Abstract
Aims
Pulmonary embolism severity index (PESI) has been developed to help physicians make decisions about the treatment of patients with pulmonary embolism (PE). The combination of echocardiographic parameters could potentially improve PESI’s mortality prediction. To assess the additional prognostic value of tricuspid annular plane systolic excursion (TAPSE) and pulmonary artery systolic pressure (PASP) when combined with the PESI score in patients with PE to predict short-term mortality.
Methods and results
A multicentric prospective study database of patients admitted with PE in 75 academic centres in Argentina between 2016 and 2017 was analysed. Patients with an echocardiogram at admission with simultaneous measurement of TAPSE and PASP were included. PESI risk score was calculated blindly and prospectively, and in-hospital all-cause mortality was assessed. Of 684 patients, 91% had an echocardiogram, PASP and TAPSE could be estimated simultaneously in 355 (57%). All-cause in-hospital mortality was 11%. The receiver operating characteristic analysis showed an area under the curve (AUC) [95% confidence interval (CI)] of 0.76 (0.72–0.81), 0.74 (0.69–0.79), and 0.71 (0.62–0.79), for the PESI score, PASP, and TAPSE parameters, respectively. When PESI score was combined with the echocardiogram parameters (PESI + PASP-TAPSE = PESI-Echo), an AUC of 0.82 (0.77–0.86) was achieved (P = 0.007). A PESI-Echo score ≥128 was the optimal cut-off point for predicting hospital mortality: sensitivity 82% (95% CI 67–90%), specificity 69% (95% CI 64–74%). The global net reclassification improvement was 9.9%.
Conclusions
PESI-Echo score is a novel tool for assessing mortality risk in patients with acute PE. The addition of echocardiographic parameters to a validated clinical score improved the prediction of hospital mortality.
Keywords: Pulmonary embolism, Mortality, Echocardiography, Argentina
Introduction
Pulmonary embolism (PE) is a major health problem worldwide, with an annual incidence of 60–70 cases per 100 000 inhabitants according to the USA and European data.1–3 However, the actual figures are likely to be higher since silent PE can develop in up to 50% of patients with deep venous thrombosis.4
There is a wide variation in its prognosis, with a mortality of less than 3% in those without haemodynamic impairment or evidence of myocardial injury, to more than 90% in those who present with cardiorespiratory arrest, all of which reinforces the concept that early diagnosis and risk stratification are key factors in the selection of an appropriate treatment.5–9
The pulmonary embolism severity index (PESI), developed more than 15 years ago, is one of the most widely validated and used risk stratification tools,6,10,11 it uses clinical bedside parameters and stratifies 30-day mortality into five groups of incremental risk from 1% to more than 10%. Being a bedside tool, it lacks of other important measures related to PE prognosis such as acute pulmonary hypertension and its impact on right ventricular dysfunction. In that sense, transthoracic echocardiography (TTE) measurements may help improving the prognosis stratification. Although not widely available (but increasing), and certainly with inter-operator variability, two TTE parameters have previously shown to have a good discriminative value: the tricuspid annular plane systolic excursion (TAPSE), as one of the most accepted right ventricular systolic function surrogate, and the estimated pulmonary artery systolic pressure (PASP) as an indirect measurement of pulmonary hypertension.
There is no information regarding the additional value of these echocardiographic parameters to the PESI score for risk assessment in these patients. The aim of this study was to evaluate the additional prognostic value of TAPSE and PASP when combined with the PESI score (PESI-Echo) to predict short-term mortality in a contemporary cohort of patients with acute PE.
Methods
An analysis of the CONAREC XX registry12 database was performed. Shortly, CONAREC XX registry was an observational study that prospectively included all PE patients admitted to hospitals with cardiology residency from Argentina, between October 2016 and November 2017. Information about the type of PE, its severity, site of hospitalization (coronary unit, intensive care unit, and general ward), as well as complementary tests performed and events was gathered prospectively using local charts. The PE had to be confirmed by at least one conventional imaging test (computed tomography, ventilation/perfusion scan, or angiography).
No diagnostic algorithms or guidelines for patient management were issued. An independent committee carried out a random cross-audit of 20% of the centres, which validated the results and no case was excluded after the audit. The study protocol and informed consent were approved by each centre’s ethics committee.12,13
The clinical endpoint evaluated for each risk score was in-hospital all-cause mortality. Additionally, we sought to compare the baseline characteristics of our population with the cohort of the original PESI study.14
The clinical characteristics of the PE, complementary studies, treatments and outcomes were resumed in Supplementary material online, Table S2.
Risk scoring systems
Risk scores were calculated blindly and prospectively, and in-hospital mortality for all-causes was assessed. The clinical variables from the original PESI study14 were: age, sex, comorbidities (chronic obstructive pulmonary disease, heart failure, and cancer), heart rate, respiratory rate, mental state, temperature, systolic blood pressure, and oxygen saturation at admission. After the collection of these variables, a sum was made to determine the risk categories (Supplementary material online, Table S1).
Echocardiographic variables
The following echocardiographic variables were studied in the original registry cohort13:
TAPSE: evaluated with M mode in apical view of four cameras, expressed in millimetres (mm) and
PASP: estimated by tricuspid regurgitation, expressed in mmHg, and an addition of the estimated right atria pressure inferred by the inferior vena cava size and collapse. The echocardiogram was performed by sonographists as standard of care, and data from the charts were uploaded by the local investigators into the electronic case report form (eCRF).
The PESI-Echo score was determined, based on the sum of the PESI variables and the continuous measurement of the systolic pressure of the pulmonary artery, subtracting the systolic excursion from the plane of the tricuspid annulus (PESI + PASP-TAPSE = PESI-Echo), as TAPSE’s value is inversely related to the severity of a PE event (Supplementary material online, Figure S1). This measurement was performed offline by the investigators of this report with a calc sheet of MS Excel and blinded to the outcomes of the patients.
Statistical analysis
Continuous variables were expressed as mean and standard deviation or median and interquartile range (IQR), according to their distribution. Categorical variables were expressed as numbers and percentages. For the comparisons between continuous variables, the Student’s t-test or the Wilcoxon rank sum test was used. Comparisons between proportions were made using the χ2 test or Fisher’s exact test, depending on the frequency of expected values. An alpha error of less than 5% was assumed to establish statistical significance.
The area under the receiver operating characteristic (ROC) curve [area under the curve (AUC)] was calculated to assess the prognostic accuracy and discriminative ability of each score, and the comparison between curves was performed with the method of DeLong et al. The Youden index was used to determine the cut-off point with the highest sensitivity and specificity; negative and positive predictive value, and likelihood ratio were also calculated.
We calculated the net reclassification improvement (NRI) to determine if the addition of echocardiographic parameters to the PESI risk score improves the proper classification capacity of the score (higher risk classification in individuals with the event, and lower risk in individuals without the event); this being the net proportion of correctly reclassified events and non-events. The NRI (>0) is defined as a change in the correct direction for any cut-off value considered.15
Kaplan–Meier curves were constructed for mortality as a function of time, and we used the log-rank test to assess differences in survival rates between PESI-Echo risk categories.
For statistical analysis, SPSS 23 and Medcalc 17.2 statistics software were used.
Results
Of all the patients included in the registry (n = 684), echocardiography was performed in 625 (91%), showing dilatation or dysfunction of the right ventricle in 41% and 35% of cases, respectively. The median PASP was 43 mmHg (IQR 35–55) and the median TAPSE was 18 (IQR 14–22) (Table 1). In 355 patients (52%), PASP and TAPSE could be simultaneously estimated, and they constitute the analysed sample. The remaining cases had not reported one or both of the parameters or couldn’t estimate the PASP due to the absence of tricuspid insufficiency.
Table 1.
Transthoracic echocardiogram of admission in the global cohort (n = 625)
| Transthoracic echocardiogram | 625 (91.37%) |
| Left ventricular systolic function | |
| Preserved | 532/620 (85.1%) |
| Mild deterioration | 42/620 (6.7%) |
| Moderate deterioration | 19/620 (3%) |
| Severe deterioration | 27/620 (4.3%) |
| Right ventricular systolic function deterioration | 206/595 (34.6%) |
| Systolic excursion of the plane of the tricuspid annulus | 18 (14–22) |
| Systolic pressure of the pulmonary artery | 43 (35–55) |
| Right ventricular dilatation | 250/610 (40.9%) |
| Right ventricular wall motion impairment | 128/580 (22%) |
| Flattening of the interventricular septum | 118/569 (20.7%) |
Mean age was 63.8 ± 17.2 years and 43.4% were female. The baseline characteristics of the population evaluated are summarized in Table 2, and they are similar to the characteristics of the original cohort. A statistically significant difference was only found in the history of infectious disease: 16.2% in global cohort vs. 11.2% in the analysed cohort (P = 0.03).
Table 2.
Baseline characteristics global cohort and subgroup with simultaneous estimation of TAPSE and PASP
| Variables | Global cohort (n = 684) | Subgroup with echocardiographic parameters (n = 355) | P-value |
|---|---|---|---|
| Age (±SD) | 63.80 (±16.7) | 63.6 (±17.2) | 0.2 |
| Male sex | 296 (43.2%) | 154 (43.4%) | 0.9 |
| Previous thromboembolic disease | 120 (17.5%) | 62 (17.5%) | 1 |
| PE | 32/120 (26.6%) | 17/62 (27.4%) | 0.7 |
| Deep venous thrombosis | 106/120 (88.3%) | 53/62 (85.4%) | 0.1 |
| Arterial hypertension | 383 (55.9%) | 199 (56.1%) | 1 |
| Diabetes | 98 (14.33%) | 55 (15.5%) | 0.6 |
| Former smoking | 188 (27.49%) | 102 (28.7%) | 0.6 |
| Smoking | 76 (11.11%) | 34 (9.4%) | 0.4 |
| Dyslipidaemia | 203 (29.6%) | 113 (31.8%) | 0.5 |
| Atrial fibrillation | 48 (7%) | 32 (9%) | 0.2 |
| Previous ischaemic heart disease | 57 (8.3%) | 35(9.9%) | 0.3 |
| Ambulation | |||
| Without help | 517 (75.58%) | 271 (76.3%) | 0.7 |
| With help | 111 (16.23%) | 55 (15.5%) | |
| Prostrate | 56 (8.19%) | 29 (8.2%) | |
| COPD | 61 (8.9%) | 29 (8.2%) | 0.7 |
| Heart failure | 78 (11.4%) | 45 (12.7%) | 0.5 |
| Hormone therapy | 51 (7.4%) | 22 (6.2%) | 0.4 |
| Ongoing malignancy | 150 (21.9%) | 66 (18.6%) | 0.2 |
| Malignancy in remission | 40 (5.8%) | 19 (5.4%) | 0.8 |
| Chemotherapy | 79 (11.5%) | 40 (11.2%) | 0.8 |
| Stroke | 41 (5.9%) | 18 (5.1%) | 0.6 |
| Procoagulant syndrome | 27 (3.9%) | 15 (4.2%) | 0.8 |
| Obesity | 232 (33.9%) | 116 (32.7%) | 0.6 |
| Major bleeding | 29 (4.26%) | 14 (4%) | 0.8 |
| Autoimmune disease | 35 (5.12%) | 15 (4.2%) | 0.5 |
| Infectious disease | 111 (16.2%) | 40 (11.2%) | 0.03 |
| Recent surgery (3 months) | 164 (23.9%) | 74 (20.7%) | 0.2 |
| Recent hospitalization (3 months) | 230 (33.6%) | 103 (28.9%) | 0.1 |
| Transitional rest >72 h | 186 (29.6%) | 84 (25.8%) | 0.2 |
| Long trip | 42 (6.1%) | 30 (8.5%) | 0.1 |
| Chronic kidney disease | |||
| No dialysis | 44 (6.4%) | 28 (7.9%) | 0.5 |
| Dialysis | 10 (1.4%) | 4 (1.1%) | |
| Previous anticoagulation | 64 (9.3%) | 35 (9.9%) | 0.7 |
| Adequate anticoagulationa | 31/64 (48.4%) | 15/62 (42.9%) | 0.1 |
Sometimes, for a factor or background, the sum of the different categories considered is greater than 100% because there are patients with more than one of them. Qualitative expressed as n (%).
COPD, chronic obstructive pulmonary disease; PE, pulmonary embolism.
RIN 2–3 on admission for vitamin K antagonists or with the correct dose of direct oral anticoagulants and low molecular weight heparin.
All-cause in-hospital mortality was 11% (n = 39). The average PESI-Echo risk score in those who presented the in-hospital event was 172 ± 61, compared to 100 ± 50 in those who did not (P < 0.001). Supplementary material online, Table S3 describes causes of death in high vs. low PESI-Echo score.
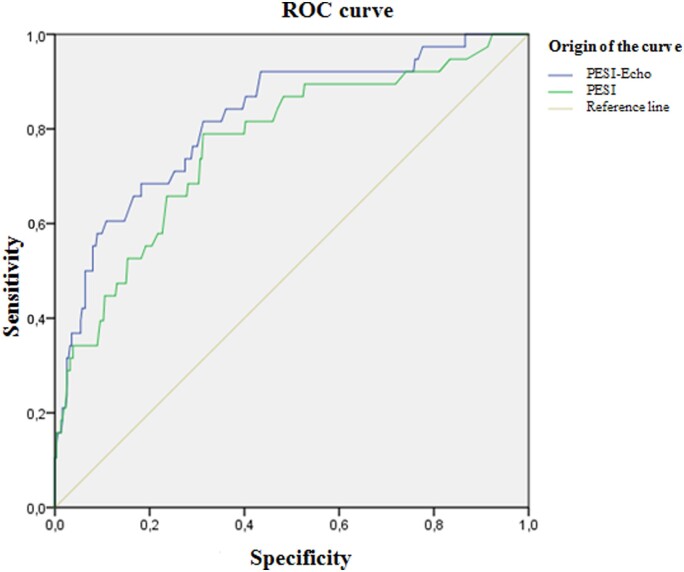
The ROC analysis showed an AUC [95% confidence interval (CI)] of 0.75 (0.67–0.83), 0.74 (0.69–0.79), and 0.71 (0.62–0, 79) for PESI score, the PASP, and the isolated TAPSE, respectively. When the PESI score was combined with the two parameters (PESI + PASP-TAPSE = PESI-Echo), an AUC curve of 0.82 (0.77–0.86) was found, which was statistically different from the area under the PESI score curve (difference between areas 0.054; 95% CI 0.0144–0.0936; P = 0.007) (Figure 1).
Figure 1.
ROC curves for PESI and PESI-Echo risk scores to predict in-hospital mortality.
Using the Youden index, a PESI-Echo score ≥128 was found to be the optimal cut-off point for the prediction of hospital mortality from all-causes: sensitivity 82% (95% CI 67–90%), and specificity 69% (95% CI 64.3–74%), with a positive likelihood ratio of 2.68 (95% CI 2.15–3.34) a negative likelihood ratio of 0.26 (95% CI 0.14–0.50), a negative predictive value of 96% (95% CI 93.7–98.3%), and a positive predictive value of 27% (95% CI 22.81–31.47%) (Table 3).
Table 3.
Sensitivity and specificity for the best cut-off point of each risk score for in-hospital mortality
| PESI | PESI-Echo | P-value | |
|---|---|---|---|
| AUC for in-hospital mortality | 0.75 (0.67–0.83) | 0.82 (0.74–0.90) | 0.007 |
| Cut-off points for the prediction of in-hospital mortality | |||
| Sensitivity (IC 95%) | S 64% (46–79%) | S 82 % (67–90%) | |
| Specificity (IC 95%) | Sp 69% (64–74%) | Sp 69% (64–74%) |
AUC, area under the curve.
When the reclassification risk index for in-hospital mortality was performed by adding echocardiographic parameters to the PESI risk score, a positive reclassification of 13% was observed in the case of events, a negative reclassification of 3.1% in the non-event group, with an NRI 9.9% overall (Table 4).
Table 4.
Net reclassification for in-hospital mortality by adding echocardiographic parameters to the PESI risk score
| In-hospital mortality | PESI | PESI-Echo |
Total | Reclassification |
||||
|---|---|---|---|---|---|---|---|---|
| Low | High | Higher | Less | Net | NRI | |||
| No | Low | 173 | 47 | 220 | 47 (14.8%) | 37 (11.7%) | −3.10% | 9.90% |
| High | 37 | 59 | 96 | |||||
| Total | 210 | 106 | 316 | |||||
| Yes | Low | 4 | 9 | 13 | 9 (23%) | 4 (10%) | 13% | |
| High | 4 | 22 | 26 | |||||
| Total | 8 | 31 | 39 | |||||
Green, correct reclassification; Grey, neutral reclassification; NRI, net reclassification improvement; Red, incorrect reclassification.
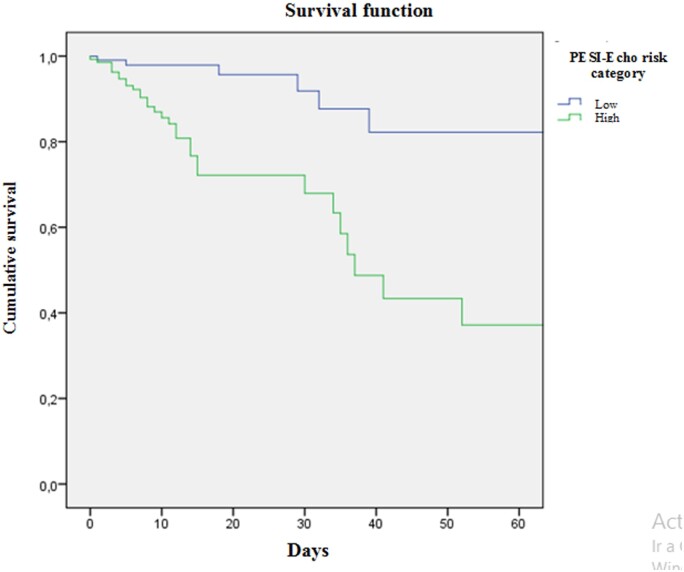
In addition, the survival curve for in-hospital mortality was analysed according to the risk category of the PESI-Echo score, with statistically significant differences between PESI Echo scores ≥128 and <128 (log-rank, P < 0.001) (Figure 2).
Figure 2.
Survival curve for in-hospital mortality according to PESI-Echo risk category. Log-rank test P < 0.001. Pesi-Echo low <128, high ≥128.
Discussion
In this registry, based on the prospective inclusion of patients admitted with PE in Argentina, we found that a risk score created from the combination of echocardiographic parameters (TAPSE and PASP) with the PESI risk score presented a better mortality prediction.
PESI is the risk score for predicting mortality in acute PE most widely validated and used.6,10 Likewise, its simplified version (sPESI) was also validated successfully and presented a similar prognostic accuracy.11 Both scores are based only on clinical parameters evaluated during the initial presentation, which allows to guide the therapy of these patients according to the estimated mortality.6,10,11
On the other hand, echocardiography, being a non-invasive technique, increasingly available, relatively inexpensive, and without side effects, is the modality of choice for the bedside evaluation of morphology and the role of right ventricle in daily clinical practice, as well as indirect measure of pulmonary hypertension and right ventricular afterload (TAPSE, PASP, strain, and tissue Doppler of the free wall of the right ventricle, among others), with a clear correlation with in-hospital all-cause mortality and PE related mortality.16–20
Prior trials had shown that right ventricular dysfunction is a frequent finding and has a clear correlation with in-hospital and 30-day mortality, so TTE is essential in the study and risk stratification of these patients.21–24 Within the specific parameters evaluated, TAPSE has demonstrated an excellent correlation with the systolic function of right ventricle in patients with PE, being easy to measure and reproducible.25–27 In those patients with acute PE, the presence of a TAPSE value ≤16 mm at the time of diagnosis was a predictor of all-cause mortality and PE-related mortality during hospitalization and at 30-day follow-up, being its predictive power even higher than other parameters such as right ventricular/left ventricular index.19,27 Also, when analysed as a continuous variable, TAPSE had the highest discriminative capacity among multiple echocardiographic predictors.19 This undoubtedly justifies its measurement for risk stratification in all patients with a recent diagnosis of PE.
It has been detected in multiple studies that the elevation of PASP during the acute event seems to have an impact on the development of long-term pulmonary hypertension,28 and also in in-hospital and 30-day mortality.19,28–30
The TAPSE and PASP relationship can be a step forward for a more efficient evaluation of the function of the right ventricle as it integrates contractility and afterload. In a previous trial, this relationship showed a negative regression line in non-survivors, who presented higher PASP and lower TAPSE more frequently.30
Although other parameters such as biomarkers (NT-proBNP, troponins) and certain CT parameters are gaining space as prognosis assessment tools for PE, echocardiography offers visual and bedside specific parameters of both right ventricular afterload and contractility, and its availability is constantly increasing hand in hand with the simplification of the devices and even pocket size echo devices.
Other investigators evaluated the use of echocardiographic parameters and serum biomarkers and showed that they improve the predictive capacity when making therapeutic decisions, when compared with the use of only the PESI score in patients with PE without haemodynamic decompensation.31 However, the actual additional value of a new risk prediction model that includes clinical variables of the PESI score and echocardiographic variables such as TAPSE and PASP had not been determined so far. Our study showed that PESI-Echo is superior to the PESI score for risk stratification of patients with acute PE.
On the other hand, the clinical diagnostic benefit of PESI-Echo was demonstrated by verifying the existence of 9% of correctly reclassified patients compared to the classification with the PESI score, especially considering that 23% of the cases were up-reclassified as high risk, which evidences its great utility. It should be also noted that it is a model that uses routine variables, both clinical and echocardiographic, and developed from a cohort of mainly intermediate and high-risk patients (75% of the cohort), which may explain the overall mortality of 11%, higher than other PE registries.
The idea and conception of this new score were performed in 2018–2019, but during publication process, the coronavirus disease 2019 (COVID-19) pandemics were increasing worldwide. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection carries the need of a proper PE stratification, as thrombotic complications are usual among high-risk COVID patients32 and elevated troponin levels are also frequent in these patients, bedside echocardiographic parameters of right ventricular dysfunction could be helpful in pulmonary risk stratification in this setting.33 In that context, the PESI-Echo score may help COVID-19 patients as well as clinicians by stratification of PE risk with more accuracy.
As limitations, we must take into account that the analysis of echocardiographic parameters was performed in a subpopulation of the cohort. Nearly 90% of the patients had an echocardiogram at admission, a use significantly higher than that reported in the literature (42.8% in the RIETE registry with 35 935 patients,17 findings consistent with recent studies of Australia34 and the USA), attributable to many potential factors, such as higher risk PE, and more widespread availability of technology in certain centres.
It is also important to mention that PASP could not be estimated in all patients, because it depends on the presence of tricuspid insufficiency, which according to studies can be found in 38–69% of patients.35–38 It is likely that the absence of tricuspid insufficiency is by itself a predictor of low pulmonary pressures and therefore of lower clinical risk, but this could not be analysed in this study.
Patients from this cohort were recruited from academic centres with cardiology residency, representing a set of patients admitted mainly in secondary or tertiary care hospitals and not the total PE population, and this may have a relation with the registered mortality rate.
However, we highlight that the PESI-Echo score described here, created from a prospective registry of patients with a confirmed diagnosis of acute PE, has achieved a greater prognostic capacity for risk stratification of in-hospital mortality of these patients, and for this reason, it could contribute to the election of the best therapy for patients with PE.
Finally, both scores only supplement the clinical judgement, and it cannot replace it, in view of the fact that the treatment of the patient with acute PE should consider multiple edges and not only the result of clinical or echocardiographic variables isolated or in combination.
Additional prospective studies are needed to validate these findings in other regions. Also, it may be used by clinicians as decision-making tools to estimate the prognosis of patients with acute PE, allowing for a more efficient use of treatment strategies, shared decision-making, and identification of high-risk patients for more intensive treatment.
Conclusion
The PESI-Echo score is a novel tool for assessing mortality risk in patients with acute pulmonary embolism. When available, the addition of echocardiographic parameters to the clinical score improved the prediction of in-hospital all mortality. Additional prospective studies are needed to validate these findings.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756–764. [DOI] [PubMed] [Google Scholar]
- 2. Oger E. Incidence of venous thromboembolism in a community-based study in Western France. Thromb Haemost 2000;83:657–660. [PubMed] [Google Scholar]
- 3. Widimský J, Malý J, Eliáš P. Doporučení pro diagnostiku a léčbu akutní plicní embolie. Vnitř Lék 2008;54:1S25–1S72. [Google Scholar]
- 4. Meignan M, Rosso J, Gauthier H, et al. Systematic lung scans reveal a high frequency of silent pulmonary embolism in patients with proximal deep venous thrombosis. Arch Intern Med 2000;160:159–164. [DOI] [PubMed] [Google Scholar]
- 5. Kurkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, Berzlanovich A, Bankl HC, Laggner AN.. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med 2000;160:1529–1535. [DOI] [PubMed] [Google Scholar]
- 6. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033–3069.25173341 [Google Scholar]
- 7. Goldhaber SZ Assessing the prognosis of acute pulmonary embolism: tricks of the trade. Chest 2008;133:334–336. [DOI] [PubMed] [Google Scholar]
- 8. Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M, Renaud B, Verhamme P, Stone RA, Legall C, Sanchez O, Pugh NA, N'gako A, Cornuz J, Hugli O, Beer HJ, Perrier A, Fine MJ, Yealy DM. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378:41–48. [DOI] [PubMed] [Google Scholar]
- 9. Zondag W, Kooiman J, Klok FA, Dekkers OM, Huisman MV.. Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta-analysis. Eur Respir J 2013;42:134–144. [DOI] [PubMed] [Google Scholar]
- 10. Aujesky D, Obrosky DS, Stone RA,Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jimenez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010;170:1383–1389. [DOI] [PubMed] [Google Scholar]
- 12. Cigalini I, Igolnikof D, Scatularo C, Jauregui J, Bernal M, Aboy JM, Garcia Zamora S, Bonorino JM, Thierer J, Zaidel EJ. Tromboembolismo pulmonar agudo en la Argentina. Registro CONAREC XX. Rev Argent Cardiol 2019;87:137–145 (12 es ex 27). [Google Scholar]
- 13. Cigalini I, Igolnikof D, Jauregui J, Ortego J, Aboy J, Cornejo D, Scatularo C, et al. Tromboembolismo de Pulmón en la República Argentina. Registro CONAREC XX: Protocolo. Revista Conarec 2018;33:244–245. [Google Scholar]
- 14. Aujesky D, Roy P, Petit C.. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J 2006;27:476–481. [DOI] [PubMed] [Google Scholar]
- 15. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS: Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 16. Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU.. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 2010;11:81–96. [DOI] [PubMed] [Google Scholar]
- 17. Bikdeli B, Lobo J.L, Jiménez D, Green P, Fernández‐Capitán C, Bura‐Riviere A, Otero R, DiTullio M.R, Galindo S, Ellis M, Parikh S.A, Monreal M; RIETE Investigators. Early use of echocardiography in patients with acute pulmonary embolism: findings from the RIETE Registry. J Am Heart Assoc 2018;7:e009042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee K, Kwon O, Lee EJ,. et al. Prognostic value of echocardiographic parameters for right ventricular function in patients with acute non-massive pulmonary embolism. Heart Vessels 2019;34:1187. [DOI] [PubMed] [Google Scholar]
- 19. Pruszczyk P, Goliszek S, Lichodziejewska B, et al. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc Imaging 2014;7:553–560. [DOI] [PubMed] [Google Scholar]
- 20. Barco S, Mahmoudpour S, Planquette B, Sánchez O, Konstantinides S.V, Meyer G.. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2019;40:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan CM, Woods C, Shorr AF.. The validation and reproducibility of the pulmonary embolism severity index. J Thromb Haemost 2010;8:1509–1514. [DOI] [PubMed] [Google Scholar]
- 22. Donzé J, Le Gal G, Fine MJ, Roy PM, Sanchez O, Verschuren F, et al. Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism. Thromb Haemost 2008;100:943–948. [DOI] [PubMed] [Google Scholar]
- 23. Kucher N, Rossi E, De Rosa M, Goldhaber SZ.. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med 2005;165:1777–1781. [DOI] [PubMed] [Google Scholar]
- 24. Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G.. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 2000;101:2817–2822. [DOI] [PubMed] [Google Scholar]
- 25. Rydman R, Larsen F, Caidahl K, Alam M.. Right ventricular function in patients with pulmonary embolism: early and late findings using Doppler tissue imaging J Am Soc Echocardiogr 2010;23:531–537. [DOI] [PubMed] [Google Scholar]
- 26. Kaul CS., Tei C, Hopkins JM, Shah PM.. Assessment of right ventricular function using two-dimensional echocardiography Am Heart J 1984;107:526–531. [DOI] [PubMed] [Google Scholar]
- 27. Lobo CJL, Holley A, Tapson V, Moores L, Oribe M, Barron M, Otero R, Nauffal D, Valle R, Monreal M, Yusen RD, Jiménez D; PROTECT and RIETE investigators Prognostic significance of tricuspid annular displacement in normotensive patients with acute symptomatic pulmonary embolism J Thromb Haemost 2014;12:1020–1027. [DOI] [PubMed] [Google Scholar]
- 28. Korkmaz A, Ozlu T, Ozsu S, et al. Long-term outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors Clin Appl Thromb Hemost 2012;18:281–288. [DOI] [PubMed] [Google Scholar]
- 29. Lafitte S, Pillois X, Reant P, et al. Estimation of pulmonary pressures and diagnosis of pulmonary hypertension by Doppler echocardiography: a retrospective comparison of routine echocardiography and invasive hemodynamics. J Am Soc Echocardiogr 2013;26:457–463. [DOI] [PubMed] [Google Scholar]
- 30. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis.Am J Physiol Heart Circ Physiol 2013;305:H1373–H1381. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez O, Trinquart L, Planquette B, et al. Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur Respir J 2013;42:681–688. [DOI] [PubMed] [Google Scholar]
- 32. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. doi:10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D.. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail 2020;26:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bing R, Chow V, Lau JK, Thomas L, Kritharides L, Ng AC.. Prevalence of echocardiography use in patients hospitalized with confirmed acute pulmonary embolism: a real‐world observational multicenter study. PLoS One 2016;11:e0168554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brittain EL, Nwabuo C, Xu M, Gupta DK, Hemnes AR, Moreira HT. et al. Echocardiographic pulmonary artery systolic pressure in the coronary artery risk development in young adults (CARDIA) study: associations with race and metabolic dysregulation. J Am Heart Assoc 2017;6:e005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lam CSP, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM.. Age‐associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009;119:2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM.. Pulmonary hypertension in heart failure with preserved ejection fraction: a community‐based study. J Am Coll Cardiol 2009;53:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armstrong DWJ, Tsimiklis G, Matangi MF.. Factors influencing the echocardiographic estimate of right ventricular systolic pressure in normal patients and clinically relevant ranges according to age. Can J Cardiol. 2010;26:e35–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.