Abstract
Background:
This study was undertaken to analyze our outcomes after robotic fundoplication for GERD in patients with failed antireflux procedures, with type IV (i.e., giant) hiatal hernias, or after extensive intra-abdominal surgery with mesh, and to compare our results to outcomes predicted by the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator and to national outcomes reported by NSQIP.
Methods:
100 patients undergoing robotic fundoplication for the aforementioned factors were prospectively followed.
Results:
100 patients, aged 67 (67 ± 10.3) years with body mass index (BMI) of 26 (25 ± 2.9) kg/m2 underwent robotic fundoplication for failed antireflux fundoplications (43%), type IV hiatal hernias (31%), or after extensive intra-abdominal surgery with mesh (26%). Operative duration was 184 (196 ± 74.3) min with an estimated blood loss of 24 (51 ± 82.9) mL. Length of stay was 1 (2 ± 3.6) day. Two patients developed postoperative ileus. Two patients were readmitted within 30 days for nausea.
Nationally reported outcomes and those predicted by NSQIP were similar. When comparing our actual outcomes to predicted and national NSQIP outcomes, actual outcomes were superior for serious complications, any complications, pneumonia, surgical site infection, deep vein thrombosis, readmission, return to OR, and sepsis (P < 0.05); our actual outcomes were not worse for renal failure, deaths, cardiac complications, and discharge to a nursing facility.
Conclusions:
Our patients were not a selective group; rather they were more complex than reported in NSQIP. Most of our results after robotic fundoplication were superior to predicted and national outcomes. The utilization of the robotic platform for complex operations and fundoplications to treat patients with GERD is safe and efficacious.
Keywords: Robotic surgery, Fundoplication, Gastroesophageal reflux, GERD, Minimally invasive surgery, Giant hiatal hernia, Reoperative fundoplication, “Redo” fundoplication, Paraesophageal hernia, Robotic-assisted laparoscopy
INTRODUCTION
Gastroesophageal reflux disease, conventionally referred to as GERD, is a common disorder within the United States. More than 30 million Americans experience symptoms of reflux at least once per month. According to the Healthcare Cost and Utilization Project (HCUP), there were 40.7 million hospitalizations and 1,653 deaths as a result of GERD in 2010.1
Traditionally, the first line treatment for GERD involves the use of antacids, H2 blockers, and proton pump inhibitors (PPIs), providing short-term alleviation for symptoms gastroesophageal reflux. Although PPIs can provide adequate heartburn control for many patients, lifelong medical therapy is expensive, compliance can be a problem, and nearly 40% of patients have persistent symptoms despite heavy use of PPIs. As well, there are long-term issues with bile reflux, carcinogenesis, osteoporosis, renal dysfunction, pneumonia and colonic infections2. Furthermore, these medications were originally approved by the FDA for only a 14-day course, up to three times per year,2 not for open-ended therapy. Persistent symptoms are a result of a mechanical problem and requires a mechanical solution. The use of antacids, H2 blockers, and PPIs neutralize or suppress gastric acid and set the stage for reflux of unopposed conjugated and unconjugated bile. Prolonged exposure to such contents can lead to esophageal injury, esophagitis, metaplasia, dysplasia, Barrett’s esophagus, and if persistent, inevitably, esophageal adenocarcinoma. Over the last four decades there has been a 57-fold increase in the incidence of esophageal adenocarcinoma.3 In the United States, it is estimated that 16,910 patients will be diagnosed with esophageal cancer in 2020, and 15,690 will die.1
Laparoscopic fundoplication, a more efficacious and durable alternative to medical therapy, is currently considered the “gold standard” surgical treatment for GERD. The fundoplication was first described by Dr. Rudolf Nissen in 1936 as a superior form of intra-thoracic reconstruction after esophagectomy. Since then, fundoplications have gained recognition for their effectiveness in treatment and amelioration of symptoms of GERD.4,5 Since its initial description, the approach to which fundoplications are undertaken has evolved from “open” to minimally invasive techniques. Minimally invasive surgery has proved to be more beneficial for patients. Since its introduction in 1991, laparoscopic fundoplication has become the surgical treatment of choice for the definitive therapy of GERD.2,6,7 Laparoscopic fundoplication is associated with excellent short- and long-term reflux resolution rates of 80% to 95%, and patients can expect less pain, shorter hospital stays, quicker returns to normal activities, and a decrease rate of incisional hernias related to the “open” technique.8 As laparoscopic fundoplications were being widely mastered and accepted, in the early 2000’s some surgeons also turned to single-site techniques, providing patients with improved cosmesis and similar salutary benefits as multi-incision and multitrocar minimally invasive surgery.9–12
Nevertheless, fundoplication has its faults. Symptom recurrence occurs in 3% to 30% of patients who have undergone fundoplication, and in approximately 3% to 6% revisional operations are required due to recalcitrant or recurrent symptoms.13,14 Recurrent and persistent symptoms occur because of several factors, including but not limited to, anatomic failures, slipped fundic wraps, and herniation of the wrap.15 Also, having a history of a previous abdominal operation and the presence of large sliding and paraesophageal hernias further complicates any operation to abate GERD. These factors make patients high risk surgical candidates and present a formidable challenge for laparoscopic surgeons.
This study was undertaken to analyze our outcomes after robotic fundoplication for GERD in patients with failed antireflux fundoplications with type IV (i.e., giant) hiatal hernias, or after extensive intra-abdominal surgery and to compare those outcomes to outcomes predicted by the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) Surgical Risk Calculator and nationally reported outcomes contained within the ACS NSQIP database. Our hypothesis in undertaking this study is that our actual patient outcomes after these complex robotic fundoplications would be similar or superior to traditional laparoscopic techniques reported for “all comers” by the ACS NSQIP, therefore, encouraging the application of the robotic platform for challenging fundoplications in patients with failed previous antireflux procedures with giant hiatal hernias, or after prior extensive intra-abdominal operations.
METHODS
With Institutional Review Board approval, from Jan 2013 to Sep 2019, 100 consecutive patients undergoing robotic fundoplication for failed antireflux fundoplications, type IV hiatal hernias, and/or after extensive intra-abdominal surgery were prospectively followed. Demographic data, including age, sex, body mass index (BMI), perioperative complications, operative duration, estimated blood loss (EBL), conversions to “open”, length of stay (LOS), in-hospital mortality, discharge to rehabilitation facility, readmissions within 30 days, and death within 30 days were recorded and analyzed. Routinely, all patients underwent preoperative upper gastrointestinal (UGI) series, and 24-hour or 48-hour ambulatory pH testing, and esophageal motility study to determine the type of valve construction (complete versus partial) necessary. Postoperatively, patients undergo repeat UGI within 24 to 48 hours to confirm resolution of symptoms. Patients were followed postoperatively in clinic, by phone, and by mail to score the frequency and severity of their symptoms.
Type IV hiatal hernia is referred to as giant hiatal hernia throughout this text and is defined as the involvement of intra-abdominal viscera migrating into the chest, including stomach, small bowel, omentum, colon, and/or spleen.16 This type of hernia also involved sliding and paraesophageal hernias. Prior extensive intra-abdominal operation is defined as any previous abdominal operation by either laparoscopy or laparotomy resulting with widespread intraperitoneal adhesions. Perioperative complications are defined as adverse events necessitating deviation from the planned procedure. Postoperative complications were defined using ACS NSQIP definitions (Table 1). Operative duration is defined as the time from the first incision to the final dressings being placed on the patient. For illustrative purposes only, patients were divided into four consecutive 25-patient cohorts.
Table 1.
Comorbidities Affecting Patients Undergoing Robotic Fundoplication
| Most common chronic preoperative conditions in patients undergoing robotic fundoplication | |
| Total patients with comorbidity | 77% |
| Hypertension | 74% |
| Diabetes | 48% |
| Hyperlipidemia | 38% |
| Cardiovascular (myocardial infarct, peripheral vascular disease, stroke) | 29% |
| Thyroid disorder (hypothyroidism, hyperthyroidism) | 17% |
| Chronic obstructive pulmonary disease | 9% |
| Other (cancer, hematologic disease, Crohn’s disease) | 9% |
| Renal failure | 6% |
Data from the ACS NSQIP, collected by the ACS from 2013 to 2017, was utilized to compare our outcomes to a national “benchmark”. Our patients’ predicted outcomes were determined using the ACS NSQIP Surgical Risk Calculator (riskcalculator.facs.org/RiskCalculator/). Our patients’ predicted outcomes were compared to national patient outcomes reported in the ACS NSQIP database. Last, our patients’ actual outcomes were compared to those predicted by ACS NSQIP Risk Calculator and compared to nationally reported outcomes in the ACS NSQIP database.
In 2013, we began to use the da Vinci® Robotic Platform to undertake minimally invasive fundoplications. Our experience began slowly. Our initial operations were undertaken utilizing the Si model of the robotic platform. All subsequent fundoplications were undertaken using the ξ model. Our standardized, institutional approach for fundoplication has been previously described.12 Our robotic approach is much the same.
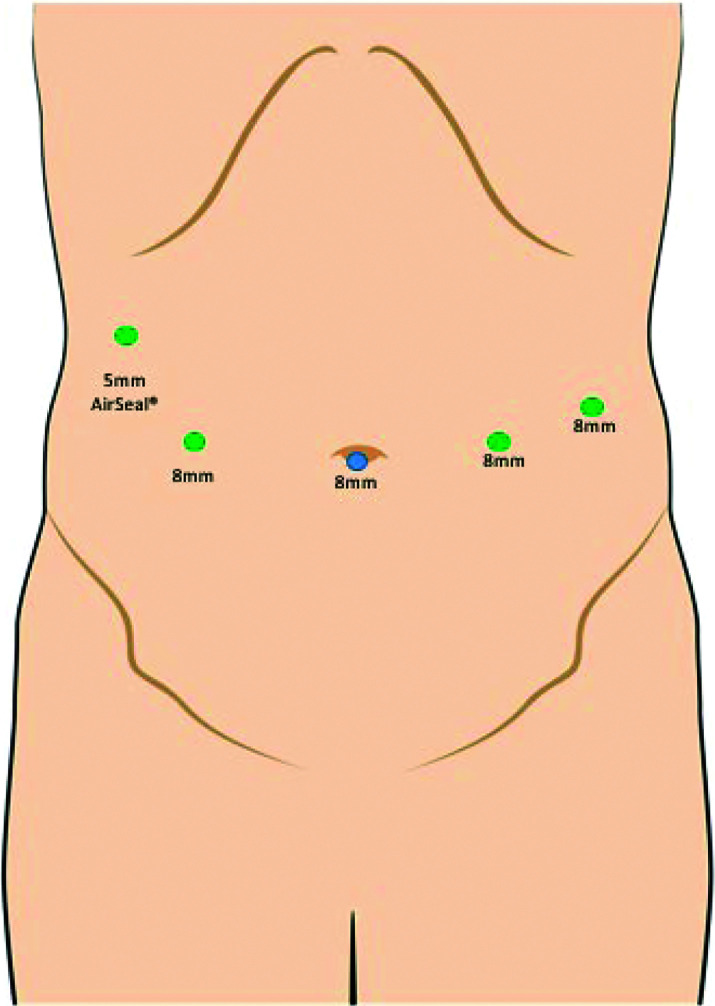
First, local anesthesia is administered at each incision site. An 8-mm incision is made at the umbilicus and with direct visualization, an 8-mm trocar is placed. Pneumoperitoneum is established. Two 8-mm robotic ports are placed at the right and left midclavicular lines slightly, just cephalad to the umbilicus. A fourth 8-mm robotic port is placed at the left anterior axillary line cephalad to the umbilicus. Finally, a 5-mm AirSeal® Access Port is placed at the right anterior axillary line at the costal margin and a liver retractor is placed through the 5-mm trocar (Figure 1).
Figure 1.
Robotic fundoplication port placement.
The operation begins by opening the gastrohepatic ligament in a stellate fashion. The opening should be generous to allow the stomach to be easily rolled/retracted/displaced to the patient’s left. This dissection continues to the right crus up and down the right crus and the mediastinum. The dissection along the greater curve begins with the most caudal short gastric vessels and is carried cephalad toward the left crus, up and down the left crus, and into the mediastinum. As is our routine, the stomach and 8 cm of esophagus are reduced into the peritoneal cavity. V-Loc™ sutures are utilized in a running fashion to bring the left and right crura nicely together and repair the hiatal hernia. Rarely do we use a mesh or anterior cruroplasty.
A 52–60 F bougie is placed per os into the stomach and the fundoplication is constructed; the size of the bougie is based on the patient’s size. For small patients and women, a 52–54 F bougie is used. For larger men, a 56–60 F bougie is used. A Nissen fundoplication is constructed using two interrupted sutures that bring the anterior fundus, esophagus, and posterior fundus together, and a third suture that brings only the anterior fundus and posterior fundus together at the GE junction. A Toupet fundoplication is constructed by placing three interrupted sutures between the posterior fundus and the right side of the esophagus at and proximal to the GE junction with three other interrupted sutures, which bring the anterior fundus and the left side of the esophagus at and proximal to the GE junction together; this results in a 270° posterior wrap or fundoplication. Once the bougie is removed, the posterior fundus is anchored to the right posterior side of the esophagus and to the right crus with one interrupted suture, to prevent tension, which might promote the wrap to come apart, or twisting of the esophagus, which would promote dysphagia. Once the fundoplication is constructed, intraoperative esophagogastroduodenoscopy (EGD) is undertaken.
Data are maintained in a secure Microsoft Excel database (Microsoft Corp, Redmond, Washington, USA). Statistical analysis was undertaken utilizing GraphPad Prism 8 software (GraphPad Software Inc., San Diego, California, USA). Nominal data were analyzed using χ-square analysis. Interval data were analyzed utilizing nonparametric testing of means through a Mann-Whitney U-test, where applicable. Patients and their outcomes were compared and analyzed utilizing regression analysis. Significance was accepted with 95% probability. For illustrative purposes, data are presented as median (mean ± SD) and in subgroups of 25 patients each.
RESULTS
Overall, 100 patients underwent robotic fundoplication after failed fundoplication, with a giant hiatal hernia, or after extensive intra-abdominal operations. The patients were a median age of 67 (67 ± 100.3) years with a median BMI of 26 (25 ± 20.9) kg/m2. Seventy-seven percent of patients had comorbidities, with the majority having more than one comorbidity (Table 1). Patients underwent robotic fundoplication for failed antireflux fundoplications (43%), giant hiatal hernias (31%), and/or after extensive intra-abdominal surgery with utilization of mesh in wound closure (26%). Two patients had open fundoplication with hiatal hernia repairs. Eighty-nine patients had previous abdominal operations. Thirty-seven patients underwent reoperative fundoplications. Of these patients, 76% received their previous fundoplications from an outside facility. Patients who experienced recurrence of symptoms underwent a reoperative fundoplication within 4 (7 50.9) years of their index fundoplication. Patients presented to clinic experiencing heart burn, upper abdominal discomfort, unintentional weight loss, regurgitation, dysphagia, and nausea.
Median operative duration was 184 (196 ± 740.3) minutes with an estimated blood loss of 24 (51 ± 820.9) mL. Patients had a length of stay of 1 (2 ± 30.6) day. There were two patients with postoperative complications. Both patients developed postoperative ileus. Two patients were readmitted within 30 days for nausea. Follow-up was continued for an average of 5 years, but up to 11 years.
The nationally reported outcomes and those predicted by NSQIP are displayed in Table 2. Our actual outcomes are also displayed in Table 2. Patients in the ACS NSQIP database were similar to our patients’ predicted outcomes. When comparing our actual outcomes to predicted and national NSQIP outcomes, actual outcomes were superior for serious complications, any complications, pneumonia, surgical site infection, deep vein thrombosis, readmission, return to OR and sepsis (P < .05) (Table 2, Table 3). Our actual outcomes were not different to predict and national outcomes for cardiac complications, renal failure, discharge to a nursing facility, and deaths.
Table 2.
Comparisons Among American College of Surgeons NSQIP Database, American College of Surgeons NSQIP-Predicted Outcomes, and Actual Outcomes
| Variable | ACS NSQIP Outcomes | ACS NSQIP Predicted Outcomes | Actual Outcomes |
|---|---|---|---|
| Patients (number) | — | 100 | 100 |
| Serious complication, % | 4 | 5 | 0*# |
| Any complication, % | 4 | 5 | 0*# |
| Pneumonia, % | 1 | 1 | 0*# |
| Cardiac complication, % | 0 | 0 | 0 |
| Surgical site infection, % | 1 | 1 | 0*# |
| Urinary tract infection, % | 1 | 1 | 0*# |
| Venous thromboembolism, % | 0 | 1 | 0*# |
| Renal failure, % | 0 | 0 | 0 |
| Sepsis, % | 0 | 1 | 0*# |
| Return to OR, % | 2 | 2 | 0*# |
| Length of stay, days | NA | 2 (2 ± 0.5) | 1 (2 ± 3.6) |
| Death, % | 0 | 0 | 0 |
| Discharge to nursing facility, % | 1 | 2 | 1 |
| Readmission, % | 4 | 5 | 2*# |
, The asterisk denotes a significant regression for all patients with a p-value ≤ 0.05. Patients were broken down into cohorts for illustrative purposes, but a regression was done to show an overall relationship between patients over time for selected variables; ACS NSQIP, American College of Surgeons National Surgical Quality Improvement Program; P < .05; #, predicted, P < .01. NA, nonapplicable.
Table 3.
Complications as Defined by American College of Surgeons NSQIP
| ACS NSQIP Complications | |
|---|---|
| Serious Complication | Any Complication |
| Cardiac arrest | Superficial incisional SSI |
| Myocardial infarction | Deep incisional SSI |
| Pneumonia | Organ space SSI |
| Progressive renal insufficiency | Wound disruption |
| Acute renal failure | Pneumonia |
| Pulmonary embolism | Unplanned intubation |
| Deep vein thrombosis | Pulmonary embolism |
| Return to operating room | Ventilator > 48 h |
| Deep incisional SSI | Progressive renal insufficiency |
| Organ space SSI | Acute renal failure |
| Systemic sepsis | Urinary tract infection |
| Unplanned intubation | Stroke |
| Urinary tract infection | Cardiac arrest |
| Wound disruption | Myocardial infarction |
| Deep vein thrombosis | |
| Return to operating room | |
| Systemic sepsis |
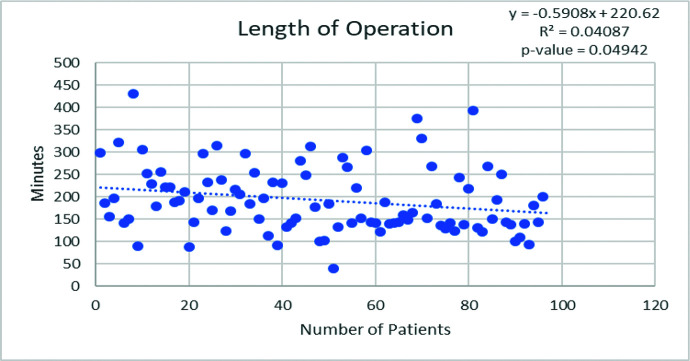
When patients are divided into quartiles for illustrative purposes, several outcomes were better appreciated. With increased experience, significantly more fundoplications were undertaken in patients with previous abdominal operations (P = .03). Operative duration decreased with experience (P = .05) and conversely, blood loss did not decrease (Figure 2). Length of stay remains steady with experience (Table 4).
Figure 2.
Regression analysis documenting a decrease in operative duration with experience.
Table 4.
Patients Stratified into 25-Patient Consecutive Cohorts for Illustrative Purposes
| Robotic Fundoplication Cohorts | |||||
|---|---|---|---|---|---|
| 0–25 | 26–50 | 51–75 | 76–100 | Total/P-Value | |
| Demographics | |||||
| Age | 61 | 36–86 (64 ± 12.9) | 66 | 59–72 (67 ± 9.6) | 67 | 47–87 (67 ± 8.4) | 67 | 39–76 (65 ± 9.7) | P = 0.67 |
| Gender, men/women | 32% M/68% W | 28% M/72% W | 36% M/64% W | 6% M/94% W | 27% M/73% W |
| BMI | 26 | 19–31 (25 ± 2.8) | 26 | 19–31 (25 ± 2.9) | 26 | 21–32 (26 ± 2.6) | 26 | 18–29 (25 ± 3.6) | P = 0.55 |
| Reoperative fundoplication | 11 patients (44%) | 11 patients (44%) | 5 patients (20%) | 10 patients (63%) | 37 | P = 0.89 |
| Giant hiatal herniam type IV | 9 patients (35%) | 9 patients (35%) | 5 patients (19%) | 3 patients (16%) | 26 | P = 0.43 |
| Previous abdominal operation | 18 patients (72%) | 23 patients (92%) | 23 patients (92%) | 25 patients (100%) | 89 | P = 0.03 * |
| Intraoperative Course | |||||
| Operative duration, min | 197 | 88–430(214 ± 76.1) | 190 | 91–315(194 ± 68.9) | 153 | 39–375(187 ± 77.2) | 143 | 121–393(186 ± 76.7) | P = 0.05* |
| Blood loss, mL | 28 | 10–350 (53 ± 72.7) | 20 | 5–350 (65 ± 92.5) | 20 | 2–500 (49 ± 104.2) | 22 | 5–150 (32 ± 35.0) | P = 0.40 |
| Conversions, to LESS | 1 | 0 | 0 | 0 | 1 | P = 0.42 |
| Concomitant procedures | 0 | 0 | 2 | 1 | 3 | P = 0.27 |
| Intraoperative complications | 0 | 0 | 0 | 0 | 0 | P = 0.99 |
| Postoperative Course | |||||
| Length of stay, days | 1 | 0–8 (2 ± 1.7) | 1 | 0–8 (2 ± 2.3) | 1 | 0–21 (3 ± 4.3) | 1 | 1–24 (4 ± 5.9) | P = 0.21 |
| Postoperative complications | 0 | 1 | 1 | 1 | 3 | P = 0.75 |
| In-hospital mortality | 0 | 0 | 0 | 0 | 0 | P = 0.99 |
| Readmission | 0 | 0 | 2 | 0 | 2 | P = 0.13 |
Values are meant as mean ± SD. BMI, body mass index.
DISCUSSION
The advent of the Intuitive Surgical Inc. da Vinci® Robotic Platform (Intuitive Surgical, Sunnyvale, California, USA) has led to a rapid paradigm shift in surgery and has opened a potential avenue that allows surgeons to undertake fundoplications in high risk patients, with the expected outcomes seen in “low risk” patients undergoing fundoplications for reflux disease. This robotic platform provides several advantages over conventional laparoscopy including high quality visualization with a near three-dimensional camera, better instrumentation, improved dexterity, and wrist articulation (with seven degrees of motion versus four degrees of motion seen with conventional laparoscopy), elimination of hand tremors, and better ergonomics leading to less fatigue. With these advantages, it seems that the robotic platform would lend itself particularly useful for the undertaking of more technically challenging fundoplications in patients that have had previous abdominal operations, are undergoing reoperative fundoplications and/or patients with giant type IV sliding and paraesophageal hiatal hernias.
Few comparative studies have been conducted to evaluate possible benefits of robot-assisted laparoscopy over traditional laparoscopy for reflux disease.17–21 To our knowledge, this study is the largest to compare an institution’s experience with robotic fundoplications to nationally reported outcomes in the ACS NSQIP database and to outcomes predicted by ACS NSQIP Risk Calculator. Although studies have looked at outcomes for robotic fundoplications, these have been generally a smaller set of patients with lower risks.22 This manuscript illustrates and supports the role of the robotic approach to challenging antireflux operations, such as patients with failed fundoplications, patients with giant hiatal hernias, and/or patients with extensive prior intra-abdominal operations often with incisional closure with mesh.
Most of our patients were women in their 60’s having a BMI within the “overweight” range. Nearly three out of four patients undergoing robotic fundoplication were for previously failed antireflux procedures and giant hiatal hernias, a challenging group of patients. Most of our patients were deconditioned with significant comorbidities including diabetes, hypertension, and vascular/coronary artery disease, which seem excessive for the average patient undergoing fundoplication in United States.
As expected, more technically complex operations inevitably take longer to complete. Prolonged operating time has been reported in all published series of robot‐assisted fundoplications.23–25 Our experience showed similar findings. In our experience there was one conversion to laparo-endoscopic single site (LESS) approach due to unanticipated need for lysis of extensive adhesions. Two patients who had prior open fundoplications and hiatal hernia repairs and/or 89 patients who had multiple previous abdominal operations were more likely to have some adhesions. However, utilization of the robotic platform made lysis of adhesions much safer and quicker. We were able to mobilize at least 8 cm of distal esophagus by dissecting more proximally into the mediastinum as necessary. This conversion occurred early in our experience, being only the third patient to undergo an attempted robotic fundoplication at this institution. For this patient the robot was scheduled, but never docked. Most conversions in laparoscopy are caused by bleeding and failure to progress, we believe that the robotic platform helps avoid unnecessary bleeding with a more precise dissection and better dexterity.22 The readmitted patients were evaluated and observed for nausea. When we stratified our patients into 25-patient cohorts, several things began to become more apparent. With increased experience, we took on more challenging patients, whereas reducing operative time, and maintaining minimal blood loss.
Our patients were not unique; they are demographically like those reported across the United States as presented in the ACS NSQIP database. They had frequent and severe comorbidities. However, our patients’ outcomes after robotic fundoplication were like or significantly superior to outcomes predicted by and reported by NSQIP as noted herein (Table 2).
It is our opinion that the best use of the robotic system is not fully appreciated in routine surgical practice, but rather for operations in more complex patients who are deemed high risk and require more technically challenging operations. Since the introduction of robotic systems, surgeons have been endeavoring to determine the right indications to apply this technology. Several small randomized studies have been undertaken to evaluate its use with primary routine antireflux surgery but failed to prove superiority of the robotic system over conventional laparoscopic surgery.18,26–28 We agree with that. Whereas these trials showed no additional value of the robotic system for primary antireflux surgery can be demonstrated, the use of the da Vinci robot may be beneficial for minimally invasive “redo” hiatal hernia and antireflux procedures in patients with prior intra-abdominal operations with incisional closures utilizing mesh due to their challenging nature, in patients with failed fundoplications, and in patients with giant hiatal hernias.29,30
CONCLUSIONS
Our patients were not a selective group; their predicted outcomes were similar to national outcomes. Most of our results after robotic fundoplication in these challenging patients were superior to predicted and national outcomes with less challenging patients. Our experience has shown that challenging robotic fundoplications are as safe and feasible as laparoscopic fundoplications reported through NSQIP, with fewer complications. Our short-term results support and promote the utilization of the robotic platform to treat patients with GERD needing challenging fundoplications for giant hiatal hernias, for revisional fundoplications, and/or after extensive intra-abdominal operations.
Footnotes
Disclosures: none.
Funding/Financial support: none.
Conflicts of Interest: Alexander Rosemurgy and Sharona Ross have educational and research relationships with Intuitive Surgical Inc. (Intuitive Corporation, Sunnyvale, CA). Kenneth Luberice, Kaitlyn Crespo, Christina De La Cruz, John-Kevin Dolce, and Iswanto Sucandy have no disclosures/conflicts of interest.
Informed consent: Dr. Rosemurgy declares that written informed consent was obtained from the patient’s for publication of this study/report and any accompanying images.
Contributor Information
Kenneth Luberice, Digestive Disorders Institute, AdventHealth Tampa, 3000 Medical Park Dr. Ste 500, Tampa, Florida 33613..
Sharona Ross, Digestive Disorders Institute, AdventHealth Tampa, 3000 Medical Park Dr. Ste 500, Tampa, Florida 33613..
Kaitlyn Crespo, Digestive Disorders Institute, AdventHealth Tampa, 3000 Medical Park Dr. Ste 500, Tampa, Florida 33613..
Christina De La Cruz, Digestive Disorders Institute, AdventHealth Tampa, 3000 Medical Park Dr. Ste 500, Tampa, Florida 33613..
John-Kevin Dolce, Digestive Disorders Institute, AdventHealth Tampa, 3000 Medical Park Dr. Ste 500, Tampa, Florida 33613..
Iswanto Sucandy, Digestive Disorders Institute, AdventHealth Tampa, 3000 Medical Park Dr. Ste 500, Tampa, Florida 33613..
Alexander S. Rosemurgy, Digestive Disorders Institute, AdventHealth Tampa, 3000 Medical Park Dr. Ste 500, Tampa, Florida 33613..
References:
- 1.US Food and Drug Administration, https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-possible-increased-risk-fractures-hip-wrist-and-spine-use-proton-pump, US Food and Drug Administration, Accessed on March 9th, 2020.
- 2.Dean BB, Gano AD, Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clini Gastroenterol Hepatol. 2004;2(8):656–664. [DOI] [PubMed] [Google Scholar]
- 3.Rosemurgy A, Wilfong C, Craigg D, Co F, Sucandy I, Ross S. The evolving landscape of esophageal cancer: a four-decade analysis. Am Surg. 2019;85(9):944–948. [PubMed] [Google Scholar]
- 4.Granderath FA, Kamolz T, Schweiger UM, Pointner R. Quality of life, surgical outcome, and patient satisfaction three years after laparoscopic Nissen fundoplication. World J Surg. 2002;26(10):1234–1238. [DOI] [PubMed] [Google Scholar]
- 5.Ross SB, Choung E, Teta AF, et al. The learning curve of laparoendoscopic single-Site (LESS) fundoplication: definable, short, and safe. JSLS. 2013;17(3):376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallemagne B, Weerts JM, Jehaes C, Markiewicz S, Lombard R. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparoscopy Endoscopy. 1991;1(3):138–143. [PubMed] [Google Scholar]
- 7.Salminen PT, Hiekkanen HI, Rantala AP, Ovaska JT. Comparison of long-term outcome of laparoscopic and conventional nissen fundoplication: a prospective randomized study with an 11-year follow-up. Ann Surg. 2007;246(2):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowgill SM, Gillman R, Kraemer E, Al-Saadi S, Villadolid D, Rosemurgy A. Ten-year follow up after laparoscopic Nissen fundoplication for gastroesophageal reflux disease. Am Surg. 2007;73(8):748–753. [PubMed] [Google Scholar]
- 9.Giovannetti A, Craigg D, Castro M, Ross S, Sucandy I, Rosemurgy A. Laparoendoscopic single-site (LESS) versus robotic “redo” hiatal hernia repair with fundoplication: which approach is better? Am Surg. 2019;85(9):978–984. [PubMed] [Google Scholar]
- 10.Rosemurgy AS, Downs D, Swaid F, Ross SB. Laparoendoscopic single-site (LESS) Nissen fundoplication: how we do it. J Gastrointest Surg. 2016;20(12):2093–2099. [DOI] [PubMed] [Google Scholar]
- 11.Sukharamwala P, Teta A, Ross S, et al. Over 250 laparoendoscopic single site (LESS) fundoplications: lessons learned. Am Surg. 2015;81(9):870–875. [PubMed] [Google Scholar]
- 12.Ross S, Roddenbery A, Luberice K, et al. Laparoendoscopic single site (LESS) vs. conventional laparoscopic fundoplication for GERD: is there a difference? Surg Endosc. 2013;27(2):538–547. [DOI] [PubMed] [Google Scholar]
- 13.Dallemagne B, Arenas Sanchez M, Francart D, et al. Long‐term results after laparoscopic reoperation for failed antireflux procedures. Br J Surg. 2011;98(11):1581–1587. [DOI] [PubMed] [Google Scholar]
- 14.Turkcapar A, Kepenekci I, Mahmoud H, Tuzuner A. Laparoscopıc fundoplicatıon with prosthetic hiatal closure. World J Surg. 2007;31(11):2169–2176. [DOI] [PubMed] [Google Scholar]
- 15.Humphries LA, Hernandez JM, Clark W, Luberice K, Ross SB, Rosemurgy AS. Causes of dissatisfaction after laparoscopic fundoplication: the impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc. 2013;27(5):1537–1545. [DOI] [PubMed] [Google Scholar]
- 16.Oleynikov D, Jolley JM. Paraesophageal hernia. Surg Clin North Am. 2015;95(3):555–565. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Zheng Q, Jin Z. Meta‐analysis of robot‐assisted versus conventional laparoscopic Nissen fundoplication for gastro‐oesophageal reflux disease. ANZ Journal of Surgery. 2012;82(3):112–117. [DOI] [PubMed] [Google Scholar]
- 18.Frazzoni M, Conigliaro R, Colli G, Melotti G. Conventional versus robot-assisted laparoscopic Nissen fundoplication: a comparison of postoperative acid reflux parameters. Surg Endosc. 2012;26(6):1675–1681. [DOI] [PubMed] [Google Scholar]
- 19.Mi J, Kang Y, Chen X, Wang B, Wang Z. Whether robot-assisted laparoscopic fundoplication is better for gastroesophageal reflux disease in adults: a systematic review and meta-analysis. Surg Endosc. 2010;24(8):1803–1814. [DOI] [PubMed] [Google Scholar]
- 20.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138(7):777–784. [DOI] [PubMed] [Google Scholar]
- 21.Ayav A, Bresler L, Brunaud L, Boissel P. Early results of one-year robotic surgery using the Da Vinci system to perform advanced laparoscopic procedures. J Gastrointest Surg. 2004;8(6):720–726. [DOI] [PubMed] [Google Scholar]
- 22.Arcerito M, Changchien E, Falcon M, Parga MA, Bernal O, Moon JT. Robotic fundoplication for gastroesophageal reflux disease and hiatal hernia: initial experience and outcome. Am Surg. 2018;84(12):1945–1950. [PubMed] [Google Scholar]
- 23.Melvin WS, Needleman BJ, Krause KR, Schneider C, Ellison EC. Computer-enhanced vs. standard laparoscopic antireflux surgery. J Gastrointest Surg. 2002;6(1):11–16. [DOI] [PubMed] [Google Scholar]
- 24.Cadière GB, Himpens J, Vertruyen M, et al. Evaluation of telesurgical (robotic) NISSEN fundoplication. Surg Endosc. 2001;15(9):918–923. [DOI] [PubMed] [Google Scholar]
- 25.Morino M, Pellegrino L, Giaccone C, Garrone C, Rebecchi F. Randomized clinical trial of robot‐assisted versus laparoscopic Nissen fundoplication. Br J Surg. 2006;93(5):553–558. [DOI] [PubMed] [Google Scholar]
- 26.Owen B, Simorov A, Siref A, Shostrom V, Oleynikov D. How does robotic anti-reflux surgery compare with traditional open and laparoscopic techniques: a cost and outcomes analysis. Surg Endosc. 2014;28(5):1686–1690. [DOI] [PubMed] [Google Scholar]
- 27.Müller-Stich BP, Reiter MA, Wente MN, et al. Robot-assisted versus conventional laparoscopic fundoplication: short-term outcome of a pilot randomized controlled trial. Surg Endosc. 2007;21(10):1800–1805. [DOI] [PubMed] [Google Scholar]
- 28.Braumann C, Jacobi CA, Menenakos C, Ismail M, Rueckert JC, Mueller JM. Robotic-assisted laparoscopic and thoracoscopic surgery with the da Vinci system: a 4-year experience in a single institution. Surg Laparosc Endosc Percutan Tech. 2008;18(3):260–266. [DOI] [PubMed] [Google Scholar]
- 29.Tolboom RC, Draaisma WA, Broeders IA. Evaluation of conventional laparoscopic versus robot-assisted laparoscopic redo hiatal hernia and antireflux surgery: a cohort study. J Robot Surg. 2016;10(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morelli L, Guadagni S, Mariniello MD, et al. Robotic giant hiatal hernia repair: 3 year prospective evaluation and review of the literature. Int J Med Robot. 2015;11(1):1–7. [DOI] [PubMed] [Google Scholar]