Abstract
Background
Hyaluronic acid (HA) is a key contributor to skin moisture (hydration), and MicroBotox demonstrates improvements for fine wrinkles of the face.
Objectives
The author sought to evaluate the safety and efficacy of intradermal injection of hydrotoxin (combined mixture of MicroHA and MicroBotox) for the treatment of skin roughness and dryness on facial dermis.
Methods
Fifty women who had thin, dry skin with fine wrinkles throughout the whole face, especially in the crow’s feet and forehead areas, were enrolled in the study. Two cc stabilized-HA filler and 1 cc (40 U) of botulinumtoxinA were mixed in the novel combined hydrotoxin mixture. Intended to hydrate the dermis and treat fine wrinkles of the face, the mixture was injected into the real dermal layer of the face. The volume of HA per site was 0.002 cc and toxin was 0.04 U. Skin roughness and stratum corneum hydration were measured at 1, 2, 3, and 6 months.
Results
One month post-treatment, skin roughness was reduced to 50.19% in topographic computer analysis utilizing 10× dermascope photos. Stratum corneum hydration on crow’s feet improved to 81.34% at 1 month and 56.12% at 2 months from pre-treatment baseline (P < 0.0001). Global Aesthetic improvement scale of Skin hydration and fine wrinkle improved.
Conclusions
The combination injection method of MicroHA and MicroBotox is not associated with side effects and showed significant synergic effect in improvement of skin roughness and moisturizing. Neuramix-hydrotoxin injection method is an easy and reproducible procedure to make constant injection depth and amount.
Level of Evidence: 4

The human dermis is a complex structure comprised of cells and extracellular matrix (ECM). The ECM is mostly comprised of protein fibers (collagen, reticular, and elastic fibers) and ground substance.1 Ground substance is a highly hydrophilic, viscous, and complex constituent such that the exchange of nutrients and metabolic waste between cells, fibers, and blood supply is best facilitated by the hydrated nature of the ECM.1 Skin aging is caused by intrinsic and extrinsic aging2,3 and also by a loss of skin moisture,4 which can be considered a major causative factor.
Hyaluronic acid (HA) plays a key role in skin health and moisture. Therefore, loss of HA can dehydrate skin, aggravate skin roughness, and cause development of wrinkles with dermal atrophy.5 Aesthetic injections for HA delivery are currently performed manually, resulting in a technique overly difficult and exhausting due to many required injections.6,7 In addition, injection of stabilized HA into the dermis can lead to long-lasting visible lumps on the skin surface.6,7 Deep injection (under the dermis) can eliminate the visible lumps on the dermis but cause only a volumizing effect with barely perceptible changes on the skin surface.8 Stabilized HA causes lumps, whereas the effects of non-stabilized HA are not prolonged. To solve this dilemma, a special injection method was designed by the author to administer stabilized HA into the true dermal layer of the face. By utilizing an automatic injector instead of manual performance, extremely small amounts of HA can be injected into each of the 1000 dermal sites.8,9 This study presents an ideal method for replenishing HA to hydrate the dermal ECM, with a focus on the ground substances. When Micro-botulinumtoxinA (MicroBotox) was utilized in combination with MicroHA, the procedure was twice as effective as a single injection. The combination method showed notable improvements of fine wrinkles on the face while reducing the injector’s effort compared with individual injections.
The objective of this study was to scientifically demonstrate and measure the effectiveness of such a technique in moisturizing skin and treating fine wrinkles. The improvement of fine wrinkles was evaluated through changes in skin roughness, measured by topographic analysis with the Gwyddion computer program.10 Dermal hydration was determined by noninvasively measuring stratum corneum hydration (SCH).7
METHODS
This was a prospective, single-center study conducted from June 2016 to December 2017. Fifty women with thin, dry skin with fine wrinkles throughout the whole face, especially in the crow’s feet and forehead areas, were enrolled in the study. All patients were treated at the author’s clinic for at least 6 months prior to the current study with only Botox injections into the entire dermis of face and were familiar with the injection processes associated with MicroBotox and MicroHA. Due to a gap longer than 6 months between the previous botulinumtoxin treatment and this study, the previously injected botulinumtoxin had no positive influence on skin roughness or hydration results. When the trial was conducted, the effects of botulinumtoxin had been exhausted and this state was set as a baseline.
Informed consent was obtained from each patient on the utilization of MicroHA and MicroBotox, and the study was conducted in accordance with the principles of the Declaration of Helsinki. Patients provided full medical histories and confirmed that they had not received any treatment involving high-intensity focused ultrasound, radiofrequency, or fractional lasers 1 year prior to and during the study. All patients received intradermal injections utilizing the novel mixture of HA and Botox.
Prior to the procedure, patients were seated in a relaxed position and their faces were first treated with a layer of 9% lidocaine topical anesthetic cream with plastic wrap for occlusive draping. After 30 minutes, the cream was removed thoroughly with facewash.
To make the novel injection solution (Neuramix hydrotoxin mixture, the author, Korea), 2 cc (40 mg) of lower cohesiveness, monophasic, stabilized HA (Neuramis Light Lidocaine, Medytox, Korea) was loaded in a 3-cc syringe, and 1 cc (40 U) of Botox (Neuronox, Medytox) in another 3-cc syringe. After carefully removing air bubbles, the two 3-cc syringes were connected by a sterilized connector, and the hydrotoxin mixture was passed 30 times through the two 3-cc syringes to mix materials evenly and homogenously (Video, available online at www.aestheticsurgeryjournal.com).
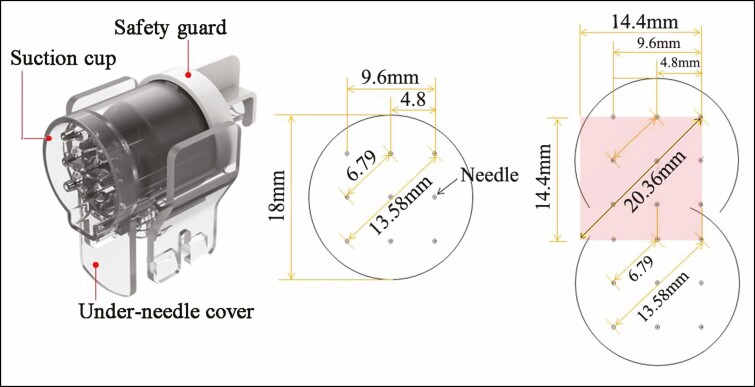
To precisely control the injection amount and depth, an automatic injector (Neurajet, Medytox) was employed. Because the automatic injector has nine 31G needles in the needle part, 111 strokes result in 999 injection sites (Figure 1). Simultaneously 0.002 cc of HA (MicroHA) and 0.04 U of Botox (MicroBotox) were delivered into 999 sites of the real dermis. Face surface area for an adult is usually approximately 450 cm2.11 Upper eyelid, eyebrow, lips, and forehead hair line are not suitable for injection, so 111 passes (230 cm2) can cover the injectable area of face uniformly (Figure 1). After injection, the patient’s face was covered by a cold wet gauze containing vitamin K to relieve post-procedural pain and ecchymosis. To facilitate spreading of the HA, molding and compression with a roller was utilized over the cold wet gauze (Video, available online at www.aestheticsurgeryjournal.com).
Figure 1.
Needle part of the injector. In the needle part, 9 needles are arranged in a 3 × 3 pattern, with each needle in 4.8-mm increments. One stroke (pass) creates 9 injection sites and covers about 2.0736 cm2 (1.44 cm × 1.44 cm) of area on the face. Thus, 111 strokes create 999 injection sites and cover 230 cm2 of area (2.0736 cm2 × 111 strokes = 230 cm2) on the patient’s entire face.
On the day of acquiring biophysical measurements, patients were instructed not to apply any product on their faces. Patients cleaned their faces utilizing the same cleansing product. Thirty minutes prior to measurements, patients remained at rest at the author’s clinic at room temperature (18-22°C). Changes in the appearance of the facial skin were assessed by topographic computer analysis of 10× dermascopic photos, and SCH was quantified and recorded at baseline and 1, 2, 3, and 6 months after the injection.
Skin Roughness and Morphology
At baseline and 1 month, skin roughness and morphology on the crow’s feet (rectangle areas vertically from 1 to 4 cm from the lateral canthi) and forehead areas (rectangular area vertically from eyebrow to trichion, horizontally between 2 pupil lines) were evaluated with a dermascope at 10 and 50 magnification employing a standard, non-contact method (Coscam, Sometech, Korea) in a closed system condition utilizing only the light of the dermascope without external light (Figure 2).
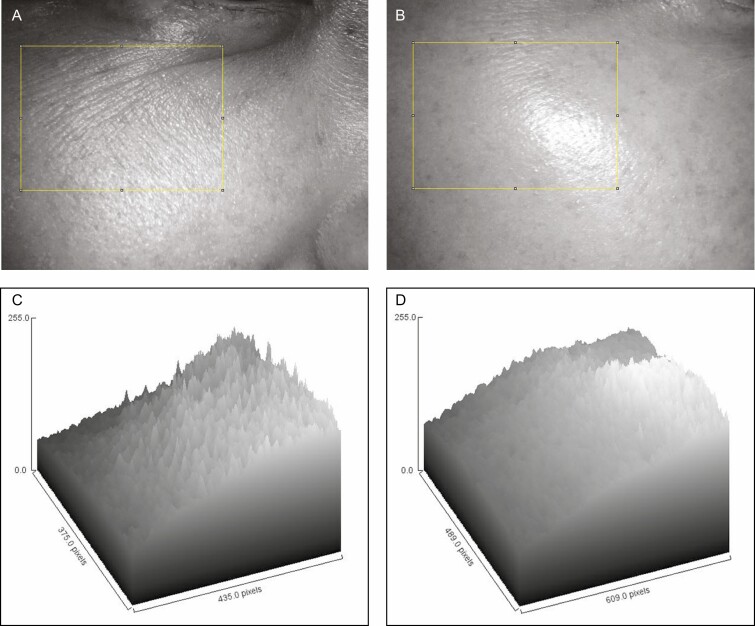
Figure 2.
The changes in roughness (A, C) before and (B, D) 1 month after the treatment of this 42-year-old woman (the same patient shown in Figure 3). (A, B) Dermatoscopic photos 10×. The yellow box above can be exhibited in computer topographic microscopic photos (C, D). This tool plots characteristics of the surface roughness for each column. Utilizing column statistics of the Gwyddion computer program in the same area, mean roughness of all patients was reduced by 51.9% at 1 month posttreatment from 7.81 ± 0.95 to 3.89 ± 0.43. (Ra: roughness average of the yellow box, a parameter of the Gwyddion computer program).
Evaluation and Quantification of Skin Roughness With Row/Column Statistics Tool
Microscopic photographs (10×) were taken of crow’s feet and forehead and utilized to quantify the skin surface roughness with a computer program (Gwyddion Statistical Analysis, Brno, Czech Republic10) at baseline and after treatment. Utilizing the computer program, the same areas (same crow’s feet and forehead areas) were chosen on the before and after microscopic photos and their measured roughness compared. The roughness can be expressed by digitalization by the computer program. The computer program can measure and calculate countless points of roughness on crow’s feet and forehead areas. This method for skin roughness measurement is very scientific and reproducible by anyone.
Roughness was evaluated and measured by the Row/Column Statistics Tool utilizing dermascopic photos by 10× before and 1 month after treatment.10 The Row/Column Statistics tool calculates numeric characteristics of each row or column and plots them as a function of its position. Roughness average () is the mean value of the height irregularities computed from data variance in a selected box area, and the standard roughness parameter Ra can be determined through the following equation:
Based on the principles and equations above, when areas of 10× microscopic photos were selected, skin roughness was calculated topographically employing Ra with the computer program (Gwyddion Statistical Analysis).10 Among countless rows/columns, individual rows showed the graph displaying the values. As a sample, the mean value and the standard deviation of the selected quantity are calculated from the set of individual row/column values (Figure 2).
Measurement of Dermal Hydration Utilizing Stratum Corneum Hydration
Using a noninvasive instrument (Gpskin-Barrier, Gpower, Korea), the effects of the treatment on dermal hydration were measured in terms of SCH at 0, 1, 2, and 6 months posttreatment. The points of measurements for the crow’s feet area on right side were 2 cm laterally and 2 cm caudally from right lateral canthus.
Patient Satisfaction Utilizing the Global Aesthetic Improvement Scale
Patient satisfaction with improvements in skin hydration and fine wrinkles was recorded using the Global Aesthetic Improvement Scale (GAIS: (0 = worse, 1 = no change, 2 = improved, 3 = much improved, 4 = very much improved) at 1, 2, and 6 months posttreatment.
RESULTS
All patients (50 women with an average age of 46.7 years; range, 32-69 years) were followed-up 6 months after the treatment without any additional cosmetic procedures on their faces. In the first week posttreatment, improvements in dryness and fine wrinkles were not perceptible by patients; more significant changes occurred a few weeks after the procedure. The delay in response may have been due to the spreading HA between collagen fibers from each injection site. Changes in fine wrinkles were more noticeable with the 10× dermascope than in physical examination at 1, 2, and 6 months. There have been cases in which the patient did not perceive much improvement in the fine wrinkles during the first 1 or 2 weeks. In these cases, a dermascope may be utilized to assure them of the improvements and allow the patients to wait with peace of mind until the improvements are more visible to the naked eye.
In all patients (100%), these morphological improvements were observed 1 month after the treatment (Figures 3-5) and lasted for up to 5 to 6 months. According to the survey, recurrence of dryness and fine wrinkles were noted by 8 patients (16%) at 4 months, by 13 patients (26%) at 5 months, and by 28 patients (56%) at 6 months. In the remaining 22 patients (44%), improvements in dryness and fine wrinkles lasted for longer than 6 months. This survey was conducted anonymously in the clinic. Survey sheets were distributed to participants and collected from them by clinic staff (Appendix).
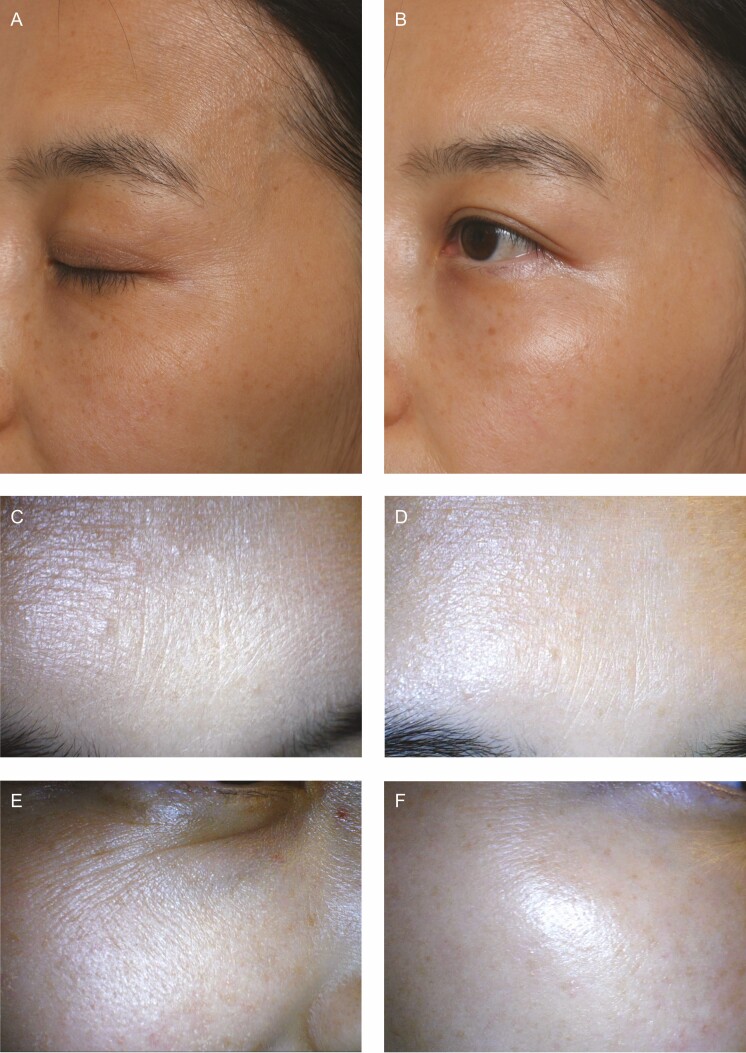
Figure 3.
(A, C, E) Before and (B, D, F) 1-month posttreatment photographs with the hydrotoxin method (MicroHA + MicroBotox) of this 42-year-old woman (the same patient shown in Figure 2). Before treatment, the skin had fine wrinkles with dryness on the forehead and crow’s feet. Improvements in fine wrinkles exhibited in these photos correspond to the improved roughness shown by the topographic computer analysis in Figure 2. The changes in roughness were always more noticeable when the face was viewed under a 10× dermascope (C-F) than when viewed with the naked eye or medical photos (A, B).
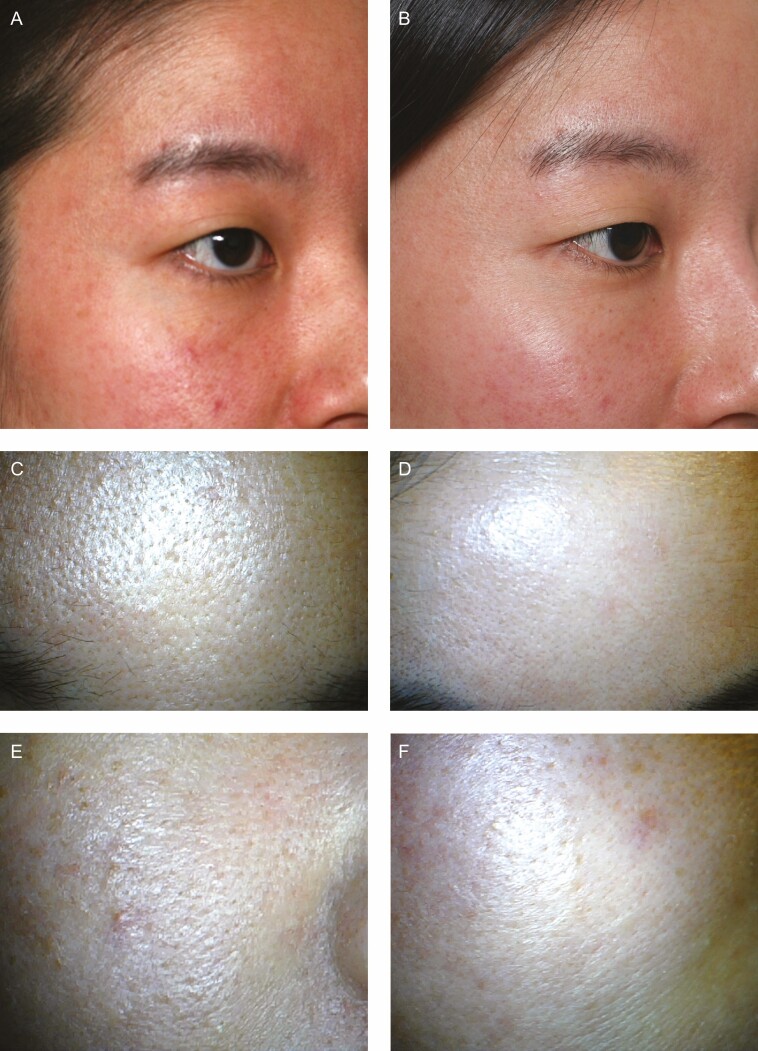
Figure 4.
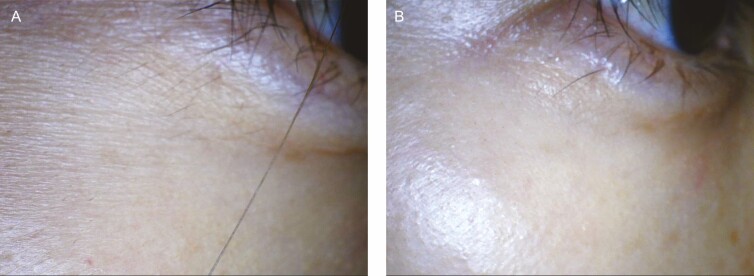
(A, C, E) Before and (B, D, F) 1-month posttreatment photographs of this 33-year-old woman. Before treatment, the patient suffered from sensitive and dehydrated skin with large skin pores. One-month posttreatment, fine wrinkles on the forehead and crow’s feet area have improved, and the skin glows with moisture. (C-F) Dermascope photos with 10× magnification.
Figure 5.
Dermascope photos with 10× magnification of this 39-year-old patient. (A) Pretreatment, the patient has severe fine wrinkles, and her skin lacks moisture. (B) At 1-month posttreatment, the skin became noticeably hydrated, and fine wrinkles improved. The skin has “hydro-glow.”
Improvement in Fine Wrinkles by Topographic Roughness
One month after treatment, roughness was reduced 50.19% (P < 0.0001) according to the computer program results employing 10× dermascopic photos from 7.81 ± 0.95 to 3.89 ± 0.43 a.u., indicating statistically significant improvements in the GAIS of fine wrinkles (Table 1). The differences in roughness (between baseline and posttreatment) showed statistical significance (P < 0.0001).
Table 1.
Changes in Roughness (Ra) and SCH
| Before (baseline) | 1 month | 2 months | 6 months | |
|---|---|---|---|---|
| Roughness (a.u.) | 7.81 ± 0.95 | 3.89 ± 0.43 | ||
| Reduced % in roughness | 50.19% (P < 0.0001) |
|||
| SCH (a.u.) on rt. crow feet | 36.24 ± 3.46 | 65.72 ± 4.78 | 56.58 ± 6.24 | 39.84 ± 3.63 |
| Increased % in SCH | 81.34% (P < 0.0001) |
56.12% (P < 0.0001) |
9.93% |
The mean roughness (Ra) decreased to 50.19% at 1 month. The mean SCH increased to 81.34% at 1 month and 56.12% at 2 months on the right side crow’s feet areas. The improvement of roughness and SCH signifies statistical significance (P < 0.0001). a.u., arbitrary unit; Ra, roughness average (a parameter of the Gwyddion computer program); SCH, stratum corneum hydration.
Improvement in Stratum Corneum Hydration
SCH on right side crow’s feet improved 81.34% (65.7 ± 4.7 a.u.) at 1 month and 56.12% (59.6 ± 6.2 a.u.) at 2 months from pretreatment baseline (36.2 ± 3.4 a.u.) (P < 0.0001). However, at 6 months, SCH improved only 9.93% (39.84 ± 3.6 a.u.), and there was no statistically significant improvement in hydration (Table 1).
Patient Satisfaction Survey Utilizing Global Aesthetic Improvement Scale
Skin hydration improvements on faces were GAIS rated as 2.78 ± 0.91 at 1 month, 2.60 ± 0.96 at 2 months, and 1.92 ± 0.65 at 6 months. Fine wrinkle improvements on faces were GAIS rated as 3.38 ± 0.53 at 1 month, 2.92 ± 0.66 at 2 months, and 2.06 ± 0.54 at 6 months.
Of the patients who participated in the study, the percentage of those willing to receive the procedure again at their expenditure was 38%.
Adverse Effects
Many lumps were found immediately after the injections, especially around the lower eyelid (Supplemental Figure 1) and were treated by molding and compressing utilizing a wide roller and compressing each lump with a cotton swab immediately, 1 by 1. Lumps were still visible in 12 patients only on the following day in the lower eyelid areas. Therefore, focused compression with a cotton ball should be performed in the clinic on the procedure day and 1 day after the procedure. Twenty-four patients (48%) experienced ecchymosis within 1 week (for 2-6 days after treatment), especially on thin skin areas (around the eyelid) (Supplemental Figure 1).
No impairment of facial expressions occurred as a result of intradermal injection with 40 U of Botox (0.04 U into 1000 sites, MicroBotox) on the entire face. No prolonged side effects were observed, indicating that the hydrotoxin injection method was safe from adverse effects such as nodules, lumps, infection, itching, irritation, immediate or delayed allergy, and prolonged bruising.
DISCUSSION
Structurally, the dermis is formed by 3 components: cells, fibers, and ground substance. The connective tissue in the dermis is responsible for protecting, providing, and maintaining the body’s structure. The ground substance in the ECM delivers metabolic support to cells as the medium for the diffusion of nutrients and waste products and also supplies moisture for cellular and fibrous components.1,5 The ECM consists of ground substance and fibers (collagen, reticular, and elastic fibers). Ground substance is a highly hydrophilic, andviscous complex of glycosaminoglycans (GAGs), proteoglycans, laminin, and fibronectin.1 GAGs are linear polysaccharides formed by repeating disaccharide units. The largest and most unique GAG is HA; HA forms a viscous network of polymers that binds a considerable amount of water, facilitates the diffusion of molecules, and maintains moisture in the dermis.5 Fibroblasts synthesize most components of the ECM, including the collagen, reticular and elastic fibers, GAGs, proteoglycans, and glycoproteins. Diminishing amounts of these dermal components is proven to cause aging of human skin.5,8 Reduced efficacy of estrogens and androgens also results in collagen degradation, dryness, loss of elasticity, epidermal atrophy, and wrinkling of the skin.5,12 Although intrinsic and extrinsic skin aging processes are distinct, they share similarities in molecular mechanisms. Among other things, skin aging is strongly associated with the loss of skin moisture.2,4,8 The HA content in the dermis is significantly higher than that in the epidermis, and the papillary dermis has much higher levels of HA than the reticular dermis.13 The most dramatic histochemical change observed in senescent skin is the marked disappearance of epidermal HA, and a progressive reduction in the size of the HA polymers in the skin as a result of aging has been reported.14 These aging or pathologic processes may be treated with the novel injection method of MicroHA into the dermis described here. Recently, studies have focused on a class of ECM molecules that exert major effects on cellular function, to which HA belongs.15 Recent reviews described the involvement of HA in angiogenesis,16 immune regulation,17,18 skin aging, and hydration.19 HA polymers occur in a vast number of configurations and conformations, depending on polymer size and salt concentration,20 and HA may form aggregates with proteoglycans.21
HA has a half-life of less than 1 day in the skin22,23 and is degraded into fragments by hyaluronidases. In humans, 6 types of hyaluronidases have been identified.24 HA can also be degraded non-enzymatically through a free-radical mechanism; 25 thus, it is easy for the HA component to be deficient in the skin. Replenishing stabilized HA in the dermis (through MicroHA) every 6 months may delay skin aging by maintaining skin hydration and regulating water balance, osmotic pressure, and ion flow. Injected stabilized HA may last longer and enhance the health of fibroblasts, collagen, and elastic fibers by moisturizing. In the dermis and epidermis, HA is co-localized with CD44. CD44-HA interactions have been reported to mediate the binding of Langerhans cells to HA in the matrix surrounding keratinocytes via their CD44-rich surfaces as they migrate through the epidermis.26,27 The replenishment of HA may stimulate dermal cells.
Manual stabilized HA injection may create dermal lumps because of its lifting capacity with low migration property. To resolve dermal lumps during dermal injection of stabilized HA, the author proposes 4 recommendations. First, inject deep into the fat layers to avoid manifestation of dermal lumps. This, however, will create a mainly volumizing effect instead of hydrating the dermis; therefore, deep injections into the fat have less effect for dermal hydration purposes.4 Skin-Booster (Galderma, Sweden), whose accurate name would be “Fat-Booster,” is currently injected into the fat layer for this reason. Second, replace mono-phasic filler with bi-phasic HA (Skin-Booster). Third, dilute HA filler with saline.4 After diluting, it is easy to control the injection amount. Finally, inject a minute quantity of filler per site and utilize an automatic injector. In this study, 0.002 cc (0.04 mg) of a monophasic HA was injected into the dermis to prevent dermal lumps. During the dermal injection, HA with a low crosslinking rate, low elasticity, low viscosity, and low cohesiveness is recommended. To inject 1000 sites with regular spacing and depth, the automatic injector is recommended over manual injection. Many types of devices can be employed for this procedure (dermal injection); any multi-needle automatic injector will suffice. The choice of which device to utilize would depend simply on the preference of the physician. What is important is that the depth of the injections is kept within the dermal layer and does not penetrate the fat layer. This injection method is more effective in fine wrinkle treatment and dermal hydration when applied to dermal injection than injection into the fat layer. This injection method is not for volumizing on the fat layer. Whereas volumizing effects of the HA filler injections are typically noticed immediately after treatment, fine wrinkle improvement and hydration after this technique utilizing MicroHA can be seen after a few weeks. Because spreading of MicroHA in dermis needs time, HA may attract more water in the dermis. HA droplets initially stay only in injected sites, and then HA spreads, later hydrating the entire dermis.
In this study, MicroBotox was added simultaneously with MicroHA and delivered together to enhance the treatment of fine wrinkles on faces. Before 2009, the author injected stabilized HA only into the dermis of faces.4 However, most of the patients had complained of little noticeable change in skin texture or hydration effect on the skin during the initial 1 or 2 weeks because the hydration effects appeared a few weeks after the injection.
The onset of MicroBotox is 5 days, and it can cover the initial gap and better satisfy patients for those first few weeks. Since 2009, after mixing MicroHA and MicroBotox, patients have reported satisfaction in 1 week due to the speed of the initial MicroBotox effect. In a few weeks after the injection utilizing the mixture, its synergic effects on dermis were more significant than MicroHA or MicroBotox alone. A total of 40 U MicroBotox for the whole face did not disturb facial expression. The author had studied the injection amount of MicroBotox for a whole face from 10, 20, 40, 60, and 80 U to 100 U. Less than 20 U MicroBotox had little improvement of fine wrinkles on the facial dermis, and more than 100 U of MicroBotox disturbed facial expression. Considering cost effectiveness, calculations, and mixing of reconstitution, 40 or 60 U of botulinum toxin is an advisable amount of MicroBotox on the whole face. The mixing of HA with saline and Botox may change the properties of stabilized HA, potentially decreasing viscosity and increasing the spreading property with a lower risk of dermal lump formation than biphasic HA. But these are easily its greatest benefit.
There are numerous benefits when MicroHA and MicroBotox are injected simultaneously. Through this method, the surgeon only has to inject the patient once and can treat dry skin, fine wrinkles, and skin texture without impairing facial expression or creating lumps. Traditionally, MicroHA8,9,28 and Micro-Botox9,28 are injected separately, therefore resulting in 2 injections and twice the pain for the patient. The procedure combining an intradermal injection of MicroHA (Neuramis) and MicroBotox (Neuronox) using an automatic injector (Neurajet) was named “Neuramix Hydrotoxin Method” by the author. Neuramix is not a product name and there is no generic term for the neuramix method. The combination method of MicroHA and MicroBotox has been utilized since 2009, and over 3000 patients were treated in the author’s clinic without occurrence of any side effects, including lumps, nodules, infection, or allergy. The author’s unique combination methods were presented in many aesthetic congresses. This method was patent certified by the Korean Intellectual Property Office29 and has become a widespread and accepted procedure within Korea. In the previous study, 1 cc stabilized HA was mixed with Botox.8,9 In this study, the amount of stabilized HA increased 2 cc to create greater effects for treating fine wrinkles with hydration. The more, the better. The human dermis is very thin, and thus HA can be injected beyond the dermal layer (into fat layer) in individuals with thin skin. In such cases, the changes made to the dermal layer are minimal. Utilizing an automatic injector to control the depth of the injections is a challenging task that requires education and training. The injection of MicroBotox into the dermal later is still a much-debated topic. Conventionally, Botox is injected into muscles. However, its effects on the sebaceous glands, sweat glands, and small muscles whose mechanism is yet unknown are continuously developing and being researched. In the 20-year experience of the author, the injection of MicroBotox into the dermis has proved effective in improving fine wrinkles and showed no signs of any side effects (Video, available online at www.aestheticsurgeryjournal.com).
A total of 48% of the patients experienced very small-sized ecchymosis on the face, and these numerous small needle marks disappeared within 1 week. It is possible to create ecchymosis after injection utilizing a needle. In this study, most ecchymoses were located in the dermal layer, because the desired injection depth was the dermis. Larger and more ecchymoses were located in thin skin areas (near the lower eyelid) than in thick skin areas (such as the cheek area). In thin skin areas such as near the lower eyelid, ecchymosis originated in the subdermal layer, because in thin skin it is difficult to inject into the real intradermal layer utilizing the injector. However, due to the smaller needles (31G), the ecchymoses were small. Because there were many injection sites, small ecchymoses appeared on many points, with severity increasing in individuals with thinner skin. Individuals with thicker skin were less likely to undergo the procedure because their skin develops less fine wrinkles, but they also experienced less ecchymosis after the procedure. If injection depth was deeper, needle marks were more visible with more bleeding during the injection. In cases of patients with thicker skin, there was less ecchymosis than in thin-skinned patients. Males usually present with thicker wrinkles with skin fold compared with fine wrinkles most often observed in females. Little improvement was expected to occur via the trialed procedure in male skin, and thus male patients were excluded from this study. The trialed procedure is most suitable for females with thin skin because it effectively provides hydration and improves fine wrinkles, primarily because monophasic-HA was utilized in this study. I am planning another study to include males utilizing biphasic HA in the future.
All patients stated that their skin began to feel less dry. MicroBotox (1000 injections of 0.04 U of Botox into the dermis) did not impair facial expression (Figures 3-5). MicroBotox may affect the facial muscles mildly through diffusion into the deep layer and may improve fine wrinkles, especially crow’s feet. Perhaps there would be some patterns of unknown very fine muscles in the dermis that may make Langerhans’s lines on facial dermis. There is a well-known pili muscle in the dermis. MicroBotox may affect the unknown fine muscle in the dermis and improve skin roughness. There are lymphatic channels in dermis and subdermis, so there would be unknown squeezing muscles in lymphatic vessels to enhance flow. MicroBotox might affect these unknown squeezing muscles in lymphatic vessels and could cause fluid retention in the dermis. Osmolality from botulinum toxin itself or artificial additives or stabilizers might cause fluid retention. This fluid retention may last longer in the dermal injection of botulinum toxin because there is more limitation of spreading of injected materials than in the fat or muscle layers. Fluid retention is frequently observed after botulinum toxin injection in the dermis. The fluid retention after MicroBotox can help to treat very fine wrinkles on the face, making it appear shiny and creating a luminous glow. MicroBotox also may affect skin appendages such as the sebaceous or sweat glands and may cause reduction of skin pore size (Figure 4). The duration of MicroBotox might be 3 or 4 months.9,28 The intention with injection of MicroBotox into the dermis is most likely to decrease sweat and sebaceous gland activity to improve skin texture and sheen30 and to target the superficial layer of muscles that find attachment to the undersurface of the dermis, causing visible rhytids.
For the measurement of hydration directly in the dermis, electric resistance was measured between two 31-gauge needles in the author’s previous study,8 but this is an invasive method inconvenient for patients. Therefore, in this study, SCH (which is an indirect, noninvasive method) was measured. Skin hydration effect (SCH) after MicroHA injection into the dermis lasted for more than 6 months. The GPSkin measures both transepidermal water loss and skin capacitance. The instrument capacitance measurement is collected from 2 sensors on the outer edge of the probe and follows the same technological principles as the Corneometer CM 825.31,32 The Corneometer31,32 is a marker of skin hydration via a high-frequency (approximately 1 MHz) capacitance measurement of a dielectric skin medium. Utilizing the dielectric medium in the skin, the instrument measures a dielectric constant, which is reported in arbitrary units of 0 to 120.33 This dielectric constant is sensitive to changes in moisture and is what allows capacitance measurements to function as a surrogate for skin hydration.33 Further specifics on probe configuration remain proprietary at this time, but GPSkin is currently being utilized for investigative purposes only and is not yet FDA approved.
For the measurement of skin roughness, certain parts of crow’s feet and forehead areas in microscopic photos were measured utilizing the computer program. Estimating skin roughness or its change by physical examination, as mentioned, lacks accuracy and cannot be considered a scientifically credible method. For this reason, roughness has been measured utilizing brightness in other articles. Convex or relatively bulging parts appear brighter, whereas indented areas appear darker. The speckle contrast method employs this mechanism to objectively measure the brightness. Furthermore, with computer programs, the roughness of large areas can be scientifically measured and automatically calculated. The speckle photography analysis technique was utilized by Lehmann et al (speckle elongation),34 Lehmnan (speckle-pattern illumination),35 Lehman (fractal diffraction gratings built),36 and Lu et al (co-occurrence matrix of speckle patterns).37 Images captured by a microscopic camera in a closed environment from external light with the same light source that illuminates the photographed area can be analyzed this way. There is a significant relationship between the technology employed to analyze skin roughness along with the depth and detail of the analysis demonstrated in this study. Although the speckle contrast method (measuring roughness and depth employing brightness) had been utilized in articles in the past, its accuracy is not consistent with today’s standards. The author’s study, however, takes advantage of newer technology that allows the most accurate measurement of brightness and thus roughness without error.
CONCLUSIONS
The hydrotoxin injection method is an easy and reproducible procedure to achieve constant injection depth and amount for every physician. The practice of intradermal injections of HA minimized the risk of vascular complications. Utilizing 0.002 cc MicroHA at 1000 injection sites did not cause visible lumps, and 0.04 U Botox (at the same 1000 sites) did not impair facial expression. This combination method showed more dramatic effects than either alone without adverse effects such as irritation, allergy, dermal lumps, or nodules. In most cases, after just 1 treatment session, the duration of clinical improvements persisted for 6 months. The hydrotoxin mixture showed improvement of both skin roughness and hydration effect together. Utilizing the hydrotoxin mixture, 2 ingredients could be injected on the exact dermal layer of the face in a session.
Supplementary Material
Acknowledgments
The author expresses thanks to Hae Jin Park for all the support.
Disclosures
The author declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The author received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1. Mescher A, ed. Chapter 5, Connective tissue. Introduction. Junqueira’s Basic Histology. 14th ed. New York: The McGraw-Hill Companies, 2015:96-119. [Google Scholar]
- 2. Berneburg M, Trelles M, Friguet B, et al. . How best to halt and/or revert UV-induced skin ageing: strategies, facts and fiction. Exp Dermatol. 2008;17(3):228-240. [DOI] [PubMed] [Google Scholar]
- 3. Makrantonaki E, Adjaye J, Herwig R, et al. . Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell. 2006;5(4):331-344. [DOI] [PubMed] [Google Scholar]
- 4. Hooper L, Bunn D, Jimoh FO, Fairweather-Tait SJ. Water-loss dehydration and aging. Mech Ageing Dev. 2014;136-137:50-58. [DOI] [PubMed] [Google Scholar]
- 5. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Micheels P, Sarazin D, Besse S, Sundaram H, Flynn TC. A blanching technique for intradermal injection of the hyaluronic acid Belotero. Plast Reconstr Surg. 2013;132(4 Suppl 2):59S-68S. [DOI] [PubMed] [Google Scholar]
- 7. Williams S, Tamburic S, Stensvik H, Weber M. Changes in skin physiology and clinical appearance after microdroplet placement of hyaluronic acid in aging hands. J Cosmet Dermatol. 2009;8(3):216-225. [DOI] [PubMed] [Google Scholar]
- 8. Kim JS. Effects of injection depth and volume of stabilized hyaluronic acid in human dermis on skin texture, hydration, and thickness. Arch Aesthetic Plast Surg. 2014;20(2):97-103. [Google Scholar]
- 9. Kim J. Clinical effects on skin texture and hydration of the face using microbotox and microhyaluronicacid. Plast Reconstr Surg Glob Open. 2018;6(11):e1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klapetek P, Necas D, Anderson C. Gwyddion User Guide. Section 4: Data Processing and Analysis, pages 30-147.http://gwyddion.net/download/user-guide/gwyddion-user-guide-en.pdf. Accessed July 28, 2020.
- 11. Yoon SH, Lee JE. Computing the surface area of three-dimensional scanned human data. Symmetry. 2016;8(7):67. [Google Scholar]
- 12. Brincat MP. Hormone replacement therapy and the skin. Maturitas. 2000;35(2):107-117. [DOI] [PubMed] [Google Scholar]
- 13. Meyer LJ, Stern R. Age-dependent changes of hyaluronan in human skin. J Invest Dermatol. 1994;102(3):385-389. [DOI] [PubMed] [Google Scholar]
- 14. Longas MO, Russell CS, He XY. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydr Res. 1987;159(1):127-136. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe K, Yamaguchi Y. Molecular identification of a putative human hyaluronan synthase. J Biol Chem. 1996;271(38):22945-22948. [DOI] [PubMed] [Google Scholar]
- 16. Slevin M, Krupinski J, Gaffney J, et al. . Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26(1):58-68. [DOI] [PubMed] [Google Scholar]
- 17. Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230(1):216-231. [DOI] [PubMed] [Google Scholar]
- 18. Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stern R, Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008;26(2):106-122. [DOI] [PubMed] [Google Scholar]
- 20. Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6(7):2397-2404. [PubMed] [Google Scholar]
- 21. Bates EJ, Harper GS, Lowther DA, Preston BN. Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochem Int. 1984;8(5):629-637. [PubMed] [Google Scholar]
- 22. Fraser JR, Laurent TC, Pertoft H, Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981;200(2):415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reed RK, Laurent UB, Fraser JR, Laurent TC. Removal rate of [3H]hyaluronan injected subcutaneously in rabbits. Am J Physiol. 1990;259(2 Pt 2):H532-H535. [DOI] [PubMed] [Google Scholar]
- 24. Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106(3):818-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lapcík L Jr, Chabrecek P, Stasko A. Photodegradation of hyaluronic acid: EPR and size exclusion chromatography study. Biopolymers. 1991;31(12):1429-1435. [DOI] [PubMed] [Google Scholar]
- 26. Weiss JM, Sleeman J, Renkl AC, et al. . An essential role for CD44 variant isoforms in epidermal Langerhans cell and blood dendritic cell function. J Cell Biol. 1997;137(5):1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiss JM, Renkl AC, Sleeman J, et al. . CD44 variant isoforms are essential for the function of epidermal Langerhans cells and dendritic cells. Cell Adhes Commun. 1998;6(2-3):157-160. [DOI] [PubMed] [Google Scholar]
- 28. Wu WT. Microbotox of the lower face and neck: evolution of a personal technique and its clinical effects. Plast Reconstr Surg. 2015;136(5 Suppl):92S-100S. [DOI] [PubMed] [Google Scholar]
- 29. Title of Invention: S-HA injection technique using an automatic injector for dermal hydration and rejuvenation. Patent number: 10-1790405, Certificate of patent of Korean; Intellectual Property Office; 2015.
- 30. Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg. 2013;39(3 Pt 1):443-448. [DOI] [PubMed] [Google Scholar]
- 31. Anthonissen M, Daly D, Peeters R, et al. . Reliability of repeated measurements on post-burn scars with corneometer CM 825(®). Skin Res Technol. 2015;21(3):302-312. [DOI] [PubMed] [Google Scholar]
- 32. Courage-Khazaka. Scientific devices: Corneometer® CM 825.https://www.courage-khazaka.de/index.php/en/products/scientific/55-corneometer-cm1. Accessed October 31, 2018.
- 33. Grinich EE, Shah AV, Simpson EL. Validation of a novel smartphone application-enabled, patient-operated skin barrier device. Skin Res Technol. 2019;25(5):612-617. [DOI] [PubMed] [Google Scholar]
- 34. Lehmann P, Patzelt S, Schöne A. Surface roughness measurement by means of polychromatic speckle elongation. Appl Opt. 1997;36(10):2188-2197. [DOI] [PubMed] [Google Scholar]
- 35. Lehmann P. Surface-roughness measurement based on the intensity correlation function of scattered light under speckle-pattern illumination. Appl Opt. 1999;38(7):1144-1152. [DOI] [PubMed] [Google Scholar]
- 36. Lehman M. Fractal diffraction gratings built through rectangular domains. Opt Commun. 2001;195(1-4):11-26. [Google Scholar]
- 37. Lu RS, Tian GY, Gledhill D, Ward S. Grinding surface roughness measurement based on the co-occurrence matrix of speckle pattern texture. Appl Opt. 2006;45(35): 8839-8847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.