Abstract
Intravenous phenylephrine (PE) is utilized commonly in critical care for cardiovascular support. Its impact on the cerebrovasculature is unclear and its use may have important implications during states of critical neurological illness. The aim of this study was to perform a scoping review of the literature on the cerebrovascular/cerebral blood flow (CBF) effects of PE in traumatic brain injury (TBI), evaluating both animal models and human studies. We searched MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and the Cochrane Library from inception to January 2020. We identified 12 studies with various animal models and 4 studies in humans with varying TBI pathology. There was a trend toward a consistent increase in mean arterial pressure (MAP) by the injection of PE systemically, and by proxy, an increase of the cerebral perfusion pressure (CPP). There was a consistent constriction of cerebral vessels by PE reported in the small number of studies documenting such a response. However, the heterogeneity of the literature on the CBF/cerebral blood volume (CBV) response makes the strength of the conclusions on PE limited. Studies were heterogeneous in design and had significant limitations, with most failing to adjust for confounding factors in cerebrovascular/CBF response. This review highlights the significant knowledge gap on the cerebrovascular/CBF effects of PE administration in TBI, calling for further study on the impact of PE on the cerebrovasculature both in vivo and in experimental settings.
Keywords: cerebral blood flow, cerebral blood volume, cerebrovascular reactivity, cerebrovascular response, phenylephrine
Introduction
l-(3-hydroxyphenyl)-N-methylethanolamine or phenylephrine (PE), is an alpha-1 adrenergic drug that is used for systemic blood pressure support in a variety of pathologies. It is one of the most commonly utilized vasopressor agents for cardiovascular support in the management of critically ill patients, through the modulation of adrenergic receptors.1 PE is well known as an effective vasopressor that triggers vascular constriction and increases blood pressure.2
Emerging methods of biomedical signal processing for recorded cerebral perfusion pressure (CPP) and intracranial pressure (ICP) monitoring have led to targeted patient therapies, with the goal of improving patient outcomes in traumatic brain injury (TBI) based on continuous measures of cerebrovascular reactivity.3,4 Vasopressors, including PE, are used to treat patients with critical neurological illness, such as TBI, with the aim of targeting specific mean arterial pressure (MAP) and CPP goals.1,5 However, it remains unclear if PE is detrimental to end-organ cerebral perfusion, or leads to impairment of cerebral autoregulation. Such impacts may have important connotations for outcomes in critically ill patients with TBI, as the effective mediation of cerebral blood flow (CBF) or cerebrovascular reactivity may be vital.6–9 As such, understanding the impact of exogenously administered PE on cerebrovascular reactivity and CBF is crucial, as individualized care and personalized targets based on cerebral autoregulation and cerebrovascular response are emerging in management strategies for TBI.8–13
The goal of this study was to perform a systematic, scoping review of the available literature on the impact of PE on cerebrovascular/CBF response in animal models and human subjects with TBI.
Methods
A systematic review of the literature was conducted using the methodology outlined in the Cochrane Handbook for Systematic Reviewers.14 The data were reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).15 Supplementary Appendix S1 provides the PRISMA checklist.
The review questions and search strategy were decided on by the supervisor (F.A.Z.) and primary author (L.F.).
Search question, population, and inclusion/exclusion criteria
The question posed for systematic review was: What is the effect of exogenous systemically administered PE on the cerebrovascular response/CBF in TBI? All studies (animal or human), prospective and retrospective, of any size were included.
The primary outcome measure was the impact on objectively measured CBF or the cerebrovascular responsiveness (i.e., cerebral vasoconstriction or cerebral autoregulation/cerebrovascular reactivity) as documented by: autoradiographic diffusible tracer technique, freely diffusible tracers, thermal diffusion probe, clearance method, laser-Doppler flow probe, flow transducer, flow meter, visual recording software, or any other objective means of CBF determination (including imaging-based techniques). Secondary outcomes included adverse effects of PE administration, including systemic or cerebral end-organ complications.
All studies whether prospective or retrospective, of all sizes, of any age category, and with the use of PE with formal documentation of cerebrovascular response/CBF during administration in the setting of TBI, were eligible for inclusion in this review. Exclusion criteria were: a non-English language study, non-TBI animal model, non-TBI human cohort, or CBF mediation with substance other than PE.
Search strategy
MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and the Cochrane Library from inception to January 2020 were searched using individualized search strategies for each database. The search strategy for MEDLINE can be reviewed in Supplementary Appendix S2; a similar search strategy was used for the other databases. In addition, the reference lists of reviewed articles on the cerebral blood vessels/CBF response to PE were examined to ensure no references were left out.
Study selection
Using two reviewers (L.F. and J.D.), a two-step review of all articles returned by our search strategy was performed. First, the reviewers independently screened all titles and abstracts of the returned articles to decide whether they met the inclusion criteria. Second, full text of each returned article was assessed to confirm whether it met the inclusion criteria and that the primary outcome of cerebrovascular/CBF response to PE was documented. Third, the attained literature was separated into TBI versus non-TBI models. Any discrepancies between the two reviewers were resolved by a third party (F.A.Z.).
Data collection
Data were extracted from the selected articles and stored in multiple electronic databases to ensure data integrity.
Animal studies
Data abstraction fields included the following: number of animals, type of study, animal model characteristics, the goal of the study, type of vasopressors administered, dose of vasopressors administered, technique of CBF/vasculature assessment, cerebrovascular/CBF response to PE, other outcomes, and general conclusions.
Human studies
Data fields included the following: number of patients, study type, article location, mean age, patient characteristics, goal of the study, dose, dose duration, technique to measure cerebrovascular/CBF response, the documented cerebrovascular/CBF response, other outcomes, and conclusion.
Statistical analysis
A meta-analysis was not performed in this study because of the heterogeneity of model types, study designs, and data.
Results
Search results and study characteristics
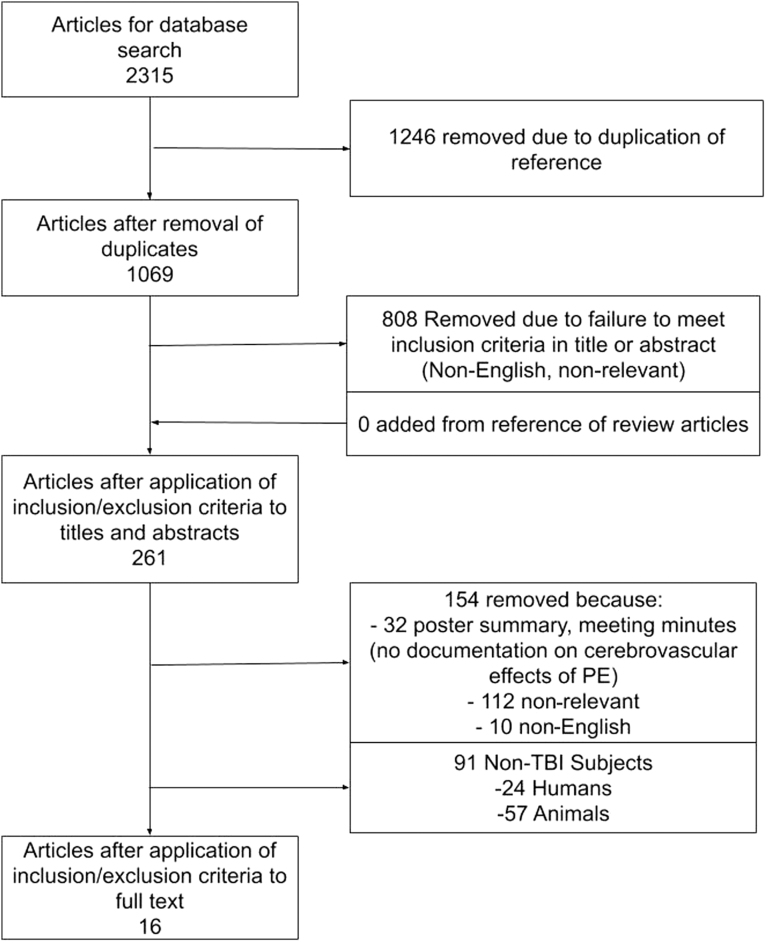
The results of the search strategy across all databases and other sources are summarized in Figure 1. Overall, 2315 articles were identified from the databases searched. Removed were 1246 articles because of duplicate references, leaving 1069 to review. By applying the inclusion/exclusion criteria to the title and abstract of these articles, we identified 261 articles that fit these criteria. No articles were added from reference sections of pertinent review articles, leaving a total of 261 articles to review. On applying the inclusion/exclusion criteria to the full-text documents, only 16 articles were found eligible for inclusion in the systematic review, all from the database search. Articles were excluded because they either did not report details around objectively measured cerebrovascular/CBF response to PE administration, were review articles, were non-relevant, or contained non-TBI cohorts.
FIG. 1.
PRISMA (Preferred Reporting in Systematic Reviews and Meta-Analysis) flow diagram of search results and filtering.
Part 1: TBI animal models
Tables 1 and 2 outline the animal TBI study characteristics and cerebrovascular/CBF responses, respectively. Of the 12 articles that had animal models with TBI, the following models were described: induced fluid percussion injury,16–22 impact-acceleration injury,23 rapid axial head rotation,24,25 and rod directed into the parietal cortex.26,27 To measure cerebrovascular/CBF response, a variety of techniques were used including: comparing ICP (found with strain gauge or catheter transducer—a surrogate metric for pulsatile cerebral blood volume [CBV]) and partial or region oxygenation (found with near infrared spectroscopy or blood samples), thermal diffusion probes, cortical laser Doppler flowmetry and direct measurement of vasculature change, or change in cerebral tissue weight (i.e., as a metric of CBV). There were 10 studies that used swine models and 2 that used rats.23,26 Of the swine model studies, 5 were conducted on newborn pigs,16–18,24,25 one had juvenile models,22 and the remaining 4 did not specify age (but were assumed to be pre-adult based on weight).19–21,27 The majority of studies controlled for both PO2 and PCO2, while administering heavy sedation.
Table 1.
TBI Animals Included Studies: General Characteristics and Study Goals
| Reference | Number of animals | Study type | Model characteristics | Primary goal of study |
|---|---|---|---|---|
| Armstead et al.16 | 40 swine | 8-arm study | Yorkshire newborn swine anesthetized initially with isoflurane then maintained with fentanyl and midazolam; TBI was induced with a lateral fluid percussion injury. Models had a craniotomy preformed to evaluate vessel change. | Primary: Examination of TBI caused cardiac dysfunction and catecholamine excess and the mediation by a vasoactive agent. |
| Secondary: Vasoactive agents' effectiveness to normalize CPP and prevent impairment of cerebral autoregulation. | ||||
| Feinstein et al.19 | 37 swine | 5-arm study | Swine anesthetized with continuous infusions of ketamine, fentanyl, and xylazine; TBI was induced with a fluid percussion injury. Models had a craniotomy preformed to evaluate vessel change. | Primary: Compare initial resuscitation of AVP, PE, or isotonic crystalloid fluid after TBI and vasodilatory shock. |
| Patel et al.20 | 26 swine | 3-arm study | Swine anesthetized with continuous infusions of ketamine, xylazine, and fentanyl with induced TBI and hemorrhage. Models had a craniotomy preformed to evaluate vessel change. | Primary: Evaluate the neurotoxicity, vasoactivity, cardiac toxicity, and inflammatory activity of hemoglobin-based oxygen carrier-201 resuscitation in TBI models. |
| Dudkiewicz et al.27 | 35 swine | Prospective randomized, blinded animal study | Swine anesthetized with ketamine and xylazine, then ketamine, xylazine, and fentanyl were used to maintain sedation; TBI was induced with blunt force. | Primary: Evaluate tissue oxygenation during management of CPP with PE or AVP. |
| Malhotra et al.21 | 45 swine | 2-arm study | Swine anesthetized with continuous infusions of ketamine, xylazine and fentanyl; TBI was induced with a fluid percussion injury, then animals were bled for 45% of their total blood volume. | Primary: Evaluate the benefits of CPP-directed therapy following TBI models. |
| Friess et al.24 | 16 piglets | 3-arm study | Four-week-old piglets were anesthetized initially with ketamine and xylazine, then maintained with isoflurane; TBI was induced with sagittal head rotations. | Primary: Evaluate PE vs. NE effect on CBF after non-invasive brain trauma. |
| Secondary: Evaluate the effects of PE and NE in targeted CPP treatment. | ||||
| Friess et al.25 | 21 swine | 2-arm study | Four-week-old female piglets were anesthetized with ketamine and xylazine, followed by inhaled 4% isoflurane; TBI was induced through a rapid axial head rotation. | Primary: Evaluate PE augmentation of CPP and its subsequent effect on ICP. |
| Cherian et al.26 | 23 rats | Prospective, randomized study | Male Long-Evans rats, weighing 300–400 g, were fasted overnight and anesthetized with isoflurane; TBI was induced by direct brain tissue impact from a rod. | Primary: Evaluate cerebral hemodynamic effects of PE and L-arginine after cortical impact injury. |
| Curvello et al22 | 30 swine | 6-arm study | Juvenile pigs anesthetized initially with isoflurane, then maintained with midazolam, fentanyl, propofol, dexmedetomidine, and saline; TBI was induced with a fluid percussion injury. | Primary: Evaluate sex and age differences in TBI models with PE treatment of CPP. |
| Talmor et al.23 | 48 rats | 4-arm study | Sprague-Dawley rats were anesthetized with halothane, with TBI induced through closed-head trauma. | Primary: Examine PE-induced hypertension to improve neurological outcomes in TBI models. |
| Armstead et al.18 | 14 groups of 5 swine | 14-arm study | Newborn swine were anesthetized with isoflurane; TBI was induced through a fluid percussion injury. | Primary: Evaluate the potassium channel impairment after TBI in the presence of PE. |
| Secondary: The previously stated assessment comparing sex-dependent responses. | ||||
| Armstead et al.17 | 49 swine | 4-arm study | Newborn swine were anesthetized with isoflurane and maintained with a-chloralosed; TBI was induced through fluid percussion injury. | Primary: Evaluate the impairment of cerebral autoregulation during hypotension after TBI through modulation of ERK MAPK. |
AVP, vasopressin; CBF, cerebral blood flow; CPP, cerebral perfusion pressure; DA, dopamine; ERK, extracellular signal-regulated kinase; ICP, intracranial pressure; MAPK, mitogen-activated protein kinase; NE, norepinephrine; PE, phenylephrine; TBI, traumatic brain injury.
Table 2.
TBI Animals Phenylephrine Treatment and Cerebrovascular Response: Study Details
| Reference | Dose | Mean administration | Technique to measure cerebrovascular response |
Cerebrovascular response | Adverse effects to phenylephrine | Conclusions |
|---|---|---|---|---|---|---|
| Armstead et al.16 | PE: 0.8–1.2 μg/kg/min DA: 0.8–1.2 μg/kg/min Papaverine: 10−8 and 10−6M |
Increase CPP to 55–60 mm Hg | Vessel diameter: Closed cranial window technique ICP: Camino transducer |
PE in males: Diameter decreased by over 5%, THRR decreased from 1.05 to 0.95(p < 0.05) PE in females: Diameter increased by 10%, THRR increased from 1.05 to 1.1 (p < 0.05) PE + papaverine: Diameter increased by 15% in both groups DA always increase artery diameter in all groups by up to 25%, THRR increased from 1.05 to 1.20 PCO2 and PO2 were not accounted for in vessel changes |
PE and DA reduced ICP in males and females after TBI to roughly the same degree | DA caused a consistent increase to artery diameter and maintained cerebral autoregulation; PE has a varied response that is sex dependent Despite the sex-dependent cerebral vessel response to PE, the increase in CPP indicates an increase in CBF |
| Feinstein et al.19 | PE: 0.05–1 mg/kg AVP: 0.1–0.4U/kg |
40–300 min | PO2: Blood samples SvO2: Oximetry ICP: Fiber optic pressure transducer |
ICP in all groups decreased by at least 10 mm Hg as compared with the 23 mm Hg of the controls For all groups PO2 fell during shock and recovered with resuscitation, which responded to CO2 challenges SvO2 in solo PE or AVP groups failed to reach 60% but when PE or AVP was given with a crystalloid solution SvO2 reached a 60% threshold CPP dropped from 80 mm Hg to 20 mm Hg in all groups during shock, then increased to above 50 mm Hg PO2 and PCO2 were controlled |
In addition to vascular hyporesponsiveness in the late stages of vasodilatory shock, catecholamines have significant side effects including tachycardia, arrhythmia, increased myocardial oxygen consumption, and ischemia | To correct vasodilatory shock after TBI, a resuscitation strategy that combined either PE or AVP plus crystalloid solution was superior to either fluid or pressor alone PE and AVP had a non-significant effect on CBF; the ICP/PO2 relationship is being used as a surrogate assessment of CBF |
| Patel et al.20 | PE: 0.1 mg/mL Mannitol: 1 g/kg |
Increase CPP to 70 mm Hg for up to 300 min | ICP: Camino transducer PbtO2: NIRS |
PE and hemoglobin-based oxygen carrier group had on average about 20 mm Hg higher CPP then control group ICP increased to 30 mm Hg in control group, whereas PE and hemoglobin-based oxygen carrier groups remained at 10 mm Hg PbtO2: Returned to baseline value of 100% in PE and control groups, hemoglobin-based oxygen carrier group only reached 50%, and PE and control group responded to CO2 challenges; however, hemoglobin-based oxygen carrier group did not PO2and PCO2 were controlled |
Not mentioned | PE appears to decrease the overall CBF (using the technique of ICP/SctO2) as compared with control group |
| Dudkiewicz et al.27 | AVP: 20 units/mL PE: 10 mg/mL |
360 min | ICP: LICOX probe SctO2: NIRS |
AVP and PE increased CVR, SctO2, and ICP AVP: SctO2 had 5 mm Hg peaks above baseline during CO2 challenges; this increase was also seen within ICP PE: SctO2 had 2 mm Hg peaks above baseline during CO2 challenges; only after ICP was above 25 mm Hg did the challenges result in peaks PE had an ICP that was higher and lower SctO2 than AVP PO2 and PCO2 were controlled |
Not mentioned | AVP was as effective as PE for maintaining CPP but at the expense of ICP and SctO2 values Cerebrovascular response was found from ICP/ SctO2, which indicates that PE improved cerebrovascular response as compared with AVP |
| Malhotra et al.21 | PE: To maintain CPP >80 mm Hg with DA at 2 μg/kg/min |
Bolus dose of DA with a continuous infusion of PE | ICP: ICP probe in superior sagittal sinus SvO2: Blood samples from catheter |
Both PE and control group had a similar rise in ICP after injury to 15 mm Hg SvO2 in PE group increased to a baseline of 80%, whereas control group only reached 65% PO2 and PCO2 were controlled |
Not mentioned | PE was used to mediate CPP therapy, which saw a significant increase in end SvO2. CBF found in the relationship of ICP/SvO2 indicates that PE decreased CBF compared with control group |
| Friess et al.24 | NE: 7.9 ± 5.2 μg/kg/min PE: 0.9 ± 0.7 μg/kg/min |
CPP >70 mm Hg for 5 h | CBF: Thermal diffusion probe ICP: Intraparenchymal monitors |
CPP and ICP had similar responses to PE and NE NE resulted in an increase in brain tissue oxygen tension to 25 torr, and PE increased to15 torr from baseline of 10 torr NE increased CBF over time up to 40 mL/100 g/min at 5 h PE increased CBF to 30 mL/100 g/min at 5 h PO2 and PCO2 were controlled |
Not mentioned | NE resulted in a greater increase in brain tissue oxygen tension than PE; along with this NE displayed a higher increase in CBF |
| Friess et al.25 | PE: Injected to maintain CPP at 40 and 70 mm Hg | 6 h | CBF: Thermal diffusion probe PO2: Microdialysis probe |
Augmentation of CPP to 70 mm Hg by PE significantly improved PO2 CBF for the control group was under 10 mL/100 g/min at 6 h, with PE groups having a CBF above 50 mL/100 g/min (p < 0.05) PO2 and PCO2 were measured, with limited change in PCO2 |
Not mentioned | The increase in CPP by PE had similar 6-h response in CBF, which was significantly higher than the control group |
| Cherian et al.26 | Saline: 1 mL L-arginine: 300 mg/kg/min PE: 0.3 μg/kg/min |
Bolus infusion for L-arginine and 4 mL of PE over 3 h | CBF, ICP, and CPP: Laser Doppler flow PO2 and :CO2: Blood samples |
Impact injury increased ICP; and a decreased MAP, CPP, and CBF Saline-treated animals: CBF decreased to 25% of the baseline values at the impact site and stayed at that level for the entire 3-h monitoring period. On the contralateral side, CBF decreased initially and recovered gradually to approximately 50% of the pre-impact baseline value PE and L-arginine increased CBF back to near-baseline levels. PE increased ICP significantly, whereas ICP with L-arginine did not change L-arginine treatment reduced the contusion volume from a median value of 5.28 mm3 to 0.63 mm3 PCO2 and PO2 were measured, with limited change in PCO2 |
Although the pressor agents are used currently to increase CBF after TBI, other strategies may also increase CBF without the potential adverse effects of induced hypertension | PE increased CBF by increasing CPP L-arginine increased CBF without changing CPP; the improvement in CBF was accompanied by a decrease in neurological injury |
| Curvello et al.22 | PE: 0.8–1.3 μg/kg/min | Not mentioned | Pial arterial diameter: Closed cranial window | In both male and female groups PE increased THRR by 0.20 (p < 0.05), with a similar increase in arterial diameter (10% for males and females) PCo2 and Po2 were measured, with limited change in PCo2 and Po2 |
Not mentioned | PE constricts brain vessels in both male and female models PE preserves cerebral autoregulation after TBI |
| Talmor et al.23 | PE: Hypertension increase of 30–35 mm Hg | 15 min | CBV and CBF: Tissue was cut and weighed to identify volume; this is correlated with CBF | Control: Tissue injury volume was 335 ± 92 mm3 PE: Tissue injury volume was 357 ± 154 mm3 (p > 0.62) PCO2 and PO2 were not accounted for |
The study indicates that post-injury treatment with 15 min of PE-induced hypertension does not attenuate brain edema, reduce tissue injury volume, or improve neurological outcome in rats | PE-induced hypertension increased CPP and blood flow in a rat model of the focal cerebral ischemia |
| Armstead et al.18 | PE: 1 mg/kg/min Cromakalim: 10−8 to 10−6 M CGRP: 10−8 to 10−6 M |
Bolus dose 30 min before and after TBI | Blood pressure, tissue oxygen concentration, and pH: Catheter was inserted into a femoral artery Pial arterial vessel diameter: Measured with a microscope, a camera, a video output screen, and a video microscaler |
Cromakalim and CGRP elicited reproducible pial small artery dilation; vasodilation was blunted by TBI PE: Prevented reductions in pial small artery cerebral vasodilation in response to cromakalim and CGRP in females, but further reduced dilation in males PE increased CPP more in females than in males, both after TBI or combined hypotension and TBI PCO2 and PO2 were not accounted for |
CPP via PE sex-dependently improves impairment of cerebral autoregulation seen after TBI through modulation of ERK MAPK upregulation, which is aggravated in males, but is blocked in females | Autoregulation of CBF is dependent on intact functioning potassium channels These data suggest a role for sex-dependent mechanisms in the treatment of the impaired cerebral autoregulation seen after pediatric TBI, therefore PE has a tendency to increase CBF in females but result in CBF being unaffected or reduced in males |
| Armstead et al.17 | PE: 1 μg/kg/min Papaverine: 10−8 and 10−6 M |
Not mentioned | Pial artery diameter: Cranial window technique for measuring pial artery diameter CBF velocity: Transcranial Doppler ICP: Camino transducer |
PE: Reductions in pial artery diameter, CBF, CPP, and elevated ICP after TBI in males compared with females PE in males resulted in CBF decreased by 20 mL/min/100 g PE in females: Decreased impairment of hypotensive pial artery dilation after TBI Papaverine-induced pial artery vasodilation not effected by TBI and PE CBF, CPP, and autoregulation index decreased markedly during hypotension and TBI in males but less in females ERK MAPK was increased more in males than females after TBI PE blunted ERK MAPK upregulation in females, but increased ERK MAPK upregulation in males PO2 and PCO2 were controlled |
Not mentioned | Data indicate elevation of CPP with PE sex dependently prevents impairment of cerebral autoregulation during hypotension after TBI through modulation of ERK MAPK In males CBF tends to decrease, whereas in females CBF remains constant |
AVP, vasopressin; CBF, cerebral blood flow; CO2, carbon dioxide; CPP, cerebral perfusion pressure; CVR, cerebrovascular resistance; DA, dopamine; ERK, extracellular signal-regulated kinase; h, hours; ICP, intracranial pressure; MAPK, mitogen-activated protein kinase; min, minutes; NE, norepinephrine; NIRS, near infrared spectroscopy; PbtO2, brain tissue partial oxygen pressure; PCO2, partial pressure of carbon dioxide; PE, phenylephrine; PO2, partial pressure of oxygen; TBI, traumatic brain injury; THRR, transient hypothermic response ratio; SctO2, tissue oxygen saturation; SvO2, venous oxygen saturation.
CBF/CBV response
A clear and consistent increase in CPP was noted in all studies in which subjects had PE injected systemically.16,19–22,24–27 However this change did not always translate to an increase in CBF or ICP (a surrogate measure of CBV), shown by the lack of impact on ICP and CBF in two studies.21,25 In the studies in which cerebral autoregulation was impaired, there was a significant increase in ICP and CBF caused by PE injection (cerebral autoregulation was impaired with a fluid percussion injury).16–18,22 These studies evaluated the CBF before and after injury, as well as before and after PE injection, with CBF increasing toward16 or exceeding baseline values post-injection.17,18,22 One study evaluated cerebral autoregulation (direct visual change in pial blood vessels) during PE injections, with cerebral autoregulation becoming impaired in male piglets only.16 Further, in the setting of a traumatic impact (rod injected to a deformation of 3 mm in parietal cortex) with sodium nitroprusside infusion, a decrease in CBF and ICP occurred, which was then reversed with PE injection back to near baseline values.26 In a study utilizing systemic injection of PE, and that measured brain tissue mass change, there was a direct increase in tissue volume mass (correlated with CBV).23
Direct effect on cerebral vessels
The studies that measured the direct effect of PE on cerebral vessels were all neonatal models. Similar effects of PE in these studies were shown within cerebral vessels, with all studies that measured cerebrovascular diameter or tension demonstrating cerebral vasoconstriction. In two studies, PE was injected directly through a cranial window on the vessels, demonstrating a reduction in pial artery diameters.17,22 One study that used the cranial window technique, but utilized systemic injection of PE, found mixed results to vessel change.16 Another study found that pial arterial diameter decreases in male piglets during systemic PE injection, but increases in female piglets.18
Part 2: TBI human models
Tables 3 and 4 outline the human TBI study characteristics and cerebrovascular/CBF responses, respectively. There are four studies with human patients with TBI who were injected with PE, and had CBF or cerebrovascular responses measured.28–31 One study did not specify the ages of the patients, who had a Glasgow Coma Scale (GCS) score ≤8.28 The other three studies all had patients with mean ages of 30–35 years. Of these three studies, one had patients with a GCS score between 3 and 14,29 one had patients with diffuse brain injury and a GCS score ≤12,30 and one had patients with a mean GCS score of 5.5 and an injury severity score of 37.31 In these studies, CBF was measured with the 133Xenon inhalation method,28,29 the arterial jugular difference in oxygen,30 or computed tomography (CT) perfusion.31 Most studies adjusted for PCO2 but failed to mention whether PO2was controlled. Sedative regiments for these studies were not clearly indicated.
Table 3.
TBI Human Included Studies: General Characteristics and Study Goals
| References | No. patients | Study type | Article location | Mean age | Patient characteristics | Goals of study |
|---|---|---|---|---|---|---|
| Bouma et al.28 | 35 patients | Prospective cohort study | Journal | Not mentioned | Severe head injury with GCS score ≤8 for at least 6 h, with no history of cardiac disease; anesthesia was not discussed | Primary: Identify the relationship between cardiac output and CBF |
| Secondary: Compare the previously describe relationship in patients with intact vs. impaired autoregulation | ||||||
| Oertel et al.29 | Transient hyperventilation: 27 patients PE: 26 patients Propofol: 21 patients |
Prospective cohort study | Journal | 33 ± 13 years | GGS score of 3–14 underwent a total of 70 vasoreactivity testing sessions from post-injury for 0–13 days; anesthesia was not discussed | Primary: Efficacy of hyperventilation, blood pressure elevation, and metabolic suppression therapy in controlling ICP after head injury |
| Secondary: Factors that are predictive of ICP reduction | ||||||
| Sahuquillo et al.30 | 46 patients | Retrospective cohort study | Journal | 29.7 ± 11 years | Moderate or Severe head trauma with diffuse brain injury type 2 or 3 and GCS score ≤12; anesthesia was not discussed | Primary: To document false autoregulation in patients with severe head injury |
| Secondary: Autoregulation's importance in CPP management | ||||||
| Peterson et al.31 | Cerebral autoregulation was intact in 25 patients | Retrospective cohort study | Journal | 35 years | Severe TBI with GCS score of 5.5 and injury severity score of 37; anesthesia was not discussed | Primary: Use computed tomography perfusion to guide blood pressure manipulation in TBI |
| Cerebral autoregulation was disrupted in 8 patients |
CBF, cerebral blood flow; CPP, cerebral perfusion pressure; DA, dopamine; E, epinephrine; GCS, Glasgow Coma Scale; NE, norepinephrine; PE, phenylephrine; TBI, traumatic brain injury.
Table 4.
TBI Human Phenylephrine Treatment and Cerebrovascular Response: Study Details
| References | Dose | Mean duration of dose administration | Technique to measure cerebrovascular response | Cerebrovascular response | Other outcome | Conclusions |
|---|---|---|---|---|---|---|
| Bouma et al.28 | PE: 80mg/500 mL Arfonad: 500 mg/500 mL Mannitol: 0.66 mg/kg was given only to patients who had not received this drug during the previous 4 h and who were not likely to need it in the next 8 h (ICP <20 mm Hg and stable) |
To raise MAP by 30%, usually for 20 min Mannitol was given in a 20% solution for 3 min |
CBF: 133Xenon inhalation or intravenous injection method Cardiac output: Thermodilution method PCO2: Capnometer |
PE:Intact autoregulation found a CBF change of -1 ± 12% Defective autoregulation found a CBF change of 53 ± 20% (p < 0.05) Arfonad: Intact autoregulation found a CBF change of -2 ± 8% Defective autoregulation found a CBF change of -31 ± 1% Mannitol: Intact autoregulation found a CBF change of 0 ± 12% Defective autoregulation found a CBF change of 40 ± 35% (p < 0.05) In all groups ICP did not significantly change PCO2 was maintained at 34 mm Hg; PO2 was not accounted for |
Cardiac output varied significantly after injection in all groups | No correlation existed between the changes in cardiac output and the changes in CBF, regardless of the status of blood pressure autoregulation A significant increase in CBF was found after administration of mannitol and PE when autoregulation was defective |
| Oertel et al.29 | Propofol: 1 mg/kg PE: 10–100 ug/kg/min |
Propofol was administered over 10 min, followed by an infusion of PE increasing every 5 min | CBF: 133Xenon assessment and transcranial Doppler ultrasonography recordings of the MCA SjvO2: Jugular and venous blood samples |
Hyperventilation therapy: Patients experienced a mean decrease in PCO2 from 35 ± 5 to 27 ± 5 mm Hg, ICP from 20 ± 11 to 13 ± 8 mm Hg (p < 0.001) CBF velocity from 111 ± 45 to 86 ± 37 cm/sec SjvO2 from 73 ± 8 to 67 ± 8% (p < 0.001) PE: ICP increased from 16 ± 9 to 19 ± 9 mm Hg (p = 0.001) CBF velocity from 71 ± 27 to 76 ± 29 cm/sec SjvO2 from 72 ± 7 to 74 ± 9% (p < 0.001) Propofol: ICP decreased from 20 ± 10 to 16 ± 11 mm Hg (p < 0.001) CBF from 39.8 ± 13.8 to 33.5 ± 12.9 mL/100g/min (p < 0.001) SjvO2 increased from 72 ± 8 to 75 ± 8% PCO2 was measured and found to decrease on average in those who underwent hyperventilation; PO2 was not accounted for |
Predictors of an effective reduction in ICP included a high PCO2 for hyperventilation, a high study GCS score for induced hypertension, and a high PCO2 and a high CBF for metabolic suppression | Of the three modalities tested to reduce ICP, hyperventilation therapy was the most consistently effective CBF velocity was increased as a result of the PE-induced hypertension therapy Metabolic suppression therapy and hyperventilation therapy demonstrated a decrease in CBF velocity |
| Sahuquillo et al.30 | PE used to induce MAP increase by 20 mmHg | Not mentioned | ICP: Camino transducer Blood oxygenation: Fiberoptic catheter CBF: Found from arterio-jugular difference of oxygen |
CBF: 50.5% of patients had a 20% increase and 13.7% of patients had a 20% decrease; the rest had a non-significant response CPP: Had an average increase of 11.5 mm Hg but significant variation Patients with constant PCO2 were included; PO2 was not accounted for |
CPP management of autoregulation is not recommended due to the various responses | Increasing MAP to obtain a better CPP in patients is not beneficial because CBF is not modified or may even be reduced PE demonstrated a variation in CBF and ICP response with limited correlation |
| Peterson et al.31 | PE infusion was used to raise the CPP by 20 mmHg | Not mentioned | CBF: CBV/mean transit time from CT scan ICP: Camino transducer |
PE resulted in minimal changes to CBF in 75.7% of patients, with 24.3% having a notable diffusion increase The mean ICP change was 3.8 mm Hg for the disrupted autoregulation group and 1.5mm Hg for the intact autoregulation group (p < 0.006) PCO2 and PO2 were not accounted for |
Using direct measurement of CBF in response to a CPP challenge found autoregulation disruption to be much less common than other reports of similar groups | PE injections increase CPP, which either increases CBF or causes no significant change |
AVP, vasopressin; CBF, cerebral blood flow; CBV, cerebral blood volume; CPP, cerebral perfusion pressure; CVR, cerebrovascular resistance; ICP, intracranial pressure; MAP, mean arterial pressure; MCA, middle cerebral artery; PCO2, partial pressure of carbon dioxide; PE, phenylephrine; PO2, partial pressure of oxygen; SjvO2, jugular venous oxygen saturation.
CBF/CBV response
All studies objectively documented the CBF response to PE injection in human patients with TBI. One study demonstrated an increase in CPP, with concordant MAP increase.30 The CBF response in this study varied and was inconsistent across the population, demonstrating both increases and decreases. A second study failed to demonstrate a significant change in CBF or ICP (i.e., surrogate of pulsatile CBV).31 A third study compared patients with intact versus impaired cerebrovascular reactivity (measured by the percentage change in CPP/CBF with the clearance method) and found that PE substantially increased CBF, by up to 53%, when regulation was impaired versus not impaired.28 Regarding the CBV response to PE administration, an effect on ICP was also shown, with those displaying impaired cerebrovascular reactivity having a higher mean change in ICP of 3.8 mm Hg versus 1.5 mm Hg to PE injection, for the impaired versus intact groups, respectively (cerebrovascular reactivity was consider intact if an increase in CPP did not change CBF as measured by CT perfusion).31
Cerebrovascular response
No human study measured the direct cerebrovascular response to PE in human subjects. Despite this, there were some studies that attempted to quantify cerebrovascular resistance (CVR) from the CPP/CBF relationship.28,30 However, there was no consensus on the CVR response to PE administration, with CVR demonstrating a variable response.
Adverse events to phenylephrine in all models
The adverse effects of PE in animal models included: bradycardia, vascular hyperresponsiveness in the late stages of vasodilatory shock, tachycardia, arrhythmia, increased myocardial oxygen consumption, and ischemia.19,23 One study showed an increased expression of cerebral V1 receptor subtype after TBI, and the subsequent formation of cerebral edema.19 In two studies, one animal and one human, cerebral autoregulation was impaired with PE administration (i.e., there was an in-phase direct increase in CBF with increased MAP).17,28 Impaired autoregulation appeared to demonstrate a sex predominance in another study, which found PE induced impaired autoregulation preferentially in male neonatal pigs, as compared with females.17
Discussion
PE is a commonly used vasopressor agent to increase MAP in a variety of treatment practices. In critical care patients, and specifically those with TBI, PE may be utilized in targeting of ICP, CPP (and hopefully CBF) to improve outcomes. Knowledge of the cerebrovascular response to various commonly utilized vasopressor agents, such as PE, is crucial for the provision of effective treatments in TBI.4,6–11,13,32,33 The current literature on the cerebrovascular effects of PE leaves uncertainty in predicting the cerebrovascular/CBF response to its administration. Through our scoping review we attempted to gather all studies that documented the administration of PE and measured its effects on the cerebrovascular response/CBF, in both animal models and human subjects afflicted with TBI. Some important points and preliminary trends can be gleaned from this review, although it must be acknowledged that, overall, the literature in this area is limited, highlighting a significant knowledge gap.
First, PE injection leads to a direct increase in MAP, and subsequently CPP. MAP increase is reported in all studies regardless of design or method, and subsequently led to a CPP increase in all studies that concurrently evaluated ICP. This general finding is not surprising, as the main role for PE in critical neurological illness is the augmentation of MAP and CPP. Particularly, in the treatment of moderate/severe TBI, this aspect of PE is attractive and is employed to maintain ICP/CPP targets, as suggested by current guideline-based therapies.4,34 However, the exact dose-response relationship between MAP and CPP was not clearly delineated in many studies, particularly in the human studies. As we transition to personalized physiological targets in TBI care, such as optimal CPP,10,11,13 we may find ourselves targeting CPP values well above the current Brain Trauma Foundation defined target range of 60–70 mm Hg.4 As such, with increasing PE dosing, the relationship between PE dose, MAP, and subsequently CPP may prove to be non-linear. In fact, with escalating doses of PE and the subsequent increase in MAP, there is the potential to overwhelm cerebrovascular reactivity and cause degeneration of the classic Lassen autoregulatory curve.35 This would expose patients to significant secondary injury, and poor long-term outcomes, as reported in the human TBI literature.7,33,36–38 The current literature in both animal and human TBI fails to inform whether such an upper limit of PE dosing exists. It is likely the specific dose-response is dependent on a variety of individual factors, including genotype.39 Thus, despite the similar results seen with PE administration in both animal and human TBI studies, a true understanding of its impact on the cerebral vasculature remains limited, necessitating future investigation.
Second, there were a limited number of TBI animal model studies that measured directed cerebral vessel response, with only four studies in neonate piglet models.16–18,22 The literature lacks homogeneity in model type and study design. However, PE administration demonstrated consistent constriction of the cerebral vessels. It must be acknowledged though that the ability to translate cerebrovascular responses to PE in immature neonatal vessels to mature adult cerebral vessels is limited. Despite the clear limitations of these data, there is sufficient homogeneity in the outcome to suggest that PE has a direct vasoconstrictive effect on the cerebral pre-capillary arterioles. This suggested effect is important; given the strong independent association between impaired cerebrovascular reactivity and poor patient outcome in TBI,37,38 it is clear that cerebrovascular reactivity is an important aspect of cerebral physiology that impacts secondary injury exposure and outcome. Administration of vasoactive compounds such as PE may directly impact cerebrovascular reactivity, given cerebrovascular reactivity is believed to occur at the pre-capillary arteriole level.35,40–42 As highlighted in this review, literature on an exact dose-response at the pre-capillary arteriole and its impact on continuously measured cerebrovascular reactivity is absent. Therefore the impact of continuous PE dosing on cerebrovascular reactivity in TBI remains unclear, supporting the need for future research.
Third, despite the general increase in CPP, the quantitative CBF response in human models was underinvestigated. Of the two studies that showed a CBF increase with PE injection, either autoregulation was impaired,28 or there was a conflicting increase and subsequent decrease in CBF.30 The animal literature also demonstrated an increase in CBF. However, in the study design PE was never the sole focus of the study, with other medications co-administered, leading to significant confounding. Without a true understanding of the impact of PE on the pre-capillary vascular response, and subsequently CBF, we remain in the dark regarding safe dosing ranges in TBI.
Finally, within the literature it is unclear what the impact of systemic PE injection is on cerebrovascular reactivity/cerebral autoregulation in animal/human TBI, as there were no studies identified that evaluated the cerebral microvascular change within human models. Thus, it remains unknown if there exists a dose-response effect on cerebral autoregulation, where specific PE dose ranges may lead to impaired autoregulation. The animal literature adds insight to the effects of PE on cerebral vessels as reported in four studies in which PE injected systemically or locally found vessels to constrict.16–18,22 Further, one animal study suggested a potential disparity in autoregulatory response to PE based on sex, with males displaying deterioration in cerebral autoregulation caused by PE injection.17 However, the lack of direct translation of these data to human models and patient care leaves limitations to be addressed in future studies. Also, there was a lack of consistency in evaluating cerebral autoregulation. Within the human studies alone, there were three different methods used to evaluate if cerebral autoregulation was intact. A refined and accurate way to quantify cerebral autoregulation in vivo is needed. Such autoregulatory monitoring should take the form of commonly adopted continuous measures of cerebrovascular reactivity, such as pressure reactivity index (PRx).
Thus, overall, given the outlined trends and limitations of the identified literature in this area, we are left with an inconclusive view of the impact of PE on CBF/cerebrovasculature in TBI. But a “negative” or “inconclusive” systematic review does provide important information regarding the current gap in knowledge. Despite PE being a commonly administered vasoactive compound in neurocritical care, we have a limited idea of its impact on the end organ of our primary interest, the brain. Aside from the general increase in MAP and trend toward an increase in CVR, when measured, the impact on cerebral autoregulation/cerebrovascular reactivity and CBF is unclear. Such a glaring hole in the available TBI literature is both important to identify/quantify—which we believe this review has done—and represents an area where future research is of utmost importance (see “Future directions” section for more details). As such, despite the limited conclusions drawn here, we believe highlighting such limitations/deficiencies will lead to future directed study of the cerebral impacts of PE.
Limitations
Although this review is comprehensive, there exist significant limitations. First, the overall literature was very heterogeneous in design. This limits how definitive we can be in our statements regarding the impact of PE on CBF, and cerebrovascular response in TBI. Similarly, there was heterogeneity in the co-administration of vasoactive compounds, sedative regimens, and documentation of PCO2 and PO2 levels during measurement of CBF/cerebrovascular response. Also, the heterogeneity in CBF/cerebrovascular response measurement techniques between studies further limits our ability to consolidate findings between studies. Thus, the conclusions regarding the impact of PE on CBF/cerebrovascular response lack strength. Second, animal models do not necessarily translate directly to human response to PE. Therefore, despite a larger volume of literature on animal TBI models, it remains unknown if any of the data can directly translate to human patients. Similarly, some models focused on young or neonatal animals, in which it is known that the cerebrovasculature is in an immature state. The response of such vessels to PE administration may not reflect the true response in mature animals, or adult humans. As such, we are again limited in our ability to extrapolate results. Third, the disparity in response shown in CBF to PE administration limits our ability to confidently state the impact of PE on CBF, both in animals and humans afflicted with TBI. This is further clouded by the different populations and models studied. Finally, almost all studies identified, both animal and human, failed to focus on the cerebrovascular response of the pre-capillary arterioles to PE administration. The animal studies that did utilized neonatal models. These pre-capillary arterioles, which are believed to be the major players in cerebral autoregulatory response, may behave differently in neonates as compared with adults. Knowledge of the impact of PE on such vessels is crucial, as impaired cerebrovascular function is a known associate of poor outcome in the setting of TBI.
Future directions
Through this review of the literature on the effect of PE on CBF/cerebral vessel response, we found a trend toward an increase in MAP, CPP, and CVR across all animal models and human subjects with TBI. However, the CBF/cerebrovascular response remains unclear, given the disparity in responses shown. Despite the limited concrete conclusions on the impact of PE on TBI derived, this review has highlighted an important knowledge gap related to a readily administered vasoactive compound in neurocritical care, and we have outlined future avenues that require investigation.
Future work on PE and the cerebrovascular response should focus on the dose response in both animals and humans, evaluating regional disparities in vascular response across the age spectrum and between sex groups, and on the pre-capillary arterioles responsible for cerebral autoregulation. Human TBI research would benefit from multi-center and comprehensive high-frequency physiological data sets. Such data would include accurate and complete treatment markups linked to high-frequency physiology, allowing assessment of the impact of PE administration/dose changes on various aspects of cerebral physiology. The high-frequency physiology data sets would need to comprise full waveform data, allowing for advanced bio-signal analytics, and derivation of continuous measures of cerebrovascular reactivity and compensatory reserve. Similarly, the monitoring modalities employed in this human TBI population would ideally include not only ICP/ICP-derived metrics, but also measures of end-organ CBF (such as regional thermal diffusion probes or multi-channel near infrared diffuse correlation/time-resolved spectroscopy), end-organ oxygen delivery (through brain tissue oxygen probes or near infrared spectroscopy), and cerebral metabolism (through microdialysis). Further, with all monitoring in place, manipulations in PE dosing would need to occur, to ensure a comprehensive understanding of the impact of changes in PE on cerebrovascular physiology in vivo.
We envision the experimental animal model work employing both healthy controls and TBI models. An important aspect of such future investigations would be controlling/adjusting for potential confounders. This would require uniform sedation regimens and accurate control of PCO2/PO2. Evaluation of CBF/cerebrovascular response in such animal models would benefit from continuous high-temporal frequency methods, using clinically applied multi-modal monitoring/signal processing techniques. Such monitoring could, in theory, deploy multiple monitors simultaneously, assessing regional CBF, cerebrovascular reactivity, oxygen delivery (using brain tissue oxygen probes or advance near infrared spectroscopy monitoring), and metabolics (using microdialysis). Within the TBI models, given regional disruption in blood–brain barrier integrity, such high-temporal and spatial continuous monitoring is crucial, as regional disparities in CBF/cerebrovascular response post-TBI may be driven by regional differences in blood–brain barrier functionality. This research should involve the application of multi-modal cerebral physiological monitoring, and the derivation of continuous metrics of cerebrovascular reactivity.
Finally, both human and animal research would benefit from concordant genotyping and assessment of vascular protein/vasoactive small-molecule biomarkers associated with cerebrovascular control.43 The outlined multi-faceted, comprehensive approach to monitoring is the only way to improve understanding of the impact of PE administration on CBF/cerebrovascular response in TBI. Such investigations are the ongoing aspirations/work of various European,44 North American,45–50 and Canadian51 collaborative efforts.
Conclusion
In TBI, the human and animal literature on the cerebrovascular/CBF effects of PE is scarce and significantly limited. Limitations include study and model design heterogeneity and failing to adjust for potential confounders. The identified literature demonstrated a trend toward increased MAP, CPP, and CVR with PE administration. The direct cerebrovascular response to PE administration demonstrated cerebral vasoconstriction in TBI animal models. However, the CBF response to PE administration was heterogeneous, in both animal and human studies identified, with no definitive conclusions. This review highlights a major knowledge gap regarding the impact of PE in TBI. Further research into the CBF and pre-capillary arteriole response to PE administration in TBI is required, as it carries important implications for treatment in moderate/severe TBI.
Supplementary Material
Acknowledgments
F.A.Z. receives research support from the Manitoba Public Insurance (MPR) Neuroscience/TBI Research Endowment, the Health Sciences Center Foundation Winnipeg, the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS), the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI), Research Manitoba, the University of Manitoba VPRI Research Investment Fund (RIF), the University of Manitoba Center on Aging, and the University of Manitoba Rudy Falk Clinician-Scientist Professorship. A.G. is supported through the University of Manitoba Clinician Investigator Program.
Abbreviations Used
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- CPP
cerebral perfusion pressure
- CT
computed tomography
- CVR
cerebrovascular resistance
- GCS
Glasgow Coma Scale
- ICP
intracranial pressure
- MAP
mean arterial pressure
- PE
phenylephrine
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRx
pressure reactivity index
- TBI
traumatic brain injury
Funding Information
This work was supported directly through the University of Manitoba, Department of Surgery GFT Research Grant (L.F.), and the University of Manitoba Office of Research Services (ORS), University Research Grant Program (URGP; L.F.).
Author Disclosure Information
No competing financial interests exist.
Supplementary Material
Cite this article as: Froese, L, Dian, J, Gomez, A, Unger, B, and Zeiler, FA (2020) Cerebrovascular response to phenylephrine in traumatic brain injury: A scoping systematic review of the human and animal literature, Neurotrauma Reports 1:1, 46–62, DOI:10.1089/neur.2020.0008.
References
- 1. Kellum, J.A., and Pinsky, M.R. (2002). Use of vasopressor agents in critically ill patients. Curr. Opin. Crit. Care 8, 236. [DOI] [PubMed] [Google Scholar]
- 2. Hollenberg, S.M. (2011). Vasoactive drugs in circulatory shock. Am. J. Respir. Crit. Care Med. 183, 847–855 [DOI] [PubMed] [Google Scholar]
- 3. Kochanek, P.M., Tasker, R.C., Bell, M.J., Adelson, P.D., Carney, N., Vavilala, M.S., Selden, N.R., Bratton, S.L., Grant, G.A., Kissoon, N., Reuter-Rice, K.E., and Wainwright, M.S. (2019). Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatr. Crit. Care Med. 20, 269–279 [DOI] [PubMed] [Google Scholar]
- 4. Carney, N., Totten, A.M., O'Reilly, C., Ullman, J.S., Hawryluk, G.W.J., Bell, M.J., Bratton, S.L., Chesnut, R., Harris, O.A., Kissoon, N., Rubiano, A.M., Shutter, L., Tasker, R.C., Vavilala, M.S., Wilberger, J., Wright, D.W., and Ghajar, J. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
- 5. Auchet, T., Regnier, M.-A., Girerd, N., and Levy, B. (2017). Outcome of patients with septic shock and high-dose vasopressor therapy. Ann. Intensive Care 7, doi: 10.1186/s13613-017-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Budohoski, K.P., Czosnyka, M., Kirkpatrick, P.J., Smielewski, P., Steiner, L.A., and Pickard, J.D. (2013). Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat. Rev. Neurol. 9, 152–163 [DOI] [PubMed] [Google Scholar]
- 7. Czosnyka, M., Smielewski, P., Kirkpatrick, P., Laing, R.J., Menon, D., and Pickard, J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41, 11–19 [DOI] [PubMed] [Google Scholar]
- 8. Zeiler, F.A., Ercole, A., Cabeleira, M., Beqiri, E., Zoerle, T., Carbonara, M., Stocchetti, N., Menon, D.K., Lazaridis, C., Smielewski, P., Czosnyka, M., and CENTER-TBI High Resolution ICU Sub-Study Participants and Investigators. (2020). Patient-specific ICP Epidemiologic Thresholds in Adult Traumatic Brain Injury: A CENTER-TBI validation study. J. Neurosurg. Anesthesiol. doi: 10.1097/ANA.0000000000000616 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9. Lazaridis, C., DeSantis, S.M., Smielewski, P., Menon, D.K., Hutchinson, P., Pickard, J.D., and Czosnyka, M. (2014). Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J. Neurosurg. 120, 893–900 [DOI] [PubMed] [Google Scholar]
- 10. Steiner, L., Czosnyka, M., Piechnik, S., Smielewski, P., Chatfield, D., Menon, D., and Pickard, J. (2002). Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 30, 733–738 [DOI] [PubMed] [Google Scholar]
- 11. Aries, M.J., Czosnyka, M., Budohoski, K., Steiner, L., Lavinio, A., Kolias, A., Hutchinson, P., Brady, K., Menon, D., Pickard, J., and Smielewski, P. (2012). Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit. Care Med. 40, 2456–2463 [DOI] [PubMed] [Google Scholar]
- 12. Zeiler, F.A., Ercole, A., Cabeleira, M., Carbonara, M., Stocchetti, N., Menon, D.K., Smielewski, P., Czosnyka, M., Anke, A., Beer, R., Bellander, B.-M., Buki, A., Chevallard, G., Chieregato, A., Citerio, G., Czeiter, E., Depreitere, B., Eapen, G., Frisvold, S., Helbok, R., Jankowski, S., Kondziella, D., Koskinen, L.-O., Meyfroidt, G., Moeller, K., Nelson, D., Piippo-Karjalainen, A., Radoi, A., Ragauskas, A., Raj, R., Rhodes, J., Rocka, S., Rossaint, R., Sahuquillo, J., Sakowitz, O., Stevanovic, A., Sundström, N., Takala, R., Tamosuitis, T., Tenovuo, O., Vajkoczy, P., Vargiolu, A., Vilcinis, R., Wolf, S., and Younsi, A. (2018). Comparison of Performance of Different Optimal Cerebral Perfusion Pressure Parameters for Outcome Prediction in Adult Traumatic Brain Injury: a Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. J. Neurotrauma 36, 1505–1517 [DOI] [PubMed] [Google Scholar]
- 13. Beqiri, E., Smielewski, P., Robba, C., Czosnyka, M., Cabeleira, M.T., Tas, J., Donnelly, J., Outtrim, J.G., Hutchinson, P., Menon, D., Meyfroidt, G., Depreitere, B., Aries, M.J., and Ercole, A. (2019). Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open 9, e030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins, J., and Thomas, J. (2019). Cochrane Handbook for Systematic Reviews of Interventions. www.training.cochrane.org/handbook/current (Last accessed January5, 2020).
- 15. Moher, D., Liberati, A., and Tetzlaff, J. (2009). Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Ann. Intern. Med. 151, 264–269 [DOI] [PubMed] [Google Scholar]
- 16. Armstead, W.M., and Vavilala, M.S. (2019). Cerebral perfusion pressure directed-therapy modulates cardiac dysfunction after traumatic brain injury to influence cerebral autoregulation in pigs. Neurocrit. Care 31, 476–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armstead, W.M., Kiessling, J.W., Kofke, W.A., and Vavilala, M.S. (2010). Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Crit. Care Med. 38, 1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstead, W.M., Kiessling, J.W., Riley, J., Kofke, W.A., and Vavilala, M.S. (2011). Phenylephrine infusion prevents impairment of ATP- and calcium-sensitive potassium channel-mediated cerebrovasodilation after brain injury in female, but aggravates impairment in male, piglets through modulation of ERK MAPK upregulation. J. Neurotrauma 28, 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feinstein, A.J., Patel, M.B., Sanui, M., Cohn, S.M., Majetschak, M., and Proctor, K.G. (2005). Resuscitation with pressors after traumatic brain injury. J. Am. Coll. Surg. 201, 536–545 [DOI] [PubMed] [Google Scholar]
- 20. Patel, M.B., Feinstein, A.J., Saenz, A.D., Majetschak, M., and Proctor, K.G. (2006). Prehospital HBOC-201 after traumatic brain injury and hemorrhagic shock in swine. J. Trauma 61, 46–56 [DOI] [PubMed] [Google Scholar]
- 21. Malhotra, A.K., Schweitzer, J.B., Fox, J.L., Fabian, T.C., and Proctor, K.G. (2003). Cerebral perfusion pressure directed therapy following traumatic brain injury and hypotension in swine. J. Neurotrauma 20, 827–839 [DOI] [PubMed] [Google Scholar]
- 22. Curvello, V., Hekierski, H., Riley, J., Vavilala, M., and Armstead, W.M. (2017). Sex and age differences in phenylephrine mechanisms and outcomes after piglet brain injury. Pediatr. Res. 82, 108–113 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Talmor, D., Roytblat, L., Artru, A.A., Yuri, O., Koyfman, L., Katchko, L., and Shapira, Y. (1998). Phenylephrine-induced hypertension does not improve outcome after closed head trauma in rats. Anesth. Analg. 87, 574–578 [DOI] [PubMed] [Google Scholar]
- 24. Friess, S.H., Bruins, B., Kilbaugh, T.J., Smith, C., and Margulies, S.S. (2015). Differing effects when using phenylephrine and norepinephrine to augment cerebral blood flow after traumatic brain injury in the immature brain. J. Neurotrauma 32, 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friess, S.H., Smith, C., Kilbaugh, T.J., Frangos, S.G., Ralston, J., Helfaer, M.A., and Margulies, S.S. (2012). Early cerebral perfusion pressure augmentation with phenylephrine after traumatic brain injury may be neuroprotective in a pediatric swine model. Crit. Care Med. 40, 2400–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cherian, L., Chacko, G., Goodman, J.C., and Robertson, C.S. (1999). Cerebral hemodynamic effects of phenylephrine and l-arginine after cortical impact injury. Crit. Care Med. 27, 2512–2517 [DOI] [PubMed] [Google Scholar]
- 27. Dudkiewicz, M., and Proctor, K.G. (2008). Tissue oxygenation during management of cerebral perfusion pressure with phenylephrine or vasopressin. Crit. Care Med. 36, 2641–2650 [DOI] [PubMed] [Google Scholar]
- 28. Bouma, G.J., and Muizelaar, J.P. (1990). Relationship between cardiac output and cerebral blood flow in patients with intact and with impaired autoregulation. J. Neurosurg. 73, 368–374 [DOI] [PubMed] [Google Scholar]
- 29. Oertel, M., Kelly, D.F., Lee, J.H., McArthur, D.L., Glenn, T.C., Vespa, P., Boscardin, W.J., Hovda, D.A., and Martin, N.A. (2002). Efficacy of hyperventilation, blood pressure elevation, and metabolic suppression therapy in controlling intracranial pressure after head injury. J. Neurosurg. 97, 1045–1053 [DOI] [PubMed] [Google Scholar]
- 30. Sahuquillo, J., Amoros, S., Santos, A., Poca, M.A., Valenzuela, H., Báguena, M., and Garnacho, A. (2000). False autoregulation (pseudoautoregulation) in patients with severe head injury. Its importance in CPP management, in: A.D., Mendelow, A. Baethmann, Z. Czernicki, J.T. Hoff, U. Ito, H.E. James, T. Kuroiwa, A. Marmarou, L.F. Marshall, and H.-J. Reulen (eds). Brain Edema XI. Springer: Vienna, pps. 485–490 [DOI] [PubMed] [Google Scholar]
- 31. Peterson, E., and Chesnut, R.M. (2009). Static autoregulation is intact in majority of patients with severe traumatic brain injury. J. Trauma 67, 944–949 [DOI] [PubMed] [Google Scholar]
- 32. Zuercher, P., Groen, J.L., Aries, M.J.H., Steyerberg, E.W., Maas, A.I.R., Ercole, A., and Menon, D.K. (2016). Reliability and validity of the therapy intensity level scale: analysis of clinimetric properties of a novel approach to assess management of intracranial pressure in traumatic brain injury. J. Neurotrauma 33, 1768–1774 [DOI] [PubMed] [Google Scholar]
- 33. Donnelly, J., Czosnyka, M., Adams, H., Cardim, D., Kolias, A.G., Zeiler, F.A., Lavinio, A., Aries, M., Robba, C., Smielewski, P., Hutchinson, P.J.A., Menon, D.K., Pickard, J.D., and Budohoski, K.P. (2019). Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: a retrospective, single-center analysis. Neurosurgery 85, E75–E82 [DOI] [PubMed] [Google Scholar]
- 34. Hawryluk, G.W.J., Aguilera, S., Buki, A., Bulger, E., Citerio, G., Cooper, D.J., Arrastia, R.D., Diringer, M., Figaji, A., Gao, G., Geocadin, R., Ghajar, J., Harris, O., Hoffer, A., Hutchinson, P., Joseph, M., Kitagawa, R., Manley, G., Mayer, S., Menon, D.K., Meyfroidt, G., Michael, D.B., Oddo, M., Okonkwo, D., Patel, M., Robertson, C., Rosenfeld, J.V., Rubiano, A.M., Sahuquillo, J., Servadei, F., Shutter, L., Stein, D., Stocchetti, N., Taccone, F.S., Timmons, S., Tsai, E., Ullman, J.S., Vespa, P., Videtta, W., Wright, D.W., Zammit, C., and Chesnut, R.M. (2019). A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 45, 1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lassen, N.A. (1974). Control of cerebral circulation in health and disease. Circ. Res. 34, 749–760 [DOI] [PubMed] [Google Scholar]
- 36. Sorrentino, E., Diedler, J., Kasprowicz, M., Budohoski, K.P., Haubrich, C., Smielewski, P., Outtrim, J.G., Manktelow, A., Hutchinson, P.J., Pickard, J.D., Menon, D.K., and Czosnyka, M. (2012). Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care 16, 258–266 [DOI] [PubMed] [Google Scholar]
- 37. Zeiler, F.A., Ercole, A., Beqiri, E., Cabeleira, M., Thelin, E.P., Stocchetti, N., Steyerberg, E.W., Maas, A.I.R., Menon, D.K., Czosnyka, M., Smielewski, P., Anke, A., Beer, R., Helbok, R., Bellander, B.-M., Nelson, D., Buki, A., Chevallard, G., Citerio, G., Chieregato, A., Czeiter, E., Depreitere, B., Eapen, G., Frisvold, S., Jankowski, S., Kondziella, D., Koskinen, L.-O., Meyfroidt, G., Moeller, K., Piippo-Karjalainen, A., Raj, R., Radoi, A., Sahuquillo, J., Ragauskas, A., Rocka, S., Rhodes, J., Rossaint, R., Stevanovic, A., Sakowitz, O., Sundström, N., Takala, R., Tamosuitis, T., Tenovuo, O., Vajkoczy, P., Vargiolu, A., Vilcinis, R., Wolf, S., and Younsi, A. (2019). Association between cerebrovascular reactivity monitoring and mortality is preserved when adjusting for baseline admission characteristics in adult traumatic brain injury: a CENTER-TBI study. J. Neurotrauma 37, 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bennis, F.C., Teeuwen, B., Zeiler, F.A., Elting, J.W., van der Naalt, J., Bonizzi, P., Delhaas, T., and Aries, M.J. (2020). Improving prediction of favourable outcome after 6 months in patients with severe traumatic brain injury using physiological cerebral parameters in a multivariable logistic regression model. Neurocrit. Care, doi: 10.1007/s12028-020-00930-6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Odekon, L., Landau, R., Blouin, J.-L., Brodow, D., Wang, S., and Smiley, R.M. (2015). The effect of β2-adrenoceptor genotype on phenylephrine dose administered during spinal anesthesia for cesarean delivery. Anesth. Analg. 120, 1309–1316 [DOI] [PubMed] [Google Scholar]
- 40. Hundley, W.G., Renaldo, G.J., Levasseur, J.E., and Kontos, H.A. (1988). Vasomotion in cerebral microcirculation of awake rabbits. Am. J. Physiol. 254, H67-H71 [DOI] [PubMed] [Google Scholar]
- 41. Halpern, W., and Osol, G. (1985). Influence of transmural pressure of myogenic responses of isolated cerebral arteries of the rat. Ann. Biomed. Eng. 13, 287–293 [DOI] [PubMed] [Google Scholar]
- 42. Auer, L.M., Ishiyama, N., and Pucher, R. (1987). Cerebrovascular response to intracranial hypertension. Acta Neurochir. (Wien) 84, 124–128 [DOI] [PubMed] [Google Scholar]
- 43. Zeiler, F.A., Thelin, E.P., Donnelly, J., Stevens, A.R., Smielewski, P., Czosnyka, M., Hutchinson, P.J., and Menon, D.K. (2019). Genetic drivers of cerebral blood flow dysfunction in TBI: a speculative synthesis. Nat. Rev. Neurol. 15, 25–39 [DOI] [PubMed] [Google Scholar]
- 44. Maas, A.I.R., Menon, D.K., Steyerberg, E.W., Citerio, G., Lecky, F., Manley, G.T., Hill, S., Legrand, V., Sorgner, A., and CENTER-TBI Participants and Investigators. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 [DOI] [PubMed] [Google Scholar]
- 45. Govindan, R.B., Brady, K.M., Massaro, A.N., Perin, J., Jennings, J.M., DuPlessis, A.J., Koehler, R.C., and Lee, J.K. (2019). Comparison of frequency- and time-domain autoregulation and vasoreactivity indices in a piglet model of hypoxia-ischemia and hypothermia. Dev. Neurosci., 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeiler, F.A., Lee, J.K., Smielewski, P., Czosnyka, M., and Brady, K. (2018). Validation of intracranial pressure-derived cerebrovascular reactivity indices against the lower limit of autoregulation, Part II: experimental model of arterial hypotension. J. Neurotrauma 35, 2812–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeiler, F.A., Donnelly, J., Calviello, L., Lee, J.K., Smielewski, P., Brady, K., Kim, D.-J., and Czosnyka, M. (2018). Validation of pressure reactivity and pulse amplitude indices against the lower limit of autoregulation, Part I: experimental intracranial hypertension. J. Neurotrauma 35, 2803–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee, J.K., Yang, Z.-J., Wang, B., Larson, A.C., Jamrogowicz, J.L., Kulikowicz, E., Kibler, K.K., Mytar, J.O., Carter, E.L., Burman, H.T., Brady, K.M., Smielewski, P., Czosnyka, M., Koehler, R.C., and Shaffner, D.H. (2012). Noninvasive autoregulation monitoring in a swine model of pediatric cardiac arrest. Anesth. Analg. 114, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brady, K.M., Lee, J.K., Kibler, K.K., Easley, R.B., Koehler, R.C., Czosnyka, M., Smielewski, P., and Shaffner, D.H. (2009). The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth. Analg. 108, 1278–1283 [DOI] [PubMed] [Google Scholar]
- 50. Brady Ken M., Lee Jennifer K., Kibler Kathleen K., Easley R. Blaine, Koehler Raymond C., and Shaffner Donald H. (2008). Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure. Stroke 39, 2531–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bernard, F., Gallagher, C., Griesdale, D., Kramer, A., Sekhon, M., and Zeiler, F.A. (2020). The CAnadian High-Resolution Traumatic Brain Injury (CAHR-TBI) Research Collaborative. Can. J. Neurol. Sci. J. Can. Sci. Neurol., 1–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.