Abstract
Dorsoventral (DV) patterning is a key landmark of embryonic development that is primarily regulated by bone morphogenetic protein (BMP) signaling. Disruption of DV patterning can result in downstream effects on cell specification and organogenesis. Zebrafish embryos have been extensively used to understand signaling pathways that regulate DV patterning, as zebrafish embryos develop ex utero and, contrary to mammalian embryos that develop in utero, can be observed in real-time using brightfield and fluorescence microscopy. Embryos with disrupted DV patterning are either dorsalized or ventralized, with lack of development of head or trunk/tail structures, respectively. Although these phenotypes are typically accompanied by effects on BMP signaling, exceptions exist where some drugs or environmental chemicals can disrupt DV patterning in the absence of effects on BMP signaling. Therefore, assessments of DV patterning should be accompanied by BMP signaling-specific readouts to confirm the role of BMP disruption. Here, we describe an exposure paradigm and steps for phenotyping zebrafish embryos for two types of DV defects, dorsalization and ventralization, with a range of severities. In addition, we describe a strategy for whole-mount immunohistochemistry of zebrafish embryos with a phospho-SMAD 1/5/9-specific antibody, as disruption in phospho-SMAD 1/5/9 localization is indicative of an effect on BMP signaling. Taken together, these protocols describe an initial strategy for evaluating DV patterning defects under various experimental conditions and confirming BMP-mediated DV patterning disruptions, which can be followed by additional studies that aim to uncover mechanisms leading to these adverse phenotypes.
Keywords: dorsoventral patterning, immunohistochemistry, phospho-SMAD, BMP signaling, zebrafish embryos
INTRODUCTION
Dorsoventral (DV) patterning is a key landmark of embryonic development when the dorsoventral axis of the body is established. This includes rapid mitotic division and regionalization of the zygotic cell mass, forming germ layers that differentiate into specific cell types through a dynamic series of signaling cascades (Pomreinke et al., 2017; Sagerstrom et al., 1996). During gastrulation, the germ layers encounter a decreasing gradient of bone morphogenetic protein (BMP) signaling from the ventral to the dorsal side of the embryo (Graff, 1997; Pomreinke et al., 2017). This gradient creates the basis for DV patterning that results in the formation of anterior structures (e.g., the head) from dorsal areas of low BMP signaling, or in the formation of posterior structures (e.g., the tail or coccyx) from ventral areas of high BMP signaling. Interestingly, disruption of DV patterning interferes with the normal trajectory of embryogenesis, leading to defects later in the process, such as a lack of normal brain and neuronal development (Bond et al., 2012). Indeed, drugs or environmental chemicals that target DV patterning during prenatal development may, as a result, induce long-term, irreversible effects later in development. However, studying chemically-induced effects on DV patterning within mammalian systems is not feasible due to in utero development.
Zebrafish have been extensively used to understand signaling pathways (including BMP signaling) that regulate DV patterning (Hammerschmidt et al., 1996). Mutations in BMP activator genes such as bmp7 result in a disrupted DV axis, leading to both excess dorsal tissues such as the head and lack of ventral tissues such as blood, muscles, and tail – a phenotype known as dorsalization (Dick et al., 2000; Yu et al., 2008). Forward genetic screens using zebrafish identified a small molecule-based disruptor of DV patterning, dorsomorphin or DMP, that acts through BMP inhibition (Yu et al., 2008). By 24 h post fertilization (hpf), DMP-exposed embryos are dorsalized, with a concentration-dependent increase in the strength of dorsalization phenotypes that is representative of the extent of BMP disruption (Cannon et al., 2010). Similar to dorsalization, zebrafish has also been used to study ventralization, a phenotype that is due to BMP overactivation and results in enhanced development of ventral tissues (e.g., the tail) at the expense of dorsal tissues (Genthe et al., 2017; Vrijens et al., 2013). Mutations in BMP inhibitor genes such as sizzled (szl) or chordin (chd), as well as exposure to small molecules such as 4’-hydroxychalcone (4’-H) – an activator of BMP signaling –, lead to ventralized embryos (Little and Mullins, 2004; Vrijens et al., 2013). BMP disruption can be confirmed by immunostaining embryos for phospho-SMAD 1/5/9 (pSMAD 1/5/9) protein, a product of BMP signaling. For example, during late gastrulation, the cell masses of normal embryos show a ventral-to-dorsal gradient of pSMAD 1/5/9 that represents the BMP signaling gradient (Pomreinke et al., 2017), whereas DMP-exposed embryos lack this gradient and, as such, exhibit disruption of BMP signaling (Dasgupta et al., 2018). The use of pSMAD 1/5/9 localization is critical to confirm the role of BMP disruption in driving DV patterning defects such as dorsalization and ventralization.
Based on prior studies from our group (Cheng et al., 2019; Dasgupta et al., 2018; Dasgupta et al., 2017), we here first provide a detailed protocol for exposure and assessment of different strengths of dorsalization and ventralization phenotypes (represented as classes) that are elicited by a range of concentrations of DMP and 4’H, respectively, or by microinjection of a chordin (chd) morpholino (Basic Protocol 1). These chemicals or morpholinos can be used as positive controls for chemical testing or functional genetics, respectively. These protocols will help users identify these phenotypes during zebrafish embryogenesis under their experimental conditions of interest. Moreover, we provide a detailed protocol for pSMAD 1/5/9 localization and imaging within an intact gastrulating embryo (Basic Protocol 2). Taken together, these two protocols present an initial strategy for confirming BMP-mediated DV patterning disruptions, which can be followed by additional studies that aim to uncover mechanisms leading to these adverse phenotypes.
STRATEGIC PLANNING
Embryo collection and exposures.
For our experiments, we obtained specific pathogen-free 5D founder fish from the Sinnhuber Aquatic Research Laboratory (Corvallis, OR) and collected embryos through batch spawning. Adult fish should be housed in optimal densities (~5 fish/L), since higher-than-normal densities may affect fish and embryonic health that may result in background malformations in normal, untreated embryos. Likewise, once collected, embryos should be incubated at an optimal temperature (~28°C) and density (~30–50 embryos in a 100-mm Petri dish). The same paradigm should be followed for exposures. If these precautions are not followed, development of embryos may be delayed or defective.
Age of the embryos at initiation of exposure will depend on the requirement of the experiment; for experiments presented here, exposures were initiated at 0.75 hpf.
Stock solutions of chemicals should be checked for proper dissolution, and working solutions (including any necessary dilution series) must be thoroughly mixed before initiation of embryonic exposures. The concentration range of chemicals will depend on the stage of exposure initiation. For our experiments, a concentration range of 0.078–0.625 μM and 0.5–10 μM were used for DMP and 4’H, respectively.
Morpholino injections.
The use of morpholinos to induce ventralization is optional. This should only be pursued as a positive control if test chemicals produce severe ventralized phenotypes. In order to minimize off-target effects of morpholinos, working concentrations of morpholinos must first be optimized based on preliminary experiments that identify the maximum tolerated concentration following injection of negative control morpholinos. For our study, negative control and chd morpholinos were used at a concentration of 0.5 mM and 0.125 mM, respectively. After preparing working stocks in molecular-grade water from a primary stock solution, stocks must be centrifuged at 1500 rpm for 5 min, and supernatant should be used. Otherwise, particles present may clog up microinjection needles. Prior to morpholino injections into embryos, the needle size and injection pressure should be optimized according to the manufacturer’s instructions. In addition, injection volume must be optimized in the following way: 1) load needles with 3 μL of morpholino; 2) inject approximately five droplets into a dish filled with mineral oil to test the injection volume; 3) measure diameter of the droplet using a scale available in the imaging software (alternatively, open source tools such as ImageJ can be used), and calculate the droplet volume using 4/3 π (d/2)3, where d = droplet diameter; and 4) estimate the injection volume based on an average volume of three replicate droplets. The typical volume per droplet should be ~3 nL. A volume of 3 μL morpholino loaded into the needle should be sufficient for injecting 1000 embryos; however, the number of actual embryos injected is typically much less due to optimization, injection errors, and volume loss. All injections must be done prior to the 8-cell stage (1 hpf) to ensure diffusion of morpholinos across all cells from the yolk sac. Please refer to Timme-Laragy et al. (2012) for additional details about microinjection of morpholinos into zebrafish embryos.
Immunohistochemistry.
Dilution of antibodies must be optimized according to the manufacturer’s instructions. Within our study, we used a dilution of 1:100 and 1:500 for pSMAD 1/5/9-specific primary antibodies and Alexa Fluor-conjugated secondary antibodies, respectively.
BASIC PROTOCOL 1: PHENOTYPING FOR DORSALIZATION AND VENTRALIZATION
Chemical and genetic insults during embryonic development can result in disruption of DV patterning, leading to dorsalized and ventralized embryos. This protocol describes materials, methods, and strategies for identifying these phenotypes during zebrafish embryogenesis. We describe protocols for chemical treatment of the embryos (Steps 1 to 5) of for using morpholinos (Steps 6 to 13). For dorsalization, embryos will be exposed to DMP (0.078–0.625 μM) from 0.75–24 hpf. For ventralization, embryos will be exposed to 4’-H (0.5–10 μM) from 0.75–24 hpf or microinjected with a fluorescein-tagged, chd-specific morpholino by 1 hpf. Since our aim is to demonstrate a range of ventralization phenotypes, we use both 4’H (which typically induces mild/moderate phenotypes) and chd knockdown (which typically induces severe phenotypes). For all chemical exposures, 0.1% dimethyl sulfoxide (DMSO) is used as a vehicle control. At 24 hpf, different strengths of dorsalization and ventralization phenotypes will appear; these strengths are represented as classes of dorsalization (class 1–5) and ventralization (class 1–4). Each phenotype is characteristic of one or more BMP signaling mutants, and a concentration-dependent increase in the class number indicates the potential for a stronger impact on BMP signaling.
Materials
Fertilized wildtype zebrafish embryos in embryo media or system water (see Strategic Planning) Embryo media (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, pH 7 dissolved in reverse osmosis water) (Dasgupta et al., 2017) Chemicals of interest (DMSO, CAS# 67–68-5; DMP, CAS# 866405–64-3 and 4’-H, CAS# 2657–25-2) 100-mm Petri dishes or 96-well plates Stereomicroscope with imaging capabilities (e.g., Leica MZ10 F stereomicroscope equipped with a GFP filter and DMC2900 camera) Microinjection needles (e.g., Pre-Pulled Glass Pipettes, Plain, 0.4 μm from World Precision Instruments, Inc.; catalog number: TIP04TW1F) Microinjection rig (e.g., a motorized Eppendorf Injectman NI2 and FemtoJet 4x) Molecular-grade water Mineral oil (commercially available) Injection plates containing solidified 1% agarose with channels for embryo placement (zebrafish embryo-specific injection plate molds are commercially available from World Precision Instruments, Inc.; catalog number: Z-MOLDS) Fluorophore (e.g., fluorescein)-tagged negative control morpholino (0.5 mM, oligo sequence: 5’-CCTCTTACCTCAGTTACAATTTATA-3’) (Gene Tools, LLC, Philomath, OR) Fluorophore (e.g., fluorescein)-tagged target morpholino (e.g., 0.125 mM chd-specific morpholino, oligo sequence: 5’-ATCCACAGCAGCCCCTCCATCATCC-3’) (Gene Tools, LLC, Philomath, OR)
Protocol Steps
Dorsalization or ventralization by chemical exposure
-
1Collect embryos immediately after spawning (0 hpf) and discard unfertilized or dead embryos. Rinse embryos with embryo media. Stage embryos according to Kimmel et al. (1995), as it is critical that all embryos are at the same developmental stage at the onset of exposure. The initiation stage will depend on the experimental design; in this example, exposures began at 0.75 hpf (2–4 cell stage).A significant percentage of control embryos may be coagulated and dead by ~2–3 hpf. This is normal, since newly fertilized embryos may not survive the maternal-to-zygotic transition (~3 hpf). This factor must be taken into consideration for exposures initiated within 0–2 hpf, as control survival in these cases may be <80%. If exposures are initiated past 3 hpf, dead embryos must be discarded prior to exposure initiation.
-
2Prepare a 96-well plate with 100 μL of test solution per well.In this protocol, test solutions contain 0.078–6.25 μM DMP or 0.5–10 μM 4’H.
-
3Transfer one embryo per well into the plate.Alternatively, exposures can be done in groups within Petri dishes (not exceeding 50 embryos in a 100-mm petri dish). Too high embryonic density may delay development. At least 30 embryos should be used per treatment group. If performed in Petri dishes, at least three replicate Petri dishes containing ~30–50 embryos per Petri dish should be used.
-
4Incubate embryos at 28°C.96-well plates should be wrapped with Parafilm to prevent solution loss due to evaporation.
-
5Image at 24 hpf by positioning embryos laterally.Positioning can be done with needles or forceps. A 2X or 10X magnification is required.
Ventralization by chordin (chd)-specific morpholino injections
-
6
Prior to collecting eggs immediately after fertilization (0 hpf), gather injection supplies and prepare working stocks of morpholinos to the desired concentration (0.5 mM for negative control and 0.125 mM for chd morpholinos) in molecular-grade water. Optimize injection volume with mineral oil as described in the Strategic Planning section.
-
7
Load first microinjection needle with 3 μL of the morpholino stock and set up injection system.
-
8Collect embryos immediately after spawning (0 hpf) and discard unfertilized or dead embryos. Stage embryos according to Kimmel et al. (1995). Sort and stage embryos immediately after rinsing with embryo media, collecting only normal embryos at or before the 1-cell stage.It is critical that all embryos are within the 1- to 8-cell stage when injected.
-
9
Divide sorted embryos into injection plates. To do this, drop a “clump” of embryos onto the injection plate and gently nudge embryos into channels using a pipette tip or spatula. Remove any embryos that are damaged as a result of this process.
-
10Begin injecting embryos with morpholinos (3 nL volume per embryo) into the yolk sac. All injections should be completed by 1 hpf.Injecting by 1 hpf ensures homogeneous distribution of morpholino across all cells since cell division is symmetrical. As embryogenesis advances, cell division becomes asymmetrical.
-
11After all embryos have been injected (at least 100 embryos per morpholino), gently wash embryos out of the injection plate and into clean Petri dishes using a wash bottle filled with embryo media or system water. Remove any embryos that were damaged during the injection process. Incubate remaining embryos until 4 hpf.There may be some embryos that are damaged during the injection process as a result of the needle breaking, incorrect injection angle, etc.
-
12At 4 hpf, examine embryos under fluorescence (e.g., a GFP filter for fluorescein) at 2X magnification. At this stage, you will be able to identify morpholino-positive embryos. Remove any embryos without fluorescence.In this case, both negative control and chd morpholinos are tagged with fluorescein. If morpholinos are not fluorescently labeled, 0.5% phenol red can be spiked into working stocks to visualize successful injection under the microscope.
-
13
Image at 24 hpf as described in Step 5.
BASIC PROTOCOL 2: WHOLE-MOUNT IMMUNOHISTOCHEMISTRY WITH PHOSPHO-SMAD 1/5/9 ANTIBODY
Canonical BMP signaling is induced by phosphorylation of SMAD proteins by BMP receptors that heterodimerize and translocate into the nucleus to drive gene expression (Laux et al., 2011). In a gastrulating embryo, the gradient of BMP signaling can be visualized by immunostaining with a pSMAD 1/5/9-specific antibody (Pomreinke et al., 2017). Therefore, disruption of this gradient, specifically by BMP inhibitors such as DMP, can easily be detected. Here we describe a protocol for whole-mount immunohistochemistry (IHC) of 8-hpf zebrafish embryos using an anti-pSMAD 1/5/9 antibody. This protocol should be used to confirm the role of BMP signaling disruption following observation of DV patterning defects under a condition of interest (Basic Protocol 1). If BMP signaling is disrupted, immunostained embryos will show a lack of the ventral-to-dorsal BMP signaling gradient within the cell mass.
Materials
Zebrafish embryos, treated with chemicals of interest. In this case, embryos were treated with 0.625 μM DMP.
IHC baskets (microcentrifuge tubes with conical portion removed and bottom fitted with mesh, sized for 24- or 48- well plates, typically manufactured in-house based on Thisse and Thisse, 2007. Please see Figure 3 within Thisse and Thisse, 2007 for more details) 48-well plates (flat-bottomed) Molecular-grade water 1X phosphate-buffered saline (PBS) 1X PBST (1X PBS + 0.1% Tween-20) 4% paraformaldehyde in 1X PBS (<2 days old) Sheep serum (frozen aliquot) (Millipore Sigma; catalog number: S3772) Bovine serum albumin (BSA) (stored at 4°C) Blocking buffer (1X PBST, 2% sheep serum, 2 mg/ml BSA) (freshly prepared) Anti-pSMAD 1/5/9 IgG (Cell Signaling Technology; catalog number: 13820S); 1:100 dilution in blocking buffer Alexa Fluor 488-conjugated goat anti-rabbit IgG (H+L) secondary antibody (Invitrogen, 2 mg/ml stock; catalog number: A11008); 1:500 dilution in blocking buffer Stereomicroscope with imaging capabilities (e.g., Leica MZ10 F stereomicroscope equipped with a GFP filter and DMC2900 camera)
Figure 3. Representative data for dorsalization phenotypes at 24 hpf (Basic Protocol 1).
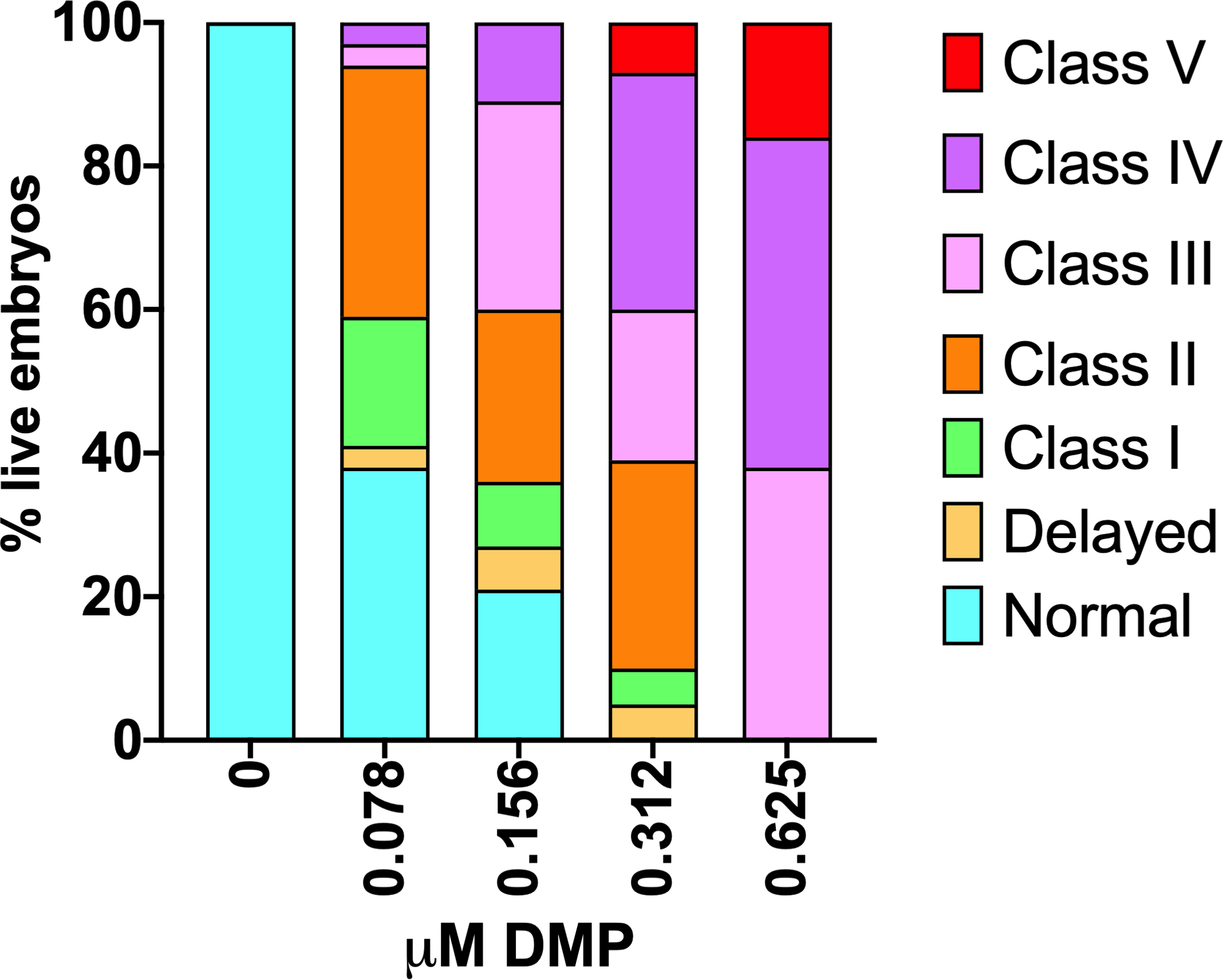
A) Concentration-response data for DMP exposures initiated at 0.75 hpf (A). Data can be represented as cumulative percentages of dorsalization strengths over multiple replicate experiments. Figure redrawn from Dasgupta et al. (2017).
Protocol Steps
Staining
Day 1
-
1
Fix 8-hpf zebrafish embryos in 4% paraformaldehyde (dissolved in 1X PBS). Use ~20 embryos per 1 mL paraformaldehyde in a 1.5-ml microcentrifuge tube. Incubate overnight at 4°C.
All incubations following fixation are performed within IHC baskets within a 48-well plate. IHC should be performed on at least 20 embryos per treatment.
Day 2
-
2
Set up 48-well plates with wells dedicated to 1X PBS (1 well), blocking buffer (1 well), primary antibody (1 well), 1X PBST (3 wells), secondary antibody (1 well), and 1X PBST (3 wells) for each treatment. Before each step, transfer 500 μL of each solution to the respective wells.
-
3
Aspirate the paraformaldehyde from the 1.5-mL tubes from Step 1 and replace with 1X PBS. Store embryos at 4°C for long-term storage.
-
4
Dechorionate embryos by removing chorions with forceps.
-
5
Transfer dechorionated embryos to IHC baskets in 48-well plates in 1X PBS with a pipette.
-
6
Move IHC baskets with embryos to blocking buffer wells. Incubate embryos for 4 h at room temperature in blocking buffer with gentle agitation (shaker at 100 rpm).
-
7
Move IHC baskets to primary antibody wells and incubate embryos in blocking buffer containing 1:100 dilution of anti-pSMAD 1/5/9 antibody overnight at 4°C with shaking (cold room or 4°C incubator).
Day 3
-
8
Wash embryos in 1X PBST at room temperature three times for 5 min each with gentle agitation. This can be performed by moving the IHC baskets serially into three different 1X PBST wells (see Step 2).
-
9
Move IHC baskets into secondary antibody wells. Incubate embryos in blocking buffer containing 1:500 dilution of Alexa Fluor-conjugated secondary antibody overnight at 4°C with gentle agitation (cold room or 4°C incubator).
Day 4
-
10
Wash embryos in 1X PBST at room temperature three times for 5 min each with gentle agitation, similar to Step 8.
-
11
Store in 1X PBS at 4°C until imaging.
Imaging
-
12
Image under a stereomicroscope equipped with a fluorescent filter that is consistent with the fluorophore used. Imaging should be done at 8–10X magnification. Embryos should be positioned with a left-right, dorsal-ventral orientation. Imaging may be done in 1X PBST but embryos should be stored in 1X PBS for long-term storage. The background fluorescence setting should be adjusted to enable efficient fluorescence detection, and identical settings must be used for all embryos imaged from the same experiment.
Embryos are spherical and will tend to roll. As a result, use of 100% glycerol or embedding in 4% agarose may be appropriate.
COMMENTARY
BACKGROUND INFORMATION
Zebrafish embryos have been widely used to study early developmental landmarks such as dorsoventral patterning. The zebrafish genome is ~80% similar to that in mammalian systems and the developmental trajectory is largely conserved, features that enable the potential translation of findings to human health. There are many advantages to using zebrafish. The ex utero development of zebrafish embryos enables real-time visualization of development and phenotyping at any stage after fertilization, a characteristic which is not feasible within mammalian models as a result of in utero development. Furthermore, ex utero development facilitates genetic manipulations (e.g., morpholino injections and gene editing) without the need for in vitro fertilization. High fecundity of zebrafish also supports large-scale experiments with multiple variables such as concentration-response, chemical comparisons, mixture studies, and variations of physical parameters (Bugel et al., 2014). Here, we demonstrate the use of this model to study DV patterning defects during early development.
Phenotyping for DV defects has been previously used for the detection of early developmental toxicity in large scale screens for drugs or small molecules that target developmental signaling pathways. Specifically, since BMP signaling primarily regulates DV patterning, studies from our lab and other groups have used dorsalization and ventralization phenotypes as indicators of BMP signaling defects by BMP inhibitors such as DMP, activators such as 4’-H (Dasgupta et al., 2018; Vrijens et al., 2013; Yu et al., 2008), or by knockdown of BMP pathway-specific genes such as szl and chd (Dasgupta et al., 2017). However, our work also showed that DV defects are not necessarily readouts of direct impacts on BMP signaling, since two structurally diverse chemicals – tris(1,3-dichloro-2-propyl)phosphate (TDCIPP, an environmental chemical) and ciglitazone (a drug) – induced dorsalization and ventralization phenotypes, respectively, without corresponding impacts on BMP signaling, based on mRNA-sequencing and pSMAD 1/5/9 staining (Cheng et al., 2019; Dasgupta et al., 2018; Dasgupta et al., 2017). Prior to dorsalization at 24 hpf, TDCIPP-treated embryos were characterized by epiboly defects, disrupted mesodermal cell localization, and lack of somites, but displayed no impacts on pSMAD 1/5/9 staining (Dasgupta et al., 2018). On the contrary, DMP-treated embryos exhibited disrupted pSMAD 1/5/9 but normal mesodermal localization and somites (albeit elongated). Ciglitazone-treated embryos did not demonstrate abnormal phenotypes nor evidence of BMP signaling disruption prior to concentration-dependent weak ventralization phenotypes at 24 hpf (Cheng et al., 2019). These data collectively suggest that the observation of DV patterning defects is not sufficient to confirm disruption of BMP signaling. To confirm the role of BMP signaling pathway, pSMAD 1/5/9 staining is essential.
pSMAD 1/5/9 staining has been used in a number of previous studies to assess the gradient of BMP signaling during gastrulation. The pSMAD gradient begins to appear in the cell mass of embryos during the epiboly stages, and this gradient is disrupted by DMP as well as within chd mutants, resulting in blastomere-wide staining (Dasgupta et al., 2018; Pomreinke et al., 2017). While pSMAD is a confirmatory indicator of BMP disruption, follow-up approaches to study mechanisms of DV patterning defects should include additional approaches such as transcriptomics (Dasgupta et al., 2018), use of transgenic and mutant fish lines (Kishimoto et al., 1997; Laux et al., 2011), and in situ hybridization for target genes (Laux et al., 2011).
CRITICAL PARAMETERS
Basic Protocol 1:
For DV patterning, it is critical that all exposures begin with embryos at an identical stage of the development. If the embryos are at different stages, embryos lagging in development will result in a false positive “delayed” phenotype. If morpholinos are used, care should be taken to ensure that the injection is robust by checking for blastomere fluorescence (for fluorophore-tagged morpholinos) and removal of dead or non-injected embryos. In addition, the needle size and injection pressure should be optimized according to the manufacturer’s instructions to ensure a consistent volume of morpholino injection per embryo (typically 3 nL per embryo). The density of embryos for batch exposures should also be monitored (as suggested in the Strategic Planning section) and the experiment should be repeated if control embryos are not at the target (Prim-5) stage at 24 hpf. Importantly, positive controls should always be included in the study design.
Basic Protocol 2:
The embryos must be dechorionated for antibodies to penetrate the tissues. Care should also be taken to ensure that, for each step, shaking is gentle and embryos are not damaged. Furthermore, the number of embryos within IHC baskets should be optimized so that all embryos are properly immersed in solution. During imaging, the background fluorescence setting should be adjusted to enable efficient fluorescence detection, and identical settings must be used for all embryos imaged from the same experiment. Positive controls (DMP or 4’-H for chemical exposures; morphants for knockdown studies) should be included in the study design. Importantly, if pSMAD 1/5/9 staining is performed in morphants, fluorophores for the morpholino and the secondary antibody should be different.
TROUBLESHOOTING
Basic Protocol 1:
In the event of unexpected phenotypes within control embryos, then 1) chemical or morpholino concentrations should be checked; 2) morpholino uptake should be confirmed by using phenol red or fluorescence; and/or 3) embryo density should be checked. For chemical exposures, proper shaking should be used to ensure homogeneous mixing of chemicals in the solution.
Basic Protocol 2:
If a normal pSMAD 1/5/9 gradient is not detected within control embryos, the composition of reagents, storage temperature, or date of expiration should be confirmed. For example, antibodies and serum can degrade at room temperature. The proportion of primary and secondary antibodies – in this case 1:100 and 1:500, respectively – should be optimized if necessary. Conversely, if the signal is distributed throughout the cell mass without a gradient within control embryos, the proportion of primary antibody as well as fluorescence settings on the stereomicroscope should be adjusted.
UNDERSTANDING RESULTS
Interpretation of phenotypic data from Basic Protocols 1 and 2 are detailed below. The different strengths of dorsalization or ventralization phenotypes are derived from specific BMP signaling genes and, therefore, a concentration-dependent increase in the percentage of phenotypes from Class 1 to 4/5 indicate a progression of disruption of BMP signaling across multiple BMP pathway components. As stated previously, it is important to note that a DV patterning phenotype does not necessarily indicate BMP signaling disruption and should be confirmed with pSMAD 1/5/9 staining. Moreover, additional experiments may be needed to understand the role of BMP signaling in driving phenotypes. Since chemical exposures typically result in a range of dorsalization or ventralization strengths within the same concentration, we only show representative phenotypes in Figures 1 and 2.
Figure 1. Representative images of dorsalization phenotypes (D1-D5) following exposures to vehicle (0.1% DMSO) or DMP (0.078–0.625 μM) with exposures initiated at 0.75 hpf (Basic Protocol 1).
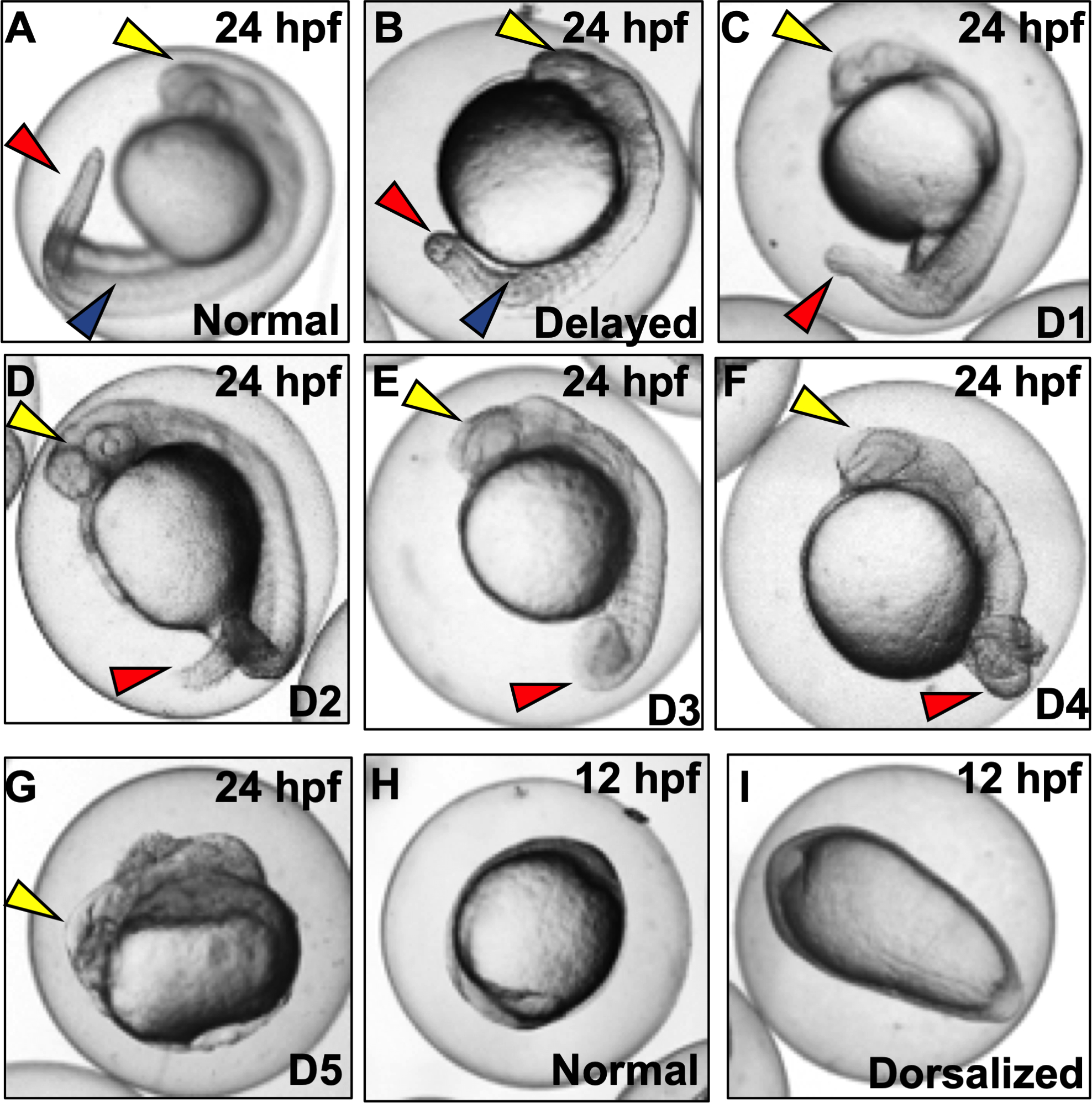
(A) Normal embryo at the prim-5 stage. Yellow arrow denotes head; blue arrow denotes somites; and red arrow denotes tail. (B) Embryo delayed in development, with lack of development of eye/otic vesicle (yellow arrow) and somite numbers (blue arrow) compared to normal embryos. (C-D) Class 1–4 dorsalized embryos with red arrows representing loss of ventral tail fin and (E and F) gradual coiling of the tail and trunk area. (G) Class 5 dorsalization with ill-developed head (yellow arrow) but no development of trunk and tail tissues. (H and I) Normal and dorsalized (ovoid) embryos at 12 hpf.
Figure 2. Representative images of ventralization phenotypes at 24 hpf following chd morpholino microinjections, or exposures to vehicle (0.1% DMSO) or 4’-H (0.5–10 μM) with exposures initiated at 0.75 hpf (Basic Protocol 1).
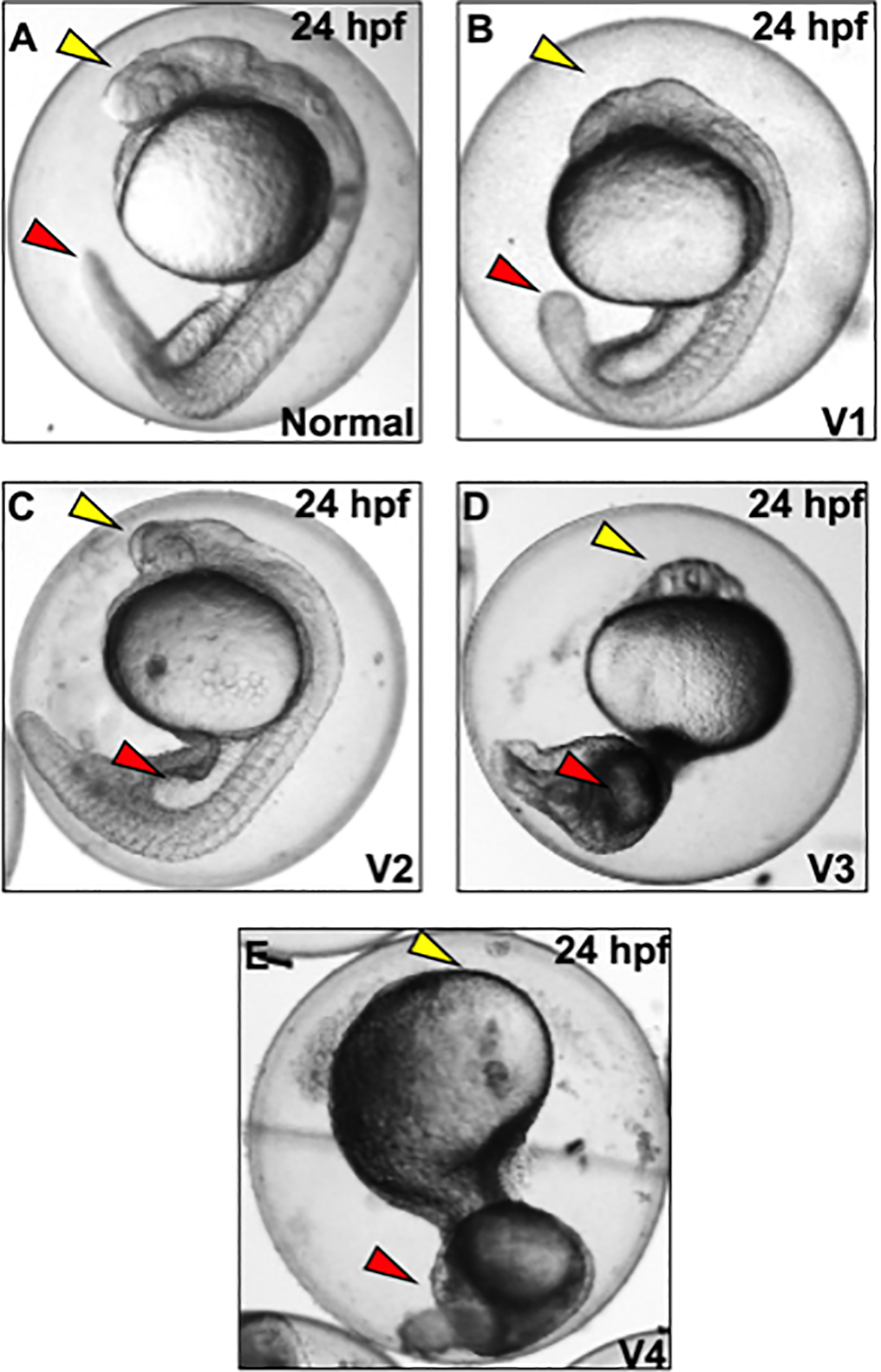
A) Normal embryo at the prim-5 stage, with yellow arrow denoting head and red arrow denoting tail. B) Class 1 ventralization with head (yellow arrow) and slightly thickened tail (red arrow). (C-D) Class 2–3 ventralization with lack of development of head (yellow arrow) and thickened blood island (red arrow) and tail. E) Class 4 ventralization with no proper head development (yellow arrow) and thick ventral tissues (red arrow) (E).
Dorsalization phenotyping at 24 hpf
Key dorsalization phenotypes range from Class 1 to 5. However, several other phenotypes (e.g., delayed progression) may also be present.
1. Normal embryos:
Normal embryos will have a developing head, trunk, and tail, and will undergo spontaneous tail contractions that are characteristic of this stage (Figure 1A).
2. Delayed:
These embryos are developmentally delayed as noted by the embryonic stage (Figure 1B), and are characterized by a lack of eye development (yellow arrows) or number of somites (blue arrows).
3. Class 1 and 2 dorsalization (D1 and D2):
These embryos are characterized by the loss of ventral tail fins (red arrow), have serrated (D1) or bent (D2) tails (Figures 1C and D), and undergo normal spontaneous tail contractions.
4. Class 3 and 4 dorsalization (D3 and D4):
These embryos are characterized by partially (D3) or fully (D4) underdeveloped trunk and tail areas where the trunk twists around itself like a snail shell (Figures 1E and F); these embryos undergo strenuous spontaneous tail contractions.
5. Class 5 dorsalization (D5):
Within these embryos, the body is never properly differentiated into the head or tail, but embryos are still alive at 24 hpf (Figure 1G). These embryos undergo occasional strenuous spontaneous contractions.
6. Ovoid embryos at ~ 10–12 hpf:
Occurrence of dorsalization can also be predicted prior to 24 hpf as ovoid embryos at ~10–12 hpf, compared to round embryos for controls (Figures 1H and I). Closer examination will show that notochord and somites within these embryos are broader and more radially extended (not shown here).
Ventralization phenotyping at 24 hpf
Key ventralization phenotypes range from Classes 1–4.
1. Normal embryos:
Normal embryos will have a developing head, trunk, and tail (Figure 2A).
2. Class 1 ventralization (V1):
These embryos have ill-developed eyes, but have a normal notochord and trunk (Figure 2B)
3. Class 2 ventralization (V2):
These embryos lack well-developed heads, have expanded somites, lack a notochord (Figure 2C), and have a thicker blood island (yellow arrow).
4. Class 3 ventralization (V3):
These embryos lack proper head structures, contain a thickened trunk area, and exhibit enlarged blood islands (Figure 2D).
5. Class 4 ventralization (V4):
These embryos lack all anterior structures but contain traces of posterior structures such as somites (Figure 2E).
Phenotypic data visualization and analysis
During observations at 24 hpf, score each embryo by class based on Figures 1 and 2. As noted, a single concentration may produce a range of DV phenotypes. Data can be plotted as stacked bar plots as shown in Figure 3, and statistical tests can be performed based on percent of normal embryos (Figure 3).
Immunohistochemistry
For pSMAD 1/5/9 immunohistochemistry, control embryos will show a ventral-to-dorsal gradient of staining in the cell mass. However, for dorsalized embryos, this gradient will be disrupted, and the staining will be present across the entire cell mass (Figure 4).
Figure 4. Representative images following pSMAD 1/5/9 localization (Basic Protocol 2).
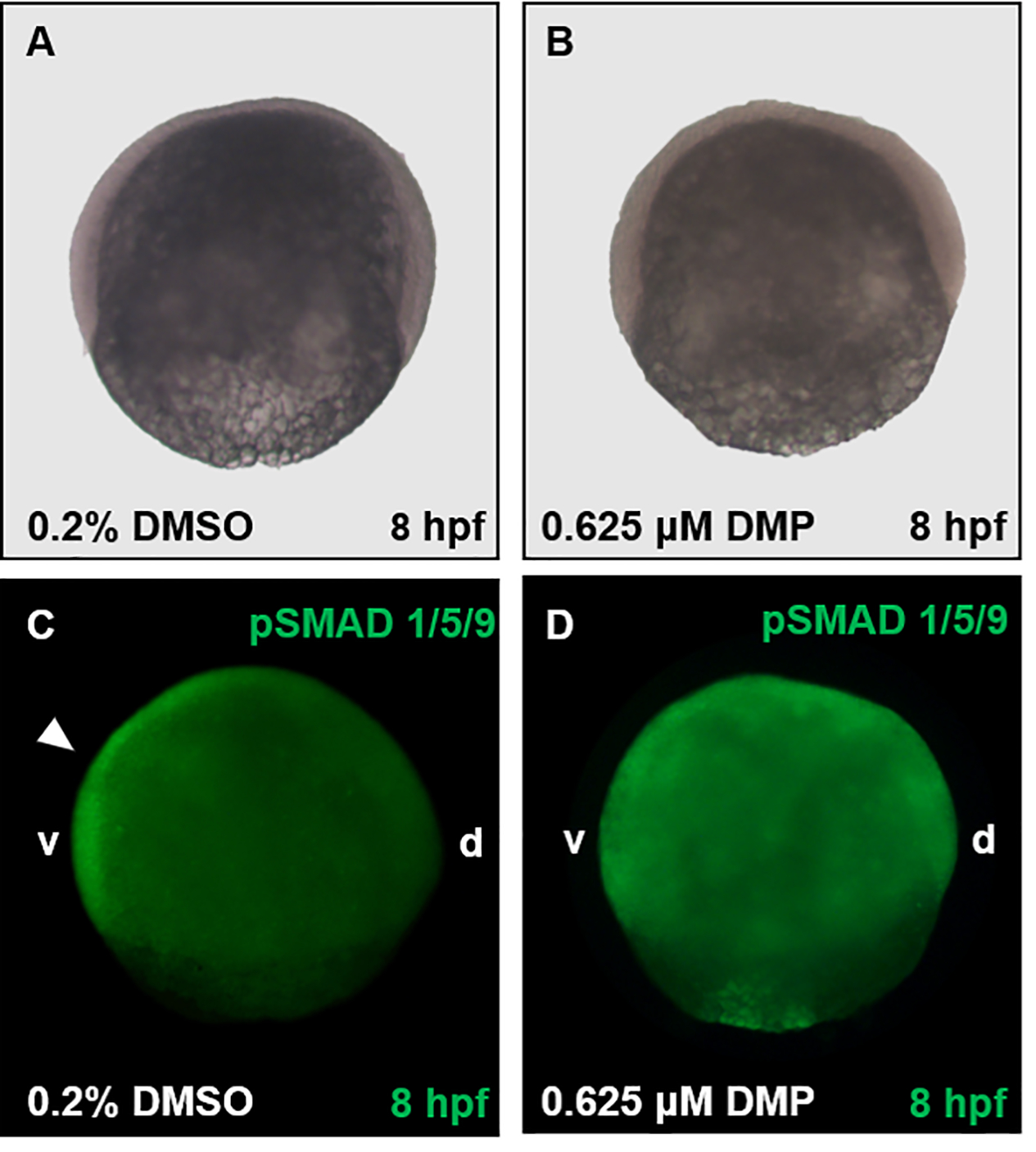
Embryos were exposed to either vehicle (0.2% DMSO) (A, C) or 0.625 μM DMP (B, D) from 0.75 hpf and fixed in 4% PFA at 8 hpf. Embryos within Panels A and B were imaged using brightfield microscopy, and embryos within Panels C and D imaged using fluorescence microscopy. Within control embryos (C), pSMAD 1/5/9 staining is localized along the ventral (v) side of the embryo (white arrowhead), with lack of staining along the dorsal (d) side. Contrary to vehicle controls (C), the gradient of pSMAD 1/5/9 is disrupted by 0.625 μM DMP and diffused throughout the embryo (D).
TIME CONSIDERATIONS
The full protocol presented here can be performed in 5 days.
Day 1:
Set up exposure or morpholino injections. Embryos can be subsampled, dechorionated, and fixed at 8 hpf (Basic Protocol 1).
Day 2:
Observation of phenotypes (Basic Protocol 1). If DV phenotypes are observed, proceed with Basic Protocol 2.
Day 3:
Embryonic exposures for Basic Protocol 2. Collect embryos at ~ 8 hpf and fix.
Days 4–6:
Complete Basic Protocol 2 and image embryos.
ACKNOWLEDGEMENTS
We thank Dr. Sara Frie Vliet for her assistance in optimization of morpholino injections. This work was supported by a NRSA T32 Training Program [T32ES018827] to V.C, and a National Institutes of Health grant [R01ES027576] and USDA National Institute of Food and Agriculture Hatch Project [1009609] to D.C.V.
Footnotes
CONFLICT OF INTEREST STATEMENT:
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT:
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
LITERATURE CITED
- Bond AM, Bhalala OG, & Kessler JA (2012). The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Developmental neurobiology, 72(7), 1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel SM, Tanguay RL & Planchart A (2014) Zebrafish: A Marvel of High-Throughput Biology for 21st Century Toxicology. Curr Envir Health Rpt 1, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J, Upton P, Smith J, & Morrell N (2010). Intersegmental vessel formation in zebrafish: requirement for VEGF but not BMP signalling revealed by selective and non-selective BMP antagonists. British Journal of Pharmacology, 161(1), 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V, Dasgupta S, Reddam A, & Volz DC (2019). Ciglitazone-a human PPARgamma agonist-disrupts dorsoventral patterning in zebrafish. PeerJ, 7, e8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Cheng V, Vliet SMF, Mitchell CA, & Volz DC (2018). Tris(1,3-dichloro-2-propyl) Phosphate Exposure During the Early-Blastula Stage Alters the Normal Trajectory of Zebrafish Embryogenesis. Environ Sci Technol, 52(18), 10820–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Vliet SM, Kupsco A, Leet JK, Altomare D, & Volz DC (2017). Tris(1,3-dichloro-2-propyl) phosphate disrupts dorsoventral patterning in zebrafish embryos. PeerJ, 5, e4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, & Hammerschmidt M (2000). Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development, 127(2), 343–354. [DOI] [PubMed] [Google Scholar]
- Genthe JR, Min J, Farmer DM, Shelat AA, Grenet JA, Lin W, Finkelstein D, Vrijens K, Chen T, Guy RK, Clements WK, & Roussel MF (2017). Ventromorphins: A New Class of Small Molecule Activators of the Canonical BMP Signaling Pathway. ACS Chem Biol, 12(9), 2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JM (1997). Embryonic patterning: to BMP or not to BMP, that is the question. Cell, 89(2), 171–174. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, & McMahon AP (1996). Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes & development, 10(19), 2452–2461. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, & Schilling TF (1995). Stages of embryonic development of the zebrafish. Developmental Dynamics, 203(3), 253–310. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Lee K-H, Zon L, Hammerschmidt M, & Schulte-Merker S (1997). The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development, 124(22), 4457–4466. [DOI] [PubMed] [Google Scholar]
- Laux DW, Febbo JA, & Roman BL (2011). Dynamic analysis of BMP-responsive smad activity in live zebrafish embryos. Dev Dyn, 240(3), 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, & Mullins MC (2004). Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development, 131(23), 5825–5835. [DOI] [PubMed] [Google Scholar]
- Pomreinke AP, Soh GH, Rogers KW, Bergmann JK, Bläßle AJ, & Müller P (2017). Dynamics of BMP signaling and distribution during zebrafish dorsal-ventral patterning. Elife, 6, e25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagerstrom C, Grinblat Y, & Sive H (1996). Anteroposterior patterning in the zebrafish, Danio rerio: an explant assay reveals inductive and suppressive cell interactions. Development, 122(6), 1873–1883. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Karchner SI, Hahn ME (2012) Gene knockdown by morpholino-modified oligonucleotides in the zebrafish (Danio rerio) model: applications for developmental toxicology. Methods Mol Biol 889, 51–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B (2007) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3, 59–69. [DOI] [PubMed] [Google Scholar]
- Vrijens K, Lin W, Cui J, Farmer D, Low J, Pronier E, Zeng F, Shelat AA, Guy K, Taylor MR, Chen T, & Roussel MF (2013). Identification of small molecule activators of BMP signaling. PLOS ONE, 8(3), e59045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, . . . Peterson RT (2008). Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nature chemical biology, 4(1), 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


