Abstract
Caregiving for a person with dementia or neurodegenerative disease (PWD) is associated with increased rates of depression and anxiety. As the population ages and dementia prevalence increases worldwide, mental health problems related to dementia caregiving will become an even more pressing public health concern. The present study assessed emotional empathy (physiological, behavioral, and self-reported emotional responses to a film depicting others suffering) and two measures of cognitive empathy (identifying the primary emotion experienced by another person; providing continuous ratings of the valence of another person’s changing emotions) in relation to mental health (standard questionnaires) in 78 caregivers of PWDs. Greater emotional empathy (self-reported emotional responses) was associated with worse mental health, even after accounting for known risk factors. Neither measure of cognitive empathy was associated with mental health. A relationship between high levels of emotional empathy and poor mental health in caregivers suggests possible risk indicators and intervention targets.
Keywords: depression, anxiety, mental health, caregiving, emotional empathy, cognitive empathy, dementia, neurodegenerative disease
Older adults generally have lower levels of mental health problems, such as depression and anxiety, compared to younger adults (Fiske, Wetherell, & Gatz, 2009; Schuurmans & van Balkom, 2011). One exception to this pattern is found in individuals providing care for a person with dementia or neurodegenerative disease (PWD). Familial caregivers of PWDs manifest up to four-fold increases in rates of depression and three-fold increases in seeking treatment for anxiety compared to same-aged non-caregiving adults (Brodaty & Donkin, 2009; Coope et al., 1995; Cuijpers, 2005; Kolanowski, Fick, Waller, & Shea, 2004). With the worldwide “graying” of the population (by 2050 11.4% of the U.S. population will be over 75; Kawas & Brookmeyer, 2001) and the increasing prevalence of dementia with age (44% of individuals between the ages of 75-84 have Alzheimer’s disease, the most common form of dementia; Herbert, Weuve, Scherr, & Evans, 2013), mental health problems associated with caregiving will be a growing major public health issue with increasing implications for clinical psychology.
Although familial PWD caregivers as a group are highly vulnerable to declining mental health, individual caregivers differ considerably. Whereas some find caregiving to be a highly rewarding experience, including feeling enhanced spirituality and a greater sense of fulfillment and purpose (Abdollahpour, Nedjat, & Salimi, 2018), others struggle with the increased burden and strain of caregiving, including being exposed to the suffering of a loved one (Monin & Schulz, 2009; Richardson, Lee, Berg-Weger, & Grossberg, 2013). This variation among caregivers underscores the importance of identifying factors that are associated with declining mental health in caregivers. Such factors can help identify caregivers who are at heightened risk for developing mental health problems and suggest potential intervention targets to prevent new mental health problems and reduce the severity of existing ones.
Vulnerabilities to poor mental health in caregivers
Studies on individual differences in the negative effects of familial caregiving have identified specific characteristics of the PWD. An emerging consensus suggests that greater severity of PWDs’ behavioral and psychological symptoms (including emotion-related behaviors such as agitation and apathy) are worse for caregiver health than cognitive (e.g., memory loss) or functional (e.g., loss of mobility) symptoms (Ornstein & Gaugler, 2012; Schulz, O’Brien, Bookwala, & Fleissner, 1995). In our research, for example, we have found that declines in PWDs’ emotional functioning, including reduced empathy (Brown et al., 2018; Brown et al., 2020), altered emotional reactivity (Chen et al., 2017; Lwi et al., 2018), and diminished emotion regulation (Otero & Levenson, 2017), are associated with poorer caregiver health and well-being. Together, these findings suggest that declining emotional functioning in PWDs is an important risk factor for poor mental health outcomes in caregivers.
In addition to these risk factors associated with the PWD, a number of demographic, financial, and social variables have also been linked to poor health in familial caregivers. Meta-analyses suggest that being the spouse of a PWD, a woman (i.e., experiencing sexism), of a systemically oppressed race (i.e., experiencing racism), low in socioeconomic status, and more socially isolated are all associated with negative outcomes in caregivers (Brodaty & Donkin, 2009; Schulz et al., 1995; Young et al., 2020).
Many studies have robustly characterized the negative psychological effects of caregiving by healthcare providers and family members of PWDs (e.g., Kokkonen, Cheston, Dallos, & Smart, 2014; Schulz, Beach, Czaja, Martire, & Monin, 2020) and characterized familial caregivers’ emotion-related experiences during caregiving (e.g., compassion fatigue, coping strategies to reduce stress; Day & Anderson, 2011; van Knippenberg, de Vugt, Ponds, Verhey, & Myin-Germeys, 2018). However, familial caregivers’ emotional experiences or emotional functioning (which we conceptualize as emotional reactivity, emotion regulation, and empathy; Levenson et al., 2008) have largely not been investigated as a basis for predicting adverse caregiver mental health outcomes. Because of the importance of one’s own emotional functioning in relation to their mental health (Gross, Uusberg, & Uusberg, 2019), we sought to examine this relationship in familial caregivers.
Studies that have examined the relationship between caregiver emotional functioning and their mental health suggest that negative emotional reactivity and poor emotion regulation relate to negative caregiver outcomes. For example, caregiver propensity to experience negative emotions or a negative attitude toward caregiving relates to worse psychological outcomes (Safavi, Berry, & Wearden, 2017; Shim, Barroso, & Davis, 2012). We have found that caregivers who have the short-short variant in the serotonin transporter gene, which is thought to be related to greater emotional reactivity (Belsky & Pluess, 2009; Gyurak et al., 2013; Haase et al., 2015), show a relationship between low empathy in the PWD and low psychological well-being in the caregiver, whereas others who do not have this variant do not show such an association (Wells et al., 2019). Moreover, work from our laboratory has shown that poor emotion regulation ability in caregivers relates to their having higher levels of anxiety (Wells, Hua, & Levenson, 2020). In the present study we focused on empathy, because despite the strong evidence for the important role that low empathy in the PWD plays in poor caregiver mental health (Brown et al., 2018; Hsieh, Irish, Daveson, Hodges, & Piguet, 2013), empathy in the familial caregiver in relation to their own mental health has not been well studied.
Empathy as a risk factor for poor caregiver mental health
The ability to know, feel, and respond appropriately to what others are feeling (Levenson & Ruef, 1992) is often referred to as empathy. Empathy can be broken down into emotional and cognitive facets. Emotional empathy refers to the ability to feel or share others’ emotional states, whereas cognitive empathy refers to the ability to know or understand another person’s emotions (Decety & Jackson, 2006; Preston & de Waal, 2002; Singer & Lamm, 2009; Zaki, Weber, Bolger, & Ochsner, 2009).
Emotional and cognitive empathy are both beneficial in many contexts (Morelli, Ong, Makati, Jackson, & Zaki, 2017; Wei, Liao, Ku, & Shaffer, 2011). However, in the context of providing care for a loved one undergoing a distressing life experience, these facets of empathy may have quite different relationships with caregiver mental health (Lee, Brennan, & Daly, 2001). When PWDs experience distress, a caregiver with high emotional empathy may feel or share the PWD’s distress, which can lead to the caregiver being overwhelmed by their own sense of distress, making high emotional empathy problematic for caregivers by increasing their distress vicariously. In contrast, a caregiver with high cognitive empathy may accurately know or understand that the PWD has a higher need for care, which can lead to more effective ways of helping the PWD and to reduced burden for caregivers.
In line with these ideas, professional healthcare providers with greater emotional empathy are especially prone to share others’ distress. When exposed to high levels of negative emotions in stressful environments, providers can develop empathy burnout and emotional exhaustion (Decety & Fotopoulou, 2014; Figley, 2011). On the other hand, greater cognitive empathy (operationalized as perspective taking or understanding others’ emotions using trait empathy measures, such as the Interpersonal Reactivity Index or Jefferson Scale of Empathy; Davis, 1983; Hojat et al., 2001) in professional healthcare providers (e.g., physicians, nurses) has been associated with beneficial patient outcomes (patient satisfaction, control of hemoglobin A1c in diabetic patients; Blatt, LeLacheur, Galinsky, Simmens, & Greenberg, 2010; Hojat et al., 2011) and provider outcomes (compassion satisfaction; Gleichgerrcht & Decety, 2013). Interestingly, these tensions between different kinds of empathy are also found in early psychotherapy theory and research (Rogers, 1951, 1957), where effective psychotherapists were thought to be able to understand client’s emotional states accurately without becoming enmeshed and emotionally over-involved.
Despite these provocative insights from previous work, researchers have rarely examined familial caregivers’ emotional and cognitive empathy in relation to their own mental health. One study found that higher emotional empathy in caregivers was associated with lower life satisfaction, whereas higher cognitive empathy was associated with greater life satisfaction (Lee et al., 2001). In another study (Jütten, Mark, & Sitskoorn, 2019), higher emotional empathy in caregivers was associated with greater anxiety and cognitive empathy was associated with depression in a curvilinear fashion, such that highest levels of cognitive empathy predicted the lowest levels of depression.
In these two prior studies of the relationship between caregiver empathy and mental health, empathy was measured using self-report inventories, which can be susceptible to several forms of bias (Levenson & Ruef, 1992; Murphy & Lilienfeld, 2019). To our knowledge, no prior studies of caregiver empathy and mental health have used laboratory-based measures of caregiver empathy. Laboratory assessments of emotional empathy typically measure participant responses (physiology, behavior, self-reported emotional experience) to viewing others who are experiencing powerful negative emotions, such as emotional pain or distress (Hein & Singer, 2008; Lamm, Decety, & Singer, 2011; Marsh, 2018). Laboratory assessments of cognitive empathy typically ask participants label or track others’ emotions, with accuracy judged against an external criterion, such as ratings by the target person or a panel of experts (Goodkind et al., 2012; Ickes, 1997; Levenson & Ruef, 1992; Ruef & Levenson, 2007; Zaki et al., 2009). While these laboratory assessments use forms of self-report responses (e.g., ratings of emotional experience in response to viewing others suffering or reporting on what they think someone else feels), these self-report empathy measures capture a more immediate feeling or understanding of others’ emotional states. Such responses may be less susceptible to variation in metacognitive insight about empathic abilities compared to traditional trait empathy measures (Murphy & Lilienfeld, 2019). Furthermore, because heightened negative emotional reactivity has been independently associated with poor caregiver mental health (Safavi et al., 2017) and greater emotional reactivity may help individuals feel or understand others’ emotional states (Rueckert, Branch, & Doan, 2011), accounting for the potential influence of caregivers’ emotional reactivity to a negative or aversive stimulus will help determine if findings are specific to empathy. Applying these approaches could greatly increase our understanding of the role that caregiver empathy plays in accounting for individual differences in caregiver mental health.
The Present Study
The present study aimed to understand the relationships between laboratory-based measures of caregiver emotional and cognitive empathy and caregiver mental health measured using standard questionnaires. We measured caregivers’ general ability to feel or share others’ emotion states (emotional empathy) and to know or understand others’ emotional states (cognitive empathy). Emotional empathy was assessed by measuring physiological, behavioral, and self-reported emotional responses to a film depicting others suffering. Cognitive empathy was assessed both by having participants identify the primary emotion experienced by a target character in a film and by having them provide continuous ratings of the valence of a person’s changing emotions. To control for individual differences in emotional reactivity, we measured caregivers’ self-reported emotional response to an aversive emotional stimulus (a sudden, unexpected loud noise) and used it as a covariate in our analyses. By examining laboratory measures of emotional and cognitive empathy in relation to caregiver mental health, our study has the potential to further caregiving research by identifying a specific aspect of caregiver emotional functioning that may place caregivers at greater risk for developing poor mental health.
Our primary hypothesis was that greater emotional empathy in caregivers would be associated with worse caregiver mental health. We reasoned that caregivers with greater emotional responses to the suffering of others in our laboratory task would also have greater emotional responses to the declines and suffering of the PWD in their care. We reasoned that greater sensitivity to the suffering of others, combined with the other stressors and burdens involved in caregiving, would create a fertile breeding ground for symptoms of anxiety and depression. Because cognitive empathy does not engender this kind of additional suffering, we did not expect greater cognitive empathy in our laboratory tasks to be related to worse caregiver mental health.
We did not have a priori hypotheses as to which aspect of emotional empathy would be most strongly associated with caregiver mental health. Because we conceptualize emotional responses as having physiological, behavioral, and self-report components (Levenson et al., 2008), and laboratory measures of emotional empathy have not yet been examined in relation to caregiver mental health, we wanted to determine which aspect of emotional empathy would be most strongly related to caregiver mental health.
Materials and Methods
Participants
Seventy-eight PWDs and their familial or close caregivers participated in a study of emotional functioning at the Berkeley Psychophysiology Laboratory at the University of California, Berkeley (UCB). Participants were recruited at the Memory and Aging Center at the University of California, San Francisco (UCSF), where PWDs underwent a full diagnostic evaluation, including neurological, neuropsychological, and neuroimaging assessment. At the UCSF assessment, caregivers were told about the Berkeley study, and, if they expressed interest, were subsequently contacted to schedule a laboratory session. All participants, or their legal guardians when appropriate, provided consent for their participation. All procedures for obtaining consent and all study procedures were approved by the Committee for the Protection of Human Subjects at UCB.
Caregivers were 64.5 years old on average, predominantly spouses of PWDs seen at UCSF (92.3%), women (60.3%), White (83.3%), and highly educated (71.7% with at least 16 years of education). At UCSF, PWDs were diagnosed according to consensus criteria (Armstrong et al., 2013; Budka et al., 1995; Gorno-Tempini et al., 2011; Klockgether, 2010; Litvan et al., 1996; McKeith, 2004; McKhann et al., 2011; Rascovsky et al., 2011). The sample of 78 PWDs included: (a) 33 with frontotemporal dementia (FTD), which includes three clinical syndromes that affect socioemotional and language functioning (16 behavioral variant FTD, 9 non-fluent variant primary progressive aphasia, 8 semantic variant primary progressive aphasia); (b) 11 with Alzheimer’s disease (AD), which predominantly affects memory functioning; (c) 25 with diagnoses that were characterized by motor symptoms (Motor), including 9 with corticobasal syndrome, 2 with dementia with Lewy body disease, 1 with Parkinson’s disease, 1 with prion disease, 11 with progressive supranuclear palsy, and 1 with spinocerebellar ataxia; and (d) 9 at risk for developing dementia, including 5 with mild cognitive impairment (MCI) and 4 relatives of a person with FTD. For more details on caregiver and PWD demographics, see Table 1.
Table 1. Sociodemographic characteristics and clinical variables.
Means and standard deviations provided, unless otherwise noted.
| PWDs | Caregivers | |
|---|---|---|
| N = | 78 | 78 |
| Age | 62.60 (8.69) | 64.52 (9.26) |
| Gender (% Women) | 43.3 | 60.3 |
| Race (n=) | ||
| Native American/Alaska Native | 2 | 0 |
| East or Southeast Asian/Asian American | 4 | 5 |
| Black/African American/Afro-Caribbean | 1 | 1 |
| Latinx/Chicanx/Hispanic | 4 | 3 |
| Multi-racial | 3 | 4 |
| Native Hawaiian/Pacific Islander | 1 | 0 |
| White | 63 | 65 |
| Education (n=) | ||
| High School | - | 7 |
| 2-Year College | - | 15 |
| 4-Year College | - | 29 |
| Master’s Degree | - | 15 |
| PhD, MD, or other professional degree | - | 12 |
| Relationship to the PWD (% Spouse) | - | 92.3 |
| Caregiver severity of anxiety symptoms (BAI) | - | 7.06 (7.60) |
| Caregiver severity of depression symptoms (CESD) | - | 12.12 (9.24) |
| PWD Diagnosis (n=) | ||
| FTD | 33 | - |
| AD | 11 | - |
| Motor | 25 | - |
| MCI or family member of person with FTD | 9 | - |
| PWD disease severity (CDR) | 3.96 (2.71) | - |
| PWD cognitive functioning (MMSE) | 24.82 (4.81) | - |
PWD = person with neurodegenerative disease or dementia; FTD = frontotemporal dementia; AD = Alzheimer’s disease; Motor = diseases that primarily impact motor functioning; MCI = mild cognitive impairment
Procedure
Upon arrival at UCB, all participants (PWDs and caregivers) reviewed the procedures for the day and completed the consent forms. PWDs and caregivers were then seated in separate rooms and non-invasive physiological sensors (see more details below) were attached to participants to monitor their physiological responses. Participants sat in a chair facing a 21-inch color monitor. Video recordings of participants’ heads and torsos were obtained using a remote-controlled camera that was partially hidden from view. Participants completed a daylong laboratory session designed to provide a comprehensive assessment of multiple aspects of emotional functioning, including emotion recognition, emotional reactivity, and emotion regulation (Levenson et al., 2008).
The present study focused on caregiver data from four specific tasks (described below), including assessments of cognitive empathy (two tasks), emotional empathy, and emotional reactivity.
Apparatus and measures
Rating dial.
The rating dial (Ruef & Levenson, 2007) consisted of a small metal box with a knob and attached pointer that rotated through a 180° semi-circle. The semi-circle was divided into 9 equal divisions labelled with descriptors of very bad (shown with a schematic frowning face) at the far left, neutral (shown with a schematic neutral face) in the middle, and very good (shown with a schematic smiling face) at the far right. The dial generated a voltage that reflected the dial position; a computer sampled the voltage every 3 milliseconds and calculated the average dial position every second. The rating dial was located near the participant’s dominant hand.
Physiology.
In line with psychophysiology standard practices (Mendes, 2009), physiological measures were monitored continuously in order to capture reactivity (e.g., differences in physiological activity during resting baseline periods and trial periods) for various tasks. We used a system consisting of Biopac amplifier modules, a computer with analog-to-digital capability, and an online data acquisition and analysis software package (written by Robert W. Levenson). The program computed second-by-second averages for the following measures: (a) heart rate—inter-beat interval was the time interval in milliseconds between successive R waves, using Beckman miniature electrodes with Redux paste that were placed on opposite sides of the participants’ chest; (b) finger pulse amplitude—a UFI photoplethysmograph recorded the amplitude of blood volume in the finger using a photocell taped to the distal phalanx of the index finger of the nondominant hand; (c) finger pulse transmission time—the time interval in milliseconds was calculated between the R wave of the electrocardiogram and the upstroke of the peripheral pulse at the finger site, recorded from the distal phalanx of the index finger of the nondominant hand; (d) ear pulse transmission time—a UFI photoplethysmograph recorded the volume of blood in the ear to measure transmission time between the R waves of the electrocardiogram signal and the upstroke of pulse at the ear; (e) systolic blood pressure and (f) diastolic blood pressure—a cuff placed on the ring finger of the participant’s nondominant hand calculated blood pressure on every heartbeat using an Ohmeda Finapress 2300; (g) skin conductance level—the electrical conductance of the skin was computed using a constant voltage device to pass voltage between Beckman regular electrodes on the ring and index fingers of the nondominant hand to calculate the sweat response; (h) somatic activity—the amount of overall movement was computed using an electromechanical transducer attached to the platform of the participant’s chair; (i) respiration rate—the inter-cycle interval was the time interval in milliseconds between breaths calculated using a pneumatic bellows stretched around the thoracic region.
These nine measures were selected to sample the major autonomic (cardiovascular, electrodermal, respiratory) and somatic systems associated with emotional responding. For each measure, the average of the resting baseline period was subtracted from the average obtained during the task period to create a difference score for physiological reactivity (length of baseline and task period detailed below). Averages for each physiological reactivity score were normalized, reverse scored if necessary (so that larger values reflected greater physiological arousal), and then averaged. The use of this kind of composite measure, which helps control for Type I error, has been described in detail in several of our other publications (Sturm, Rosen, Allison, Miller, & Levenson, 2006; Verstaen et al., 2016).
Facial Behavior.
Trained coders rated recordings of participants’ facial behavior using the Emotional Expressive Behavior coding system (Gross & Levenson, 1993). Facial behavior was coded second by second for nine emotional facial behaviors (anger, disgust, happiness/amusement, contempt, sadness, embarrassment, fear, surprise, and confusion) on an intensity scale ranging from 0-3.
Laboratory Tasks
Cognitive empathy: Emotion Recognition Task.
Participants watched a series of 11 film clips that were developed to assess ability to recognize specific emotions (Goodkind et al., 2015). Each film clip (approximately 35 seconds in length) showed a character experiencing a positive (affection, amusement, calmness, enthusiasm), negative (anger, disgust, fear, sadness), or self-conscious emotion (embarrassment, pride, shame) and was preceded by a 30 second baseline period during which an “X” was displayed on the monitor. After watching each film clip, participants were shown a picture of the target character displaying a neutral expression and were asked to identify the emotion the target character felt most strongly from a list of the 11 emotions.
Accuracy on this task was calculated by summing correct answers across film clips, with a minimum score of 0 and a maximum score of 11.
Cognitive empathy: Dynamic Tracking Task.
Participants watched videos of two different heterosexual married couples having conversations. These conversations were selected from a study that followed couples longitudinally (Levenson, Carstensen, & Gottman, 1993; Verstaen, Haase, Lwi, & Levenson, 2018) and had been used previously to study empathy in young, middle-aged, and older adults (Sze, Goodkind, Gyurak, & Levenson, 2012). For each video, participants were asked to focus on rating the emotions of a target spouse (i.e., the wife or husband) who was highlighted with a green dot above his/her head. Using the rating dial, participants rated the valence of the emotion being experienced by the target person by moving the rating dial continuously to indicate how positive or negative they believed the target person was feeling. Each video lasted approximately 3 minutes.
Accuracy on this task was calculated using time-lagged cross correlations to determine the agreement between a caregiver’s moment-to-moment ratings of the target person’s emotions and the averaged ratings from an expert panel of graduate students trained in the Facial Action Coding, Emotional Expressive Behavioral Coding, and Specific Affect Coding systems (Coan & Gottman, 2007; P. Ekman, 1977; Gross & Levenson, 1993). To allow for differences in processing speed, the maximum correlation coefficient was selected for lags between −10 or +10 seconds following methods previously used with this task (Brown et al., 2018). Because performances for both videos on the dynamic tracking task were correlated (r = .59, t = 6.37, p < .001, 95% CI [.42, .72]), a composite accuracy score was calculated by averaging the maximum cross correlation coefficient for the two videos. Higher averaged cross correlation coefficients indicated greater accuracy on this task.
Emotional empathy: Film depicting suffering.
Participants watched a film clip that has been found to induce concern and distress in young, middle-aged, and older adults (Sze, Gyurak, Goodkind, & Levenson, 2012). The film consists of images of people in Darfur suffering from starvation and disease. The film lasted 120 seconds and was preceded by a 60 second baseline period during which an “X” was displayed on the monitor. After the film, participants rated on a 0-2 scale how much they felt 10 positive and negative emotions (affection, fear, amusement, anger, shame, disgust, embarrassment, enthusiasm, pride, surprise) as well as concern and distress.
Physiological responses to the film were computed by subtracting the average level of each measure during the pre-film baseline from the average level during the last 80 seconds of the film, which had previously been found to produce the most intense emotional facial responses (Sze, Gyurak, et al., 2012). The responses were combined into a composite score as described above. Facial behavior was also coded during the final 80 seconds of the film. A composite measure of negative facial behavior was obtained by summing the intensity scores for seven negative emotion codes (sadness, confusion, anger, fear, surprise, contempt, and disgust). Inter-coder reliability was high (intraclass correlation coefficient = .83). Self-reported emotional experience was calculated by summing the total reported intensity for seven negative and two caring emotions (fear, anger, surprise, sadness, disgust, shame, distress, affection, concern).
Emotional reactivity: Acoustic startle task.
Participants were told to relax and watch the computer screen. An “X” was displayed on the screen when the pre-trial baseline began and remained in view for 60 seconds. A loud startle stimulus (115 dB, 100 ms burst of white noise) was then presented without warning using speakers located behind the participant. Participants sat through a 60 second post startle period during which an “X” was presented on the screen. After the post startle period, participants rated on a 0-2 scale how much they felt 10 positive and negative emotions (affection, fear, amusement, anger, shame, disgust, embarrassment, enthusiasm, pride, surprise). We used this task, which has been used previously with participants of all ages (Levenson et al., 2008; Soto, Levenson, & Ebling, 2005; Sturm et al., 2006), to provide a measure of emotional reactivity to an aversive stimulus that is experienced directly, rather than vicariously (as with the film depicting suffering).
Self-reported emotional experience was calculated by summing the total intensity for the seven emotions that are typical responses to the startle task (Sturm et al., 2006): surprise, sadness, anger, fear, disgust, embarrassment, and amusement. Due to laboratory sessions ending early because of PWD fatigue or caregivers declining to participate in all tasks, data on this task were obtained from only 68 participants. Physiology and facial behavior were also recorded during this task; however, this data was not used in analyses (see below).
Correlations between laboratory tasks.
Table 2 displays correlations between laboratory measures. Supplemental Figure 1 displays the distributions of these laboratory measures.
Table 2.
Correlation coefficients between measures of caregiver mental health (depression and anxiety composite), caregiver cognitive empathy (accuracy on films or tracking tasks), caregiver emotional empathy (responses to film of suffering), and caregiver emotional reactivity (responses to acoustic startle task).
| Cognitive Empathy | Emotional Empathy | Emotional Reactivity | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1. Depression and anxiety | .1 | −.01 | −.2 | .29* | −.07 | .15 | |
| 2. Cognitive Empathy (emotion recognition) | .09 | .1 | −.2 | .03 | .03 | ||
| 3. Cognitive Empathy (dynamic tracking) | .1 | −.1 | .13 | .16 | |||
| 4. Emotional Empathy (physiological) | −.1 | .09 | .01 | ||||
| 5. Emotional Empathy (self-reported) | 0 | .22 | |||||
| 6. Emotional Empathy (facial behavior) | −.01 | ||||||
| 7. Emotional Reactivity (self-reported) | |||||||
p < .05
Validity for laboratory tasks.
The emotion recognition task has criterion validity as it is correlated with performance on standardized emotion recognition tasks using standardized photographs of emotional facial expressions (Goodkind et al., 2015). Whereas the emotion recognition film task captures recognition of discrete emotion states, the dynamic tracking task captures recognition of emotional valence over time. We expect these two different cognitive empathy tasks to capture different aspects of recognizing emotion. The dynamic tracking task shows discriminant validity as it is not correlated with performance on the emotion recognition task (as noted above). This discriminant validity has also been demonstrated in a previous study, in which performance on the dynamic tracking task is not correlated with performance on recognizing emotion from standardized photographs of emotional facial expressions (Sze, Gyurak, et al., 2012). The emotional empathy task has content validity as the film was selected to induce negative and caring emotions for others, and this is film has been shown to effective induce these emotional experiences (Lwi, Haase, Shiota, Newton, & Levenson, 2019; Sze, Gyurak, et al., 2012). Furthermore, the emotional reactivity task has content validity as responses to the acoustic startle have well-characterized and well-documented emotional responses (P. Ekman, Friesen, & Simons, 1985; Roberts et al., 2004; Sturm et al., 2006).1
Other Measures
PWD disease severity.
At UCSF, the Clinical Dementia Rating Scale (CDR) was completed using a semi-structured interview conducted by clinicians with caregivers (Morris, 1993). The CDR assesses functional performance in six domains: (1) memory, (2) orientation, (3) judgement and problem-solving, (4) community affairs, (5) home and hobbies, and (6) personal care. Scores in each domain range from 0 (none) to 3 (severe) and are summed to create a composite score, ranging from 0 to 18, with higher scores indicating greater disease severity. This measure is often used to stage disease severity in individuals with dementia (Morris, 1997; M. M. Williams, Storandt, Roe, & Morris, 2013). The CDR has been validated against neuropathology data (Berg, McKeel, Miller, Baty, & Morris, 1993) and demonstrates good reliability (Burke et al., 1988).
PWD cognitive impairment.
At UCSF, the Mini-Mental State Examination (MMSE) was administered to assess the severity and progression of cognitive impairment (Folstein, Folstein, & McHugh, 1975). This exam evaluates several domains of cognitive functioning: (1) orientation, (2) visuospatial construction, (3) language, (4) concentration or attention, (5) working memory, and (6) memory recall. A total score is calculated, ranging from 0-30, with lower scores indicating greater cognitive impairment. This measure is often used to detect dementia and stage disease course (O’Bryant, Humphreys, et al., 2008; O’Bryant, Waring, et al., 2008). The MMSE has demonstrated good reliability and validity for grading cognitive impairment (Tombaugh & McIntyre, 1992).
Caregiver mental health.
Within a month following the laboratory session at UCB, caregivers completed two questionnaires online to assess their mental health. Depression was measured using the Center for Epidemiological Studies Depression Scale (CESD; Radloff, 1977), which has respondents rate themselves over the past week on a scale from 0 (“rarely or none of the time”) to 3 (“most or all of the time”) for 20 items (e.g., “I felt sad”, “I felt lonely”). Four items were reverse scored and then all items were summed, with higher scores indicating greater levels of depression symptoms. The CESD has been previously validated for measuring depression in older adults (Beekman et al., 1997; Haringsma, Engels, Beekman, & Spinhoven, 2004). Anxiety was measured using the Beck Anxiety Inventory (BAI; Steer & Beck, 1997), which has respondents rate themselves over the past month on a scale from 0 (“not at all”) to 3 (“a lot”) for 21 items (e.g., “Unable to relax”). Scores were summed, with higher scores indicating greater levels of anxiety symptoms. The BAI has demonstrated reasonable test-retest reliability and validity when used with individuals with anxiety disorders (Beck, Epstein, Brown, & Steer, 1988; Fydrich, Dowdall, & Chambless, 1992).
Because the CESD and BAI were significantly correlated in our sample (r = .68, t = 8.14, p < .001, 95% CI [.54, .79]), and to reduce the risk of Type I errors from multiple comparisons, a composite of caregiver mental health symptoms was computed by z-scoring the CESD and BAI and averaging these z-scores. Higher scores on the composite of mental health indicate greater severity of averaged depression and anxiety symptoms.
Sensitivity power analyses
Because we recruited a convenience sample to maximize our sample size, we could not conduct a priori power analyses. To determine if our study had adequate power to detect effects, we conducted two sensitivity power analyses. For analyses with our full sample size of 78, a maximum of 5 predictors, alpha level = .05, and power = .80, we computed a medium effect size of f2 = .18 (Cohen, 1988). Only 68 participants completed the acoustic startle task (see above). For analyses with a sample size of 68, a maximum of 5 predictors, alpha level = .05, and power = .80, we computed a medium effect size of f2 = .21. Thus, our study was adequately powered to detect medium effect sizes.
Analytic approach
Bivariate Pearson correlations were conducted to examine: (a) the relationship between caregiver cognitive empathy and caregiver mental health, and (b) the relationship between caregiver emotional empathy and caregiver mental health. Then, if significant associations were found, linear regression analyses were conducted with inclusion of covariates, including relevant demographic or clinical variables (identified below) and caregiver emotional reactivity. We focused on independent significant associations from correlations in order to avoid potential suppressor effects in multivariate linear regression (Beckstead, 2012). Physiology, facial behavior, or self-reported emotional responses to the emotional reactivity task were used as a covariate, depending on the type of empathy response (i.e., physiological, behavioral, or self-report) that emerged as being significantly associated with caregiver mental health.
Identifying covariates
We calculated correlations between caregiver demographic or PWD clinical variables and caregiver mental health to identify covariates to include in our primary analyses of the relationship between caregiver emotional functioning (cognitive empathy, emotional empathy, emotional reactivity) and caregiver mental health. Potential covariates included caregiver age; caregiver gender (0 = man, 1 = woman); caregiver race, as a crude index for systemic oppression based on race (given the small number of People of Color, this variable was coded as 0 = White, 1 = non-White); caregiver education (0 = High School, 1 = 2-year college, 2 = 4-year college, 3 = Master’s degree, 4 = MD, PhD, or other professional degree); caregiver relationship to PWD (1 = spouse, 0 = non-spouse); PWD diagnosis (FTD, AD, or Motor; three variables coded as 1 = yes, 0 = no); PWD disease severity; and PWD cognitive functioning.
Caregiver race (non-White), greater PWD disease severity, and lower PWD cognitive functioning were correlated with worse caregiver mental health in our sample, thus, they were included as covariates in analyses (race: r = .23, t = 2.05, p = .04, 95% CI [.007, .43], disease severity: r = .37, t = 3.57, p < .001, 95% CI [.17, .55]; cognitive functioning: r = −.28, t = −2.50, p = .01, 95% CI [−.47, −.06]).
Caregiver age, gender, education, relationship to PWD, and PWD diagnosis variables were not correlated with caregiver mental health in our sample, thus they were not included as covariates in analyses (caregiver age: r = −.19, t = −1.60, p = .11, 95% CI [−.41, .05]; caregiver gender: r = .18, t = 1.48, p = .14, 95% CI [−.06, .40]; caregiver education: r = .02, t = .17, p = .87, 95% CI [−.20, .24]; caregiver relationship: r = −.10, t = −.85, p = .40, 95% CI [−.31, .13]; FTD diagnosis: r = −.02, t = −.15, p = .88, 95% CI [−.24, .21]; AD diagnosis: r = −.07, t = −.62, p = .54, 95% CI [−.29, .15]; Motor diagnosis: r = .10, t = .91, p = .37, 95% CI [−.12, .32]).
Results
Caregiver cognitive empathy and caregiver mental health
Neither caregiver accuracy on the emotion recognition task (r = .13, t = 1.12, p = .27, 95% CI [−.10, .34]) nor on the dynamic tracking task (r = −.01, t = −.10, p = .91, 95% CI [−.23, .21]) was related to caregiver mental health.
Caregiver emotional empathy and caregiver mental health
Caregiver self-reported emotional experience to the film depicting suffering was associated with caregiver mental health, such that greater experience of negative and caring emotions was related to lower mental health (r = .29, t = 2.66, p = .009, 95% CI [.07, .48]). See Figure 1. In contrast, caregiver physiological responses (r = −.14, t = −1.24, p = .22, 95% CI [−.35, .09]) and facial behavior responses (r = −.07, t = −.61, p = .55, 95% CI [−.29, .16]) to the film depicting suffering were not related to caregiver mental health.2
Figure 1.
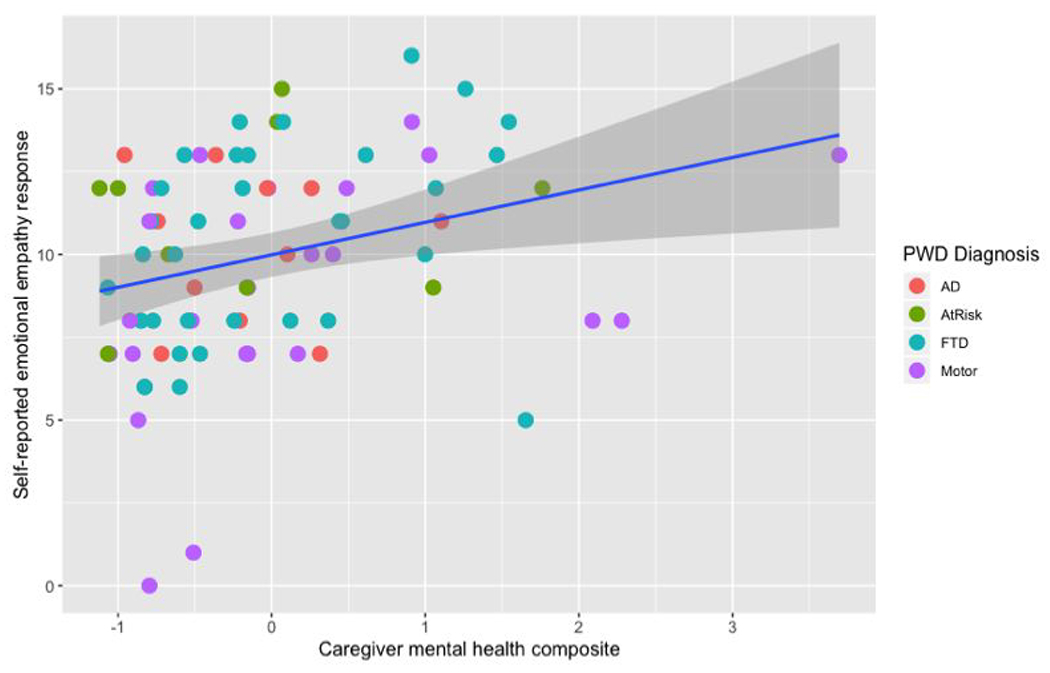
Scatterplot of the sum of intensity for self-reported emotional empathy (in response to the Darfur film depicting suffering) and caregiver mental health (composite of caregiver depression and anxiety symptoms). Greater self-reported emotional empathy was associated with worse caregiver mental health. This relationship was significant in a zero-order Pearson correlation and in linear regressions accounting for caregiver race, PWD disease severity, PWD cognitive functioning, and caregiver self-reported emotional reactivity (in response to the acoustic startle task). Note: one caregiver had a depression and anxiety composite score greater than three standard deviations from the mean (z = 4.03). All findings remained the same without this outlier.
We evaluated the robustness of the relationship between greater emotional empathy (i.e., self-reported emotional experience to film depicting suffering) and lower caregiver mental health in two ways: (a) accounting for covariates, and (b) accounting for covariates and for caregiver emotional reactivity (i.e., self-reported emotional experience to the acoustic startle task).
Accounting for covariates.
We conducted a linear regression accounting for caregiver race, PWD disease severity, and PWD cognitive functioning (the variables found to be independently predictive of caregiver mental health above). When these variables were entered as covariates, greater emotional empathy (self-reported emotional experience) was still related to worse caregiver mental health (t = 2.62, β = .25, p = .01).
Accounting for covariates and caregiver emotional reactivity.
We conducted a linear regression accounting for self-reported emotional experience to the acoustic startle task as well as caregiver race, PWD disease severity, and PWD cognitive functioning. In this analysis, caregiver emotional reactivity was not associated with caregiver mental health (t = .59, β = .06, p = .55). However, greater emotional empathy (self-reported emotional experience) remained associated with worse caregiver mental health (t = 2.74, β = .30, p = .008).
Caregiver emotional empathy and mental health: Depression and anxiety considered separately
Although measures of caregiver depression and anxiety were highly correlated in our sample, for transparency, we examined relationships between caregiver emotional empathy (self-reported emotional experience) and depression and anxiety considered separately. Linear regressions accounting for caregiver emotional reactivity, caregiver race, PWD disease severity, and PWD cognitive functioning revealed that greater emotional empathy was associated with greater depression symptoms (t = 2.97, β = .33, p = .004) and with greater anxiety symptoms at trend level (t = 1.94, β = .23, p = .057). In contrast, caregiver emotional reactivity was not associated with either depression (t = −.28, β = −.03, p = .78) or anxiety (t = 1.26, β = .14, p = .21) symptoms.
Discussion
The present study examined the relationship that laboratory measures of emotional and cognitive empathy have with mental health in a sample of PWD caregivers. Results were partially consistent with our hypothesis that laboratory measures of emotional empathy would be associated with poor caregiver mental health. We found an association between one of the three emotional empathy measures (self-reported emotional experience to the emotional empathy task) and caregiver mental health, using a composite measure of depression and anxiety symptoms. Given the heightened depression and anxiety found in PWD caregivers (Brodaty & Donkin, 2009; Coope et al., 1995; Cuijpers, 2005; Kolanowski et al., 2004) and the increasing prevalence of caregiving for PWDs due to the aging population (Schulz et al., 2020), this finding suggests an important risk factor and possible intervention target for clinical psychologists and other health professionals who are concerned with late-life mental health issues.
Emotional empathy and caregiver mental health
Historically, clinical psychologists have considered empathy to be a highly desirable quality that is associated with desirable outcomes (Elliott, Bohart, Watson, & Murphy, 2018; Rogers, 1957). However, in the present study we found the opposite, with high levels of a particular aspect of empathy (i.e., emotional empathy as measured by self-reported negative and caring emotions in response to viewing the suffering of others) associated with an undesirable outcome (i.e., greater severity of mental health symptoms) in PWD caregivers. Of course, these findings are not without precedent, but rather are consistent with prior research indicating that high levels of emotional empathy are associated with poorer mental health in the context of others’ suffering. For example, prior research has found that too much empathy leads to empathy burnout and emotional distress in nurses, doctors, and other healthcare providers who regularly interact with distressed or suffering individuals (Decety & Fotopoulou, 2014; Figley, 2011). Similarly, an optimal level of empathy (i.e., not too much or too little) is thought to be critical for having a better psychological distinction between oneself and another’s distress (E. Ekman & Halpern, 2015). Having too much emotional empathy runs the risk of reducing this psychological distinction (Lee et al., 2001) and is considered an important cause of over-identification with patients in healthcare professionals (Decety & Fotopoulou, 2014). Our findings similarly exemplify the adages that “it depends on the context” and that one can have “too much of a good thing.” In the context of caring for a PWD, caregivers high in emotional empathy may become overly enmeshed, taking on the added burden of feeling the distress and suffering experienced by a loved one who is dealing with the ravages of a cruel, progressive, and ultimately terminal illness. For these caregivers, chronically experiencing a combination of their own distress and that of the person in their care could greatly heighten risk for developing symptoms of depression and anxiety.
Previous psychological and neuroscience research suggests that individuals who can regulate their own emotional responses to others’ suffering (and thus have optimal levels of emotional responses) can express greater concern for others instead of feeling overwhelming emotional distress (Decety & Meyer, 2008; Ho, Konrath, Brown, & Swain, 2014; A. Williams, O’Driscoll, & Moore, 2014). Caregivers may similarly benefit from evidence-based interventions that help them manage their negative emotional responses to the suffering of others in order to help maintain the distinction between self and other. Emotional responses, including those elicited as a function of empathy, are amenable to influence by emotion regulation processes (Thompson, Uusberg, Gross, & Chakrabarti, 2019; Zaki, 2014; Zaki, Bolger, & Ochsner, 2008). One well-studied regulation strategy is to engage in self-distancing, a form of adaptive self-reflection in which a “fly on the wall” approach is taken to process one’s emotional experiences (Kross, Gard, Deldin, Clifton, & Ayduk, 2012; Verduyn, Van Mechelen, Kross, Chezzi, & Van Bever, 2012). This approach has been demonstrated to be helpful in the context of relationships (Ayduk & Kross, 2010) in which high self-distancers respond to negative emotions from partners with less reciprocation of negative emotions, thus allowing for reconstrual of difficult situations. Therapists who use a similar distancing approach (e.g., imagining greater psychological distance from overwhelming client distress and the client themselves) report greater psychological well-being (Weilenmann et al., 2018). In the caregiver-PWD dyad, a caregiver may be advised to observe mentally their own emotional responses “from afar” in response to the PWDs’ distress. Slowing down the pace and reducing the magnitude of the immediate negative emotional responses may reduce distress levels and allow for greater psychological distinction between caregivers and PWDs. Of course, compared to healthcare professionals and their patients, familial caregivers and PWDs are likely to share a much longer, more intimate, and more personal history. This can make separating oneself from the PWD’s suffering particularly difficult, especially for caregivers who are high in emotional empathy. Future research should examine whether helping caregivers who are high in emotional empathy learn to regulate their emotions to others’ suffering and maintain distinction between self and other has preventative and/or therapeutic value for protecting mental health.
Robustness of findings
Our findings were robust to a number of covariates found to be related to caregiver mental health (caregiver race, PWD disease severity, and PWD cognitive functioning) and a measure of caregiver emotional reactivity (self-reported emotional experience to an acoustic startle stimulus). There is a wealth of literature on caregivers’ demographic and PWDs’ clinical variables that are associated with poor caregiver mental health (Cooper, Balamurali, & Livingston, 2007; Schulz et al., 2020). However, even after accounting for these potential influences in our sample, the relationship between caregiver emotional empathy and mental health remained. Moreover, several prior studies have found that caregivers who experience more negative emotions are more vulnerable to negative mental health outcomes (Brodaty & Donkin, 2009; Safavi et al., 2015). However, in our study, even after controlling for caregivers’ emotional responses to an aversive stimulus, the relationship between caregiver emotional empathy and mental health remained. We conclude from these findings that, in our study sample, emotional empathy plays an important role in caregivers’ mental health above and beyond the role of other well-established factors related to patient functioning, caregiver demographics, and caregiver emotional reactivity.
Limited to self-report aspect of emotional empathy
It is important to note that among the multiple aspects of caregivers’ emotional empathy (physiological, behavioral, self-reported emotional experience) that we assessed, only greater self-reported emotional experience in response to a film depicting suffering was associated with worse caregiver mental health. This specificity in findings may have implications for identifying caregivers most at risk for poor mental health. Because emotional empathy behavior was not associated with caregiver mental health, it may be difficult for clinicians and outside observers to recognize if caregivers are not faring well.3 Clinicians and outside observers may ask caregivers directly about their emotional experiences (particularly those that are relevant to another person’s suffering) in order to identify those who may be at greater risk.
Although physiological, behavioral, and self-report aspects of emotional responding can cohere in certain situations (Brown et al., 2019; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005), this is certainly not always the case (Evers et al., 2014; Reisenzein, Studtmann, & Horstmann, 2013). We have argued previously that self-reported emotional experience is much more malleable to contextual influences than physiological and expressive aspects of emotion (e.g., culture; Levenson, Soto, & Pole, 2007; Soto et al., 2005). Self-reported emotional experience arises from complex appraisals that include both contextual and interoceptive processes (Levenson, 1999; Levenson, 2003; Levenson et al., 2017) and thus may tap into some of the same processes that contribute to symptoms of anxiety and depression. Consequently, although some PWD caregivers may respond to a film depicting suffering with heightened facial expressions of negative emotions and autonomic nervous system activation, it may be those who report actually “feeling” high levels of negative emotions and concern for whom the worries, loneliness, burdens, and grief associated with caregiving are most profound and developing symptoms of depression and anxiety is most likely. Conversely, those who have more severe depression and anxiety symptoms may be more likely to report “feeling” high levels of negative emotions and concerns for others.
Cognitive empathy and caregiver mental health
In contrast to the robust relationship we found between greater emotional empathy and worse mental health in PWD caregivers, we found no relationship between either of the two measures of cognitive empathy and caregiver mental health. Prior literature suggests that professional healthcare providers (e.g., physicians, nurses) with greater cognitive empathy experience better psychological outcomes by increasing emotional distance and focusing on how the distressed person feels rather than sharing that distress (Cusi, Macqueen, Spreng, & McKinnon, 2011; E. Ekman & Halpern, 2015; Halpern, 2003). Although a similar association could be expected for familial caregivers who are high in cognitive empathy, we found no evidence supporting this in our sample of caregivers and PWDs. Nonetheless, additional research with caregivers for individuals with other disorders, at other stages of caregiving, and with yet other measures of cognitive empathy would be worthwhile.
Causality
Because the present study utilized a cross-sectional design, findings raise important questions regarding the direction of influence. It is impossible to know from our data whether caregiver emotional empathy influences caregiver mental health or vice versa. Indeed, similar associations between emotional empathy and mental health have been found in research for individuals with depression, anxiety, and other forms of psychopathology. For example, individuals with more severe psychopathology symptoms have been shown to have greater emotional empathy (O’Connor, Berry, Weiss, & Gilbert, 2002; Thoma, Schmidt, Juckel, Norra, & Suchan, 2015; Tibi-Elhanany & Shamay-Tsoory, 2011) and have trouble effectively regulating their emotional states (Sheppes, Suri, & Gross, 2015; Thompson et al., 2019). Future research using longitudinal and experimental designs would be critical for understanding the directional influences between caregiver emotional empathy and caregiver mental health.
Strengths and Limitations
Strengths of this study include: using laboratory-based measures of emotional and cognitive empathy; measuring physiological, behavioral, and self-reported aspects of emotional empathy; including two measures of cognitive empathy (emotion recognition and dynamic tracking tasks); examining and accounting for demographic factors, PWD characteristics, and caregiver emotional reactivity, all of which could influence caregiver mental health; and including caregivers who were providing care for PWDs with heterogenous diagnoses to increase generalizability.
Limitations of the study include: using self-report measures of caregiver anxiety and depression rather than structured clinical diagnostic interviews; using stimuli that activated different emotions across cognitive empathy and emotional empathy tasks, rather than consistently using stimuli showing others’ suffering, which limits our ability to compare across facets of empathy; difficulty ruling out spontaneous emotion regulation used by caregivers, which may have impacted their emotional responses to our laboratory tasks; using a cross-sectional design that limits ability to determine causal and directional influences; lack of comparison groups (e.g., caregivers of persons with other illnesses, healthy controls); and lack of diversity in race, socioeconomic status, and type of relationship to the PWD in our sample which limit the generalizability of our findings.
Conclusions
We examined the relationships between two facets of empathy and mental health in a sample of familial caregivers of PWDs with a number of different kinds of dementia and neurodegenerative diseases. Our findings indicate that greater emotional empathy in caregivers (as indicated by greater self-reported experience of negative emotions and concern in response to viewing the suffering of others) is associated with worse mental health (i.e., greater severity of depression and anxiety symptoms) in caregivers. Given the enormous number of PWDs and family caregivers worldwide and projections that this number will increase dramatically in the future, depression, anxiety, and other mental health problems in caregivers will undoubtedly become an increasingly important concern on the research, assessment, and intervention agendas for clinical psychology. Recognizing factors that increase caregiver vulnerability to poor mental health can help identify caregivers at heightened risk who may benefit from existing interventions and point toward targets for developing new interventions. Our findings suggest that it might be useful to design and evaluate interventions that help PWD caregivers regulate their emotional responses to the distress of the person in their care. Given findings that poor mental health in caregivers is also associated with greater mortality in PWDs (Lwi, Ford, Casey, Miller, & Levenson, 2017), finding ways to reduce mental health problems in caregivers could greatly improve the quality of life for both caregivers and the persons in their care.
Supplementary Material
Acknowledgements
We would like to thank our participants, Scott Newton, Deepak Paul, and all of the past and present members of the Berkeley Psychophysiology lab.
Funding
Preparation of this manuscript was supported by a National Institute of Aging grants awarded to R.W.L. (4R01AG041762-05 and 1R01AG062639-01).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest with respect to the authorship or the publication of this article.
Test re-test reliability for laboratory tasks. Given the nature of our participant pool (PWDs’ diseases are progressive), we typically only have one opportunity to enroll PWDs and their caregivers in our laboratory study. Thus, we do not have the ability to demonstrate test-retest reliability of our laboratory tasks with this sample.
To ensure we did not inadvertently influence our results by removing caregiver emotional empathy task measures from our analyses too soon, we conducted an additional analysis to include caregiver physiological responses and facial behavior to the emotional empathy task as additional covariates. We conducted a linear regression accounting for caregiver race, PWD disease severity, PWD cognitive functioning, caregiver physiological response, and caregiver facial behavior response as covariates. When these variables were entered as covariates, greater emotional empathy (self-reported emotional experience) was still related to worse caregiver mental health (t = 2.60, β = .26, p = .01), whereas caregiver physiological responses to the emotional task (t = −.52, β = −.05, p =.61) and caregiver facial behavior to the emotional empathy task were still not associated with caregiver mental health (t = −.72, β = −.07, p = .47).
We would like to thank and recognize Reviewer 2 as the source of this comment.
References
- Abdollahpour I, Nedjat S, & Salimi Y (2018). Positive Aspects of Caregiving and Caregiver Burden: A Study of Caregivers of Patients With Dementia. J Geriatr Psychiatry Neurol, 31(1), 34–38. doi: 10.1177/0891988717743590 [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, … Weiner WJ (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, & Kross E (2010). From a distance: implications of spontaneous self-distancing for adaptive self-reflection. JPers Soc Psychol, 98(5), 809–829. doi: 10.1037/a0019205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer R (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. [DOI] [PubMed] [Google Scholar]
- Beckstead JW (2012). Isolating and Examining Sources of Suppression and Multicollinearity in Multiple Linear Regression. Multivariate Behav Res, 47(2), 224–246. doi: 10.1080/00273171.2012.658331 [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJH, van Limbeerk J, Braam AW, de Vries MZ, & van Tilburg W (1997). Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D) results from a community-based sample of older subjects in the Netherlands. Psychological Medicine, 27, 231–235. [DOI] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull, 135(6), 885–908. doi: 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW Jr., Miller JP, Baty J, & Morris JC (1993). Neuropathological indexes of Alzheimer’s disease in demented and nondemented persons aged 80 years and older. ArchNeurol, 50(4), 349–358. doi: 10.1001/archneur.1993.00540040011008 [DOI] [PubMed] [Google Scholar]
- Blatt B, LeLacheur SF, Galinsky AD, Simmens SJ, & Greenberg L (2010). Does perspective-taking increase patient satisfaction in medical encounters? Acad Med, 55(9), 1445–1452. doi: 10.1097/ACM.0b013e3181eae5ec [DOI] [PubMed] [Google Scholar]
- Brodaty H, & Donkin M (2009). Family caregivers of people with dementia. Dialogues in clinical neuroscience, 22(2), 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Lwi SJ, Goodkind MS, Rankin KP, Merrilees J, Miller BL, & Levenson RW (2018). Empathic Accuracy Deficits in Patients with Neurodegenerative Disease: Association with Caregiver Depression. Am J Geriatr Psychiatry, 26(4), 484–493. doi: 10.1016/j.jagp.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Van Doren N, Ford BQ, Mauss FB, Sze JW, & Levenson RW (2019). Coherence between subjective experience and physiology in emotion: Individual differences and implications for well-being. Emotion. doi: 10.1037/emo0000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Wells JL, Hua AY, Chen KFL, Merrilees J, Miller BL, & Levenson RW (2020). Emotion Recognition and Reactivity in Persons with Neurodegenerative Disease Are Differentially Associated with Caregiver Health. The Gerontologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H, Aguzzi A, Brown P, Brucher J-M, Bugiani O, Gullotta F, … Weller RO (1995). Neuropathological Diagnostic Criteria for Creutzfeldt-Jakob Disease (CJD) and Other Human Spongiform Encephalopathies (Prion Diseases). Brain Pathology, 5, 459–466. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, … Berg L (1988). Reliability of the Washington University Clinical Dementia Rating. Arch Neurol, 45(1), 31–32. doi: 10.1001/archneur.1988.00520250037015 [DOI] [PubMed] [Google Scholar]
- Chen KH, Lwi SJ, Hua AY, Haase CM, Miller BL, & Levenson RW (2017). Increased subjective experience of non-target emotions in patients with frontotemporal dementia and Alzheimer’s disease. Curr Opin Behav Sci, 15, 77–84. doi: 10.1016/j.cobeha.2017.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Gottman JM (2007). The specific affect coding system (SPAFF). In Handbook of emotion elicitation and assessment (pp. 267–285). [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. New Jersey: Lawrence Erlbaum Associates. [Google Scholar]
- Coope B, Ballard C, Saad K, Patel A, Bentham P, Bannister C, … Wilcock G (1995). The Prevalence of Depression in the Carers of Dementia Sufferers. International Journal of Geriatric Psychiatry, 10, 237–242. [Google Scholar]
- Cooper C, Balamurali TB, & Livingston G (2007). A systematic review of the prevalence and covariates of anxiety in caregivers of people with dementia. Int Psychogeriatr, 19(2), 175–195. doi: 10.1017/S1041610206004297 [DOI] [PubMed] [Google Scholar]
- Cuijpers P (2005). Depressive disorders in caregivers of dementia patients: a systematic review. Aging Ment Health, 9(4), 325–330. doi: 10.1080/13607860500090078 [DOI] [PubMed] [Google Scholar]
- Cusi AM, Macqueen GM, Spreng RN, & McKinnon MC (2011). Altered empathic responding in major depressive disorder: relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res, 188(2), 231–236. doi: 10.1016/j.psychres.2011.04.013 [DOI] [PubMed] [Google Scholar]
- Davis MH (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44(1), 113–126. [Google Scholar]
- Day JR, & Anderson RA (2011). Compassion fatigue: an application of the concept to informal caregivers of family members with dementia. Nurs Res Pract, 2011, 408024. doi: 10.1155/2011/408024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Fotopoulou A (2014). Why empathy has a beneficial impact on others in medicine: unifying theories. Front Behav Neurosci, 8, 457. doi: 10.3389/fnbeh.2014.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Jackson PL (2006). A Social-Neuroscience Perspective on Empathy. Current Directions in Psychological Science, 15(2), 54–58. [Google Scholar]
- Decety J, & Meyer M (2008). From emotion resonance to empathic understanding: a social developmental neuroscience account. Dev Psychopathol, 20(4), 1053–1080. doi: 10.1017/S0954579408000503 [DOI] [PubMed] [Google Scholar]
- Ekman E, & Halpern J (2015). Professional Distress and Meaning in Health Care: Why Professional Empathy Can Help. Soc Work Health Care, 54(7), 633–650. doi: 10.1080/00981389.2015.1046575 [DOI] [PubMed] [Google Scholar]
- Ekman P (1977). Facial action coding system. [Google Scholar]
- Ekman P, Friesen WV, & Simons RC (1985). Is the Startle Reaction an Emotion? Journal of Personality and Social Psychology, 49(5), 1416–1426. doi: 10.1037//0022-3514.49.5.1416 [DOI] [PubMed] [Google Scholar]
- Elliott R, Bohart AC, Watson JC, & Murphy D (2018). Therapist Empathy and Client Outcome An Updated Meta-analysis. Psychotherapy, 55, 399–410. [DOI] [PubMed] [Google Scholar]
- Figley CR (2011). The Empathic Response in Clinical Practice: Antecedents and Consequences. In Empathy: From bench to bedside (pp. 263). Cambridge, Massachusetts London, England: The MIT Press. [Google Scholar]
- Fiske A, Wetherell JL, & Gatz M (2009). Depression in older adults. Annu Rev Clin Psychol, 5, 363–389. doi: 10.1146/annurev.clinpsy.032408.153621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J psychiat Res, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Fydrich T, Dowdall D, & Chambless DL (1992). Reliability and validity of the beck anxiety inventory. Journal of Anxiety Disorders, 6, 55–61. [Google Scholar]
- Gleichgerrcht E, & Decety J (2013). Empathy in clinical practice: how individual dispositions, gender, and experience moderate empathic concern, burnout, and emotional distress in physicians. PLoS One, 8(4), e61526. doi: 10.1371/journal.pone.0061526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, & Levenson R (2012). Tracking emotional valence: the role of the orbitofrontal cortex. Hum Brain Mapp, 33(4), 753–762. doi: 10.1002/hbm.21251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sturm VE, Ascher EA, Shdo SM, Miller BL, Rankin KP, & Levenson RW (2015). Emotion recognition in frontotemporal dementia and Alzheimer’s disease: A new film-based assessment. Emotion, 15(4), 416–427. doi: 10.1037/a0039261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez MF, Cappa SF, … Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1993). Emotional Suppression Physiology, Self-Report, and Expressive Behavior. Journal of Personality and Social Psychology, 64(6), 970–986. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Uusberg H, & Uusberg A (2019). Mental illness and well-being-an affect regulation perspective. World Psychiatry, 18(2), 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Haase CM, Sze J, Goodkind MS, Coppola G, Lane J, … Levenson RW (2013). The effect of the serotonin transporter polymorphism (5-HTTLPR) on empathic and self-conscious emotional reactivity. Emotion, 13(1), 25–35. doi: 10.1037/a0029616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase CM, Beermann U, Saslow LR, Shiota MN, Saturn SR, Lwi SJ, … Levenson RW (2015). Short alleles, bigger smiles? The effect of 5-HTTLPR on positive emotional expressions. Emotion, 15(4), 438–448. Retrieved from http://search.proquest.com/docview/1685830561/abstract/embedded/BD1R9E2JSTRDN7AX?source=fedsrch [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://search.proquest.com/docview/1685830561/fulltextwithgraphics/embedded/BD1R9E2JSTRDN7AX?source=fedsrch.
- http://search.proquest.com/docview/1685830561/fulltextPDF/embedded/BD1R9E2JSTRDN7AX?source=fedsrch.
- http://psvcnet.apa.org/journals/emo/15/4/438/
- Halpern J (2003). What is clinical empathy? J Gen Intern Med, 18, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman AT, & Spinhoven P (2004). The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry, 19(6), 558–563. doi: 10.1002/gps.1130 [DOI] [PubMed] [Google Scholar]
- Hein G, & Singer T (2008). I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol, 18(2), 153–158. doi: 10.1016/j.conb.2008.07.012 [DOI] [PubMed] [Google Scholar]
- Herbert LE, Weuve J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Konrath S, Brown S, & Swain JE (2014). Empathy and stress related neural responses in maternal decision making. Front Neurosci, 8, 152. doi: 10.3389/fnins.2014.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, & Gonnella JS (2011). Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med, 86(3), 359–364. doi: 10.1097/ACM.0b013e3182086fe1 [DOI] [PubMed] [Google Scholar]
- Hojat M, Mangione S, Nasca TJ, Cohen MJM, Gonella JS, Erdmann JB, & Veloski J (2001). The Jefferson Scale of Physician Empathy: development and preliminary psychometric data. Educational and psychological measurement, 61(2), 349–365. [Google Scholar]
- Hsieh S, Irish M, Daveson N, Hodges JR, & Piguet O (2013). When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol, 26(3), 174–184. doi: 10.1177/0891988713495448 [DOI] [PubMed] [Google Scholar]
- Ickes WJ (Ed.) (1997). Empathic accuracy. New York: Guilford Press. [Google Scholar]
- Jütten LH, Mark RE, & Sitskoorn MM (2019). Empathy in informal dementia caregivers and its relationship with depression, anxiety, and burden. Int J Clin Health Psychol, 19(1), 12–21. doi: 10.1016/j.ijchp.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, & Brookmeyer R (2001). Aging and the public health effects of dementia. The New England Journal of Medicine, 344(15), 1160–1161. doi: 10.1056/NEJM200104123441509 [DOI] [PubMed] [Google Scholar]
- Klockgether T (2010). Sporadic ataxia with adult onset classification and diagnostic criteria. Lancet Neurol, 9, 94–104. [DOI] [PubMed] [Google Scholar]
- Kokkonen TM, Cheston RI, Dallos R, & Smart CA (2014). Attachment and coping of dementia care staff: The role of staff attachment style, geriatric nursing self-efficacy, and approaches to dementia in burnout. Dementia (London), 13(4), 544–568. doi: 10.1177/1471301213479469 [DOI] [PubMed] [Google Scholar]
- Kolanowski AM, Fick D, Waller JL, & Shea D (2004). Spouses of persons with dementia: their healthcare problems, utilization, and costs. Res Nurs Health, 27(5), 296–306. doi: 10.1002/nur.20036 [DOI] [PubMed] [Google Scholar]
- Kross E, Gard D, Deldin P, Clifton J, & Ayduk O (2012). “Asking why” from a distance: Its cognitive and emotional consequences for people with major depressive disorder. Journal of Abnormal Psychology, 121(3), 559–569. doi: 10.1037/a0028808 [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, & Singer T (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54(3), 2492–2502. doi: 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Lee HS, Brennan PF, & Daly BJ (2001). Relationship of empathy to appraisal, depression, life satisfaction, and physical health in informal caregivers of older adults. Research in Nursing & Health, 24, 44–56. [DOI] [PubMed] [Google Scholar]
- Levenson RW (1999). The intrapersonal functions of emotion. Cognition and Emotion, 13(5), 481–504. [Google Scholar]
- Levenson RW (2003). Blood, sweat, and fears: the autonomic architecture of emotion. Annals of the New York Academy of Science, 1000, 348–366. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14766648 [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ascher EA, Goodkind MS, McCarthy M, Sturm VE, & Werner KH (2008). Laboratory testing of emotion and frontal cortex. In Goldenberg G & Miller BL (Eds.), Handbook of Clinical Neurology (Vol. 88): Elsevier. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, & Gottman JM (1993). Long-Term Marriage Age, Gender, and Satisfaction. Psychology and Aging, 8(2), 301–313. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Lwi SJ, Brown CL, Ford BQ, Otero MC, & Verstaen A (2017). Emotion. In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.), Handbook of psychophysiology (4th edition) (pp. 444–464). New York: Cambridge University Press. [Google Scholar]
- Levenson RW, & Ruef AM (1992). Empathy: A Physiological Substrate. Journal of Personality and Social Psychology, 63(2), 234–246. [PubMed] [Google Scholar]
- Levenson RW, Soto J, & Pole N (2007). Emotion, biology, and culture. In Kitayama S & Cohen D (Eds.), Handbook of cultural psychology (pp. 780–796). New York: Guilford Press. [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, … Zee DS (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology, 47(1), 1–9. doi: 10.1212/wnl.47.1.1 [DOI] [PubMed] [Google Scholar]
- Lwi SJ, Casey JJ, Verstaen A, Connelly DE, Merrilees J, & Levenson RW (2018). Genuine Smiles by Patients During Marital Interactions are Associated with Better Caregiver Mental Health. J Gerontol B Psychol Sci Soc Sci, 00(00), 1–13. doi: 10.1093/geronb/gbx157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwi SJ, Ford BQ, Casey JJ, Miller BL, & Levenson RW (2017). Poor caregiver mental health predicts mortality of patients with neurodegenerative disease. Proc Natl Acad Sci U S A, 114(28), 7319–7324. doi: 10.1073/pnas.1701597114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwi SJ, Haase CM, Shiota MN, Newton SL, & Levenson RW (2019). Responding to the emotions of others: Age differences in facial expressions and age-specific associations with relational connectedness. Emotion, 19(8), 1437–1449. doi: 10.1037/emo0000534 [DOI] [PubMed] [Google Scholar]
- Marsh AA (2018). The neuroscience of empathy. Current Opinion in Behavioral Sciences, 19, 110–115. doi: 10.1016/j.cobeha.2017.12.016 [DOI] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5(2), 175–190. [DOI] [PubMed] [Google Scholar]
- McKeith IG (2004). Dementia with Lewy bodies. Dialogues in clinical neuroscience, 6(3), 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, … Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB (2009). Assessing autonomic nervous system activity. In Methods in Social Neuroscience (pp. 118–147).19851940 [Google Scholar]
- Monin JK, & Schulz R (2009). Interpersonal effects of suffering in older adult caregiving relationships. Psychol Aging, 24(3), 681–695. doi: 10.1037/a0016355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli SA, Ong DC, Makati R, Jackson MO, & Zaki J (2017). Empathy and well-being correlate with centrality in different social networks. Proc Natl Acad Sci U S A, 114(37), 9843–9847. doi: 10.1073/pnas.1702155114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Morris JC (1997). Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr, 9 Suppl 1, 173–176; discussion 177-178. doi: 10.1017/s1041610297004870 [DOI] [PubMed] [Google Scholar]
- Murphy BA, & Lilienfeld SO (2019). Are self-report cognitive empathy ratings valid proxies for cognitive empathy ability? Negligible meta-analytic relations with behavioral task performance. Psychol Assess, 31(8), 1062–1072. doi: 10.1037/pas0000732 [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, & Lucas JA (2008). Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol, 65(7), 963–967. doi: 10.1001/archneur.65.7.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, … Doody R (2008). Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol, 65(8), 1091–1095. doi: 10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor LE, Berry JW, Weiss J, & Gilbert P (2002). Guilt, fear, submission, and empathy in depression. Journal of Affective Disorders, 71, 19–27. [DOI] [PubMed] [Google Scholar]
- Ornstein K, & Gaugler JE (2012). The problem with “roblem behaviors”: a systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient-caregiver dyad. Int Psychogeriatr, 24(10), 1536–1552. doi: 10.1017/S1041610212000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero MC, & Levenson RW (2017). Lower Visual Avoidance in Dementia Patients Is Associated with Greater Psychological Distress in Caregivers. Dement Geriatr Cogn Disord, 43(5-6), 247–258. doi: 10.1159/000468146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, & de Waal FB (2002). Empathy: Its ultimate and proximate bases. Behavioral and Brain Sciences, 25, 21–72. doi: 10.1017/s0140525x02230010 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, … Miller BL (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(Pt 9), 2456–2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson TJ, Lee SJ, Berg-Weger M, & Grossberg GT (2013). Caregiver health: health of caregivers of Alzheimer’s and other dementia patients. Curr Psychiatry Rep, 15(7), 367. doi: 10.1007/s11920-013-0367-2 [DOI] [PubMed] [Google Scholar]
- Roberts NA, Beer JS, Werner KH, Scabini D, Levens SM, Knight RT, & Levenson RW (2004). The impact of orbital frontal prefrontal cortex damage on emotional activation to unancitipated and anticipated acoustic startle stimuli. Cognitive, Affective, & Behavioral Neuroscience, 4(3), 307–316. [DOI] [PubMed] [Google Scholar]
- Rogers CR (1951). Client-centered therapy. Boston: Houghton Mifflin. [Google Scholar]
- Rogers CR (1957). The necessary and sufficient conditions of therapeutic personality change. Journal of Consulting Psychology, 21(2), 95–103. Retrieved from http://search.proquest.com/docview/615327271/abstract/embedded/GCP29XIB0CZ3LGD4?source=fedsrch [DOI] [PubMed] [Google Scholar]
- http://search.proquest.com/docview/615327271/fulltext/embedded/GCP29XIB0CZ3LGD4?source=fedsrch.
- http://search.proquest.com/docview/615327271/fulltextPDF/embedded/GCP29XIB0CZ3LGD4?source=fedsrch.
- Rueckert L, Branch B, & Doan T (2011). Are Gender Differences in Empathy Due to Differences in Emotional Reactivity? Psychology, 02(06), 574–578. doi: 10.4236/psych.2011.26088 [DOI] [Google Scholar]
- Ruef A, & Levenson R (2007). Continuous Measurement of Emotion: The affect rating dial In Coan JA, Allen JJB, editors. Handbook of Emotion Elicitation and Assessment. In: New York: Oxford University Press. [Google Scholar]
- Safavi R, Berry K, & Wearden A (2017). Expressed Emotion in relatives of persons with dementia: a systematic review and meta-analysis. Aging Ment Health, 21(2), 113–124. doi: 10.1080/13607863.2015.1111863 [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Czaja SJ, Martire LM, & Monin JK (2020). Family Caregiving for Older Adults. Annu Rev Psychol, 71, 635–659. doi: 10.1146/annurev-psych-010419-050754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, & Fleissner K (1995). Psychiatric and Physical Morbidity Effects of Dementia Caregiving Prevalence, Correlates, and Causes. The Gerontological Society of America, 35(6), 771–791. [DOI] [PubMed] [Google Scholar]
- Schuurmans J, & van Balkom A (2011). Late-life anxiety disorders: a review. Curr Psychiatry Rep, 13(4), 267–273. doi: 10.1007/s11920-011-0204-4 [DOI] [PubMed] [Google Scholar]
- Sheppes G, Suri G, & Gross JJ (2015). Emotion regulation and psychopathology. Annu Rev Clin Psychol, 11, 379–405. doi: 10.1146/annurev-clinpsy-032814-112739 [DOI] [PubMed] [Google Scholar]
- Shim B, Barroso J, & Davis LL (2012). A comparative qualitative analysis of stories of spousal caregivers of people with dementia: negative, ambivalent, and positive experiences. Int JNurs Stud, 49(2), 220–229. doi: 10.1016/j.ijnurstu.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Singer T, & Lamm C (2009). The social neuroscience of empathy. Ann N Y Acad Sci, 1156, 81–96. doi: 10.1111/j.1749-6632.2009.04418.x [DOI] [PubMed] [Google Scholar]
- Soto JA, Levenson RW, & Ebling R (2005). Cultures of Moderation and Expression: Emotional Experience, Behavior, and Physiology in Chinese Americans and Mexican Americans. Emotion, 5(2), 154–165. [DOI] [PubMed] [Google Scholar]
- Steer RA, & Beck AT (1997). Beck Anxiety Inventory. [Google Scholar]
- Sturm VE, Rosen HJ, Allison S, Miller BL, & Levenson RW (2006). Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain, 129(Pt 9), 2508–2516. doi: 10.1093/brain/awl145 [DOI] [PubMed] [Google Scholar]
- Sze JA, Goodkind MS, Gyurak A, & Levenson RW (2012). Aging and emotion recognition: not just a losing matter. Psychol Aging, 27(4), 940–950. doi: 10.1037/a0029367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JA, Gyurak A, Goodkind MS, & Levenson RW (2012). Greater emotional empathy and prosocial behavior in late life. Emotion, 12(5), 1129–1140. doi: 10.1037/a0025011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Schmidt T, Juckel G, Norra C, & Suchan B (2015). Nice or effective? Social problem solving strategies in patients with major depressive disorder. Psychiatry Res, 228(3), 835–842. doi: 10.1016/j.psychres.2015.05.015 [DOI] [PubMed] [Google Scholar]
- Thompson NM, Uusberg A, Gross JJ, & Chakrabarti B (2019). Empathy and emotion regulation: An integrative account. Prog Brain Res, 247, 273–304. doi: 10.1016/bs.pbr.2019.03.024 [DOI] [PubMed] [Google Scholar]
- Tibi-Elhanany Y, & Shamay-Tsoory SG (2011). Social cognition in social anxiety: First evidence for increased empathic abilities. Isr J Psychiatry Relat Sci, 48(2), 98–106. [PubMed] [Google Scholar]
- Tombaugh TN, & McIntyre NJ (1992). The mini-mental state examination: a comprehensive review. J Am Geriatr Soc, 40(9), 922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- van Knippenberg RJM, de Vugt ME, Ponds RW, Verhey FRJ, & Myin-Germeys I (2018). Emotional reactivity to daily life stress in spousal caregivers of people with dementia: An experience sampling study. PLoS One, 13(4), e0194118. doi: 10.1371/journal.pone.0194118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduyn P, Van Mechelen I, Kross E, Chezzi C, & Van Bever F (2012). The Relationship Between Self-Distancing and the Duration of Negative and Positive Emotional Experiences in Daily Life. Emotion. doi: 10.1037/a0028289 [DOI] [PubMed] [Google Scholar]
- Verstaen A, Eckart JA, Muhtadie L, Otero MC, Sturm VE, Haase CM, … Levenson RW (2016). Insular atrophy and diminished disgust reactivity. Emotion, 16(6), 903–912. doi: 10.1037/emo0000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstaen A, Haase CM, Lwi SJ, & Levenson RW (2018). Age-related changes in emotional behavior: Evidence from a 13-year longitudinal study of long-term married couples. Emotion. doi: 10.1037/emo0000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Liao KY, Ku TY, & Shaffer PA (2011). Attachment, self-compassion, empathy, and subjective well-being among college students and community adults. JPers, 79(1), 191–221. doi: 10.1111/j.1467-6494.2010.00677.x [DOI] [PubMed] [Google Scholar]
- Weilenmann S, Schnyder U, Parkinson B, Corda C, von Kanel R, & Pfaltz MC (2018). Emotion Transfer, Emotion Regulation, and Empathy-Related Processes in Physician-Patient Interactions and Their Association With Physician Well-Being: A Theoretical Model. Front Psychiatry, 9, 389. doi: 10.3389/fpsyt.2018.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JL, Brown CL, Hua AY, Soyster PD, Chen KH, Dokuru DR, … Levenson RW (2019). Neurodegenerative Disease Caregivers’ 5-HTTLPR Genotype Moderates the Effect of Patients’ Empathic Accuracy Deficits on Caregivers’ Well-Being. Am J Geriatr Psychiatry, 27(10), 1046–1056. doi: 10.1016/j.jagp.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JL, Hua AY, & Levenson RW (2020). Poor disgust suppression is associated with increased anxiety in caregivers of persons with neurodegenerative disease. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, O’Driscoll K, & Moore C (2014). The influence of empathic concern on prosocial behavior in children. Front Psychol, 5, 425. doi: 10.3389/fpsyg.2014.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MM, Storandt M, Roe CM, & Morris JC (2013). Progression of Alzheimer’s disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement, 9(1 Suppl), S39–44. doi: 10.1016/j.jalz.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM, Bell JF, Whitney RL, Ridberg RA, Reed SC, & Vitaliano PP (2020). Social Determinants of Health: Underreported Heterogeneity in Systematic Reviews of Caregiver Interventions. Gerontologist, 60(Supplement_1), S14–S28. doi: 10.1093/geront/gnz148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J (2014). Empathy: a motivated account. Psychol Bull, 140(6), 1608–1647. doi: 10.1037/a0037679 [DOI] [PubMed] [Google Scholar]
- Zaki J, Bolger N, & Ochsner KN (2008). It takes two: The interpersonal nature of empathic accuracy. Psychological Science, 19(4), 399–404. [DOI] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, & Ochsner KN (2009). The neural bases of empathic accuracy. Proc Natl Acad Sci U S A, 106(27), 11382–11387. doi:10.1073 pnas.0902666106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


