Abstract
Relatively little is known about associations between peripheral inflammation and neural function in humans. Neuroimaging studies in adults have suggested that elevated peripheral inflammatory markers are associated with altered resting state functional connectivity (rsFC) in several brain networks associated with mood and cognition. Few studies have examined these associations in adolescents, yet scarce data from adolescents point to different networks than adult studies. The current study examined the associations between peripheral inflammation and rsFC in a community sample of adolescents (n = 70; age, 12–15 years; 32 female, 36 male, 2 nonbinary). After blood sampling, an fMRI scan was performed to assess rsFC. Assay for serum inflammatory markers, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), was performed. Results indicated that higher TNF-α was associated with altered rsFC between the right amygdala and left striatum and between the right inferior frontal gyrus and left parietal cortex (p < 0.05 whole-brain corrected). Associations with IL-6 and CRP were not significant. In contrast with findings in adults, inflammation may have unique links with the connectivity of the developing adolescent brain. Results have implications for understanding how peripheral inflammation may influence connectivity during adolescence, when neural networks are undergoing major developmental changes.
Keywords: Inflammation, fMRI, Adolescence, Resting state connectivity, Brain, Amygdala
1. Introduction
The neuroimmune network hypothesis proposes that bidirectional communication between the immune system and the brain alters neural function, with implications for mental health (Nusslock and Miller, 2016). Emerging evidence in adults supports this hypothesis and suggests that higher peripheral inflammation is linked to specific alterations in brain function, but relatively little is known about how peripheral inflammation is associated with brain function during adolescence, when the brain is still undergoing major developmental changes and may be particularly sensitive to both exogenous and endogenous influences. The neuroimmune network hypothesis integrates evidence from animal models that shows that peripheral inflammation can reach the central nervous system through several different routes (Nusslock and Miller, 2016). Specifically, peripheral cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) can access the brain through several mechanisms, including carrier-mediated transport, via leaky regions in the blood–brain barrier, or activating receptors on afferent nerves (e.g., the vagus nerve) that stimulate the release of cytokines centrally (Felger and Miller, 2012; Capuron and Miller, 2011). Through these routes, peripheral inflammatory signaling is relayed centrally through the brainstem to other regions in the brain including the hypothalamus, amygdala, hippocampus, striatum, and thalamus (Kraynak et al., 2018). Thus, peripheral inflammatory markers could affect central nervous system function through a variety of routes.
In adults, studies have used functional magnetic resonance imaging (fMRI) to examine associations between peripheral inflammatory markers and resting state functional connectivity (rsFC). This research has identified several patterns of altered rsFC associated with peripheral inflammation. First, cross-sectional research in adults with depression has shown that higher levels of the peripheral inflammatory marker, C-reactive protein (CRP), are associated with reduced rsFC between the amygdala and ventromedial prefrontal cortex (Mehta et al., 2018). Second, in adults with depression, elevated CRP levels are correlated with reduced rsFC between the ventral striatum and ventromedial prefrontal cortex (Felger et al., 2016). Third, in adults with depression, higher CRP is associated with decreased connectivity between the ventromedial prefrontal cortex and a range of regions, including the striatum, insula, orbital and dorsolateral prefrontal cortex, and temporal and associative cortices (e.g., temporal gyrus, postcentral gyrus, precuneus) (Yin et al., 2019). Fourth, in a community sample of adults, higher levels of the inflammatory marker IL-6 are associated with increased connectivity between the subgenual anterior cingulate cortex and default mode network, as well as decreased connectivity between the dorsomedial prefrontal cortex and default mode network (Marsland et al., 2017). Finally, studies that use an acute inflammatory challenge to transiently increase inflammation show that higher inflammation (as indexed by increased IL-6, TNF-α, and/or IL-8) is associated with increased connectivity between the anterior insula and midcingulate cortex (Lekander et al., 2016), decreased connectivity between salience network regions, including the insula, amygdala, dorsal anterior cingulate cortex, and anterior prefrontal cortex (Labrenz et al., 2019), and increased thalamus-cerebellum connectivity as well as decreased connectivity between the amygdala and fronto-parietal regions, between the insula and frontal, temporal, and parietal regions, and between the anterior cingulate cortex and frontal and temporal regions (Labrenz et al., 2016). The pattern of results in adults generally suggests that higher peripheral inflammation is associated with decreased subcortico-cortical as well as decreased cortico-cortical connectivity; however, most studies conducted in adults have examined different regions of interest, making it difficult to draw strong conclusions across studies.
In comparison to adults, findings in adolescent participants are limited, which stands in sharp contrast to the salient developmental changes of these resting state networks during the period of adolescence (van Duijvenvoorde et al., 2019). It is currently unknown whether the associations between inflammation and rsFC in adolescents are similar to findings in adults. Only one study to our knowledge has examined the association between peripheral inflammation and rsFC in adolescents. Specifically, Nusslock et al. (2019) examined the association between an inflammation composite variable that included CRP, IL-6, TNF-α, and IL-10 and resting state connectivity in two samples, a sample of 82 African American adolescents aged 13–14 years old and a sample of 90 African American young adults aged 25 years old. In the sample of adolescents, they found that higher inflammation was associated with lower rsFC within an “emotion regulation” network comprising the somatomotor area, inferior frontal gyrus, precentral gyrus, middle temporal gyrus, and angular gyrus, and lower rsFC within a “central executive” network comprising the inferior parietal lobule, middle frontal gyrus, middle temporal gyrus, medial frontal gyrus, and right caudate. In the sample of young adults, they found that higher inflammation was associated with lower rsFC in the emotion regulation network. Contrary to some results observed in adults (Yin et al., 2019; Marsland et al., 2017; Lekander et al., 2016; Labrenz et al., 2019, 2016), findings in the adolescent and young adult samples showed no significant associations between inflammation and rsFC within the salience network or default mode network. Taken together with results of studies in adults, the results in adolescents also suggest that higher inflammation is associated with decreased cortico-cortical connectivity, although generally in different regions than those identified in adults.
In sum, in both adolescents and adults, there is evidence that higher peripheral inflammation is associated with decreased cortico-cortical connectivity, but the specific regions where connectivity differences are observed may differ. In adults, there is also evidence that higher peripheral inflammation is associated with decreased subcortico-cortical connectivity. These initial results suggest that more research is needed in adolescent samples to determine the associations between peripheral inflammation and rsFC during this crucial developmental stage. To address this gap in knowledge, the primary goal of the current study was to examine whether peripheral inflammatory markers are associated with rsFC in adolescents aged 12 to 15 years old. Based on prior research in adolescents and adults, we chose 11 seed regions of interest (ROIs) to examine how peripheral inflammation is associated with rsFC between these regions and the rest of the brain. First, we chose the left and right amygdala and left and right nucleus accumbens as ROIs, based on research in adults finding that inflammation is associated with decreased connectivity between these regions and prefrontal regions (Mehta et al., 2018; Felger et al., 2016; Labrenz et al., 2016). Next, we chose the ventromedial prefrontal cortex as an ROI, based on research in adults finding reduced connectivity between the ventromedial prefrontal cortex and a range of other regions (Yin et al., 2019). We chose the left and right insula as ROIs based on research in adults finding that higher inflammation is associated with both increased insula connectivity (Lekander et al., 2016) and decreased insula connectivity with other regions (Labrenz et al., 2019, 2016). Finally, to limit the number of comparisons performed, we selected one ROI from each of the networks associated with peripheral inflammation in the adolescent sample (Nusslock et al., 2019) based on theoretical grounds and our secondary goal of examining associations with symptoms of depression (see next paragraph). For the “emotion regulation” network, we selected the left and right inferior frontal gyrus based on meta-analytic evidence that the ventrolateral prefrontal cortex/inferior frontal gyrus is abnormally activated across a range of psychiatric disorders (McTeague et al., 2020). For the “central executive” network, we chose the left and right middle frontal gyrus (corresponding to the dorsolateral prefrontal cortex), based on meta-analytic evidence that this region is abnormally activated across a range of psychiatric disorders (McTeague et al., 2020). We hypothesized that higher inflammation would be associated with decreased subcortico-cortical as well as decreased cortico-cortical connectivity.
Although not a primary goal of the study, we also planned to test whether the inflammatory markers or any of the patterns of rsFC associated with inflammatory markers in this sample were cross-sectionally associated with depressive symptoms. This secondary goal was motivated by research in adults (Dowlati et al., 2010; Zorrilla et al., 2001; Valkanova et al., 2013; Fraguas et al., 2019) and adolescents (Miller and Cole, 2012; Khandaker et al., 2014; Slavich et al., 2020; Kim et al., 2014; Flouri et al., 2020) demonstrating that higher peripheral inflammation can increase risk for several psychiatric disorders including depression; we therefore sought to test whether any of the patterns of connectivity associated with inflammation were also associated with depressive symptoms in this sample.
2. Methods and materials
2.1. Participants
Participants were from the ongoing Adolescent Health and Brain (AHB) Study, which was designed to examine associations between peripheral inflammation, brain function, and mental health. Participants were recruited from the community through a variety of methods, including mailing postcard advertisements to households, posting fliers in community settings (e.g., coffee shops) and adult mental health clinics, advertising online (e.g., Facebook and Craigslist), and setting up recruitment tables at the local mall, farmer’s market, and other community events. Non-overlapping analyses using other measures from a subset of the participants in this study have been reported in prior published research (Swartz et al., 2019).
Inclusion criteria were that participants were between 12 and 15 years old; could speak, read, and write in English; were capable of understanding all study procedures, providing informed assent, and remaining supine and still in the MRI scanner. Exclusion criteria included a diagnosis of autism spectrum disorder, attention-deficit/hyperactivity disorder, schizophrenia, or bipolar disorder (based on parent report of whether their child had ever received one of these diagnoses from a mental health professional); chronic disease or condition that could affect cerebral blood flow such as hypertension or diabetes; use of psychotropic medications such as selective serotonin reuptake inhibitors, medications for high blood pressure (e.g., beta blockers), glucocorticoids (e.g., dexamethasone), or medications for high cholesterol (e.g., statins); and any contraindications to MRI scanning (e.g., braces, metal in the body, pregnancy, etc.). All procedures were approved by the University of California, Davis Institutional Review Board; parents provided informed consent and adolescents provided informed assent before beginning study procedures.
The AHB study is ongoing; the current paper reports on the results of the first 104 participants recruited for the study through November 15, 2019, for which inflammatory marker data have been assayed. Of these 104 participants enrolled in the study through November 15, 2019, 5 participants refused to provide a blood sample, 2 participants did not attend the MRI scan, and 7 participants attended the MRI but did not complete a resting state scan due to ending the MRI scan early, technical difficulties with the stimulus computer, or running out of time during the scanning appointment, leaving 90 participants with both a blood sample and resting state MRI scan. Of these participants, 19 did not meet quality control criteria for the resting state scan (see quality control section below), resulting in a total of 71 participants who had usable rsFC data meeting quality control criteria and a blood sample. In addition, one participant was removed from the current analyses because they reported use of antibiotics for an infection, resulting in a final sample size of 70 participants. We examined whether the final sample of 70 participants differed significantly from the excluded participants on any key study variables. There was no difference between included and excluded participants in age, t(102) = −0.25, p = 0.80, gender, χ2(2) = 1.70, p = 0.43, depressive symptoms, t(100) = 0.65, p = 0.521, or the IL-6/CRP inflammation composite, t(97) = −0.15, p = 0.88.2 There was a marginally significant difference for TNF-α, in which included participants had higher TNF-α (M = 0.13, SD = 1.01) compared to excluded participants (M = −0.31, SD = 0.92), t(97) = −2.02, p = 0.046.
Participants were 12 to 15 years old (M = 13.60, SD = 1.04); 32 were female, 36 were male, and 2 identified as non-binary. There were 4 sets of siblings in the sample. Given the small number of sibling sets in the sample, we did not statistically adjust for clustering within families for this paper. Participants’ race/ethnicity was assessed through self-report: 6 self-reported as Asian, 3 as Black or African American, 5 as Hispanic/Latino, 3 as Native Hawaiian or Pacific Islander, 26 as White, 24 as two or more races, 1 as other, and 2 did not report their race. Parents were asked to report whether their child or any of the child’s first-degree relatives had ever received a psychiatric diagnosis from a health professional such as a psychologist or psychiatrist. One parent reported that their child enrolled in the study had received a diagnosis of a mood disorder. Fourteen participants (20% of the sample) had a first-degree relative that had received a diagnosis (past or current) of a mood disorder according to parent report. Participant characteristics are reported in Table 1 and bivariate correlations between key study variables and nuisance regressors are reported in Table 2.
Table 1.
Participant Characteristics.
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age | 13.60 | 1.04 | 12 | 15 |
| BMI | 22.08 | 5.34 | 14.8 | 43.6 |
| IL-6 (pg/mL) | 0.49 | 0.48 | 0.10 | 3.00 |
| TNF-α (pg/mL) | 2.49 | 0.62 | 1.33 | 4.35 |
| CRP (mg/L) | 1.60 | 4.12 | 0.06 | 23.90 |
| CDI-2 Total | 7.45 | 6.40 | 0 | 28 |
| CESDS-C Total | 11.74 | 8.75 | 0 | 39 |
| Inflammation composite (Z-score) | 0.00 | 1.81 | −2.87 | 5.23 |
| Depressive symptoms (Z-score) | 0.00 | 1.82 | −4.51 | 3.56 |
Note: SD = Standard deviation; Min = Minimum; Max = Maximum. BMI = Body mass index; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-alpha; CRP = C-reactive protein; CDI-2 = Children’s Depression Inventory-2; CESDS-C = Center for Epidemiologic Studies Depression Scale for Children. Values reported in table for BMI, IL-6, TNF-α, CRP, CDI, and CESDS-C are reported for non-transformed data for ease of interpretation. These variables were transformed before statistical analysis. The inflammation composite was obtained by summing Z-score values for IL-6 and CRP. The depressive symptoms composite was obtained by summing Z-score values for the CDI-2 and CESDS-C.
Table 2.
Bivariate correlations.
| IL-6 | CRP | TNF-α | Inflammation composite |
Depressive symptoms |
Gender | Age | |
|---|---|---|---|---|---|---|---|
| IL-6 | 1 | ||||||
| CRP | 0.64*** | ||||||
| TNF-α | 0.18 | 0.18 | |||||
| Inflammation composite | 0.91*** | 0.91*** | 0.20 | ||||
| Depressive symptoms | 0.27* | 0.08 | −0.19 | 0.19 | |||
| Gender | −0.001 | 0.07 | 0.25* | 0.04 | −0.11 | ||
| Age | 0.22 | 0.20 | 0.05 | 0.23 | 0.22 | −0.02 | |
| BMI | 0.61*** | 0.53*** | 0.03 | 0.63*** | 0.25* | 0.03 | 0.19 |
| Time of day of blood draw | 0.04 | −0.11 | −0.18 | −0.04 | 0.003 | −0.24* | 0.08 |
| Days between visits | −0.13 | −0.13 | −0.07 | −0.14 | −0.15 | 0.11 | 0.03 |
| Length of rsFC scan | −0.20 | −0.01 | 0.13 | −0.12 | 0.01 | −0.02 | 0.06 |
| Mean FD | −0.08 | −0.07 | −0.02 | −0.08 | −0.19 | −0.02 | −0.21 |
| Med use | 0.13 | 0.02 | 0.04 | 0.08 | 0.07 | −0.08 | 0.21 |
Note:
p < 0.05
p < 0.01
p < 0.001. The inflammation composite is the sum of Z-scored and log-transformed IL-6 and CRP. The BMI variable was log-transformed. The gender variable included in this table is the dummy-coded variable for female vs. male gender (dummy-coded female = 0; male = 1). Mean FD = mean framewise displacement. Med use = medication use (dummy-coded 0 = no meds; 1 = meds).
2.2. Procedure
The study was conducted during two visits. During the first visit, which was conducted at the UC Davis CTSC Clinical Research Center (CCRC), participants provided informed consent/assent. Next, nurses at the CCRC took anthropometric measurements to calculate body mass index (BMI) and the temperature of the adolescent, to confirm absence of a fever. Adolescents then provided one non-fasting blood sample of 6 ml drawn by a CCRC certified phlebotomist via antecubital venipuncture. Visits generally occurred in the afternoon (mean time of day for blood sample was 2:00 pm, although they ranged from 8:44 am to 5:15 pm). Time of day of the blood sample was included as a covariate in analyses, as described below. After this, participants and their accompanying parent completed self-report measures on tablets using Qualtrics survey software to assess depressive symptoms and other measures.
The second study visit included the MRI scan and was conducted at the UC Davis Imaging Research Center. This visit generally took place within a week of the first visit (mean time between visits = 4.16 days; SD = 6.48; range = 0 to 30 days). During this visit, all participants first completed a practice scan in a mock scanner to become accustomed to the MRI scanner and to learn and practice each MRI task. During MRI scanning, participants completed three tasks, followed by a resting state scan. For the present paper, we only report results from the resting state scan.
2.3. Inflammatory markers
CCRC staff processed the blood samples within 2 h of collection, including centrifuging and serum aliquoting, and samples were stored on site in a −80C freezer located in the CCRC. Frozen serum samples were then shipped to the UCLA Cousins Center for Psychoneuroimmunology to be assayed. A panel of six immune markers was assayed from serum samples, including IL-6, IFN-g, IL-10, IL-8, TNF-α (using Multiplex assays by Meso Scale Discovery, Rockville, Maryland), and CRP (using a high-sensitivity ELISA assay by R&D Systems, Minneapolis, MN). Mean inter-assay CVs for IL-6, IFN-g, IL-10, IL-8, TNF-α, and CRP were 4.0%, 6.1%, 11.3%, 3.6%, 5.6%, and 1.2%, respectively. Mean intra-assay CVs were 1.0%, 1.2%, 12.5%, 0.5%, 4.9%, 1.0%, respectively. Samples were assayed in duplicate. Since the primary hypotheses were focused on inflammation, we selected the inflammation markers IL-6, TNF-α, and CRP for the present analyses. We selected these three markers based on theoretical grounds. Specifically, IL-6 and TNF- α are both pro-inflammatory cytokines and both regulate CRP production (Baumann and Gauldie, 1994; Calabró et al., 2003), and thus they are closely linked biologically. Moreover, these are the markers that have most frequently been examined in relation to rsFC in prior research, facilitating comparison with other studies. Future analyses will consider the roles of IFN-g, which plays important roles in regulating antiviral immunity, the chemokine IL-8, and the anti-inflammatory cytokine IL-10. Because IL-6 and CRP were significantly correlated (Table 2), we combined these into a composite variable. Because TNF-α was not significantly correlated with the other markers, we examined this as a separate variable. We log-transformed all markers before Z-scoring them. We then summed the log-transformed and Z-scored IL-6 and CRP to create the composite.
2.4. fMRI data acquisition
Scanning took place at the UC Davis Imaging Research Center on a research-dedicated 3 T Siemens TIM Trio MRI system. After acquiring localizer scans, a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence was applied to obtain high-resolution structural images with the following parameters: TR/TE/flip angle = 2500 ms/4.33 ms/7; FOV = 243 mm; 208 slices; slice thickness = 0.95 mm, voxel size: 0.9 mm isotropic. After high-resolution structural images were acquired, a semi-automated high-order shimming program was used to ensure global field homogeneity. After the shimming program, participants completed the tasks and the resting state scan while blood-oxygen-level dependent (BOLD) images were collected. In between tasks, a field map was collected with slices prescribed along the EPI slices, with TE 1 = 2.46 ms and TE 2 = 4.92 ms, for static magnetic field inhomogeneity correction.
For the BOLD sequence, a series of 35 interleaved transversal slices were acquired for full-brain coverage with a gradient-echo based, echo planar imaging (EPI) sequence with the following parameters: TR/TE/flip angle = 2000 ms/27 ms/80; FOV = 224 mm; slice thickness = 3.5 mm, voxel size: 3.5 mm isotropic. For the first 13 participants reported on in this paper, 200 volumes of rsFC data were collected, for a total rsFC length of 6.7 min. For the remaining participants, the rsFC collection was shortened to allow for the addition of a new modality (diffusion tensor imaging), and so for the remaining 57 participants in the sample, 168 volumes of rsFC data were collected, for a total rsFC length of 5.6 min. We included a covariate in all statistical analyses to control for the difference in rsFC length, as described below in the covariates section. Participants viewed a white fixation cross on a black screen during the resting state acquisition.
2.5. Resting state functional connectivity
The rsFC images were pre-processed using the CONN toolbox v.17.f (https://web.conn-toolbox.org) with the ‘default_mniphase’ option because high-quality field maps were available. Pre-processing steps included realignment and unwarping using the field map, slice-timing correction, detection of outlier scans, segmentation and normalization (resampled to 2 mm isotropic voxels) of functional images into a standard stereotaxic space (Montreal Neurological Institute; MNI), and smoothing with a 6 mm FWHM Gaussian kernel. In addition, images were processed with the CONN toolbox’s default denoising pipeline, including censoring (i.e., scrubbing) volumes according to the conservative criteria within the CONN toolbox (any volumes exceeding a global-signal Z-value threshold of 3 or a 0.5 mm volume-to-volume motion threshold), and adding nuisance regressors for noise components from white matter and cerebrospinal areas (using the anatomical component-based noise correction procedure) and subject motion parameters (3 translation, 3 rotation, and their first-order derivatives). During this step, BOLD images were bandpass filtered to a 0.01 Hz-0.1 Hz window. Participants with more than 15% of volumes censored by motion scrubbing were excluded from analyses; this resulted in the removal of 18 participants from the data set. In addition, images were visually inspected for quality control, and one participant was removed due to artifact in the images, resulting in the removal of a total of 19 participants for quality control, as described above in the participants section.
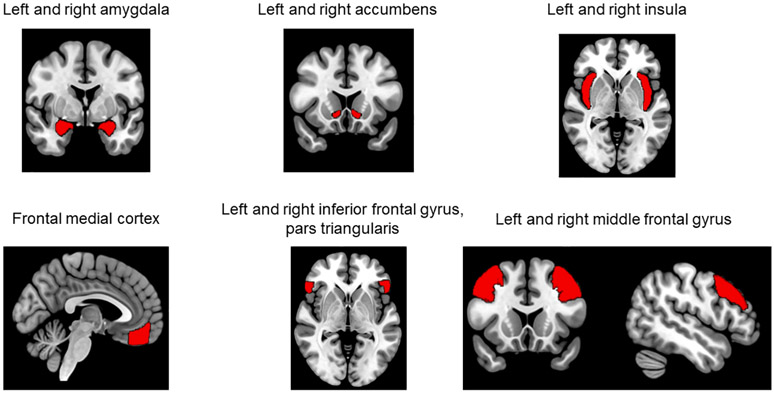
To test our hypotheses regarding rsFC, we used seed-to-voxel analysis. The CONN toolbox calculates seed-based connectivity maps with Fisher-transformed bivariate correlation coefficients. To limit the number of comparisons performed, we selected 11 seed ROIs based on prior research and theoretical considerations. ROIs were all selected from the standard CONN toolbox cortical atlas, which is derived from the Harvard-Oxford structural atlas (Fig. 1). ROIs with the following names from the CONN toolbox atlas were selected: left and right amygdala (327 and 342 voxels, respectively), left and right accumbens (107 and 84 voxels, respectively), left and right insular cortex (1334 and 1344 voxels, respectively), frontal medial cortex (984 voxels), left and right inferior frontal gyrus, pars triangularis (650 and 555 voxels, respectively), and left and right middle frontal gyrus (2924 and 2740 voxels, respectively).
Fig. 1.
Regions of interest for seed-to-whole brain resting state functional connectivity analysis. Note: Regions of interest were obtained from the CONN toolbox atlas, which is derived from the Harvard-Oxford atlas. Coronal and sagittal views of the middle frontal gyrus are both shown to better depict this ROI.
2.6. Depressive symptoms
Depressive symptoms were measured through adolescent self-report with the well-validated Children’s Depression Inventory 2 (CDI-2) (Kovacs, 2010) and the Center for Epidemiologic Studies Depression Scale for Children (CESDS-C) (Faulstich et al., 1986). The CDI assesses depressive symptoms in the past two weeks with 28 items on a scale of 0 to 2. This measure demonstrated good reliability in this sample, Cronbach’s alpha = 0.88. This variable was skewed and so was log-transformed. The CESDS-C assesses depression symptoms in the past week with 20 items on a scale of 0 to 3. This measure demonstrated good reliability, Cronbach’s alpha = 0.87, and was also log-transformed to adjust for skewness. Scores on the CDI and CESDS-C were significantly correlated, r = 0.66, p < 0.001, and so they were Z-scored and summed into a single depressive symptom variable for analyses. One participant was excluded from this analysis because their Qualtrics data were missing due to technical difficulties with the tablets, and one participant was excluded because they skipped half of the questions on the CDI-2, resulting in 68 participants available to examine associations between rsFC and depressive symptoms.
2.7. Covariates
Covariates included in all analyses were gender (dummy-coded for male, female, and non-binary groups, with female as the reference group); age; BMI, to control for correlations with both inflammation and depressive symptoms (see Table 2); time of day for the blood draw, to control for circadian variation in the inflammatory markers; number of days between blood draw and MRI scan; length of resting state scan (0 = longer length; 1 = shorter length); mean framewise displacement from the rsFC scan (Power et al., 2012); and medication use (0 = no medications reported; 1 = medications). The following medications (based on participant self-report) were coded as 1 for this single variable: antihistamines, oral contraceptives, nonsteroidal antinflammatory drugs such as ibuprofen, and bronchodilators such as albuterol. Sixteen participants reported they were taking one or more of these medications. One participant was missing medication data due to technical difficulties with the Qualtrics questionnaire, so was coded as 0 for this variable.
2.8. Statistical analyses: Peripheral inflammation and rsFC
Our primary goal was to examine the association between peripheral inflammatory markers and resting state connectivity with the selected ROIs. To achieve this, we conducted whole-brain multiple regressions in SPM for each seed region selected. The main variable of interest in this regression was the inflammation variable, either the IL-6/CRP composite or TNF-α.
We used 3dClustSim in AFNI to calculate cluster-size thresholds to achieve a whole-brain correction of p < 0.05. To conduct 3dClustSim cluster size calculations, we saved out residuals from the second-level group analysis (the multiple regression), which creates one residual file for each participant in the analysis. We then used 3D to 4D file conversion in SPM to create one 4D residual file containing the residuals for each participant in the analysis. This residual file was then entered into AFNI’s 3DFWHMx using AFNI version 20.3.02 to estimate the smoothness in the residuals of the data with the ‘acf’ option. Cluster size threshold was determined using 2-sided thresholding with the first-nearest neighbor clustering (NN1) option. To examine whole-brain effects, we used an uncorrected voxelwise p-value of p < 0.002 and a cluster extent threshold based on the 3dClustSim results (see Table 3). One of the limitations of 3dClustSim is that it determines whether an observed cluster is below a specified significance threshold, but does not provide an exact p-value. Therefore, in order to conduct a Bonferroni correction for the number of comparisons tested, we used the cluster-level q (FDR-corrected p-value) provided by SPM to conduct a multiple comparisons correction. Specifically, a cluster-level q < 0.0022 from the SPM output was determined to meet correction for 22 comparisons (11 seed regions × 2 inflammation variables).
Table 3.
Cluster extent threshold for 3dClustSim correction.
| Seed region | Required cluster size for whole-brain p < 0.05 for IL-6/CRP composite | Required cluster size for whole-brain p < 0.05 for TNF-α |
|---|---|---|
| Left Amygdala | 267 | 275 |
| Right Amygdala | 238 | 225 |
| Left Accumbens | 250 | 240 |
| Right Accumbens | 251 | 239 |
| Left Insula | 191 | 213 |
| Right Insula | 230 | 229 |
| Medial Prefrontal Cortex | 219 | 214 |
| Left Inferior Frontal Gyrus | 240 | 250 |
| Right Inferior Frontal Gyrus | 250 | 252 |
| Left Middle Frontal Gyrus | 215 | 225 |
| Right Middle Frontal Gyrus | 175 | 184 |
Note: Residuals for 3dClustSim were obtained by saving residuals from the multiple regression group analysis for each seed region. Resulting smoothness estimates were used for conducting 3dClustSim in AFNI. Cluster size extent thresholds in this table are based on an uncorrected p-value of p < 0.002. Cluster size was determined using 2-sided thresholding with −NN 1.
2.9. Statistical analyses: Inflammatory markers, rsFC, and depressive symptoms
A secondary goal was to examine whether the inflammatory markers or any of the patterns of connectivity associated with peripheral inflammation were also associated with depressive symptoms in the sample. To test the association between the inflammation variables and depressive symptoms, we conducted a multiple regression analysis including all covariates described above. To examine the association between rsFC and depressive symptoms, we extracted connectivity values for rsFC from any clusters found to be significant in the whole-brain rsFC analyses described above using the eigenvariate function in SPM12. These connectivity values were then imported into SPSS for further analyses. To examine the association between rsFC and depressive symptoms, we conducted a multiple regression analysis with the rsFC connectivity values as a predictor variable and depressive symptoms as the dependent variable, including all covariates described above.
3. Results
3.1. Peripheral inflammation and rsFC
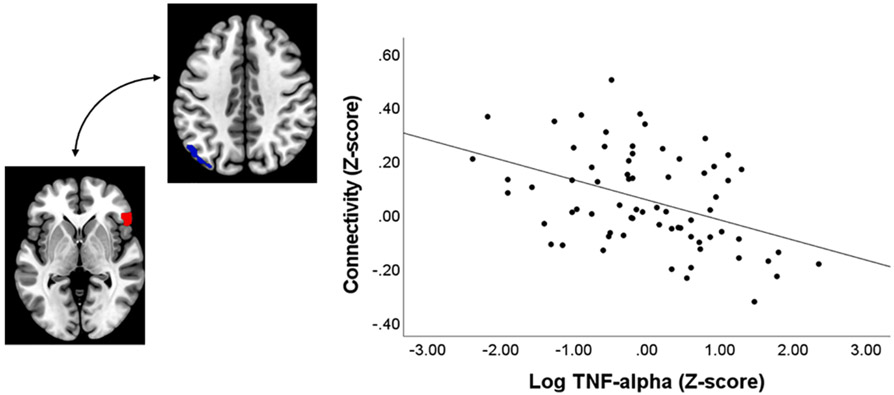
We identified two patterns of connectivity associated with TNF-α. First, when examining results for the amygdala, we observed a significant positive association between TNF-α and right amygdala connectivity with the left striatum, including the ventral striatum, caudate, and putamen (Table 4; Fig. 2), and this effect survived correction for multiple comparisons. In addition, TNF-α was negatively associated with connectivity between the right inferior frontal gyrus (IFG) and left parietal cortex (Table 4; Fig. 3), and this effect survived correction for multiple comparisons. There were no significant effects surviving correction for the IL-6/CRP composite variable.
Table 4.
Associations between TNF-α and resting state connectivity.
| Seed Region |
t (59) |
Voxelwise p- uncorrected (peak voxel) |
Cluster Size (voxels) |
Cluster-level q-FDR corrected |
Coordinates at peak local maxima within cluster |
Label of cluster | Direction of association |
|---|---|---|---|---|---|---|---|
| Left amygdala | No clusters reaching significance | ||||||
| Right amygdala | 4.84 | <0.001 | 706 | <0.001* | (−18, 14, −8) | Left putamen/caudate/ventral striatum | Positive |
| 4.49 | <0.001 | (−20, 2, −8) | |||||
| 4.05 | <0.001 | (−28, −8, −6) | |||||
| Left accumbens | No clusters reaching significance | ||||||
| Right accumbens | No clusters reaching significance | ||||||
| Left insula | No clusters reaching significance | ||||||
| Right insula | No clusters reaching significance | ||||||
| Medial prefrontal cortex | No clusters reaching significance | ||||||
| Left inferior frontal gyrus | No clusters reaching significance | ||||||
| Right inferior frontal gyrus | 5.48 | <0.001 | 353 | 0.0015* | (−50, −78, 30) | Left angular gyrus/middle occipital gyrus/parietal cortex | Negative |
| 4.47 | <0.001 | (−54, −68, 38) | |||||
| 4.42 | <0.001 | (−50, −68, 46) | |||||
| Left middle frontal gyrus | No clusters reaching significance | ||||||
| Right middle frontal gyrus | No clusters reaching significance | ||||||
Note:
signifies that this cluster reaches a Bonferroni-corrected p-value < 0.0022 and is significant adjusting for 22 comparisons. Labels for coordinates are determined based on the AAL atlas in the WFU Pickatlas tool as well as from labels derived from Neurosynth.org.
Fig. 2.
Association between TNF-α and right amygdala connectivity. Note: Region where TNF-α is significantly associated with resting state connectivity using right amygdala as seed. Cluster in red depicts right amygdala seed ROI and cluster in blue depicts the striatal region where a significant effect was observed, thresholded at p < 0.05 whole-brain corrected using the 3dClustSim correction.
Fig. 3.
Association between TNF-α and right inferior frontal gyrus connectivity. Note: Region where TNF-α is significantly associated with resting state connectivity using right inferior frontal gyrus as seed. Cluster in red depicts right inferior frontal gyrus seed ROI and cluster in blue depicts the parietal cortex region where a significant effect was observed, thresholded at p < 0.05 whole-brain corrected using the 3dClustSim correction.
3.2. Associations with depressive symptoms
Neither the IL-6/CRP composite or TNF-α were associated with depressive symptoms in the multiple regression analysis (all p’s greater than 0.05). We extracted rsFC values from all significant clusters identified above and imported them into SPSS to examine whether connectivity in any of these clusters was associated with depressive symptoms. Results of the multiple regressions indicated that amygdala-striatum connectivity was not significantly associated with depressive symptoms when not controlling for TNF-α in the regression (p = 0.33), and when controlling for TNF-α in the regression (p = 0.89). There was an association between IFG-parietal connectivity and depressive symptoms when not controlling for TNF-α in the regression, B = 2.93, SE = 1.29, p = 0.03; however, when controlling for TNF-α in the regression, this association was no longer significant, B = 2.52, SE = 1.52, p = 0.10.
4. Discussion
The goal of this paper was to examine cross-sectional associations between peripheral inflammation and rsFC of 11 ROIs that included cortical and subcortical regions in adolescents 12 to 15 years old. We observed two significant effects that survived correction for multiple comparisons. First, there was a positive association between TNF-α and right amygdala connectivity with the left striatum. Second, there was a negative association between TNF-α and right inferior frontal gyrus connectivity with the left parietal cortex. Below, we discuss each of these results separately, their implications for emotional and cognitive functioning, and how these compare to prior research conducted in adolescents and adults.
First, we found that higher TNF-α was associated with increased rsFC between the right amygdala and left striatum in a cluster that encompassed the ventral striatum, caudate, and putamen. This is different from what has been observed in adults, in that studies in adults have found that inflammation is associated with altered connectivity between the amygdala and mPFC (Mehta et al., 2018) and between the ventral striatum and mPFC (Felger et al., 2016), but not between the amygdala and striatum. This could be because subcortico-cortical and cortico-subcortical connectivity exhibit protracted courses of development extending across the period of adolescence, so that associations between inflammation and altered connectivity between subcortical regions and the medial prefrontal cortex may not be evident until later in adolescence (Casey et al., 2019). The one prior study conducted in adolescents did not test for associations between inflammation and connectivity between the amygdala and striatum (Nusslock et al., 2019). Thus, a novel implication of the current results is that, in adolescents, higher peripheral inflammation may be associated with heightened subcortico-subcortical connectivity, potentially because these connections are still developing during adolescence.
This novel finding aligns with what is known about the development of these regions from childhood to adulthood. Specifically, prior research has shown that significant positive coupling between the ventral striatum and amygdala is evident starting in childhood and that these regions remain positively coupled across adolescence and early adulthood (Fareri et al., 2015). These regions work together to process information about reward and punishment. The amygdala responds to all salient and emotional stimuli, both positive and negative, but tends to respond most strongly to threatening or stressful stimuli. The striatum is involved in reward learning and reward processing and integrates information about reward and punishment. It is possible that increased connectivity between these regions could shift processing towards more aversive or punishing stimuli during reward learning. If the integration of information about rewards and punishments is shifted towards the processing of more aversive information, this could contribute to the enhanced sensitivity towards punishment that has been observed in depressed individuals (Hevey et al., 2017), a possibility that should be tested in future research with behavioral measures of reward learning. Consistent with this possibility, animal research has demonstrated that amygdala-striatum connections are involved in connecting incentive value to stimulus-outcome associations, including connecting negative incentive value to stimuli paired with stressful outcomes such as foot-shock (Zorrilla and Koob, 2013).
The mechanisms through which higher TNF-α relates to increased amygdala-striatum connectivity remain to be determined. In an animal model, induction of chronic pain resulted in increased TNF-α expression in the basolateral amygdala (BLA) (Chen et al., 2013). This study also found that TNF-α increased excitatory signaling and decreased inhibitory signaling in the BLA. The authors proposed that this imbalance between excitation and inhibition in the BLA could contribute to the development of anxiety-related behaviors. Other tracing studies have shown that there are extensive connections from the amygdala to the striatum, and that the BLA is one of the primary amygdala sub-regions that sends inputs to the striatum (Zorrilla and Koob, 2013). All together, these studies suggest that altered BLA excitation could be a potential neurobiological mechanism through which higher inflammation could influence connectivity between the amygdala and striatum. However, it is important to keep in mind the limitations of this study, including that it is unclear if levels of peripheral inflammation reflect levels of inflammation in the central nervous system, and that the correlational results preclude drawing firm conclusions regarding the direction of associations between peripheral inflammation and altered neural connectivity. Future inflammatory challenge paradigms in human research could be useful for examining changes in amygdala-striatum connectivity resulting from experimentally-induced increases in inflammation, as well as testing the hypothesis that this could alter sensitivity to noxious or stressful stimuli.
We did not find evidence for cross-sectional associations between depressive symptoms and amygdala-ventral striatum connectivity or inflammatory markers in this sample. This is perhaps not entirely surprising given the relatively young age of the sample (mean age of 13.6). Research in longitudinal samples such as the Dunedin study suggests the lifetime prevalence of depression is relatively low by the age of 13 (3.2%), but rises substantially by age 18 (to 20.7%), suggesting that depression becomes much more prevalent towards the end of adolescence (Thapar et al., 2012). Thus, further research is needed to test whether the pattern of connectivity observed here may predict higher risk for developing depression later in adolescence, when symptoms begin to rise. Indeed, the present sample is being followed longitudinally, which will allow us to test this possibility. It is also possible that this pattern of connectivity only predicts the development of depressive symptoms within stressful environmental contexts; and this question will also be examined. Prior research in other samples suggests higher amygdala-ventral striatum connectivity may be associated with depression risk. For example, in a large sample of 926 children, adolescents, and adults between 8 and 22 years old, Barber et al. (Barber et al., 2019) found that depression, but not other forms of psychopathology such as psychosis or ADHD, was associated with accelerated development of connectivity between the striatum and subcortical limbic regions including the amygdala. Thus, accelerated development of connectivity between these regions could represent a potential neurodevelopmental mechanism through which higher peripheral inflammation increases depression risk. Although our data are correlational and cannot address the causal relationship between inflammation and amygdala-striatum connectivity, some research in adults supports the possibility that inflammation may cause changes in function within these regions. For example, prior research in adults that has used brief inflammatory challenges to induce inflammation has found that the induction of inflammation results in increased amygdala activity to socially threatening images (Inagaki et al., 2012).
Results also indicated that TNF-α was associated with decreased connectivity between the right IFG and left parietal cortex, with peak coordinates in the angular gyrus. Although this exact pattern of connectivity has not been observed in relation to peripheral inflammation in adult samples, this is in accordance with our general prediction that higher inflammation would be associated with decreased cortico-cortical connectivity. This finding also agrees with that of Nusslock et al. (2019), who found that higher peripheral inflammation was associated with decreased connectivity in an emotion regulation network that included the right inferior frontal gyrus and left angular gyrus, among other regions. Consistent with the idea that these regions form part of an emotion regulation network, other research has shown that the inferior frontal gyrus plays an important role in inhibitory control (Taylor et al., 2018) and emotion regulation (Hallam et al., 2015), and has functional connections with the parietal cortex (Du et al., 2020). Some research frameworks suggest that parietal regions including the angular gyrus are involved in embodied emotion regulation and executing regulatory processes directed by prefrontal regions (Kohn et al., 2014). Thus, a correlation between higher TNF-α and decreased connectivity between these regions could suggest decreased emotion regulation or ability to inhibit undesirable behaviors, which could have implications for the future development of psychopathology. Interestingly, there was an association between IFG-parietal connectivity and depressive symptoms, but the positive association was in the opposite direction than would be expected. However, this effect would not survive correction for multiple comparisons and was no longer significant when controlling for TNF-α in the regression, overall suggesting this association was relatively weak and not robust. This highlights the need for future longitudinal research to examine how observed patterns of rsFC relate to depressive symptoms later in adolescence, when depression prevalence rises markedly.
Because this study is correlational, there is an alternative explanation that should be considered in interpreting this pattern of results. Specifically, in addition to the afferent pathways described in the introduction through which peripheral inflammation can influence brain function, there are also efferent pathways through which brain function regulates peripheral inflammation and can stimulate an increase in peripheral inflammation in response to stress (Marsland et al., 2017; Miller et al., 2009; Rohleder, 2014). Thus, it is possible that higher peripheral inflammation could lead to the observed patterns of rsFC, or alternatively that the observed patterns of rsFC could influence levels of peripheral inflammation. Specifically, heightened amygdala-striatal connectivity could shift processing towards more threatening stimuli (as postulated above), which could in turn lead to heightened stress sensitivity and higher peripheral inflammation in response to stress. Further longitudinal research with multiple time points of data and/or experimental paradigms that include a stress induction are needed to help determine the direction of these effects.
Overall, some of these patterns of connectivity in adolescents were different from those observed in adult samples and in one prior study conducted in adolescents. There were several differences in methodology between the present study and the study conducted by Nusslock et al. (Nusslock et al., 2019) that may have contributed to these differences. First, the participants in that study were limited to urban African American adolescents. The present paper examined these associations in a diverse community sample of adolescents that includes most major racial/ethnic groups. Second, that study did not include many of the seed regions examined in the present analysis, including the amygdala. Third, that study only examined associations between peripheral inflammatory markers and within-network connectivity. Fourth, that study did not control for body mass index (BMI), even though this is a potential confound of associations between inflammation, brain function, and psychiatric symptoms (Byrne et al., 2015). Indeed, the significant correlation between IL-6/CRP and BMI in our sample could explain why we did not observe robust associations between these markers and rsFC, given that we did control for BMI in our analyses. All of these factors may have contributed to differences in results across the studies.
In addition, results of the present study should be interpreted with respect to several limitations. First, there was some variability in the time of day of blood sampling and the amount of time between the blood sample and MRI scan. Although we controlled for these factors with covariates, it is possible this could have led to some noise in our estimates. Second, some participants were using medications which could have impacted inflammation levels or rsFC. However, we included a covariate in all analyses to control for medication use. Third, this was a community sample of adolescents with relatively low levels of depressive symptoms. This could explain why we did not observe robust associations between depressive symptoms and inflammatory markers or rsFC. As described above, we plan to follow these participants longitudinally, which will allow us to examine whether any of these patterns of rsFC are prospectively related to changes in depressive symptoms. Fourth, some of the ROIs provided in the CONN toolbox which were used as seed regions for the rsFC analyses are relatively large, and may have prevented our ability to detect patterns of rsFC within sub-regions of these larger regions.
In sum, our study identified several patterns of rsFC associated with higher TNF-α, including increased amygdala-striatal connectivity and decreased IFG-parietal connectivity. Further research is needed to examine how these patterns of connectivity relate to cognitive and emotional processing, and which of these patterns of connectivity mediate the association between higher TNF-α and changes in depressive symptoms later in adolescence. If future research finds that increased amygdala-striatum connectivity is associated with enhanced sensitivity to punishment during reward processing, this could help identify ways to target treatments for adolescents with elevated TNF-α, such as using cognitive treatments that alter reward learning to shift processing towards positive outcomes (Craske et al., 2016).
Acknowledgments
This work was supported by UC Davis, Prop. 63, the Mental Health Services Act and the Behavioral Health Center of Excellence at UC Davis (JRS) and the USDA National Institute of Food and Agriculture, Hatch project 1013485 (JRS). AFC was supported by the Eugene Cota Robles Fellowship from UC Davis. Part of this research was conducted at the UC Davis Clinical and Translational Science Center Clinical Research Center, which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. LMT was supported by a Building Interdisciplinary Research Careers in Women’s Health award (K12 HD051958, PI: Ellen B. Gold, PhD), funded by the National Institute of Child Health and Human Development (NICHD), Office of Research on Women’s Health, Office of Dietary Supplements, and the National Institute of Aging. The authors would like to thank the participants of the study for their time.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Degrees of freedom are lower for this t-test because two participants in the included sample were missing depressive symptoms, as described below.
Degrees of freedom are lower for this t-test because five participants were excluded from analyses because they did not provide a blood sample.
References
- Barber AD, Sarpal DK, John M, Fales CL, Mostofsky SH, Malhotra AK, et al. , 2019. Age-normative pathways of striatal connectivity related to clinical symptoms in the general population. Biol. Psychiatry 85, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Gauldie J, 1994. The acute phase response. Immunol. Today 15 (2), 74–80. [DOI] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson NM, Mitchell SA, Allen NB, 2015. Adolescent-onset depression: Are obesity and inflammation developmental mechanisms or outcomes? Child Psychiatry Hum. Dev 46, 839–850. [DOI] [PubMed] [Google Scholar]
- Calabró P, Willerson JT, Yeh ETH, 2003. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation 108 (16), 1930–1932. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH, 2011. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther 130 (2), 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Heller AS, Gee DG, Cohen AO, 2019. Development of the emotional brain. Neurosci. Lett 693, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu X, et al. , 2013. The contribution of TNF-a in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci. Lett 541, 275–280. [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ, 2016. Treatment for anhedonia: A neuroscience driven approach. Depress Anxiety. 33 (10), 927–938. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL, 2010. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67 (5), 446–457. [DOI] [PubMed] [Google Scholar]
- Du J, Rolls ET, Cheng W, Li Y.u., Gong W, Qiu J, Feng J, 2020. Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex. 123, 185–199. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, Caldera C, Tottenham N, 2015. Normative development of ventral striatal resting state connectivity in humans. NeuroImage. 118, 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich ME, Carey MP, Ruggiero L, Enyart P, Gresham F, 1986. Assessment of depression in childhood and adolescence: An evaluation of the Center for Epidemiological Studies Depression Scale for Children (CES-DC). Am. J. Psychiatry 143, 1024–1027. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21 (10), 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH, 2012. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front. Neuroendocrinol 33 (3), 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouri E, Francesconi M, Midouhas E, Lewis G, 2020. Prenatal and childhood adverse life events, inflammation and depressive symptoms across adolescence. J. Affect. Disord 260, 577–582. [DOI] [PubMed] [Google Scholar]
- Fraguas D, Diaz-Caneja CM, Ayora M, Hernandez-Alvarez F, Rodriguez-Quiroga A, Recio S, et al. (2019): Oxidative stress and inflammation in first-episode psychosis: A systematic review and meta-analysis. Schizophr Bull. 45:742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam GP, Webb TL, Sheeran P, Miles E, Wilkinson ID, Hunter MD, Barker AT, Woodruff PWR, Totterdell P, Lindquist KA, Farrow TFD, Pourtois G, 2015. The neural correlates of emotion regulation by implementation intentions. PLoS ONE 10 (3), e0119500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevey D, Thomas KM, Laureano-Schelten S, Looney K, Booth R, 2017. Clinical depression and punishment sensitivity on the BART. Front. Psychol 8, 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI, 2012. Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage. 59 (4), 3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB, 2014. Association of serum interleukin 6 and c-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry. 71 (10), 1121. 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-W, Szigethy EM, Melhem NM, Saghafi EM, Brent DA, 2014. Inflammatory markers and the pathogenesis of pediatric depression and suicide: A systematic review of the literature. J. Clin. Psychiatry 75 (11), 1242–1253. [DOI] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U, 2014. Neural network of cognitive emotion regulation – An ALE meta-analysis and MACM analysis. NeuroImage. 87, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, 2010. Children’s Depression Inventory, 2nd Edition. [Google Scholar]
- Kraynak TE, Marsland AL, Wager T, Gianaros PJ, 2018. Functional neuroanatomcy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neurosci. Biobehav. Rev 94, 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrenz F, Wrede K, Forsting M, Engler H, Schedlowski M, Elsenbruch S, Benson S, 2016. Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain Behav. Immun 54, 17–26. [DOI] [PubMed] [Google Scholar]
- Labrenz F, Ferri F, Wrede K, Forsting M, Schedlowski M, Engler H, Elsenbruch S, Benson S, Costantini M, 2019. Altered temporal variance and functional connectivity of BOLD signal is associated with state anxiety during acute systemic inflammation. Neuroimage. 184, 916–924. [DOI] [PubMed] [Google Scholar]
- Lekander M, Karshikoff B, Johansson E, Soop A, Fransson P, Lundström JN, Andreasson A, Ingvar M, Petrovic P, Axelsson J, Nilsonne G, 2016. Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain Behav. Immun 56, 34–41. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Kuan D-H, Sheu LK, Krajina K, Kraynak TE, Manuck SB, Gianaros PJ, 2017. Systemic inflammation and resting state connectivity of the default mode network. Brain Behav. Immun 62, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, Chick CF, Eickhoff SB, Etkin A, 2020. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 177 (5), 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC, 2018. Inflammation negatively correlates with amygdala-ventromedial prefrontal connectivity in association with anxiety in patients with depression: Preliminary results. Brain Behav. Immun 73, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW, 2012. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol. Psychiatry 72 (1), 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65 (9), 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Brody GH, Armstrong CC, Carroll AL, Sweet LH, Yu T, Barton AW, Hallowell ES, Chen E, Higgins JP, Parrish TB, Wang L, Miller GE, 2019. Higher peripheral inflammatory signaling associated with lower resting-state functional brain connectivity in emotion regulation and central executive networks. Biol. Psychiatry 86 (2), 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Miller GE, 2016. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry. 80 (1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 59 (3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, 2014. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med 76 (3), 181–189. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Giletta M, Helms SW, Hastings PD, Rudolph KD, Nock MK, et al. , 2020. Intepersonal life stress, inflammation, and depression in adolescence: Testing Social Signal Transduction Theory of Depression. Depress Anxiety. 37, 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carranza AF, Knodt AR, 2019. Amygdala activity to angry and fearful faces relates to bullying and victimization in adolescents. Social Cognitive and Affective Neuroscience 14 (10), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Robertson A, Keller AE, Sato J, Urbain C, Pang EW, 2018. Inhibition in the face of emotion: Characterization of the spatial-temporal dynamics that facilitate automatic emotion regulation. Hum. Brain Mapp 39 (7), 2907–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, Thapar AK, 2012. Depression in adolescence. Lancet 379 (9820), 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord 150 (3), 736–744. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Westhoff B, de Vos F, Wierenga LM, Crone EA, 2019. A three-wave longitudinal study of subcortical-cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum. Brain Mapp 40, 3769–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Xu X, Chen G, Mehta ND, Haroon E, Miller AH, Luo Y, Li Z, Felger JC, 2019. Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain Behav. Immun 80, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF, 2013. Amygdalostriatal projections in the neurocircuitry for motivation: A neuroanatomical thread through the career of Ann Kelley. Neursoci. Biobehav. Rev 37 (9), 1932–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K, 2001. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav. Immun 15 (3), 199–226. [DOI] [PubMed] [Google Scholar]