Abstract
Nanotechnology can offer different solutions for enhancing the therapeutic efficiency of polyphenols, a class of natural products widely explored for a potential applicability for the treatment of different diseases including cancer. While possessing interesting anticancer properties, polyphenols suffer from low stability and unfavorable pharmacokinetics, and thus suitable carriers are required when planning a therapeutic protocol. In the present review, an overview of the different strategies based on polymeric materials is presented, with the aim to highlight the strengths and the weaknesses of each approach and offer a platform of ideas for researchers working in the field.
Keywords: Nanocarrier, polyphenols, cancer therapy, polymeric materials
Introduction
In cancer therapy, the efficacy of conventional cytotoxic agents is dramatically reduced due to their unfavorable physico-chemical and pharmacological properties, consisting in poor water affinity, non-specific distribution within the body, severe toxic effects to healthy cells and tissues, inadequate drug concentrations at tumors sites, and the development of multi-drug resistance (Luo & Prestwich, 2002; Luo et al., 2009; Jemal et al., 2010).
In the last decades, tremendous efforts have been made to overcome these drawbacks by two main approaches:
Exploration of alternative anticancer agents
Fabrication of targeted nanocarriers
Naturally occurring polyphenols: therapeutic opportunities
Among the different bioactive food components with healthy effect on humans, antioxidants and polyphenols in particular are under investigation as both nutraceutical supplement and/or therapeutic agents undergoing intravenous administration (Cirillo et al., 2016).
Polyphenols consist of at least one aromatic ring with one or more hydroxyl groups and differ in terms of number of aromatic rings and number and position of phenolic groups (Bravo, 1998; Nichenametla et al., 2006). They can be preliminarily classified in (Del Rio et al., 2013):
Flavonoids: 15 carbon atoms with two aromatic rings connected by a three-carbon bridge
Non-flavonoids: heterogeneous class of compounds, with the C6–C1 phenolic acids as the most interesting.
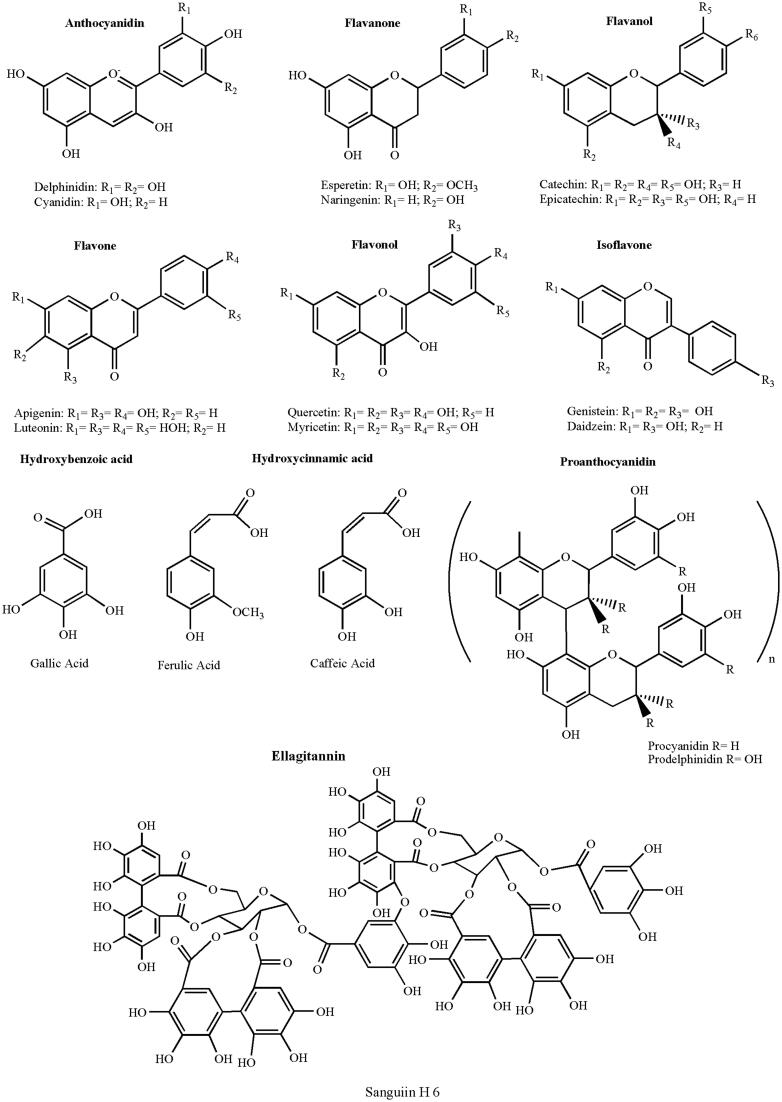
Polyphenols possess different biological activities and have been proposed to exert beneficial effects in a multitude of pathological states, including cancer, cardiovascular, and neurodegenerative diseases (Williams et al., 2004). The antioxidant properties of the polyphenols influencing the intracellular redox status have been hypothesized as the molecular mechanism of action at the basis of their biological functions (Yao et al., 2004). Nevertheless, it was recently speculated that this is unlikely to be the sole explanation for cellular effects (Schroeter et al., 2001; Spencer et al., 2001). It was supposed that they might exert modulatory effects in cells through selective actions on intracellular signaling cascades at different level (Kobuchi et al., 1999; Kong et al., 2000). The structures of polyphenols of biological relevance are sketched in Figure 1 (Dai & Mumper, 2010).
Figure 1.
Chemical structure of main polyphenols of biological relevance.
Anticancer activity of polyphenol: modulation of cell redox state
Extensive laboratory and epidemiological studies have suggested that the cellular damage caused by aberrant production of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radicals is recognized to be the primary cause of aging and age-related diseases, including cancer (Fresco et al., 2006; Hail Jr et al., 2008). Thus, the systematic use of antioxidant molecules to reduce oxidative stress seems to be the ideal approach for cancer prevention (Hou et al., 2005; Khan et al., 2006; Lambert & Elias, 2010). Consistently, extensive epidemiology evidence both in animal and in human studies, suggested that specific dietary antioxidants could be effective in preventing cancer incidence and mortality (Dai & Mumper, 2010). A 5–7% increase in mean survival time and 25–39% decrease in tumor load was recorded in mammary adenocarcinoma bearing mice treated with 0.05% green tea polyphenols as the sole source of drinking fluid, and this was correlated with reduced level of malonyldialdehyde–DNA adduct (a marker of oxidative stress) (Kaur et al., 2007).
Freeze-dried black raspberries were also found to possess high chemoprevention activity in a 6-month chemopreventive pilot study involving patients with Barrett’s esophagus, a premalignant esophageal condition in which the normal stratified squamous epithelium changes to a metaplastic columnar-lined epithelium with increased oxidative stressing conditions (Wang & Sampliner, 2008) by monitoring the urine content, it was proved that high consumption of black raspberries reduced the oxidative stress and the tumor incidence (Kresty et al., 2006). Nevertheless, there are a plenty of research studies with controversial results and a strong debate has started about the real usefulness of polyphenols as therapeutic agents for cancer prevention and/or treatment (Howes, 2006).
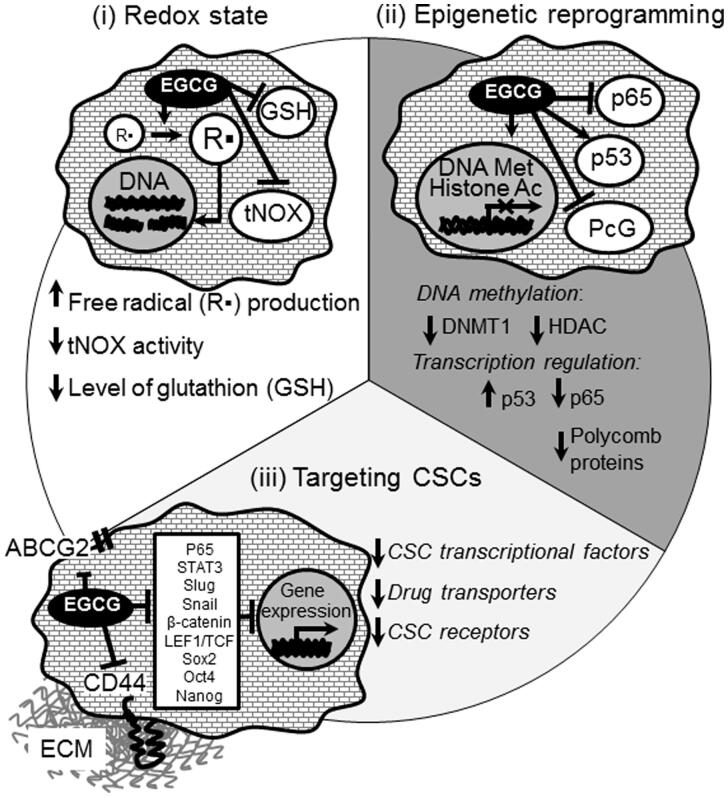
Although the cancer prevention properties of polyphenols are attributed to their anti-oxidant activity, it has been recently shown that the anti-tumor activity arises from their pro-oxidant, rather than antioxidant properties (Hou et al., 2005; Shen et al., 2005) via both direct and indirect mechanisms. Direct pro-oxidant activity involves the formation of liable chemical species (e.g. aroxyl radical and redox complex with a metal cation) under certain conditions, such as high concentration of transition metals and high pH (Procházková et al., 2011; Park & Pezzuto, 2012). Polyphenols can also activate the intracellular production of ROS by indirect mechanisms via interaction with specific cellular pathways (e.g. NOX) (León-González et al., 2015) (Figure 2).
Figure 2.
Molecular mechanisms of the biological action of EGCG on tumor cells.
Cancer cells frequently exhibit high oxidative stress, suggesting that it might be possible to preferentially eliminate these cells by pharmacologically increasing the ROS insults (Gupte & Mumper, 2009). However, advanced stage tumor cells developed the ability to adapt to ROS insult by the upregulation of intracellular antioxidant capacity which in turn confers chemo-resistance (Furfaro et al., 2016).
Glutathione (GSH) is an important antioxidant capable of preventing damage to important cellular components caused by reactive oxygen species. Cancer cells usually show elevated GSH level increasing their antioxidant capacity and the resistance to oxidative stress (Ortega et al., 2011). Abrogation of such drug-resistant mechanisms by redox modulation could have significant therapeutic implications. Our recent work has demonstrated that a chemically modified form of polyphenol, called dextran–catechin (Dex–CA), induces oxidative stress in cancer cells and simultaneously increases their sensitivity to intracellular ROS damages by downregulating cellular glutathione. In particular, Dex–CA showed anticancer activity inducing apoptosis in pancreatic ductal adenocarcinoma cells (Vittorio et al., 2012) and was effective in the treatment of neuroblastoma in vivo by enhancing ROS generation (Vittorio et al., 2016a). Specifically, the anticancer activity was related to the elevate cellular copper content, which is a common features found in many tumors (Brady et al., 2014). It was demonstrated that neuroblastoma cells display significantly high expression levels of the copper transport 1 (CTR1) and intracellular copper levels resulting in high sensitivity to Dex–CA. In sharp contrast, the depletion of CTR1 expression reduced intracellular copper levels and led to a decrease in neuroblastoma cell sensitivity to the conjugate. In particular, Dex–CA reacts with intracellular copper generating ROS by Fanton reaction (Vittorio et al., 2016a).
In recent studies, the pro-apoptotic effects of the epigallocatechin gallate (EGCG) and other tea polyphenols against H1299 human non-small lung carcinoma (Khan & Mukhtar, 2015) and HL-60 human promyelocytic leukemia cells (Elbling et al., 2005) were related to the induction of ROS production, mainly H2O2: the co-treatment with exogenous catalase, indeed, resulted in a consistent reduction of EGCG-mediated apoptosis. This confirms that EGCG may undergo intracellular oxidation, and suggest that EGCG-mediated pro-oxidant activity may have anticancer relevance in vivo.
Other studies have proved that low concentrations of curcumin promote apoptosis in human cutaneous squamous cell carcinoma by altering of cellular redox homeostasis via depletion of reduced glutathione (Numsen Jr, 2008), and by obstructing mitochondrial function via enhanced ROS production and consequent dissipation of ΔΨm (Scott & Loo, 2004; Atsumi et al., 2006; Su et al., 2006). It was supposed that curcumin promotes the one-electron reduction of superoxide to form the highly biologically reactive hydroperoxyl radical (Mishra et al., 2004; Shen & Ji, 2007) or the reduction of transition metals (e.g. Iron) which in turns catalyze the formation of hydroxyl radical via Fenton chemistry (Galati & O'Brien, 2004; Ligeret et al., 2004; Kawanishi et al., 2005). It is clear indeed that polyphenols play an important role in the regulation of the ROS homeostasis in cancer cells and their combination with standard chemotherapies could potentially overcome drug resistance and improve outcome in patients.
Anticancer activity of polyphenols: administration of polyphenols for epigenetic reprogramming
Epigenetic modifications are heritable alteration in the structure and function of the chromatin that occur without changes in the DNA sequences. In mammalian cells, these modifications consist of dysregulation of DNA methylation and post-translational histone modifications such as phosphorylation, methylation, acetylation, or ubiquitination, which orchestrate gene expression in various cell types and at the different stages of embryonic development. Furthermore, a view is emerging that epigenetic alteration of the progenitor cells may be an initial step in the development of various diseases including autoimmune diseases, neurodegenerative diseases, and tumor malignancies. In cancer, chromatin reorganization driven by DNA methylation and histone modifications might induce transcriptional silencing of tumor suppressor genes and reactivate transcription of epigenetically silenced oncogenes. A growing body of evidence suggests that epigenetic therapy including DNA-demethylation agents and the drugs targeting histone methylation and acetylation is emerging as an effective and valuable approach for cancer treatment (Yoo & Jones, 2006; Peitzsch et al., 2016; Zahnow et al., 2016). Cancer-specific epigenetic modulators are a unique model for the investigation of cancer development and serve as an attractive therapeutic strategy for cancer treatment. Currently, epigenetic effect of green tea polyphenols such as catechin, curcumin, epigallocatechin-3-gallate (EGCG), and their more stable nano-modified analogs are widely recognized, and their effect on histone modifications and DNA methylation is reported for the various types of cancer cells (Gao & Tollefsbol, 2015). Although the fact that green tea polyphenols such as EGCG are transported to the cell nucleus has been known for a decade, only recently the specific genes in which expression is affected by the polyphenols as well as mechanisms of this regulation appeared in the focus of cancer research (Polster et al., 2003). An effect of the natural polyphenols on the chromatin structure is mediated by inhibition of the key enzymatic proteins involved in DNA and histone modifications such as DNA methyltransferases, histone deacetylases, and histone methyltransferases. Methylation of DNA at the C5-position of cytosine within the CpG islands around the gene promoters is an important mechanism for epigenetic control of gene expression (Gao & Tollefsbol, 2015). Hypomethylation of these CpG sites is associated with gene activation whereas hypermethylation leads to the gene silencing. Recent studies have proven that green tea catechin EGCG reduces methylation of CpG islands within the promoter of tumor suppressor genes p16INK4a, retinoic acid receptor β (RARβ), O6-methylguanine methyltrasferase (MGMT), and human mrtL homolog 1 (hMLH1), and, therefore, reactivates expression of these genes in cancer cells (Fang et al., 2003; Wu et al., 2013). The mechanisms of the EGCG-dependent DNA demethylation are poorly understood. The study of Fang et al. showed that EGCG can inhibit DNA demethylation by forming hydrogen bonds in the catalytic pocket of DNA methyltransferases such as DNMT1 (Lee et al., 2005). The follow-up investigations demonstrated that other dietary polyphenols such as epicatechin, catechin (CA), quercetin (Q), fisetin, and myricetin also have a strong inhibition activity toward DNMT-mediated DNA methylation with IC50 values ranged from one to a few μM, but EGCG was the most potent inhibitor among them with IC50 value of less than 1 μM (Lee et al., 2005). Epigenetic alteration induced by the natural polyphenols can also be mediated by the inhibition of histone acetyltransferases (HAT) or histone deacetylases (HDAC) by direct binding to the substrate pocket of these enzymes (Choi et al., 2009; Kim & Kim, 2013; Khan et al., 2015). HDACs play an important role in transcription repression by inducing chromatin compaction (Zahnow et al., 2016). Several studies have demonstrated that EGCG-mediated HDAC inhibition is associated with cell-cycle arrest, apoptosis, and metastasis mediated by upregulation of the genes which suppress cancer pathogenesis including Raf kinase inhibitor protein (RKIP) in pancreatic cancer cells, tissue inhibitor of matrix metalloprotease-3 (TIMP3) in breast cancer cell lines, and estrogen receptor α (ESR1) in ER-negative breast tumor cells (Meeran et al., 2012; Kim & Kim, 2013; Deb et al., 2015).
Furthermore, green tea polyphenols can directly activate transcription factors such as tumor suppressor TP53 by the activation of its acetylation that results in increased expression of p21/Waf1 and Bax proteins leading to the growth arrest and apoptosis (Thakur et al., 2012). In contrast, other studies of Choi et al. (2009) demonstrated that EGCG treatment abrogates acetylation of another transcriptional regulator, NFκB-p65, which is mediated by p300/CBP acetyltransferase, that results in suppressing of the NFκB-dependent gene expression and B-cell malignant transformation by Epstein–Barr virus (EBV). Noteworthy, EGCG reduces protein level of some other transcriptional regulators such as polycomb group (PcG) proteins through their proteasome-dependent degradation. PcG proteins are associated with epigenetic gene silencing and implicated in the neoplastic development and metastatic spread (Sparmann & Van Lohuizen, 2006). Treatment of cancer cells with EGCG reduces the level of PcG proteins such as Ezh2, Eed, and Suz12 and decreases the formation of repressive histone marks, H3K27me3 and H2AK119ub (Choudhury et al., 2011).
Taken together, dietary polyphenols might influence the epigenetic landscape and gene expression in cancer cells by several mechanisms that include direct regulation of gene transcription by inhibition of the key enzymes involved in DNA and histone modification, or indirect effect on gene expression by regulation of the multiple transcription factors.
Biological activity of polyphenols: administration of polyphenols for the targeting of cancer stem cell
Nowadays, the failure of an anticancer treatment is widely attributed to cancer stem cells (CSCs) or tumor initiating cells (TICs), a heterogeneous cell fraction within the tumor which is able to divide asymmetrically and give rise to an identical cell and an intermediate progenitor able to differentiate and repopulate the tumor mass, and is also responsible for chemo and radiotherapy resistance. The capability of these cells to escape treatment has been linked to their specific properties such as increased DNA damage response reflected through higher activation of DNA repair by early triggering of Chk 1 and 2 (Bao et al., 2006), higher levels of anti-apoptotic molecules, and lower levels of the reactive oxygen species (ROS) mediated by the higher presence of free radical-scavenging machinery (Diehn et al., 2009; Cojoc et al., 2015). Together with the above-mentioned intrinsic mechanisms of resistance to therapy, CSCs undergo accumulation of gene alterations such as loss of TP53, RB1, p16, and gain of telomerase, Ras, ERBB2, Braf, c-Myc and WNT/β-catenin signaling mutations (Hanahan & Weinberg, 2011; Baccelli & Trumpp, 2012; Cojoc et al., 2015). The phytochemicals are intensively reported for their cancer preventive and CSCs targeting effects by modulating and regulating multiple self-renewal signaling pathways and transcription factors, fatty acid metabolism and lipid rafts with induction of a ROS-dependent cell proliferation arrest and premature senescence, as well as apoptotic pathway activity.
The green tea polyphenol (GTP) (−)-epigallocatechin-3-gallate (EGCG) has gained considerable attention for its chemo-preventive properties. It has been reported to inhibit nasopharyngeal carcinoma (NPC) CSCs through attenuation of STAT3 (Lin et al., 2014) or NF-κB p65 activity (Li et al., 2015). It has been also shown that synergistic treatment of EGCG with quercetin led to a decrease of CD44+/CD133 + prostate cells through inhibition of Vimentin, Slug, Snail, and nuclear β-catenin expression and LEF-1/TCF responsive reporter activity, affecting the CSC migration and invasion (Tang et al., 2010), and in combination with curcumin, it led to a reduction of sphere formation from breast tumor cells by inhibition of STAT3 phosphorylation and retention of STAT3-NFkB interaction (Chung & Vadgama, 2015). Moreover, in head and neck squamous cell carcinoma (HNSCC), EGCG treatment has been reported to downregulate the expression of stem cell markers, such as Oct4, Sox2, Nanog, and CD44 and to suppress HNSCC cells sphere forming capacity, augmenting their cisplatin (CP)-mediated chemosensitivity by inhibiting ABCC2 and ABCG2 transporter genes (Lee et al., 2013b). In the colorectal tumor model, EGCG induced sensitization of the 5-fluorouracil (5FU)-resistant colorectal cancer cells and spheroid-derived colorectal CSCs to 5FU treatment that was associated with suppression of Notch1, Bmi1, Suz12, and Ezh2, upregulation of self-renewal suppressive-miRNAs, miR-34a, miR-145, and miR-200c and enhancement of apoptosis and cell-cycle arrest (Toden et al., 2016). Catechin is another flavonoid present in a variety of plant sources including grape seeds, tea leaves, apples, apricots, pears, or plums. Catechin is proven to sensitize prostate CSCs to radiotherapy, with reduction of aldehyde dehydrogenase (ALDH) positive fraction, as well as downregulation of stemness factors Nanog and Oct4 (Castro Nava et al., 2016).
Sulforaphane, a dietary component of broccoli or broccoli sprouts, demonstrated its efficacy in targeting CSCs by decreasing of the ALDH positive cell population in human breast cancer cells and reducing the primary mammospheres formation in vitro that is accompanied by the downregulation of Wnt/β-catenin self-renewal pathway (Li et al., 2010). The same effect was reported also for the studies on ALDH1 and CD44 positive populations in oral squamous cell carcinomas (OSCCs) where sulforaphane induced miR-200c expression (Liu et al., 2015) and on ALDH1+, CD49f + prostate CSCs with where sulforaphane led to the c-Myc inhibition (Vyas et al., 2016). In the same manner, sulforaphane attenuated expression of pluripotency maintaining transcription factors Nanog and Oct-4 and angiogenic markers VEGF and PDGFRα in human pancreatic CSCs through dysregulation of the sonic hedgehog (Shh)-GLI signaling pathway (Rodova et al., 2012; Li et al., 2013).
The selective efficacy of curcumin and piperine on CSCs, with little or no effect on differentiated cells, has been documented for MCF7 breast tumor cells, where polyphenol treatment led to the inhibition of Wnt signaling pathway and decrease in mamosphere formation by ALDH1 positive cell population (Kakarala et al., 2010). For CD44 positive colorectal CSCs, curcumin might couple with CD44 at the cell membrane with some blocking effect on the transport of glutamine into the cells, decreasing the glutamine content in the CD44(+) cells, and inducing apoptosis (Huang et al., 2016). In H460 lung cancer cells, curcumin was able to reduce the tumor spheres and repress tumor growth in xenograft mouse model via inhibiting the JAK2/STAT3 signaling pathway (Wu et al., 2015).
To β-carotene have been attributed to many anticancer activities, but its exact molecular mechanisms of action on CSCs is not clear yet. Treatment of the neuroblastoma cells with β-carotene led to the induction of neuronal cell differentiation (Lee et al., 2013a) and inhibiting CSCs in vivo, while tumor incidence in murine model was significantly inhibited comparing to the untreated group (Lim et al., 2014).
Quercetin, a polyphenol present in many fruit and vegetables, has been studied in different in vitro models for tumor entities like head and neck, where it was able to reduce the ALDH1 positive fraction and inhibit expression of the stemness signatures in head and neck cancer-derived spheres (Chang et al., 2013b) and pancreas cancer, where it chemosensitized the pancreatic CSCs to gemcitabine treatment through β-catenin inhibition (Cao et al., 2015).
The use of soy derivatives has been shown to be beneficial for the reduction of tumor risk for many tumor types. Thus, genistein, a predominant isoflavone found in soy products, has been reported to decrease the CD44/CD24-positive CSC population in MCF7 breast tumor cells and mammosphere formation by downregulating Hedgehog-Gli1 signaling pathway (Fan et al., 2013). Acting on the same signaling pathway in prostate cancer, genistein was shown to suppress prostatosphere and colony formation in vitro and tumorigenicity in vivo (Zhang et al., 2012a). In gastric cancer cells, genistein was reducing the chemoresistance of CSCs to 5-FU and CP by targeting ABCG2 expression and ERK 1/2 activity (Huang et al., 2014).
The correlation of cancer stem cells and resistance to chemo and radiotherapy encourages the critical consideration for efficient prooxidant CSC-targeted therapy by employing the polyphenols which enable to interfere with the levels of reactive species within these cells, rendering them more sensitive to conventional therapies.
Nanotechnology and cancer therapy
Cancers originate from alterations in biologic processes at the molecular level, thus strategies acting at the nanoscale are an emerging and promising approach for cancer diagnosis and treatment (Kim et al., 2010; Wang et al., 2012a; Gharpure et al., 2015; Pacardo et al., 2015; Stylianopoulos & Jain, 2015). Different systems have been proposed as drug nanocarriers, including polymeric nanoparticles, vesicles, lipid nanoparticles, nanohybrids, with outstanding results in preclinical research and early clinical studies (Eetezadi et al., 2015; Fernandes et al., 2015; Johnstone et al., 2016).
Nanotherapeutics possess unique properties such as high surface area to volume ratio, and controllable physico-chemical and biological properties via surface functionalization offering solutions to the current obstacles in cancer therapies and allowing the preparation of multiple drug delivery with synergistic therapeutic response or the combination of therapeutic agents and diagnostic probes into theranostic platforms (Kumari et al., 2016). Further advantages related to carrier performance within the physiological environment are the improvement of the therapeutic index, the reduction of the drug toxicity by controlled delivery rate, the improvement of drug’s pharmacokinetic profile by enhanced drug stability and minimized systemic clearance and the obtainment of steady state levels (Kumari et al., 2016). More importantly, the site-specificity of the drug release can be achieved by both, passive (e.g. magnetic field or EPR effect) or active (via suitable surface functionalities) targeting.
These features and benefits can be of dramatic significance for therapeutics agents such as polyphenols whose translation from “bench to bedside” for human use is limited by inefficient systemic delivery, poor bioavailability, and high dosage typically required for optimum response limiting, despite the outstanding advancement in fundamental cancer biology in preclinical settings (Siddiqui et al., 2012). Notably, the majority of in vitro studies employ the high concentration of the polyphenols which are not achievable in vivo due to their fast degradation caused by the pH or temperature fluctuations. Therefore, development of the nano-modified dietary polyphenols brings a promise to improve their stability and therapeutic performance. Thus, the combination of the favorable biological activity of polyphenols with the technological performance of nanocarriers, overcoming the limitations of conventional chemotherapy at different levels, could represent a fascinating and promising scenario for developing high effective and clinically relevant anticancer therapy.
Polymeric nanoparticles
Polymeric nanocarriers, defined as colloidal systems with irregular or spherical shape encapsulating or physically entrapping a bioactive molecule (Pérez-Herrero & Fernández-Medarde, 2015), show peculiar properties such as high stability, homogeneous size distribution, controllable physicochemical properties, high drug payload, and controlled drug release (Hu et al., 2010). Different biocompatible polymeric materials from synthetic and/or natural origin with different structure have been employed as nanocarriers in cancer therapy (Tsouris et al., 2014; Estanqueiro et al., 2015). Some key examples of nanoparticulate polymer systems proposed for the delivery of polyphenols are summarized in Table 1. The main attention is devoted to the materials coupling biocompatibility and biodegradability such as naturally occurring chitosan (Jayakumar et al., 2010) and synthetic PLGA (Danhier et al., 2012), while PEG derivatives are widely explored for the surface functionalization preventing macrophage uptake of nanoparticles (Dong et al., 2015).
Table 1. Composition and activity of main polyphenol nanocarriers employed in cancer therapy.
| Polyphenol |
||||||||
|---|---|---|---|---|---|---|---|---|
| Carrier |
Anticancer activity |
|||||||
| Type | Composition | Preparation | Type | Cancer type | Cells | Synergism | In vivo model | References |
| NPs | PLGA-PEG | ESE | PmPs | Breast | MCF-7, Hs578T | – | – | Shirode et al. (2015) |
| NPs | CT | IG | TPs | Hepatic | HepG2 | – | – | Liang et al. (2014) |
| NPs | CT | IG | TPs | Hepatic | HepG2 | – | – | Liang et al. (2011) |
| NF | PCL | ESP | PPs | Gastric | MKN28 | – | – | Kim et al. (2012) |
| NPs | ALG-CT-PX F127 | IG | CUR | Cervice | HeLa | – | – | Das et al. (2010) |
| NPs | PLGA | ESE | CUR | Oral | CAL27 | – | – | Chang et al. (2013a) |
| NPs | FB | CaCl2 CK | CUR | Prostate | PC3 | – | – | Sanoj Rejinold et al. (2011) |
| Breast | MCF-7 | |||||||
| NPs | PLGA | ESE | CUR | Prostate | LNCaP, PC-3, DU-145 | – | – | Mukerjee & Vishwanatha (2009) |
| NPs | PLGA | ESE | CUR | Osteosarcoma | U2OS | – | – | Peng et al. (2014) |
| HYs | CHT | IG | CUR | Melanoma | A375 | – | – | Mangalathillam et al. (2012) |
| NPs | Peptide | SA | CUR | Medulloblastoma | DAOY | – | – | Altunbas et al. (2011) |
| NPs | NIPAA-VP-PEG-MA | RP | CUR | Pancreas | MiaPaCa2, Su86.86, BxPC-3, Capan-1, PANC-1, E3LZ10.7, PL-5, PL-8 | – | HM | Bisht et al. (2007) |
| NPs | CT-PBCA | EP | CUR | Hepatic | HepG2, Bel7402, Huh7 | – | HepG2 XM | Duan et al. (2010) |
| HYs | PX 407–PX F188 | SA | CUR | Melanoma | B16-F10 | – | – | Sun et al. (2014b) |
| NPs | CL | NPR | CUR | Prostate | C4-2, LNCaP, DU-145, PC-3 | – | – | Yallapu et al. (2012) |
| NPs | PLGA | ESE | CUR | Cervice | HeLa | – | – | Nair et al. (2012) |
| NPs | PLGA–PX F127 | NPR | CUR | Cervice | KB-V1, KB-3-1 | – | – | Punfa et al. (2012) |
| HYs | PEG-DA-AA | RP | CUR | Cervice | HeLa | – | – | Deepa et al. (2012) |
| HYs | FA-PEG-DA-AA | RP | CUR | Cervice | HeLa | – | – | Pillai et al. (2014) |
| NPs | PLGA | NPR | CUR | Ovarian | A2780, A2780CP | – | – | Yallapu et al. (2010a) |
| NPs | PLGA-PEG | ROP | CUR | Breast | MCF-7 | – | – | Mirakabad et al. (2016) |
| NPs | PLGA | ESE | CUR | Breast | MCF-7 | – | – | Verderio et al. (2013) |
| NPs | EU-S100 | ESE | CUR | Colon | HT-29 | – | – | Prajakta et al. (2009) |
| NPs | PLGA-PEG | NPR | CUR | Colon | HT29 | – | HM | Li et al. (2014) |
| NPs | HSA | ESE | CUR | Colon | HCT116 | – | HCT116 XM | Kim et al. (2011) |
| Pancreas | MiaPaCa2 | |||||||
| HYs | CT, GL, HA | EFS | CUR | Lung | A549 | – | – | Teong et al. (2015) |
| NPs | NIPAA-VP-AA | RP | CUR | Medulloblastoma | DAOY, D283Med | – | – | Lim et al. (2011) |
| Glioblastoma | HSR-GBM1, JHH-GBM14 | |||||||
| HYs | CT | IG | CUR | Oral | SCC25 | – | – | Popat et al. (2014) |
| NPs | PLGA | NPR | CUR | Prostate | DU-145, PC-3 | – | XM | Yallapu et al. (2014) |
| HYs | GL-PAGA | EP | CUR | Colon | HCT-116 | – | – | Madhusudana Rao et al. (2015) |
| NPs | PLGA | ESE | CUR | Breast | MCF-7 | 5-FU | – | Balasubramanian et al. (2014) |
| NPs | CT-PBCA | EP | CUR | Breast | MCF-7 | DOX | – | Duan et al. (2012) |
| NPs | PLGA | NPR | CUR | Ovarian | A2780CP | CP | – | Yallapu et al. (2010b) |
| Breast | MDA-MB-231 | |||||||
| NPs | NIPAA-VP-AA | RP | CUR | Pancreas | – | GM | Pa03C XM | Bisht et al. (2010) |
| NPs | GL-PEC | LbL | EGCG | Breast | MBA-MD-231 | – | – | Shutava et al. (2009) |
| NPs | CS-pp-CT | GP-CK | EGCG | Hepatic | HepG2 | – | – | Hu et al. (2014) |
| Gastric | BGC823 | |||||||
| NPs | CS-pp-CT | GP-CK | EGCG | Colon | Caco-2 | – | – | Hu et al. (2015) |
| NPs | PLGA-PEG | NPR | EGCG | Prostate | LNCaP | – | – | Sanna et al. (2011) |
| NPs | HA | SA | EGCG | EAC | EAC | DOX | – | Ray et al. (2013) |
| NPs | CT | IG | EGCG | Melanoma | Mel928 | – | Mel928 XM | Siddiqui et al. (2014) |
| NPs | PLGA-PEG | NPR | EGCG | Prostate | PC-3 | – | 22Rυ1 XM | Siddiqui et al. (2009) |
| NPs | CT | IG | EGCG | Prostate | – | – | 22Rυ1 XM | Khan et al. (2014) |
| NPs | CT-GL-PEG | IG | EGCG | Gastric | Luc MKN45 | – | XM | Lin et al. (2015) |
| NPs | PLGA | NPR | EGCG | Lung | A549 | CP | – | Singh et al. (2011) |
| Cervice | HeLA | |||||||
| TF | Leukemia | THP-1 | ||||||
| NPs | PLGA | SE | EGCG | Lung | A549 | CP | EAC XM | Singh et al. (2015) |
| Cervice | HeLA | |||||||
| TF | Leukemia | THP-1 | ||||||
| EAC | – | |||||||
| NPs | BSA | NPR | RV | Lung | NCI-H460 | – | – | Karthikeyan et al. (2015) |
| NPs | BSA | NPR | RV | Ovarian | SKOV3 | – | – | Guo et al. (2015) |
| NPs | PLGA | EM | RV | Breast | MCF-7 | – | – | Kumar et al. (2016) |
| NPs | PLGA-PEG | NPR | RV | Prostate | DU-145, LNCaP | – | – | Sanna et al. (2013) |
| NPs | MI-PEG-PLA | SA | RV | Glioblastoma | CT26, U87 | – | CT26 XM | Guo et al. (2013) |
| NPs | CT | IG | Q | Pancreas | MiaPaCa2, | 5-FU | – | David et al. (2015) |
| NPS | PLGA | ESE | Q | Breast | MCF-7 | TM | XM | Jain et al. (2013) |
| Colon | Caco2 | |||||||
| NPs | HA- PBCA-α-TPh-PEG-SC | RP | MH | Lung | A549 | – | S180 XM | Abbad et al. (2015) |
| Hepatic | L02 | |||||||
| PC | PEG | CM | CUR | Glioma | C6 | – | – | Dey et al. (2015) |
| PC | PEG | CM | CUR | Prostate | PC-3 | – | – | Safavy et al. (2007) |
| PC | PEG | CM | CUR | Pancreas | MiaPaCa2, PANC-1, BxPC-3, AsPC-1 | GM | – | Li et al. (2009) |
| PC | PEG | CM | RV | Cervice | HeLa | BT | – | Wang et al. (2016) |
| Breast | MCF-7 | |||||||
| PC | CMCT | CM | Q | Hepatic | HepG2 | PTX | HepG2 XM | Wang et al. (2014) |
| PC | PEG | CM | CUR | Cervice, Breast | HeLa, EMT6 | – | EMT6 XM | Lv et al. (2015) |
| PC | PEG-DTE | CM | CUR | Breast | MDA-MB-231 | – | – | Shpaisman et al. (2012) |
| PC | PEG | CM | CA | Breast | MDA-MB-231 | BZ | – | Su et al. (2011) |
| PC | HA-PEI | CM | EGCG | Colon | HCT-116 | GB | – | Liang et al. (2016) |
| PC | Dex | FRG | CA | Pancreas | MiaPaca-2, PL45 | – | – | Vittorio et al. (2012) |
| PC | Dex | FRG | CA | Neuroblastoma | IMR-32, IMR-32-CisRes, BE(2)-C | – | XM | Vittorio et al. (2016a) |
| PC | Dex | ELC | CA | Neuroblastoma | IMR-32 | – | – | Vittorio et al. (2016b) |
| PC | GL | FRG | GA | Prostate | DU-145, PC-3 | – | – | Cirillo et al. (2010) |
| Kidney | A498 | |||||||
| PC | PMAA | FRG | Q | Cervice | HeLa | – | – | Puoci et al. (2012) |
| MCs | GL-Dex | SA-GP-CK | PPs | Breast cancer | MCF-7 | – | – | Zhou et al. (2012) |
| PMs | PVP-PEG | EE | PPs | Glioblastoma | DBTRG-05MG | – | – | Wang et al. (2015a) |
| MCs | GL-Dex | SA-GP-CK | CUR | Cervice | HeLa | – | HM | Zhang et al. (2014a) |
| PMs | KT | SE | CUR | Cervice | HeLa | – | – | Curcio et al. (2015a) |
| PMs | GL | Se | CUR | Lung | H1299 | – | – | Curcio et al. (2015b) |
| MCs | CS | SA | CUR | Cervice | HeLa | – | – | Sahu et al. (2008b) |
| MCs | ZN-PEG | SA | CUR | Ovarian | NCI | – | HM | Podaralla et al. (2012) |
| MCs | CT-STA | SA | CUR | Colon | Primary | – | XM | Wang et al. (2012b) |
| MCs | PEG-PAE | SE | CUR | Cervice | HeLa | – | – | Lv et al. (2013) |
| MCs | PEG-PLA | SE | CUR | Breast | MCF-7 | DOX | XM | Lv et al. (2016) |
| MCs | PEG-PCL | TLE | CUR | Ovarian | A2780t | – | – | Gou et al. (2015) |
| MCs | PEG-PCL | TLE | CUR | Breast | MDA-MB-436 | – | – | Zhu et al. (2015) |
| MCs | PEG-PCL | SA | CUR | Breast | 4T1 | – | 4T1 XM | Liu et al. (2013) |
| MCs | PEG-PCL | TLE | CUR | Cervice | HeLa | – | XM | Mikhail et al. (2014) |
| Colon | HT-29 | |||||||
| MCs | PEG-PCL | TLE | CUR | Lung | LL/2 | DOX | XM | Wang et al. (2013) |
| MCs | PEG-PCL | SA | CUR | Lung | LL/2 | – | ZF | Gong et al. (2013) |
| MCs | LNA-PEG-PCL | SA | CUR | Cervice | HeLa | – | HM | Song et al. (2014) |
| Lung | A549 | |||||||
| MCs | PEG -PAC | SA | CUR | Cervice | HeLa | – | – | Sahu et al. (2008a) |
| PMs | PEG-OA | TLE | CUR | Brain | U87MG | – | – | Erfani-Moghadam et al. (2014) |
| MCs | PEG2000-DSPE | TLE | CUR | Ovarian | SK-OV-3TR | PTX | – | Abouzeid et al. (2014) |
| MCs | PEG2000-DSPE | TLE | CUR | Ovarian | NCI | PTX | SK-OV-3TR XM | Sarisozen et al. (2014) |
| MCs | PEG2000-DSPE | TLE | CUR | Colon | HCT-116 | DOX | XM | Abouzeid et al. (2013) |
| MCs | PEG-DOX | SA | CUR | Cervice | HeLa | DOX | HepG2 XM | Zhang et al. (2016) |
| Hepatic | HepG2 | |||||||
| MCs | PX-PEG-SC | SE | CUR | Ovarian | NCI | – | – | Saxena & Hussain (2013) |
| MCs | PX F127 F68 | TLE | CUR | Cervice | HeLa | – | – | Sahu et al. (2011) |
| MCs | PX F127 | TLE | RV, CUR | Ovarian | SKOV-3 | DOX | HM | Carlson et al. (2014) |
| MCs | PX F127 | TLE | RV, Q | Ovarian | SKOV-3 | DOX | HM | Cote et al. (2015) |
| MCs | PCL-PEG-SC | TLE | RV | Breast | MCF-7 | – | – | Wang et al. (2015b) |
| MCs | AP-E3 | rDNA | RV | Glioblastoma | A-172 | – | – | Kim et al. (2015a) |
| MCs | PLA-PEG | TLE | EGCG | Pancreas | MiaPaca-2 | – | – | Sun et al. (2014a) |
| MCs | CS | SA | EGCG | Colon | HT-29 | – | – | Haratifar et al. (2014b) |
| MCs | CS | SA | EGCG | Colon | – | – | XM | Haratifar et al. (2014a) |
| CNs | GO | RM | TPs | Colon | HT29, SW48 | – | – | Abdolahad et al. (2013) |
| CNs | GO | RM | RV | Ovarian | A2780 | – | – | Gurunathan et al. (2015) |
| CNHs | PCL-MWNT | ESP | TPs | Lung | A549 | – | – | Shao et al. (2011) |
| Hepatic | HepG2 | |||||||
| CNHs | GL-MWNT | CG | CA | Prostate | DY-145, PC-3, LNCap | RT | – | Castro Nava et al. (2016) |
| CNHs | GL-MWNT | CG | CA | Cervice | HeLa | – | – | Cirillo et al. (2013b) |
| CNHs | PMAA-MWNT | RC | Q | Cervice | HeLa | – | – | Cirillo et al. (2013a) |
| CNHs | PMAA-MWNT | RC | Q | Neuroblastoma | IMR-32 | CP | – | Vittorio et al. (2014a) |
| MNPs | Fe-CA | CPM | CUR | Breast | MCF-7 | – | – | Wani et al. (2011) |
| MNPs | HA-Fe | LbL | CUR | Colon | Caco-2 | – | – | Manju & Sreenivasan (2011) |
| PVP-Fe | Glioma | C6 | ||||||
| MNPs | Fe-PX F127 | NPR | CUR | Pancreas | HPAF-II, Panc-1 | – | XM | Yallapu et al. (2013) |
| MNPs | Fe-DEX | SM | CA | Pancreas | MIA Paca2 | – | – | Vittorio et al. (2014b) |
| MNPs | Fe | KL | EGCG | Colon | CT-26 | – | XM | Xiao et al. (2015) |
| MNPs | Ni | ED | Q | Hepatic | SMMC-7721 | – | – | Guo et al. (2009) |
PEG2000-DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; 5-FU, 5-fluorouracil; AA, acrylic acid; ALG, alginate; AP, apolipoprotein; BT, bicalutamide; BZ, bortezomib; BSA, bovine serum albumin; CPM, capping method; CNHs, carbon nanohybrids; CNs, carbon nanostructures; CMCT, carboxymethyl chitosan; CS, casein; CA, catechin; CL, cellulose; KM, chelation method; CHT, chitin; CT, chitosan; CP, cisplatin; CG, coating; CM, condensation method; CK, crosslinking; CUR, curcumin; DTE, desaminotyrosyl-tyrosine ethyl ester; Dex, dextran; DOX, doxorubicin; EAC, Ehrlich ascites carcinoma; ED, electrochemical deposition; ESP, electrospinning; EFS, electrostatic field system; ESE, elmusion solvent evaporation; EE, emulsion evaporation; EM, emulsion method; EP, emulsion polymerization; ELC, enzyme laccase catalysis; EGCG, epigallocatechin gallate; EU, eudragit; FB, fibrinogen; FA, folic acid; FRG, free radical grafting; GA, gallic acid; GL, gelatin; GM, gemcitabine; GP, genipin; GB, granzyme B; GO, graphene oxide; GG, guar gum; HM, healthy mice; HSA, human serum albumin; HA, hyaluronic acid; HYs, hydrogels; IG, ionic gelation; KT, keratin; LbL, layer-by-layer; LNA, linoleic acid; MNPs, magnetic nanoparticles; MI, maleimide; MCs, micelles; MH, morin hydrate; MWNT, multi-walled carbon nanotubes; NFs, nanofibers; NPs, nanoparticles; NPR, nanoprecipitation; NIPAA, N-isopropylacrylamide; VP, N-vinyl-2-pyrrolidone; OA, oleic acid; PTX, paclitaxel; PAC, palmitic acid; pp, phosphopeptide; PPs, plant polyphenols; PX, poloxamers; PAGA, poly(acrylamidoglycolic acid); PA, poly(acrylates); PAE, poly(anhydride esters); PBCA, poly(butyl cyanoacrylate); PCL, poly(caprolactone); PCA, poly(cyanoacrylates); PEC, polyelectrolyte; PEG-mA, poly(ethylene glycol) acrylate; PEG-DA, poly(ethylene glycol) diacrylate; PEG, poly(ethylene glycols); PEI, poly(ethyleneimine); PLA, poly(lactic acid); PLGA, poly(lactide-co-glycolide) acid; PMAA, poly(methacrylic acid) ; PVP, poly(vinyl pyrrolidone); PC, polymer conjugate; PMs, polymersomes; PmPs, pomgranade polyphenols; Q, quercetin; RC, radical coupling; RP, radical polymerization; RT, radiotherapy; rDNA, recombinant DNA; RM, reduction method; RV, resveratrol; ROP, ring opening polymerization; SA, self-assembly; SM, solvation method; SE, solvent evaporation; STA, stearic acid; SC, succinate; TM, tamoxifen; TPs, tea polyphenols; TF, theaflavin; TLE, thin layer evaporation; TPh, tocopheryl; XM, xenograft mice; ZF, zebrafish; ZN, Zein.
Different synthetic strategies have been proposed for the preparation of effective delivery vehicles, depending on the physico-chemical properties of the starting polymer, mainly retained in the final product, and on the desired technological features of the carriers effecting their performance both in vitro and in vivo experiments. The widely explored methodologies involved polar interaction, emulsion solvent evaporation, and radical polymerization (Table 1).
In vitro experiments showed that the loading into Chitosan (CT), poly(caprolactone) (PCL), and poly(lactide-co-glycolide) acid (PLGA) nanoparticles carried out to the enhancement of the antiproliferative effects of polyphenols extracts through effective cell internalization (Shirode et al., 2015), necrosis and/or apoptosis induction in hepatic (Liang et al., 2011,2014), gastric (Kim et al., 2012), and breast (Shirode et al., 2015) cancer cell lines with superior compared with their free counterparts.
As before mentioned, curcumin (CUR) is one of the most promising polyphenols with biological activity, and several efforts have been performed in pointing out an effective formulation overcoming its poor water solubility. The most of the proposed delivery nano-systems show mechanisms of action mirror that of free curcumin, allowing an effective passive targeting on different cancer cells, including cervical (Das et al., 2010), oral (Chang et al., 2013a), prostate (Mukerjee & Vishwanatha, 2009; Sanoj Rejinold et al., 2011), breast (Sanoj Rejinold et al., 2011) cancers, osteosarcoma (Peng et al., 2014), melanoma (Mangalathillam et al., 2012), and medulloblastoma (Altunbas et al., 2011) by controlling the CUR release over time. Furthermore, in vivo studies proved that nanoparticles prepared by free radical polymerization of N-isopropylacrylamide (NIPAAm), N-vinyl-2-pyrrolidone (VP), and poly(ethylene glycol) acrylate (PEG-mA), proposed for the treatment of pancreatic cancer, show negligible toxicity in mouse model (Bisht et al., 2007), while the emulsion polymerization of butyl-cyanoacrylate in the presence of CT allows the obtainment of a CUR delivery vehicles suitable for the treatment of hepatic cancer with favorable pharmacokinetic profiles (Duan et al., 2010).
In addition to these features, improved cellular uptake and/or apoptosis induction by CUR nanoformulations result in enhanced anticancer activity in melanoma (Sun et al., 2014b), prostate (Yallapu et al., 2012), cervical (Deepa et al., 2012; Nair et al., 2012; Punfa et al., 2012; Pillai et al., 2014), ovarian (Yallapu et al., 2010a), breast (Yallapu et al., 2010a; Verderio et al., 2013; Mirakabad et al., 2016), colon (Prajakta et al., 2009; Li et al., 2014), pancreatic (Kim et al., 2011), lung (Teong et al., 2015) medulloblastoma (Lim et al., 2011), glioblastoma (Lim et al., 2011), and oral carcinoma (Popat et al., 2014) cancer cell lines with superior pharmacokinetics (Li et al., 2014). Furthermore, PLGA-PEG nanoparticles are able to enhance the anticancer efficiency of CUR in prostate (Yallapu et al., 2014) and colon (Kim et al., 2011) cancer cell both in vitro and in vivo. The enhancement of CUR activity was also reached by encapsulation into GL-based nanogels able to target the deliver the polyphenol to colon cancer due to pH responsivity (Madhusudana Rao et al., 2015).
CUR nanocarriers have been also proposed for the treatment of breast cancer in combination therapy with conventional cytotoxic agents such as 5-FU (Balasubramanian et al., 2014), Doxorubicin (DOX) (Duan et al., 2012), and CP (Yallapu et al., 2010b) with synergistic activity. The association with CP is also useful for ovarian cancer treatment (Yallapu et al., 2010b), while in vivo studies proved the synergism of CUR nanoformulation prepared by free radical polymerization of NIPAAm, VP, and AA with Gemcitabine in Pa03C Xenograft mouse model (Bisht et al., 2010).
EGCG stability in physiological environments and in vitro anticancer activity against breast (Shutava et al., 2009), hepatic (Hu et al., 2014), gastric (Hu et al., 2014), colon (Hu et al., 2015), and prostate cancer (Sanna et al., 2011) were improved in nanoparticulate systems based on naturally occurring polymers, including (gelatin – GL) (Shutava et al., 2009), casein–chitosan (CS–CT) derivative (Hu et al., 2014,2015), or synthetic PLGA-PEG derivative (Sanna et al., 2011). Synergistic effect was recorded by using EGCG-hyaluronic acid (HA) nanoparticles in combination with DOX for the treatment of Ehrlich ascites carcinoma (EAC) (Ray et al., 2013).
The effective suitability of EGCG as anticancer agent was proved by in vivo experiments involving EGCG nanoformulations and xenograft mice models of melanoma (Siddiqui et al., 2014), prostate (Siddiqui et al., 2009; Khan et al., 2014) and gastric (Lin et al., 2015) cancers. Furthermore, the in vitro (Singh et al., 2011), and in vivo (Singh et al., 2015) synergism recorded when EGCG was delivered in association with CP, offers new therapeutic opportunities for some invasive human cancer diseases.
Resveratrol (RV) was effectively released from GL (Karthikeyan et al., 2015), Bovine Serum Albumin (BSA) (Guo et al., 2015), PLGA (Kumar et al., 2016), PLGA-PEG (Sanna et al., 2013), nanoparticles, showing enhanced anticancer efficiency for the treatment of lung (Karthikeyan et al., 2015), ovarian (Guo et al., 2015), breast (Kumar et al., 2016), and prostate (Sanna et al., 2013) cancers. The insertion of transferrin units on PLGA-PEG nanoparticles allows the obtainment of RV nanovehicles with favorable pharmacokinetic profiles and active targeting effect on glioma cancer cells in vivo (Guo et al., 2013).
When Q is loaded on CT nanoparticles show synergistic effect with 5-FU towards pancreatic cancer cells both in the 2D and in the 3D cultures (David et al., 2015), while its encapsulation into PLGA was suitable for the treatment of breast cancer in xenograft mouse model (Jain et al., 2013).
Finally, a hyaluronic acid-polybutyl cyanoacrylate-α-tocopheryl-PEG-succinate (HA-PBCA-α-TPh-PEG) carrier system was proposed as delivery vehicle for the treatment with MH of lung and hepatic cancer in vitro and sarcoma in vivo (Abbad et al., 2015).
Polymer conjugates
Polymer conjugates, composed of a drug covalently linked to a water-soluble macromolecular system, are emerging tools as anticancer therapeutics, and relevant research activity are exploring their impact in novel and performing therapies. The high molecular weight confers the same favorable pharmacokinetic and toxicological features discussed for polymeric nanoparticles, and the ability to overcome some mechanisms of drug resistance (Minko et al., 1998) and to elicit immunostimulatory effects (Říhová et al., 2003; Sirova et al., 2013).
According to the Ringsdorf model (Ringsdorf, 1975), a water-soluble polymeric–drug conjugate consist of three different units (Sobczak et al., 2013): the drug-linking portion, a moiety responsible for the physicochemical properties; a third unit incorporating an active targeting element (e.g. monoclonal antibody) allowing site-specificity at the cellular level (Allen, 2002). The concept of polymer therapeutics can be extended to antioxidant polymers, obtained either by the polymerization of monomeric polyphenols or their conjugation to natural or synthetic macromolecules. Three main approaches have been proposed for the synthesis of high molecular weight antioxidants, namely condensation, radical grafting, and enzymatic catalysis (Spizzirri et al., 2014).
The condensation methods involve reactions between the chemical functionalities within the antioxidant molecules and those inside the polymeric backbones (e.g. acylation, esterification, etc.), allowing the possibility to modulate the properties of the final products (e.g. mechanical, physical, etc.). The methods are very versatile in terms of substrate availability (e.g. chemical composition and type of the coupling linkage), but the overall reaction process is often a multi-step reaction and is mainly employed in the case of functionalization of synthetic polymers, since the mechanical properties remain almost unchanged compared to the parent material.
The radical grafting approach works in mild reaction conditions almost completely preserving the chemical integrity of the polyphenol moiety and can be applied to polymeric materials (e.g. natural polymers like proteins and polysaccharides) possessing chemical functionalities able to undergo free radical coupling (e.g. heteroatoms and/or aromatic rings) (Oliver et al., 2016).
Finally, the third approach involves a coupling reaction in the presence of a suitable enzyme (laccases, peroxidases, or tyrosinases) as a catalyst and allows synthesizing well-defined structures with controlled stereo- and chemo-selectivity in milder reactions conditions (temperature, pressure, pH) and without using toxic reagents (Nyanhongo et al., 2012; Ravichandran et al., 2012; Zhang et al., 2012b).
Curcumin conjugates have been synthesized by condensation reaction with PEG via carbodiimide chemistry, and proposed as a polymer therapeutics for the treatment of glioma (Dey et al., 2015), prostate (Safavy et al., 2007), and pancreatic cancer in combination with gemcitabine (Li et al., 2009). Carbodiimide was also employed for the preparation of PEG-RV conjugate showing cytotoxic activity in cervical and breast cancer cells in synergism with Bicalutamide (Wang et al., 2016), and carboxymethyl chitosan-Q conjugate for the treatment of hepatic cancer in vitro and in vivo in combination with Paclitaxel (PTX) (Wang et al., 2014). An interesting upgrade of carbodiimide chemistry was proposed in (Lv et al., 2015), where CUR was at first reacted with dithiopropionic acid, and the resulting hydrophobic co-polymer was conjugated with PEG in the presence of biotin as targeting element. Then, emulsion solvent evaporation method allows the obtainment of nanoparticles employed for the treatment of cervical (in vitro) and breast (in vitro and in vivo) cancers in synergism with DOX. A different approach for CUR conjugation was proposed when triphosgene chemistry was employed for the preparation of CUR-PEG hydrogels with enhanced anti-proliferative activity in breast cancer cells (Shpaisman et al., 2012).
By amidation with PEG (Su et al., 2011) and hyaluronic acid (Liang et al., 2016), the anticancer activity of CA and EGCG against breast and colon cancer was significantly enhanced in synergism with Bortezomib and Granzyme B, respectively. The conjugation of CA to Dex by free radical (Vittorio et al., 2012, 2016a) or laccase catalysis (Vittorio et al., 2016b) allows the obtainment of polymer conjugates suitable for the treatment of pancreatic cancer in vitro (Vittorio et al., 2012) and neuroblastoma in vivo (Vittorio et al., 2016a) with higher efficiency when compared with the free flavonoid. A free radical approach was also explored for the synthesis of a GL–gallic acid (GL–GA) (Cirillo et al., 2010) and polymethacrylic acid-Quercetin (PMAA-Q) (Puoci et al., 2012) conjugates showing anticancer activity towards prostate and cervical cancer, respectively.
Polymeric vesicular systems
Amphiphilic block copolymers, composed of a hydrophobic and a hydrophilic portion, are able to form self-assembled structures with different size and shape, including spherical micelles (Discher et al., 2000), worm-like micelles, and closed bi- or multilayer structures (Kumar et al., 2007), named polymersomes (Brinkhuis et al., 2011). Both micelles and polymersomes are promising candidate for drug delivery applications, offering some peculiar advantages.
Polymeric micelles can delivery both water soluble and insoluble molecules by encapsulation in the hydrophobic core or inclusion in the hydrophilic shell (Onaca et al., 2009). They show reduced size (20–80 nm), long circulation times in the bloodstream (Tong & Cheng, 2007; Torchilin, 2007), while suffer from insufficient stability in systemic circulation and premature drug leakage which may cause side effects and a decrease of effectiveness (Lu & Park, 2013).
Polymersomes are bilayered systems with size and shape close to liposomes but showing key advantages for the modulation of the delivery of an encapsulated drug related to the lower permeability since they are composed of amphiphiles with higher molecular weight and conformational freedom, the higher mechanical stability due to the thicker membrane, and the possibility to tune the membrane properties through the polymer chemistry (Onaca et al., 2009). In addition, the interaction of polymersomes with macrophages is reduced due to the conveying surface-protective properties of the employed shell-forming hydrophilic flexible polymers (Brož et al., 2005).
As previously discussed, the use of biodegradable or at least biocompatible polymers is obligatory for biomedical applications, thus polymeric micelles and polymersomes suitable for polyphenol delivery are formed by naturally occurring polysaccharides (e.g. Dex and CT), proteins (e.g. GL, CS) of synthetic building blocks mainly composed of PEG, PCL, PX, and their derivatives (e.g. fatty acid esters), see Table 1.
Plant polyphenols were successfully encapsulated into GL-Dex (Zhou et al., 2012) micelles and PVP-PEG (Wang et al., 2015a) polymersomes and explored in vitro for the treatment of breast cancer with higher efficiency than their free counterpart (Zhou et al., 2012) and glioblastoma by caspase-dependent activation of both the intrinsic and extrinsic signaling pathways (Wang et al., 2015a).
The imprinted GL-Dex micelles prepared in the presence of tea polyphenols (Zhou et al., 2012) were also proposed as nanocarriers for CUR and tested for the treatment of HeLa cancer cells (Zhang et al., 2014a). Interestingly, such vehicle improved the CUR pharmacokinetics profile with considerable therapeutic benefits.
Proteins from both animal (keratin – KT, GL, and CS) and vegetal (Zein) sources were explored as starting materials for the preparation of CUR delivery devices by solvent evaporation (Curcio et al., 2015a,b) and self-assembly (Sahu et al., 2008b; Podaralla et al., 2012). The main advantages of such materials are the high biocompatibility and improved CUR efficiency on cervical (Sahu et al., 2008b; Curcio et al., 2015a) and lung (Curcio et al., 2015b) cancer cells in vitro and ovarian cancer with negligible immunogenicity in mice (Podaralla et al., 2012). Amphiphilic behavior was conferred to CT by conjugation with STA, allowing the obtainment of CUR carrier for the treatment of colon cancer both in vitro (primary cancer cells) and in vivo (orthotopic mice) with improved efficiency (Wang et al., 2012b). The high anticancer efficiency of CUR is responsible for the wide researches devoted to the preparation of micelles and polymersomes able to enhance its performance. PEG is the synthetic polymers at the basis of most of the proposed vesicular delivery systems, due to its biocompatibility and stealth properties. By solvent evaporation, PEG in combination with poly(anhydride esters) (PAE) (Lv et al., 2013) and poly(lactic acid) (PLA) (Lv et al., 2016) carried out to micelles employed as CUR vehicle in HeLa (Lv et al., 2013) in vitro and in MCF-7 cancer cells with considerable results in xenograft mouse model in synergism with DOX (Lv et al., 2016). In different studies including in vitro (Gou et al., 2015) or in vivo cancer models, PEG-PCL micelles prepared by self-assembly or thin layer evaporation were proposed for the treatment of ovarian (Gou et al., 2015) and breast (Zhu et al., 2015) cancer cells, breast (Liu et al., 2013), cervical (Mikhail et al., 2014), colon (Mikhail et al., 2014), and lung (Gong et al., 2013; Wang et al., 2013) xenograft mouse model, showing anti-angiogenesis properties in zebrafish (Gong et al., 2013; Wang et al., 2013). A further modification of PEG-PCL micelles by introduction of fatty acids residues (e.g. linoleic acid – LNA, palmitic acid – PAC, and oleic acid – OA) allows the obtainment of CUR delivery nanocarriers suitable for the treatment of cervical (Sahu et al., 2008a; Song et al., 2014), brain (Erfani-Moghadam et al., 2014), and lung tumors (Erfani-Moghadam et al., 2014), with superior efficiency and pharmacokinetics (Song et al., 2014). A different approach involves the employment of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] in a thin-layer evaporation methods for the preparation of CUR micelles able to treat ovarian (Abouzeid et al., 2014; Sarisozen et al., 2014) and colon (Abouzeid et al., 2013) cancer cells in vitro (Abouzeid et al., 2014) and in vivo (Abouzeid et al., 2013; Sarisozen et al., 2014), conferring synergistic effect to PTX- (Sarisozen et al., 2014) and DOX- (Abouzeid et al., 2013) based therapies.
Micelles composed of PEG-DOX conjugate were also prepared by self-assembly and proposed in a combination therapy for cervical cancer in vitro and hepatic cancer in xenograft model with favorable biodistribution and accumulation in toumors (Zhang et al., 2016).
The encapsulation of CUR into PX-based micelles was found to be highly toxic towards ovarian (Saxena & Hussain, 2013) and HeLa cancer cells (Sahu et al., 2011), while in association with RV, show a synergistic effect with DOX for the treatment of ovarian cancer and cardioprotective effects in mic (Carlson et al., 2014). The same effect was recorded by a RV-Q combination in ovarian tumors (Cote et al., 2015).
RV was proposed as therapeutic agent for breast cancer (Wang et al., 2015b), and glioblastoma (Kim et al., 2015a) treatment after encapsulation in PEG-PCL micelles bearing α-tocopheryl units (Wang et al., 2015b) or apolipoprotein obtained by recombinant DNA technique (Kim et al., 2015a).
Finally, some key literature data involve the use of micelles based on synthetic PEG-PLA (Sun et al., 2014a) or naturally occurring casein (Haratifar et al., 2014a,b) for the efficient EGCG delivery in pancreas (Sun et al., 2014a) and colon cancers in vitro (Haratifar et al., 2014a) and in vivo (Haratifar et al., 2014b).
Carbon nanostructures and nanohybrids
Carbon nanostructures are a class on nanoscaled materials showing different sizes and shapes widely explored for use in biomedicine (Bhattacharya et al., 2016). Among them, carbon nanotubes (CNT) and graphene (G) gained a significant interest in the scientific community, due to their superior physico-chemical properties, large surface area available for interaction with biologically active agents, low cost, and ability in negotiating biological barriers (Bianco et al., 2005; Feng & Liu, 2011).
CNT are condensed benzene rings composed of sp2 carbons rolled up into a tubular structure with a single (SWNT) or multiple layer (MWNT) (Lin et al., 2004; Liu et al., 2009). They undergo membrane penetration and cell internalization through a spiraling or winding motion as well as through strong interactions with various proteins (Zhang et al., 2014b). Graphene (G) is single-layered six- sp2-carbon atom organized in a two-dimensional honeycombed network (Rao et al., 2009; Wang et al., 2011), up taken by cells via direct penetration events (e.g. endocytosis) or energy-dependent pathways (Peng et al., 2010). In biomedicine, G-based delivery vehicle is mainly obtained by using the oxidized derivative of G, named graphene oxide (GO), since the presence of abundant functional groups (epoxy, hydroxyl, carboxylic groups) has led to a surge of important potential in drug loading and delivery (Makharza et al., 2013).
The suitability of CNT and GO as drug carrier in cancer therapy is related to their pharmacokinetic profiles. CNT and GO distribute quickly throughout the body regardless of the administration route, accumulate in kidney, stomach and bone (CNT) or lungs, liver, and spleen (GO), and are excreted via urine and biliary pathways (Wang et al., 2004; Chaudhuri et al., 2010; Kiew et al., 2016). Nevertheless, the toxicity concerns dramatically reduce the potential application, mainly in the case of CNT (Lam et al., 2006), due to the fiber-like structure causing inflammation, fibrosis, granulomas, and necrosis, as a consequence of strong interference with the cellular redox state (Van Berlo et al., 2014). GO show improved biocompatibility features, even if cytotoxic effects can arise from impurities produced during synthesis or generation of intracellular ROS at the edge/internal defects (Bagri et al., 2010; Ambrosi et al., 2012).
The whole of the toxicity drawbacks can be successfully overcome by covalent or non-covalent surface functionalization with biocompatible hydrophilic compounds, mainly polymeric materials, with the generation of carbon nanohybrids (Spizzirri et al., 2015b).
In the literature, several different CNT and GO delivery vehicles for improved cancer therapy are reported (Spizzirri et al., 2015a), while only few examples of polyphenol carrier are available. To this regard, an interesting approach involves the reduction of GO by polyphenols and the simultaneous establishment of π interaction between the two species (Kim et al., 2015b). The resulting nanocarrier, obtained by using tea polyphenol extract (Abdolahad et al., 2013) or RV (Gurunathan et al., 2015), show high antiproliferative activity against colon and ovarian cancer cells, respectively.
In the case of CNT, although some researches report on polyphenol nanocarriers based on pristine CNT (Cirillo et al., 2011), biologically relevant materials are obtained only when they are coated with polymeric materials, such as GL and PMAA or PCL due to the toxicity problems above discussed (Table 1). By this approach, lung and hepatic cancer can be effectively treated by loading tea polyphenols onto PCL-MWNT nanoformulation prepared by electrospinning (Shao et al., 2011).
A different approach involves the preparation of functional nanohybrids, where the polyphenol is covalently attached to the polymeric counterpart by radical reaction. More in detail, CA was conjugated to GL and the obtained polymer therapeutic employed as coating for MWNT (Cirillo et al., 2013b; Castro Nava et al., 2016), while Q was employed in a one-step radical polymerization process in the presence of MWNT and methacrylic acid (MAA) (Cirillo et al., 2013a; Vittorio et al., 2014a). This approach was found top drastically enhance the anticancer activity of both flavonoids by means of improved stability and cell uptake features on HeLa cancer cells (Cirillo et al., 2013a,b). Interestingly, CA and Q nanohybrids can be employed in combination therapy with radiotherapy on prostate cancer (Castro Nava et al., 2016), and CP chemotherapy (Vittorio et al., 2014a) on neuroblastoma, showing considerable synergistic effects in both cases.
Magnetic nanoparticles
Magnetic nanoparticles (MNPs) have shown promissory results as drug delivery nanosystems for anti-inflammatory, antitumor, or regenerative therapies. The main advantage of MNPs as carrier nanosystems is related to the possibility of being remotely directed toward a target tissue or organ by means of a magnetic field gradient (Riggio et al., 2014). Additionally, magnetic nanoparticles can be irradiated with an alternate magnetic field in the radiofrequency range to produce heat, increasing the possibility of synergic therapeutic action (Hernández et al., 2010; Asín et al., 2013). Both types of magnetic interactions (remote actuation and heating) require the MNPs to have an acceptable cytotoxic profile for in vivo applications. Moreover, a careful control of the magnetic properties such as magnetic moment and magnetic anisotropy must be provided for optimal performance. These properties are best realized when the magnetic colloids contain MNPs that are chemically stable and inert, and a narrow size distribution (Lima Jr et al., 2013).
There have been recently several attempts to use MNPs as carriers of polyphenols due to their known antitumor activity. In vitro tests using CUR conjugates as a cytotoxic agent on breast (Wani et al., 2011), glioma, and Caco-2 cells (Manju & Sreenivasan, 2011) have reported promising results, while nanoparticles prepared in the presence of PX show high anticancer activity with favorable pharmacokinetics and negligible immunogenicity and toxicity in pancreas xenograft models (Yallapu et al., 2013). MNPs were also prepared by coating with DEX–CA conjugate (Vittorio et al., 2014b) and reduction/chelation method with EGCG, and the resulting nanomaterials found to be effective on pancreas (Vittorio et al., 2014b) and colon cancer both in vitro (Vittorio et al., 2014b; Xiao et al., 2015) and in vivo (Xiao et al., 2015).
Furthermore, the in vitro cytotoxicity of Q delivered to SMMC-7721 tumor cells by Ni nanoparticles has been reported to operate on a synergistic effect between the Ni nanoparticles (increasing cell permeability) and the therapeutic Q (Guo et al., 2009). Although the magnetic properties of Ni particles were not exploited in this work, the potential uses of this property in addition to the synergistic effects described are obvious.
There are few reported works on polyphenols-delivery in vivo using nanoparticles as carriers, but it is currently accepted that the main limitations for any nanoparticle-based system such as non-specificity and short systemic circulation times also apply to polyphenol therapeutic strategies (Xiao et al., 2015).
Conclusions and perspectives
The research for novel and effective therapeutic protocols for fighting cancer is still a challenge for researchers in different fields, from chemistry, biology, and physics to nanotechnology, engineering, and medicine. An overview of the literature clearly highlights the need to work in a multidisciplinary context to reach the expected results.
In oncology, naturally derived products such as polyphenols have a long-time history of promises and encouraging preliminary results, but their real applicability is still debated, since most of the in vitro and in vivo results have been not transferred to clinic. While it is clear that a therapeutic protocol for cancer treatment cannot be exclusively based on the employment of polyphenols, they can be proposed in combination with standard gene-, chemo-, and/or radio- therapies by virtue of the strong synergism found in different tumor models. Similarly, the wide range of nanomaterials available for the development of multiple delivery vehicles able to provide effective and non-toxic cancer treatment opens new opportunities for the clinical applicability of polyphenols.
Declaration of interest
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of this article
References
- Abbad S, Waddad AY, Lv H, Zhou J. (2015). Preparation, in vitro and in vivo evaluation of Polymeric nanoparticles based on hyaluronic acid-Poly(butyl cyanoacrylate) and D-alpha-tocopheryl Polyethylene glycol 1000 succinate for tumor-targeted delivery of Morin hydrate. Int J Nanomed 10:305–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolahad M, Janmaleki M, Mohajerzadeh S, et al. (2013). Polyphenols attached graphene nanosheets for high efficiency NIR mediated photodestruction of cancer cells. Mater Sci Eng C 33:1498–505 [DOI] [PubMed] [Google Scholar]
- Abouzeid AH, Patel NR, Rachman IM, et al. (2013). Anti-cancer activity of anti-GLUT1 antibody-targeted polymeric micelles co-loaded with curcumin and doxorubicin. J Drug Target 21:994–1000 [DOI] [PubMed] [Google Scholar]
- Abouzeid AH, Patel NR, Sarisozen C, Torchilin VP. (2014). Transferrin-targeted polymeric micelles co-loaded with curcumin and paclitaxel: efficient killing of paclitaxel-resistant cancer cells. Pharm Res 31:1938–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM. (2002). Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2:750–63 [DOI] [PubMed] [Google Scholar]
- Altunbas A, Lee SJ, Rajasekaran SA, et al. (2011). Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 32:5906–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi A, Chua CK, Khezri B, et al. (2012). Chemically reduced graphene contains inherent metallic impurities present in parent natural and synthetic graphite. Proc Natl Acad Sci U S A 109:12899–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asín L, Goya GF, Tres A, Ibarra MR. (2013). Induced cell toxicity originates dendritic cell death following magnetic hyperthermia treatment. Cell Death Disease 4:e596. doi: 10.1038/cddis.2013.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi T, Tonosaki K, Fujisawa S. (2006). Induction of early apoptosis and ROS-generation activity in human gingival fibroblasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Arch Oral Biol 51:913–21 [DOI] [PubMed] [Google Scholar]
- Baccelli I, Trumpp A. (2012). The evolving concept of cancer and metastasis stem cells . J Cell Biol 198:281–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Mattevi C, Acik M, et al. (2010). Structural evolution during the reduction of chemically derived graphene oxide. Nat Chem 2:581–7 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Ravindran Girija A, Nagaoka Y, et al. (2014). Curcumin and 5-Fluorouracil-loaded, folate-and transferrin-decorated polymeric magnetic nanoformulation: a synergistic cancer therapeutic approach, accelerated by magnetic hyperthermia. Int J Nanomed 9:437–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, Mclendon RE, et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–60 [DOI] [PubMed] [Google Scholar]
- Bhattacharya K, Mukherjee SP, Gallud A, et al. (2016). Biological interactions of carbon-based nanomaterials: from coronation to degradation. Nanomed: Nanotechnol Biol Med 12:333–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A, Kostarelos K, Partidos CD, Prato M. (2005). Biomedical applications of functionalized carbon nanotubes. Chem Commun 5:571–7 [DOI] [PubMed] [Google Scholar]
- Bisht S, Feldmann G, Soni S, et al. (2007). Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol 5:3. doi: 10.1186/1477-3155-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S, Mizuma M, Feldmann G, et al. (2010). Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther 9:2255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady DC, Crowe MS, Turski ML, et al. (2014). Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509:492–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo L. (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:317–33 [DOI] [PubMed] [Google Scholar]
- Brinkhuis RP, Rutjes FPJT, Van Hest JCM. (2011). Polymeric vesicles in biomedical applications. Polym Chem 2:1449–62 [Google Scholar]
- Brož P, Benito SM, Saw C, et al. (2005). Cell targeting by a generic receptor-targeted polymer nanocontainer platform. J Control Release 102:475–88 [DOI] [PubMed] [Google Scholar]
- Cao C, Sun L, Mo W, et al. (2015). Quercetin mediates β-catenin in pancreatic cancer stem-like cells. Pancreas 44:1334–19 [DOI] [PubMed] [Google Scholar]
- Carlson LJ, Cote B, Alani AW, Rao DA. (2014). Polymeric micellar co-delivery of resveratrol and curcumin to mitigate in vitro doxorubicin-induced cardiotoxicity. J Pharm Sci 103:2315–22 [DOI] [PubMed] [Google Scholar]
- Castro Nava A, Cojoc M, Peitzsch C, et al. (2016). Development of novel radiochemotherapy approaches targeting prostate tumor progenitor cells using nanohybrids. Int J Cancer 137:2492–503 [DOI] [PubMed] [Google Scholar]
- Chang PY, Peng SF, Lee CY, et al. (2013a). Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int J Oncol 43:1141–50 [DOI] [PubMed] [Google Scholar]
- Chang WW, Hu FW, Yu CC, et al. (2013b). Quercetin in elimination of tumor initiating stem-like and mesenchymal transformation property in head and neck cancer. Head Neck 35:413–19 [DOI] [PubMed] [Google Scholar]
- Chaudhuri P, Soni S, Sengupta S. (2010). Single-walled carbon nanotube-conjugated chemotherapy exhibits increased therapeutic index in melanoma. Nanotechnology 21:025102. doi: 10.1088/0957-4484/21/2/025102 [DOI] [PubMed] [Google Scholar]
- Choi KC, Myung GJ, Lee YH, et al. (2009). Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res 69:583–92 [DOI] [PubMed] [Google Scholar]
- Choudhury SR, Balasubramanian S, Chew YC, et al. (2011). (–)-Epigallocatechin-3-gallate and DZNep reduce polycomb protein level via a proteasome-dependent mechanism in skin cancer cells. Carcinogenesis 32:1525–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Vadgama JV. (2015). Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFκB signaling. Anticancer Res 35:39–46 [PMC free article] [PubMed] [Google Scholar]
- Cirillo G, Curcio M, Vittorio O, et al. (2016). Polyphenol conjugates and human health: a perspective review. Crit Rev Food Sci Nutr 56:326–37 [DOI] [PubMed] [Google Scholar]
- Cirillo G, Hampel S, Klingeler R, et al. (2011). Antioxidant multi-walled carbon nanotubes by free radical grafting of gallic acid: new materials for biomedical applications. J Pharm Pharmacol 63:179–88 [DOI] [PubMed] [Google Scholar]
- Cirillo G, Kraemer K, Fuessel S, et al. (2010). Biological activity of a gallic acid–gelatin conjugate. Biomacromolecules 11:3309–15 [DOI] [PubMed] [Google Scholar]
- Cirillo G, Vittorio O, Hampel S, et al. (2013a). Quercetin nanocomposite as novel anticancer therapeutic: improved efficiency and reduced toxicity. Eur J Pharm Sci 49:359–65 [DOI] [PubMed] [Google Scholar]
- Cirillo G, Vittorio O, Hampel S, et al. (2013b). Incorporation of carbon nanotubes into a gelatin-catechin conjugate: innovative approach for the preparation of anticancer materials. Int J Pharm 446:176–82 [DOI] [PubMed] [Google Scholar]
- Cojoc M, Peitzsch C, Kurth I, et al. (2015). Aldehyde dehydrogenase is regulated by β-Catenin/TCF and promotes radioresistance in prostate cancer progenitor cells. Cancer Res 75:1482–94 [DOI] [PubMed] [Google Scholar]
- Cote B, Carlson LJ, Rao DA, Alani AWG. (2015). Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J Control Release 213:128–33 [DOI] [PubMed] [Google Scholar]
- Curcio M, Blanco-Fernandez B, Diaz-Gomez L, et al. (2015a). Hydrophobically modified keratin vesicles for GSH-responsive intracellular drug release. Bioconjug Chem 26:1900–7 [DOI] [PubMed] [Google Scholar]
- Curcio M, Cirillo G, Vittorio O, et al. (2015b). Hydrolyzed gelatin-based polymersomes as delivery devices of anticancer drugs. Eur Polym J 67:304–13 [Google Scholar]
- Dai J, Mumper RJ. (2010). Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhier F, Ansorena E, Silva JM, et al. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–22 [DOI] [PubMed] [Google Scholar]
- Das RK, Kasoju N, Bora U. (2010). Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed: Nanotechnol Biol Med 6:e153–60 [DOI] [PubMed] [Google Scholar]
- David KI, Jaidev LR, Sethuraman S, Krishnan UM. (2015). Dual drug loaded chitosan nanoparticles–sugar-coated arsenal against pancreatic cancer. Colloids Surf B: Biointerfaces 135:689–98 [DOI] [PubMed] [Google Scholar]
- Deb G, Thakur VS, Limaye AM, Gupta S. (2015). Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol Carcinog 54:485–99 [DOI] [PubMed] [Google Scholar]
- Deepa G, Thulasidasan AKT, Anto RJ, et al. (2012). Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy. Int J Nanomed 7:4077–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio D, Rodriguez-Mateos A, Spencer JPE, et al. (2013). Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18:1818–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Ambattu LA, Hari PR, et al. (2015). Glutathione-bearing fluorescent polymer–curcumin conjugate enables simultaneous drug delivery and label-free cellular imaging. Polymer (United Kingdom) 75:25–33 [Google Scholar]
- Diehn M, Cho RW, Lobo NA, et al. (2009). Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458:780–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher BM, Hammer DA, Bates FS, Discher DE. (2000). Polymer vesicles in various media. Curr Opin Colloid Interface Sci 5:125–31 [Google Scholar]
- Dong H, Tang M, Li Y, et al. (2015). Disulfide-bridged cleavable PEGylation in polymeric nanomedicine for controlled therapeutic delivery. Nanomedicine 10:1941–58 [DOI] [PubMed] [Google Scholar]
- Duan J, Mansour HM, Zhang Y, et al. (2012). Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm 426:193–201 [DOI] [PubMed] [Google Scholar]
- Duan J, Zhang Y, Han S, et al. (2010). Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm 400:211–20 [DOI] [PubMed] [Google Scholar]
- Eetezadi S, Ekdawi SN, Allen C. (2015). The challenges facing block copolymer micelles for cancer therapy: in vivo barriers and clinical translation. Adv Drug Deliv Rev 91:7–22 [DOI] [PubMed] [Google Scholar]
- Elbling L, Weiss RM, Teufelhofer O, et al. (2005). Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J 19:807–9 [DOI] [PubMed] [Google Scholar]
- Erfani-Moghadam V, Nomani A, Zamani M, et al. (2014). A novel diblock copolymer of (monomethoxy poly [ethylene glycol]-oleate) with a small hydrophobic fraction to make stable micelles/polymersomes for curcumin delivery to cancer cells. Int J Nanomed 9:5541–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estanqueiro M, Amaral MH, Conceição J, Sousa Lobo JM. (2015). Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B: Biointerfaces 126:631–48 [DOI] [PubMed] [Google Scholar]
- Fan P, Fan S, Wang H, et al. (2013). Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res Therapy 4:146. doi: 10.1186/scrt357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang MZ, Wang Y, Ai N, et al. (2003). Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 63:7563–70 [PubMed] [Google Scholar]
- Feng L, Liu Z. (2011). Graphene in biomedicine: opportunities and challenges. Nanomedicine (Lond) 6:317–24 [DOI] [PubMed] [Google Scholar]
- Fernandes E, Ferreira JA, Andreia P, et al. (2015). New trends in guided nanotherapies for digestive cancers: a systematic review. J Control Release 209:288–307 [DOI] [PubMed] [Google Scholar]
- Fresco P, Borges F, Diniz C, Marques MPM. (2006). New insights on the anticancer properties of dietary polyphenols. Med Res Rev 26:747–66 [DOI] [PubMed] [Google Scholar]
- Furfaro AL, Piras S, Domenicotti C, et al. (2016). Role of Nrf2, HO-1 and GSH in neuroblastoma cell resistance to Bortezomib. PLoS One 11:e0152465. doi: 10.1371/journal.pone.0152465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati G, O'brien PJ. (2004). Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med 37:287–303 [DOI] [PubMed] [Google Scholar]
- Gao Y, Tollefsbol TO. (2015). Impact of epigenetic dietary components on cancer through histone modifications. Curr Med Chem 22:2051–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure KM, Wu SY, Li C, et al. (2015). Nanotechnology: future of oncotherapy. Clin Cancer Res 21:3121–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Deng S, Wu Q, et al. (2013). Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 34:1413–32 [DOI] [PubMed] [Google Scholar]
- Guo D, Wu C, Li J, et al. (2009). Synergistic effect of functionalized nickel nanoparticles and quercetin on inhibition of the SMMC-7721 cells proliferation. Nanoscale Res Lett 4:1395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Q, Liu L, Wang C, et al. (2015). Polymeric nanoassemblies entrapping curcumin overcome multidrug resistance in ovarian cancer. Colloids Surf B: Biointerfaces 126:26–34 [DOI] [PubMed] [Google Scholar]
- Guo L, Peng Y, Li Y, et al. (2015). Cell death pathway induced by resveratrol-bovine serum albumin nanoparticles in a human ovarian cell line. Oncol Lett 9:1359–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Li A, Jia Z, et al. (2013). Transferrin modified PEG-PLA-resveratrol conjugates: in vitro and in vivo studies for glioma. Eur J Pharmacol 718:41–7 [DOI] [PubMed] [Google Scholar]
- Gupte A, Mumper RJ. (2009). Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 35:32–46 [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Han JW, Kim ES, et al. (2015). Reduction of graphene oxide by resveratrol: a novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int J Nanomed 10:2951–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hail N Jr, Cortes M, Drake EN, Spallholz JE. (2008). Cancer chemoprevention: a radical perspective. Free Radic Biol Med 45:97–110 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell 144:646–74 [DOI] [PubMed] [Google Scholar]
- Haratifar S, Meckling KA, Corredig M. (2014a). Antiproliferative activity of tea catechins associated with casein micelles, using HT29 colon cancer cells. J Dairy Sci 97:672–8 [DOI] [PubMed] [Google Scholar]
- Haratifar S, Meckling KA, Corredig M. (2014b). Bioefficacy of tea catechins encapsulated in casein micelles tested on a normal mouse cell line (4D/WT) and its cancerous counterpart (D/v-src) before and after in vitro digestion. Food Funct 5:1160–6 [DOI] [PubMed] [Google Scholar]
- Hernández R, Sacristán J, Asín L, et al. (2010). Magnetic hydrogels derived from polysaccharides with improved specific power absorption: potential devices for remotely triggered drug delivery. J Phys Chem B 114:12002–7 [DOI] [PubMed] [Google Scholar]
- Hou Z, Sang S, You H, et al. (2005). Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res 65:8049–56 [DOI] [PubMed] [Google Scholar]
- Howes RM. (2006). The free radical fantasy: a panoply of paradoxes. Ann N Y Acad Sci 1067:22–6 [DOI] [PubMed] [Google Scholar]
- Hu B, Wang Y, Xie M, et al. (2015). Polymer nanoparticles composed with gallic acid grafted chitosan and bioactive peptides combined antioxidant, anticancer activities and improved delivery property for labile polyphenols. J Funct Foods 15:593–603 [Google Scholar]
- Hu B, Xie M, Zhang C, Zeng X. (2014). Genipin-structured peptide – polysaccharide nanoparticles with significantly improved resistance to harsh gastrointestinal environments and their potential for oral delivery of polyphenols. J Agric Food Chem 62:12443–52 [DOI] [PubMed] [Google Scholar]
- Hu CMJ, Aryal S, Zhang L. (2010). Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv 1:323–34 [DOI] [PubMed] [Google Scholar]
- Huang W, Wan C, Luo Q, Huang Z. (2014). Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int J Mol Sci 15:3432–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YT, Lin YW, Chiu HM, Chiang BH. (2016). Curcumin induces apoptosis of colorectal cancer stem cells by coupling with CD44 marker. J Agric Food Chem 64:2247–53 [DOI] [PubMed] [Google Scholar]
- Jain AK, Thanki K, Jain S. (2013). Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol Pharm 10:3459–74 [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Menon D, Manzoor K, et al. (2010). Biomedical applications of chitin and chitosan based nanomaterials – a short review. Carbohydr Polym 82:227–32 [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. (2010). Cancer statistics, 2010. CA Cancer J Clin 60:277–300 [DOI] [PubMed] [Google Scholar]
- Johnstone TC, Suntharalingam K, Lippard SJ. (2016). The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev 116:3436–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarala M, Brenner DE, Korkaya H, et al. (2010). Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122:777–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan S, Hoti SL, Prasad NR. (2015). Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed Pharmacotherapy 70:274–82 [DOI] [PubMed] [Google Scholar]
- Kaur S, Greaves P, Cooke DN, et al. (2007). Breast cancer prevention by green tea catechins and black tea theaflavins in the C3(1) SV40 T,t antigen transgenic mouse model is accompanied by increased apoptosis and a decrease in oxidative DNA adducts. J Agric Food Chem 55:3378–85 [DOI] [PubMed] [Google Scholar]
- Kawanishi S, Oikawa S, Murata M. (2005). Evaluation for safety of antioxidant chemopreventive agents. Antioxid Redox Signal 7:1728–39 [DOI] [PubMed] [Google Scholar]
- Khan MA, Hussain A, Sundaram MK, et al. (2015). (−)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol Rep 33:1976–84 [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, et al. (2006). Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res 66:2500–5 [DOI] [PubMed] [Google Scholar]
- Khan N, Bharali DJ, Adhami VM, et al. (2014). Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 35:415–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Mukhtar H. (2015). Dietary agents for prevention and treatment of lung cancer. Cancer Lett 359:155–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiew SF, Kiew LV, Lee HB, et al. (2016). Assessing biocompatibility of graphene oxide-based nanocarriers: a review. J Control Release 226:217–28 [DOI] [PubMed] [Google Scholar]
- Kim BYS, Rutka JT, Chan WCW. (2010). Current concepts: nanomedicine . N Engl J Med 363:2434–43 [DOI] [PubMed] [Google Scholar]
- Kim SH, Adhikari BB, Cruz S, et al. (2015a). Targeted intracellular delivery of resveratrol to glioblastoma cells using apolipoprotein E-containing reconstituted HDL as a nanovehicle. PLoS One 10:e0135130. doi: 10.1371/journal.pone.0135130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee JM, Kumer RA, et al. (2015b). Environmentally friendly synthesis of P-doped reduced graphene oxide with high dispersion stability by using red table wine. Chem – Asian J 10:1192–7 [DOI] [PubMed] [Google Scholar]
- Kim SO, Kim MR. (2013). (−)-Epigallocatechin 3-gallate inhibits invasion by inducing the expression of Raf kinase inhibitor protein in AsPC-1 human pancreatic adenocarcinoma cells through the modulation of histone deacetylase activity. Int J Oncol 42:349–58 [DOI] [PubMed] [Google Scholar]
- Kim TH, Jiang HH, Youn YS, et al. (2011). Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm 403:285–91 [DOI] [PubMed] [Google Scholar]
- Kim YJ, Park MR, Kim MS, Kwon OH. (2012). Polyphenol-loaded polycaprolactone nanofibers for effective growth inhibition of human cancer cells. Mater Chem Phys 133:674–80 [Google Scholar]
- Kobuchi H, Roy S, Sen CK, et al. (1999). Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am J Physiol – Cell Physiol 277:C403–11 [DOI] [PubMed] [Google Scholar]
- Kong ANT, Yu R, Chen C, et al. (2000). Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch Pharmacal Res 23:1–16 [DOI] [PubMed] [Google Scholar]
- Kresty LA, Frankel WL, Hammond CD, et al. (2006). Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer 54:148–56 [DOI] [PubMed] [Google Scholar]
- Kumar M, Grzelakowski M, Zilles J, et al. (2007). Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc Natl Acad Sci U S A 104:20719–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Lather V, Pandita D. (2016). A facile green approach to prepare core–shell hybrid PLGA nanoparticles for resveratrol delivery. Int J Biol Macromol 84:380–4 [DOI] [PubMed] [Google Scholar]
- Kumari P, Ghosh B, Biswas S. (2016). Nanocarriers for cancer-targeted drug delivery. J Drug Target 24:179–91 [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, Mccluskey R, et al. (2006). A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol 36:189–217 [DOI] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ. (2010). The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HA, Park S, Kim Y. (2013a). Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells. Oncol Rep 30:1869–77 [DOI] [PubMed] [Google Scholar]
- Lee SH, Nam HJ, Kang HJ, et al. (2013b). Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur J Cancer 49:3210–18 [DOI] [PubMed] [Google Scholar]
- Lee WJ, Shim JY, Zhu BT. (2005). Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol 68:1018–30 [DOI] [PubMed] [Google Scholar]
- León-González AJ, Auger C, Schini-Kerth VB. (2015). Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem Pharmacol 98:371–80 [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Yang C, et al. (2009). Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol Pharmacol 76:81–90 [DOI] [PubMed] [Google Scholar]
- Li L, Xiang D, Shigdar S, et al. (2014). Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int J Nanomed 9:1083–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Fu J, Watkins DN, et al. (2013). Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem 373:217–27 [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Korkaya H, et al. (2010). Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 16:2580–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Wu SL, Lu SM, et al. (2015). (−)-Epigallocatechin-3-gallate inhibits nasopharyngeal cancer stem cell self-renewal and migration and reverses the epithelial–mesenchymal transition via NF-κB p65 inactivation. Tumor Biol 36:2747–61 [DOI] [PubMed] [Google Scholar]
- Liang J, Li F, Fang Y, et al. (2011). Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles. Colloids Surf B: Biointerfaces 82:297–301 [DOI] [PubMed] [Google Scholar]
- Liang J, Li F, Fang Y, et al. (2014). Cytotoxicity and apoptotic effects of tea polyphenol-loaded chitosan nanoparticles on human hepatoma HepG2 cells. Mater Sci Eng C 36:7–13 [DOI] [PubMed] [Google Scholar]
- Liang K, Ng S, Lee F, et al. (2016). Targeted intracellular protein delivery based on hyaluronic acid-green tea catechin nanogels. Acta Biomater 33:142–52 [DOI] [PubMed] [Google Scholar]
- Ligeret H, Barthelemy S, Zini R, et al. (2004). Effects of curcumin and curcumin derivatives on mitochondrial permeability transition pore. Free Radic Biol Med 36:919–29 [DOI] [PubMed] [Google Scholar]
- Lim JY, Kim YS, Kim KM, et al. (2014). β-Carotene inhibits neuroblastoma tumorigenesis by regulating cell differentiation and cancer cell stemness. Biochem Biophys Res Commun 450:1475–80 [DOI] [PubMed] [Google Scholar]
- Lim KJ, Bisht S, Bar EE, et al. (2011). A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther 11:464–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima E Jr, Torres TE, Rossi LM, et al. (2013). Size dependence of the magnetic relaxation and specific power absorption in iron oxide nanoparticles. J Nanopart Res 15:1654. doi: 10.1007/s11051-013-1654-x [DOI] [Google Scholar]
- Lin CH, Chao LK, Hung PH, Chen YJ. (2014). EGCG inhibits the growth and tumorigenicity of nasopharyngeal tumor-initiating cells through attenuation of STAT3 activation. Int J Clin Exp Pathol 7:2372–81 [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Taylor S, Li H, et al. (2004). Advances toward bioapplications of carbon nanotubes. J Mater Chem 14:527–41 [Google Scholar]
- Lin YH, Chen ZR, Lai CH, et al. (2015). Active targeted nanoparticles for oral administration of gastric cancer therapy. Biomacromolecules 16:3021–32 [DOI] [PubMed] [Google Scholar]
- Liu CM, Peng CY, Liao YW, et al. (2015). Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J Formosan Med Assoc. [Epub ahead of print]. doi: 10.1016/j.jfma.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Liu L, Sun L, Wu Q, et al. (2013). Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int J Pharm 443:175–82 [DOI] [PubMed] [Google Scholar]
- Liu Z, Tabakman S, Welsher K, Dai H. (2009). Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res 2:85–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Park K. (2013). Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm 453:198–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. (2009). Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136:823–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Prestwich GD. (2002). Cancer-targeted polymeric drugs. Curr Cancer Drug Targets 2:209–26 [DOI] [PubMed] [Google Scholar]
- Lv L, Guo Y, Shen Y, et al. (2015). Intracellularly degradable, self-assembled amphiphilic block copolycurcumin nanoparticles for efficient in vivo cancer chemotherapy. Adv Healthcare Mater 4:1496–501 [DOI] [PubMed] [Google Scholar]
- Lv L, Qiu K, Yu X, et al. (2016). Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer. J Biomed Nanotechnol 12:973–85 [DOI] [PubMed] [Google Scholar]
- Lv L, Shen Y, Li M, et al. (2013). Novel 4-arm poly(ethylene glycol)-block-poly(anhydride-esters) amphiphilic copolymer micelles loading curcumin: preparation, characterization, and in vitro evaluation. BioMed Res Int 2013:507103. doi: 10.1155/2013/507103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudana Rao K, Krishna Rao KS, Ramanjaneyulu G, Ha CS. (2015). Curcumin encapsulated pH sensitive gelatin based interpenetrating polymeric network nanogels for anti cancer drug delivery. Int J Pharm 478:788–95 [DOI] [PubMed] [Google Scholar]
- Makharza S, Cirillo G, Bachmatiuk A, et al. (2013). Graphene oxide-based drug delivery vehicles: functionalization, characterization, and cytotoxicity evaluation. J Nanopart Res 15:2099. doi: 10.1007/s11051-013-2099-y [DOI] [Google Scholar]
- Mangalathillam S, Rejinold NS, Nair A, et al. (2012). Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 4:239–50 [DOI] [PubMed] [Google Scholar]
- Manju S, Sreenivasan K. (2011). Enhanced drug loading on magnetic nanoparticles by layer-by-layer assembly using drug conjugates: blood compatibility evaluation and targeted drug delivery in cancer cells. Langmuir 27:14489–96 [DOI] [PubMed] [Google Scholar]
- Meeran SM, Patel SN, Li Y, et al. (2012). Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS One 7:e37748. doi: 10.1371/journal.pone.0037748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhail AS, Eetezadi S, Ekdawi SN, et al. (2014). Image-based analysis of the size-and time-dependent penetration of polymeric micelles in multicellular tumor spheroids and tumor xenografts. Int J Pharm 464:168–77 [DOI] [PubMed] [Google Scholar]
- Minko T, Kopečková P, Pozharov V, Kopeček J. (1998). HPMA copolymer bound adriamycin overcomes MDR1 gene encoded resistance in a human ovarian carcinoma cell line. J Control Release 54:223–33 [DOI] [PubMed] [Google Scholar]
- Mirakabad FST, Akbarzadeh A, Milani M, et al. (2016). A comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif Cells Nanomed Biotechnol 44:423–30 [DOI] [PubMed] [Google Scholar]
- Mishra B, Priyadarsini KI, Bhide MK, et al. (2004). Reactions of superoxide radicals with curcumin: probable mechanisms by optical spectroscopy and EPR. Free Radic Res 38:355–62 [DOI] [PubMed] [Google Scholar]
- Mukerjee A, Vishwanatha JK. (2009). Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res 29:3867–75 [PubMed] [Google Scholar]
- Nair KL, Thulasidasan AKT, Deepa G, et al. (2012). Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm 425:44–52 [DOI] [PubMed] [Google Scholar]
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH. (2006). A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr 46:161–83 [DOI] [PubMed] [Google Scholar]
- Numsen H. Jr (2008). Mitochondrial reactive oxygen species affect sensitivity to curcumin-induced apoptosis. Free Radic Biol Med 44:1382–93 [DOI] [PubMed] [Google Scholar]
- Nyanhongo GS, Nugroho Prasetyo E, Herrero Acero E, Guebitz GM. (2012). Engineering strategies for successful development of functional polymers using oxidative enzymes. Chem Eng Technol 35:1359–72 [Google Scholar]
- Oliver S, Vittorio O, Cirillo G, Boyer C. (2016). Enhancing the therapeutic effects of polyphenols with macromolecules. Polym Chem 7:1529–44 [Google Scholar]
- Onaca O, Enea R, Hughes DW, Meier W. (2009). Stimuli-responsive polymersomes as nanocarriers for drug and gene delivery. Macromol Biosci 9:129–39 [DOI] [PubMed] [Google Scholar]
- Ortega AL, Mena S, Estrela JM. (2011). Glutathione in cancer cell death. Cancers 3:1285–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacardo DB, Ligler FS, Gu Z. (2015). Programmable nanomedicine: synergistic and sequential drug delivery systems. Nanoscale 7:3381–91 [DOI] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM. (2012). Flavonoids in cancer prevention. Anti-Cancer Agents Med Chem 12:836–51 [DOI] [PubMed] [Google Scholar]
- Peitzsch C, Cojoc M, Hein L, et al. (2016). An epigenetic reprograming strategy to resensitize radioresistant prostate cancer cells. Cancer Res 76:2637–51 [DOI] [PubMed] [Google Scholar]
- Peng C, Hu W, Zhou Y, et al. (2010). Intracellular imaging with a graphene-based fluorescent probe. Small 6:1686–92 [DOI] [PubMed] [Google Scholar]
- Peng SF, Lee CY, Hour MJ, et al. (2014). Curcumin-loaded nanoparticles enhance apoptotic cell death of U2OS human osteosarcoma cells through the Akt-Bad signaling pathway. Int J Oncol 44:238–46 [DOI] [PubMed] [Google Scholar]
- Pillai JJ, Thulasidasan AKT, Anto RJ, et al. (2014). Folic acid conjugated cross-linked acrylic polymer (FA-CLAP) hydrogel for site specific delivery of hydrophobic drugs to cancer cells. J Nanobiotechnol 12:25. doi: 10.1186/1477-3155-12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podaralla S, Averineni R, Alqahtani M, Perumal O. (2012). Synthesis of novel biodegradable methoxy poly(ethylene glycol)-zein micelles for effective delivery of curcumin. Mol Pharm 9:2778–86 [DOI] [PubMed] [Google Scholar]
- Polster J, Dithmar H, Feucht W. (2003). Are histones the targets for flavan-3-ols (catechins) in nuclei? Biol Chem 384:997–1006 [DOI] [PubMed] [Google Scholar]
- Popat A, Karmakar S, Jambhrunkar S, et al. (2014). Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity. Colloids Surf B: Biointerfaces 117:520–7 [DOI] [PubMed] [Google Scholar]
- Prajakta D, Ratnesh J, Chandan K, et al. (2009). Curcumin loaded pH-sensitive nanoparticles for the treatment of colon cancer. J Biomed Nanotechnol 5:445–55 [DOI] [PubMed] [Google Scholar]
- Procházková D, Boušová I, Wilhelmová N. (2011). Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82:513–23 [DOI] [PubMed] [Google Scholar]
- Punfa W, Yodkeeree S, Pitchakarn P, et al. (2012). Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin 33:823–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoci F, Morelli C, Cirillo G, et al. (2012). Anticancer activity of a quercetin-based polymer towards HeLa cancer cells. Anticancer Res 32:2843–7 [PubMed] [Google Scholar]
- Pérez-Herrero E, Fernández-Medarde A. (2015). Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 93:52–79 [DOI] [PubMed] [Google Scholar]
- Rao CNR, Sood AK, Subrahmanyam KS, Govindaraj A. (2009). Graphene: the new two-dimensional nanomaterial. Angew Chem – Int Ed 48:7752–77 [DOI] [PubMed] [Google Scholar]
- Ravichandran S, Nagarajan S, Ku BC, et al. (2012). Halogen-free ultra-high flame retardant polymers through enzyme catalysis. Green Chem 14:819–24 [Google Scholar]
- Ray L, Kumar P, Gupta KC. (2013). The activity against Ehrlich's ascites tumors of doxorubicin contained in self assembled, cell receptor targeted nanoparticle with simultaneous oral delivery of the green tea polyphenol epigallocatechin-3-gallate. Biomaterials 34:3064–76 [DOI] [PubMed] [Google Scholar]
- Riggio C, Calatayud MP, Giannaccini M, et al. (2014). The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field. Nanomed: Nanotechnol Biol Med 10:1549–58 [DOI] [PubMed] [Google Scholar]
- Říhová B, Strohalm J, Prausová J, et al. (2003). Cytostatic and immunomobilizing activities of polymer-bound drugs: experimental and first clinical data. J Control Release 91:1–16 [DOI] [PubMed] [Google Scholar]
- Ringsdorf H. (1975). Structure and properties of pharmacologically active polymers. J Polym Sci Polym Symp 51:135–53 [Google Scholar]
- Rodova M, Fu J, Watkins DN, et al. (2012). Sonic Hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS One 7:e46083. doi: 10.1371/journal.pone.0046083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavy A, Raisch KP, Mantena S, et al. (2007). Design and development of water-soluble curcumin conjugates as potential anticancer agents. J Med Chem 50:6284–8 [DOI] [PubMed] [Google Scholar]
- Sahu A, Bora U, Kasoju N, Goswami P. (2008a). Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater 4:1752–61 [DOI] [PubMed] [Google Scholar]
- Sahu A, Kasoju N, Bora U. (2008b). Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 9:2905–12 [DOI] [PubMed] [Google Scholar]
- Sahu A, Kasoju N, Goswami P, Bora U. (2011). Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J Biomater Appl 25:619–39 [DOI] [PubMed] [Google Scholar]
- Sanna V, Pintus G, Roggio AM, et al. (2011). Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem 54:1321–32 [DOI] [PubMed] [Google Scholar]
- Sanna V, Siddiqui IA, Sechi M, Mukhtar H. (2013). Resveratrol-loaded nanoparticles based on poly(epsiloncaprolactone) and poly(d,l-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol Pharm 10:3871–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanoj Rejinold N, Muthunarayanan M, Chennazhi KP, et al. (2011). Curcumin loaded fibrinogen nanoparticles for cancer drug delivery. J Biomed Nanotechnol 7:521–34 [DOI] [PubMed] [Google Scholar]
- Sarisozen C, Abouzeid AH, Torchilin VP. (2014). The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm Biopharm 88:539–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V, Hussain MD. (2013). Polymeric mixed micelles for delivery of curcumin to multidrug resistant ovarian cancer. J Biomed Nanotechnol 9:1146–54 [DOI] [PubMed] [Google Scholar]
- Schroeter H, Spencer JPE, Rice-Evans C, Williams RJ. (2001). Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J 358:547–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DW, Loo G. (2004). Curcumin-induced GADD153 gene up-regulation in human colon cancer cells. Carcinogenesis 25:2155–64 [DOI] [PubMed] [Google Scholar]
- Shao S, Li L, Yang G, et al. (2011). Controlled green tea polyphenols release from electrospun PCL/MWCNTs composite nanofibers. Int J Pharm 421:310–20 [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, et al. (2005). Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J Mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res 22:1805–20 [DOI] [PubMed] [Google Scholar]
- Shen L, Ji HF. (2007). Theoretical study on physicochemical properties of curcumin. Spectrochim Acta A Mol Biomol Spectrosc 67:619–23 [DOI] [PubMed] [Google Scholar]
- Shirode AB, Bharali DJ, Nallanthighal S, et al. (2015). Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int J Nanomed 10:475–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpaisman N, Sheihet L, Bushman J, et al. (2012). One-step synthesis of biodegradable curcumin-derived hydrogels as potential soft tissue fillers after breast cancer surgery. Biomacromolecules 13:2279–86 [DOI] [PubMed] [Google Scholar]
- Shutava TG, Balkundi SS, Vangala P, et al. (2009). Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 3:1877–85 [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Adhami VM, Bharali DJ, et al. (2009). Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res 69:1712–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui IA, Adhami VM, Chamcheu CJ, Mukhtar H. (2012). Impact of nanotechnology in cancer: emphasis on nanochemoprevention. Int J Nanomed 7:591–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui IA, Bharali DJ, Nihal M, et al. (2014). Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomed: Nanotechnol Biol Med 10:1619–26 [DOI] [PubMed] [Google Scholar]
- Singh M, Bhatnagar P, Mishra S, et al. (2015). PLGA-encapsulated tea polyphenols enhance the chemotherapeutic efficacy of cisplatin against human cancer cells and mice bearing Ehrlich ascites carcinoma. Int J Nanomed 10:6789–809 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Singh M, Bhatnagar P, Srivastava AK, et al. (2011). Enhancement of cancer chemosensitization potential of cisplatin by tea polyphenols poly(lactide-co-glycolide) nanoparticles. J Biomed Nanotechnol 7:202. doi: 10.1166/jbn.2011.1268 [DOI] [PubMed] [Google Scholar]
- Sirova M, Kabesova M, Kovar L, et al. (2013). HPMA copolymer-bound doxorubicin induces immunogenic tumor cell death. Curr Med Chem 20:4815–26 [DOI] [PubMed] [Google Scholar]
- Sobczak M, Debek C, Oledzka E, Kozłowski R. (2013). Polymeric systems of antimicrobial peptides-strategies and potential applications. Molecules 18:14122–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Zhu W, Liu N, et al. (2014). Linolenic acid-modified PEG-PCL micelles for curcumin delivery. Int J Pharm 471:312–21 [DOI] [PubMed] [Google Scholar]
- Sparmann A, Van Lohuizen M. (2006). Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6:846–56 [DOI] [PubMed] [Google Scholar]
- Spencer JPE, Schroeter H, Crossthwaithe AJ, et al. (2001). Contrasting influences of glucuronidation and O-methylation of epicatechin on hydrogen peroxide-induced cell death in neurons and fibroblasts. Free Radic Biol Med 31:1139–46 [DOI] [PubMed] [Google Scholar]
- Spizzirri UG, Cirillo G, Picci N, Iemma F. (2014). Recent development in the synthesis of eco-friendly polymeric antioxidants. Curr Org Chem 18:2912–27 [Google Scholar]
- Spizzirri UG, Curcio M, Cirillo G, et al. (2015a). Recent advances in the synthesis and biomedical applications of nanocomposite hydrogels. Pharmaceutics 7:413–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizzirri UG, Hampel S, Cirillo G, et al. (2015b). Functional gelatin–carbon nanotubes nanohybrids with enhanced antibacterial activity. Int J Polym Mater Polym Biomater 64:439–47 [Google Scholar]
- Stylianopoulos T, Jain RK. (2015). Design considerations for nanotherapeutics in oncology. Nanomed: Nanotechnol Biol Med 11:1893–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CC, Lin JG, Li TM, et al. (2006). Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res 26:4379–89 [PubMed] [Google Scholar]
- Su J, Chen F, Cryns VL, Messersmith PB. (2011). Catechol polymers for pH-responsive, targeted drug delivery to cancer cells. J Am Chem Soc 133:11850–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhang C, Li P. (2014a). Copolymeric micelles for delivery of EGCG and cyclopamine to pancreatic cancer cells. Nutr Cancer 66:896–903 [DOI] [PubMed] [Google Scholar]
- Sun Y, Du L, Liu Y, et al. (2014b). Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-β-cyclodextrin for melanoma treatment. Int J Pharm 469:31–9 [DOI] [PubMed] [Google Scholar]
- Tang SN, Singh C, Nall D, et al. (2010). The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal 5:14. doi: 10.1186/1750-2187-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teong B, Lin CY, Chang SJ, et al. (2015). Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J Mater Sci: Mater Med 26:5357. doi: 10.1007/s10856-014-5357-3 [DOI] [PubMed] [Google Scholar]
- Thakur VS, Gupta K, Gupta S. (2012). Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int J Oncol 41:353–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toden S, Tran HM, Tovar-Camargo OA, et al. (2016). Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer Oncotarget 7:16158–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong R, Cheng J. (2007). Anticancer polymeric nanomedicines. Polym Rev 47:345–81 [Google Scholar]
- Torchilin VP. (2007). Micellar nanocarriers: pharmaceutical perspectives. Pharm Res 24:1–16 [DOI] [PubMed] [Google Scholar]
- Tsouris V, Joo MK, Kim SH, et al. (2014). Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv 32:1037–50 [DOI] [PubMed] [Google Scholar]
- Van Berlo D, Wilhelmi V, Boots AW, et al. (2014). Apoptotic, inflammatory, and fibrogenic effects of two different types of multi-walled carbon nanotubes in mouse lung. Arch Toxicol 88:1725–37 [DOI] [PubMed] [Google Scholar]
- Verderio P, Bonetti P, Colombo M, et al. (2013). Intracellular drug release from curcumin-loaded PLGA nanoparticles induces G2/M block in breast cancer cells. Biomacromolecules 14:672–82 [DOI] [PubMed] [Google Scholar]
- Vittorio O, Brandl M, Cirillo G, et al. (2016a). Dextran–catechin: an anticancer chemically-modified natural compound targeting copper that attenuates neuroblastoma growth. Oncotarget 7:47479–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittorio O, Brandl M, Cirillo G, et al. (2014a). Novel functional cisplatin carrier based on carbon nanotubes-quercetin nanohybrid induces synergistic anticancer activity against neuroblastoma in vitro. RSC Adv 4:31378–84 [Google Scholar]
- Vittorio O, Cirillo G, Iemma F, et al. (2012). Dextran-catechin conjugate: a potential treatment against the pancreatic ductal adenocarcinoma. Pharm Res 29:2601–14 [DOI] [PubMed] [Google Scholar]
- Vittorio O, Cojoc M, Curcio M, et al. (2016b). Polyphenol conjugates by immobilized laccase: the green synthesis of dextran-catechin. Macromol Chem Phys 217:1488–92 [Google Scholar]
- Vittorio O, Voliani V, Faraci P, et al. (2014b). Magnetic catechin-dextran conjugate as targeted therapeutic for pancreatic tumour cells. J Drug Target 22:408–15 [DOI] [PubMed] [Google Scholar]
- Vyas AR, Moura MB, Hahm ER, et al. (2016). Sulforaphane inhibits c-Myc-mediated prostate cancer stem-like traits. J Cell Biochem. 117:2482–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AZ, Langer R, Farokhzad OC. (2012a). Nanoparticle delivery of cancer drugs. Annu Rev Med 63:185–98 [DOI] [PubMed] [Google Scholar]
- Wang BL, Shen YM, Zhang QW, et al. (2013). Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int J Nanomed 8:3521–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang JJ, To TSS, et al. (2015a). Role of SIRT1-mediated mitochondrial and Akt pathways in glioblastoma cell death induced by Cotinus coggygria flavonoid nanoliposomes. Int J Nanomed 10:5005–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang J, Deng X, et al. (2004). Biodistribution of carbon single-wall carbon nanotubes in mice. J Nanosci Nanotechnol 4:1019–24 [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang T, Liu L, et al. (2012b). Novel micelle formulation of curcumin for enhancing antitumor activity and inhibiting colorectal cancer stem cells. Int J Nanomed 7:4487–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Sampliner RE. (2008). Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus . Am J Gastroenterol 103:788–97 [DOI] [PubMed] [Google Scholar]
- Wang S, Chen R, Morott J, et al. (2015b). MPEG-b-PCL/TPGS mixed micelles for delivery of resveratrol in overcoming resistant breast cancer. Expert Opin Drug Deliv 12:361–73 [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang L, Le Y, et al. (2016). Synergistic effect of PEGylated resveratrol on delivery of anticancer drugs. Int J Pharm 498:134–41 [DOI] [PubMed] [Google Scholar]
- Wang X, Chen Y, Dahmani FZ, et al. (2014). Amphiphilic carboxymethyl chitosan–quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 35:7654–65 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Z, Wang J, et al. (2011). Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol 29:205–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani KD, Kitture R, Ahmed A, et al. (2011). Synthesis, characterization and in vitro study of curcumin-functionalized citric acid-capped magnetic (CCF) nanoparticles as drug delivery agents in cancer. J Bionanosci 5:59–65 [Google Scholar]
- Williams RJ, Spencer JPE, Rice-Evans C. (2004). Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med 36:838–49 [DOI] [PubMed] [Google Scholar]
- Wu DS, Shen JZ, Yu AF, et al. (2013). Epigallocatechin-3-gallate and trichostatin A synergistically inhibit human lymphoma cell proliferation through epigenetic modification of p16 INK4a. Oncol Rep 30:2969–75 [DOI] [PubMed] [Google Scholar]
- Wu L, Guo L, Liang Y, et al. (2015). Curcumin suppresses stem-like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncol Rep 34:3311–17 [DOI] [PubMed] [Google Scholar]
- Xiao L, Mertens M, Wortmann L, et al. (2015). Enhanced in vitro and in vivo cellular imaging with green tea coated water-soluble iron oxide nanocrystals. ACS Appl Mater Interfaces 7:6530–40 [DOI] [PubMed] [Google Scholar]
- Yallapu MM, Dobberpuhl MR, Maher DM, et al. (2012). Design of curcumin loaded cellulose nanoparticles for prostate cancer. Curr Drug Metab 13:120–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu MM, Ebeling MC, Khan S, et al. (2013). Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol Cancer Ther 12:1471–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. (2010a). Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci 351:19–29 [DOI] [PubMed] [Google Scholar]
- Yallapu MM, Khan S, Maher DM, et al. (2014). Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 35:8635–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu MM, Maher DM, Sundram V, et al. (2010b). Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J Ovarian Res 3:11. doi: 10.1186/1757-2215-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Jiang YM, Shi J, et al. (2004). Flavonoids in food and their health benefits. Plant Foods Hum Nutr 59:113–22 [DOI] [PubMed] [Google Scholar]
- Yoo CB, Jones PA. (2006). Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5:37–50 [DOI] [PubMed] [Google Scholar]
- Zahnow CA, Topper M, Stone M, et al. (2016). Inhibitors of DNA methylation, histone deacetylation, and histone demethylation: a perfect combination for cancer therapy. Adv Cancer Res 130:55–111 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li L, Jiao M, et al. (2012a). Genistein inhibits the stemness properties of prostate cancer cells through targeting Hedgehog-Gli1 pathway. Cancer Lett 323:48–57 [DOI] [PubMed] [Google Scholar]
- Zhang L, Qi Z, Huang Q, et al. (2014a). Imprinted-like biopolymeric micelles as efficient nanovehicles for curcumin delivery. Colloids Surf B: Biointerfaces 123:15–22 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao W, Ma Z, et al. (2012b). Enzymatic polymerization of phenol catalyzed by horseradish peroxidase in aqueous micelle system. Eur Polym J 48:580–5 [Google Scholar]
- Zhang Y, Petibone D, Xu Y, et al. (2014b). Toxicity and efficacy of carbon nanotubes and graphene: the utility of carbon-based nanoparticles in nanomedicine. Drug Metab Rev 46:232–46 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang C, Wang W, et al. (2016). Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep 6:21225. doi: 10.1038/srep21225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Sun X, Zhang L, et al. (2012). Fabrication of biopolymeric complex coacervation core micelles for efficient tea polyphenol delivery via a green process. Langmuir 28:14553–61 [DOI] [PubMed] [Google Scholar]
- Zhu W, Song Z, Wei P, et al. (2015). Y-shaped biotin-conjugated poly (ethylene glycol)-poly (epsilon-caprolactone) copolymer for the targeted delivery of curcumin. J Colloid Interface Sci 443:1–7 [DOI] [PubMed] [Google Scholar]