Abstract
We performed a retrospective study to determine the epidemiology of Rothia mucilaginosa infections among pediatric cancer patients. Over 20 years, 37 cases were identified; 27% developed complications, but there was no infection-related mortality. All cases were successfully treated with vancomycin.
Keywords: bacteremia, cancer, children, infection, Rothia mucilaginosa
Rothia mucilaginosa is an opportunistic pathogen identified primarily in immunocompromised patients [1] with neutropenia, indwelling central venous catheters (CVCs), and oral mucosal breakdown [2, 3]. While uncommon, infections are associated with complications such as sepsis, pneumonia, and meningitis [4, 5] and may result in death. Few studies have described the incidence, clinical presentation, and treatment of R mucilaginosa infection (RMI) in pediatric cancer patients [6, 7]. Most of the current knowledge comes from relatively small case series [4, 8].
To better understand the epidemiology and treatment of RMI among pediatric cancer patients, we retrospectively reviewed records from hospitalized patients at St Jude Children’s Research Hospital (St Jude) with a diagnosis of RMI.
METHODS
Study Population and Design
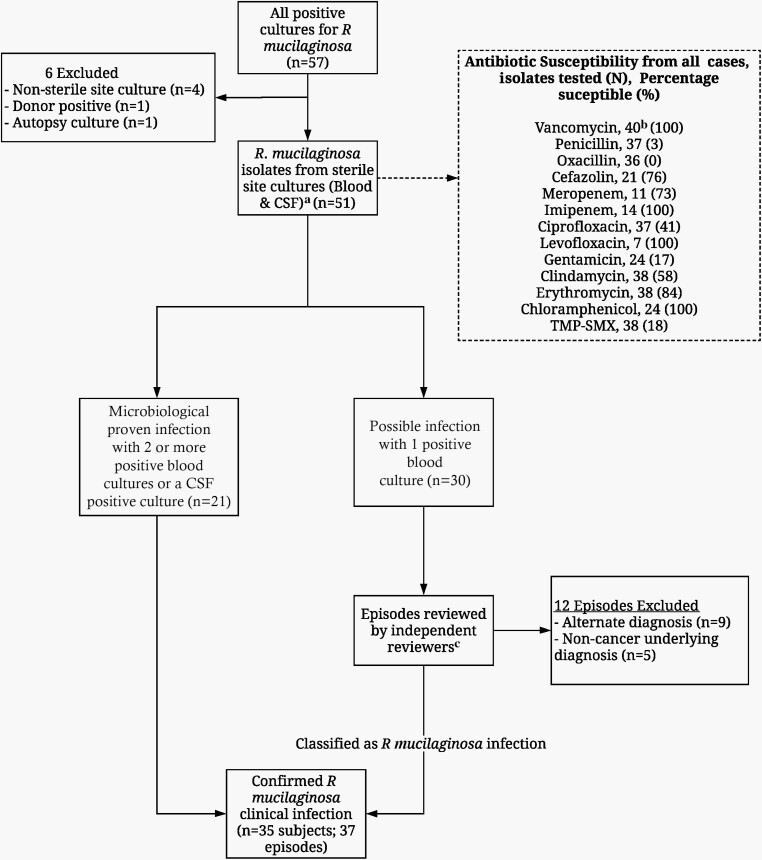
We reviewed and abstracted clinical information, microbiology and radiology results, medication exposures, and outcomes from medical records of pediatric cancer patients at St. Jude who had a positive blood or cerebrospinal fluid (CSF) culture for R mucilaginosa between 1 January 1995 and 31 December 2015. Proven RMI was defined as clinical symptoms of infection in the setting of > 1 positive blood culture (CVC, peripheral, or both) or CSF culture for R mucilaginosa in the absence of other infections or alternative causes of fever. Cases with only 1 positive culture at the onset of symptoms (possible RMI) were further reviewed to rule out an alternate diagnosis by 2 independent physician reviewers (G. M., J. W.), with a third reviewer for any discrepancies (H. H.) (Figure 1). This study was approved by the St Jude Institutional Review Board.
Figure 1.
Flowchart of subject selection included in final analysis. aSeventy-one percent of patients had positive blood cultures only from a central venous catheter (CVC); 29% had positive blood cultures from both CVC and peripheral sites. bSusceptibility analysis was performed, but is not reported in 66 isolates due to poor growth in culture media. cPhysicians reviewed clinical signs and symptoms at the time of infection, number and time to positivity of blood cultures, and alternative diagnosis. Abbreviations: CVC, central venous catheter; TMP-SMX, trimethoprim-sulfamethoxazole.
RESULTS
Cohort
Thirty-seven distinct episodes of RMI in 35 patients were included (Figure 1). Median incidence of RMI was 1.11 cases per 1000 patients per year. Cohort characteristics are described in Table 1. Median age was 9 years (range, 0.61–10.92 years). Most patients had an underlying hematological malignancy, and all had CVCs at the time of infection (Table 1). Susceptibility testing was available for only 40% of the samples due to poor growth on subculture (Figure 1).
Table 1.
Clinical and Demographic Characteristics of All Episodes With Clinically Positive Rothia mucilaginosa Infections With Complications Versus Those Without Complications
| Characteristic | Overall (N = 37) | Patients With Complications (n = 10) | Patients Without Complications (n = 27) |
|---|---|---|---|
| Age, y, median (range) | 9 (0.61–16.92) | 8.44 (2.23–16.67) | 9.00 (0.61–16.92) |
| Sex | |||
| Female | 12 (2.4) | 3 (30.0) | 9 (33.3) |
| Male | 25 (67.6) | 7 (70.0) | 18 (66.7) |
| Diagnosis category | |||
| Hematologic malignancy | 29 (78.4) | 8 (80.0) | 21 (77.8) |
| Solid tumor | 6 (16.2) | 1 (10.0) | 5 (18.5) |
| Brain tumor | 2 (5.4) | 1 (10.0) | 1 (3.7) |
| Diagnosis | |||
| ALL | 16 (43.2) | 5 (50.0) | 11 (40.7) |
| AML | 10 (27.0) | 3 (30.0) | 7 (25.9) |
| Wilms tumor | 1 (2.7) | 0 (0.0) | 1 (3.7) |
| Non-Hodgkin lymphoma | 3 (8.1) | 0 (0.0) | 3 (11.1) |
| Posterior medulloblastoma | 1 (2.7) | 0 (0.0) | 1 (3.7) |
| Brainstem glioma | 1 (2.7) | 1 (10.0) | 0 (0.0) |
| Ewing sarcoma | 2 (5.4) | 0 (0.0) | 2 (7.4) |
| Neuroblastoma | 2 (5.4) | 1 (10.0) | 1 (3.7) |
| Retinoblastoma | 1 (2.7) | 0 (0.0) | 1 (3.7) |
| Relapse/recurrence | 8 (21.6) | 4 (40.0) | 4 (14.8) |
| Hematopoietic cell transplant | |||
| Allogeneic | 7 (63.6) | 1 (50.0) | 6 (66.7) |
| Autologous | 4 (36.4) | 1 (50.0) | 3 (33.3) |
| Catheter at time of infection | |||
| Yes | 37 (100.0) | 10 (100.0) | 27 (100.0) |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neutropenic in 15 d prior to bacteremia | |||
| Yes | 28 (75.7) | 8 (80.0) | 20 (74.1) |
| No | 10 (26.3) | 2 (20.0) | 8 (28.6) |
| Days of neutropenia prior to bacteremia, median (range) | 9.00 (0.00–15.00) | 15.00 (0.00–15.00) | 7.00 (1.00–15.00) |
| Antibacterial therapy | |||
| Yes | 33 (89.2) | 10 (100.0) | 23 (85.2) |
| No | 4 (10.8) | 0 (0.0) | 4 (14.8) |
| Use of steroids or immunosuppressive therapy | 4 (10.8) | 2 (20.0) | 2 (7.4) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia.
Clinical Characteristics
Fever occurred in 34 (91.9%) episodes, 76% occurring in the setting of neutropenia. Median duration of fever was 3 days (range, 0–25 days). Mucositis was present at the time of infection in 10 (27%) patients. Ten patients (27%) presented with lower respiratory symptoms and 3 (8.1%) had headache and mental status changes. Only 3 patients had microbiological and radiological evidence of localized disease: 2 had meningitis and 1 had pneumonia.
Antibacterial prophylaxis at our institution is considered for patients with hematological malignancies when absolute neutrophil count (ANC) is < 500 and continues until the ANC is > 100 and rising. Regimens consist of vancomycin plus ciprofloxacin, or levofloxacin alone. In the 15 days prior to RMI, 6 patients (16.2%) received quinolone prophylaxis and 10 (27%) received vancomycin prophylaxis (dose: 15 mg/kg/dose intravenously every 12 hours).
Complications related to RMI included admission to the intensive care unit (ICU) (n = 8 [21.6%]), respiratory distress (n = 6 [16.2%]), and sepsis (n = 4 [10.8%]) (Table 1). Median length of ICU stay was 2.5 days (range, 1–75 days). Patients with complications had longer duration of neutropenia prior to onset of RMI (median, 15 days) compared to those who did not (median, 7 days). The median hospital stay was 10 days (range, 1–190 days). No deaths were attributed to RMI.
Treatment
Median duration of treatment was 14 days (range, 3–114 days). Vancomycin treatment was initiated at the onset of fever in 34 (97.14%) episodes. Patients who were receiving prophylactic vancomycin were switched to treatment doses (intravenous vancomycin every 6 or 8 hours with target trough concentrations of 10–20 μg/mL). Additional antibiotics were given in 32 episodes with the most common being cefepime (n = 26), tobramycin (n = 15), meropenem (n = 14), and ceftazidime (n = 7).
Two patients were treated for central nervous system (CNS) infection. Patient 1 developed meningitis in the setting of relapsed biphenotypic leukemia 8 months after haploidentical hematopoietic cell transplantation (HCT). Treatment consisted of vancomycin for 113 days plus ampicillin for 90 days and rifampin for 23 days. This patient underwent a second haploidentical HCT after 8 weeks of antibiotic therapy, and continued treatment until 3 weeks after neutrophil engraftment. Patient 2 had acute lymphoblastic leukemia and was diagnosed during induction chemotherapy. He was initially treated with vancomycin for 17 days plus ampicillin for 14 days and received 1 dose of intrathecal vancomycin 15 days after the initial positive CSF culture. He recovered neutrophils on day 14 of treatment and completed 49 days of linezolid treatment. In both patients, CSF cultures were negative after initial sampling, and CSF parameters over time were used to guide length of therapy (Supplementary Table 1). Neither of these patients had neurological sequelae.
DISCUSSION
We report the epidemiology, treatment, and outcomes of infections caused by R mucilaginosa at a single center over a 20-year period. To our knowledge, this is the largest cohort of R mucilaginosa infections in pediatric cancer to date. Consistent with previous reports, we found that most RMIs are seen in patients with leukemia [2] and that neutropenia, indwelling CVC, and mucositis are well-established risk factors [3, 4]. In our cohort, all patients had an indwelling CVC.
One-third of patients were receiving vancomycin prophylaxis at the time of RMI. We cannot determine if these infections were related to subtherapeutic levels of vancomycin as levels are not collected while patients are receiving prophylaxis. All patients, however, had an appropriate clinical response and achieved negative blood cultures when switched to treatment-dose vancomycin. Furthermore, no microbiological resistance to vancomycin was noted in the available isolates.
Ten patients received quinolones prior to RMI, half of them for prophylaxis. Exposure to quinolones has been described as a possible risk factor for developing RMI [3]. Levofloxacin was established as preferred prophylaxis for hematological malignancies at St Jude in 2014. Within a year of this measure, 5 of 9 cases of RMI occurred in patients receiving levofloxacin prophylaxis. An association between quinolone exposure and increased risk of RMI cannot be examined with our small cohort. In the setting of recent recommendations for levofloxacin prophylaxis in pediatric cancer patients, monitoring for RMI may be warranted. The isolates for which susceptibility results were available showed no resistance to levofloxacin, although more than half were resistant to ciprofloxacin, which has been described in the literature [9]. Susceptibility patterns may change in the pediatric cancer population as use of levofloxacin prophylaxis increases.
The clinical course of RMI is often characterized by prolonged fever and a slow response to antibiotic therapy [10], but fulminant infection and fatality can occur [11, 12]. In our cohort, most patients presented with febrile neutropenia, had favorable response to treatment, and had a benign clinical course with the majority not experiencing prolonged fevers. Complications such as ICU admission or respiratory distress were identified in approximately one-quarter of our patients. Whether these complications were related to RMI or to patients’ underlying diagnosis cannot be established; however, the proportion of complications in our cohort was lower to that previously reported in the literature [3, 4].
Optimal treatment for RMI has not yet been established [3]. Rothia mucilaginosa isolates with susceptibility results available were uniformly susceptible to vancomycin, consistent with previous literature [3, 4]. In all published case series, vancomycin has been successfully used as treatment, often with an additional antibiotic active against gram-positive organisms such as cephalosporins, ampicillin, or linezolid [2, 10]. Based on our experience, our current practice is to treat all RMI infections with vancomycin, alone or in combination with ampicillin.
For CNS infection, previous literature has reported 13–71 days of antibiotic treatment [4]. Both of our patients with meningitis were initially treated with vancomycin in combination with a β-lactam. In addition, patient 2 received intrathecal antibiotics, and vancomycin was switched to linezolid due to renal toxicity. The role of intrathecal antibiotics is controversial and has not been widely used. We successfully treated both patients until count recovery, complete resolution of symptoms, and normal CSF parameters. Since completing this review, we have successfully treated a third patient with CNS RMI undergoing HCT using the same therapy as patient 1.
Our study has several limitations. The retrospective design and small sample size at a single center limit the ability to generalize findings and find significant associations. Despite using independent physician reviewers to assess episodes, clinical judgment was used to determine the presence of RMI. Further multicenter studies with larger sample sizes are needed to fully understand the epidemiology and treatment of RMI.
CONCLUSIONS
Despite the reported severity of R mucilaginosa as an emerging pathogen, little is known about its clinical course in pediatric cancer patients. Our findings suggest that CVCs may be an important risk factor for RMI; that complications seem to be less frequent than previously reported; and that overall outcome with prompt initiation of therapy is favorable, including for those with meningitis. Larger studies with more patients are needed to identify those at the highest risk for developing complications and to define the most effective therapeutic approaches.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was financially supported by the St Jude Pediatric Oncology Education Program and American Lebanese Syrian Associated Charities.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Luna RA, Fasciano LR, Jones SC, et al. DNA pyrosequencing-based bacterial pathogen identification in a pediatric hospital setting. J Clin Microbiol 2007; 45:2985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McWhinney PH, Kibbler CC, Gillespie SH, et al. Stomatococcus mucilaginosus: an emerging pathogen in neutropenic patients. Clin Infect Dis 1992; 14:641–6. [DOI] [PubMed] [Google Scholar]
- 3. Ramanan P, Barreto JN, Osmon DR, Tosh PK. Rothia bacteremia: a 10-year experience at Mayo Clinic, Rochester, Minnesota. J Clin Microbiol 2014; 52:3184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chavan RS, Pannaraj PS, Luna RA, et al. Significant morbidity and mortality attributable to Rothia mucilaginosa infections in children with hematological malignancies or following hematopoietic stem cell transplantation. Pediatr Hematol Oncol 2013; 30:445–54. [DOI] [PubMed] [Google Scholar]
- 5. Lee AB, Harker-Murray P, Ferrieri P, et al. Bacterial meningitis from Rothia mucilaginosa in patients with malignancy or undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer 2008; 50:673–6. [DOI] [PubMed] [Google Scholar]
- 6. Maraki S, Papadakis IS. Rothia mucilaginosa pneumonia: a literature review. Infect Dis (Lond) 2015; 47:125–9. [DOI] [PubMed] [Google Scholar]
- 7. Ascher DP, Zbick C, White C, Fischer GW. Infections due to Stomatococcus mucilaginosus: 10 cases and review. Rev Infect Dis 1991; 13:1048–52. [DOI] [PubMed] [Google Scholar]
- 8. Wang JY, Brossard J, Cellot S, et al. Invasive Rothia infections in children with acute myeloid leukemia: a report from the Canadian infections in AML research group. Pediatr Hematol Oncol 2016; 33:277–81. [DOI] [PubMed] [Google Scholar]
- 9. Bruminhent J, Tokarczyk MJ, Jungkind D, DeSimone JA Jr. Rothia mucilaginosa prosthetic device infections: a case of prosthetic valve endocarditis. J Clin Microbiol 2013; 51:1629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaufhold A, Reinert RR, Kern W. Bacteremia caused by Stomatococcus mucilaginosus: report of seven cases and review of the literature. Infection 1992; 20:213–20. [DOI] [PubMed] [Google Scholar]
- 11. Henwick S, Koehler M, Patrick CC. Complications of bacteremia due to Stomatococcus mucilaginosus in neutropenic children. Clin Infect Dis 1993; 17:667–71. [DOI] [PubMed] [Google Scholar]
- 12. Lowry TR, Brennan JA. Stomatococcus mucilaginosis infection leading to early cervical necrotizing fasciitis. Otolaryngol Head Neck Surg 2005; 132:658–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.