Summary
Background:
There are few long-term studies of interventions to reduce weight gain in low-socioeconomic status children with overweight or obesity. the Stanford GOALS trial evaluated a 3-year, community-based multi-level, multi-setting, multi-component (MMM) systems intervention, to reduce weight gain among low socioeconomic status, Latinx children with overweight or obesity.
Methods:
Two-arm, parallel group, randomized, open label, active placebo-controlled trial with blind assessment over three years. Families from low-income, primarily Latinx communities in California, USA, with 7–11 year old children with overweight or obesity were randomized to a multi-level, multi-setting, multi-component (MMM) intervention or a Health Education (HE) comparison intervention. The MMM intervention included home environment changes and behavioral counseling, community after school team sports, and reports to primary care providers. The primary outcome was child body mass index (BMI) trajectory over three years. Secondary outcomes included one- and two-year changes in BMI. ClinicalTrials.gov NCT01642836.
Findings:
241 families were randomized to MMM (n=120) or HE (n=121). Children were mean ± SD = 9.5 ± 1.4 years of age, 134 (55.6%) female, and 236 (97.9%) Latinx. 238 (98.8%), 233 (96.7%), and 227 (94.2%) participated in 1-, 2-, and 3-year follow-up assessments, respectively. In intention-to-treat analysis, over three years the difference between groups in BMI trajectory was not statistically significant (mean adjusted difference (95% Confidence Interval [CI]) = −0.25 (−0.90, 0.40) kg/m2, Cohen’s d = −.10, p= 0.45). Children in the MMM intervention gained less BMI over one year (mean adjusted difference (95% CI) = −0.73 (−1.07, −0.39) kg/m2, d = −.55) and over 2 years (mean adjusted difference (95% CI) = −0.63 (−1.13, −0.14) kg/m2, d = −.33). Differential adverse events were not observed.
Interpretation:
The MMM intervention did not reduce BMI gain versus HE over 3 years but the effects over 1 and 2 years in this rigorous trial show the promise of this systems intervention approach for reducing weight gain and cardiometabolic risk factors in low-socioeconomic status communities.
Funding:
U.S. National Institutes of Health.
Introduction
The United States has experienced dramatic increases in obesity. The prevalence of child and adolescent obesity has more than tripled since the 1960’s and 1970’s, with the highest rates in 2015–2018 among non-Hispanic Black and Mexican-American youth.1 Obesity in children and adolescents is associated with hypertension, dyslipidemias, early atherosclerotic lesions, prediabetes and type 2 diabetes, and many other medical, psychological, and social complications.2 While some effective behavioral treatments exist, clinical childhood obesity treatment programs are often relatively costly and time-consuming to implement, serve only limited numbers of children, and not available in many communities.2,3 Further, outcomes beyond 6 or 12 months are rarely reported.4–7 To address some of these challenges, the Stanford GOALS trial evaluated a 3-year, community-based multi-level, multi-setting, multi-component (MMM) systems intervention, to reduce weight gain among low socioeconomic status, Latinx children with overweight or obesity, compared to a nutrition and health education intervention. The GOALS MMM intervention was designed to deliver treatment in settings where children and families live and play, and to interactively target multiple eating, physical activity and screen media behaviors at multiple levels in multiple settings.
Methods
Stanford GOALS was a two-arm, parallel group, randomized, open label, active placebo-controlled trial with blind assessment. The methods have been described previously.8 Stanford GOALS was part of the NIH-sponsored Childhood Obesity Prevention and Treatment Research (COPTR) consortium.9 The study protocol and procedures were approved by the Stanford University Administrative Panel on Human Subjects in Medical Research (#19311) and an independent, NIH-appointed, Data and Safety Monitoring Board (DSMB). The DSMB also met twice yearly throughout the trial to monitor study progress, data quality and completeness, and participant safety. Participants were randomized to either the MMM intervention or a community-based nutrition and health education intervention for three years. Participants were assessed at baseline and annually. The primary outcome measure was BMI trajectory during the three-year study.
Participant Recruitment, Eligibility, Informed Consent and Assent, Randomization and Masking
Participants were recruited from July 2012 through October 2013, through primary care providers, clinics, schools, community centers, churches, and other community locations in low-income, primarily Latinx neighborhoods in Northern California, USA. Potentially eligible families were scheduled for an initial assessment. Signed consent/assent and HIPAA authorization were required prior to data collection.
Children were eligible if they were 7–11 years of age on the date of randomization with BMI ≥ 85th percentile for age and sex on the 2000 U.S. Centers for Disease Control and Prevention (CDC) BMI reference. Children were not eligible if they were diagnosed with a medical condition or taking a medication affecting growth; had a condition limiting participation in interventions or assessments; they or their parent/guardian were unable to read, understand and complete informed consent in English or Spanish; planned to move from the area within 36 months; or deemed to have another characteristic that made them unsuitable for the study.
After completing all baseline measures, households were randomized by computer by a study statistician to the MMM or Health Education conditions. Efron’s biased coin randomization10 was used within strata defined by BMI percentile for age and sex at baseline (≥ 85th and < 95th percentile, ≥ 95th percentile). For households with more than one eligible child, one child was randomly selected at the time of randomization to include in the analysis. Only the statisticians were aware of which child in a multi-child household was in the analysis sample. The investigators and all assessment staff remained masked to experimental assignment until after the final study follow-up assessments and data cleaning were completed.
The MMM Intervention
The MMM intervention was multi-level, intervening directly with individual children, parents and families, peer groups, primary care clinics, and the home and community environments, multi-component, intervening on eating behaviors, physical activity, screen time and parenting, via behavioral and environmental interventions, and multi-setting, intervening in homes, community-based after school programs, and primary care clinics.8 The MMM intervention was grounded in Bandura’s social cognitive model11 and delivered over three years for each family. It was designed based on past research and refined with 18-months of community-engagement, formative research, and pilot studies.8 Latinx cultural values were incorporated into the intervention, considering both surface structure and deep structure12 regarding psychological and socio-cultural influences on health and behavior.8 We included strategies from recent social psychological science to promote intrinsic motivation,13 alter implicit mindsets,14 and affirm values,15 to address psychological threats to behavior change. Further, we designed specific intervention content and activities to maximize motivation for participation in the process of behavior change with stealth intervention principles.16 The MMM intervention was conceptualized holistically as a complex systems intervention, including planned interactions, opportunities for mutual reinforcement, repetition, and positioning complementary elements across the different levels, settings and components of the intervention, to generate synergistic effects and accommodate individual and family preferences and life experiences over their three years of participation.8
GOALS@home included environmental and behavioral interventions delivered to the children, parents/guardians, and other family members in their home by trained, bilingual (Spanish and English) health educators, following a protocol. Five modules were designed to span the three years, and each module included a number of levels requiring mastery of specific skills before moving forward. First, a module to alter the home environment (4 levels), by replacing plates, bowls, glasses, and serving utensils to promote smaller serving sizes,17 followed by three modules to promote behavior changes in eating (8 levels), physical activity (7 levels) and screen time (6 levels), delivered in an order chosen by the family, and a fifth module on problem solving and maintenance (5 levels). The content was adapted from prior interventions,18–20 and consistent with U.S. Preventive Services Task Force recommendations.21
Team GOALS was a community-based after school team sports program designed for children with overweight and obesity from low socioeconomic status families.22 Team GOALS was offered weekdays, year-round including a 5–6 week summer program, except school holidays, in partnership with four community centers run by the local Boys and Girls Clubs, the County Parks and Recreation Department, and the Police Activities League. Team GOALS was an environmental intervention, made available in the neighborhoods where participants were recruited for MMM children to attend as often as they wished. Each session included 1–1.5 hours of activity plus time for homework. Four sports, soccer, flag football, basketball, and lacrosse, were rotated seasonally throughout the year with additional sports introduced with community partners during summers (e.g., track and field, volleyball, rugby, ultimate frisbee, swimming). Sessions and activities were structured to keep children moving and coaches were trained by the investigators to provide feedback promoting intrinsic motivation13 and a growth mindset14 for activity.
Primary Care GOALS provided brief, self-guided, semi-annual reports of GOALS@Home and Team GOALS progress to help primary care medical providers counsel their participating patients and reinforce participation.8 Health educators also reviewed copies of the progress reports with families during GOALS@home visits and encouraged participants to take their copies to primary care medical visits to review with their providers.
The Health Education Comparison Intervention
The comparison intervention was also delivered over the entire 3 years of participation for each family, and included two home counseling visits per year, monthly health education newsletters mailed individually to parents and to children, quarterly neighborhood-based health education/family fun nights, and 1–2 social field trips per year to the Stanford campus/athletic events.8 Health Education content focused on nutrition, physical activity, screen time, chronic disease prevention and general health topics. It was designed as a rigorous and ethical active placebo comparison intervention, to limit risks of resentful demoralization or compensatory rivalry, and include active ingredients which may influence obesity-related behavior but differed from the conceptually relevant ingredients in the MMM intervention.
Primary care providers and participating families in both the MMM and Health Education conditions also received children’s annual blood test results with explanations appropriate for readers with low health literacy.
Assessments
Assessments were performed in a clinic, community or home setting at baseline, one, two and three years, by bilingual English/Spanish, trained and certified data collectors, masked to experimental assignment. Data collectors followed standardized protocols and were monitored for measurement quality and intra- and inter-rater reliability for key measures (Supplementary material, Figure S1).8 To enhance participation and limit attrition over the three-year study we emphasized establishing trust, sensitivity to families’ individual needs, identify and belonging in Stanford GOALS, and contributing to research. Follow-up visits were conducted in convenient locations and families were compensated with gift cards for completing measures ($50 at baseline, 1, and 2 years, and $100 at 3 years) and non-monetary tokens of appreciation (e.g., t-shirt, drawstring bag, duffel/gym bag).
Primary Outcome Measure
The primary outcome, trajectory of BMI over time, is a measure of change in BMI and derived by computing a slope for each child by regressing BMI on time, where each child may have up to 4 BMI measurements, taken at the baseline and 3 annual follow up visits that contribute to the outcome calculation. BMI was calculated as weight in kilograms divided by the square of height in meters. Weight and height were measured with the participant in light clothing without shoes, weight to the nearest 0.1 kg using research precision grade, calibrated, digital scales and height to the nearest 0.1 cm using a free-standing stadiometer.
Secondary Outcome Measures
Trajectories of percent of the median BMI for age and sex,23,24 waist circumference, waist-to-height ratio, triceps skinfold thickness, and estimated percent body fat25 were included as secondary measures of body composition changes for children. Waist circumference was measured to the nearest 0.1 cm just above the uppermost lateral border of the right ilium. Triceps skinfold was measured to the nearest 1 mm in the midline of the posterior aspect of the arm, over the triceps muscle, at a point midway between the lateral projection of the acromion process of the scapula and the inferior margin of the olecranon process of the ulna. Trajectories of BMI and waist circumferences were used to assess changes among parents/guardians but excluded from analysis when a parent reported being pregnant or within three months after childbirth or miscarriage.
Children’s resting systolic and diastolic blood pressure and heart rate were measured three times using an automated device (Carescape v100, GE Healthcare) with an appropriate-sized cuff. The average of the second and third measures were used in analysis.
Fasting (≥ 8 hours) blood samples were collected by venipuncture from children at each assessment. Hemoglobin A1c, glucose, insulin, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, high-sensitivity c-reactive protein (hs-CRP), and alanine aminotransferase (ALT) were measured using standardized protocols with high quality control.8 Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated from glucose and insulin measures.26
Children wore triaxial accelerometers (Actigraph GT3X+) on the right hip for seven complete days, including while sleeping, except during water activity, and repeated if not worn for at least 6 hours between 5:00am and 11:59pm on at least 3 weekdays and 1 weekend day. Accelerations were assessed at 40-Hertz and processed in 15 second epochs, and nonwear time was excluded using a previously validated algorithm.27 Weighted (5:2 weekday to weekend day) average percent of time per day and average after school minutes per day (3pm-6pm, Monday through Friday) of moderate to vigorous physical activity (MVPA) and sedentary behavior were calculated using previously validated triaxial vector magnitude thresholds for children in this age group.28 Parents/guardians reported their own and the other parent’s/guardian’s (if applicable) physical activity.29
Children reported their screen media use and frequencies of eating breakfasts and dinners with the television turned on. Parents/guardians reported typical total household television use.30 Estimates of average total daily energy intake, percent of dietary energy from fat, average daily added sugar, fiber, servings of sugar-sweetened beverages, and percent of daily energy consumed while watching screens, were obtained from 24-hour dietary recalls with children on three randomly selected nonconsecutive days, including two weekdays and one weekend day, > 7 but ≤ 30-days apart, using the Minnesota Nutrition Data System for Research (NDS-R) software (University of Minnesota Nutrition Coordinating Center). The first dietary recall was collected face-to-face and the second and third by phone.
Children reported their overconcern with weight and shape,31 depressive symptoms,32 recent school grades, and implicit theories of body weight, habit formation, sports ability and eating habits. Pubertal stages were self-reported,33 and girls reported their age at menarche. Parents/guardians were assessed for health literacy,34 and reported child and household sociodemographics (Table 1).
Table 1.
Baseline sample characteristics
| All | MMM | Health Education | |
|---|---|---|---|
| Number | 241 | 120 | 121 |
| Age in years, mean (sd) | 9.5 (1.4) | 9.5 (1.4) | 9.5 (1.5) |
| Female, n (%) | 134 (55.6%) | 69 (57.5%) | 65 (53.7%) |
| Race/Ethnicity, n (%) | |||
| Latinx | 236 (97.9%) | 117 (97.5%) | 119 (98.3%) |
| Black | 4 (1.7%) | 2 (1.7%) | 2 (1.7%) |
| Other | 1 (0.4%) | 1 (0.8%) | 0 |
| Number of adults living in the household. n (%) | |||
| 1 Adult | 13 (5.4%) | 8 (6.7%) | 5 (4.1%) |
| 2 Adults | 137 (56.8%) | 69 (57.5%) | 68 (56.2%) |
| 3 Adults | 56 (23.2%) | 28 (23.3%) | 28 (23.1%) |
| 4 or more Adults | 35 (14.5%) | 15 (12.5%) | 20 (16.5%) |
| Number of children living in the household, n (%) | 2.7 (1.1) | 2.7 (1.1) | 2.7 (1.1) |
| 1 Child | 26 (10.8%) | 8 (6.7%) | 5 (4.1%) |
| 2 children | 90 (37.3%) | 69 (57.5%) | 68 (56.2%) |
| 3 children | 83 (34.4%) | 28 (23.3%) | 28 (23.1%) |
| 4 children | 30 (12.4%) | 15 (12.5%) | 20 (16.5%) |
| 5 or more children | 12 (5.0%) | 8 (6.7%) | 5 (4.1%) |
| Families who own their own home, n (%) | 42 (17.4%) | 23 (19.2%) | 19 (15.7%) |
| Index Parent/guardian age in years, mean (sd) | 37.2 (6.9) | 37.4 (7.0) | 37.0 (6.8) |
| Index Parent/Guardian female, n (%) | 229 (95.0%) | 114 (95.0%) | 115 (95.0%) |
| Index Parent/Guardian relationship to child n (%) | |||
| Biological Mother | 226 (93.8%) | 112 (93.3%) | 114 (94.2%) |
| Step Mother | 1 (0.4%) | 1 (0.8%) | 0 |
| Grandmother | 1 (0.4%) | 0 | 1 (0.8%) |
| Aunt | 1 (0.4%) | 1 (0.8%) | 0 |
| Biological Father | 12 (5.0%) | 6 (5.0%) | 6 (5.0%) |
| Index Parent/Guardian race/ethnicity, n (%) | |||
| Latinx | 234 (97.1%) | 115 (95.8%) | 119 (98.3%) |
| Black | 4 (1.7%) | 2 (1.7%) | 2 (1.7%) |
| white | 1 (0.4%) | 1 (0.8%) | 0 |
| other | 2 (0.8%) | 2 (1.7%) | 0 |
| Parent/Guardian marital status, n (%) | |||
| Married | 207 (85.9%) | 99 (82.5%) | 108 (89.3%) |
| Single-Never Married, Divorced/Separated or Widowed | 34 (14.1%) | 21 (17.5%) | 13 (10.7%) |
| Maximum household education level, n (%) | |||
| 8th grade or less | 96 (39.8%) | 51 (42.5%) | 45 (37.2%) |
| Attended some high school | 57 (23.7%) | 26 (21.7%) | 31 (25.6%) |
| High School graduate | 41 (17.0%) | 20 (16.7%) | 21 (17.4%) |
| Some College/Technical School or Associate’s degree | 42 (17.4%) | 20 (16.7%) | 22 (18.2%) |
| Bachelor’s degree or higher | 5 (2.1%) | 3 (2.5%) | 2 (1.7%) |
| English spoken at home, n (%) | |||
| Never | 99 (41.1%) | 58 (48.3%) | 41 (33.9%) |
| Sometimes | 98 (40.7%) | 42 (35.0%) | 56 (46.3%) |
| About half the time | 28 (11.6%) | 11 (9.2%) | 17 (14.0%) |
| Most of the time or always | 16 (6.6%) | 9 (7.5%) | 7 (5.8%) |
| One or more parent born in U.S., n (%) | 22 (9.1%) | 11 (9.2%) | 11 (9.1%) |
| Female caregiver’s employment status, n (%) | |||
| Working full time | 73 (30.3%) | 36 (30.0%) | 37 (30.6%) |
| Working part time | 62 (25.7%) | 31 (25.8%) | 31 (25.6%) |
| Not working for pay | 106 (44.0%) | 53 (44.2%) | 53 (43.8%) |
| Male caregiver’s employment status, n (%) | |||
| Working full time | 155 (64.3%) | 74 (61.7%) | 81 (66.9%) |
| Working part time | 39 (16.2%) | 19 (15.8%) | 20 (16.5%) |
| Not working for pay | 10 (4.1%) | 3 (2.5%) | 7 (5.8%) |
| (No male caregiver in household) | 37 (15.4%) | 24 (20.0%) | 13 (10.8%) |
| Annual total household income, n (%) | |||
| Refusal to respond, prefer not to answer | 60 (24.9%) | 29 (24.2%) | 31 (25.6%) |
| Less than $15,000 | 37 (15.4%) | 19 (15.8%) | 18 (14.9%) |
| $15,000–24,999 | 60 (24.9%) | 35 (29.2%) | 25 (20.7%) |
| $25,000–$34,999 | 36 (14.9%) | 16 (13.3%) | 20 (16.5%) |
| $35,000–$49,999 | 32 (13.3%) | 12 (10.0%) | 20 (16.5%) |
| $50,000 or more | 16 (6.6%) | 9 (7.5%) | 7 (5.8%) |
| Food Insecurity n (%) | |||
| High food security | 139 (57.7%) | 70 (58.3%) | 69 (57.0%) |
| Low food security | 75 (31.1%) | 35 (29.2%) | 40 (33.1%) |
| Very low food security | 27 (11.2%) | 15 (12.5%) | 12 (9.9%) |
| Receives Supplemental Nutrition Assistance Program (SNAP) benefits, n (%) | 98 (40.7%) | 52 (43.3%) | 46 (38.0%) |
| Receives Unemployment, Social Security or Disability benefits, n (%) | 15 (6.2%) | 9 (7.5%) | 6 (5.0%) |
| Child receives free or reduced price school breakfast or lunch, n (%) | 218 (90.5%) | 107 (89.2%) | 111 (91.7%) |
| Child medical insurance, n (%) | |||
| Uninsured | 15 (6.2%) | 10 (8.3%) | 5 (4.1%) |
| Public insurance | 186 (77.2%) | 88 (73.3%) | 98 (81.0%) |
| Private insurance (Kaiser or other private medical insurance) | 40 (16.6%) | 22 (18.3%) | 18 (14.9%) |
| Child born in the U.S., n (%) | 217 (90.0%) | 107 (89.2%) | 110 (90.9%) |
| TV in the room where child sleeps, n (%) | 161 (66.8%) | 87 (72.5%) | 74 (61.2%) |
| Number of Televisions in home, n (sd) | 2.3 (1.0) | 2.3 (0.9) | 2.3 (1.1) |
| Girls: Self-assessed breast maturation, n (% of girls) | |||
| Stage 1 | 38 (28.4%) | 22 (31.9%) | 16 (24.6%) |
| Stage 2 | 44 (32.8%) | 21 (30.4%) | 23 (35.4%) |
| Stage 3 | 48 (35.8%) | 25 (36.2%) | 23 (35.4%) |
| Stage 4 | 4 (3.0%) | 1 (1.4%) | 3 (4.6%) |
| Stage 5 | 0 | 0 | 0 |
| Girls: Self-assessed pubic hair maturation, n (% of girls) | |||
| Stage 1 | 78 (58.2%) | 40 (58.0%) | 38 (58.5%) |
| Stage 2 | 26 (19.4%) | 16 (23.2%) | 10 (15.4%) |
| Stage 3 | 21 (15.7%) | 8 (11.6%) | 13 (20.0%) |
| Stage 4 | 8 (6.0%) | 4 (5.8%) | 4 (6.2%) |
| Stage 5 | 1 (0.7%) | 1 (1.4%) | 0 |
| Girls: Entered puberty (stage 2 or greater for breast and/or pubic hair), n (% of girls) | 100 (74.6%) | 50 (72.5%) | 50 (76.9%) |
| Girls: Menarche, n (%) | 12 (9.0%) | 6 (8.7%) | 6 (9.2%) |
| Boys: Self-assessed testes maturation, n (% of boys) | |||
| Stage 1 | 44 (41.1%) | 23 (45.1%) | 21 (37.5%) |
| Stage 2 | 53 (49.5%) | 23 (45.1%) | 30 (53.6%) |
| Stage 3 | 10 (9.3%) | 5 (9.8%) | 5 (8.9%) |
| Stage 4 | 0 | 0 | 0 |
| Stage 5 | 0 | 0 | 0 |
| Boys: Self-assessed pubic hair maturation, n (% of boys) | |||
| Stage 1 | 52 (48.6%) | 25 (49.0%) | 27 (48.2%) |
| Stage 2 | 44 (41.1%) | 20 (39.2%) | 24 (42.9%) |
| Stage 3 | 10 (9.3%) | 5 (9.8%) | 5 (8.9%) |
| Stage 4 | 1 (0.9%) | 1 (2.0%) | 0 |
| Stage 5 | 0 | 0 | 0 |
| Boys: Entered puberty (stage 2 or greater for testes and/or pubic hair), n (% of boys) | 74 (69.2%) | 33 (64.7%) | 41 (73.2%) |
| Index Parent Health Literacy (NVS), n (%) | |||
| High likelihood of limited literacy | 130 (53.9%) | 62 (51.7%) | 68 (56.2%) |
| Possibility of limited literacy | 77 (32.0%) | 41 (34.2%) | 36 (29.8%) |
| Adequate literacy | 34 (14.1%) | 17 (14.2%) | 17 (14.0%) |
| Child’s unsupervised time at home, mean hours per week (sd) | 1.1 (3.4) | 1.4 (3.9) | 0.9 (2.8) |
| ≥ 1 Life events experienced in past year, n (%) | 59 (24.5%) | 32 (26.7%) | 27 (22.3%) |
Process Measures
Intervention staff collected implementation data to assess the success of intervention delivery and individual-level intervention participation (reach, dose delivered, dose received, and fidelity).
Adverse Events
Parents/guardians were queried about all child and parent/guardian injuries, illnesses or other medical problems requiring a visit to a medical care provider and related to participation in the study, and all serious adverse events, since the prior assessment visit. Adverse events were also recorded and evaluated when they came to study staff attention between assessment visits.
Analysis
We hypothesized that children randomized to the MMM intervention would have statistically significantly attenuated BMI trajectories over three years compared to children randomized to the Health Education intervention.8 Linear trajectories of change in BMI (slopes) were calculated from all available BMI measures for each individual and regressed on intervention group assignment (centered), with the baseline value of BMI (centered at its mean) and the Intervention x baseline BMI interaction as covariates, using standard maximum likelihood linear regression techniques. A two-sided Wald Test was conducted at the 0.05 level of significance. Consistent with intent-to-treat principles, trajectories of BMI for children with only a baseline BMI measure were imputed using a joint modeling multiple imputation approach as implemented in SAS using PROC MI (version 9.4) (Supplementary material). This analysis also accommodated deviations from the ideal measurement schedule. The same analytic approach with multiple imputation was used to assess changes in the a priori specified secondary outcomes. We also explored baseline (pre-randomization) sociodemographic, psychological, behavioral, and physiological/physical measures as possible moderators of treatment effects on BMI trajectories.8,35
Detectable Difference, Sample Size, and Power
The planned sample of 120 children per group provided approximately 90% power to detect intervention effects of Cohen’s d= 0.40 or greater, for a two-sided α = 0.05, across a variety of assumptions regarding missing data, correlations of serial BMI measures, and intervention x baseline BMI interactions (Supplementary material).8
Role of the funding source
The NIH was represented on the Steering and Executive Committees and subcommittees of the COPTR consortium that contributed to the study design. Dr. Pratt (NIH) participated in critical revision of this report and the decision to submit the paper for publication. All authors had full access to the full data in the study and accept responsibility to submit for publication.
Results
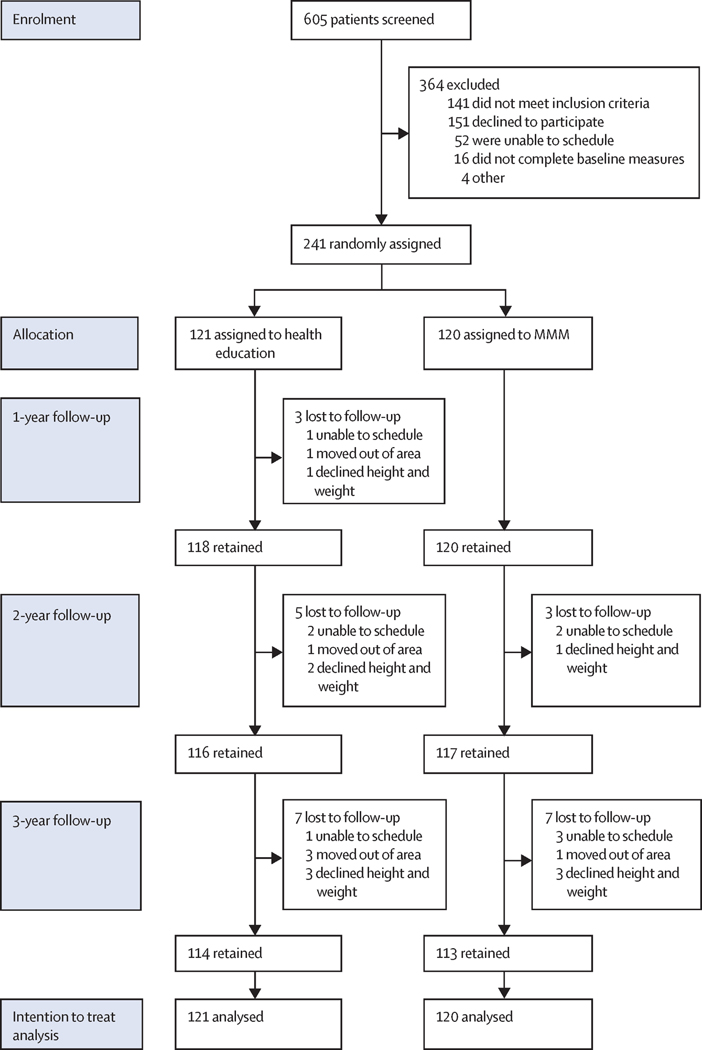
Participant recruitment and flow are displayed in Figure 1. Retention was high, 238 (98.8%) over 1 year, 233 (96.7%) over 2 years, and 227 (94.2%) over 3 years, with 225 (93.4%) completing all four measures, 10 (4.1%) completing three, and 3 (1.2%) completing two and one. Sample baseline demographic characteristics are reported in Table 1 and baseline values of outcome measures in Tables 2, 3, and Supplementary Tables S1 and S2. Baseline characteristics show a low socioeconomic status, high-risk, predominantly Latinx sample of 241 families with children with overweight and obesity. Children averaged 9.5 years of age and 134 (55.6%) were female. About three-quarters had BMI ≥ 95th percentile for age and sex, and more than half had evidence of dyslipidemias and/or pre-diabetes. Parents/guardians were predominantly immigrants, reporting low levels of education, income and health literacy, and moderate to high use of public assistance programs. 201 (83%) of participating parents/guardians (mostly mothers) had a BMI ≥ 25 kg/m2 and 128 (53%) had a BMI ≥ 30 kg/m2.
Figure 1:
Trial profile
Table 2.
Baseline values, changes, and group differences in body mass index and secondary body composition measures.
| Changes over 1 year | Changes over 2 years | Changes over 3 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, Mean (SD)a | Slope, Mean (SD)b | Adjusted MMM-HE difference over 1 year (95% CI)c | Standardized Effect Size (Cohen’s d) | Slope, Mean (SD) b | Adjusted MMM-HE difference over 2 years (95% CI) c | Standardized Effect Size (Cohen’s d) | Slope, Mean (SD) b | Adjusted MMM-HE difference over 3 years (95% CI) c | Standardized Effect Size (Cohen’s d) | |||||
| MMM | HE | MMM | HE | MMM | HE | MMM | HE | |||||||
| Body Mass Index, BMI (kg/m2) | 25.26 (4.04) | 24.86 (3.87) | 0.47 (1.49) | 1.17 (1.15) | −0.73 (−1.07, −0.39) | d=0.55 | 0.81 (1.01) | 1.10 (0.95) | −0.63 (−1.13, −0.14) | d=0.33 | 1.05 (0.88) | 1.11 (0.83) | −0.25 (−0.90, 0.40) | d=0.10 |
| Percent of median BMI for age and sex | 152.64 (23.78) | 150.03 (22.31) | −1.95 (8.83) | 2.25 (6.63) | −4.33 (−6.32, −2.34) | d=0.55 | −0.17 (5.72) | 1.58 (5.34) | −3.68 (−6.49, −0.87) | d=0.33 | 0.92 (4.80) | 1.40 (4.51) | −1.60 (−5.18, 1.98) | d=0.11 |
| Waist Circumference (cm) | 86.18 (11.00) | 84.68 (10.61) | 2.62 (4.19) | 4.34 (3.48) | −1.68 (−2.65, −0.70) | d=0.44 | 3.20 (2.78) | 4.00 (2.76) | −1.56 (−2.96, −0.15) | d=0.28 | 3.42 (2.46) | 3.72 (2.37) | −0.93 (−2.79, 0.93) | d=0.13 |
| Waist-to-Height Ratio | 0.62 (0.06) | 0.61 (0.06) | −0.0069 (0.0300) | 0.0051 (0.0251) | −0.0116 (−0.0185, −0.0046) | d=0.42 | −0.0018 (0.0191) | 0.0032 (0.0192) | −0.0095 (−0.0191, 0.0002) | d=0.25 | 0.0010 (0.0157) | 0.0026 (0.0155) | −0.0050 (−0.0170, 0.0070) | d=0.11 |
| Triceps Skinfold (mm) | 24.02 (5.52) | 22.86 (5.05) | −0.88 (3.91) | 1.04 (3.74) | −1.63 (−2.57, −0.69) | d=0.44 | 0.04 (2.30) | 0.77 (2.24) | −1.14 (−2.25, −0.03) | d=0.26 | 0.42 (1.82) | 0.65 (1.61) | −0.47 (−1.78, 0.84) | d=0.09 |
| Estimated Percent Body Fat | 41.39 (4.27) | 40.57 (4.20) | −0.66 (2.55) | 0.68 (2.34) | −1.29 (−1.91, −0.67) | d=0.53 | −0.08 (1.81) | 0.44 (1.84) | −1.06 (−1.99, −0.13) | d=0.29 | 0.13 (1.48) | 0.30 (1.46) | −0.50 (−1.63, 0.63) | d=0.11 |
Baseline data from all participants, N = 120 for MMM and N= 121 for Health Education for all variables at baseline
Unadjusted slopes calculated from all participants with follow-up data, N= 120 for MMM and N = 118 for Health Education for BMI measures, N= 120 for MMM and N = 117 for Health Education for waist circumference, triceps skinfold thickness, estimated percent body fat.
Adjusted MMM minus Health Education differences from linear trajectories (slopes) for each individual regressed on intervention group assignment (centered), with the baseline value (centered at its mean) and the Intervention x baseline interaction as covariates, using standard maximum likelihood linear regression techniques. Consistent with intent-to-treat principles, trajectories for children with only a baseline measure were imputed using a joint modeling multiple imputation approach as implemented in SAS using PROC MI (version 9.4). Thus, N= 120 for MMM and 121 for Health Education, for all outcomes.
Table 3.
Baseline values, changes, and group differences in physiological, physical activity, diet, screen use and psychosocial secondary outcome measures.
| Changes over 1 year | Changes over 2 years | Changes over 3 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, Mean (SD)a | Slope, Mean (SD)b | Adjusted MMM-HE difference over 1 year (95% CI)c | Standardized Effect Size (Cohen‘s d) | Slope, Mean (SD) b | Adjusted MMM-HE difference over 2 years (95% CI) c | Standardized Effect Size (Cohen‘s d) | Slope, Mean (SD) b | Adjusted MMM-HE difference over 3 years (95% CI) c | Standardized Effect Size (Cohen‘s d) | |||||
| MMM | HE | MMM | HE | MMM | HE | MMM | HE | |||||||
| Systolic Blood Pressure (mmHg) | 96.82 (9.36) | 96.18 (9.76) | 3.00 (7.92) | 3.95 (9.51) | −0.41 (−2.27, 1.45) | d=0.06 | 2.60 (5.23) | 3.35 (5.01) | −0.89 (−3.10, 1.31) | d=0.10 | 2.17 (4.04) | 1.92 (3.81) | 1.34 (−1.33, 4.01) | d=0.13 |
| Diastolic Blood Pressure (mmHg) | 51.88 (5.74) | 52.48 (6.97) | 0.50 (5.67) | 1.68 (7.36) | −1.37 (−2.68, −0.06) | d=0.27 | 0.94 (3.76) | 1.55 (3.85) | −1.45 (−3.03, 0.13) | d=0.24 | 0.65 (2.41) | 0.67 (2.81) | −0.21 (−1.89, 1.47) | d=0.03 |
| Total Cholesterol (mg/dL) | 155.26 (27.72) | 152.58 (29.52) | −7.70 (20.86) | 0.55 (21.72 ) | −6.77 (−11.99, −1.55) | d=0.34 | −2.19 (11.14) | −1.12 (12.76) | −1.22 (−6.87, 4.44) | d=0.05 | −1.66 (7.98) | −0.92 (7.14) | −0.93 (−6.42, 4.56) | d=0.05 |
| HDL-Cholesterol (mg/dL) | 44.57 (10.06) | 44.66 (9.78) | −0.41 (6.45) | 0.24 (5.74) | −0.34 (−1.89, 1.21) | d=0.06 | 0.19 (3.43) | −0.59 (3.92) | 1.73 (−0.12, 3.58) | d=0.24 | 0.26 (2.70) | −0.22 (2.46) | 1.82 (−0.15, 3.79) | d=0.24 |
| LDL-Cholesterol (mg/dL) | 89.71 (23.94) | 89.19 (26.31) | −6.30 (15.95) | −0.67 (16.42 ) | −5.05 (−9.09, −1.02) | d=0.33 | −2.42 (9.17) | −1.42 (9.11) | −1.96 (−6.29, 2.37) | d=0.12 | −2.27 (6.66) | −1.44 (5.76) | −2.12 (−6.41, 2.18) | d=0.13 |
| Triglycerides (mg/dL) | 106.44 (66.37) | 93.63 (45.76) | −5.65 (46.87) | 5.05 (43.14 ) | −5.88 (−16.00, 4.23) | d=0.16 | −0.56 (27.53) | 5.22 (27.15) | −7.25 (−20.20, 5.71) | d=0.15 | 1.27 (20.74) | 3.85 (16.26) | −2.71 (−15.99, 10.57) | d=0.05 |
| Glucose (mg/dL)d | 92.48 (5.80) | 92.21 (5.88) | −0.62 (6.29) | −0.37 (6.03) | −0.20 (−1.66, 1.26) | d=0.03 | −0.55 (3.40) | −0.26 (3.20) | −0.33 (−1.92, 1.26) | d=0.05 | −0.59 (2.73) | −0.70 (2.67) | 0.56 (−1.48, 2.59) | d=0.07 |
| Insulin (uIU/ml) | 15.84 (10.35) | 16.17 (12.40) | 1.45 (9.63) | 0.92 (10.25 ) | 0.55 (−1.85, 2.95) | d=0.06 | 1.82 (5.75) | 1.84 (5.73) | −0.36 (−3.13, 2.41) | d=0.03 | 2.04 (5.32) | 2.26 (4.57) | −0.85 (−4.62, 2.93) | d=0.06 |
| HOMA_IRd | 3.67 (2.51) | 3.72 (2.90) | 0.29 (2.27) | 0.19 (2.58) | 0.08 (−0.47, 0.64) | d=0.04 | 0.38 (1.42) | 0.41 (1.48) | −0.17 (−0.87, 0.54) | d=0.06 | 0.42 (1.23) | 0.49 (1.15) | −0.19 (−1.09, 0.71) | d=0.05 |
| Hemoglobin A1c (percent)d | 5.09 (0.28) | 5.07 (0.26) | −0.05 (0.15) | −0.04 (0.15) | −0.01 (−0.05, 0.03) | d=0.07 | −0.02 (0.09) | −0.01 (0.09) | −0.02 (−0.07, 0.02) | d=0.14 | 0.01 (0.08) | 0.01 (0.06) | −0.02 (−0.07, 0.04) | d=0.08 |
| Alanine Aminotransferase (IU/L) | 28.92 (19.47) | 33.50 (25.39) | 1.18 (30.21) | −2.09 (23.17 ) | 1.56 (−5.01, 8.13) | d=0.06 | −1.07 (8.77) | −0.51 (13.45) | −3.68 (−8.82, 1.46) | d=0.18 | −0.43 (9.72) | −1.14 (9.56) | 0.06 (−6.86, 6.98) | d=0.00 |
| High sensitivity C-Reactive Protein (mg/dL) | 0.23 (0.31) | 0.25 (0.31) | 0.04 (0.35) | 0.03 (0.48) | −0.01 (−0.11, 0.10) | d=0.01 | 0.00 (0.25) | 0.02 (0.23) | −0.05 (−0.16, 0.06) | d=0.11 | −0.01 (0.14) | −0.01 (0.12) | −0.05 (−0.13, 0.04) | d=0.15 |
| Resting Heart Rate (beats per minute) | 84.20 (11.05) | 84.36 (10.57) | 0.90 (11.66) | 1.63 (10.40 ) | −0.84 (−3.26, 1.59) | d=0.09 | −0.29 (6.45) | 0.18 (5.62) | −0.94 (−3.62, 1.74) | d=0.09 | −0.60 (4.07) | −0.38 (4.01) | −0.53 (−3.39, 2.32) | d=0.05 |
| Accelerometer average percent of day spent in moderate-to-vigorous physical activity e | 9.65 (2.89) | 9.71 (3.12) | −0.83 (2.60) | −1.09 (2.17) | 0.29 (−0.28, 0.86) | d=0.13 | −0.94 (1.27) | −0.92 (1.42) | −0.03 (−0.65, 0.59) | d=0.01 | −0.88 (1.01) | −0.77 (1.04) | −0.35 (−1.06, 0.37) | d=0.13 |
| Accelerometer Average percent of day spent sedentary e | 71.35 (5.63) | 71.12 (5.46) | 2.42 (5.38) | 3.00 (4.51) | −0.67 (−1.85, 0.50) | d=0.15 | 2.37 (3.12) | 2.53 (2.93) | −0.19 (−1.64, 1.27) | d=0.03 | 2.39 (2.42) | 2.39 (2.33) | 0.48 (−1.33, 2.28) | d=0.07 |
| Accelerometer average after school minutes of moderate-to-vigorous physical activity (3–6pm weekdays) | 26.08 (13.07) | 25.04 (10.93) | 3.14 (17.60) | −2.74 (10.85 ) | 6.88 (3.69, 10.07) | d=0.57 | −0.73 (8.85) | −1.78 (6.81) | 2.83 (−0.50, 6.16) | d=0.22 | −1.77 (5.88) | −1.48 (4.93) | −0.23 (−3.83, 3.37) | d=0.02 |
| Accelerometer average after school minutes sedentary (3–6pm weekdays) | 108.27 (26.34) | 106.00 (19.61) | −2.89 (30.81) | 9.58 (29.18 ) | −11.95 (−18.78, −5.12) | d=0.47 | 3.64 (17.99) | 8.27 (25.50) | −7.94 (−18.19, 2.31) | d=0.20 | 5.95 (12.98) | 6.82 (10.19) | −0.28 (−7.20, 6.65) | d=0.01 |
| Weekly hours of total screen time | 20.73 (14.61) | 19.82 (18.33) | 0.20 (15.31) | 0.29 (17.14 ) | 0.21 (−3.33, 3.74) | d=0.02 | 0.33 (10.76) | 1.28 (10.11) | −1.16 (−5.79, 3.47) | d=0.06 | 0.36 (8.14) | 1.77 (8.56) | −3.40 (−9.12, 2.32) | d=0.15 |
| Weekly hours of television viewing | 9.48 (8.24) | 8.97 (8.68) | −0.72 (10.23) | 0.25 (8.95) | −0.91 (−3.17, 1.34) | d=0.11 | −1.13 (5.45) | −0.27 (5.20) | −1.33 (−3.36, 0.70) | d=0.17 | −1.30 (3.88) | −0.76 (4.17) | −1.18 (−3.54, 1.18) | d=0.13 |
| Typical household TV use (0 low – 4 high) | 2.15 (0.96) | 2.07 (1.06) | −0.45 (1.07) | −0.45 (0.89) | 0.03 (−0.19, 0.26) | d=0.04 | −0.20 (0.56) | −0.20 (0.53) | 0.05 (−0.19, 0.28) | d=0.05 | −0.10 (0.43) | −0.14 (0.37) | 0.14 (−0.12, 0.40) | d=0.14 |
| Days per week ate breakfast with TV | 1.97 (2.32) | 2.04 (2.35) | −0.78 (2.62) | −0.11 (2.47) | −0.76 (−1.29, −0.24) | d=0.39 | −0.44 (1.44) | −0.39 (1.28) | −0.23 (−0.72, 0.26) | d=0.12 | −0.32 (1.02) | −0.35 (0.85) | −0.10 (−0.62, 0.43) | d=0.05 |
| Days per week ate dinner with TV | 2.54 (2.61) | 2.37 (2.38) | −0.92 (2.85) | −0.33 (2.65) | −0.47 (−1.03, 0.08) | d=0.23 | −0.53 (1.47) | −0.30 (1.51) | −0.31 (−0.83, 0.21) | d=0.15 | −0.41 (0.98) | −0.18 (0.97) | −0.61 (−1.15, −0.08) | d=0.29 |
| Total daily energy intake (Kcal) | 1214.97 (421.96) | 1173.12 (388.53) | −126.69 (416.25) | −12.55 (378.5 2) | −91.27 (−180.01, −2.52) | d=0.26 | −106.76 (262.89) | −42.14 (185.21) | −98.84 (−192.64, −5.05) | d=0.27 | −66.86 (189.82 ) | −26.93 (164.07) | −79.23 (−193.11, 34.64) | d=0.18 |
| Percent of dietary energy from fat | 30.26 (5.80) | 29.97 (5.86) | −2.44 (7.03) | −1.07 (6.99) | −0.94 (−2.48, 0.61) | d=0.16 | −1.18 (4.34) | −0.87 (3.48) | −0.33 (−2.01, 1.35) | d=0.05 | −0.81 (2.83) | −0.06 (2.61) | −1.95 (−3.73, −0.16) | d=0.28 |
| Added sugar (gm) | 33.32 (19.09) | 33.34 (20.27) | −2.52 (22.75) | −0.17 (23.74 ) | −2.34 (−7.75, 3.07) | d=0.12 | −2.73 (14.38) | −0.69 (11.65) | −4.13 (−10.06, 1.80) | d=0.18 | −1.23 (11.97) | −1.38 (9.12) | 0.15 (−7.40, 7.69) | d=0.00 |
| Fiber (gm) | 11.90 (4.43) | 10.89 (4.08) | −0.55 (5.40) | 1.03 (4.81) | −1.02 (−2.25, 0.20) | d=0.23 | −0.80 (2.77) | −0.02 (2.64) | −0.89 (−2.04, 0.26) | d=0.20 | −0.56 (1.97) | −0.10 (2.02) | −0.72 (−2.04, 0.60) | d=0.14 |
| Percent of daily energy consumed while watching screens | 24.32 (20.78) | 22.75 (21.48) | −7.30 (22.43) | 0.21 (21.54 ) | −6.55 (−11.55, −1.55) | d=0.36 | −5.30 (12.61) | 1.15 (12.54) | −11.51 (−16.53, −6.48) | d=0.59 | −3.78 (9.70) | 0.15 (8.97) | −10.71 (−16.42, −5.01) | d=0.48 |
| Sugar sweetened beverages (servings) | 1.16 (0.81) | 1.11 (0.90) | −0.19 (0.87) | −0.04 (1.04) | −0.12 (−0.34, 0.10) | d=0.15 | −0.12 (0.56) | −0.06 (0.53) | −0.08 (−0.33, 0.17) | d=0.08 | −0.08 (0.39) | −0.05 (0.39) | −0.06 (−0.33, 0.22) | d=0.05 |
| Overconcern with weight and shape (5 items, 1 low – 3 high) | 2.11 (0.50) | 2.08 (0.47) | −0.09 (0.52) | −0.15 (0.48) | 0.08 (−0.04, 0.19) | d=0.18 | −0.09 (0.31) | −0.10 (0.27) | 0.05 (−0.08, 0.18) | d=0.09 | −0.08 (0.23) | −0.09 (0.20) | 0.02 (−0.13, 0.16) | d=0.03 |
| School Performance (1 = mostly F’s to 9 = mostly A’s) | 7.31 (1.39) | 7.55 (1.14) | 0.08 (1.40) | −0.35 (1.39) | 0.28 (−0.04, 0.60) | d=0.23 | −0.08 (0.84) | −0.15 (0.77) | −0.00 (−0.38, 0.37) | d=0.00 | −0.03 (0.59) | −0.09 (0.45) | 0.02 (−0.36, 0.39) | d=0.01 |
| Depressive Symptoms (0 low – 20 high) | 2.68 (2.33) | 3.24 (3.29) | −0.53 (2.57) | −1.03 (3.01) | 0.06 (−0.46, 0.57) | d=0.03 | −0.45 (1.56) | −0.64 (1.72) | −0.08 (−0.71, 0.56) | d=0.03 | −0.36 (1.24) | −0.57 (1.31) | 0.10 (−0.63, 0.83) | d=0.03 |
| Total Child Implicit Theories (1 fixed – 6 growth) | 3.72 (1.17) | 3.78 (1.07) | 0.53 (1.12) | 0.48 (1.04) | −0.00 (−0.25, 0.25) | d=0.00 | 0.46 (0.74) | 0.46 (0.60) | −0.08 (−0.34, 0.19) | d=0.07 | 0.41 (0.49) | 0.37 (0.39) | 0.06 (−0.19, 0.32) | d=0.06 |
Baseline data from all participants, N = 120 for MMM and N= 121 for Health Education for all variables at baseline
Unadjusted slopes calculated from all participants with follow-up data, N= 120 for MMM and N = 117 for Health Education for blood pressures and resting heart rate, N= 114 for MMM and N = 116 for Health Education for all blood measures, N= 117 for MMM and N = 116 for Health Education for accelerometer measures, N = 119 for MMM and N = 116 – 117 for Health Education for all survey measures.
Adjusted MMM minus Health Education differences from linear trajectories (slopes) for each individual regressed on intervention group assignment (centered), with the baseline value (centered at its mean) and the Intervention x baseline interaction as covariates, using standard maximum likelihood linear regression techniques. Consistent with intent-to-treat principles, trajectories for children with only a baseline measure were imputed using a joint modeling multiple imputation approach as implemented in SAS using PROC MI (version 9.4). Thus, N= 120 for MMM and 121 for Health Education, for all outcomes.
For outcome analyses, glucose values trimmed to 126 mg/dL and Hemoglobin A1c values trimmed to 6.5% for three participants diagnosed with diabetes (1 child at years 2 and 3, and 2 children at year 3).
Daily accelerometer estimates between 5:00 AM and 11:59pm for all days, weighted 5:2 weekdays to weekend days.
Intervention Implementation and Participation
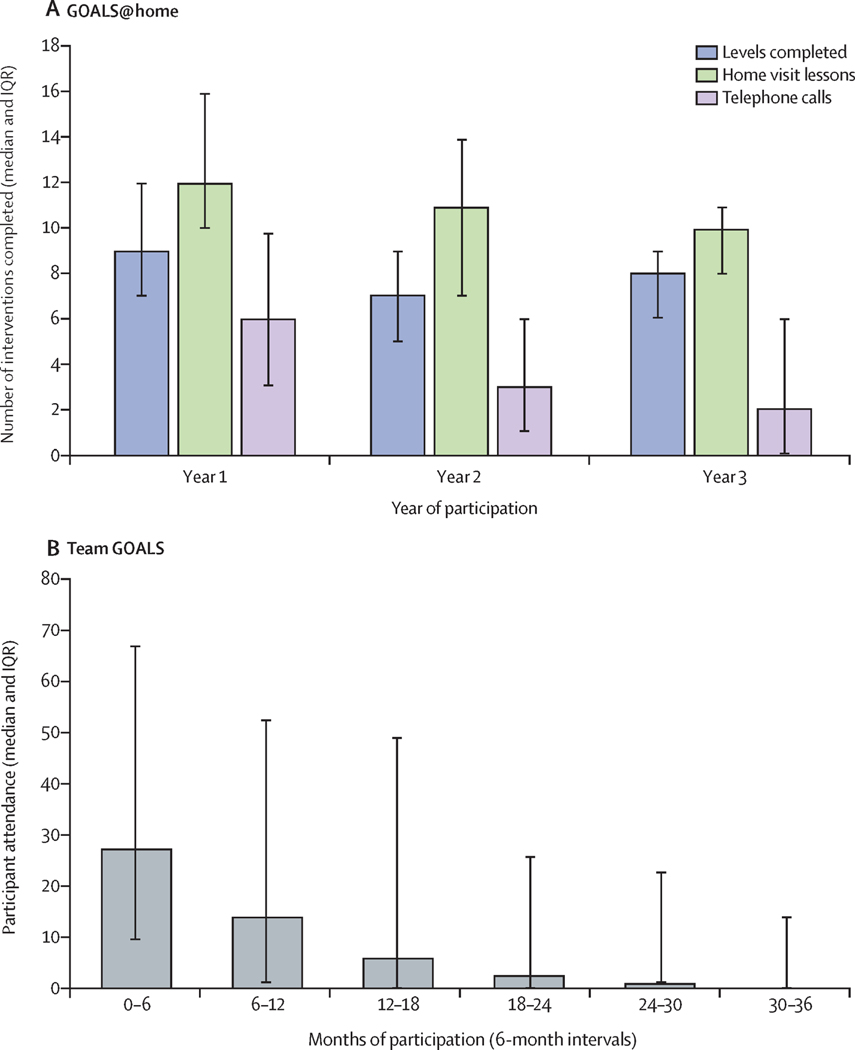
The overall MMM intervention dose received by participants was high but generally fell over the course of 3 years. Families received a median (interquartile range, IQR) of 35 (27.5 – 39) home visits and 11 (6 – 18) phone calls, respectively, completing 26.5 (21 – 30) or 88.3% (70% −100%) of 30 possible GOALS@Home mastery levels. The number of visits, phone calls and levels completed fell over the course of the three years (Figure 2). 120 (100%) MMM families completed the first module, 114 (95.0%) a second module, 105 (87.5%) a third module, 72 (60.0%) a fourth module and 47 (39.2%) the fifth module. Home visits were attended by a median (IQR) of 2.43 (2.20 – 2.83) family members; 0.96 (0.93 – 0.98) index children, 0.97 (0.88 – 1.00) index parents, and 0.60 (0.31 – 1.03) other family members. 120 (100%) MMM families received dishware during the first GOALS@Home module. Compared to the dishware children were using before, the non-rim surface area of new plates and volumes of new bowls, glasses and mugs decreased from a mean ± SD of 201.8 ± 72.1 to 172.9 ± 4.4 cm2 (3.3 ± 37.0% smaller), 749.0 ± 199.5 to 323.5 ± 39.2 ml (53.3 ± 15.6% smaller), 445.9 ± 128.0 to 284.9 ± 11.8 ml (30.7 ± 20.3% smaller), and 369.9 ± 86.8 to 236.0 ± 8.0 ml (32.5 ±16.3% smaller), respectively. For adults, the non-rim surface area of new plates and volumes of new bowls, glasses and mugs decreased from a mean ± SD of 230.4 ± 56.0 to 172.9 ± 4.4 cm2 (20.1 ± 23.5% smaller), 844.7 ± 229.8 to 323.1 ± 39.4 ml (58.9 ± 12.3% smaller), 504.0 ± 149.0 to 284.9 ± 11.8 ml (39.2 ± 16.3% smaller), and 427.0 ± 102.9 to 236.1 ± 8.0 ml (41.5 ± 14.1% smaller), respectively. After 3 years, 100 (83%) families reported children were still using all small dishware – 104 (87%) plates, 101 (84%) bowls, 104 (87%) glasses, 103 (86%) mugs, 93 (78%) other family members were still using all small dishware – 96 (80%) plates, 94 (78%) bowls, 96 (80%) glasses, 100 (83%) mugs, and 13 (11%) of families were not assessed.
Figure 2:
Trends in participation over 3 years in the GOALS@home and Team GOALS interventions
Children attended Team GOALS after school team sports sessions an overall mean of 137.9 or 22.2%, median (IQR) = 86 (18 – 224) or 14.1% (2.9% - 36.5%) of a possible average 621.1 days, median (IQR) = 613.0 (606 – 626) days, over 3 years (Figure 2). Overall, 51 (42.5%) MMM participants averaged at least one day per week over the entire three years, falling from 71 (59.2%) during their first six months to 24 (20.0%) in the final sixth six months of participation. Team GOALS sessions were delivered with high fidelity and produced substantial activity rates. Systematic, time-sampled direct observations36 demonstrated out of a mean ± SD length of 55.3 ± 9.6 minutes, 41.0 ± 7.9 minutes (74.6 ± 9.9%) were devoted to fitness, skills practice and game play activities instead of group management and knowledge, and children were moving 33.8 ± 6.9 minutes (61.8 ±10.7%), from when they arrived to when they departed. All five Primary Care GOALS progress reports were delivered to primary care medical providers for 114 participants (mean 98.2%, median (IQR) = 100% (100% - 100%)) and shared with 116 families by health educators (mean 99.0%, median (IQR) = 100% (100% - 100%)).
The Health Education comparison intervention was also delivered with high fidelity. 120 (99.2%), 118 (97.5%), 115 (95.0%), 114 (94.2%), 114 (94.2%), and 112 (92.6%) families received Health Education home visits one through six, respectively, and a mean of 35.5 of 36 (98.6%, median (IQR) = 100% (100% - 100%) of the monthly Health Education newsletters were delivered to children and parents/guardians. While not unexpected,20 family attendance at quarterly neighborhood health education/family fun nights was low, averaging 0.38 events per family (4.3%, median (IQR) = 0.0% (0.0% - 11.1%) of families).
Outcomes
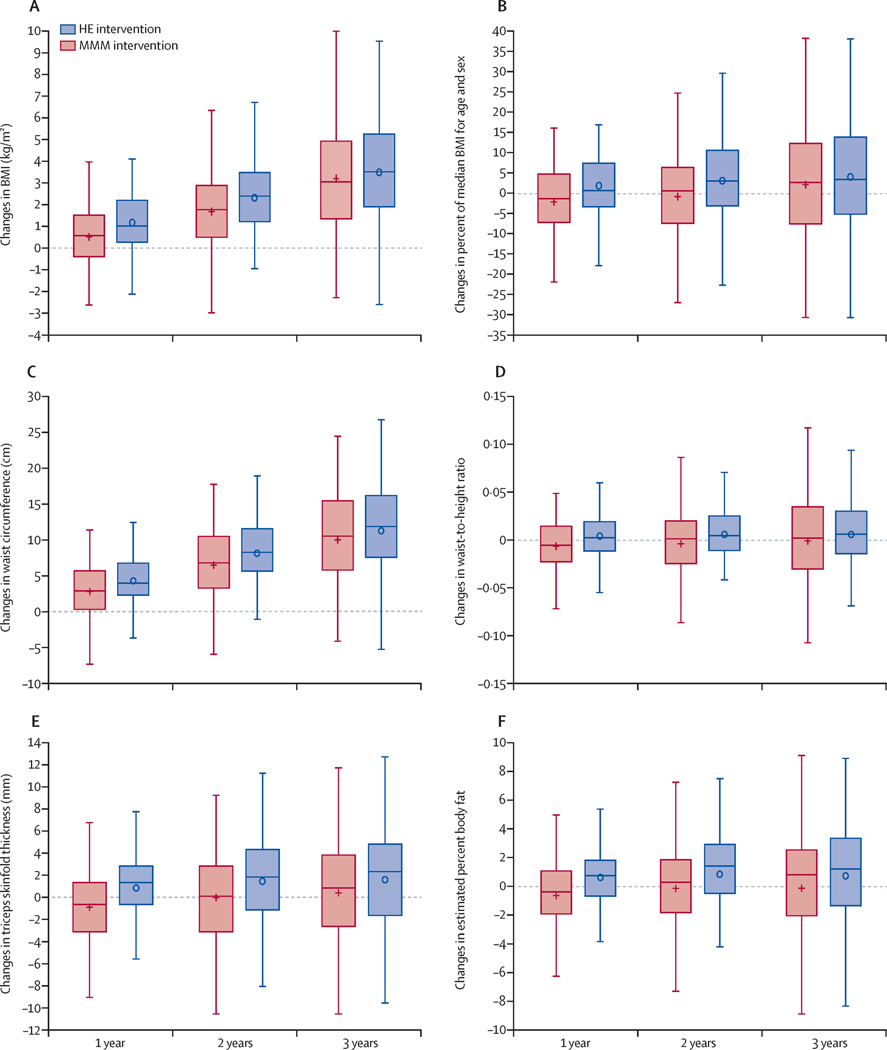
Changes in child outcomes over 1, 2 and 3 years are reported in Tables 2 and 3, Figure 3, and Supplementary Table S1 and Figures S2, S3 and S4, and changes in parent/guardian outcomes are reported in Table S2. The difference between children randomized to MMM and Health Education in BMI trajectory over 3 years, the primary outcome, was not statistically significant (mean adjusted difference (95% Confidence Interval [CI]) = −0.25 (−0.90, 0.40) kg/m2, Cohen’s d = −.10, p= 0.45). Children randomized to the MMM intervention gained less BMI over one year (mean adjusted difference (95% CI) = −0.73 (−1.07, −0.39) kg/m2, d = −.55) and over 2 years (mean adjusted difference (95% CI) = −0.63 (−1.13, −0.14) kg/m2, d = −.33) than children randomized to Health Education. Similar patterns of change, with substantial differences between groups over one and two years but not over three years, were seen for secondary measures of adiposity, percent of median BMI for age and sex, waist circumference, waist-to-height ratio, triceps skinfold thickness, and estimated percent body fat (Table 2 and Figure 3), and for rates remission from severe obesity at baseline (Supplementary Table S1). Favorable changes in children randomized to the MMM intervention compared to Health Education also were observed over one and/or two years for other cardiometabolic risk factor changes (diastolic blood pressure, total cholesterol, LDL cholesterol), physical activity changes (after school minutes of moderate-to-vigorous physical activity, after school minutes of sedentary behavior, parent/guardian physical activity), diet changes (total daily energy intake, percent of daily energy consumed while watching screens, days of eating breakfast while watching TV), and changes in parent/guardian health literacy. Average daily percent of dietary energy from fat, percent of daily energy consumed while watching screens, and days per week eating dinner while watching TV improved more in MMM compared to Health Education children over three years, (Table 3).
Figure 3: Changes in measures of adiposity over over 1 year, 2 years, and 3 years.
Box and whisker plots of changes in the MMM group (shown in red) and the Health Education group (shown in blue) over 1,2, and 3 years. The mean value is shown as a + (MMM) or o (Health Education) and the median is shown as a horizontal line across the centre of the box. The box shows IQR, bounded by the 75th percentile above and the 25th percentile below. The whiskers show the highest observed value at or within 1–5 times the IQR above the 75th percentile and below the 25th percentile. From all available data (238 individuals over lyear, 233 individuals over 2 years, 227 individuals over 3 years). Means and SD at each assessment timepoint and statistical significance for adjusted differences from the intention-to-treat analysis (241 individuals) are reported in table 2. HE=Health Education. MMM=multi-level, multi-settinq, multi-component.
Sensitivity Analysis
Sensitivity analyses for the primary outcome that varied assumptions regarding method of estimation and missingness yielded results consistent with our primary findings. A sensitivity analysis that relaxed linearity assumptions regarding BMI changes over time, however, demonstrated that the BMI trajectory for the MMM group was statistically significantly attenuated over three years compared to that of the Health Education group, in contrast to our primary analysis that constrained BMI changes to be linear over time (Supplementary material).
Moderator Analysis
In pre-specified exploratory analyses, all baseline sociodemographic, psychological, behavioral and physiological/physical measures were tested as possible moderators of intervention effects on trajectories of children’s BMI. Over three years, the MMM intervention had greater effects than Health Education on BMI trajectories (p<0.05) among children with married parents and lower baseline waist-to-height ratios.
Dose-Response Analysis
Among families randomized to the MMM intervention, number of completed GOALS@Home mastery levels were statistically significantly correlated with BMI changes during year 1 (Spearman r= −0.27) and year 3 (r= −0.21) and the combined number of completed home visits and phone calls were correlated with BMI changes in year 3 (r= −0.20). Team GOALS attendance was associated with BMI changes over 1, 2 and 3 years (r= −0.36, r= −0.32, and r= −0.19, respectively), where greater participation was associated with less BMI gain (Supplementary Figure S6).
Adverse Events
Systematic annual monitoring of adverse events did not find evidence of differential risks associated with the MMM and Health Education interventions in children or parents/guardians (Supplementary material). In addition, changes in overconcern for weight and shape did not statistically significantly differ between groups (Table 2).
Discussion
The Stanford GOALS randomized controlled trial tested a three-year, community-based, culturally-tailored, multi-level, multi-setting, multi-component systems intervention, designed to reduce weight gain among low socioeconomic status, predominantly Latinx children with overweight or obesity, compared to an active-placebo nutrition and health education comparison intervention. In an intent-to-treat analysis, children in families randomized to the MMM group gained an average of about 0.25 kg/m2 less than those randomized to Health Education children over three years, the primary outcome, but the difference was not statistically significant. Among secondary outcomes, children randomized to the MMM intervention gained an average of about 0.73 kg/m2 less over one year and about 0.63 kg/m2 less over two years than children randomized to Health Education. Differences between the MMM intervention compared to Health Education in changes over one and two years were also evident in secondary measures of adiposity and, over one year for several important obesity-related cardiometabolic risk factor measures, diastolic blood pressure and total- and LDL-cholesterol. A number of behaviors targeted by the interventions also changed more in the MMM group over one and/or two years, including changes in children’s dietary energy intakes and meals eaten with screens, after school physical activity and sedentary behavior, and parent/guardian physical activity and health literacy. Over three years, percent of daily energy consumed while watching screens, dinners eaten with television, and percent of children’s dietary energy from fat fell more in the MMM group. Parent/guardian marital status and baseline waist-to-height ratio moderated the effects of the MMM versus Health Education interventions on BMI change over three years. These and possible moderators of intervention effects on BMI changes over one and two years may be useful for designing future studies.35
The one- and two-year outcomes are notable for the effect sizes and length of follow-up, particularly for a community-based trial in a low socioeconomic status, high risk sample. Judging the clinical significance of these findings must take into account the effect sizes as well as potential harms, costs, acceptability and availability of this and alternative interventions for the intended population and setting. Most prior intervention studies for children with overweight and obesity have been in smaller, less socioeconomically-challenged samples in clinical settings, and rarely report outcomes beyond 6–12 months.4–7 The observed BMI changes and standardized effect sizes compare favorably to the results reported in recent systematic reviews and meta-analyses of lifestyle/behavioral interventions for children with overweight and obesity.4–7 Prior randomized trials of community-based interventions to prevent or control obesity in children from racial/ethnic minority, low-socioeconomic status families have generally failed to find effects on weight-related measures, even over shorter periods of study.3 In contrast, compared to the Health Education intervention, the MMM intervention resulted in large relative average changes in body composition and cardiometabolic risk factors over one and/or two years. These findings and the observed changes in eating, activity and screen media behaviors suggest it is possible to substantially reduce trajectories of weight-gain and weight-related risk factors over one and two years in low-socioeconomic status, Latinx children with theory-driven, family and community-based environmental and behavioral interventions.
Important features of the MMM intervention may have led to the one- and two-year results compared to prior studies. The MMM intervention was grounded in social cognitive theory, emphasizing the interdependent, triadic reciprocal influences of personal, behavioral and environmental factors on behavior change,11 it was multi-level, multi-setting and multi-component, culturally-tailored, and designed from substantial formative research and community input, it included several components previously demonstrated to reduce weight gain or weight on their own, incorporated innovative strategies from social psychological science and principles of stealth interventions, and was designed as a systems intervention to promote synergistic effects and accommodate individualized pathways through the intervention.8
The rigor of the Stanford GOALS trial bolsters confidence in the results. The outstanding participant retention, measurement completeness and quality, fidelity of intervention delivery, intent-to-treat analysis with multiple imputation for missing outcomes, and active control intervention, all enhanced internal validity. We attribute the high study engagement in both groups to our strong foundation in theory applied to all aspects of the study implementation. Because there was no untreated comparison group, however, it is possible that effect sizes were attenuated by effects of the Health Education comparison intervention. Implementing the trial in low socioeconomic status neighborhoods using existing community infrastructure and partnerships, and developing the interventions with community and participant input, all enhance the potential generalizability of the results.
The substantial group differences observed over one and two years but lack of sustained statistically significant effects over three years may be explained in part by the fall in intervention participation over the course of the study. The MMM intervention was designed to try to overcome some barriers to children’s participation and adherence in clinical and behavioral obesity treatment programs and included environmental interventions such as smaller dishware in the home and availability of neighborhood after school team sports, and behavioral interventions designed to personalize, evolve and maintain motivation over the entire three years for each family. Yet, there were still steady drop-offs in average participation over time. Dose-response analyses lend partial support to this explanation. It is difficult to maintain participation of children and families in even much shorter behavioral interventions,4–7 particularly among socioeconomically-stressed families with many competing priorities. Interestingly, age and sex were not found to be possible moderators of intervention response. It is possible that additional community-level changes can improve outcomes. These results suggest that developing interventions, or series of complementary interventions, for low socioeconomic status children that can sustain participation and effects on body composition over even longer periods of time should be a priority for future research.
Supplementary Material
Research in context.
Evidence before this study
Systematic reviews and meta-analyses find that intensive behavioral interventions can reduce weight gain in children with overweight and obesity over six to 12 months. However, these programs are often expensive and time-consuming to implement, not available in many communities, able to serve only limited numbers of children, and may be inconvenient for children and families to attend. Prior randomized trials of community-based interventions to prevent or control obesity in children from low socioeconomic status, racial/ethnic minority families have generally failed to find effects on weight-related measures.
Added value of this study
There are few long-term studies of interventions to reduce weight gain in children with overweight or obesity from low-socioeconomic status families. The Stanford GOALS randomized controlled trial rigorously tested a novel community-based, multi-level, multi-setting, multi-component (MMM) intervention model, grounded in behavior change theory and incorporating cultural tailoring, recent advances in psychological science, principles of stealth interventions, and a systems perspective, to reduce weight gain among low socioeconomic status Latinx children with overweight and obesity over three years. The MMM intervention reduced body mass index (BMI) trajectory over one and two years, compared to a nutrition and health education control intervention, but the differences were not statistically significant over three years. Changes were also observed in additional measures of adiposity, cardiometabolic risk factors, and eating and physical activity behaviors. Effect sizes were similar to those estimated from meta-analyses of interventions with shorter follow-up periods.
Implications of all the available evidence
These findings suggest that the community-based MMM systems intervention model has the potential to successfully reduce weight gain and cardiometabolic risk factors for at least two years among children with overweight or obesity from high risk, low socioeconomic status, racial/ethnic minority families. Future research is needed to develop similar interventions and replicate these findings in other high risk populations and communities, and to develop additional strategies to sustain impacts over longer periods.
Acknowledgements:
This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers U01HL103629 and T32 HL007034, and from the Stanford Maternal and Child Health Research Institute and the Department of Pediatrics, Stanford University, with additional support from the other members of the Childhood Obesity Prevention and Treatment Research (COPTR) Consortium (awards U01HL103622, U01HL103620, U01HD068990, U01HL103561) from the National Heart, Lung, and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Office of Behavioral and Social Sciences Research, National Institutes of Health. The content expressed in this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Office of Behavioral and Social Sciences Research, the National Institutes of Health, the U.S. Department of Health and Human Services, and the U.S. Government.
We thank the children and families who participated, the Stanford GOALS research staff, including health educators, coaches and data collectors, community advisors and community partner organizations, the Boys and Girls Clubs of the Peninsula, The Police Activities League of Redwood City, CA, and the City of Redwood City, CA Parks, Recreation, and Community Services Department, and the participating children and families who participated in the Stanford GOALS formative and pilot studies, for their contributions to the Stanford GOALS trial. We also thank our COPTR consortium collaborators and Data and Safety Monitoring Board, Stephen Daniels, MD, PhD (Chair), Tom Baranowski, PhD, John D. Lantos, MD, Kiang J Liu, PhD, Robert P. Schwartz, MD, and Deborah Rohm Young, PhD, for their valuable input throughout the planning and conduct of this study.
Declaration of Interests:
TNR serves on scientific advisory boards for WW International, Inc. DMW has received grant funding and serves on a scientific advisory board for Tolerion, on an advisory board for the California Institute for Regenerative Medicine, and on a data and safety monitoring board for Intrexon T1D Partners, LLC. JS has received funding from Weight Watchers.
Footnotes
Data Sharing:
Investigators may request the public access data set from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BIOLINCC) https://biolincc.nhlbi.nih.gov/studies/?q=coptr (last accessed February 17, 2021)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Thomas N. Robinson, Stanford Solutions Science Lab, Division of General Pediatrics, Departments of Pediatrics, Medicine, and Epidemiology and Population Health, Stanford University, Stanford, California, USA.
Dr Donna Matheson, Stanford Solutions Science Lab, Division of General Pediatrics, Department of Pediatrics, Stanford University, Stanford, California, USA.
Darrell M. Wilson, Division of Endocrinology and Diabetes, Department of Pediatrics, Stanford University, Stanford, California, USA.
Dana L. Weintraub, Stanford Solutions Science Lab, Division of General Pediatrics, Department of Pediatrics, Stanford University, Stanford, California, USA.
Jorge A. Banda, Department of Public Health, Purdue University, West Lafayette, Indiana, USA.
Dr. Arianna McClain, Cruise, LLC, San Francisco, California, USA.
Lee M. Sanders, Division of General Pediatrics, Department of Pediatrics, Stanford University, Stanford, California, USA.
William L. Haskell, Department of Medicine, Stanford University, Stanford, California, USA.
Ms. K. Farish Haydel, Stanford Solutions Science Lab, Division of General Pediatrics, Department of Pediatrics, Stanford University, Stanford, California, USA.
Mr Kristopher I. Kapphahn, Quantitative Sciences Unit, Department of Medicine, Stanford University, Stanford, California, USA.
Dr Charlotte Pratt, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, Maryland, USA.
Kimberly P. Truesdale, Department of Nutrition, University of Carolina, Chapel Hill, Chapel Hill, North Carolina, USA.
June Stevens, Departments of Nutrition and Epidemiology, University of Carolina, Chapel Hill, Chapel Hill, North Carolina, USA.
Manisha Desai, Quantitative Sciences Unit, Departments of Medicine, Biomedical Data Science, and Epidemiology and Population Health, Stanford University, Stanford, California, USA.
References
- 1.Ogden CL, Fryar CD, Martin CB, et al. Trends in Obesity Prevalence by Race and Hispanic Origin-1999–2000 to 2017–2018. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz WH, Robinson TN. Overweight Children and Adolescents. New England Journal of Medicine 2005;352:2100–9. [DOI] [PubMed] [Google Scholar]
- 3.Dietz WH. We Need a New Approach to Prevent Obesity in Low-Income Minority Populations. Pediatrics 2019;143. [DOI] [PubMed] [Google Scholar]
- 4.Ho M, Garnett SP, Baur L, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics 2012;130:e1647–71. [DOI] [PubMed] [Google Scholar]
- 5.Mead E, Brown T, Rees K, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev 2017;6:CD012651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017;317:2427–44. [DOI] [PubMed] [Google Scholar]
- 7.Peirson L, Fitzpatrick-Lewis D, Morrison K, Warren R, Usman Ali M, Raina P. Treatment of overweight and obesity in children and youth: a systematic review and meta-analysis. CMAJ Open 2015;3:E35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TN, Matheson D, Desai M, et al. Family, community and clinic collaboration to treat overweight and obese children: Stanford GOALS-A randomized controlled trial of a three-year, multi-component, multi-level, multi-setting intervention. Contemp Clin Trials 2013;36:421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pratt CA, Boyington J, Esposito L, et al. Childhood Obesity Prevention and Treatment Research (COPTR): interventions addressing multiple influences in childhood and adolescent obesity. Contemp Clin Trials 2013;36:406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efron B. Forcing a sequential experiment to be balanced. Biometrika 1971;58:403–17. [Google Scholar]
- 11.Bandura A. Self-Efficacy: The Exercise of Control. New York: W.H. Freeman and Company; 1997. [Google Scholar]
- 12.Resnicow K, Baranowski T, Ahluwalia JS, Braithwaite RL. Cultural sensitivity in public health: defined and demystified. Ethn Dis 1999;9:10–21. [PubMed] [Google Scholar]
- 13.Lepper MR, Master A, Yow WQ. Intrinsic motivation in education. In: Maehr ML, Karabenick SA, Urdan TC, eds. Advances in Motivation in Education. Bingley, UK: Emerald Group Publishing Limited; 2008:521–56. [Google Scholar]
- 14.Blackwell LS, Trzesniewski KH, Dweck CS. Implicit theories of intelligence predict achievement across an adolescent transition: a longitudinal study and an intervention. Child Dev 2007;78:246–63. [DOI] [PubMed] [Google Scholar]
- 15.Cohen GL, Garcia J, Purdie-Vaughns V, Apfel N, Brzustoski P. Recursive processes in self-affirmation: intervening to close the minority achievement gap. Science 2009;324:400–3. [DOI] [PubMed] [Google Scholar]
- 16.Robinson TN. Stealth interventions for obesity prevention and control: motivating behavior change. In: Dube L, Bechara A, Dagher A, et al. , eds. Obesity Prevention: The Role of Brain and Society on Individual Behavior. New York, NY: Elsevier, Inc; 2010:319–27. [Google Scholar]
- 17.Robinson TN, Matheson DM. Environmental strategies for portion control in children. Appetite 2015;88:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson TN. Behavioural treatment of childhood and adolescent obesity. Int J Obes Relat Metab Disord 1999;23:S52–S7. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TN. Reducing children’s television viewing to prevent obesity. JAMA 1999;282:1561–7. [DOI] [PubMed] [Google Scholar]
- 20.Robinson TN, Matheson DM, Kraemer HC, et al. A randomized controlled trial of culturally tailored dance and reducing screen time to prevent weight gain in low-income African American girls: Stanford GEMS. Arch Pediatr Adolesc Med 2010;164:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U. S. Preventive Services Task Force, Grossman DC, Bibbins-Domingo K, et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317:2417–26. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub DL, Tirumalai EC, Haydel KF, Fujimoto M, Fulton JE, Robinson TN. Team sports for overweight children: the Stanford Sports to Prevent Obesity Randomized Trial (SPORT). Arch Pediatr Adolesc Med 2008;162:232–7. [DOI] [PubMed] [Google Scholar]
- 23.Freedman DS, Woo JG, Ogden CL, Xu JH, Cole TJ. Distance and percentage distance from median BMI as alternatives to BMI z score. Br J Nutr 2020;124:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. Am J Hum Biol 2007;19:487–94. [DOI] [PubMed] [Google Scholar]
- 25.Stevens J, Cai J, Truesdale KP, Cuttler L, Robinson TN, Roberts AL. Percent body fat prediction equations for 8- to 17-year-old American children. Pediatr Obes 2014;9:260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- 27.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 2011;43:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romanzini M, Petroski EL, Ohara D, Dourado AC, Reichert FF. Calibration of ActiGraph GT3X, Actical and RT3 accelerometers in adolescents. Eur J Sport Sci 2014;14:91–9. [DOI] [PubMed] [Google Scholar]
- 29.Washburn RA, Adams LL, Haile GT. Physical activity assessment for epidemiologic research: the utility of two simplified approaches. Prev Med 1987;16:636–46. [DOI] [PubMed] [Google Scholar]
- 30.Medrich EA. Constant television: A background to daily life. J Communication 1979;29:171–6. [Google Scholar]
- 31.Shisslak CM, Rneger R, Sharpe T, et al. Development and evaluation of the McKnight Risk Factor Survey for assessing potential risk factors for disordered eating in preadolescent girls. Int J Eat Disord 1999;25:195–214. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs M. The Children’s Depression Inventory (CDI) Manual. Toronto, Ontario: Multi-Health Systems, Inc.; 1992. [Google Scholar]
- 33.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 1980;9:271–80. [DOI] [PubMed] [Google Scholar]
- 34.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877–83. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie TL, Sallis JF, Nader PR. SOFIT: System for observing fitness instruction time. Journal of Teaching in Physical Education 1991;11:195–205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.