Abstract
Background
The goals of abdominoplasty in massive weight loss (MWL) patients are often functional, with a greater emphasis on safety than on aesthetic rejuvenation. As important as functional improvements and safety are, however, there may be room for increasing the aesthetic potential of abdominoplasties in these patients.
Objectives
To determine the safety of the pursuit of aesthetic goals for abdominoplasty in MWL patients.
Methods
This is a retrospective study examining 910 consecutive female patients consisting of 3 groups: postpartum (n = 718), dietary MWL (n = 65), and bariatric MWL (n = 127). All patients were approached with a well-defined set of aesthetic goals which were pursued as needed and as feasible.
Results
The utilization of aesthetic abdominoplasty components was similar in all groups, supporting the assertion that the groups were subjected to a similar aesthetic emphasis. Logistic regression showed that a history of bariatric MWL was an independent risk factor for multiple complications (odds ratio 2.738, P = 0.014) and that elevated body mass index (BMI), smoking, diabetes, and age were likewise independent risk factors for complications. Propensity score-matched case-control pairs showed that bariatric MWL patients were more likely than dietary MWL patients to experience multiple complications (9.52% vs 0%, P = 0.031).
Conclusions
Bariatric MWL patients but not dietary weight loss patients seem to have a higher risk than postpartum patients. Other comorbidities (elevated BMI, smoking, diabetes, and age) seem to be more important predictors of complications than MWL status. Select MWL patients can likely be approached with an emphasis on aesthetic goals, without increasing risks as compared with the postpartum population.
Level of Evidence: 2

Abdominoplasty in the massive weight loss (MWL) patient tends to be different from abdominoplasty in the typical postpartum patient. MWL patients are more likely to suffer from functional problems such as intertrigo1 and back pain2 and are more likely to be anemic or suffer from nutritional deficiencies.3,4 Even though they have lost a good deal of weight, their body mass index (BMI) tends to remain higher, putting them at higher risk for surgical, anesthetic, and thromboembolic complications.5,6 The degree of abdominal soft tissue deformity tends to be greater.7 Psychological problems related to body image may be severe.8 There may be technical considerations such as preexisting abdominal scars or laparoscopic adjustable gastric band ports.9 It is fair to say that abdominoplasty in the MWL patient can present a series of challenges.
Although there is much written about enhancing the aesthetic outcome of abdominoplasty, most of the available literature on abdominoplasty in the MWL population focuses on the optimization of safety, minimization of complications, and functional or quality-of-life outcomes.10-14 There is relatively little written about strictly aesthetic issues in the MWL population. Clearly, there are extreme cases of “panniculectomy” in which aesthetic outcomes are remote considerations.15,16 In less extreme cases, it is important to consider the patient’s objectives: will the procedure be done for mainly aesthetic or mainly functional reasons? For many MWL patients, the aesthetic result is very important, as they are often burdened by powerful body image issues.17 In a heavier patient, it is more difficult to achieve a good aesthetic outcome, although the amount of aesthetic improvement tends to be greater,18 and this concept of maximal improvement would seem to apply especially to the MWL population. An important question is this: If one decides to strive for maximal aesthetic improvement in a patient who is so motivated, what are the specific goals? One can identify a series of key aesthetic elements in abdominoplasty and this represents a good conceptual starting point.19 It has been this current author’s practice to implement a similar series of objectives, an aspirational “aesthetic checklist,” to whatever extent is feasible in selected MWL patients (Appendix). These are the same objectives that are applied to postpartum abdominoplasties. Obviously, not all of these will be needed or even possible in all MWL patients, yet they are part of a mindset that takes the patient’s aesthetic goals seriously. However, another important question is this: what are the consequences, in terms of complications, of pushing an aesthetic agenda in the MWL population?
This “safety vs aesthetics” question presents a bit of a conundrum, as these 2 goals may be at cross purposes. Indeed, the reason that aesthetics do not seem to be emphasized in the literature on MWL abdominoplasties may stem from a need to focus on safety, as there are many studies that support the notion that these patients have higher complication rates.20-25 Although these studies do not provide absolute clarity, in the aggregate, they would certainly give a rational surgeon pause and would tend to temper enthusiasm for aesthetic goals when contemplating abdominoplasty in an MWL patient. This study represents an attempt to define the fraught relationship between aesthetic goals and safety in the MWL population. Since the focus of this study is on aesthetics, it makes sense to compare complications in the MWL population to a “control” group of purely aesthetic abdominoplasties, for example, the postpartum population that, aside from the fluctuations of pregnancy, has not lost a significant amount of weight. Thus, for this study, strictly postpartum vs MWL (many of which are also postpartum) women were compared. Since there is some speculation, as noted above, that the method of weight loss—either by bariatric surgery or through diet and exercise—may be an important factor for complication rates, this distinction was invoked and the MWL population was divided into the 2 types. This study aimed to compare complications among these groups, and since the baseline characteristics of these 3 groups were not equal, certain statistical analyses were undertaken in an attempt to control for preoperative differences in risk factors.
METHODS
The author’s database of 955 consecutive abdominoplasties over a 12-year period from January 2008 to December 2019 was examined. The exclusion of men yielded 910 women who had abdominoplasty with and without simultaneous aesthetic procedures (eg, breast surgery or other body contouring). These patients were then considered as 3 groups: a “control” group of postpartum patients without significant weight loss (postpartum, n = 718), a group with a history of greater than 50 pounds weight loss realized through diet and exercise (dietary MWL, n = 65), and a group with a history of greater than 50 pounds weight loss following bariatric surgery (bariatric MWL, n = 127). It is the author’s current practice to exclude abdominoplasties from all patients of any category until a goal of BMI < 35 is reached and until smoking cessation for 3 months is achieved. The database was queried for patient demographic and physical data as well as postoperative information regarding complications and operative reports were examined to gain information about the technique. Preoperative photographs were examined to determine deformity grade26 (Table 1). The study followed the guiding principles of the Belmont report, insofar as this study falls under the category of practice of “accepted therapy,” which is “designed solely to enhance the well-being of an individual patient and that has a reasonable expectation of success.” Furthermore, all provisions of the Declaration of Helsinki were followed in the conduct of this study, and written informed consent was obtained for all patients.
Table 1.
Pittsburgh Rating Scale for Abdominal Deformity
| Appearance | Classification | Numerical score |
|---|---|---|
| Normal | 0 | 0 |
| Moderate adiposity without overhang | 1 | 1 |
| Overhanging pannus | 2 | 2 |
| Double roll confined to panty/girdle line | 3a | 3 |
| Double roll extending to the midaxillary line | 3b | 4 |
| Double roll extending to back | 3c | 5 |
| Triple roll | 3d | 6 |
Operative Technique
Abdominoplasties in the postpartum group were performed in a fairly standard manner with flap undermining, fascial plication (repair of rectus diastasis plus paramedian fascial tightening as indicated), and flank liposuction and sub-Scarpa’s thinning as indicated.27 All operations were performed under general anesthesia. Sequential compression devices were applied to the lower extremities of all patients, and almost all patients had preoperative subcutaneous heparin. Postoperative chemoprophylaxis with either enoxaparin or rivaroxaban was administered for patients with a Caprini (2005) score of 4 or above. Progressive tension sutures were not used.
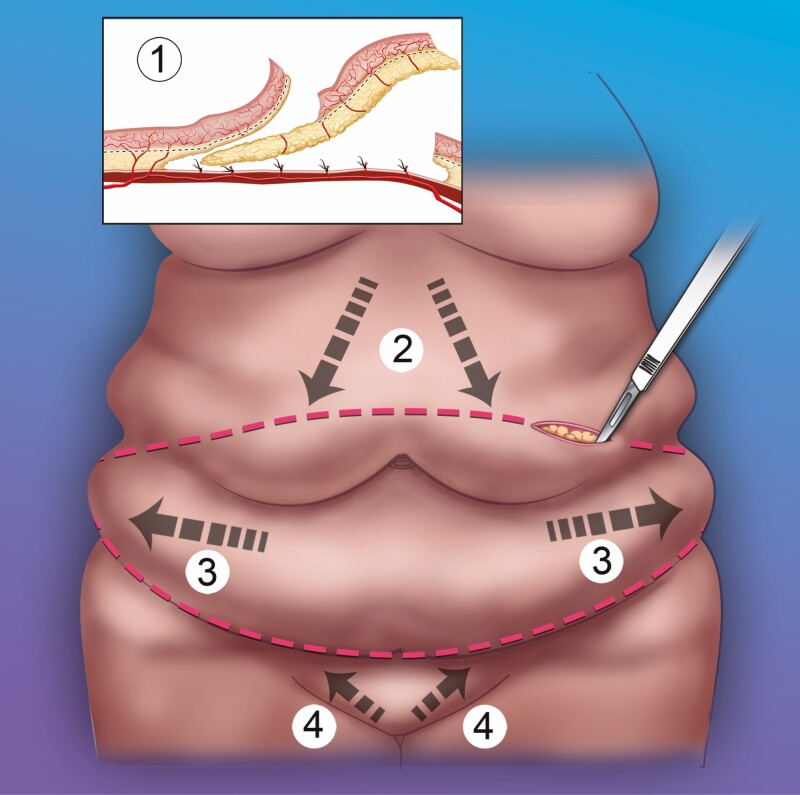
The operative approach in the MWL groups was somewhat different from that in the postpartum group. The MWL patients tended to have a greater degree of deformity (Table 2) with greater degrees of tissue laxity, including in the transverse direction, which underlies the utility of “fleur-de-lis” variants in the MWL patient population. However, in this series, the vertical scar was avoided by adherence to several principles (Figure 1), as follows:
Table 2.
Baseline Characteristics of Abdominoplasty Patients: Postpartum vs Dietary MWL vs Bariatric MWL
| Postpartum | Dietary MWL | Bariatric MWL | P-valuea | |
|---|---|---|---|---|
| No. of patients | 718 | 65 | 127 | NA |
| Weight loss (pounds) | NA | 85.49 | 107.74 | Dietary vs bariatric <0.001b |
| BMI (at abdominoplasty) | 26.74 | 28.28 | 30.12 | Postpartum vs dietary 0.039b
Postpartum vs bariatric <0.005b Dietary vs bariatric 0.032b |
| Deformity grade | 2.56 | 3.17 | 3.52 | Postpartum vs dietary <0.001b
Postpartum vs bariatric <0.001b Dietary vs bariatric 0.039b |
| Age (mean) | 43.27 | 41.12 | 44.75 | Postpartum vs dietary 0.212 Postpartum vs bariatric 0.268 Dietary vs bariatric 0.043b |
| % Smokers | 11.84(85) | 12.31(8) | 16.54(21) | Postpartum vs dietary 0.911 Postpartum vs bariatric 0.141 Dietary vs bariatric 0.439 |
| % Diabetic | 2.23(16) | 3.08(2) | 7.09(9) | Postpartum vs dietary 0.662 Postpartum vs bariatric 0.003b Dietary vs bariatric 0.258 |
ANOVA, analysis of variance; BMI, body mass index; MWL, massive weight loss; NA, not applicable. aCalculated using ANOVA with post hoc analysis, unpaired t test and χ² test. bStatistically significant difference between groups.
Figure 1.
Principles of abdominoplasty flap contouring in the massive weight loss population. 1. Flap itself may need improvement (thinning, release, and possible tightening), which can be achieved by sub-Scarpa’s resection with electrocautery. 2. Higher upper incision results in strong downwards/lateral tension vector. 3. Extended, upwards curving lower incision provides lateral tension vector. 4. Mons rejuvenation through a lower incision placement (approximately 6-7 cm from introitus) with upwards/lateral tension vector as well as mons thinning and fascial suspension.
The flap itself may need to be rehabilitated. Often, even after undermining, the flap will have multiple rolls, retractions, and contour irregularities, which prevent its smooth redraping onto the abdominal wall. Resection of the sub-Scarpa’s fat pad will not only thin the flap but also release the flap and render it smoother and more pliable. There may also be a significant element of flap retraction and tightening through the thorough application of electrocautery to the undersurface of Scarpa’s fascia. Although this assertion of controlled thermal injury is unproven, conceptually, it is analogous to skin shrinkage with laser28 and ultrasound-assisted29 lipoplasty, flap retraction with VASER lipoabdominoplasty (Solta Medical, Pleasanton, CA),30 thermal capsulorhaphy for breast implant revision,31 and orbital septum shrinkage with electrocautery in blepharoplasty.28-32 It is important to note that the sub-Scarpa’s resection must be done in the appropriate plane to avoid devascularizing the flap.27
Due to the increase in skin/tissue laxity, the downwards tension vector needs to be stronger than it does in the typical postpartum patient. This vector also has a lateral component. This means that the transverse cut delineating the lower edge of the flap may need to be higher than usual; almost always the umbilical donor site is removed with the resection specimen. There is little concern for excess tension on closure or vulvar distortion because, again, skin/tissue laxity is typically substantial and the mons ptosis that is the usual finding in these patients actually needs the upwards tension vector that is the complement of the stronger downwards tension vector. This approach results in the removal of greater amounts of skin and tissue, and since the tension vector has a lateral component, this alleviates some of the side-to-side laxity by distributing excess skin away from the midline.
The MWL patient typically needs an “extended” abdominoplasty with the incision extending back to the mid-axillary line or further; indeed, some will have circumferential or near circumferential approaches. The lateral extent of this extended incision curves upwards. This extension achieves 2 advantages. Firstly, there is a longer incision through which skin/tissue excess can be distributed laterally, and secondly, the upwards curve facilitates a lateral tension vector, which, again, can be used to help alleviate side-to-side tissue laxity by distributing excess skin away from the midline. This technique shares some of the concepts of the high-lateral-tension abdominoplasty,33 namely the recognition of side-to-side skin/tissue excess, an upwards curving lateral incision with significant lateral skin resection, an inferolateral tension vector, and superficial fascial system suspension.
The mons region deserves special attention in the MWL patient.34,35 Mons ptosis and tissue excess predispose to a globular postoperative appearance of the mons. This tendency can be counteracted by placing the lower incision at a lower point, typically measured 6 or 7 cm from the apex of the introitus with the skin under maximal tension, trimming the fat from the undersurface of the mons flap (or alternatively, mons liposuction), and applying upwards and lateral suturing of the mons to the fascia before skin closure.
This operative sequence is demonstrated in the Video. MWL patients will have a propensity toward seroma formation because BMI often remains high and because flank liposuction and sub-Scarpa’s lipectomies may be frequently utilized. Two and sometimes 4 Jackson-Pratt (JP) drains were placed. JP drains were removed once the effluent output dropped below 25 mL per day.
For the purposes of this study, major flap necrosis was defined as full-thickness loss of greater than 5 cm2, minor flap necrosis as full-thickness loss, epidermolysis or wound separation of less than 5 cm2, and fat necrosis as any palpable nodule present more than 3 months postoperatively. Infection was defined as any cellulitis, wound infection, or cloudiness of JP effluent.
Statistical Analysis
Univariate comparisons for continuous variables were performed using unpaired t tests and one-way analysis of variance (ANOVA) with post hoc analysis and for categorical variables with χ 2 tests. For multivariate comparisons, binary logistic regression models were created for each dependent variable (each complication or combination of complications and the need for revision). The principle of parsimony was applied, and the independent regression variables were taken from variables that on univariate analysis demonstrated a significance of 0.10. For case-control studies, a propensity score matching strategy was used to pair patients from the postpartum and weight loss groups that had similar baseline characteristics (the covariates of age, BMI, history of smoking, history of diabetes, fascial plication, flank liposuction, sub-Scarpa’s fat resection, and a simultaneous aesthetic procedure). Propensity scores were estimated by logistic regression followed by a nearest-neighbor matching algorithm seeking 1:1 matches with no replacement and a caliper of 0.2 of the standard deviation of the logit of the propensity score. Standardized mean differences for all covariates were calculated before and after matching to assess the adequacy of the matching process. After the matching process, dichotomous dependent variables (ie, the presence or absence of each complication or combination of complications and the need for revision) were compared by McMemar’s test. The above analyses were performed in SPSS version 27 (IBM Corporation, Armonk, NY) with the PS Matching extension (developed by Felix Thoemmes at Cornell University, Ithaca, NY) and R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Follow-up ranged between 6 months and 10 years (mean 17 months). The mean age of all patients was 43.27 years (range 16.68 to 74.51 years). Baseline characteristics of the 3 groups and the utilization of cosmetic components are summarized in Tables 2 and 3, respectively. Statistical significance was reached for the amount of weight loss, BMI, age, deformity grade, flank liposuction, and sub-Scarpa’s fat pad resection between dietary MWL and bariatric MWL. Statistical significance was reached for BMI, deformity grade, percentage of diabetic patients, sub-Scarpa’s fat pad resection, fascial plication, and flank liposuction between postpartum and bariatric MWL. Statistical significance was reached for BMI, deformity grade, percentage with a simultaneous aesthetic procedure, sub-Scarpa’s fat pad resection, and fascial plication between postpartum and dietary MWL. Any aesthetic component, defined as either flank liposuction or sub-Scarpa’s lipectomy or fascial plication or a combination of these, was performed in 86.35% of the postpartum group, 80% of the dietary MWL group, and 80.31% of the bariatric MWL group; these differences were not statistically significant.
Table 3.
Utilization of Aesthetic Components and Concomitant Aesthetic Procedures: Postpartum vs Dietary MWL vs Bariatric MWL
| Postpartum | Dietary MWL | Bariatric MWL | P-valuea | |
|---|---|---|---|---|
| %Simultaneous aesthetic procedure | 63.37(455) | 78.46(51) | 65.35(83) | Postpartum vs dietary 0.015b
Postpartum vs bariatric 0.668 Dietary vs bariatric 0.061 |
| % Flank liposuction | 21.87(157) | 16.92(11) | 4.72(6) | Postpartum vs dietary 0.353 Postpartum vs bariatric <0.001b Dietary vs bariatric 0.005b |
| %Sub-Scarpa’s fat resection | 33.84(243) | 46.15(30) | 48.82(62) | Postpartum vs dietary 0.046b
Postpartum vs bariatric 0.001b Dietary vs bariatric 0.726 |
| %Fascial plication | 83.28(598) | 61.54(40) | 64.57(82) | Postpartum vs dietary <0.005b
Postpartum vs Bariatric <0.005b Dietary vs bariatric 0.680 |
| Any aesthetic componentc | 86.35(620) | 80.00(52) | 80.31(102) | Postpartum vs dietary 0.150 Postpartum vs bariatric 0.075 Dietary vs bariatric 0.959 |
MWL, massive weight loss. aCalculated using χ² test. bStatistically significant difference between groups. cFlank liposuction, sub-Scarpa’s fat resection, plication, or combination.
Univariate analysis of complications is presented in Table 4. Statistical significance was reached for the following comparisons: seroma formation: postpartum vs dietary MWL (P = 0.038) and postpartum vs bariatric MWL (P = 0.008); any complication: postpartum vs bariatric MWL (P = 0.030); and multiple complications: postpartum vs bariatric MWL (P < 0.001) and dietary MWL vs bariatric MWL (P = 0.010). No other comparison reached statistical significance.
Table 4.
Univariate Analysis of Complications
| Total | Postpartum | Dietary massive weight loss | Bariatric massive weight loss | P-valuea | |
|---|---|---|---|---|---|
| No. of patients | 910 | 718 | 65 | 127 | — |
| Major flap necrosis (%) | 1.65(15) | 1.39(10) | 1.54(1) | 3.15(4) | Postpartum vs dietary 0.924 Postpartum vs bariatric 0.153 Dietary vs bariatric 0.507 |
| Minor flap necrosis (%) | 3.96(36) | 4.04(29) | 1.54(1) | 4.72(6) | Postpartum vs dietary 0.315 Postpartum vs bariatric 0.721 Dietary vs bariatric 0.265 |
| Fat necrosis (%) | 3.29(30) | 2.92(21) | 3.08(2) | 5.51(7) | Postpartum vs dietary 0.945 Postpartum vs bariatric 0.133 Dietary vs bariatric 0.450 |
| Seroma (%) | 13.08(119) | 11.28(81) | 20.00(13) | 19.68(25) | Postpartum vs dietary 0.038b
Postpartum vs bariatric 0.008b Dietary vs bariatric 0.959 |
| Infection (%) | 3.52(32) | 3.76(27) | 0 | 3.93(5) | Postpartum vs dietary 0.112 Postpartum vs bariatric 0.923 Dietary vs bariatric 0.105 |
| Hematoma (%) | 0.88(8) | 0.84(6) | 0 | 1.57(2) | Postpartum vs dietary 0.459 Postpartum vs bariatric 0.428 Dietary vs bariatric 0.309 |
| Venous thromboembolism (%) | 1.54(14) | 1.25(9) | 1.54(1) | 3.15(4) | Postpartum vs dietary 0.845 Postpartum vs bariatric 0.110 Dietary vs bariatric 0.507 |
| Any complication (%) | 24.28(221) | 22.70(163) | 27.69(18) | 31.49(40) | Postpartum vs dietary 0.361 Postpartum vs bariatric 0.030b Dietary vs bariatric 0.587 |
| Ischemic complicationc (%) | 8.24(75) | 7.79(56) | 6.15(4) | 11.81(15) | Postpartum vs dietary 0.663 Postpartum vs bariatric 0.133 Dietary vs bariatric 0.214 |
| Multiple complications (%) | 3.19(29) | 2.36(17) | 0 | 9.45(12) | Postpartum vs dietary 0.361 Postpartum vs bariatric <0.001b Dietary vs bariatric 0.010b |
| Revision (%) | 4.51(41) | 4.59(33) | 3.08(2) | 4.72(6) | Postpartum vs dietary 0.570 Postpartum vs bariatric 0.949 Dietary vs bariatric 0.589 |
aCalculated by χ² test. bStatistically significant difference between groups. cAny flap necrosis or fat necrosis.
For multivariate analyses, all the logistic regression models were statistically significant, indicating that each model was able to distinguish between patients with and without each complication. The models identified BMI as a predictor of major flap necrosis (odds ratio [OR] 1.163, 95% CI: 1.063-1.270, P = 0.001), fat necrosis (OR 1.083, 95% CI: 1.010-1.161, P = 0.025), seroma formation (OR 1.049, 95% CI: 1.008-1.092, P = 0.020), any complication (OR 1.066, 95% CI: 1.032-1.102, P < 0.001), any ischemic complication (1.060, 95% CI: 1.012-1.112, P = 0.015), and multiple complications (OR 1.099, 95% CI: 1.024-1.181, P = 0.009). A history of smoking was identified as a predictor of major flap necrosis (OR 4.619, 95% CI: 1.562-13.661, P = 0.006), minor flap necrosis (OR 2.599, 95% CI 1.168-5.782, P = 0.019), and any ischemic complication (OR 2.418, 95% CI: 1.320-4.431, P = 0.004). A history of diabetes was identified as a predictor of minor flap necrosis (OR 6.926, 95% CI: 2.355-20.363, P < 0.001) and any ischemic complication (OR 5.750, 95% CI: 2.361-14.004, P < 0.001). Age was identified as a predictor of infection (OR 1.061, 95% CI: 1.024-1.100, P = 0.001), venous thromboembolism (OR 1.054, 95% CI 1.003-1.108, P = 0.038), and multiple complications (OR 1.039, 95% CI: 1.002-1.078, P = 0.041). A history of bariatric MWL was an independent predictor of multiple complications (OR 2.738, 95% CI: 1.231-6.090, P = 0.014). These results are summarized in Table 5.
Table 5.
Predictors of Complications via Binary Logistic Regression
| Complication | Predictor | Odds ratio | 95% Confidence interval | P-value |
|---|---|---|---|---|
| Major flap necrosis (>5 cm2) | BMI | 1.163 | 1.063-1.270 | 0.001a |
| Smoking | 4.619 | 1.562-13.661 | 0.006a | |
| Minor flap necrosis(<5 cm2) | Diabetes mellitus | 6.926 | 2.355-20.363 | <0.001a |
| Smoking | 2.599 | 1.168-5.782 | 0.019a | |
| Fat necrosis | BMI | 1.083 | 1.010-1.161 | 0.025a |
| Seroma | BMI | 1.049 | 1.008-1.092 | 0.020a |
| Infection | Age | 1.061 | 1.024-1.100 | 0.001a |
| Venous thromboembolism | Age | 1.054 | 1.003-1.108 | 0.038a |
| Any complication | BMI | 1.066 | 1.032-1.102 | <0.001a |
| Ischemic complicationb | BMI | 1.060 | 1.012-1.112 | 0.015a |
| Smoking | 2.418 | 1.320-4.431 | 0.004a | |
| Diabetes mellitus | 5.750 | 2.361-14.004 | <0.001a | |
| Multiple complications | Age | 1.039 | 1.002-1.078 | 0.041a |
| BMI | 1.099 | 1.024-1.181 | 0.009a | |
| Bariatric MWL | 2.738 | 1.231-6.090 | 0.014a |
BMI, body mass index; MWL, massive weight loss. aStatistically significant. bAny flap necrosis or fat necrosis.
The matching algorithm was successful in that it yielded 61 case-control pairs comparing postpartum with dietary MWL, 120 case-control pairs comparing postpartum with bariatric MWL, and 63 case-control pairs comparing bariatric MWL with dietary MWL. These case-control pairs were all balanced with respect to the covariates of age, BMI, history of smoking, history of diabetes, fascial plication, flank liposuction, sub-Scarpa’s resection, and a simultaneous aesthetic procedure as reflected by a standardized mean difference of less than 0.25 for all of these covariates. A comparison of complications in these matched and balanced pairs showed no statistically significant differences between the postpartum group and either the dietary MWL group or the bariatric MWL group on any complication/group of complications/need for revision (Tables 6 and 7). However, when the bariatric MWL and the dietary MWL were compared directly, there was a statistically significant higher rate of multiple complications in the bariatric MWL group (Table 8).
Table 6.
Comparison of Complications in Propensity Score-Matched Pairs: Postpartum vs Dietary Massive Weight Loss
| Postpartum | Dietary massive weight loss | P-valuea | |
|---|---|---|---|
| No. of patients | 61 | 61 | — |
| % Major flap necrosis | 1.7(1) | 1.7(1) | 1.0 |
| % Minor flap necrosis | 3.3(2) | 1.7(1) | 1.0 |
| % Fat necrosis | 3.3(2) | 3.3(2) | 1.0 |
| % Seroma | 14.8(9) | 16.4(10) | 1.0 |
| % Infection | 0 | 0 | NS |
| % Hematoma | 0 | 0 | NS |
| % VTE | 0 | 0 | NS |
| % Any complication | 18.0(11) | 19.7(12) | 1.0 |
| % Ischemic complicationb | 8.19(5) | 6.56(4) | 1.0 |
| % Multiple complications | 1.64(1) | 0(0) | 1.0 |
| % Revision | 8.2(5) | 3.3(2) | .453 |
aCalculated using McNemar’s test. bAny flap necrosis or fat necrosis. NS, non significant; VTE, venous thromboembolism.
Table 7.
Comparison of Complications in Propensity Score-Matched Pairs: Postpartum vs Bariatric Massive Weight Loss
| Postpartum | Bariatric massive weight loss | P-valuea | |
|---|---|---|---|
| No. of patients | 120 | 120 | — |
| % Major flap necrosis | 2.50(3) | 3.33(4) | 1.00 |
| % Minor flap necrosis | 5.00(6) | 4.17(5) | 1.00 |
| % Fat necrosis | 5.00(6) | 5.00(6) | 1.00 |
| % Seroma | 12.5(15) | 19.16(23) | 0.229 |
| % Infection | 5.83(7) | 4.17(5) | 0.758 |
| % Hematoma | 0.83(1) | 1.66(2) | 1.0 |
| % VTE | 5.00(6) | 4.17(5) | 1.0 |
| % Any complication | 26.67(32) | 30.83(37) | 0.567 |
| % Ischemic complicationb | 10.83(13) | 11.67(14) | 1.0 |
| % Multiple complications | 5.83(7) | 8.33(10) | 0.629 |
| % Revision | 5.00(6) | 4.17(5) | 1.0 |
aCalculated using McNemar’s test. bAny flap necrosis or fat necrosis.
Table 8.
Comparison of Complications in Propensity Score-Matched Pairs: Dietary Weight Loss vs Bariatric Weight Loss
| Dietary weight loss | Bariatric weight loss | P-valuea | |
|---|---|---|---|
| No. of patients | 63 | 63 | — |
| % Major flap necrosis | 1.59(1) | 1.59(1) | 1.0 |
| % Minor flap necrosis | 1.59(1) | 4.76(3) | 0.617 |
| % Fat necrosis | 3.17(2) | 6.35(4) | 0.683 |
| % Seroma | 20.65(13) | 20.65(13) | 1.0 |
| % Infection | 0 | 3.17(2) | NS |
| % Hematoma | 0 | 0 | NS |
| % VTE | 0 | 1.59(1) | NS |
| % Any complication | 26.70(17) | 30.16(19) | 0.851 |
| % Ischemic complicationc | 6.35(4) | 14.29(9) | 0.267 |
| % Multiple complications | 0(0) | 9.52(6) | 0.031b |
| % Revision | 3.17(2) | 7.94(5) | 0.450 |
aCalculated using McNemar’s test. bStatistically significant difference between groups. cAny flap necrosis or fat necrosis. NS, non significant.
DISCUSSION
In this study, the bariatric MWL group but not the dietary MWL group had higher complication rates than the postpartum controls. This was true on univariate analysis, where the bariatric MWL group had a statistically higher rate of seroma formation, any complication, and multiple complications (Table 4); on multivariate analysis, where the bariatric MWL group had an increased risk of multiple complications (Table 5); and in case-control pairs, where the bariatric MWL group also had a higher rate of multiple complications (Table 8). The dietary MWL group did have a higher seroma rate than the postpartum group but this was only true on univariate analysis. These findings agree with studies by Greco et al,20 Lievain et al,21 Staalesen et al,22 Breiting et al,23 Vico et al24, and Constantine et al,36 which implied that bariatric MWL patients have a higher risk than dietary MWL patients, as well as with Hasenbegovic’s meta-analysis25 of these studies that concluded that a history bariatric MWL presented a 60% greater risk than a history of dietary MWL. These findings are at odds, however, with those of de Kerviler37 and Gusenoff et al,38 who found no difference between these 2 MWL groups. If the increase in multiple complications in the bariatric MWL group in this current study is true, one can hypothesize malnutrition as a possible cause. Bariatric MWL patients tend to have severe nutritional deficiencies39 (particularly hypoalbuminemia), even more so than dietary MWL38 patients, and in fact protein supplementation has been shown to ameliorate the risk of complications in bariatric MWL patients3 as well as in both bariatric and dietary MWL patients.40 This current study compared the MWL groups not only to each other but also to postpartum controls, because if we are to assess the safety of abdominoplasty in the MWL population, it seems that the postpartum, non-weight loss, “purely aesthetic” abdominoplasty is the low complication gold standard against which the MWL populations should be compared.
It is important to put these findings into the context of accompanying comorbidities. Any increased risk of the postbariatric population is not well defined and likely multifactorial; could there be other important risk factors besides the fact that the patient has lost a lot of weight? Probably, the strongest candidate for an additional important risk factor is residual obesity, as the MWL groups had statistically significant higher BMIs in spite of the MWL. For example, in this current study, logistic regression gives an OR of 1.163 for BMI and the complication of major flap necrosis, which suggests a 16.3% risk increase for each unit increase in the BMI. Since the mean BMI of the bariatric weight loss group was 3.38 units greater than the postpartum controls, this suggests a more than 60% increase in major flap necrosis due to BMI alone. A similar conclusion could be reached on the contribution of BMI to fat necrosis, seroma formation, any complication, any ischemic complication, or multiple complications in this current study (Table 5). Other authors have pointed to residual obesity as a principal culprit for the increased complication rate in the MWL population. For example, papers by Greco et al,20 Lievain et al,21 Coon et al,41 Vastine et al,42 and Winocour et al43 all concluded that elevated BMI at the time of abdominoplasty contributed, in various degrees, to an elevated complication rate. Au et al,44 in a study that examined postbariatric and dietary weight loss patients who had undergone a variety of body contouring procedures, concluded that BMI (post weight loss) at the time of body contouring was a predictor of postoperative complications. The previously referenced Arthurs et al5 article, looking at postbariatric panniculectomies using logistic regression, found that elevated BMI was an independent predictor for developing a postoperative complication, with an OR of 3.3. In a recent study also looking at just postbariatric abdominoplasties, Schlosshauer et al45 concluded that elevated BMI, age, and resection weights were risk factors for total complications. In addition to obesity, the MWL population may have a greater incidence of other risk factors, such as diabetes and smoking; this was the case in the previously referenced Greco et al’s study,20 and this would be expected to impact complication rates.46-48 In this current study, the bariatric MWL group had a statistically significant higher rate of diabetes as well as a statistically insignificant trend toward a greater percentage of smokers as compared with postpartum controls (Table 2). Also in this current study, the other baseline risk factors of diabetes, smoking, age, as well as elevated BMI were important, independent and statistically significant contributors to increased complications (Table 5). Along these lines, Dutot et al,49 in a recent large long-term study, concluded that the other risk factors of age, obesity, and smoking were more important factors than a history of weight loss. The evidence in this current paper as well as the just cited evidence in the literature raises the possibility that although a history of bariatric MWL may present independent risk, there is a collinearity of risk factors that may contribute substantially to the increased complication rates, and these additional comorbidities should be included in the risk calculus.50
What evidence is there that the MWL groups in this study were subject to the same “aesthetic emphasis” as the postpartum group? This is obviously highly subjective and difficult to quantify with anything even approaching scientific rigor. However, an overall sense can be obtained by looking at the rates of various “aesthetic components” of the abdominoplasties and the rates of performance of simultaneous aesthetic procedures. For example, in the author’s practice, fascial plication is performed solely to effect a change in the shape of the abdominal wall and never for a functional reason (although there is some evidence that fascial plication can have a functional benefit).51,52 Thus, for the purposes of this study, plication can be considered as an aesthetic component of abdominoplasty. Likewise, a sub-Scarpa’s fat resection and flank liposuction are done only for aesthetic reasons. The addition of a separate, simultaneous aesthetic procedure (eg, aesthetic breast surgery or other body contouring procedures) implies that an operation was done for at least partly aesthetic reasons. The percentages of patients in each group that had one or more of these aesthetic components (“any aesthetic component”, Table 3) were very similar, and the percentage of patients having a simultaneous aesthetic procedure was actually higher in the dietary weight loss group (Table 3), suggesting that the 3 groups (postpartum, dietary MWL, and bariatric MWL) were treated at least somewhat similarly with respect to aesthetic concerns.
There were 4 patients in the bariatric MWL group that had a thromboembolic event, and although this rate of 3.15% was not statistically different from postpartum controls or the dietary MWL group in any of the statistical analyses, this rate does seem high and is worthy of comment. There were no deaths in this series, but venous thromboembolism certainly carries that risk. Many of the MWL patients undergo simultaneous procedures (69.8% of the patients in this series) and this adds to the time under anesthesia, adding to the thromboembolic risk presented by the higher BMI in these patients. Although the data presented in this paper cannot provide recommendations for abatement of this risk, it does seem that chemoprophylaxis and/or staging53 of multiple procedures should be contemplated and weighed in many of these cases, and numerous studies offer recommendations for risk stratification and prophylaxis against the serious issue of thromboembolism in the abdominoplasty patient population.54-58
The zero implementation rate of a “fleur-de-lis” technique in this study is at odds with much of the literature on abdominoplasty in MWL patients and is also worthy of comment. Other authors22,26,41,54,59,60 report performing a substantial percentage of abdominoplasties in the MWL patient population as combined horizontal and vertical excisions and offer the rationale of correction of side-to-side skin/tissue laxity as an acceptable trade-off for the increased scar burden and potentially higher wound complication rate. However, the author of this current study has found it difficult to convince patients to accept the vertical scar component as many of them have the lofty aspiration to wear 2-piece bathing suits, and this patient reluctance regarding the vertical midline scar has been experienced by other authors.61 This provides the impetus for avoidance, but in which patients is it possible to avoid the vertical scar and still obtain acceptable side-to-side skin tightening? Patients with a Pittsburgh deformity rating26 of 2 or less will not likely need the additional skin excision but this represents only a small percentage of this current study in which the average deformity rating was 3.17 for dietary MWL and 3.52 for bariatric MWL patients. In spite of these high deformity ratings, a “fleur-de-lis” approach was not deemed to be necessary for any patient in this study. There are several possible reasons for this. Firstly, 11% of the MWL patients in this current study were circumferential “belt” lipectomies, and virtually all of the others were “extended” abdominoplasties where the posterior scar extended past the midaxillary line, providing greater latitude in reconciling the transverse skin excess. Other authors have likewise stated that “extended” or circumferential/near circumferential abdominoplasties can avoid the vertical skin excision by virtue of the greater incisional length to distribute the side-to-side skin laxity,61-63 even papers that frequently employ a “fleur-de-lis” approach state that circumferential lipectomies can avoid it.26,63 Secondly, other factors in this current author’s technique, namely the flap thinning,27,61 flap redraping, and possible flap tightening principles as outlined above (Methods and Figure 1), may allow for avoidance of the vertical excision even in some cases where preoperatively it might seem to be appropriate (Figures 2 and 3). Although the “fleur-de-lis” is a well-established technique with equivalent complication rates reported in some centers,64 other authors find the complication rates to be higher.49,54,65 It is the author’s opinion that in many cases the aesthetics are better (Figures 2 and 3) without the vertical midline scar, even if some minor side-to-side laxity remains. One center concurs with this assessment and has largely ceased the performance of the “fleur-de-lis” due to concerns regarding the aesthetics of the “hard to disguise” vertical scar as well as, in their experience, a higher complication rate.49
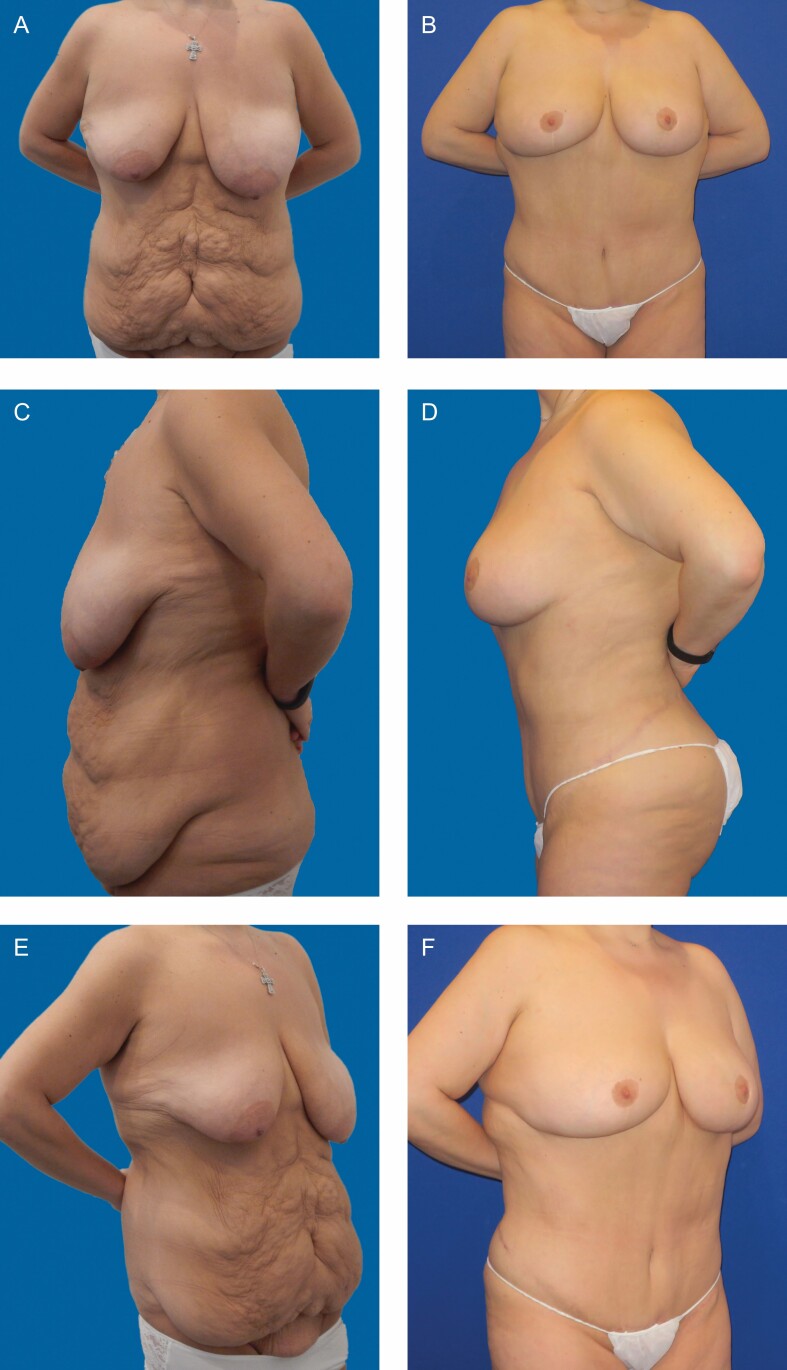
Figure 2.
A 31-year-old nonsmoking, nondiabetic woman with body mass index of 30.84 and a deformity rating of 3(b) had lost 140 pounds following gastric bypass and then underwent abdominoplasty with “belt” lipectomy and breast reduction. The anterior abdominal specimen weighed 4038 g. Photographs taken at 3 years postoperative follow-up. (A) Frontal preoperative view. (B) Frontal postoperative view. (C) Lateral preoperative view. (D) Lateral postoperative view. (E) Oblique preoperative view. (F) Oblique postoperative view.
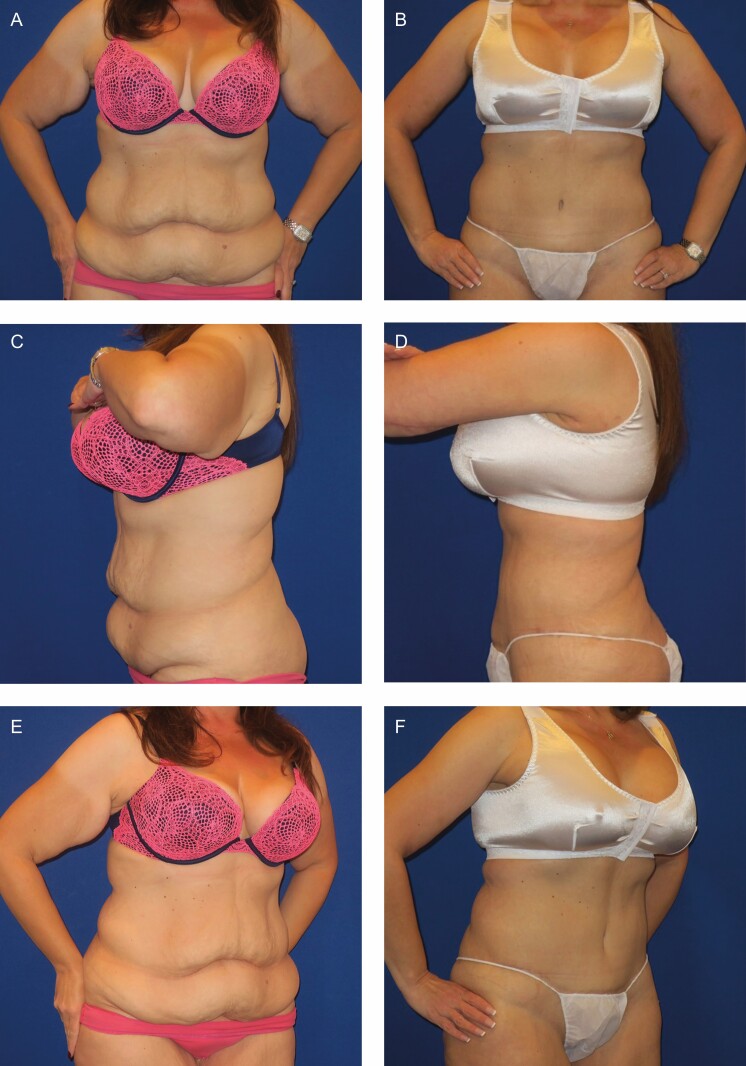
Figure 3.
A 43-year-old nonsmoking, nondiabetic woman with a body mass index of 32.89 and a deformity rating of 3(c) had lost 120 pounds by a program of diet and exercise and then underwent abdominoplasty and brachioplasty. Photographs taken at 1 year postoperative follow-up. (A) Frontal preoperative view. (B) Frontal postoperative view. (C) Lateral preoperative view. (D) Lateral postoperative view. (E) Oblique preoperative view. (F) Oblique postoperative view.
The strengths of this study are that it is a large, consecutive, single-surgeon study, so that relative uniformity of technique and assessment can be assumed. One weakness is that it is a retrospective study that relies on chart/electronic medical record review, which could miss or misclassify information. Another weakness is that the ability to record or even define “aesthetic intent” is by nature very subjective, as noted above. A third weakness is that there is no consideration given to the nutritional status of the patients, which may be a factor in wound healing in the MWL population.3,38-40 This study contributes to the knowledge base and differs from the majority of the literature in that it compares the MWL groups to postpartum patients and also attempts to introduce rarely mentioned aesthetic concepts in the MWL population.
CONCLUSIONS
The MWL abdominoplasty candidate often presents a challenge in risk mitigation as well as in the achievement of aesthetic goals. The risks may be higher due to comorbidities (chiefly residual obesity), which makes an argument for consideration of all risk factors as opposed to a focus merely on the history of weight loss. In addition, the data herein suggest that a history of bariatric MWL may be an independent risk factor for complications, whereas a history of dietary MWL seems not to be. Thus, postbariatric patients should be approached with greater caution. In both MWL groups, the deformities are greater so that the path to a good aesthetic outcome is longer (and potentially more perilous). However, select MWL candidates (lower BMI, nonsmoking, nondiabetic, and dietary MWL) can probably be approached with a mindset to optimize aesthetics, as in this study the prioritization of aesthetic goals in these patients did not increase the risk of major complications as compared with postpartum controls. This mindset might include attention to aesthetic details as previously delineated as well as careful reconsideration as to whether concessions to safety such as sacrifice of the umbilicus63,66 or unnatural skin patterns such as the W-plasty61,67-69 are actually necessary. In the author’s opinion, the common “fleur-de-lis” pattern may sometimes, perhaps often, be avoided as well. In this day and age of ubiquitous self-publication by patients of results on the internet, there is a better general awareness of plastic surgical techniques and outcomes; patients know what they want and especially what they don’t want. For this reason, it seems that aesthetic standards have been raised; thus, we are in a sense forced to reassess some of our old habits. “So, we should not be ashamed to change our methods; rather we should be ashamed not to do so.” 70 MWL patients have high expectations, just like postpartum patients, and so long as we can do it safely we should strive to meet those expectations.
Supplementary Material
Presented at: The abstract of this manuscript was presented at The Aesthetic Meeting 2021, Miami, FL.
Disclosures
The author declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The author received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1.Bossert RP, Rubin JP. Evaluation of the weight loss patient presenting for plastic surgery consultation. Plast Reconstr Surg. 2012;130(6):1361-1369. [DOI] [PubMed] [Google Scholar]
- 2.Staalesen T, Olsén MF, Elander A. The effect of abdominoplasty and outcome of rectus fascia plication on health-related quality of life in post-bariatric surgery patients. Plast Reconstr Surg. 2015;136(6):750e-761e. [DOI] [PubMed] [Google Scholar]
- 3.Austin RE, Lista F, Khan A, Ahmad J. The impact of protein nutritional supplementation for massive weight loss patients undergoing abdominoplasty. Aesthet Surg J. 2016;36(2):204-210. [DOI] [PubMed] [Google Scholar]
- 4.Michaels J 5th, Coon D, Rubin JP. Complications in postbariatric body contouring: strategies for assessment and prevention. Plast Reconstr Surg. 2011;127(3):1352-1357. [DOI] [PubMed] [Google Scholar]
- 5.Arthurs ZM, Cuadrado D, Sohn V, et al. Post-bariatric panniculectomy: pre-panniculectomy body mass index impacts the complication profile. Am J Surg. 2007;193(5):567-570; discussion 570. [DOI] [PubMed] [Google Scholar]
- 6.Colwell AS, Borud LJ. Optimization of patient safety in postbariatric body contouring: a current review. Aesthet Surg J. 2008;28(4):437-442. [DOI] [PubMed] [Google Scholar]
- 7.Song AY, Jean RD, Hurwitz DJ, Fernstrom MH, Scott JA, Rubin JP. A classification of contour deformities after bariatric weight loss: the Pittsburgh Rating Scale. Plast Reconstr Surg. 2005;116(5):1535-1544; discussion 1545. [DOI] [PubMed] [Google Scholar]
- 8.Song A, Fernstrom MH. Nutritional and psychological considerations after bariatric surgery. Aesthet Surg J. 2008;28(2):195-199. [DOI] [PubMed] [Google Scholar]
- 9.Wu AY, Coon JL, Huerta S, Condé-Green A, Morrow DM. The post-adjustable gastric band abdominoplasty. Aesthet Surg J. 2012;32(5):634-646. [DOI] [PubMed] [Google Scholar]
- 10.Coriddi MR, Koltz PF, Chen R, Gusenoff JA. Changes in quality of life and functional status following abdominal contouring in the massive weight loss population. Plast Reconstr Surg. 2011;128(2):520-526. [DOI] [PubMed] [Google Scholar]
- 11.van der Beek ESJ, Geenen R, de Heer FAG, van der Molen ABM, van Ramshorst B. Quality of life long-term after body contouring surgery following bariatric surgery: sustained improvement after 7 years. Plast Reconstr Surg. 2012;130(5):1133-1139. [DOI] [PubMed] [Google Scholar]
- 12.Masoomi H, Rimler J, Wirth GA, Lee C, Paydar KZ, Evans GRD. Frequency and risk factors of blood transfusion in abdominoplasty in post-bariatric surgery patients: data from the nationwide inpatient sample. Plast Reconstr Surg. 2015;135(5):861e-868e. [DOI] [PubMed] [Google Scholar]
- 13.Smith OJ, Hachach-Haram N, Greenfield M, et al. Body contouring surgery and the maintenance of weight-loss following Roux-En-Y gastric bypass: a retrospective study. Aesthet Surg J. 2018;38(2):176-182. [DOI] [PubMed] [Google Scholar]
- 14.Michaels J 5th, Coon D, Rubin JP. Complications in postbariatric body contouring: postoperative management and treatment. Plast Reconstr Surg. 2011;127(4):1693-1700. [DOI] [PubMed] [Google Scholar]
- 15.Manahan MA, Shermak MA. Massive panniculectomy after massive weight loss. Plast Reconstr Surg. 2006;117(7):2191-2197; discussion 2198. [DOI] [PubMed] [Google Scholar]
- 16.Matory WE Jr, O′Sullivan J, Fudem G, Dunn R. Abdominal surgery in patients with severe morbid obesity. Plast Reconstr Surg. 1994;94(7):976-987. [DOI] [PubMed] [Google Scholar]
- 17.Song AY, Rubin JP, Thomas V, Dudas JR, Marra KG, Fernstrom MH. Body image and quality of life in post massive weight loss body contouring patients. Obesity (Silver Spring). 2006;14(9):1626-1636. [DOI] [PubMed] [Google Scholar]
- 18.Saldanha OR, Salles AG, Ferreira MC, et al. Aesthetic evaluation of lipoabdominoplasty in overweight patients. Plast Reconstr Surg. 2013;132(5):1103-1112. [DOI] [PubMed] [Google Scholar]
- 19.Patronella CK. Redefining abdominal anatomy: 10 key elements for restoring form in abdominoplasty. Aesthet Surg J. 2015;35(8):972-986. [DOI] [PubMed] [Google Scholar]
- 20.Greco JA 3rd, Castaldo ET, Nanney LB, et al. The effect of weight loss surgery and body mass index on wound complications after abdominal contouring operations. Ann Plast Surg. 2008;61(3):235-242. [DOI] [PubMed] [Google Scholar]
- 21.Lievain L, Aktouf A, Auquit-Auckbur I, Coquerel-Beghin D, Scotte M, Milliez PY. [Abdominoplasty complications: particularities of the post-bariatric patients within a 238 patients series]. Ann Chir Plast Esthet. 2015;60(1):26-34. [DOI] [PubMed] [Google Scholar]
- 22.Staalesen T, Olsén MF, Elander A. Complications of abdominoplasty after weight loss as a result of bariatric surgery or dieting/postpregnancy. J Plast Surg Hand Surg. 2012;46(6):416-420. [DOI] [PubMed] [Google Scholar]
- 23.Breiting LB, Lock-Andersen J, Matzen SH. Increased morbidity in patients undergoing abdominoplasty after laparoscopic gastric bypass. Dan Med Bull. 2011;58(4):A4251. [PubMed] [Google Scholar]
- 24.Vico PG, De Vooght A, Nokerman B. Circumferential body contouring in bariatric and non-bariatric patient. J Plast Reconstr Aesthet Surg. 2010;63(5):814-819. [DOI] [PubMed] [Google Scholar]
- 25.Hasanbegovic E, Sørensen JA. Complications following body contouring surgery after massive weight loss: a meta-analysis. J Plast Reconstr Aesthet Surg. 2014;67(3):295-301. [DOI] [PubMed] [Google Scholar]
- 26.Zammerilla LL, Zou RH, Dong ZM, Winger DG, Rubin JP, Gusenoff JA. Classifying severity of abdominal contour deformities after weight loss to aid in patient counseling: a review of 1006 cases. Plast Reconstr Surg. 2014;134(6):888e-894e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restifo RJ. Sub-Scarpa’s lipectomy in abdominoplasty: an analysis of risks and rewards in 723 consecutive patients. Aesthet Surg J. 2019;39(9):966-976. [DOI] [PubMed] [Google Scholar]
- 28.DiBernardo BE. Randomized, blinded split abdomen study evaluating skin shrinkage and skin tightening in laser-assisted liposuction versus liposuction control. Aesthet Surg J. 2010;30(4):593-602. [DOI] [PubMed] [Google Scholar]
- 29.Nagy MW, Vanek PF Jr. A multicenter, prospective, randomized, single-blind, controlled clinical trial comparing VASER-assisted lipoplasty and suction-assisted lipoplasty. Plast Reconstr Surg. 2012;129(4):681e-689e. [DOI] [PubMed] [Google Scholar]
- 30.Hoyos A, Perez ME, Guarin DE, Montenegro A. A report of 736 high-definition lipoabdominoplasties performed in conjunction with circumferential VASER liposuction. Plast Reconstr Surg. 2018;142(3):662-675. [DOI] [PubMed] [Google Scholar]
- 31.Harris R, Raphael P, Harris SW. Thermal capsulorrhaphy: a modified technique for breast pocket revision. Aesthet Surg J. 2014;34(7):1041-1049. [DOI] [PubMed] [Google Scholar]
- 32.Prado A, Andrades P, Danilla S, Castillo P, Benitez S. Nonresective shrinkage of the septum and fat compartments of the upper and lower eyelids: a comparative study with carbon dioxide laser and Colorado needle. Plast Reconstr Surg. 2006;117(6):1725-1735; discussion 1736. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood T. High-lateral-tension abdominoplasty with superficial fascial system suspension. Plast Reconstr Surg. 1995;96(3):603-615. [DOI] [PubMed] [Google Scholar]
- 34.Bloom JM, Van Kouwenberg E, Davenport M, Koltz PF, Shaw RB Jr, Gusenoff JA. Aesthetic and functional satisfaction after monsplasty in the massive weight loss population. Aesthet Surg J. 2012;32(7):877-885. [DOI] [PubMed] [Google Scholar]
- 35.Michaels J 5th, Friedman T, Coon D, Rubin JP. Mons rejuvenation in the massive weight loss patient using superficial fascial system suspension. Plast Reconstr Surg. 2010;126(1):45e-46e. [DOI] [PubMed] [Google Scholar]
- 36.Constantine RS, Davis KE, Kenkel JM. The effect of massive weight loss status, amount of weight loss, and method of weight loss on body contouring outcomes. Aesthet Surg J. 2014;34(4):578-583. [DOI] [PubMed] [Google Scholar]
- 37.de Kerviler S, Hüsler R, Banic A, Constantinescu MA. Body contouring surgery following bariatric surgery and dietetically induced massive weight reduction: a risk analysis. Obes Surg. 2009;19(5):553-559. [DOI] [PubMed] [Google Scholar]
- 38.Gusenoff JA, Coon D, Rubin JP. Implications of weight loss method in body contouring outcomes. Plast Reconstr Surg. 2009;123(1):373-376. [DOI] [PubMed] [Google Scholar]
- 39.Agha-Mohammadi S, Hurwitz DJ. Enhanced recovery after body-contouring surgery: reducing surgical complication rates by optimizing nutrition. Aesthetic Plast Surg. 2010;34(5):617-625. [DOI] [PubMed] [Google Scholar]
- 40.Agha-Mohammadi S, Hurwitz DJ. Nutritional deficiency of post-bariatric surgery body contouring patients: what every plastic surgeon should know. Plast Reconstr Surg. 2008;122(2):604-613. [DOI] [PubMed] [Google Scholar]
- 41.Coon D, Gusenoff JA, Kannan N, El Khoudary SR, Naghshineh N, Rubin JP. Body mass and surgical complications in the postbariatric reconstructive patient: analysis of 511 cases. Ann Surg. 2009;249(3):397-401. [DOI] [PubMed] [Google Scholar]
- 42.Vastine VL, Morgan RF, Williams GS, et al. Wound complications of abdominoplasty in obese patients. Ann Plast Surg. 1999;42(1):34-39. [DOI] [PubMed] [Google Scholar]
- 43.Winocour J, Gupta V, Ramirez JR, Shack RB, Grotting JC, Higdon KK. Abdominoplasty: risk factors, complication rates, and safety of combined procedures. Plast Reconstr Surg. 2015;136(5):597e-606e. [DOI] [PubMed] [Google Scholar]
- 44.Au K, Hazard SW 3rd, Dyer AM, Boustred AM, Mackay DR, Miraliakbari R. Correlation of complications of body contouring surgery with increasing body mass index. Aesthet Surg J. 2008;28(4):425-429. [DOI] [PubMed] [Google Scholar]
- 45.Schlosshauer T, Kiehlmann M, Jung D, Sader R, Rieger UM. Post-bariatric abdominoplasty: analysis of 406 cases with focus on risk factors and complications. Aesthet Surg J. 2021;41(1):59-71. [DOI] [PubMed] [Google Scholar]
- 46.Bamba R, Gupta V, Shack RB, Grotting JC, Higdon KK. Evaluation of diabetes mellitus as a risk factor for major complications in patients undergoing aesthetic surgery. Aesthet Surg J. 2016;36(5):598-608. [DOI] [PubMed] [Google Scholar]
- 47.Kantar RS, Rifkin WJ, Wilson SC, et al. Abdominal panniculectomy: determining the impact of diabetes on complications and risk factors for adverse events. Plast Reconstr Surg. 2018;142(4):462e-471e. [DOI] [PubMed] [Google Scholar]
- 48.Manassa EH, Hertl CH, Olbrisch RR. Wound healing problems in smokers and nonsmokers after 132 abdominoplasties. Plast Reconstr Surg. 2003;111(6):2082-2087; discussion 2088. [DOI] [PubMed] [Google Scholar]
- 49.Dutot MC, Serror K, Al Ameri O, Chaouat M, Mimoun M, Boccara D. Improving safety after abdominoplasty: a retrospective review of 1128 cases. Plast Reconstr Surg. 2018;142(2):355-362. [DOI] [PubMed] [Google Scholar]
- 50.Vu MM, Ellis MF, Blough JT, Gutowski KA, Kim JYS. Development and internal validation of the abdominoplasty risk calculator. Plast Reconstr Surg. 2018;141(1):34e-45e. [DOI] [PubMed] [Google Scholar]
- 51.Temel M, Türkmen A, Berberoğlu Ö. Improvements in vertebral-column angles and psychological metrics after abdominoplasty with rectus plication. Aesthet Surg J. 2016;36(5):577-587. [DOI] [PubMed] [Google Scholar]
- 52.Oneal RM, Mulka JP, Shapiro P, Hing D, Cavaliere C. Wide abdominal rectus plication abdominoplasty for the treatment of chronic intractable low back pain. Plast Reconstr Surg. 2011;127(1):225-231. [DOI] [PubMed] [Google Scholar]
- 53.Coon D, Michaels J 5th, Gusenoff JA, Purnell C, Friedman T, Rubin JP. Multiple procedures and staging in the massive weight loss population. Plast Reconstr Surg. 2010;125(2):691-698. [DOI] [PubMed] [Google Scholar]
- 54.Seth AK, Lin AM, Austen WG Jr, Gilman RH, Gallico GG 3rd, Colwell AS. Impact of patient subtype and surgical variables on abdominoplasty outcomes: a 12-year Massachusetts General Hospital experience. Plast Reconstr Surg. 2017;140(5):899-908. [DOI] [PubMed] [Google Scholar]
- 55.Hatef DA, Trussler AP, Kenkel JM. Procedural risk for venous thromboembolism in abdominal contouring surgery: a systematic review of the literature. Plast Reconstr Surg. 2010;125(1):352-362. [DOI] [PubMed] [Google Scholar]
- 56.Pannucci C. Evidence-based recipes for venous thromboembolism prophylaxis: a practical safety guide. Plast Reconstr Surg. 2017;139(2):520-532e. [DOI] [PubMed] [Google Scholar]
- 57.Murphy RX Jr, Alderman A, Gutowski K, et al. Evidence-based practices for thromboembolism prevention: summary of the ASPS venous thromboembolism task force report. Plast Reconstr Surg. 2012;130(1):168e-175e. [DOI] [PubMed] [Google Scholar]
- 58.Somogyi RB, Ahmad J, Shih JG, Lista F. Venous thromboembolism in abdominoplasty: a comprehensive approach to lower procedural risk. Aesthet Surg J. 2012;32(3):322-329. [DOI] [PubMed] [Google Scholar]
- 59.Mendes FH, Viterbo F, Luna ALAP. Inner scar umbilicus: new horizons for vertical abdominoplasty. Plast Reconstr Surg. 2018;141(4):507e-516e. [DOI] [PubMed] [Google Scholar]
- 60.Leahy PJ, Shorten SM, Lawrence WT. Maximizing the aesthetic result in panniculectomy after massive weight loss. Plast Reconstr Surg. 2008;122(4):1214-1224. [DOI] [PubMed] [Google Scholar]
- 61.Strauch B, Herman C, Rohde C, Baum T. Mid-body contouring in the post-bariatric surgery patient. Plast Reconstr Surg. 2006;117(7):2200-2211. [DOI] [PubMed] [Google Scholar]
- 62.Aly AS, Cram AE, Chao M, Pang J, McKeon M. Belt lipectomy for circumferential truncal excess: the University of Iowa experience. Plast Reconstr Surg. 2003;111(1):398-413. [DOI] [PubMed] [Google Scholar]
- 63.Borud LJ, Warren AG. Modified vertical abdominoplasty in the massive weight loss patient. Plast Reconstr Surg. 2007;119(6):1911-1921. [DOI] [PubMed] [Google Scholar]
- 64.Friedman T, O′Brien Coon D, Michaels V J, et al. Fleur-de-Lis abdominoplasty: a safe alternative to traditional abdominoplasty for the massive weight loss patient. Plast Reconstr Surg. 2010;125(5):1525-1535. [DOI] [PubMed] [Google Scholar]
- 65.Chaouat M, Levan P, Lalanne B, Buisson T, Nicolau P, Mimoun M. Abdominal dermolipectomies: early postoperative complications and long-term unfavorable results. Plast Reconstr Surg. 2000;106(7):1614-1618; discussion 1619. [DOI] [PubMed] [Google Scholar]
- 66.Fernando da Costa L, Landecker A, Manta AM. Optimizing body contour in massive weight loss patients: the modified vertical abdominoplasty. Plast Reconstr Surg. 2004;114(7):1917-1923. [DOI] [PubMed] [Google Scholar]
- 67.Wilkinson TS. Mini-abdominoplasty. Plast Reconstr Surg. 1988;82(5):917-918. [DOI] [PubMed] [Google Scholar]
- 68.Wilkinson TS. Reply: about the W abdominoplasty. Plast Reconstr Surg. 1989;83(6):1084. [DOI] [PubMed] [Google Scholar]
- 69.Heller JB, Teng E, Knoll BI, Persing J. Outcome analysis of combined lipoabdominoplasty versus conventional abdominoplasty. Plast Reconstr Surg. 2008;121(5):1821-1829. [DOI] [PubMed] [Google Scholar]
- 70.Rosenfield LK. Commentary on: Post-bariatric abdominoplasty: analysis of 406 cases with focus on risk factors and complications. Aesthet Surg J. 2021;41(1):72-73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.