Abstract
In advanced Chronic Kidney Disease, patients require renal replacement therapy (dialysis or transplantation) for clearance of toxins, electrolyte and acid-base balance and removal of excess fluid. Dialysis adequacy should be taken into consideration in the adjustment of the dialysis prescription. Kt/Vurea is one method of measuring dialysis adequacy that is commonly used in clinical practice. Different formulae for calculating Kt/V are available. The appropriate Kt/V formula to be used depends on the clinical scenario, as well as parameters such as gender and size of patient, frequency of dialysis, mode of dialysis (ie hemodialysis vs, peritoneal dialysis), inter-dialysis weight gain, clinical symptoms, complications (fluid overload, hyperkalemia, intolerance to dialysis, etc), and residual kidney function. Nutrition parameters including serum protein and albumin levels, vitamin B12 and β2-microglobulin levels should be factored into the assessment of dialysis adequacy. In this review, we have described how Kt/Vurea is calculated in hemodialysis and peritoneal dialysis with examples. We reviewed the available literature by searching for papers related to calculating Kt/Vurea, single pool Kt/V, double pool Kt/V, weekly Kt/V, standard Kt/V, surface area normalized Kt/V, and various equations commonly practiced in clinical practice. We found several original articles, some review articles along with detailed information from manufacturers of different dialyzers published on their websites or as package inserts. Understanding the different equations available for calculating Kt/Vurea and the application of these results in the clinical setting is important for refining patient care and for designing clinical studies.
Keywords: Dialysis adequacy, Kt/V, spKt/V, standard Kt/V, surface area normalized Kt/V
Introduction
Dialysis adequacy can be assessed using several parameters. Clearance of waste products and toxins such as urea, creatinine and β2-microglobulin may be directly measured. Clinical parameters including presence of uremic symptoms, and nutrition markers such as serum protein and albumin levels should also be monitored.[1,2,3] Combined assessment of a patient's laboratory markers and overall clinical status should be taken into consideration in the determination of adequacy and adjustment of the dialysis prescription.
Kt/Vurea ('K' is dialyzer urea clearance, 't' is total dialysis session time, and 'V' is volume of distribution of urea which is approximately equal to total body water) is the most commonly used method of measurement of dialysis adequacy worldwide. The term 'Kt' (for urea) depicts volume of fluid completely cleared of urea in a single dialysis session. Kt/Vurea was developed by Frank Gotch and John Sargent.[4,5,6]
There is some controversy regarding whether Kt/Vurea is the most appropriate measure of adequacy as it is a surrogate for small molecule clearance and does not provide information on middle and large molecule clearance. The Hemo study showed that patients undergoing hemodialysis thrice a week, with higher Kt/V than that recommended (single pool Kt/Vurea of at least 1.2), or use of high flux membranes (that give better clearance of larger molecules like β2-microglobulin) have no major impact on mortality or on serious adverse events like hospitalization for infection or cardiac causes.[7] Though the studies have shown conflicting results regarding whether more frequent dialysis improves mortality, there are other benefits including regression of left ventricular mass (if it was abnormal earlier), improved blood pressure control, reduced use of antihypertensives, and better pregnancy outcomes.[8]
Several formulae to calculate Kt/Vurea have been developed for use in different clinical settings. These include single pool Kt/V (non-equilibrated), double pool Kt/V (equilibrated), estimated weekly Kt/V, standard Kt/V and surface area normalized standard Kt/V.
In this article we will discuss these different equations and specific settings in which each is used with examples.
Section 1 Hemodialysis
Calculating residual kidney function (RKF)
Mathew A.T, Fishbane S, Obi Y and Kalantar-Zadeh K proposed that significant residual kidney function should be defined as >500 ml of urine output per day. They also proposed that RKF should be monitored regularly until urine volume is <100 mL/day or KRU (residual urea clearance by kidneys) is <2 mL/min/1.73m2.[9]
Residual kidney function is calculated as follows [Box 1]:[10]
Box 1.
Example- calculating residual kidney function
| Problem: A 46-year-old female patient with plasma BUN 74 (average of post dialysis and pre-dialysis BUN described above) and 24-h urine output of 400 ml, urine BUN 350 mg/dl. Height is 165 cm, and weight 54 Kg. Calculate her residual kidney function (Krt/V). |
| Solution: |
| Step 1: Calculate urea clearance or BUN clearance (urea clearance and BUN clearance is equal) |
| BUN clearance=BUNurine x Vurine (in ml/minute)/BUNplasma |
| BUNurine=350 mg/dl |
| BUNplasma=74 mg/dl |
| Vurine in ml/minute=400 ml/(24 x 60) = 400/1440 ml/min |
| BUN clearance = (350/74) x (400/1440) = 1.314 ml/min |
| Step 2: Calculate Krt/V |
| Kr (L/week) = Urea clearance (ml/min) X 10.08 |
| Kr=1.314 x 10.08 |
| Kr=13.245 L/week |
| V = -2.097 + (0.2466 X Weight in Kg) + (0.1069 X Height in cm) |
| = - 2.097 + (0.2466 x 54) + (0.1069 x 165) |
| =-2.097+13.3164+17.6385=28.8579 L=approximately 28.86 L |
| Krt/V=13.245 x 1/28.86=0.4589 |
Step 1: Calculate urea clearance:
Parameters to be measured:
Post- dialysis BUN (mg/dL): BUN (Blood Urea Nitrogen) measured from sample collected at the end of a session of hemodialysis (arbitrarily named as session A). If post-dialysis urea is being measured (and not BUN), then pre-dialysis urea (and not BUN), and urine urea (and not urine urea nitrogen) concentrations should be measured.
Pre-dialysis BUN (mg/dL): BUN measured before next session of hemodialysis (arbitrarily named as session B).
Mean of pre-dialysis and post-dialysis BUN: The mean is calculated and is called plasma BUN concentration (mg/dL) (BUNplasma).
24-hours urine volume (Vurine measured in ml/24 hours) and urine urea nitrogen concentration (mg/dL) (UNurine): A 24-hour urine is collected between sessions A and B (on a non-dialysis day). 24-hour urine Volume and urine urea nitrogen concentration is measured from this sample.
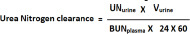
Urea nitrogen is the amount of nitrogen in urea. Molecular weight of urea is 60, and of nitrogen is 28. If we calculate Urea/Nitrogen, it is 60/28 = 2.14.
BUN is Blood Urea Nitrogen, the amount of nitrogen in urea present in blood. So, urea is equal to BUN x 2.14. In calculations, either urea nitrogen level or urea level should be used (not both).
Urea Nitrogen clearance and Urea clearances are same (see the equation below).
Urea clearance = (Ureaurine X Vurine)/ (Ureaplasma X 24 X 60)
If Urea Nitrogen is measured, and not urea, then:
Urea clearance = UNurine x 2.14 x Vurine/[(BUNplasma x 2.14) X 24 X 60]
The 2.14 in numerator and denominator cancel each other.
Step 2: Calculate residual kidney function Krt/V as follows:
'Kr' is urea clearance per week ('r' refers to 'residual')
The term 't' is time = 1 week
'V' is volume measured as total body water.
Calculating Kr
Kr is calculated in liters per week. Therefore, convert urea clearance obtained above from ml/min to liters/week.
Kr (L/week) = Urea clearance (ml/min) X [(60 X 24 X 7)/1000] = Urea clearance (ml/min) X 10.08
Calculating V (total body water):
In practice 'V' is usually calculated using Watson's equation or Hume's equation.
Watson's equation for calculating the Total Body Water (TBW) or 'V':[11,12]
Male TBW or V (in liters) = 2.447 + (0.3362 X Weight in Kg) + (0.1074 X Height in cm) – (0.09156 X Age)
Female TBW or V (in liters) = -2.097 + (0.2466 X Weight in Kg) + (0.1069 X Height in cm)
Hume-Weyers:[11]
Male TBW or V (in liters) = (0.194786 x height) + (0.296785 x weight) - 14.012934
-
Female TBW or V (in liters) = (0.34454 x height) + (0.183809 x weight) - 35.270121
The Watson formulas overestimates TBW in obese patients and underestimates TBW in overhydrated people.[13] The accurate measurement of total body water involves use of isotope dilution techniques including deuterium isotope dilution or tritium dilution.[13,14] Since it is difficult to use isotope dilution technique on a larger number of patients, investigators have used bioelectrical impedance analysis (BIA) technique to calculate total body water.[15,16]
BIA technique has been shown to accurately and reliably measure TBW.[15,16] As compared to single frequency BIA, multi-frequency BIA (MFBIA) distinguishes intracellular and extracellular water, and is more precise in measuring body water.[16] A study involving 2943 healthy Korean subjects used BIA to calculate total body water, and made a comparative study with formulae using anthropometric measurements. The investigators found that TBW calculated using Watsons formula for women, and Humes formula for men correlated better with TBW calculated using BIA.[15]
Calculating Kt/V in hemodialysis
Calculating delivered hemodialysis dose
Scenario 1: A 33-year-old female patient from Manipur is undergoing hemodialysis twice a week, each session of 4 hours duration. She has a small body size. She complains of pleuritic chest pain and breathlessness. On examination, she is found to be having pericardial friction rub. There is no history of fever. Past history: CKD, cause recurrent pyelonephritis. There is no past history suggestive of malignancy, autoimmune diseases, or tuberculosis.
Dialysis records show that her hemoglobin level is 11 gm%. BP is 138/78 mm Hg, pulse 68/min, respiratory rate 34/min. She has good 24-hour urine output (1-1.5 liters). URR is good (71%), interdialytic weight gain is 1-1.5 kg. There is no sign of fluid overload. Uremic pericarditis is suspected.
Scenario 2: A 44-year-old man, with big and strong built body structure, is receiving hemodialysis twice a week. His urine output is a little less than 1 liter per day. The regular investigations show normal serum albumin, serum creatinine 9.2 mg/dl, serum phosphorus 6.7 mg/dl, serum calcium 9.8 mg/dl, uric acid 10.2 mg/dl. Patient has no complaints. Treating nephrologist wonders whether he is receiving adequate dialysis.
Discussion: In scenario 1, calculating Kt/V may reveal that subject is being underdialysed, despite a normal URR. This subject may need increased dialysis dose, and regular monitoring of adequacy of dialysis (Kt/V), and the regular protocol of dialysis advised to manage uremic pericarditis patient. In scenario 2, the subject's serum creatinine is 9.8 mg/dl. His other labs are also high (high serum phosphorus and uric acid). This may raise the suspicion of delivering inadequate dialysis dose. Assessing dialysis adequacy by measuring Kt/V can be helpful in this case.
Single pool Kt/V is most commonly measured parameter for measuring dialysis adequacy. Double pool Kt/V is more accurate but needs the patient to wait for 30-60 minutes post dialysis.
Advantage: Kt/V calculation assures clinicians, patients, insurance companies, and any concerned authority that adequate dialysis dose is being given to a patient. Also, it may help in timely intervention to modify dialysis dose prescription.
Single pool Kt/Vurea (spKt/Vurea): In this model, it is assumed that urea is in only one compartment or 'pool' of the body and that there is a linear decline in urea level during dialysis, and an immediate equilibration occurs between the blood and tissue compartments after dialysis. This is not true, as urea exists in intracellular and extracellular spaces (different compartments). Urea from intracellular space diffuses out into the extracellular space, but complete equilibration is not immediate as it is assumed in this model and takes 30 to 60 minutes after the end of dialysis session. As the test is performed before equilibration can occur, spKt/V is also referred to as 'non-equilibrated Kt/V'.[17] Please see Figure 1 for understanding the basic concept of single pool Kt/Vurea.
Figure 1.
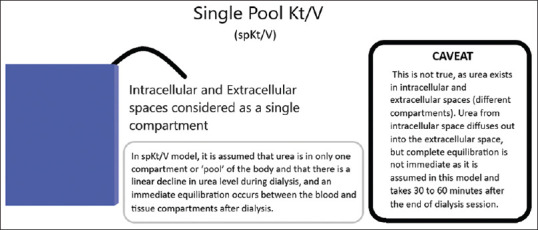
Understanding single pool Kt/V
Towards the end of dialysis session, the blood flow pump is slowed to a speed of 100 ml/min for 15 seconds and then stopped, and blood sample is collected for post-dialysis BUN. This method helps in preventing hemodialysis access recirculation.[17]
The following equation for calculating spKt/V gives reliable results when hemodialysis is being given three times per week [Box 2].[18]
Box 2.
Example- calculating spKt/V when dialysis sessions are more than 3 times per week
| Problem: A 38-year old female is receiving hemodialysis 6 hours per day, 5 times a week. The dialysis days are Monday, Tuesday, Thursday, Friday, Saturday. Calculate spKt/V from the samples taken on Friday. Post dialysis blood urea (from sample taken on Thursday) is 18 mg/dl. Pre-dialysis blood urea (from sample taken on Friday) is 52 mg/dl. Weight loss is 1 L. Her weight is 57 Kg and height 165 cm. |
| Solution: |
| We will use equation 2 (given above) for calculating spKt/V, as hemodialysis frequency is high (5 times per week). |
| Equation 2: spKt/V = - ln (R - GFAC x T) + (4-3.5R) x 0.55 x Weight loss/V |
| Step 1: Here, PIDI is 1 day (Thursday to Friday). |
| G-Factor (GFAC) is 0.0175+0.001=0.0185 [from Table 1]. |
| Step 2: Calculating ‘V’ by Watson’s formula for women: |
| V = -2.097 + (0.2466 X Weight in Kg) + (0.1069 X Height in cm) |
| = -2.097 + (0.2466 X 57) + (0.1069 X 165) |
| = -2.097+14.0562+17.6385=29.5977 L |
| Step 3: Calculating R |
| R = post-dialysis blood urea/pre-dialysis blood urea=18/52=0.3462 |
| Using these values in equation 2: |
| Equation 2: spKt/V = - ln (R - GFAC x T) + (4-3.5R) x 0.55 x Weight loss/V |
| spKt/V = -In (0.3462-0.0185 x 6) + (4-3.5 x 0.3462) x 0.55 x (1/29.5977) |
| = - ln (0.3462-0.111) + (4-1.2117) x 0.55 x 0.0338 |
| =-ln 0.2352+2.7883 x 0.0186 |
| =-ln 0.2352+0.052 |
| Using Ln calculator available on Rapid Tables (link provided in appendix): |
| = - (-1.4473) + 0.052=1.4473+0.052=1.4993 |
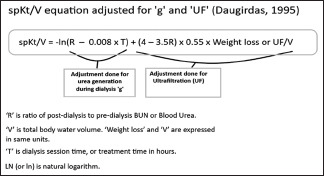
Equation 1: spKt/V = -ln (R – 0.008 x T) + (4 – 3.5R) x 0.55 x Weight loss/V
'R' is ratio of post-dialysis to pre-dialysis BUN or Blood Urea. In some parts of the world (for example, America) BUN is assayed; and in other parts (for example, Europe), blood urea is assayed [Urea = BUN x 2.14].[18,19,20]
'V' is total body water volume.
'Weight loss' and 'V' are expressed in same units.
'T' is dialysis session time, or treatment time in hours.
LN (or ln) is natural logarithm. Ln = loge. This means that natural log is equal to log to the base of e. The value of 'e' is 2.178.
Equation 1 for calculating spKt/V does not give accurate results when hemodialysis sessions are fewer than 3 times per week (or more - up to 7 times per week) due to differences in inter dialytic urea generation. Following equation is more accurate in these cases [18]:
Equation 2: spKt/V = -ln (R – GFAC x T) + (4 – 3.5R) x 0.55 x Weight loss/V
GFAC or G-factor (urea generation factor that adjusts for urea generation) value depends on frequency of dialysis and preceding interdialysis interval (PIDI). John T Daugirdas, et al. have given a table [see Table 1] to help find the value of GFAC.[14] Roughly it is 0.175 divided by the preceding inter-dialysis interval in days.[18]
Table 1.
GFAC (G-Factor) Value
| Dialysis sessions per week | Preceding Inter-Dialysis Interval (PIDI) in days | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 2 | 0.0055 | 0.0045 | ||
| 3 | 0.008 | 0.006 | ||
| 4 | 0.0155 | 0.009 | ||
| 5* | 0.0175 | 0.0095 | ||
| 6* | 0.0175 | 0.0095 | ||
| 7* | 0.0175 | 0.0175 | ||
*If dialysis session is more than 300 min, add 0.001 to GFAC value given above. Reproduced with permission from John T. Daugirdas. Reference: Daugirdas J.T, et al. Improved equation for estimating single-pool Kt/V at higher dialysis frequencies. Nephrology Dialysis Transplantation. August 2013;28 (8):2156-2160
Calculating weekly standard Kt/V is more appropriate in more frequent dialysis. Standard Kt/V is explained later in the text.
Urea reduction ratio (URR) refers to reduction in urea due to dialysis, a commonly used parameter to quantify efficacy of dialysis. URR of more than 65% is recommended as a marker of adequacy for patients who are stable on hemodialysis three times a week schedule.[21,22] URR is calculated using following equation:

Mathematical relationship between URR and spKt/V, the basic equation
Here is the mathematical equation expressing how URR and spKt/V are related:[23]
spKt/V = -ln (1-URR)
The expression 'ln' represents 'natural logarithm'.
If URR is 60%, let's calculate spKt/V.
spKt/V = -ln (1-0.6)
= -ln (0.4)
Value of 'ln 0.4' from rapid tables is -0.916
Hence, spKt/V = - (-0.916)
spKt/V = 0.916
spKt/V and URR, correction for urea generation and ultrafiltration (UF)
The basic equation given above does not consider the impact of urea generation and ultrafiltration on spKt/V during dialysis. During dialysis, the body continues to generate urea. Also, for a given value of URR, the actual urea removal is more due to ultrafiltration.[23]
Let's understand this with an example.
Patient 'A' has pre-dialysis 'V' = 45 L, UF is 3 L, so post-dialysis 'V' is 42 L.
Pre-dialysis BUN concentration is 80 mg/dl or 0.8 g/L.
So total BUN is 0.8 x 45 = 36 g.
Post dialysis BUN concentration is 40 mg/dl or 0.4 g/L. So, total BUN post-dialysis is 0.4 x 42 = 16.8 g. Total urea reduction is 36 – 16.8 = 19.2 g.
URR is [(0.8 – 0.4)/0.8] x 100 = 50%
Patient 'B' has pre-dialysis 'V' = 45 L, UF is Nil, so post-dialysis 'V' is 45 L.
Pre-dialysis BUN concentration is 80 mg/dl or 0.8 g/L, so total BUN pre-dialysis is 0.8 x 45 = 36 g.
Post dialysis BUN concentration is 40 mg/dl or 0.4 g/L. So, total BUN post-dialysis is 0.4 x 45 = 18 g. Total urea reduction is 36 – 18 = 18 g.
URR is [(0.8 – 0.4)/0.8] x 100 = 50%
So, in this example we see that for the same URR (50%), actual urea removal is more when UF is more. This implicates that spKt/V is more when UF is more, as spKt/V has 'V' in denominator, so if UF is more, the post dialysis 'V' will be lesser giving a higher value of spKt/V.
Deriving spKt/V from URR (including effect of urea generation and ultrafiltration)
From the discussion above, we see that a simple or basic mathematical equation representing the relation between URR and spKt/V is not sufficient. We need to include the effect of urea generation (g) and Ultrafiltration (UF) in the mathematical equation. Here is how we can do it.[23]
If 'R' = (predialysis BUN/post-dialysis BUN).
URR = [(predialysis BUN – Post-dialysis BUN)/pre-dialysis BUN] x 100
URR = [(predialysis BUN/predialysis BUN) – (Post-dialysis BUN/pre-dialysis BUN)] x 100
URR (expressed as a percentage) = [1 – (Post-dialysis BUN/pre-dialysis BUN)] x 100
Substituting (pre-dialysis BUN/postdialysis BUN) by 'R', we get:
URR (expressed as a percentage) = (1 – R) x 100 URR (not expressed as percentage) = 1 - R
Substituting this value of URR in the basic spKt/V equation:
Basic equation: spKt/V = -ln (1-URR)
spKt/V = -ln (1- (1-R)
spKt/V = -ln (R)
If 'V' is not known, 'V' can be assumed to be 55% of post-dialysis weight (W)
Then the above equation becomes:
spKt/V = -ln (R – 0.008 x T) + (4 – 3.5 R) x UF/W
Estimated (theoretical) dialysis dose:
Clearance of urea varies between different models of dialyzers and is dependent on-
Blood flow rate, dialysate flow rate and ultrafiltration flow rates
Surface area of the membrane, and
Intrinsic diffusive capacity of the membrane.
Surface area and intrinsic diffusive capacity of the membrane are measured as KoA (mass transfer-area coefficient)
Estimated dialysis dose can be calculated by Michaels equation shown below.[24]

KoA is the urea mass transfer-area coefficient.
'Exp' is natural exponential function. The mathematical expression exp (n) means en, where 'e' is the base of natural logarithm. Its value is equal to 2.71828 (Euler's number).[25] The number, 2.71828, a constant, is called Euler's number after the Swiss mathematician Leonhard Euler. The constant is also called Napier's constant after John Napier.[26,27] Exp is the inverse function of natural logarithm function (LN).[25]
KoA was considered as a constant for a given dialyzer-solute combination in Michaels equation.[24] However, a study by Leypoldt et al. showed that KoA value may vary at higher blood flow rates. Twenty-two different models of commercial hollow fiber dialyzers were evaluated. In-vitro testing of clearances at three countercurrent dialysate flow (QD) and blood flow (QB) rate combinations at 37°C temperature were observed. The effect of changes in QD and QB were found to be similar in different models of dialyzers used. The investigators observed that Urea KoA did not change when QB changed from 306 + - 7 to 459 + - 10 ml/min at QD 500 ml/min, and 0 UF rate. However, when QD was increased from 504 + - 6 to 819 + -8 ml/min, keeping QB at 450 ml/min and UF rate 0, urea KoA increased by 14 + - 7% (range 3 to 33% depending on the model of the dialyzer) to 780 + - 150 ml/min. The investigators proposed that small solute clearance might be limited due to stagnant fluid layers in both blood and dialysate flow systems.[28]
On realizing that stagnation fluid layers may affect dialyzer performance, manufacturers introduced better dialyzer models with better features like hollow fiber undulations, spacer yarns, and changes in fiber packing density.[24] Ward et al. investigated single-use dialyzers with hollow fiber undulations and increased packing density (Polyflux Revaclear and Revaclear MAX dialyzers) for effect of increasing dialysate flow on Kt/V. Dialysate flow rates were kept at 600 and 800 ml/min. The investigators found that increasing dialysate flow rate did not have statistically significant effect on delivered Kt/Vurea.[24]
Table 2 shows KoA (urea) values for different brands of dialyzers; and Table 3 shows urea clearance achieved from different dialyzers at different flow rates.
Table 2.
| Manufacturer | Brand of dialyzer | KoA for urea |
|---|---|---|
| Baxter | Revaclear 300 | 1186 |
| Baxter | Revaclear 400 | 1438 |
| Frasenius | Optiflux F160NR | 1064 |
| Frasenius | Optiflux F180NR | 1239 |
| Baxter | Polyflux 6H (for pediatric application) | 465 |
| Asahi Kasei Medical Co., Ltd. | Rexeed-13 L | 888 |
| Asahi Kasei Medical Co., Ltd. | Rexeed-15 L | 1045 |
| Asahi Kasei Medical Co., Ltd. | Rexeed-18 L | 1145 |
| Asahi Kasei Medical Co., Ltd. | Rexeed-21 L | 1321 |
Table 3.
| Manufacturer | Brand of Dialyzer | Membrane Material | Membrane Surface Area (m2) | K (urea) in ml/min when QB is 200 ml/min, and QD is 500 ml/min | K (urea) in ml/min when QB is 400 ml/min, and QD is 500 ml/min |
|---|---|---|---|---|---|
| Baxter | Revaclear 300 | PAES/PVP | 1.4 | 196 | 323 |
| Baxter | Revaclear 400 | PAES/PVP | 1.8 | 198 | 338 |
| Fresenius | Hemoflow F6 | Fresenius Polysulfone® polymer | 1.3 | 171 | 252 |
| Fresenius | Hemoflow F8 | Fresenius Polysulfone® polymer | 1.8 | 177 | 280 |
| Fresenius | Optiflux F180A; F180B | Fresenius Polysulfone® polymer | 1.8 | 194 | 314 |
| Fresenius | Optiflux F200A, F200B | Fresenius Polysulfone® polymer | 2.0 | 194 | 320 |
QB is blood flow rate, QD is dialysate flow rate, K is clearance, PAES is Polyarylethersulfone, PVP is Polyvinylpyrrolidone
The clearance K for urea decreases only slightly (1-2% per 10 re-uses) with reuse. By contrast, the clearance of β2-microglobulin may decrease, remain unchanged, or increase substantially with re-use depending on the membrane material and reprocessing technique used.[29]
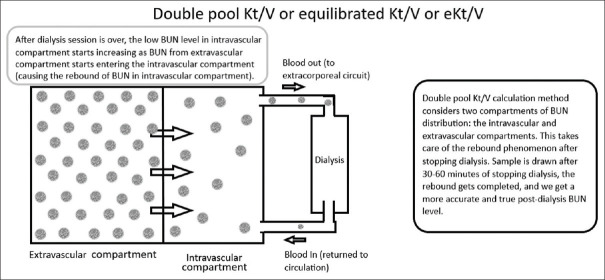
Double pool Kt/Vurea
In single pool Kt/V calculation, BUN is assumed to be distributed in a single compartment. This is far from reality. Double pool Kt/V calculation method takes into account two compartments of BUN distribution: the intravascular and extravascular compartments. After dialysis session is over, the low BUN level in intravascular compartment starts increasing as BUN from extravascular compartment starts entering the intravascular compartment (causing the rebound of BUN in intravascular compartment). If sample is drawn after 30-60 minutes of stopping dialysis, the rebound gets completed, and we get a more accurate and true post-dialysis BUN level. Please see Figure 2 for understanding the basic concept of double pool Kt/Vurea.
Figure 2.
Understanding the concept of double pool Kt/V or eKt/V
Hence, double pool Kt/Vurea, also termed equilibrated Kt/Vurea or eKt/Vurea and is more accurate as compared with single pool Kt/Vurea as it eliminates the inaccuracy of post-dialysis BUN level due to BUN rebound post-dialysis. This calculation requires obtaining a post dialysis BUN 30 minutes after the end of dialysis session.
Waiting for 30-60 minutes post dialysis for collecting post dialysis sample for estimating BUN allows enough time for urea to equilibrate from extravascular compartment to vascular compartment (the rebound). This equilibration occurs approximately 30 minutes after dialysis session is over.[17] Besides this, there is no hemodialysis access recirculation to affect BUN levels 30-60 minutes post-dialysis. Most patients do not want to wait for a test after dialysis is over, hence single pool approach for calculating Kt/V is more commonly practiced.
Equilibrated Kt/Vurea is usually 0.15 to 0.20 lower than the single-pool Kt/V. This means that spKt/Vurea of 1.2 (minimum recommended dose), is equal to equilibrated Kt/Vurea of 1.00 to 1.05.[7] This difference is due tothe 'rebound'. 'Rebound' is the urea rebound after hemodialysis session is over. For deriving eKt/Vurea from spKt/Vurea, the 'rebound' is subtracted from spKt/Vurea to get eKt/Vurea.[30]
The magnitude of 'rebound' depends on the rate at which the dialysis dose is delivered. The 'rate of dialysis' is defined as spKt/V divided by the dialysis session duration 'T', and equals to 'K/V', where 'K' is the delivered clearance, and 'V' is the volume of distribution of urea or total body water. K/V is directly proportional to rebound (greater the K/V, greater is the rebound). Consequently, the smallest patients (with smallest 'V') will have highest K/V, and highest rebounds. This means that spKt/V may be higher for small patients, and eKt/V much lower due to higher rebounds. Such patients may be getting less dialysis than that inferred from the spKt/V value.[30]
The following equations help in calculating eKt/V from spKt/V (hence a relief for clinicians and researchers as subjects do not have to wait for 30 minutes or more for the sample collection after dialysis). Two equations are available, depending on whether AV fistula (arterial access) is being used for dialysis, or a central venous catheter (venous access).[17]
The Hemo study, a multicenter trial sponsored by National Institutes of Health (NIH), demonstrated that the kinetically derived rate equation gives reasonably good prediction of eKt/V.[31] The equations are given below:[32]
AV Fistula (Arterial Access)
eKt/V = spKt/V – rebound*
Rebound = 0.6 x hemodialysis rate – 0.03**
Replacing 'hemodialysis rate' in above equation with (spKt/V) T, we get:
eKt/V = spKt/V – [0.6 x (spKt/V)/T - 0.03]
eKt/V = spKt/V – 0.6 x (spKt/V)/T + 0.03
Central Venous Catheter (Venous access)
eKt/V = spKt/V – rebound*
Rebound = 0.47x hemodialysis rate – 0.02**
Replacing 'hemodialysis rate in above equation with (spKt/V)/T, we get:
eKt/V = spKt/V – [0.47 x (spKt/V)/T - 0.02]
eKt/V = spKt/V – 0.47 x (spKt/V)/T + 0.02
*'Rebound' is 'urea rebound after hemodialysis session'
**Hemodialysis rate = (spKt/V)/T, where 'T' is hemodialysis session time in hours.
eKt/V can also be calculated using the Tattersall equation. The equation is given below.[33,34]
eKt/V = spKt/V x T/(T + C), where 'T' is dialysis session time in minutes, and 'C' is a constant and its value depends on the solute and the vascular access. When solute under question is urea, 'C' is equal to 35 minutes for arterial access, and 22 minutes for venous access.
AV Fistula (Arterial Access)
eKt/V = spKt/V x T/(T + C)*
Replacing value of 'C' we get:
eKt/Vurea = spKt/Vurea x T/(T + 35)
*'T' is dialysis session time in minutes.
*'C' is a constant and its value depends on the solute and the vascular access. When solute under question is urea, 'C' is equal to 35 minutes for arterial access.
Central Venous Catheter (Venous access)
eKt/V = spKt/V x T/(T + C)*
Replacing value of 'C' we get:
eKt/Vurea = spKt/Vurea x T/(T + 22)
*'T' is dialysis session time in minutes.
*'C' is a constant and its value depends on the solute and the vascular access. When solute under question is urea, 'C' is equal to 22 minutes for venous access.
Standard Kt/V
Scenario 3: A 45-year-old female was unable to tolerate more than 2 hours hemodialysis sessions, so she was started with 5 hemodialysis sessions per week, each of 2 hours duration. The patient is worried whether the dialysis dose is adequate.
Discussion: Standard Kt/V is recommended for calculating dialysis adequacy in more frequent hemodialysis.
Advantage: While single pool and double pool Kt/V calculate adequacy of single dialysis sessions, standard Kt/V calculates cumulative adequacy of multiple dialysis sessions over a week. This makes it a more reliable parameter of dialysis adequacy.
Disadvantage: Standard Kt/V may not be a very reliable parameter of dialysis adequacy in smaller subjects and females receiving hemodialysis more than 3 times per week. Surface area adjusted standard Kt/V is more appropriate in these settings.
Equilibrated and non-equilibrated Kt/V are used to assess the impact of a single dialysis session. Standard Kt/V (stdKt/V) is useful when the need is to calculate the efficacy of multiple sessions of dialysis over a span of a week. This is more useful in patients receiving frequent (more than 3 times a week) hemodialysis, continuous or intermittent peritoneal dialysis, or continuous renal replacement therapy (CRRT) in acute kidney injury. Standard Kt/V is also useful when the aim is to compare different dialysis treatment regimens and methods.[17]
Fixed volume model and Variable volume model for calculating stdKt/V
Standard Kt/Vurea is a representation of hypothetical continuous clearance in patients receiving intermittent hemodialysis. It takes into account the averaged pre-dialysis BUN levels, and urea nitrogen generation rate.[35] The fixed volume model does not incorporate the effect of fluid removal during dialysis and hence underestimates the delivered dialysis dose. The variable volume model takes into account the effect of fluid removal and the residual kidney function too, and hence gives more accurate estimates.[35]
Method of calculating stdKt/Vurea.
Standard Kt/Vurea can be calculated as follows [Box 3].[36]
Box 3.
Example- calculating stdKt/V
| Problem: Let us take the same patient as described in box 2 for calculating stdKt/V. Recalling the problem from box three: a 38-year old female is receiving hemodialysis 6 hours per day, 5 times a week. The dialysis days are Monday, Tuesday, Thursday, Friday, Saturday. Calculate spKt/V from the samples taken on Saturday. Post dialysis blood urea (from sample taken on Friday) is 18 mg/dl. Pre-dialysis blood urea (from sample taken on Saturday) is 52 mg/dl. Weight loss is 1 L. Her weight is 57 Kg and height 165 cm. Additional information: Vascular access is AV Fistula. Weekly ultrafiltration volume is 5400 ml. Residual kidney function (urea clearance, Kru) is 1.4 ml/min. |
| Solution: We will follow the steps as given below. |
| Step1: calculate single pool Kt/V: spKt/V has already been calculated for this patient in box 3. It is 1.4993 |
| Step 2: calculate eKt/V. |
| Using Tattersall equation: eKt/V=spKt/V x T/(T+C) |
| =1.4993x [(6 x 60)/[(6 x 60) + 35] |
| =1.4993x [360/(360+35)] |
| =1.4993x 360/395=1.3665 |
| Step 3: calculate fixed volume model std Kt/V as follows: |
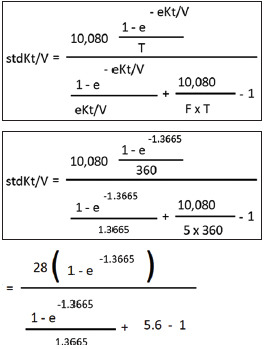 |
| As mentioned earlier, ‘e’ is 2.71828 (Euler’s number). |
| [We will use scientific calculator to calculate the value of e-1.3665 or 2.71828-1.3665] |
| e-1.3665 or 2.71828-1.3665=0.255 |
| Putting this value in the equation above, we get: |
| =28 (1-0.255)/[(1-0.255)/1.3665] + 4.6=28 x 0.745/[(0.745/1.3665) + 4.6] |
| =20.86/(0.5452+4.6) |
| =20.86/5.1452=4.0543 |
| Step 4: calculate variable volume model stdKt/V by following equation: |
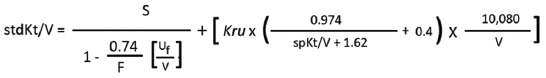 |
| S=4.0543; F=5; Uf=5400 ml; Kru=1.4 ml/min; spKt/V=1.4993; V calculated by Watson’s equation=29597.7 ml (already calculated in box 3). |
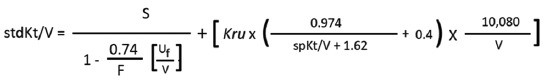 |
| =[(4.0543/(1-0.027)] + [ 1.4 x {(0.974/3.1193) +0.4} x 0.34056 |
| =(4.0543/0.973) + [1.4 x (0.3122+0.4) x 0.34056=4.1668 + (1.4 x 0.7122 x 0.34056) |
| =4.1668+0.3396=4.5064 |
| StdKt/V is 4.5064 |
Step 1: calculate spKt/V by equation used for higher frequency hemodialysis (more than 3 times per week). The equation is: spKt/V = -ln (R – GFAC x T) + (4 – 3.5R) x 0.55 x Weight loss/V
Step 2: calculate eKt/V, for example by Tattersall equation: eKt/V = spKt/V x T/(T + C), where 'T' is dialysis session time in minutes, and 'C' is a constant. When solute under question is urea, 'C' is equal to 35 minutes for arterial access, and 22 minutes for venous access.
Step 3: calculate fixed volume model stdKt/V by this equation:
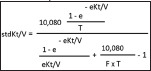
Here 'T' is treatment time in minutes; and 'F' is number of dialysis sessions in a week preceding collection of blood samples for analysis.
Step 4: calculate variable volume model stdKt/V by following equation:
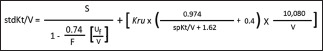
Here, 'S' is stdKt/V calculated from equation for fixed volume model; 'F' is number of dialysis sessions per week; Uf is weekly ultrafiltration volume in milliliters (ml), 'Kru' is residual urea clearance by kidneys (residual kidney function) in ml/min; and 'V' is urea distribution volume in ml.[36]
Surface area normalized standard Kt/V
Studies have shown that BSA (body surface area) seems to be more closely correlated to actual TBW.[13,37] Instead of 'V', or volume of distribution of urea as a denominator for calculating 'Kt/V', use of BSA may be more appropriate for women and smaller patients, as using 'V' results in underestimation of stdKt/V and spKt/V.[18] Moreover, 'V' changes when a patient loses weight, or gains weight (as in edema), and this affects spKt/V and stdKt/V. Using BSA instead of 'V' eliminates error due to fluctuations in 'V'.
This concept has led to the development of surface area normalized standard Kt/V (SAstdKt/V). The equation is given below:

Here, Vw is patient's volume of distribution of urea in liters and is calculated using Watson's formula. BSA is body surface area in m2 calculated using DuBois and Dubois formula. The denominator 'Nf' is 'normalizing factor', a population mean V/BSA, and its value is 20 L/m2. Its value can change if formulae other than Walson's for Vw and DuBois and DuBois for BSA.[18]
DuBois and DuBois formula for calculating BSA is given below:
BSA = (W0.425 x H0.725) x 0.007184
Here, BSA is Body Surface Area in m2, 'W' is weight in kilograms and 'H' is height in centimeters.
Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines
KDOQI guidelines for thrice weekly hemodialysis KDOQI guidelines recommend that patients with residual kidney function of less than 2 ml/min on thrice a week hemodialysis should be prescribed at least 3 hours each dialysis session.[18] Target single pool Kt/Vurea (spKt/Vurea) is recommended to be between 1.2 to 1.4 per dialysis session, spKt/Vurea of 1.2 is considered minimally adequate, and 1.4 is the target recommended dose.[18]
Many randomized control trials, including the Hemo study, showed that patients undergoing hemodialysis thrice a week, with higher Kt/V than that recommended (single pool Kt/Vurea of more than 1.4) have no survival benefit.[7,38,39] Some studies have shown that Kt/V lower than 1.2 is associated with increased mortality.[40,41] This implies that target spKt/V of 1.2 to 1.4 is reasonable.[7]
As discussed earlier, Urea Reduction Ratio (URR) is another method of measuring delivered dialysis dose. URR of 63% roughly corresponds with spKt/Vurea of 1.2.[42] Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology recommends minimum Kt/V of 1.2 or minimum URR of 65% for thrice a week hemodialysis.[43] For a given value of Kt/V, URR values may differ significantly. General consensus is that URR should be phased out and replaced with more precise methods of measuring dialysis adequacy.[18] Kt/V is more precise than URR as unlike URR, Kt/V takes into account the urea clearance due to ultrafiltration, and urea generated during hemodialysis.[18,42] Moreover, patient's residual kidney function cannot be incorporated with URR to prescribe adequate dialysis dose.[18]
KDOQI guidelines for hemodialysis less than thrice weekly
The dose of hemodialysis may be reduced in patients with significant residual kidney function. Residual kidney function needs to be monitored closely, and dialysis dose adjusted accordingly.[18] Most centers measure the residual kidney function monthly.
KDOQI guidelines for hemodialysis other than thrice weekly (more frequent and continuous dialysis treatments)
Target standard Kt/V of 2.3 per week, with a minimum of 2.1 (this includes contribution of ultrafiltration and residual kidney function) is recommended.[18]
Section 2 Peritoneal dialysis
Residual Kidney Function (RKF) when to measure and when not to measure
If peritoneal Kt/Vurea is more than 1.7, residual kidney function need not be monitored to estimate peritoneal dialysis dose.
Anuria is defined as urine output of less than 100 ml per day. If 24-hour urine output is less than 100 ml, residual kidney function need not be calculated to monitor peritoneal dialysis dose.[44]
Calculating Kt/Vurea
Kt/Vurea in peritoneal dialysis can be calculated as follows [Box 4].[45]
Box 4.
Example- calculate total weekly Kt/V in a patient receiving CAPD
| A 54-year old man is on CAPD for 2 years. He is on 3 exchanges per day with night time dwell. His weight is 65 Kg and height 168 cm. 24-hour peritoneal dialysate drain volume is 10 L, pooled dialysate urea is 42 mg/dL, and plasma urea is 48 mg/dl. Calculate his Kt/V peritoneal dialysis. If his 24-hour urine output is 450 ml, and urine urea concentration is 550 mg/dL, then calculate his residual kidney function, and total weekly Kt/V. Is he receiving adequate CAPD based on your result? |
| Answer: |
| Step 1: Calculating daily peritoneal urea clearance or Kt |
| Kt in Liters = (Durea/Purea) x VD (measured in Liters) |
| =(42/48) x 10=0.875 x 10=8.75 L |
| Step 2: Calculating V (volume of distribution of urea, that is total body water, by Watson Equation) |
| Watson: Male TBW or Volume of distribution of urea in Liters=2.447 - (0.09156 x age) + (0.1074 x height) + (0.3362 x weight) |
| = 2.447 - (0.09156 x 54) + (0.1074 x 168) + (0.3362 x 65) |
| =2.447-4.9442+18.0432+21.853=37.399 L |
| Step 3: Calculating daily peritoneal dialysis achieved (Kt/Vurea) |
| =8.75/37.399=0.234 |
| Weekly peritoneal dialysis=daily peritoneal dialysis x 7=0.234 x 7=1.638 |
| Step 4: Calculating residual kidney function Krt/V: |
| Calculate Kr, t, and V (Kr is urea clearance per week, t is time=1 week, and V is volume measured as total body water). |
| Kr is calculated in liters per week so we need to convert urea clearance obtained above from ml/min to liters/week. |
| Kr (L/week)= Urea clearance (ml/min) X [60 X 24 X 7/1000] = Urea clearance (ml/min) X 10.08 |
| V (total body water or volume of distribution of urea) is already calculated above (37.399L) |
| Urine volume in 24 hours is 450 ml. |
| Urine volume per minute=450/(24 x 60) = 450/1440=0.3125 ml/min |
| Urea clearance=Ureaurine X Vurine (in ml/min)/Ureaplasma=550 x 0.3125/48=3.5807 ml/min |
| Kr in Liters/week=urea clearance (ml/min) x 10.08=3.5807 x 10.08=36.0935 |
| Krt/V = (36.0935 x 1)/37.399=0.9651 |
| Step 5: Total Kt/V (Peritoneal dialysis+Residual kidney function) = Kt/V peritoneal + Krt/V=1.638+0.9651=2.6031 |
| Based on this result (total Kt/V=2.6031), this patient is receiving adequate peritoneal dialysis [minimum Kt/V urea should be more than 1.7 as per guidelines]. |
Step 1: calculate Kt: Kt is the daily peritoneal urea clearance.
It is calculated by following equation:
Kt = (Durea/Purea) x VD
Durea is urea concentration in pooled drain dialysate (dialysate from all exchanges in 24 hours is pooled, mixed properly, and then sample is collected from this to assess Durea.
Purea is urea concentration in plasma.
VD is 24-hour peritoneal dialysate drain volume.
D/P of small solutes is not affected by body size.[44]
Step 2: calculate 'V'. 'V' is volume of distribution of urea and is equal to total body water.
Calculating 'V' in patients with adequate nutrition and weight close to dry weight
In adults whose weight is equal to or approaching dry weight, either Watson or Hume equation should be used to calculate 'V' when calculating Kt/Vurea.[11,12,44]
Male TBW = 2.447 - (0.09156 x age) + (0.1074 x height) + (0.3362 x weight)
Female TBW = -2.097 + (0.1069 x height) + (0.2466 x weight)
Hume-Weyers:[11]
Male TBW = (0.194786 x height) + (0.296785 x weight) - 14.012934
Female TBW = (0.34454 x height) + (0.183809 x weight) - 35.270121
Step 3: calculate daily peritoneal dialysis achieved (Kt/Vurea) and weekly peritoneal dialysis (Kt/Vurea x 7)
Step 4: calculate residual kidney function (if required):
Method of calculating residual kidney function is described above in hemodialysis section.
When to increase the dose of dialysis
If a patient fails to thrive despite an adequate calculated Kt/Vurea, and there is no identifiable cause other than kidney failure (such as peritonitis, comorbid conditions), an increase in dialysis dose should be considered.[44]
Patient's symptoms should be considered in the decision to adjust dialysis prescriptions. Studies have suggested that patients with lower clearance require more erythropoietin for management of anemia, are more prone for inadequate ultrafiltration, and are more likely to be uremic, hyperkalemic, acidemic, and suffer death from congestive heart failure.[44]
Uremic pericarditis, uremic neuropathy, nausea and vomiting (no other cause identified other than uremia), hyperkalemia, pruritis, sleep disturbance, restless leg syndrome, volume overload, metabolic acidosis unresponsive to oral bicarbonate therapy and anemia are other reasons for increasing the dialysis dose.[44]
If residual kidney function is minimal, intermittent peritoneal dialysis may not be adequate. Continuous peritoneal dialysis (24 hours per day) is recommended in this situation to achieve adequate middle molecule clearance.[44]
Conclusion
The choice formula of Kt/V (whether spKt/V, eKt/V, stdKt/V, or SAstdKt/V) appropriate for calculating adequacy depends on several parameters including gender, size of the patient, frequency of dialysis, dialysis modality (hemodialysis versus peritoneal dialysis), inter-dialysis weight gain, clinical symptoms and rate of complications (like fluid overload, hyperkalemia, and intolerance to dialysis). Residual kidney function (if urine output is more than 100 ml/day) should not be ignored when calculating adequacy of dialysis. Kt/Vurea is not a perfect measure of dialysis adequacy, as it does not measure the clearance of middle molecules like β2-microglobulin. Also, uremic toxins generation rate is different for different individuals, so it is more appropriate to measure dialysis adequacy as clearance with uremic toxins generation rate as denominator.[18] Factors like uremic symptoms, nutrition parameters like serum protein and albumin levels, vitamin B12 and β2-microglobulin levels are also important for measuring dialysis adequacy.
Hemodialysis machines with online clearance monitoring facility have recently created an interest. These machines allow frequent assessment of dialysis adequacy (Kt/V) without the need for taking blood samples. It is done by measuring ionic dialysance through conductivity measurements of dialysate using online clearance monitor.[46]
Appendix: Links to online calculators
Residual Kidney Function
Volume of distribution (V) or Total Body Water (TBW)
Medcalc: http://www.medcalc.com/tbw.html
QxMD: https://qxmd.com/calculate/calculator_344/total-body-water-watson-formula
Merck manuals: https://www.merckmanuals.com/medical-calculators/TBW_M_Watson.htm
Natural logarithms Tutorials
Nippissing University: https://calculus.nipissingu.ca/tutorials/logarithms.html
Khan Academy: https://www.khanacademy.org/math/algebra2/exponential-and-logarithmic-functions/introduction-to-logarithms/v/logarithms
Natural Logarithm calculators
Rapid Tables: https://www.rapidtables.com/calc/math/Ln_Calc.html
Kt/V Calculators
QxMD : Kt/V Daugirdas. Link: https://qxmd.com/calculate/calculator_128/kt-v-daugirdas
Merck Manuals. Kt/V Dialysis Dose Formulae MultiCalc: https://www.merckmanuals.com/medical-calculators/Kt_V_Formulae_Multi.htm
Easy calculation: Kt/V Calculator - Dialysis Dose Calculation. https://www.easycalculation.com/medical/dialysis.php
MedCalc : Hemodialysis – Kt/V and URR. http://www.medcalc.com/hd.html
Touchcalc: http://touchcalc.com/calculators/ktv_quick
HDCN: www.hdcn.com
Body surface area
Cornell University: http://www-users.med.cornell.edu/~spon/picu/calc/bsacalc.htm
Merck Manuals: https://www.merckmanuals.com/medical-calculators/BodySurfaceArea.htm
Competing Interests: None
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stolic R, Trajkovic G, Stolic D, Peric V, Subaric-Gorgieva G. Nutrition parameters as hemodialysis adequacy markers. Hippokratia. 2010;14:193–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Canaud B, Morena M, Cristol JP, Krieter D. β2-microglobulin, a uremic toxin with a double meaning. Kidney Int. 2006;69:1297–9. doi: 10.1038/sj.ki.5000389. [DOI] [PubMed] [Google Scholar]
- 3.Casino FG, Mostacci SD, Santarsia G, Lopez T. [Vitamin B12 clearance (Kd-B12) in hemodialysis (HD) and hemodiafiltration (HDF)] (Abstract) G Ital Nefrol. 2004;21(Suppl 30):S217–22. [PubMed] [Google Scholar]
- 4.Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS) Kidney Int. 1985;28:526–34. doi: 10.1038/ki.1985.160. [DOI] [PubMed] [Google Scholar]
- 5.Wikipedia. Kt/V. Wikipedia: The free encyclopedia [Internet] 2019. [Last accessed on 2019 Jun 19]. Available from: https://en.wikipedia.org/wiki/Kt/V .
- 6.Gotch FA, Sargent JA, Keen ML. Whither goest Kt/V? 2000 Aug;58(Supplement 76):S3–18. doi: 10.1046/j.1523-1755.2000.07602.x. doi: 10.1046/j. 1523-1755.2000.07602.x. [DOI] [PubMed] [Google Scholar]
- 7.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–9. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 8.Suri RS, Kliger AS. When is more frequent hemodialysis beneficial.(Abstract)? Semin Dial. 2018;31:332–42. doi: 10.1111/sdi.12688. [DOI] [PubMed] [Google Scholar]
- 9.Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: Reviving an old concept for contemporary practice. Kidney Int. 2016;90:262–71. doi: 10.1016/j.kint.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleyer A, Golper TA. Incorporating residual kidney function into the dosing of intermittent hemodialysis. [Last accessed on 2019 Jan 22];UpToDate. 2018 [Google Scholar]
- 11.MedCalc. Total Body Water/Urea Volume of Distribution. 2010. [Last accessed on 2019 May 06]. Available from: http://www.medcalc.com/tbw.html .
- 12.QxMD. Total Body Water (Watson Formula) 2019. [Last acessed on 2019 May 06]. Available from: https://qxmd.com/calculate/calculator_344/total-bodywater-watson-formula .
- 13.Johansson AC, Samuelsson O, Attman PO, Bosaeus I, Haraldsson B. Limitations in anthropometric calculations of total body water in patients on peritoneal dialysis. J Am Soc Nephrol. 2001;12:568–73. doi: 10.1681/ASN.V123568. [DOI] [PubMed] [Google Scholar]
- 14.Kushner RF, Schoeller DA. Estimation of total body water by bioelectrical impedance analysis [Abstract] Am J Clin Nutr. 1986;44:417–24. doi: 10.1093/ajcn/44.3.417. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Lee SW, Kim GA, Lim HJ, Lee SY, Park GH, et al. Development of anthropometry-based equations for the estimation of the total body water in Koreans. J Korean Med Sci. 2005;20:445–49. doi: 10.3346/jkms.2005.20.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SW, Song JH, Kim GA, Lee KJ, Kim MJ. Assessment of total body water from anthropometry-based equations using bioelectrical impedance as reference in Korean adult control and haemodialysis subjects. Nephrol Dial Transplant. 2001;16:91–7. doi: 10.1093/ndt/16.1.91. [DOI] [PubMed] [Google Scholar]
- 17.Fresenius Medical Care. Measuring hemodialysis dose. Advanced renal education program. 2015. [Last accessed on 2019 May 07]. Available from: https://www.advancedrenaleducation.com/content/measuring-hemodialysisdose .
- 18.National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. [Last accessed on 2019 May 03]. Available from: https://www.ajkd.org/article/S0272-6386 (15) 01019-7/pdf . [DOI] [PubMed]
- 19.Geddes CC, Traynor J, Walbaum D, Fox JG, Mactier RA. A new method of post-dialysis blood urea sampling: The 'stop dialysate flow' method. Nephrol Dial Transplant. 2000;15:517–23. doi: 10.1093/ndt/15.4.517. [DOI] [PubMed] [Google Scholar]
- 20.Hosten AO. BUN and creatinine. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. 193. Boston: Butterworths; 1990. [Last accessed on 2019 May 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK305/ [PubMed] [Google Scholar]
- 21.Adequacy of haemodialysis (Urea reduction ratio) UK renal Registry. [Last accessed on 2019 Jun 19]. Available from: https://www.renalreg.org/wp-content/uploads/2014/09/Chapter-6.pdf .
- 22.Pyart R, Magadi W, Steenkamp R, Davenport A. UK Renal Registry 20th Annual Report: Chapter 6: Adequacy of haemodialysis in UK adult patients in 2016: National and centre-specific analyses. Nephron. 2018;139(Suppl 1):151–64. doi: 10.1159/000490964. [DOI] [PubMed] [Google Scholar]
- 23.Daugirdas JT. Physiologic principles and urea kinetic modeling. In: Daugirdas JT, Blake PG, Ing TS, editors. Handbook of Dialysis. 4th ed. New Delhi, India: Wolters Kluwer (India) pvt. Ltd; 2007. pp. 25–58. [Google Scholar]
- 24.Ward RA, Idoux JW, Hamdan H, Ouseph R, Depner TA, Golper TA. Dialysate flow rate and delivered Kt/Vurea for dialyzers with enhanced dialysate flow distribution. CJASN. 2011;6:2235–9. doi: 10.2215/CJN.02630311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exp function. Medcalc. 2019. [Last accessed on 2019 May 09]. Internet. Available from: https://www.medcalc.org/manual/exp_function.php .
- 26.e (mathematical constant) Wikipedia, the free encyclopedia. 2019. [Last accessed on 2019 May 09]. Internet. Available from: https://en.wikipedia.org/wiki/E_(mathematical_constant)
- 27.Shell-Gellasch A. Napier's e-Napier. Mathematical society of America. 2019. [Last accessed on 2019 May 09]. Internet. Available from: https://www.maa.org/publications/periodicals/convergence/napiers-e-napier .
- 28.Leypoldt JK, Cheung AK, Agodoa LY, Daugirdas JT, Greene T, Keshaviah PR. Hemodialyzer mass transfer-area coefficients for urea increase at high dialysate flow rates. Kidney Int. 1997;51:2013–7. doi: 10.1038/ki.1997.274. [DOI] [PubMed] [Google Scholar]
- 29.Cheung AK, Agodoa LY, Daugirdas JT, Depner TA, Gotch FA, Greene T, et al. The hemodialysis (HEMO) study group. Effects of Hemodialyzer Reuse on Clearances of Urea and β2-Microglobulin. JASN. 1999;10:117–27. doi: 10.1681/ASN.V101117. [DOI] [PubMed] [Google Scholar]
- 30.Fresenius Medical Care. Urea Kinetics: Impact of Urea Rebound and Residual Renal Function on the spKt/V and eKt/V Relationship. Advanced Renal Education Program. 2015. [Last accessed on 2019 Jun 21]. Internet. Available from: https://www.advancedrenaleducation.com/content/urea-kinetics-impacturea-rebound-and-residual-renal-function-spktv-and-ektvrelationship .
- 31.HEMO Study Group. Daugirdas JT, Depner TA, Gotch FA, Greene T, Keshaviah P, et al. Comparison of methods to predict equilibrated Kt/V in the HEMO Pilot Study. Kidney Int. 1997;52:1395–405. doi: 10.1038/ki.1997.467. [DOI] [PubMed] [Google Scholar]
- 32.Zyga S, Sarafis P. Haemodialysis adequacy – contemporary trends. Health Sci J. 2009;3:209–15. [Google Scholar]
- 33.Standardized Kt/V. 2018. Wikipedia, the free encyclopedia. [Last accessed on 2019 May 22]. Available from: https://en.wikipedia.org/wiki/Standardized_Kt/V .
- 34.Calculating standardised Kt/V for the Scottish Renal Registry Annual Report. The Scottish Renal Registry. 2019. [Last accessed on 2019 Jun 09]. Internet. Available from: https://www.srr.scot.nhs.uk/projects/PDF/Calculating-standardised-KtV_SRR.pdf .
- 35.Daugirdas JT, Depner TA, Greene T, Levin NW, Chertow GM, Rocco MV for the Frequent Hemodialysis Network (FHN) Trial Group. Standard Kt/Vurea: A method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 2010;77:637–44. doi: 10.1038/ki.2009.525. [DOI] [PubMed] [Google Scholar]
- 36.Rivara MB, Ravel V, Streja E, Obi Y, Soohoo M, Cheung AK, et al. Weekly standard Kt/Vurea and clinical outcomes in home and in-center hemodialysis. CJASN. 2018;13:445–55. doi: 10.2215/CJN.05680517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hume R, Weyers E. Relationship between total body water and surface area in normal and obese subjects. J Clin Pathol. 1971;24:234–8. doi: 10.1136/jcp.24.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan N. Limitations of Kt/V. Hemodialysis Kinetics 101. 2017. [Last accessed on 2019 Oct 15]. Internet. Available from: https://hemodialysiskinetics.coursepress.yale.edu/category/ktv/
- 39.Held PJ, Port FK, Wolfe RA, Stannard DC, Carroll CE, Daugirdas JT, et al. The dose of hemodialysis and patient mortality (Abstract) Kidney Int. 1996;50:550–6. doi: 10.1038/ki.1996.348. [DOI] [PubMed] [Google Scholar]
- 40.Collins AJ, Ma JZ, Umen A, Keshaviah P. Urea index and other predictors of hemodialysis patient survival (Abstract) Am J Kidney Dis. 1994;23:272–82. doi: 10.1016/s0272-6386(12)80984-x. [DOI] [PubMed] [Google Scholar]
- 41.Owen WF, Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis (Abstract) N Engl J Med. 1993;329:1001–6. doi: 10.1056/NEJM199309303291404. [DOI] [PubMed] [Google Scholar]
- 42.U.S. Department of Health and Human Services. Hemodialysis: Dose & adequacy. NIH. June 2014 [Internet] [Last accessed on 2019 Oct 15]. Available from: https://www.niddk.nih.gov/health-information/communicationprograms/nkdep/identify-manage-patients/manage-ckd/hemodialysis-dose-adequacy .
- 43.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, et al. CHAPTER 1: Hemodialysis adequacy in adults. JASN. 2006;17(3 suppl 1):S4–7. doi: 10.1681/ASN.2005121372. [DOI] [PubMed] [Google Scholar]
- 44.NKF KDOQI guidelines (2006) Clinical practice guidelines and clinical practice recommendations. Clinical practice guidelines for peritoneal dialysis adequacy. [Last accessed on 2019 Apr 30]. Available from: http://kidneyfoundation.cachefly.net/professionals/KDOQI/guideline_upHD_PD_VA/pd_rec2.htm .
- 45.Watnick S Peritoneal Dialysis Adequacy. International society for peritoneal dialysis. April 2011. [Last accessed on 2019 Jun 08]. Available from: https://ispd.org/NAC/wp-content/uploads/2010/11/Peritoneal-Dialysis-Adequacy-Watnick-April-2011-Notes.pdf .
- 46.Alayoud A, Montassir D, Hamzi A, Zajjari Y, Bahadi A, El Kabbaj D, et al. The Kt/V by ionic dialysance: Interpretation limits. Indian J Nephrol. 2012;22:333–9. doi: 10.4103/0971-4065.103906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baxter. Revaclear dialyzer technology. n.d. [Internet] [Last accessed on 2019 May 08]. Available from: https://www.baxter.com/sites/g/files/ebysai746/files/2018-08/USMP%20G82%2014-0032%284%29-Revaclear%20Spec%20Sheet_Single%20Page%20FINAL.pdf .
- 48.Baxter. Polyflux 6H dialyzer. 2018. [Internet] [Last accessed on 2019 May 08]. Available from: https://www.esrddialysis.com/sites/g/files/ebysai876/files/2018-06/USMP%20MG160%2014-0002%282%29_POLYFLUX%206H%20SPEC%20SHEET_FINAL.pdf .
- 49.Rexeed L. Asahi Kasei Medical Co., Ltd. [Internet]; [Last accessed on 2019 May 08]. Available from: http://www.asahi-kasei.co.jp/medical/en/dialysis/product/rexeedl/performance.html . [Google Scholar]
- 50.Commonly used dialyzer specifications and substitution chart for visiting patients BCL renal Agency. [Internet] [Last accessed on 2019 May 08]. Available from: http://www.bcrenalagency.ca/resource-gallery/Documents/Commonly%20Used%20Dialyzer%20Specifications%20and%20Subs%20Chart.pdf .
- 51.Fresenius Medical Care North America. Fresenius Optiflux, Hemoflow hollow fiber dialyzer. Package Insert. 2009 [Internet] [Last accessed on 2019 May 08]. Available from: https://fmcna.com/wp-content/uploads/documents/89-700-48_rev_11-09.pdf .