Abstract
Objective
To describe the laboratory parameters and biomarkers of the cytokine storm syndrome associated with severe and fatal COVID-19 cases.
Methods
A search with standardized descriptors and synonyms was performed on November 28th, 2020 of the MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, LILACS, and IBECS to identify studies of interest. Grey literature searches and snowballing techniques were additionally utilized to identify yet-unpublished works and related citations. Two review authors independently screened the retrieved titles and abstracts, selected eligible studies for inclusion, extracted data from the included studies, and then assessed the risk of bias using the Newcastle-Ottawa Scale. Eligible studies were those including laboratory parameters—including serum interleukin-6 levels—from mild, moderate, or severe COVID-19 cases. Laboratory parameters, such as interleukin-6, ferritin, hematology, C-Reactive Protein, procalcitonin, lactate dehydrogenase, aspartate aminotransferase, creatinine, and D-dimer, were extracted from the studies. Meta-analyses were conducted using the laboratory data to estimate mean differences with associated 95% confidence intervals.
Data synthesis
The database search yielded 9,620 records; 40 studies (containing a total of 9,542 patients) were included in the final analysis. Twenty-one studies (n = 4,313) assessed laboratory data related to severe COVID-19 cases, eighteen studies (n = 4,681) assessed predictors for fatal COVID-19 cases and one study (n = 548) assessed laboratory biomarkers related to severe and fatal COVID-19 cases. Lymphopenia, thrombocytopenia, and elevated levels of interleukin-6, ferritin, D-dimer, aspartate aminotransferase, C-Reactive-Protein, procalcitonin, creatinine, neutrophils and leucocytes were associated with severe and fatal COVID-19 cases.
Conclusions
This review points to interleukin-6, ferritin, leukocytes, neutrophils, lymphocytes, platelets, C-Reactive Protein, procalcitonin, lactate dehydrogenase, aspartate aminotransferase, creatinine, and D-dimer as important biomarkers of cytokine storm syndrome. Elevated levels of interleukin-6 and hyperferritinemia should be considered as red flags of systemic inflammation and poor prognosis in COVID-19.
Introduction
In December 2019, a new strain of coronavirus, severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, and starting in March of 2020, the world was plunged into a pandemic due to the disease (COVID-19) caused by this new coronavirus. As of March 11th, COVID-19 had been detected in 221 countries, with 117,799,584 confirmed cases and more than 2,615,018 deaths [1]. The clinical spectrum of this disease ranges from asymptomatic cases to severe pulmonary involvement with respiratory failure, systemic involvement with sepsis, septic shock, and multiple organ failure [2]. Accumulating evidence suggests that a subgroup of patients with severe COVID-19 might have cytokine storm syndrome (CSS). CSS and secondary hemophagocytic lymphohistiocytosis (sHLH) are both associated with cytopenia, hyperferritinemia, and elevated interleukin-6 (IL-6) due to a viral driven hyper-inflammation and amplification of the immune response [3–5].
Elevated serum concentrations of IL-6 and other inflammatory cytokines are hallmarks of CSS and correlate with poor clinical outcomes [3]. Elevated serum C-reactive protein (CRP), a protein whose expression is driven by IL-6, is also a biomarker of severe clinical manifestations of COVID-19. This infection results in monocyte, macrophage, and dendritic cell activation. IL-6 release instigates an amplification cascade that results in cis signaling with TH17 differentiation and trans-signaling in many cell types, such as endothelial cells [3, 5]. The increased systemic cytokine production contributes to the pathophysiology of severe COVID-19 and acute respiratory distress syndrome (ARDS). Recognizing and treating CSS in a timely fashion is of paramount importance, as this information could lead to better outcomes in patients who present this complication as part of their clinical course of COVID-19. We have therefore designed and carried out this living systematic review to guide the evaluation and early recognition of CSS by:
Identifying the laboratory parameters correlated with severe and fatal cases of COVID-19;
Estimating the role of IL-6 and ferritin as potential biomarkers related to cytokine storm development.
Methods
Protocol and registration
This systematic review protocol was registered within the International Prospective Register of Systematic Reviews (PROSPERO), under the code CRD42020190021. We followed the recommendations proposed by the Cochrane Collaboration Handbook [6] and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [7].
Search strategy
We searched MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, and IBECS using relevant descriptors and synonyms, adapting the search to the specifics of each database (S1 Table). We also searched the Open Grey database, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), and ClinicalTrials.gov to identify published, ongoing, and unpublished studies. Finally, we used the technique of snowballing, searching the lists of references of the included studies.
Eligibility criteria and study selection
Studies were included if they presented laboratory data—including serum IL-6 levels—from mild-to-moderate, severe or critical patients with COVID-19, according to the National Health Commission of China (NHCC) Guidelines for Diagnosis and Management of COVID-19 [8] or World Health Organization Interim Guidance for COVID-19 [9]. Exclusion criteria consisted of studies that assessed pregnant women, pediatric patients, individuals co-infected with other microorganisms, or populations exclusively related to oncological, rheumatological, transplants, or chronic renal disease. All studies published before November 28th, 2020 were included, and no language restrictions were implemented for electronic search.
Data extraction and quality appraisal
We used a predefined form to extract data from included studies. Specifically, we extracted participants and studies characteristics, including: age, gender, diagnostic criteria, and the severity of condition, details on the number of participants screened, randomized, analyzed, excluded, lost to follow-up and dropped out, setting, duration of studies, laboratorial biomarkers, outcome measures, and time points reported. We then assessed the risk of bias inherent to each of the included studies using the modified Newcastle-Ottawa Scale (NOS) [10]. We attempted to contact the authors of the included studies when any study data or other details were missing.
Summary measures and synthesis of results
Study selection, data extraction, and assessment of the risk of bias were performed independently by two review authors. Disagreements were resolved through discussion or, if required, by consulting a third author. Studies were assessed into two separate groups for analysis: severity group and mortality group. Laboratory parameters, such as IL-6, ferritin, hemoglobin, leukocytes, lymphocytes, neutrophils, platelets, CRP, procalcitonin, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), creatinine, and D-dimer were analyzed. The assays for detecting laboratory tests were similar between studies.
Statistical analysis
After data was extracted, we used the “meta” package (version 3.4.1) [11] of R software to perform the meta-analysis. For studies presenting continuous data as medians and inter-quartile ranges, we estimated the means and standard deviations according to the method described by Hozo SP et al. [12]. A meta-analysis was performed, with a calculation of mean difference (MD) and 95% confidence interval (95% CI) for each of the laboratory parameters related to patients with confirmed COVID-19 with or without severe disease and non-survivors or survivors. Heterogeneity across the included studies was assessed using a chi-square (χ2) test and the I2 statistic. A random effects model was employed to calculate pooled results. A sensitivity analysis was performed to explore the sources of heterogeneity when I2 > 50%. We explored the sources of heterogeneity by excluding studies with unclear timepoints of collection and studies that collected blood parameters after seven days of hospital admission. When at least 10 studies were included in a meta-analysis, we assessed the possibility of publication bias using Egger’s test (S2A and S2B Table).
Results
Study selection
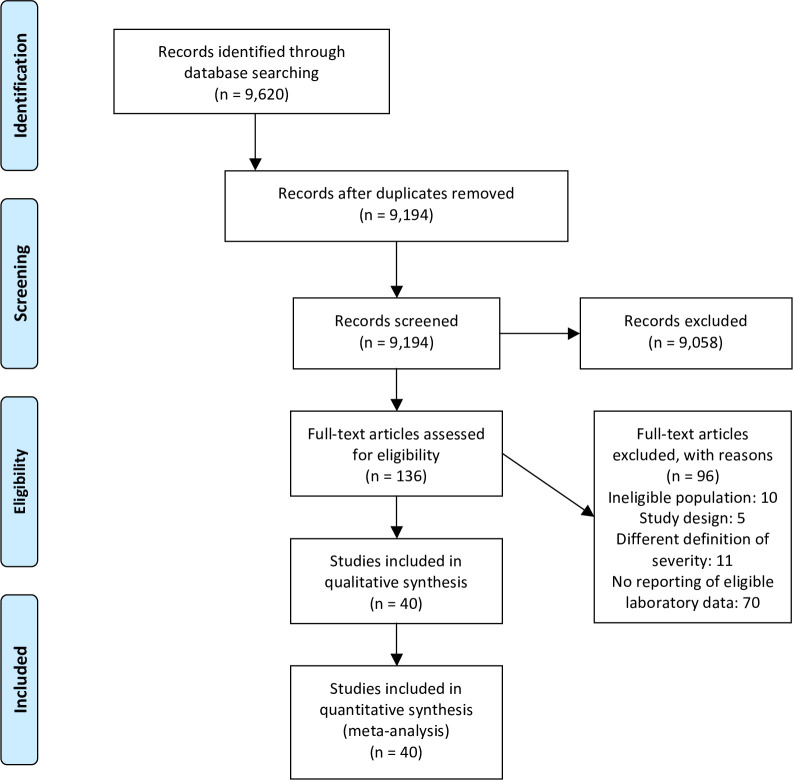
The database search yielded 9,620 records. After removing duplicates, 9,194 titles and abstracts were examined. We retrieved 136 full-text articles for further scrutiny; of those, 96 studies were excluded due to: ineligible population(n = 10), study design (n = 5), different definition of severity (n = 11) or not reporting the laboratory data of interest for this meta-analysis (n = 70) (S3 Table). We finally included 40 studies in this review (Fig 1).
Fig 1. Study selection process flow chart.
Study characteristics
Thirty-seven eligible studies consisted of retrospective case series and three were prospective cohorts [13–15], conducted in various hospital settings: China (n = 35) [16–31], Israel (n = 1) [32], Brazil (n = 1) [14], Spain (n = 1) [33], Norway (n = 1) [13] and Turkey (n = 1) [34].
The participants (n = 9,542) all received a positive COVID-19 diagnosis through 2019-nCoV RNA detection by SARS-CoV-2 real-time polymerase chain reaction nasopharyngeal swab (RT-PCR) or specific virus IgM and IgG antibodies. Twenty-one studies [16–26] (n = 4,313) assessed laboratory data related to the severity of COVID-19, comparing severe and non-severe cases. Eighteen studies (n = 4,681) [13–15, 27–33, 35–42] evaluated the association between laboratory parameters and mortality in patients with COVID-19 and one study (n = 548) [43] assessed serum biomarkers related to severe and fatal COVID-19 cases. The participants’ baseline and demographic characteristics are presented in Table 1. The descriptive analysis indicates that patients with severe disease were older than non-severe patients, except in two studies [43, 44]. Mortality was also higher in the elderly. Most patients with severe disease and higher mortality were male. Most studies showed a higher prevalence of comorbidities in patients with the severe form of COVID-19.
Table 1. Baseline and demographic characteristics of the included studies.
| Assessed outcome: Severity | ||||||||||
| Study ID | Severity criteria | Total enrolled patients | Severe group | Non-Severe group | ||||||
| No. (%) | Age* | Male (%) | Comorbidity (%) | No. (%) | Age* | Male (%) | Comorbidity (%) | |||
| Guang Chen, 2020 [16] | Severe vs Moderate cases according to NHCC COVID-19 Guideline (6th Edition) a | 21 | 11 (52.38) | 61.0 (56.5–66.0) | 10 (90.9) | 5 (45.5) | 10 (47.62) | 52.0 (42.8–56.0) | 7 (70.0) | 2 (20.0) |
| Yong Gao, 2020 [18] | Severe vs Mild cases according to WHO Interim Guidance for COVID‐19c | 43 | 15 (53.88) | 45.20 (± 7.68) | 9 (60) | ** | 28 (46.12) | 42.96 (± 14.00) | 17 (60.71) | ** |
| Zhongliang Wang, 2020 [19] | Patients with SpO2<90% (Severe) vs Patients with SpO2≥90% (Non-severe) according to NHCC COVID-19 Guidelines (3rd edition)a | 69 | 14 (20.29) | 70.5 (62.0–77.0) | 7 (50) | ** | 55 (79.71) | 37.0 (32.0–51.0) | 25 (45) | ** |
| Chuan Qin, 2020 [20] | Severe vs Moderate cases according to NHCC COVID-19 Guidelines (5th Edition)a | 452 | 286 (63.27) | 61 (51–69) | 155 (54.2) | 146 (51) | 166 (36.73) | 53 (41.25–62) | 80 (48.2) | 55 (33.1) |
| Chen Lei, 2020 [22] | Severe/Critical vs Mild cases according to NHCC COVID-19 Guidelines (4th Edition)a | 29 | 14 (48.28) | NR | NR | ** | 15 (51.72) | NR | NR | ** |
| Ruirui Wang, 2020 [23] | Critical (Severe or critical cases) vs Non-critical (mild or moderate) cases according to NHCC COVID-19 Guidelines (5th Edition)a | 125 | 25 (20) | 49.40 (±13.64) | 16 (64) | 12 (48) | 100 (80) | 39.47 (±14.84) | 55 (55) | 22 (22) |
| Zhe Zhu, 2020 [24] | Severe vs Non-severe cases according to NHCC COVID-19 Guidelines (6th Edition)a | 127 | 16 (12.6) | 57.50 (±11.70) | 9 (56.25) | 12 (75) | 111 (87.4) | 49.95 (±15.52) | 73 (65.77) | 40 (36.04) |
| Xiaohua Chen, 2020 [25] | Moderate vs Severe vs Critically ill cases according to NHCC COVID-19 Guidelines (6th Edition)a | 48 | Severe: 10 (20.83) Critically ill: 17 (35.42) | Severe: 63.9 (±15.2) Critically ill: 79.6 (±12.6) | Severe: 9 (90) Critically ill: 15 (88.2) | ** | 21 (43.75) | 52.8 (±14.2) | 13 (61.9) | ** |
| Ming Ding, 2020 [26] | Mild vs Severe vs Critical cases according to NHCC COVID-19 Guidelines (7th Edition)a | 32 | Severe: 10 (31.25) Critical: 11 (34.37) | Severe:61.3 (±17.9) Critical: 73.5 (±12.3) | Severe: 5 (50) Critical: 7 (63.63) | Severe: 3 (30) Critical: 4 (36.36) | 11 (34.37) | 54.9 (±11.3) | 1 (9.09) | 3 of 5 (60) |
| Chen LD, 2020 [46] | Mild (without pneumonia) vs Moderate cases with pneumonia (Non-severe) vs Severe cases with pneumonia according to NHCC COVID-19 Guidelines (7th Edition)a | 106 | 25 (23.6) | 60.68 (± 15.23) | 15 (60) | 11 (44.0) | Mild: 12 (11.3) Moderate: 69 (65.1) | Mild: 43.92 (± 13.73) Moderate: 51.41 (± 15.77) | Mild: 4 (33.3) Moderate: 34 (49.3) | Mild: 0 (0.0) Moderate: 14 (20.3) |
| Chen R, 2020 [43] | Mild/Moderate cases vs Severe cases vs Critical cases according to NHCC COVID-19 Guidelines (7th Edition)a | 548 | Severe: 155 (28.3) Critical: 48 (8.8) | Severe: 60.9 (± 13.8) Critical: 61.4 (±13.6) | Severe: 93 (60) Critical: 38 (79.2) | ** | 345 (62.9) | 67.3 (±12.1) | 182 (52.75) | ** |
| Chi Y, 2020 [79] | Mild vs Moderate vs Severe cases according to NHCC COVID-19 Guidelines (5th Edition)a | 66 | 8 (12.1) | 54.0 (± 12.38) | 5 (62.5) | 4 (50) | Mild: 22 (33.4) Moderate: 36 (54.5) | Mild: 43.32 (± 18.38) Moderate: 40.81 (± 11.8) | Mild: 13 (59.1) Moderate: 19 (53) | Mild: 6 (27) Moderate: 8 (22) |
| Hu ZJ, 2020 [80] | Severe vs Non-severe cases according to NHCC COVID-19 Guidelines (7th Edition)a | 76 | 13 (17.2) | 61.5 (57.1–65.9) | 8 (61.5) | ** | 63 (82.8) | 48.2 (46.0–50.4) | 26 (41.3) | ** |
| Huang Z, 2020 [44] | Moderate (Non-severe) vs Severe vs Critical cases according to NHCC COVID-19 Guidelines (7th Edition)a | 83 | Severe: 29 (35) Critical: 33 (39.7) | Severe: 67 (60–79) Critical: 58 (49–62) | Severe: 16 (55.2) Critical: 26 (78.8) | Severe: 20 (69) Critical: 21 (63.6) | 21 (25.3) | 68 (57–69) | 12 (57.14) | 9 (42.86) |
| Li X, 2020 [47] | Severe (Severe pneumonia/ ARDS) vs Non-severe cases (Mild/ Common pneumonia) according to NHCC COVID-19 Guidelines (7th Edition)a and WHO Interim Guidance for COVID‐19c | 215 | 56 (26.1) | 56.5 (20–72) | 36 (64.3) | ** | 159 (73.9) | 44 (32–52) | 91 (57.2) | ** |
| Liu D, 2020 [81] | Moderate vs Severe vs Critical cases according to NHCC COVID-19 Guidelines (7th Edition)a | 2044 | Severe: 689 (33.7) Critical: 268 (13.1) | Severe: 64.0 (54.0–71.0) Critical: 69.0 (62.0–77.0) | Severe: 349 (50.65) Critical: 176 (65.67) | Severe: 423/687 (61.57) Critical: 212/266 (79.7) | 1087 (53) | 59 (46–67) | 475 (43.7) | 540/1086 (49.72) |
| Ozsurekci Y, 2020 [34] | Mild vs Moderate vs Severe/ Critical cases according to WHO Interim Guidance for COVID‐19c | 30 | 11 (36.7) | NR | NR | ** | Mild: 4 (13.4) Moderate: 15 (50) | NR | NR | ** |
| Xu X, 2020 [48] | Moderate (non-severe) vs Severe vs Critically ill cases according to NHCC COVID-19 Guidelines (7th Edition)a | 88 | Severe: 32 (36.4) Critically ill: 9 (10.2) | Severe: 59.94 (±13.96)Critically ill: 74.78 (±10.06) | Severe: 8 (25) Critically ill: 7 (77.78) | Severe: 17 (53.13) Critically ill: 7 (77.78) | 47 (53.4) | 52.49 (±14.62) | 21 (44.68) | 17 (36.17) |
| Zeng YL, 2020 [45] | Ordinary (Non-severe) vs Severe vs Critical cases according to NHCC COVID-19 Guidelines (6th Edition)a | 49 | Severe: 16 (32.7) Critical: 5 (10.2) | Severe: 60 (±16) Critical: 68 (±20) | Severe: 8 (50) Critical: 3 (60) | ** | 28 (57.1) | 46 (±19) | 15 (53.6) | ** |
| Zeng Z, 2020 [49] | Moderate (Non-severe) vs Severe / Critical cases according to NHCC COVID-19 Guidelines (6th Edition)a | 317 | Severe: 167 (52.68) Critical: 57 (17.98) | Severe: 62.0 (51.0–69.0) Critical: 68.0 (57.0–77.0) | Severe: 90 (53.9) Critical: 31 (54.4) | ** | 93 (29.34) | 59.0 (46.0–68.5) | 41 (44.1) | ** |
| Zhao C, 2020 [82] | Mild (Non-severe) vs Severe cases according to WHO Interim Guidance for COVID‐19c | 172 | 60 (34.8) | 70.6 (±11.6) | 37 (61.7) | 38 (63.3) | 112 (65.2) | 64 (50–67) | 45 (40.2) | 57 (50.9) |
| Zou L, 2020 [83] | Severe vs Non-severe cases according to NHCC COVID-19 Guidelines (3rd-6th Edition)a | 121 | 52 (42.98) | 69.5 (61.5–79.75) | 32 (61.5) | 52 (88.5) | 69 (57.02) | 60.0 (52.0–68.0) | 34 (49.3) | 39 (56.5) |
| Assessed outcome: Mortality | ||||||||||
| Study ID | Total enrolled patients | Death | Survival | |||||||
| No. (%) | Age* | Male (%) | Comorbidity (%) | No. (%) | Age* | Male (%) | Comorbidity (%) | |||
| Tao Chen, 2020 [27] | 274 | 113 (41.24) | 68 (62–77) | 83 (73) | 71 (63) | 161 (58.76) | 51 (37–66) | 88 (55) | 62 (39) | |
| Lang Wang, 2020 [28] | 339 | 65 (19.18) | 76 (70–83) | 39 (60) | ** | 274 (80.82) | 68 (64–74) | 127 (46.4) | ** | |
| Fei Zhou [29], 2020 | 191 | 54 (28.2) | 69 (63–76) | 38 (70) | 36 (67) | 137 (71.8) | 52 (45–58) | 81 (59) | 55 (40) | |
| Haiying Sun, 2020 [30] | 244 | 121 (49.59) | 72 (66–78) | 82 (67.8) | ** | 123 (50.41) | 67 (64–72) | 51 (41.5) | ** | |
| Junli Fan, 2020 [31] | 21 | 4 (19.05) | 79.7 (±14.3) | 2 (50) | 4 (100) | 17 (80.95) | 61.5 (±9.5) | 9 (53.9) | 9 (53.9) | |
| Laguna-Goya R, 2020 [15] | 501 | 36 (7.18) | 65 (57–72) | 25 (69.4) | ** | 465 (92.82) | 52 (44–58) | 292 (62.9) | ** | |
| Chen F, 2020 [36] | 660 | 82 (12.42) | 71 (63‐83) | 58 (70.7) | 64 (78) | 578 (87.58) | 54 (37‐66) | 237 (41) | 262 (45.3) | |
| Chen H, 2020 [37] | 172 | 87 (50.58) | 71 (61–78) | 50 (57.47) | ** | 85 (49.42) | 53 (43–65) | 44 (51.76) | ** | |
| Gadotti AC, 2020 [14] | 56 | 18 (32.14) | 66 (56–77) | 16 (88.8) | ** | 38 (67.86) | 56 (43–72) | 23 (60.52) | ** | |
| Chen R, 2020 [43] | 548 | 103 (18.8) | 66.9 (±12.1) | 69 (67) | ** | 445 (81.2) | 53.5 (±13.9) | 244 (54.83) | ** | |
| Guirao JJ, 2020 [33] | 50 | 14 (28) | 69.00 (± 3.09) | 11 (78.57) | ** | 36 (72) | 61.36 (± 1.72) | 30 (83.3) | ** | |
| Ke C, 2020 [35] | 194 | 46 (23.7) | 69.76 (±10) | 32 (69.57) | ** | 148 | 60.30 (±14.56) | 83 (56.08) | ** | |
| Li C, 2020 [38] | 476 | 183 (38.4) | 71 (62–79) | 121 (66.12) | 123 (67.2) | 293 (61.6) | 68 (59–77) | 161 (54.95) | 196 (66.9) | |
| Luo M, 2020 [39] | 1018 | 201 (19.7) | 69 (62–78) | 133 (66.2) | ** | 817 (80.3) | 57 (46–66) | 388 (47.5) | ** | |
| Mandel M, 2020 [32] | 71 | 12 (16.9) | NR | NR | NR | 59 (83.1) | NR | NR | NR | |
| Wang ZH, 2020 [40] | 59 | 41 (69.5) | 70.2 (±9.0) | 26 (63.4) | ** | 18 (30.5) | 61 (±13.5) | 12 (66.7) | ** | |
| Zhang B, 2020 [41] | 98 | 36 (36.7) | 70.5 (±1.7) | 23 (64) | ** | 62 (63.3) | 60.0 (±1.9) | 35 (56.5) | ** | |
| Zhang L, 2020 [42] | 134 | 101 (75.37) | 65.46 (±9.74) | 64 (63.4) | ** | 33 (24.63) | 46.45 (±11.09) | 23 (69.70) | ** | |
| Myhre PL, 2020 [13] | 123 | 8 (6.5) | NR | NR | ** | 115 (93.5) |
NR | NR | ** | |
SpO2 = Blood oxygen saturation level, NR = Not reported.
a National Health Commission of China Guidelines for Diagnosis and Management of COVID-19 [84].
c World Health Organization Interim Guidance for COVID‐19 [85].
* Values expressed in Median (interquartile range) or Mean ± SD (standard deviation).
** study does not describe the exact prevalence of overall comorbidities in each group.
Risk of bias assessment
The studies’ risk of bias, as measured on the Newcastle-Ottawa Scale, as suggested by Bazerbachi 2018 [10], ranged from 2/5 [19, 45] to 5/5 [15, 20, 23, 24, 29, 37, 43, 46–49], with higher scores indicating lower risk of bias (Table 2). All evaluated studies succeeded in excluding cases that were not pertinent to the research question. The domain that revealed the highest frequency of negative assessment was the one related to the representativeness of the exposed group. Detailed information on each of the domains is provided in Table 2.
Table 2. Risk of bias assessment—Modified Newcastle-Ottawa Scale [10].
| Study ID, Year | Selection | Outcome | NOS | |||
|---|---|---|---|---|---|---|
| Did the sample represent the whole cases of interest? | Was the diagnosis correctly made | Was the other important diagnosis excluded? | Were all the important data cited in the report | Was the outcome correctly ascertained | ||
| Guang Chen [16], 2020 | - | + | + | + | + | 4/5 |
| Yong Gao [18], 2020 | - | + | + | + | - | 3/5 |
| Zhongliang Wang [19], 2020 | - | + | + | - | - | 2/5 |
| Chuan Qin [20], 2020 | + | + | + | + | + | 5/5 |
| Chen Lei [22], 2020 | - | + | + | + | + | 4/5 |
| Ruirui Wang [23], 2020 | + | + | + | + | + | 5/5 |
| Zhe Zhu [24], 2020 | + | + | + | + | + | 5/5 |
| Xiaohua Chen [25], 2020 | - | + | + | + | - | 3/5 |
| Ming Ding [26], 2020 | - | + | + | + | + | 4/5 |
| Tao Chen [27], 2020 | + | - | + | + | + | 4/5 |
| Lang Wang [28], 2020 | + | - | + | + | + | 4/5 |
| Fei Zhou [29], 2020 | + | + | + | + | + | 5/5 |
| Haiying Sun [30], 2020 | + | + | + | - | - | 3/5 |
| Junli Fan [31], 2020 | - | - | + | + | + | 3/5 |
| Chen LD, 2020 [46] | + | + | + | + | + | 5/5 |
| Chen R, 2020 [43] | + | + | + | + | + | 5/5 |
| Chi Y, 2020 [79] | - | + | + | + | + | 4/5 |
| Guirao JJ, 2020 [33] | - | + | + | - | + | 3/5 |
| Hu ZJ, 2020 [80] | - | + | + | + | - | 3/5 |
| Huang Z, 2020 [44] | - | + | + | + | - | 3/5 |
| Li X, 2020 [47] | + | + | + | + | + | 5/5 |
| Liu D, 2020 [81] | + | + | + | + | - | 4/5 |
| Ozsurekci Y, 2020 [34] | - | + | + | + | + | 4/5 |
| Xu X, 2020 [48] | + | + | + | + | + | 5/5 |
| Zeng YL, 2020 [45] | - | + | + | - | - | 2/5 |
| Zeng Z, 2020 [49] | + | + | + | + | + | 5/5 |
| Zhao C, 2020 [82] | + | + | + | + | - | 4/5 |
| Zou L, 2020 [83] | + | + | + | + | - | 4/5 |
| Myhre PL, 2020 [13] | - | + | + | + | + | 4/5 |
| Laguna-Goya R, 2020 [15] | + | + | + | + | + | 5/5 |
| Chen F, 2020 [36] | + | - | + | + | + | 4/5 |
| Chen H, 2020 [37] | + | + | + | + | + | 5/5 |
| Gadotti AC, 2020 [14] | - | + | + | + | - | 3/5 |
| Ke C, 2020 [35] | - | - | + | + | + | 3/5 |
| Li C, 2020 [38] | + | + | + | - | + | 4/5 |
| Luo M, 2020 [39] | + | - | + | + | + | 4/5 |
| Mandel M, 2020 [32] | - | + | + | - | + | 3/5 |
| Wang ZH, 2020 [40] | - | + | + | + | - | 3/5 |
| Zhang L, 2020 [42] | - | + | + | + | + | 4/5 |
| Zhang B, 2020 [41] | - | + | + | + | - | 3/5 |
NOS = Newcastle-Ottawa Scale
Synthesis of results
Laboratory parameters in severe versus non-severe patients and non-surviving versus surviving patients with COVID-19 are described in Table 3. Lymphopenia, thrombocytopenia and higher levels of IL-6, ferritin, D-dimer, aspartate aminotransferase, lactate dehydrogenase, CRP, procalcitonin, creatinine, neutrophils and leukocytes were associated with severe and fatal cases of COVID-19. Abnormalities of hemoglobin were not associated with disease severity or increased mortality. We explored the sources of heterogeneity by excluding studies with unclear timepoints of collection and studies that collected blood parameters after 7 days of hospital admission. However, no difference in the statistical heterogeneity across studies could be detected in this sensitivity analysis, except for d-dimer [MD 5.94; CI 5.16 to 6.73; N = 2,459; I2 = 0.19] and creatinine [MD 19.44; CI 16.79 to 22.10; N = 1,689; I2 = 0.49] in the mortality group.
Table 3. Results of meta-analysis comparing laboratory parameters in COVID-19 patients.
| SEVERE versus NON-SEVERE | FATAL versus NON-FATAL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Number of studies | MD | Random; 95% CI | I2 | Number of participants | Number of studies | MD | Random; 95% CI | I2 | Number of participants |
| Haemoglobin | 9 | -0.04 | -0.37; 0.29 | 0.84 | 3,523 | 7 | -0.10 | -0.45; 0.25 | 0.91 | 2,378 |
| White Blood Cell (WBC) | 17 | 1.63 | 0.98; 2.27 | 0.97 | 4,655 | 13 | 3.71 | 3.04; 4.38 | 0.94 | 2,990 |
| Lymphocytes | 19 | -0.4 | -0.48; -0.32 | 0.97 | 4,733 | 13 | -0.43 | -0.45; -0.40 | 0.59 | 3,435 |
| Neutrophils | 13 | 2.12 | 1.18; 3.07 | 0.98 | 2,268 | 8 | 3.99 | 2.85; 5.13 | 0.96 | 2,613 |
| Platelets | 11 | -24.22 | -36.26; -12.18 | 0.93 | 3,726 | 9 | -46.68 | -49.99; -43.37 | 0 | 2,571 |
| C-Reactive Protein | 14 | 53.54 | 39.79; 67.29 | 0.97 | 4,138 | 15 | 58.48 | 43.35; 73.61 | 0.99 | 3,755 |
| Procalcitonin | 11 | 0.08 | 0.03; 0.14 | 0.99 | 3,480 | 11 | 0.24 | 0.13; 0.36 | 0.96 | 2,845 |
| LDH | 10 | 153.58 | 87.09; 220.08 | 0.95 | 3,050 | 10 | 230.99 | 192.29; 269.70 | 0.96 | 2,622 |
| Creatinine | 10 | 8.07 | 4.28; 11.87 | 0.85 | 3,036 | 9 | 17.93 | 11.89; 23.98 | 0.91 | 2,174 |
| AST | 8 | 8.29 | 0.88; 15.7 | 0.90 | 649 | 8 | 16.81 | 8.08; 25.54 | 0.88 | 1,843 |
| D-Dimer | 9 | 2.15 | 0.68; 3.63 | 0.99 | 3,181 | 14 | 4.64 | 3.03; 6.24 | 0.97 | 3,965 |
| Ferritin | 6 | 654.4 | 383.48; 925.33 | 0.96 | 3,470 | 9 | 853.43 | 601.20; 1105.67 | 0.94 | 2,088 |
| IL-6 | 22 | 28.93 | 18.18; 39.69 | 0.99 | 4,861 | 19 | 70.82 | 45.24; 96.41 | 0.96 | 5,229 |
MD = mean difference, CI = confidence interval, LDH = lactate dehydrogenase, AST = aspartate aminotransferase, IL-6 = interleukin-6
Meta-analysis of laboratory data
Nineteen studies assessed fatal and non-fatal groups of patients with COVID-19 and reported IL-6. Pooled results from these studies showed that the patients who progressed to death had higher levels of IL-6 in comparison with those who survived [MD 70.82; 95% CI 45.24 to 96.41; N = 5,229] (Fig 2A). Pooled results from twenty-two studies of both severe and non-severe cases revealed higher levels of Il-6 in patients with severe COVID-19 in comparison to the group of mild COVID-19 cases [MD 28.99; 95% CI 18.24 to 39.75; N = 4,861] (Fig 2B). The pooled analysis of nine studies showed that patients with COVID-19 who did not survive also had higher levels of ferritin [MD 853.43; 95% CI 601.20 to 1105.67; N = 2,088] (Fig 2C). Ferritin levels were reported in six studies [16, 20, 21], with the meta-analysis of severe and non-severe patients showing that patients with severe COVID-19 had higher levels of ferritin [MD 654.40; 95% CI 383.48 to 925.33; N = 3,470] (Fig 2D). Sensitivity analysis was conducted on the IL-6 parameters. Five studies were found to be outliers in the mortality group [14, 29, 32, 33, 43], and three studies in the severity group [20–22]. There was no change in the direction of the effect of the meta-analysis after their exclusion from this calculation [MD 75.15; 95% CI 56.04 to 94.26; N = 4,313] and [MD 33.01; 95% CI 21.79 to 44.24; N = 4,200], respectively. We used the Egger’s test to assess publication bias, and the test suggested the presence of publication bias in the meta-analysis on the association between IL-6 and severity (p<0.001). No publication bias was detected in any other analyses (S2A and S2B Table).
Fig 2.
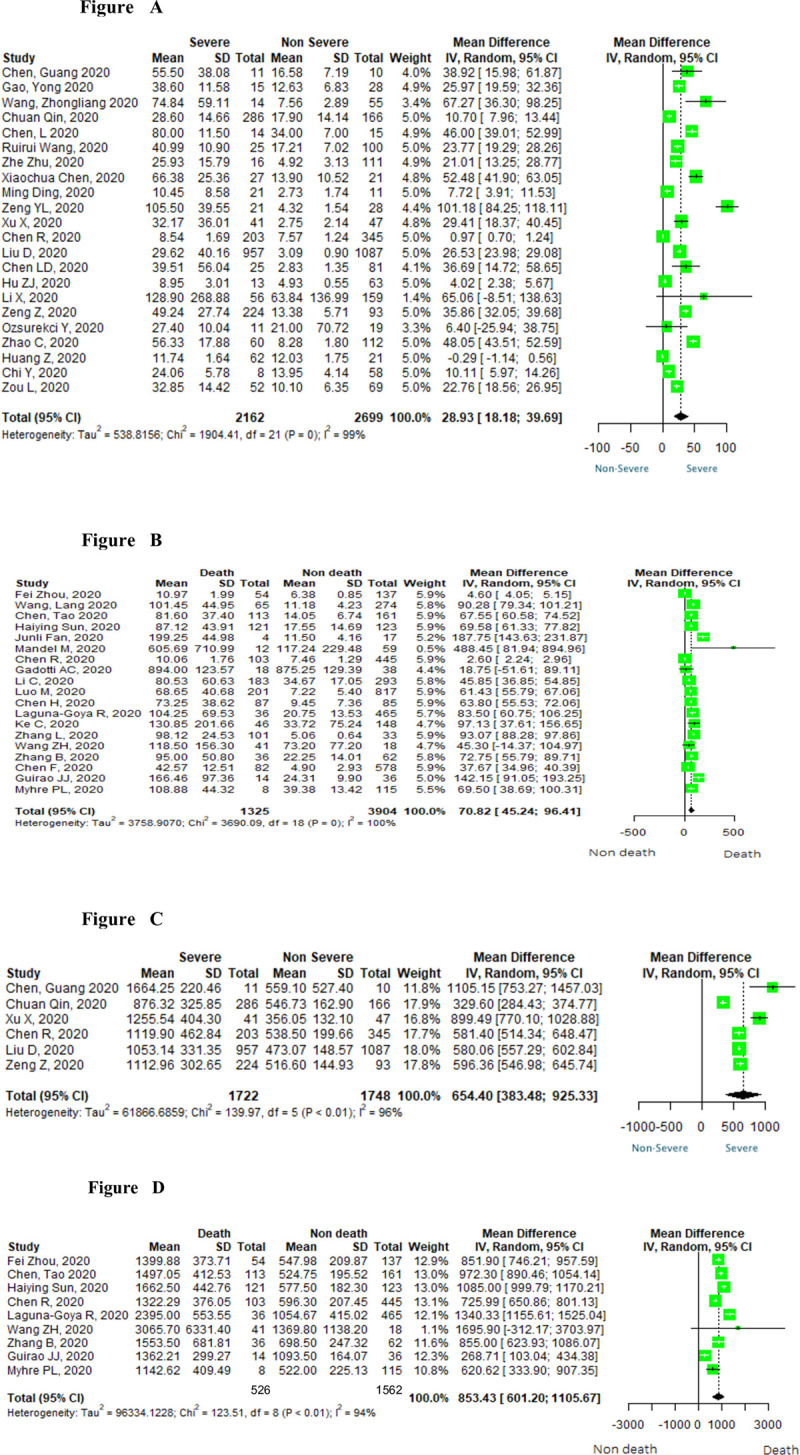
Forest plots of interleukin-6 (2A and 2B) and of ferritin (2C and 2D) in severe and fatal cases of COVID-19.
Discussion
A cytokine profile resembling secondary hemophagocytic lymphohistiocytosis is associated with severe COVID-19 (2–4, [50]. In this living systematic review with meta-analysis, a clear pattern of hematological, biochemical, inflammatory, and immune biomarker abnormalities could be found between patients with or without severe disease. These differences could allow for the identification of predictors of severe and fatal cases. We found that lymphopenia, thrombocytopenia and high levels of ferritin, D-dimer, aspartate aminotransferase, lactate dehydrogenase, C-Reactive Protein, neutrophils, procalcitonin and creatinine are good indicators of both severe and fatal cases of COVID-19 during the first days from illness onset, as is interleukin-6, the main cytokine involved in the CSS.
As high inflammation is a primary cause of pathology in COVID-19, targeted anti-inflammatory treatments are being evaluated to reduce inflammation-induced damage to the respiratory tract and to mitigate the cytokine storm [51, 52]. Based on observational studies [53, 54], COVID-19 progression has now been divided into three clinical phases: the viremia phase, the acute phase (pneumonia), and the recovery phase. During the progression of the phases, as mentioned by Lin L et al. [54], cells T and B reduce, while inflammatory cytokines and D-Dimer increase in severe patients. This progression has led some authors to suggest that anti-inflammatory treatment should be started in the acute phase to inhibit the inflammatory storms [54, 55]. One of the central challenges to defining initial treatment for patients with COVID-19 is the early identification of those individuals who will evolve into more severe forms of the disease and of those who require specific interventions or treatments.
Several laboratory differences were observed between severe and non-severe disease. Yuan X et al. [56] demonstrated that patients with severe or critical COVID-19 had decreased red blood cells compared to non-severe patients. Anemia occurs in approximately 80% of patients with sHLH, plays an important role in its pathophysiology, and can be a cause of poor prognosis on these patients [57]. Nonetheless, our meta-analysis failed to find an association of low hemoglobin levels with severity or mortality rates among patients with COVID-19-related cytokine storms.
The increased production (myelopoiesis) and mobilization of monocyte and neutrophil populations from the bone marrow is a response to many acute infections—including viral infections—and cytokines [58]. These cells are typically considered proinflammatory and are recruited to the sites of inflammation where they can respond to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) by producing interleukin-1, interleukin-6, interleukin-12, and tumor necrosis factor (TNF) [59]. Our data indicate that the increase in white blood cells is associated with elevated neutrophils, and this finding may signify clinical deterioration and increased risk of a poor outcome.
Despite an increase in white blood cell count, we found that lymphocytes were significantly decreased in patients with severe COVID-19. This result is consistent with those reported by Chen G et al. [16], who demonstrated that the total lymphocyte count, and specifically CD4+ T cells and CD8+ T cells, were slightly lower in moderate cases and significantly decreased in severe COVID-19. This study concluded that the cytokine storm is associated with COVID-19 severity, likely through increased pulmonary pathology, T cell depletion, and CD4+ T cell dysfunction.
Our meta-analysis also found an association between lymphopenia and higher mortality. Wang F et al. [60] assessed the levels of peripheral lymphocyte subsets by flow cytometry in 60 hospitalized patients with COVID-19 before and after treatment and their association with clinical characteristics and treatment efficacy. The authors reported that total lymphocytes, CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells were decreased among patients with COVID-19, more so in severe cases than in mild cases. In their analysis, the subsets of lymphocytes showed a significant association with inflammatory status in COVID-19, especially CD8+ T cells and CD4+/CD8+ ratio.
Thrombocytopenia is a recurrent complication among patients requiring intensive care, regardless of the cause of hospitalization, and represents a marker of poor prognosis [61]. In COVID-19, it is speculated that SARS-CoV-2 may inhibit hematopoiesis in the bone marrow, decreasing platelet production [62]. This phenomenon, combined with sepsis-induced disseminated intravascular coagulation, may justify the occurrence of thrombocytopenia in a subgroup of patients with COVID-19 [63]. Our meta-analysis demonstrated that thrombocytopenia could be a predictor of severity and mortality in patients with COVID-19.
Many inflammatory factors can cause systemic damage and multi-organ failure. High serum levels of CRP, AST, LDH, and ferritin in patients with COVID-19 may indicate that liver dysfunctions have been involved, and target treatments should be taken in time to prevent irreversible organ damage and increased risk of mortality [64]. Liver damage in these patients might be directly caused by the viral infection of liver cells; indeed, pathological studies [65, 66] have confirmed the presence of the SARS-CoV in liver tissues.
Procalcitonin levels are generally undetectable in healthy individuals and remain unchanged or moderately increase in those with virus infection or systemic inflammatory diseases. However, its levels increase significantly in cases of generalized infection, mainly bacterial or fungal [67]. In this systematic review, procalcitonin levels increased significantly in non-survivors but only showed a small effect size in studies comparing severe versus non-severe patients. Considering that procalcitonin is a good predictor of bacterial infection, the non-survivors possibly progressed to sepsis, an often-fatal complication in patients with COVID-19. In line with this hypothesis, in the meta-analysis published by Lippi G and Plebani M [68], a significant increase in procalcitonin levels was associated with bacterial co-infection, progression to severe forms of COVID-19, and death.
The activation of the coagulation process is a fundamental mechanism of the etiopathogenesis of SARS-CoV-2 infections and often leads to poor outcomes. Ultimately fatal cases often evolve from life-threatening disseminated intravascular coagulation requiring prompt intervention [69]. Recent clinical experiences with anticoagulants suggest that these substances are associated with a lower risk of thromboembolic disease and severe ischemic manifestations in some patients. Thus, it is imperative to identify patients at high risk for early anticoagulation in COVID-19. Tang N et al. [70] reported that the non-surviving patients with COVID-19 had higher fibrin-related markers (D-dimer and fibrin degradation product) at admission compared with survivors. The same authors also reported that administering low molecular weight heparin in severe patients infected with SARS-CoV-2 with elevated D-dimer or those with sepsis-induced disseminated intravascular coagulation was significantly associated with improved survival. In the present systematic review, increased levels of D-dimer at hospital admission could be a good predictor of severe and fatal cases of COVID-19. Another meta-analysis showed a similar result of the discriminatory ability of D-dimer in patients with and without severe forms of COVID-19 but did not provide data on mortality [71].
Several studies suggested that markedly elevated ferritin in hospitalized patients is often associated with hemophagocytic lymphohistiocytosis syndrome, but cannot be considered as a specific marker [72–74]. Ferritin is an acute protein that increases in response to a broad spectrum of inflammatory states, including infections, malignancy, iron overload, and liver or kidney disease [75, 76]. In our meta-analysis, the findings clarify the role of ferritin in the assessment of the systemic hyper-inflammation and can explain the relationship between hyperferritinemia and high levels of IL-6 among adult patients with COVID-19. Regarding immunological biomarkers, we believe that both parameters can be used as red flags for severe and fatal cases of COVID-19 infection at hospital admission. Additionally, ferritin and C-reactive protein appear to be screening tools for the early diagnosis of a systemic inflammatory response syndrome in patients with a severe form of COVID-19 (denominated CSS), with lower cost and wider availability in frontline clinical practice than IL-6.
Despite these rigorous efforts to guide clinicians in diagnosing CSS, discriminating this pathology from other conditions, particularly sepsis or disseminated intravascular coagulation, remains challenging due to the large degree of overlap in clinical presentation [77]. Therefore, biomarkers that identify which patients will evolve with the severe forms of COVID-19 have substantial clinical applicability. Our review demonstrated that older age, male gender, and comorbidities represent potential risk factors for severe and fatal cases, as has already been reported in the scientific literature. This review also confirms that certain laboratory tests that are part of routine care have also been reliably associated with severe and fatal cases of COVID-19, and including serum IL-6 could be relevant for prognosis but could also improve therapeutic decision making.
Limitations
The main limitations of this review stem from the nature of the included studies; all included studies were observational as they were published with the inherent urgency of a global pandemic. Although these are study designs with a lower level of evidence, the Newcastle-Ottawa Scale assessment of the risk of bias of the included studies did not show any salient risks. Another chief limitation lies in the fact that most patients are of Asian origin, which may compromise the external validity of this meta-analysis.
Assessing the statistical heterogeneity of the included studies is an essential component of interpreting the results of any meta-analysis. In the present study, the meta-analysis of most outcomes showed considerable heterogeneity, requiring a more detailed examination of the studies in order to determine possible causes for this issue. We attempted to explore sources of statistical heterogeneity by thoroughly examining clinical and methodological heterogeneity across studies and by performing sensitivity analysis. While we explored the sources of heterogeneity in the meta-analyses, we could not find differences that could explain the sources of heterogeneity across studies.
As the clinical course of patients with COVID-19 may widely vary across individuals, unexplored clinical parameters may explain this heterogeneity. Different definitions of the term “severity” adopted by each author, some variables such as the time taken to collect laboratory tests or the number of the days from symptoms onset of COVID-19 to hospital admission may also have notably contributed to this circumstance. Discrepancies in reporting and detailing patients’ comorbidities in each study may also have reinforced heterogeneity. Although considerable, the heterogeneity does not compromise the robustness of this meta-analysis, as it did not interfere with the direction and the magnitude of effects. As this study is underpowered to investigate the underlying mechanism of these inflammatory markers with the severity of COVID-19, further studies aiming at analyzing pathophysiological mechanisms of COVID-19 are needed.
We also examined evidence of publication bias in our meta-analyses, and the presence of non-reporting bias was suggested in the meta-analysis on the association of IL-6 with severity. As we found important heterogeneity in this meta-analysis and tests for adjusting for funnel plot asymmetry such as the trim and fill method are known to perform poorly when there is large between-study heterogeneity [78], we did not perform trim and fill test. Of note, small-study effects may be due to reasons other than publication bias, such as heterogeneity [6]. It is likely that asymmetry in this meta-analysis is also due to heterogeneity. We believe that IL-6 acts as a major pro-inflammatory mediator for the acute severe systemic inflammatory response, leading to a wide range of local and systemic changes, leucocytes recruitment and activation and hemodynamic effects. Thus, unexplored pathophysiological mechanisms may justify this heterogeneity and those results.
Systematic reviews and meta-analysis of prospective studies, with standardized COVID-19 severity criterion in a more global population sample, could be the key to better understanding the cytokine storm influences in patients infected with SARS-CoV-2 and their outcomes. We believe that the results of our review and meta-analysis including a relevant number of patients, especially those related to IL-6 and ferritin, can be extrapolated to the real world, supporting clinical practice in coping with COVID-19 and may help decision-making processes. As this is a living review, we are committed to keeping it updated as new evidence is published.
Conclusions
This review points to lymphopenia, thrombocytopenia, neutrophilia, leukocytosis as well as increased levels of IL-6, ferritin, D-dimer, aspartate aminotransferase, lactate dehydrogenase, procalcitonin, creatinine and CRP as indicators of severe and fatal cases of COVID-19. Ferritin and IL-6 are significant biomarkers of CSS, and increases in levels of either could be considered red flags of systemic inflammation and poor prognosis in COVID-19, especially in the oldest patients and those with comorbidities.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We graciously thank Cochrane Brazil for their assistance in accessing the electronic databases. We especially thank the individual group members for their rich contributions to the discussions on clinical experiences with patients with COVID-19.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization 2020. Available from: www.who.int.
- 2.Soy M, Atagündüz P, Atagündüz I, Sucak GT. Hemophagocytic lymphohistiocytosis: a review inspired by the COVID-19 pandemic. Rheumatol Int. 2020:1–12. Epub 2020/06/27. doi: 10.1007/s00296-020-04636-y ; PubMed Central PMCID: PMC7315691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–4. Epub 2020/04/19. doi: 10.1126/science.abb8925 . [DOI] [PubMed] [Google Scholar]
- 4.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. Epub 2020/03/21. doi: 10.1016/S0140-6736(20)30628-0 ; PubMed Central PMCID: PMC7270045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–16. Epub 2013/12/03. doi: 10.1016/S0140-6736(13)61048-X . [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). 2021.
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–30. Epub 2009/01/01. ; PubMed Central PMCID: PMC3090117. [PMC free article] [PubMed] [Google Scholar]
- 8.Commission CNH. Chinese Clinical Guidance For COVID-19 Pneumonia Diagnosis and Treatment 2020.
- 9.Patel A, Jernigan DB. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak—United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(5):140–6. Epub 2020/02/07. doi: 10.15585/mmwr.mm6905e1 ; PubMed Central PMCID: PMC7004396 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazerbachi F, Haffar S, Hussain MT, Vargas EJ, Watt KD, Murad MH, et al. Systematic review of acute pancreatitis associated with interferon-α or pegylated interferon-α: Possible or definitive causation? Pancreatology. 2018;18(7):691–9. Epub 2018/08/01. doi: 10.1016/j.pan.2017.08.012 . [DOI] [PubMed] [Google Scholar]
- 11.R software. Available from: (https://cran.r-project.org/bin/windows/base/old/3.4.1/)
- 12.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. Epub 2005/04/21. doi: 10.1186/1471-2288-5-13 ; PubMed Central PMCID: PMC1097734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myhre PL, Prebensen C, Strand H, Røysland R, Jonassen CM, Rangberg A, et al. Growth Differentiation Factor 15 Provides Prognostic Information Superior to Established Cardiovascular and Inflammatory Biomarkers in Unselected Patients Hospitalized With COVID-19. Circulation. 2020;142(22):2128–37. Epub 2020/10/16. doi: 10.1161/CIRCULATIONAHA.120.050360 ; PubMed Central PMCID: PMC7688084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AC G, M dCD, JP T, R W, M G, R GCO, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus research. 2020;289:198171. rayyan-111781195. doi: 10.1016/j.virusres.2020.198171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(4):799–807.e9. Epub 2020/07/28. doi: 10.1016/j.jaci.2020.07.009 ; PubMed Central PMCID: PMC7375283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–9. Epub 2020/03/29. doi: 10.1172/JCI137244 ; PubMed Central PMCID: PMC7190990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742–52. Epub 2020/04/03. doi: 10.1111/all.14309 . [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–6. Epub 2020/03/18. doi: 10.1002/jmv.25770 ; PubMed Central PMCID: PMC7228247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–77. Epub 2020/03/17. doi: 10.1093/cid/ciaa272 ; PubMed Central PMCID: PMC7184452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8. Epub 2020/03/13. doi: 10.1093/cid/ciaa248 ; PubMed Central PMCID: PMC7108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. Epub 2020/03/14. doi: 10.1001/jamainternmed.2020.0994 ; PubMed Central PMCID: PMC7070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E005. Epub 2020/02/07. doi: 10.3760/cma.j.issn.1001-0939.2020.0005 . [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Pan M, Zhang X, Han M, Fan X, Zhao F, et al. Epidemiological and clinical features of 125 Hospitalized Patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–8. Epub 2020/04/15. doi: 10.1016/j.ijid.2020.03.070 ; PubMed Central PMCID: PMC7151431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–9. Epub 2020/04/26. doi: 10.1016/j.ijid.2020.04.041 ; PubMed Central PMCID: PMC7195003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020. Epub 2020/04/18. doi: 10.1093/cid/ciaa449 ; PubMed Central PMCID: PMC7184354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding M, Zhang Q, Li Q, Wu T, Huang YZ. Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir Med. 2020;167:105981. Epub 2020/05/19. doi: 10.1016/j.rmed.2020.105981 ; PubMed Central PMCID: PMC7167578 of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091. Epub 2020/03/29. doi: 10.1136/bmj.m1091 ; PubMed Central PMCID: PMC7190011 www.icmje.org/coi_disclosure.pdf and declare: support from the Tongji Hospital for Pilot Scheme Project and the Chinese National Thirteenth Five Years Project in Science and Technology, National Commission of Health, People’s Republic of China, for the submitted work; no financial relationships with any organisation that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–45. Epub 2020/04/03. doi: 10.1016/j.jinf.2020.03.019 ; PubMed Central PMCID: PMC7118526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. Epub 2020/03/15. doi: 10.1016/S0140-6736(20)30566-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H, Ning R, Tao Y, Yu C, Deng X, Zhao C, et al. Risk Factors for Mortality in 244 Older Adults With COVID-19 in Wuhan, China: A Retrospective Study. J Am Geriatr Soc. 2020;68(6):E19–e23. Epub 2020/05/10. doi: 10.1111/jgs.16533 ; PubMed Central PMCID: PMC7267277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan J, Wang H, Ye G, Cao X, Xu X, Tan W, et al. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020;107:154243. Epub 2020/04/23. doi: 10.1016/j.metabol.2020.154243 ; PubMed Central PMCID: PMC7166305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandel M, Harari G, Gurevich M, Achiron A. Cytokine prediction of mortality in COVID19 patients. Cytokine. 2020;134:155190. Epub 2020/07/17. doi: 10.1016/j.cyto.2020.155190 ; PubMed Central PMCID: PMC7351379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guirao JJ, Cabrera CM, Jiménez N, Rincón L, Urra JM. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Mol Immunol. 2020;128:64–8. Epub 2020/10/20. doi: 10.1016/j.molimm.2020.10.006 ; PubMed Central PMCID: PMC7556792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozsurekci Y, Aykac K, Er AG, Halacli B, Arasli M, Oygar PD, et al. Predictive value of cytokine/chemokine responses for the disease severity and management in children and adult cases with COVID-19. J Med Virol. 2020. Epub 2020/11/24. doi: 10.1002/jmv.26683 ; PubMed Central PMCID: PMC7753701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke C, Yu C, Yue D, Zeng X, Hu Z, Yang C. Clinical characteristics of confirmed and clinically diagnosed patients with 2019 novel coronavirus pneumonia: a single-center, retrospective, case-control study. Med Clin (Barc). 2020;155(8):327–34. Epub 2020/08/13. doi: 10.1016/j.medcli.2020.06.055 ; PubMed Central PMCID: PMC7386390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F, Sun W, Sun S, Li Z, Wang Z, Yu L. Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China. Clin Transl Med. 2020;10(2):e40. Epub 2020/06/09. doi: 10.1002/ctm2.40 ; PubMed Central PMCID: PMC7300688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Wang J, Su N, Bao X, Li Y, Jin J. Simplified immune-dysregulation index: a novel marker predicts 28-day mortality of intensive care patients with COVID-19. Intensive Care Med. 2020;46(8):1645–7. Epub 2020/05/22. doi: 10.1007/s00134-020-06114-2 ; PubMed Central PMCID: PMC7237798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Jiang J, Wang F, Zhou N, Veronese G, Moslehi JJ, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. Epub 2020/08/23. doi: 10.1016/j.yjmcc.2020.08.008 ; PubMed Central PMCID: PMC7438272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo M, Liu J, Jiang W, Yue S, Liu H, Wei S. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight. 2020;5(13). Epub 2020/06/17. doi: 10.1172/jci.insight.139024 ; PubMed Central PMCID: PMC7406244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang ZH, Shu C, Ran X, Xie CH, Zhang L. Critically Ill Patients with Coronavirus Disease 2019 in a Designated ICU: Clinical Features and Predictors for Mortality. Risk Manag Healthc Policy. 2020;13:833–45. Epub 2020/08/09. doi: 10.2147/RMHP.S263095 ; PubMed Central PMCID: PMC7381092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Dong C, Li S, Song X, Wei W, Liu L. Triglyceride to High-Density Lipoprotein Cholesterol Ratio is an Important Determinant of Cardiovascular Risk and Poor Prognosis in Coronavirus Disease-19: A Retrospective Case Series Study. Diabetes Metab Syndr Obes. 2020;13:3925–36. Epub 2020/10/31. doi: 10.2147/DMSO.S268992 ; PubMed Central PMCID: PMC7591232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Huang B, Xia H, Fan H, Zhu M, Zhu L, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiol Infect. 2020;148:e199. Epub 2020/09/04. doi: 10.1017/S0950268820002010 ; PubMed Central PMCID: PMC7487751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146(1):89–100. Epub 2020/05/15. doi: 10.1016/j.jaci.2020.05.003 ; PubMed Central PMCID: PMC7212968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Z, Xu S, Chen J, Wei Q, Lin L, Ye X, et al. Predictive value of laboratory tests on severity of newly hospitalized patients with COVID-19. 新型冠状病毒肺炎患者入院时检验指标在病情严重程度中的预测价值. 2020;43(10):973–7. rayyan-111796663. [Google Scholar]
- 45.Zeng YL, Zhang C, Gao F, Ma L, Ding GG, Guo EE, et al. [Analysis of clinical characteristics of 49 cases of COVID-19]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(8):654–8. Epub 2020/07/31. doi: 10.3760/cma.j.cn112147-20200225-00184 . [DOI] [PubMed] [Google Scholar]
- 46.Chen LD, Zhang ZY, Wei XJ, Cai YQ, Yao WZ, Wang MH, et al. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir Res. 2020;21(1):201. Epub 2020/07/31. doi: 10.1186/s12931-020-01465-2 ; PubMed Central PMCID: PMC7389162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Liu Y, Li J, Sun L, Yang J, Xu F, et al. Immune characteristics distinguish patients with severe disease associated with SARS-CoV-2. Immunol Res. 2020;68(6):398–404. Epub 2020/09/30. doi: 10.1007/s12026-020-09156-2 ; PubMed Central PMCID: PMC7521864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, Yu MQ, Shen Q, Wang LZ, Yan RD, Zhang MY, et al. Analysis of inflammatory parameters and disease severity for 88 hospitalized COVID-19 patients in Wuhan, China. Int J Med Sci. 2020;17(13):2052–62. Epub 2020/08/14. doi: 10.7150/ijms.47935 ; PubMed Central PMCID: PMC7415392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Z, Yu H, Chen H, Qi W, Chen L, Chen G, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit Care. 2020;24(1):525. Epub 2020/08/29. doi: 10.1186/s13054-020-03255-0 ; PubMed Central PMCID: PMC7450961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinha P, Matthay MA, Calfee CS. Is a "Cytokine Storm" Relevant to COVID-19? JAMA Intern Med. 2020. Epub 2020/07/01. doi: 10.1001/jamainternmed.2020.3313 . [DOI] [PubMed] [Google Scholar]
- 51.Schijns V, Lavelle EC. Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity. Eur J Immunol. 2020. Epub 2020/05/22. doi: 10.1002/eji.202048693 ; PubMed Central PMCID: PMC7280664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckley LF, Wohlford GF, Ting C, Alahmed A, Van Tassell BW, Abbate A, et al. Role for Anti-Cytokine Therapies in Severe Coronavirus Disease 2019. Critical Care Explorations. 2020;2(8):e0178. doi: 10.1097/CCE.0000000000000178 02107256-202008000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. Jama. 2020. Epub 2020/03/18. doi: 10.1001/jama.2020.4344 . [DOI] [PubMed] [Google Scholar]
- 54.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–32. Epub 2020/03/21. doi: 10.1080/22221751.2020.1746199 ; PubMed Central PMCID: PMC7170333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020. Epub 2020/04/27. doi: 10.1016/j.jinf.2020.04.021 ; PubMed Central PMCID: PMC7177098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan X, Huang W, Ye B, Chen C, Huang R, Wu F, et al. Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol. 2020:1–7. Epub 2020/07/14. doi: 10.1007/s12185-020-02930-w ; PubMed Central PMCID: PMC7354745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon JH, Park SS, Jeon YW, Lee SE, Cho BS, Eom KS, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269–76. Epub 2018/09/15. doi: 10.3324/haematol.2018.198655 ; PubMed Central PMCID: PMC6355492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19(6):102537. Epub 2020/04/07. doi: 10.1016/j.autrev.2020.102537 ; PubMed Central PMCID: PMC7195002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mangalmurti N, Hunter CA. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53(1):19–25. Epub 2020/07/02. doi: 10.1016/j.immuni.2020.06.017 ; PubMed Central PMCID: PMC7321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J Infect Dis. 2020;221(11):1762–9. Epub 2020/04/01. doi: 10.1093/infdis/jiaa150 ; PubMed Central PMCID: PMC7184346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28(6):1871–6. Epub 2000/07/13. doi: 10.1097/00003246-200006000-00031 . [DOI] [PubMed] [Google Scholar]
- 62.Amgalan A, Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: Unanswered questions. J Thromb Haemost. 2020;18(6):1514–6. Epub 2020/04/12. doi: 10.1111/jth.14832 ; PubMed Central PMCID: PMC7262247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–8. Epub 2020/04/17. doi: 10.1007/s00277-020-04019-0 ; PubMed Central PMCID: PMC7156897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020. Epub 2020/04/03. doi: 10.1111/liv.14455 . [DOI] [PubMed] [Google Scholar]
- 65.Ridruejo E, Soza A. The liver in times of COVID-19: What hepatologists should know. Ann Hepatol. 2020. Epub 2020/05/20. doi: 10.1016/j.aohep.2020.05.001 ; PubMed Central PMCID: PMC7233236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52(2):267–75. Epub 2020/05/14. doi: 10.1111/apt.15813 ; PubMed Central PMCID: PMC7272838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delèvaux I, André M, Colombier M, Albuisson E, Meylheuc F, Bègue RJ, et al. Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis. 2003;62(4):337–40. Epub 2003/03/14. doi: 10.1136/ard.62.4.337 ; PubMed Central PMCID: PMC1754509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta. 2020;505:190–1. Epub 2020/03/08. doi: 10.1016/j.cca.2020.03.004 ; PubMed Central PMCID: PMC7094472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. Epub 2020/02/20. doi: 10.1111/jth.14768 ; PubMed Central PMCID: PMC7166509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–9. Epub 2020/03/29. doi: 10.1111/jth.14817 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–8. Epub 2020/04/15. doi: 10.1515/cclm-2020-0369 . [DOI] [PubMed] [Google Scholar]
- 72.Belfeki N, Strazzulla A, Picque M, Diamantis S. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199–202. Epub 2020/01/31. doi: 10.4081/reumatismo.2019.1221 . [DOI] [PubMed] [Google Scholar]
- 73.Moore C Jr., Ormseth M, Fuchs H. Causes and significance of markedly elevated serum ferritin levels in an academic medical center. J Clin Rheumatol. 2013;19(6):324–8. Epub 2013/08/24. doi: 10.1097/RHU.0b013e31829ce01f . [DOI] [PubMed] [Google Scholar]
- 74.Schram AM, Campigotto F, Mullally A, Fogerty A, Massarotti E, Neuberg D, et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood. 2015;125(10):1548–52. Epub 2015/01/13. doi: 10.1182/blood-2014-10-602607 . [DOI] [PubMed] [Google Scholar]
- 75.Gualdoni GA, Hofmann GA, Wohlfarth P, Winkler HM, Winkler S, Haslacher H, et al. Prevalence and Outcome of Secondary Hemophagocytic Lymphohistiocytosis Among SIRS Patients: Results from a Prospective Cohort Study. J Clin Med. 2019;8(4). Epub 2019/04/24. doi: 10.3390/jcm8040541 ; PubMed Central PMCID: PMC6518152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. A possible role for secreted ferritin in tissue iron distribution. J Neural Transm (Vienna). 2011;118(3):337–47. Epub 2011/02/08. doi: 10.1007/s00702-011-0582-0 . [DOI] [PubMed] [Google Scholar]
- 77.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–25. Epub 2020/04/27. doi: 10.1016/j.jinf.2020.04.021 ; PubMed Central PMCID: PMC7177098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Statistics in Medicine. 2007;26(25):4544–62. doi: 10.1002/sim.2889 [DOI] [PubMed] [Google Scholar]
- 79.Chi Y, Ge Y, Wu B, Zhang W, Wu T, Wen T, et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J Infect Dis. 2020;222(5):746–54. Epub 2020/06/21. doi: 10.1093/infdis/jiaa363 ; PubMed Central PMCID: PMC7337752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu ZJ, Xu J, Yin JM, Li L, Hou W, Zhang LL, et al. Lower Circulating Interferon-Gamma Is a Risk Factor for Lung Fibrosis in COVID-19 Patients. Front Immunol. 2020;11:585647. Epub 2020/11/03. doi: 10.3389/fimmu.2020.585647 ; PubMed Central PMCID: PMC7550399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu D, Cui P, Zeng S, Wang S, Feng X, Xu S, et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: A multicenter, retrospective, cohort study. EClinicalMedicine. 2020;25:100471. Epub 2020/08/26. doi: 10.1016/j.eclinm.2020.100471 ; PubMed Central PMCID: PMC7391125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao C, Bai Y, Wang C, Zhong Y, Lu N, Tian L, et al. Risk factors related to the severity of COVID-19 in Wuhan. Int J Med Sci. 2021;18(1):120–7. Epub 2021/01/05. doi: 10.7150/ijms.47193 ; PubMed Central PMCID: PMC7738952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zou L, Dai L, Zhang Y, Fu W, Gao Y, Zhang Z, et al. Clinical Characteristics and Risk Factors for Disease Severity and Death in Patients With Coronavirus Disease 2019 in Wuhan, China. Front Med (Lausanne). 2020;7:532. Epub 2020/09/10. doi: 10.3389/fmed.2020.00532 ; PubMed Central PMCID: PMC7438719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. China National Health Commission.
- 85.World Health O. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. Geneva: World Health Organization, 2020. 2020. Report No.: Contract No.: WHO/nCoV/Clinical/2020.3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.