary hypertension after myocardial infarction: pathogenic role of reduced caveolin expression. Cardiovascular Research 2004. 63 747–755. ( 10.1016/j.cardiores.2004.05.018) [DOI] [PubMed] [Google Scholar]
- 26.Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. Journal of Clinical Endocrinology and Metabolism 2008. 93 1143–1152. ( 10.1210/jc.2007-1817) [DOI] [PubMed] [Google Scholar]
- 27.Tripodi A, Mannucci PM. Laboratory investigation of thrombophilia. Clinical Chemistry 2001. 47 1597–1606. ( 10.1093/clinchem/47.9.1597) [DOI] [PubMed] [Google Scholar]
- 28.Bravo-Perez C, Vicente V, Corral J. Management of antithrombin deficiency: an update for clinicians. Expert Review of Hematology 2019. 12 397–405. ( 10.1080/17474086.2019.1611424) [DOI] [PubMed] [Google Scholar]
- 29.Ebara S, Marumo M, Mukai J, Ohki M, Uchida K, Wakabayashi I.Relationships of oxidized HDL with blood coagulation and fibrinolysis in patients with type 2 diabetes mellitus. Journal of Thrombosis and Thrombolysis 2018. 45 200–205. ( 10.1007/s11239-017-1594-x) [DOI] [PubMed] [Google Scholar]
- 30.Nobukata H, Ishikawa T, Obata M, Shibutani Y. Age-related changes in coagulation, fibrinolysis, and platelet aggregation in male WBN/Kob rats. Thrombosis Research 2000. 98 507–516. ( 10.1016/s0049-3848(0000196-1) [DOI] [PubMed] [Google Scholar]
- 31.Addai-Mensah O, Annani-Akollor ME, Nsafoah FO, Fondjo LA, Owiredu EW, Danquah KO, Duneeh RV, Amponsah FA. Effect of poor glycaemic control on plasma levels and activity of protein C, protein S, and antithrombin III in type 2 diabetes mellitus. PloS One 2019. 14 e0223171. ( 10.1371/journal.pone.0223171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haffner SM, Miettinen H. Insulin resistance implications for type II diabetes mellitus and coronary heart disease. American Journal of Medicine 1997. 103 152–162. ( 10.1016/s0002-9343(9700027-2) [DOI] [PubMed] [Google Scholar]
- 33.Roffi M, Angiolillo DJ, Kappetein AP.Current concepts on coronary revascularization in diabetic patients. European Heart Journal 2011. 32 2748–2757. ( 10.1093/eurheartj/ehr305) [DOI] [PubMed] [Google Scholar]
- 34.Radke PW, Schunkert H. Diabetics with acute coronary syndrome: advances, challenges, and uncertainties. European Heart Journal 2010. 31 2971–2973. ( 10.1093/eurheartj/ehq347) [DOI] [PubMed] [Google Scholar]
- 35.Picard F, Adjedj J, Varenne O.Diabetes Mellitus, a prothrombotic disease. Annales de Cardiologie et d'Angiologie 2017. 66 385–392. ( 10.1016/j.ancard.2017.10.011) [DOI] [PubMed] [Google Scholar]
- 36.Vazzana N, Ranalli P, Cuccurullo C, Davi G.Diabetes mellitus and thrombosis. Thrombosis Research 2012. 129 371–377. ( 10.1016/j.thromres.2011.11.052) [DOI] [PubMed] [Google Scholar]
- 37.Calleja AI, Garcia-Bermejo P, Cortijo E, Bustamante R, Rojo Martinez E, Gonzalez, Sarmiento E, Fernandez-Herranz R, Arenillas JF.Insulin resistance is associated with a poor response to intravenous thrombolysis in acute ischemic stroke. Diabetes Care 2011. 34 2413–2417. ( 10.2337/dc11-1242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002. 287 2570–2581. ( 10.1001/jama.287.19.2570) [DOI] [PubMed] [Google Scholar]
- 39.Hori Y, Gabazza EC, Yano Y, Katsuki A, Suzuki K, Adachi Y, Sumida Y. Insulin resistance is associated with increased circulating level of thrombin-activatable fibrinolysis inhibitor in type 2 diabetic patients. Journal of Clinical Endocrinology and Metabolism 2002. 87 660–665. ( 10.1210/jcem.87.2.8214) [DOI] [PubMed] [Google Scholar]
- 40.Adali E, Yildizhan R, Kurdoglu M, Bugdayci G, Kolusari A, Sahin HG. Increased plasma thrombin-activatable fibrinolysis inhibitor levels in young obese women with polycystic ovary syndrome. Fertility and Sterility 2010. 94 666–672. ( 10.1016/j.fertnstert.2009.03.037) [DOI] [PubMed] [Google Scholar]
- 41.Gazzaruso C, Paolozzi E, Valenti C, Brocchetta M, Naldani D, Grignani C, Salvucci F, Marino F, Coppola A, Gallotti P. Association between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutrition, Metabolism, and Cardiovascular Diseases 2020. 30 1914–1919. ( 10.1016/j.numecd.2020.07.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardina E, Ferrer R, Rivero J, Baena-Fustegueras JA, Lecube A, Fort JM, Vargas V, Catalan R, Peinado-Onsurbe J. Alterations in the common pathway of coagulation during weight loss induced by gastric bypass in severely obese patients. Obesity 2012. 20 1048–1056. ( 10.1038/oby.2011.361) [DOI] [PubMed] [Google Scholar]
- 43.Ragab A, Abousamra NK, Higazy A, Saleh O.Relationship between insulin resistance and some coagulation and fibrinolytic parameters in patients with metabolic syndrome. Laboratory Hematology 2008. 14 1–6. ( 10.1532/LH96.07017) [DOI] [PubMed] [Google Scholar]
- 44.Mihara M, Aihara K, Ikeda Y, Yoshida S, Kinouchi M, Kurahashi K, Fujinaka Y, Akaike M, Matsumoto T. Inhibition of thrombin action ameliorates insulin resistance in type 2 diabetic db/db mice. Endocrinology 2010. 151 513–519. ( 10.1210/en.2009-0661) [DOI] [PubMed] [Google Scholar]
- 45.Jagroop IA, Daskalopoulou SS, Mikhailidis DP.Endothelin-1 and human platelets. Current Vascular Pharmacology 2005. 3 393–399. ( 10.2174/157016105774329453) [DOI] [PubMed] [Google Scholar]
- 46.Boden G. Rao AK.Effects of hyperglycemia and hyperinsulinemia on the tissue factor pathway of blood coagulation. Current Diabetes Reports 2007. 7 223–227. ( 10.1007/s11892-007-0035-1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The current data are available to all interested researchers upon reasonable request. Requests for access to data should be made to the principal investigators of the study.



 This work is licensed under a
This work is licensed under a