Abstract
Background
Studies of patients admitted to hospital with COVID-19 have found varying mortality outcomes associated with underlying respiratory conditions and inhaled corticosteroid use. Using data from a national, multicentre, prospective cohort, we aimed to characterise people with COVID-19 admitted to hospital with underlying respiratory disease, assess the level of care received, measure in-hospital mortality, and examine the effect of inhaled corticosteroid use.
Methods
We analysed data from the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) study. All patients admitted to hospital with COVID-19 across England, Scotland, and Wales between Jan 17 and Aug 3, 2020, were eligible for inclusion in this analysis. Patients with asthma, chronic pulmonary disease, or both, were identified and stratified by age (<16 years, 16–49 years, and ≥50 years). In-hospital mortality was measured by use of multilevel Cox proportional hazards, adjusting for demographics, comorbidities, and medications (inhaled corticosteroids, short-acting β-agonists [SABAs], and long-acting β-agonists [LABAs]). Patients with asthma who were taking an inhaled corticosteroid plus LABA plus another maintenance asthma medication were considered to have severe asthma.
Findings
75 463 patients from 258 participating health-care facilities were included in this analysis: 860 patients younger than 16 years (74 [8·6%] with asthma), 8950 patients aged 16–49 years (1867 [20·9%] with asthma), and 65 653 patients aged 50 years and older (5918 [9·0%] with asthma, 10 266 [15·6%] with chronic pulmonary disease, and 2071 [3·2%] with both asthma and chronic pulmonary disease). Patients with asthma were significantly more likely than those without asthma to receive critical care (patients aged 16–49 years: adjusted odds ratio [OR] 1·20 [95% CI 1·05–1·37]; p=0·0080; patients aged ≥50 years: adjusted OR 1·17 [1·08–1·27]; p<0·0001), and patients aged 50 years and older with chronic pulmonary disease (with or without asthma) were significantly less likely than those without a respiratory condition to receive critical care (adjusted OR 0·66 [0·60–0·72] for those without asthma and 0·74 [0·62–0·87] for those with asthma; p<0·0001 for both). In patients aged 16–49 years, only those with severe asthma had a significant increase in mortality compared to those with no asthma (adjusted hazard ratio [HR] 1·17 [95% CI 0·73–1·86] for those on no asthma therapy, 0·99 [0·61–1·58] for those on SABAs only, 0·94 [0·62–1·43] for those on inhaled corticosteroids only, 1·02 [0·67–1·54] for those on inhaled corticosteroids plus LABAs, and 1·96 [1·25–3·08] for those with severe asthma). Among patients aged 50 years and older, those with chronic pulmonary disease had a significantly increased mortality risk, regardless of inhaled corticosteroid use, compared to patients without an underlying respiratory condition (adjusted HR 1·16 [95% CI 1·12–1·22] for those not on inhaled corticosteroids, and 1·10 [1·04–1·16] for those on inhaled corticosteroids; p<0·0001). Patients aged 50 years and older with severe asthma also had an increased mortality risk compared to those not on asthma therapy (adjusted HR 1·24 [95% CI 1·04–1·49]). In patients aged 50 years and older, inhaled corticosteroid use within 2 weeks of hospital admission was associated with decreased mortality in those with asthma, compared to those without an underlying respiratory condition (adjusted HR 0·86 [95% CI 0·80−0·92]).
Interpretation
Underlying respiratory conditions are common in patients admitted to hospital with COVID-19. Regardless of the severity of symptoms at admission and comorbidities, patients with asthma were more likely, and those with chronic pulmonary disease less likely, to receive critical care than patients without an underlying respiratory condition. In patients aged 16 years and older, severe asthma was associated with increased mortality compared to non-severe asthma. In patients aged 50 years and older, inhaled corticosteroid use in those with asthma was associated with lower mortality than in patients without an underlying respiratory condition; patients with chronic pulmonary disease had significantly increased mortality compared to those with no underlying respiratory condition, regardless of inhaled corticosteroid use. Our results suggest that the use of inhaled corticosteroids, within 2 weeks of admission, improves survival for patients aged 50 years and older with asthma, but not for those with chronic pulmonary disease.
Funding
National Institute for Health Research, Medical Research Council, NIHR Health Protection Research Units in Emerging and Zoonotic Infections at the University of Liverpool and in Respiratory Infections at Imperial College London in partnership with Public Health England.
Introduction
COVID-19, caused by SARS-CoV-2, was declared a pandemic by WHO on March 11, 2020. By the end of September, 2020, there were more than 1 million deaths worldwide, a major concern for further waves of infections, and a belief that the pandemic will not end for another 2 years.
People with chronic obstructive pulmonary disease (COPD) and asthma were initially considered to be at substantial risk of developing severe COVID-19.1, 2 These putative risk factors were based on the knowledge that respiratory viruses are a major cause of disease exacerbations and because both diseases are associated with deficient innate immune responses to respiratory viral infections, which are likely to be important in defence against a novel virus.1, 3 The increasing amount of observational data accumulated over the past year, as the pandemic has progressed, indicates that COPD is a definitive risk factor for severe COVID-19 outcomes.4, 5 For people with asthma, however, the predisposition to morbidity and mortality from COVID-19 is less straightforward. Early large case series of patients admitted to hospital with COVID-19 found the proportion of patients with asthma to be lower than that of the local population,6, 7, 8 unlike during the 2009 influenza pandemic.9 These findings suggested that asthma was associated with reduced susceptibility to severe COVID-19, with behavioural, pharmacological, and immunopathological explanatory mechanisms proposed.10, 11 More recent epidemiological studies have further complicated our understanding of the impact of COVID-19 on people with asthma, as some studies suggest an association with severe COVID-19,5, 12, 13 while others do not.4, 14, 15, 16, 17
Research in context.
Evidence before this study
Early case series at the beginning of the COVID-19 pandemic suggested that the prevalence of patients with chronic pulmonary disease admitted to hospital with COVID-19 was lower than the prevalence of chronic pulmonary disease in the local population. Subsequently, several studies have specifically investigated the risk of adverse COVID-19 outcomes, including admission to hospital and death, in patients with asthma and chronic obstructive pulmonary disease (COPD), with results suggesting COPD is a significant risk factor, whereas susceptibility is less clear cut for asthma. However, few observational studies have examined the effect of inhaled corticosteroids on mortality. One large study, based on the OpenSafely consortium, which included primary care records linked to COVID-19 mortality data, found that high-dose inhaled corticosteroid use in patients with asthma and any inhaled corticosteroid use in patients with COPD was associated with an increased risk of mortality from COVID-19. The authors of the study suggested that the association could be explained, fully or in part, by unmeasured confounding due to disease severity.
Added value of this study
To the best of our knowledge, our study uses the largest cohort of patients admitted to hospital with COVID-19 worldwide: the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) study. We inform on two major questions for people with underlying respiratory conditions. First, we described patients admitted to hospital with COVID-19, including the level of care they received and their mortality risk, comparing patients across age groups and common respiratory conditions. We found that patients with asthma were significantly more likely than those with no underlying respiratory condition to receive critical care and ventilatory support even after adjusting for severity on admission, age, and comorbidities; by contrast, patients with chronic pulmonary disease were significantly less likely than those with no underlying respiratory condition to receive critical care. Patients with chronic pulmonary disease and those with severe asthma had an increased risk of mortality. Second, we measured the association of mortality with inhaled corticosteroid use. In contrast to the OpenSafely study, we showed that use of inhaled corticosteroids within 2 weeks of admission was associated with decreased mortality in patients with asthma but had no effect on mortality in patients with chronic pulmonary disease.
Implications of all the available evidence
In the UK, there appears to be a disparity between the level of in-hospital care for COVID-19 received by patients with different respiratory conditions, which is not accounted for by clinical severity, age, or comorbidities. Both our secondary care study and the primary care OpenSafely study found that patients with severe asthma were at risk of increased mortality. Our study suggests that the use of inhaled corticosteroids within 2 weeks of admission might be beneficial in patients with asthma, perhaps via a putative anti-inflammatory effect. The evidence from these two large observational cohorts reports contrasting effects of inhaled corticosteroids, although the timing of inhaled corticosteroid use might have differed. Moreover, both studies are at risk of misclassification and confounding; the results of ongoing prospective randomised clinical trials evaluating the efficacy of inhaled corticosteroids in this setting will therefore be informative.
One mechanism postulated to correlate with protection is inhaled corticosteroid use.10, 18 Experimental clinical and preclinical studies have shown that inhaled corticosteroid use attenuates the expression of key genes that are thought to facilitate SARS-CoV-2 infection, in both asthma and COPD.19, 20, 21 Although analysis of data from two asthma cohorts in the USA found no association between inhaled corticosteroid use and admission to hospital for COVID-19, data from a large UK cohort found an increase in COVID-19-related mortality associated with high-dose inhaled corticosteroid use in people with asthma, and with any inhaled corticosteroid use in people with COPD.15, 22, 23 Understanding the impact of inhaled corticosteroid use on COVID-19 outcomes is important for patients with underlying respiratory conditions. Notably, at the beginning of the pandemic, in the UK the demand for inhaled corticosteroid prescriptions escalated to such an extent that it caused distressing shortages.24
Using data from the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) study, we aimed to characterise people admitted to hospital with COVID-19 who had underlying respiratory disease, assess the level of care received, measure in-hospital mortality, and examine the effect of inhaled corticosteroid use.
Methods
Data sources
The ISARIC WHO CCP-UK protocol was developed in 2009, with regular review and updates in 2012, 2013, and 2016 in response to potential threats posed by emerging infections. The ISARIC study was activated in response to the SARS-CoV-2 outbreak on Jan 17, 2020. ISARIC WHO CCP-UK is an actively recruiting, prospective cohort study of patients admitted to hospital with strongly suspected or confirmed COVID-19 across England, Scotland, and Wales. The protocol, revision history, case report forms, study information, and consent forms are available online. Data and analysis scripts are available on request.
Study population
From Jan 17 to Aug 3, 2020, all patients admitted to hospital with a confirmed or highly suspected SARS-CoV-2 infection leading to COVID-19 were eligible for inclusion in this study. Confirmation of SARS-CoV-2 was done with RT-PCR, the only testing modality available in the UK during the study period. Highly suspected but unproven cases of COVID-19 were eligible for inclusion, given that SARS-CoV-2 was an emergent pathogen at the time of protocol activation and confirmatory RT-PCR was contingent on local availability of tests. Patients who were admitted to hospital after Aug 3, 2020, were excluded to avoid bias from those who had not had adequate time to accrue an outcome. Follow-up of patients ended on Aug 17, 2020.
Ethical approval was given by the South Central-Oxford C Research Ethics Committee in England (reference 13/SC/0149), and by the Scotland A Research Ethics Committee (reference 20/SS/0028). The study is registered with the ISRCTN registry, ISRCTN66726260. All patients who provided biological samples gave informed consent. For patients who did not provide samples, some gave informed consent but for some the requirement for consent was waived.
Variables
Data were collected by clinical research staff by use of a standardised case report form and entered into a secure Research Electronic Data Capture (REDCap) secure online database. Data were collected across multiple timepoints, including at admission, during hospital stay (days 1, 3, 6, and 9) and at discharge. Characteristics including age, sex, comorbidities (asthma, chronic pulmonary disease, chronic cardiac disease, chronic haematological disease [excluding malignancy], chronic kidney disease, chronic neurological disease, HIV/AIDs, malignancy, liver disease, obesity, or rheumatological disorder) and smoking history were also recorded. Physiological variables at admission were recorded, including components of the National Early Warning Score 2 (NEWS2), which were categorised as low (<5), moderate (5–6), and severe (≥7) clinical risk. We also collected information about medications, entered as free text, and subsequently mapped this information to drug preparations using data provided by the UK National Health Service (NHS) Technology Reference data Update Distribution Service. Postal codes of patients' home addresses were used to record the Index of Multiple Deprivation (IMD), an area-based measure of relative deprivation (with the least deprived areas represented by the first quintile and the most deprived represented by the fifth). Where this information was missing, a hospital-weighted average IMD score was used.
Patients with respiratory disease, recorded in the case report form as “asthma” or “chronic pulmonary disease (no asthma)”, were stratified into three age groups: younger than 16 years, 16–49 years, and 50 years and older. Asthma spans all ages but the potential for misclassification due to other similar conditions increases from around 50 years of age. Stratification by age reduces the risk of this bias and helps control for age, one of the main confounders for assessing COVID-19 outcomes. Because of the small numbers of children (ie, patients <16 years) admitted to hospital with COVID-19, only a descriptive analysis was done for this age group. Patients younger than 50 years with asthma were identified with the case report form, or by identification of patients without chronic pulmonary disease who were taking inhaled asthma medication (inhaled corticosteroids, short-acting β-agonists [SABAs], long-acting β-agonists [LABAs], or theophylline or leukotriene receptor antagonists [LTRAs]) within 2 weeks of admission. Patients prescribed an inhaled corticosteroid plus LABA plus another maintenance asthma medication (a LAMA, LTRA, or theophylline) were considered to have severe asthma (equivalent to the highest steps in the asthma management stepwise approach). Patients with chronic pulmonary disease were those for whom “chronic pulmonary disease (no asthma)” was entered on the case report form. In patients older than 50 years, those for whom “asthma” or “chronic pulmonary disease (no asthma)” was not entered on their forms were classified as having no respiratory condition (appendix p 10).
Further breakdown of the respiratory diagnoses within the chronic pulmonary disease category was not recorded but was mostly likely to be predominantly COPD.
Outcomes
The outcomes of interest were hospital survival from the onset of symptoms (measured as time in days to death) and during hospital admission: any admission to critical care (level 3: intensive care unit; or level 2: high dependency unit), and whether the patient received invasive mechanical ventilation, non-invasive ventilation, or oxygen.
Statistical analysis
Categorical data were summarised as frequencies and percentages, and tested with the χ2 statistic, except for when cell counts were five or fewer, in which case Fisher's exact test was used (all p values are based on non-missing values). For continuous data, normally distributed variables were summarised as mean (SD) and non-normally distributed variables summarised as median (IQR). Continuous variables were analysed, where appropriate, with a two-sample Welch's t-test for normally distributed data, or with a Mann-Whitney U test or a Kruskall-Wallis test for data that were not normally distributed.
Time to in-hospital death (survival) was modelled with Cox proportional hazards regression and logistic regression was used for modelling of binary outcomes. For survival models, the reported date of symptom onset was taken as day zero and discharge from hospital was considered an absorbing state (once discharged, patients were considered to no longer be at risk of death). Discharge did not compete with death, as discharged patients were not censored and included in the risk set until the end of the follow-up (Aug 17, 2020). After building the survival models, we confirmed the assumptions of proportional hazards were met by plotting the Schoenfeld residuals and inspecting for symmetry over time. An iterative modelling approach was adopted, where clinically plausible variables that could explain differences in mortality were entered into preliminary multivariable models. We selected the final models through minimisation of the Akaike Information Criterion (AIC). Variables that did not reduce AIC were dropped to minimise overfitting. Where data on predictor (explanatory) variables were missing, we used multiple imputation by chained equations to generate five imputed datasets with five iterations of imputation per dataset. Models were then fitted on each imputed dataset and the final models pooled with Rubin's rules.
First order interactions were checked, and any significant interactions retained. Covariates included were age, sex, ethnicity, deprivation, obesity, smoking, chronic cardiac disease, chronic kidney disease, and malignancy.
Both survival models and logistic regression models took variation across health-care facilities into account by use of mixed-effects models. Patient-level risk factors were modelled as fixed effects and the health-care facility as a random effect. We did three post-hoc sensitivity analyses: first, excluding patients with obesity; second, excluding patients without a positive RT-PCR test result; and third, in patients aged 50 years and older, we included all medication strata (not only inhaled corticosteroids) but excluded patients with missing medication data for asthma or chronic pulmonary disease. Effect estimates are presented as hazard ratios (HRs) for time to death and odds ratios (ORs) for binary outcome data, alongside their corresponding 95% CIs. Statistical significance was taken at the level of p less than 0·05.
Data were analysed with R, version 3.6.3, using the tidyverse, finalfit, and mice packages.
Role of the funding source
The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
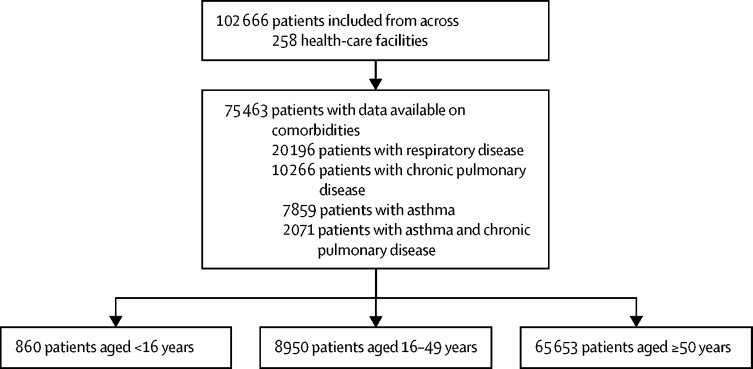
By Aug 17, 2020, 78 674 patients were enrolled into the ISARIC CCP-UK cohort across 258 health-care facilities; comorbidity data were available for 75 463 patients (figure 1 ). Chronic respiratory disease (including asthma or chronic pulmonary disease, or both) was the most prevalent comorbidity, in 20 196 (26·8%) of 75 463 patients. 73 500 (97·4%) patients had RT-PCR-positive SARS-CoV-2 infection.
Figure 1.
Flow diagram of patients by inclusion and exclusion criteria
There were 860 children younger than 16 years, 786 (91·4%) without asthma and 74 (8·6%) with asthma. Patients younger than 16 years with asthma were older and more likely to be of white ethnicity than those without asthma, but had similar clinical characteristics to those without asthma (appendix p 10). 26 (41·9%) of the 74 patients with asthma were using inhaled corticosteroids. Five patients were using monoclonal antibodies for severe asthma.
There were 8950 patients aged 16–49 years: 7083 (79·1%) without asthma and 1867 (20·9%) with asthma. The mean age was similar in both groups (38·7 [SD 8·6] years for patients with asthma and 38·9 [8·6] years for those without), as was the proportion of smokers, but patients with asthma were more likely to be female, of white ethnicity, and obese (table 1 ; appendix p 12). The prevalence of other comorbidities in patients aged 16–49 years was low, with small differences between patients with and without asthma. More than half of patients aged 16–49 years with asthma were using inhaled corticosteroids (alone, in combination with a LABA, or in combination with a LABA plus another asthma medication; table 1). Five patients were using monoclonal antibodies for asthma.
Table 1.
Characteristics of patients aged 16–49 years
| No asthma (n=7083) | Asthma (n=1867) | ||
|---|---|---|---|
| Demographic characteristics | |||
| Mean age, years | 38·9 (8·6) | 38·7 (8·6) | |
| Age group, years | |||
| 16–24 | 618 (8·7%) | 152 (8·1%) | |
| 25–39 | 2700 (38·1%) | 734 (39·3%) | |
| 40–49 | 3765 (53·2%) | 981 (52·5%) | |
| Sex at birth | |||
| Female | 3056 (43·1%) | 1031 (55·2%) | |
| Male | 4015 (56·7%) | 830 (44·5%) | |
| Data missing | 12 (0·2%) | 6 (0·3%) | |
| Ethnicity | |||
| Asian | 986 (13·9%) | 221 (11·8%) | |
| Black | 555 (7·8%) | 64 (3·4%) | |
| White | 3606 (50·9%) | 1182 (63·3%) | |
| Other | 968 (13·7%) | 176 (9·4%) | |
| Data missing | 968 (13·7%) | 224 (12·0%) | |
| Deprivation (IMD quintile) | |||
| 1 | 909 (12·8%) | 258 (13·8%) | |
| 2 | 1098 (15·5%) | 301 (16·1%) | |
| 3 | 1213 (17·1%) | 306 (16·4%) | |
| 4 | 1591 (22·5%) | 397 (21·3%) | |
| 5 | 2264 (32·0%) | 605 (32·4%) | |
| Data missing | 8 (0·1%) | 0 | |
| Smoking | |||
| Never smoked | 3606 (50·9%) | 908 (48·6%) | |
| Former smoker | 753 (10·6%) | 243 (13·0%) | |
| Current smoker | 607 (8·6%) | 187 (10·0%) | |
| Data missing | 2117 (29·9%) | 529 (28·3%) | |
| Clinical characteristics | |||
| Obesity (as defined by clinical staff) | |||
| No | 5581 (78·8%) | 1228 (65·8%) | |
| Yes | 1017 (14·4%) | 461 (24·7%) | |
| Data missing | 485 (6·8%) | 178 (9·5%) | |
| Chronic pulmonary disease (not asthma) | |||
| No | 6869 (97·0%) | 1736 (93·0%) | |
| Yes | 214 (3·0%) | 76 (4·1%) | |
| Data missing | 0 | 55 (2·9%) | |
| Chronic cardiac disease | |||
| No | 6746 (95·2%) | 1720 (92·1%) | |
| Yes | 306 (4·3%) | 89 (4·8%) | |
| Data missing | 31 (0·4%) | 58 (3·1%) | |
| Malignancy | |||
| No | 6840 (96·6%) | 1763 (94·4%) | |
| Yes | 190 (2·7%) | 34 (1·8%) | |
| Data missing | 53 (0·7%) | 70 (3·7%) | |
| Chronic kidney disease | |||
| No | 6753 (95·3%) | 1731 (92·7%) | |
| Yes | 295 (4·2%) | 69 (3·7%) | |
| Data missing | 35 (0·5%) | 67 (3·6%) | |
| Diabetes without complications | |||
| No | 6261 (88·4%) | 1559 (83·5%) | |
| Yes | 608 (8·6%) | 205 (11·0%) | |
| Data missing | 214 (3·0%) | 103 (5·5%) | |
| Diabetes with complications | |||
| No | 6612 (93·4%) | 1692 (90·6%) | |
| Yes | 267 (3·8%) | 73 (3·9%) | |
| Data missing | 204 (2·9%) | 102 (5·5%) | |
| Mild liver disease | |||
| No | 6913 (97·6%) | 1766 (94·6%) | |
| Yes | 109 (1·5%) | 26 (1·4%) | |
| Data missing | 61 (0·9%) | 75 (4·0%) | |
| Moderate or severe liver disease | |||
| No | 6890 (97·3%) | 1763 (94·4%) | |
| Yes | 139 (2·0%) | 32 (1·7%) | |
| Data missing | 54 (0·8%) | 72 (3·9%) | |
| Chronic neurological disorder | |||
| No | 6576 (92·8%) | 1671 (89·5%) | |
| Yes | 460 (6·5%) | 130 (7·0%) | |
| Data missing | 47 (0·7%) | 66 (3·5%) | |
| Chronic haematological disease | |||
| No | 6846 (96·7%) | 1749 (93·7%) | |
| Yes | 191 (2·7%) | 49 (2·6%) | |
| Data missing | 46 (0·6%) | 69 (3·7%) | |
| Rheumatological disease | |||
| No | 6828 (96·4%) | 1698 (90·9%) | |
| Yes | 195 (2·8%) | 99 (5·3%) | |
| Data missing | 60 (0·8%) | 70 (3·7%) | |
| Medication characteristics | |||
| Medication | |||
| Reported | 7083 (100·0%) | 1721 (92·2%) | |
| Data missing | 0 | 146 (7·8%) | |
| Type of inhaler therapy | |||
| No therapy | 6997 (98·8%) | 327 (17·5%) | |
| SABA | 24 (0·3%) | 346 (18·5%) | |
| ICS only | 31 (0·4%) | 423 (22·7%) | |
| LABA plus ICS | 22 (0·3%) | 424 (22·7%) | |
| ICS plus LABA plus another asthma medication* | 9 (0·1%) | 201 (10·8%) | |
| Data missing | 0 | 146 (7·8%) | |
| ICS components | |||
| No steroid | 7022 (99·1%) | 706 (37·8%) | |
| Beclometasone | 39 (0·6%) | 636 (34·1%) | |
| Budesonide | 6 (0·1%) | 151 (8·1%) | |
| Ciclesonide | 0 (0·0%) | 4 (0·2%) | |
| Fluticasone | 16 (0·2%) | 224 (12·0%) | |
| Data missing | 0 | 146 (7·8%) | |
| Oral corticosteroids | |||
| No oral steroids | 6844 (96·6%) | 1671 (89·5%) | |
| Oral steroids | 139 (2·0%) | 174 (9·3%) | |
| Data missing | 100 (1·4%) | 22 (1·2%) | |
Data are n (%) or mean (SD), unless otherwise indicated. IMD=Index of Multiple Deprivation. SABA=short-acting β-agonist. ICS=inhaled corticosteroid. LABA=long-acting β-agonist.
Other asthma medication: long-acting muscarinic antagonist, leukotriene receptor antagonist, or theophylline.
There were 65 653 patients aged 50 years and older: 47 398 (72·2%) without a respiratory condition, 5918 (9·0%) with asthma alone, 10 266 (15·6%) with chronic pulmonary disease (no asthma), and 2071 (3·2%) with a co-diagnosis of asthma and chronic pulmonary disease. Patients with asthma were slightly younger than patients with chronic pulmonary disease alone, and more likely to be female and obese, but less likely to be of white ethnicity or a current smoker (table 2 ; appendix p 14). Patients with asthma were less likely than those with chronic pulmonary disease to have chronic cardiac disease, malignant neoplasm, or chronic kidney disease, but the prevalence of other comorbidities was similar across groups. Inhaled corticosteroid use was higher among patients with asthma (3481 [60·6%] of 5745) and among patients with asthma and chronic pulmonary disease (1373 [67·5%] of 2033) than among patients with chronic pulmonary disease alone (4009 [40·0%] of 10 033).
Table 2.
Characteristics of patients aged 50 years and older
| No respiratory condition (n=47 398) | Asthma (n=5918) | Chronic pulmonary disease, no asthma (n=10 266) | Asthma and chronic pulmonary disease (n=2071) | ||
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Mean age, years | 75·7 (12·5) | 72·1 (12·8) | 77·8 (9·6) | 76·1 (10·6) | |
| Age group, years | |||||
| 50–69 | 15 523 (32·8%) | 2625 (44·4%) | 2008 (19·6%) | 560 (27·0%) | |
| 70–80 | 11 323 (23·9%) | 1373 (23·2%) | 3638 (35·4%) | 690 (33·3%) | |
| >80 | 20 552 (43·4%) | 1920 (32·4%) | 4620 (45·0%) | 821 (39·6%) | |
| Sex at birth | |||||
| Female | 19 977 (42·1%) | 3289 (55·6%) | 4195 (40·9%) | 1090 (52·6%) | |
| Male | 27 291 (57·6%) | 2618 (44·2%) | 6053 (59·0%) | 974 (47·0%) | |
| Data missing | 130 (0·3%) | 11 (0·2%) | 18 (0·2%) | 7 (0·3%) | |
| Ethnicity | |||||
| Asian | 2255 (4·8%) | 377 (6·4%) | 184 (1·8%) | 67 (3·2%) | |
| Black | 1565 (3·3%) | 187 (3·2%) | 105 (1·0%) | 30 (1·4%) | |
| White | 35 481 (74·9%) | 4431 (74·9%) | 8738 (85·1%) | 1710 (82·6%) | |
| Other | 2620 (5·5%) | 340 (5·7%) | 349 (3·4%) | 83 (4·0%) | |
| Data missing | 5477 (11·6%) | 583 (9·9%) | 890 (8·7%) | 181 (8·7%) | |
| Deprivation (IMD quintile) | |||||
| 1 | 8586 (18·1%) | 1026 (17·3%) | 1503 (14·6%) | 258 (12·5%) | |
| 2 | 9138 (19·3%) | 1096 (18·5%) | 1843 (18·0%) | 366 (17·7%) | |
| 3 | 8906 (18·8%) | 1080 (18·2%) | 1766 (17·2%) | 350 (16·9%) | |
| 4 | 9413 (19·9%) | 1196 (20·2%) | 2150 (20·9%) | 431 (20·8%) | |
| 5 | 11 336 (23·9%) | 1519 (25·7%) | 3001 (29·2%) | 666 (32·2%) | |
| Data missing | 19 (<0·1%) | 1 (<0·1%) | 3 (<0·1%) | 0 | |
| Smoking | |||||
| Never smoked | 15 661 (33·0%) | 2352 (39·7%) | 1661 (16·2%) | 420 (20·3%) | |
| Former smoker | 9114 (19·2%) | 1226 (20·7%) | 4088 (39·8%) | 800 (38·6%) | |
| Current smoker | 1707 (3·6%) | 197 (3·3%) | 1116 (10·9%) | 192 (9·3%) | |
| Data missing | 20 916 (44·1%) | 2143 (36·2%) | 3401 (33·1%) | 659 (31·8%) | |
| Clinical characteristics | |||||
| Obesity | |||||
| No | 35 123 (74·1%) | 4136 (69·9%) | 7584 (73·9%) | 1425 (68·8%) | |
| Yes | 3872 (8·2%) | 876 (14·8%) | 955 (9·3%) | 304 (14·7%) | |
| Data missing | 8403 (17·7%) | 906 (15·3%) | 1727 (16·8%) | 342 (16·5%) | |
| Chronic cardiac disease | |||||
| No | 28 755 (60·7%) | 3836 (64·8%) | 4964 (48·4%) | 1039 (50·2%) | |
| Yes | 15 232 (32·1%) | 1816 (30·7%) | 4876 (47·5%) | 945 (45·6%) | |
| Data missing | 3411 (7·2%) | 266 (4·5%) | 426 (4·1%) | 87 (4·2%) | |
| Malignant neoplasm | |||||
| No | 38 274 (80·8%) | 5131 (86·7%) | 8302 (80·9%) | 1717 (82·9%) | |
| Yes | 5036 (10·6%) | 441 (7·5%) | 1336 (13·0%) | 229 (11·1%) | |
| Data missing | 4088 (8·6%) | 346 (5·8%) | 628 (6·1%) | 125 (6·0%) | |
| Chronic kidney disease | |||||
| No | 35 149 (74·2%) | 4689 (79·2%) | 7464 (72·7%) | 1493 (72·1%) | |
| Yes | 8530 (18·0%) | 911 (15·4%) | 2264 (22·1%) | 468 (22·6%) | |
| Data missing | 3719 (7·8%) | 318 (5·4%) | 538 (5·2%) | 110 (5·3%) | |
| Diabetes with complications | |||||
| No | 34 606 (73·0%) | 4348 (73·5%) | 7801 (76·0%) | 1501 (72·5%) | |
| Yes | 7436 (15·7%) | 1056 (17·8%) | 1568 (15·3%) | 388 (18·7%) | |
| Data missing | 5356 (11·3%) | 514 (8·7%) | 897 (8·7%) | 182 (8·8%) | |
| Diabetes without complications | |||||
| No | 38 457 (81·1%) | 4988 (84·3%) | 8568 (83·5%) | 1703 (82·2%) | |
| Yes | 3489 (7·4%) | 394 (6·7%) | 785 (7·6%) | 188 (9·1%) | |
| Data missing | 5452 (11·5%) | 536 (9·1%) | 913 (8·9%) | 180 (8·7%) | |
| Mild liver disease | |||||
| No | 42 430 (89·5%) | 5471 (92·4%) | 9400 (91·6%) | 1892 (91·4%) | |
| Yes | 638 (1·3%) | 90 (1·5%) | 184 (1·8%) | 40 (1·9%) | |
| Data missing | 4330 (9·1%) | 357 (6·0%) | 682 (6·6%) | 139 (6·7%) | |
| Moderate or severe liver disease | |||||
| No | 42 365 (89·4%) | 5476 (92·5%) | 9373 (91·3%) | 1886 (91·1%) | |
| Yes | 814 (1·7%) | 93 (1·6%) | 237 (2·3%) | 55 (2·7%) | |
| Data missing | 4219 (8·9%) | 349 (5·9%) | 656 (6·4%) | 130 (6·3%) | |
| Chronic neurological disorder | |||||
| No | 37 113 (78·3%) | 4969 (84·0%) | 8376 (81·6%) | 1703 (82·2%) | |
| Yes | 6323 (13·3%) | 621 (10·5%) | 1297 (12·6%) | 234 (11·3%) | |
| Data missing | 3962 (8·4%) | 328 (5·5%) | 593 (5·8%) | 134 (6·5%) | |
| Chronic haematological disease | |||||
| No | 41 246 (87·0%) | 5344 (90·3%) | 9159 (89·2%) | 1832 (88·5%) | |
| Yes | 1991 (4·2%) | 222 (3·8%) | 482 (4·7%) | 118 (5·7%) | |
| Data missing | 4161 (8·8%) | 352 (5·9%) | 625 (6·1%) | 121 (5·8%) | |
| Rheumatological disorder | |||||
| No | 37 847 (79·8%) | 4790 (80·9%) | 8266 (80·5%) | 1598 (77·2%) | |
| Yes | 5217 (11·0%) | 778 (13·1%) | 1377 (13·4%) | 353 (17·0%) | |
| Data missing | 4334 (9·1%) | 350 (5·9%) | 623 (6·1%) | 120 (5·8%) | |
| Medication characteristics | |||||
| Medication | |||||
| Reported | 47 398 (100·0%) | 5745 (97·1%) | 10 033 (97·7%) | 2033 (98·2%) | |
| Data missing | 0 | 173 (2·9%) | 233 (2·3%) | 38 (1·8%) | |
| Salbutamol | 1805 (3·8%) | 3077 (52·0%) | 4543 (44·3%) | 1223 (59·1%) | |
| ICS components | |||||
| No steroid | 46 182 (97·4%) | 2264 (38·3%) | 6024 (58·7%) | 661 (31·9%) | |
| Beclometasone | 712 (1·5%) | 2000 (33·8%) | 2195 (21·4%) | 738 (35·6%) | |
| Budesonide | 153 (0·3%) | 578 (9·8%) | 421 (4·1%) | 192 (9·3%) | |
| Ciclesonide | 26 (0·1%) | 12 (0·2%) | 19 (0·2%) | 12 (0·6%) | |
| Fluticasone | 325 (0·7%) | 891 (15·1%) | 1374 (13·4%) | 431 (20·8%) | |
| Data missing | 0 | 173 (2·9%) | 233 (2·3%) | 37 (1·8%) | |
| LABA | 355 (0·7%) | 1583 (26·7%) | 1704 (16·6%) | 676 (32·6%) | |
| LAMA | 312 (0·7%) | 404 (6·8%) | 1954 (19·0%) | 450 (21·7%) | |
| LTRAs | 45 (0·1%) | 524 (8·9%) | 165 (1·6%) | 227 (11·0%) | |
| Theophylline | 14 (0·0%) | 88 (1·5%) | 310 (3·0%) | 103 (5·0%) | |
| Oral corticosteroids | |||||
| No | 44 440 (93·8%) | 5434 (91·8%) | 9132 (89·0%) | 1794 (86·6%) | |
| Yes | 1371 (2·9%) | 436 (7·4%) | 1051 (10·2%) | 261 (12·6%) | |
| Data missing | 1587 (3·3%) | 48 (0·8%) | 83 (0·8%) | 16 (0·8%) | |
Data are n (%) or mean (SD), unless otherwise indicated. IMD=Index of Multiple Deprivation. ICS=inhaled corticosteroid. LABA=long-acting β-agonist. LAMA=long-acting muscarinic antagonist. LTRA=leukotriene receptor antagonist.
Among all age groups, patients with a respiratory condition were significantly more likely to have dyspnoea, wheeze, and cough than those without asthma or with no respiratory condition but, in those aged 16–49 years, only around one in seven presented with wheeze (table 3 ; appendix p 15). In children (patients <16 years) with asthma, 30 (40·5%) of 74 presented with wheeze (appendix p 15). In patients aged 50 years and older, more than two-thirds of patients with a respiratory condition presented with dyspnoea, compared to slightly more than half of patients without a respiratory condition (table 3).
Table 3.
Symptoms and severity on admission, and maximal level of care received during admission, by age and respiratory condition
|
Patients aged 16–49 years |
Patients aged ≥50 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No asthma (n=7083) | Asthma (n=1867) | p value | No respiratory condition (n=47 398) | Asthma (n=5918) | Chronic pulmonary disease (n=10 266) | Chronic pulmonary disease and asthma (n=2071) | p value | ||
| Symptoms on admission | |||||||||
| Fever | 5039 (71·1%) | 1325 (71·0%) | 0·93 | 25 864 (54·6%) | 3548 (60·0%) | 5150 (50·2%) | 1096 (52·9%) | <0·0001 | |
| Dyspnoea | 4501 (63·5%) | 1388 (74·3%) | <0·0001 | 26 479 (55·9%) | 4058 (68·6%) | 7108 (69·2%) | 1479 (71·4%) | <0·0001 | |
| Wheeze | 233 (3·3%) | 271 (14·5%) | <0·0001 | 1988 (4·2%) | 729 (12·3%) | 1305 (12·7%) | 374 (18·1%) | <0·0001 | |
| Cough | 4847 (68·4%) | 1427 (76·4%) | <0·0001 | 26 054 (55·0%) | 3965 (67·0%) | 6399 (62·3%) | 1387 (67·0%) | <0·0001 | |
| Severity on admission | |||||||||
| Days of symptoms before admission | |||||||||
| Median (IQR) | 6 (2–9) | 6 (2–9) | 0·22 | 2 (0–7) | 4 (0–8) | 2 (0–7) | 2 (0–7) | <0·0001 | |
| NEWS2 | |||||||||
| Median (IQR) | 4 (2–6) | 4 (2–6) | 0·00020 | 4 (1–6) | 4 (2–6) | 5 (3–6) | 4 (2–7) | <0·0001 | |
| 0–4 | 3495 (49·3%) | 846 (45·3%) | 0·00020 | 23 992 (50·6%) | 3026 (51·1%) | 4251 (41·4%) | 918 (44·3%) | <0·0001 | |
| 5–6 | 1219 (17·2%) | 410 (22·0%) | .. | 7270 (15·3%) | 1015 (17·2%) | 1989 (19·4%) | 421 (20·3%) | .. | |
| ≥7 | 1198 (16·9%) | 320 (17·1%) | .. | 8398 (17·7%) | 1043 (17·6%) | 2714 (26·4%) | 452 (21·8%) | .. | |
| Data missing | 1171 (16·5%) | 291 (15·6%) | .. | 7738 (16·3%) | 834 (14·1%) | 1312 (12·8%) | 280 (13·5%) | .. | |
| Outcomes | |||||||||
| Survival | |||||||||
| Alive | 6594 (93·1%) | 1714 (91·8%) | 0·063 | 30 600 (64·6%) | 4188 (70·8%) | 5873 (57·2%) | 1288 (62·2%) | <0·0001 | |
| Died | 382 (5·4%) | 122 (6·5%) | .. | 16 106 (34·0%) | 1670 (28·2%) | 4269 (41·6%) | 753 (36·4%) | .. | |
| Data missing | 107 (1·5%) | 31 (1·7%) | .. | 692 (1·5%) | 60 (1·0%) | 124 (1·2%) | 30 (1·4%) | .. | |
| Critical care | 1542 (22·0%) | 451 (24·5%) | 0·025 | 6135 (13·3%) | 1023 (17·5%) | 735 (7·2%) | 184 (9·0%) | <0·0001 | |
| Invasive mechanical ventilation | 953 (13·7%) | 269 (14·8%) | 0·24 | 3889 (8·6%) | 611 (10·6%) | 305 (3·0%) | 82 (4·1%) | <0·0001 | |
| Non-invasive ventilation | 1159 (16·7%) | 385 (21·2%) | <0·0001 | 6565 (14·5%) | 1086 (18·8%) | 1427 (14·3%) | 309 (15·4%) | <0·0001 | |
| Oxygen therapy | 3878 (55·6%) | 1099 (60·5%) | 0·00020 | 29 954 (65·9%) | 3991 (68·7%) | 7291 (72·5%) | 1392 (68·8%) | <0·0001 | |
| Median length of stay (IQR) | 3 (0–27) | 3 (0–29) | 0·50 | 6 (1–33) | 4 (1–31) | 6 (1–32) | 5 (1–30) | 0·0043 | |
| Median length of stay, survivors (IQR) | 3 (0–27) | 3 (0–29) | 0·42 | 5 (1–33) | 4 (1–32) | 5 (1–31) | 5 (1–29) | 0·039 | |
Data are n (%) or median (IQR). NEWS2=National Early Warning Score 2.
Patients aged 16–49 years with asthma, and patients aged 50 years and older with chronic pulmonary disease (with or without asthma), had a higher percentage of patients who presented with a NEWS2 score greater than 4, compared to those with no respiratory condition (table 3).
No children (aged <16 years) with asthma died, but ten were transferred to critical care (appendix p 15). Patients with asthma aged 16–49 years and those aged 50 years and older were significantly more likely to receive critical care, non-invasive ventilation, and oxygen than patients without asthma after adjusting for age, sex, ethnicity, obesity, smoking, and comorbidities (table 4 ). Patients aged 50 years and older with chronic pulmonary disease (with or without asthma) were significantly less likely to receive critical care or invasive mechanical ventilation than patients without a respiratory condition, but were more likely to receive non-invasive ventilation and oxygen supplementation (table 4). After adjusting for NEWS2 scores, there was minimal change in effect estimates, except for a reduction in the odds of non-invasive ventilation in patients with chronic pulmonary disease with no asthma (adjusted OR 1·04 [95% CI 0·97–1·12]) and in those with chronic pulmonary disease and asthma (adjusted OR 1·09 [0·95–1·25]).
Table 4.
Association between maximum level of care received during admission and respiratory condition
|
Critical care |
Invasive mechanical ventilation |
Non-invasive ventilation |
Oxygen |
|||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR* (95% CI) | p value | Adjusted OR* (95% CI) | p value | Adjusted OR* (95% CI) | p value | Adjusted OR* (95% CI) | p value | |
| Patients aged 16–49 years | ||||||||
| No asthma | Ref | .. | Ref | .. | Ref | .. | Ref | .. |
| Asthma | 1·20 (1·05–1·37) | 0·0080 | 1·17 (1·00–1·38) | 0·053 | 1·36 (1·18–1·57) | <0·0001 | 1·33 (1·17–1·50) | <0·0001 |
| Patients aged ≥50 years | ||||||||
| No respiratory condition | Ref | .. | Ref | .. | Ref | .. | Ref | .. |
| Asthma | 1·17 (1·08–1·27) | <0·0001 | 1·07 (0·97–1·18) | 0·207 | 1·18 (1·09–1·28) | <0·0001 | 1·08 (1·02–1·15) | 0·012 |
| Chronic pulmonary disease (no asthma) | 0·66 (0·60–0·72) | <0·0001 | 0·49 (0·43–0·57) | <0·0001 | 1·14 (1·06–1·22) | 0·0012 | 1·35 (1·28–1·42) | <0·0001 |
| Chronic pulmonary disease and asthma | 0·74 (0·62–0·87) | <0·0001 | 0·56 (0·44–0·72) | <0·0001 | 1·15 (1·00–1·31) | 0·043 | 1·14 (1·03–1·26) | 0·014 |
OR=odds ratio.
Adjusted for age, sex, ethnicity, smoking, obesity, malignancy, chronic cardiac disease, and centre. In patients aged 50 years and older, the model was also adjusted for chronic kidney disease.
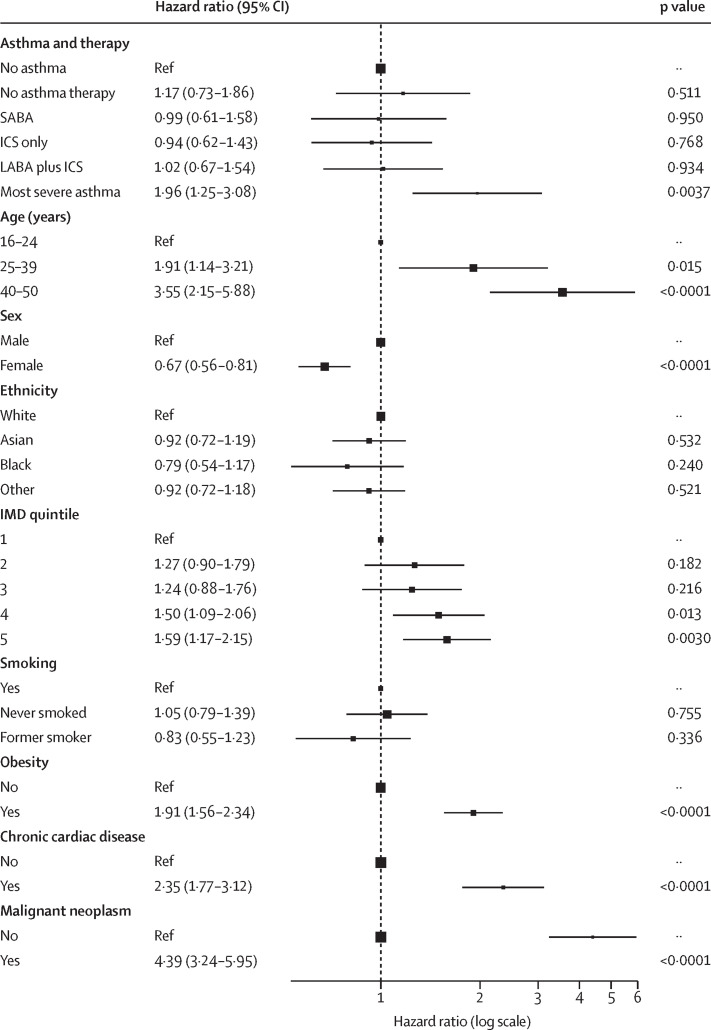
In patients aged 16–49 years, 122 (6·5%) of 1867 patients with asthma and 382 (5·4%) of 7083 patients without asthma died (table 3). After adjusting for multiple risk factors, only patients with severe asthma had increased rates of in-hospital mortality (adjusted HR 1·17 [95% CI 0·73–1·86] for patients on no asthma therapy, 0·99 [0·61–1·58] for those on SABAs only, 0·94 [0·62–1·43] for those on inhaled corticosteroids only, 1·02 [0·67–1·54] for those on inhaled corticosteroids plus LABAs, and 1·96 [1·25–3·08] for those with severe asthma—ie, on inhaled corticosteroids in combination with a LABA plus another asthma medication; figure 2 ; appendix p 16).
Figure 2.
Association between asthma and death from COVID-19 in patients aged 16–49 years
Lines are 95% CIs. SABA=short-acting β-agonist. ICS=inhaled corticosteroid. LABA=long-acting β-agonist. IMD=Index of Multiple Deprivation.
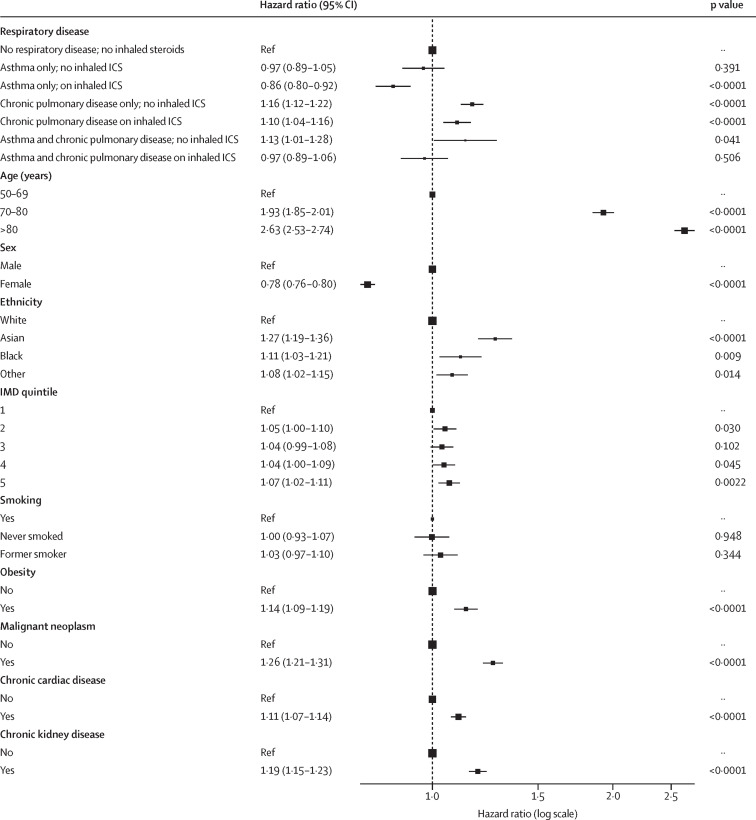
In patients aged 50 years and older, 4269 (41·6%) of 10 266 patients with chronic pulmonary disease died during their hospital stay, compared to 1670 (28·2%) of 5918 with asthma and 16 106 (34·0%) of 47 398 with no underlying respiratory condition (table 3). Patients aged 50 years and older with asthma who were using inhaled corticosteroids had a 14% reduction in mortality risk compared to those with no underlying respiratory condition (adjusted HR 0·86 [95% CI 0·80–0·92]; p<0·0001), but those who were not using inhaled corticosteroids had no significant reduction in mortality risk (adjusted HR 0·97 [95% CI 0·89–1·05]; p=0·391; figure 3 ; appendix p 17). When stratifying patients with asthma alone, patients aged 50 years and older with severe asthma had an increased mortality risk compared to those on no asthma therapy (adjusted HR 1·24 [95% CI 1·04–1·49]; p=0·020; appendix pp 3, 17). Patients aged 50 years and over with chronic pulmonary disease alone (no asthma) had a significant increase in mortality risk, regardless of inhaled corticosteroid use, compared to patients without an underlying respiratory condition (adjusted HR 1·16 [95% CI 1·12–1·22], p<0·0001, for those not on inhaled corticosteroids; and adjusted HR 1·10 [95% CI 1·04–1·16], p<0·0001, for those on inhaled corticosteroids; figure 3; appendix p 17). A significant increase in mortality risk was observed in patients aged 50 years and older with chronic pulmonary disease and asthma who were not using inhaled corticosteroids, compared to patients without an underlying respiratory condition (adjusted HR 1·13 [95% CI 1·01–1·28]; p=0·041; figure 3).
Figure 3.
Association of ICS use with respiratory condition and in-hospital mortality in patients aged 50 years and older
Lines are 95% CIs. ICS=inhaled corticosteroid. IMD=Index of Multiple Deprivation.
When stratifying by NEWS2 score, in all groups and respiratory conditions there was a lower mortality rate in patients who were on inhaled corticosteroids than in patients not on inhaled corticosteroids, except in patients with chronic pulmonary disease and no asthma who had a NEWS2 score of 5–6 (appendix p 19).
In adults, after excluding patients with obesity, mortality associations did not differ significantly from those found in the main analyses (appendix pp 4–5). Only including patients with positive RT-PCR test results did not affect associations found in the main analyses either (appendix pp 6–7). Bronchodilators, or the number of long-acting inhalers used, were not associated with mortality risk in patients with chronic pulmonary disease (appendix p 8). Modelling age as a continuous transformed variable did not affect associations compared to including age as a categorical variable (appendix pp 20, 21).
After adjusting for mortality risk factors, beclometasone was associated with a 7% reduction in the risk of in-hospital mortality compared to no inhaled corticosteroid use (adjusted HR 0·93 [95% CI 0·89–0·98]); fluticasone and ciclesonide did not have a significant association with mortality (appendix pp 9, 22).
Discussion
ISARIC CCP-UK is, to our knowledge, the largest cohort of hospital admissions for COVID-19 worldwide. In contrast to some other cohorts, we found that the proportion of people with asthma admitted to hospital with COVID-19 (8·6% aged <16 years, 20·9% aged 16–49 years, and 12·2% aged ≥50 years) was considerably higher than the national prevalence of asthma, which is around 7% for each age group.25 Patients with underlying respiratory conditions were more likely to present with respiratory symptoms, dyspnoea, and cough, but wheeze was uncommon except in children with asthma (patients <16 years).
Patients with chronic pulmonary disease had a high mortality rate, of around 40%, but were significantly less likely than patients with no underlying respiratory condition to receive critical care or invasive ventilatory support, even after considering age and comorbidities. By contrast, adult patients with asthma were significantly more likely to receive critical care and non-invasive ventilatory support. This observation did not seem to be associated with clinical severity on admission, as measured by NEWS2 scores, suggesting that other factors were contributing to the decision to provide critical care. For example, it is likely these markers did not fully account for frailty, when treatment escalation discussions were held, and a low threshold for admitting patients with asthma to critical care for observation, given their potential to deteriorate quickly. All patients with underlying respiratory conditions were more likely to receive non-invasive ventilation and oxygen supplementation than patients without an underlying respiratory condition, even after accounting for age, comorbidities, and severity of symptoms at admission.
Patients with severe asthma (denoted by their maintenance medication use before admission to hospital) had worse survival than patients without an underlying respiratory condition. In all other patients with asthma, there was no significant difference in mortality except in those using inhaled corticosteroids, aged 50 years or older, who had decreased mortality. These differential effects with age and mortality might be related to immune senescence, which can occur from 55 years of age.26 One aspect of immune senescence includes an increase in inflammation that might inhibit immunity to viruses or worsen hyperinflammation at the time of infection; inhaled corticosteroid use has been postulated to reverse this effect.27, 28 Another possibility is that inhaled corticosteroid use exhibits its apparent age preferential effect through inhibition of angiotensin-converting enzyme 2 (ACE2), since expression of ACE2 increases with age.29
Our findings in patients with asthma correspond to work from the OpenSafely primary care consortium.23 Based on more than 17 million UK medical records linked to COVID-19 deaths, the OpenSafely consortium found that only patients with severe asthma (defined by the prescription of oral corticosteroids in the past year) were predisposed to increased mortality.23 Another large study, based on UK Biobank data from more than 65 000 people with asthma (enrolled at ages 40–59 years), found non-allergic asthma, or asthma with a co-diagnosis of COPD, to be associated with increased risk of admission to hospital for COVID-19, while allergic asthma showed a non-significant association with reduced admission to hospital.13 These studies included large numbers of patients but because they were observational they are all susceptible to inherent misclassification of asthma, due to self-reporting or diagnosis by a non-specialist, as well as limited information about underlying asthma severity and phenotypes. Small case series, based on well-defined patients with asthma (diagnosed by specialists with detailed medical records) found asthma to be associated with an increased risk of severe COVID-19.30, 31 We found asthma to be associated with an increased level of in-hospital care, even after adjusting for clinical severity on admission, but not with an increased risk of death (except for patients with severe asthma), similar to what was observed in the 2009 influenza pandemic.9 In our study, we attempted to further refine asthma classification using an age cutoff and, in the older cohort, by separating patients documented as having asthma alone from patients with asthma and another chronic pulmonary disease.
The first published cohorts of patients admitted to hospital with COVID-19 in China, the USA, and Europe found variable prevalence of COPD, sometimes lower than the expected population prevalence, leading to an initial debate about possible protective mechanisms for this observation.10 Subsequently, the OpenSafely collaborative found COPD to be a significant risk factor, seemingly in agreement with the findings from ISARIC-CCP-UK, which analysed patients with chronic pulmonary disease, rather than COPD.4, 5 A possible mechanism for the increased susceptibility to COVID-19 might be related to the elevated expression of ACE2 in the airway epithelial cells in patients with COPD and smokers.32, 33
Studies showing decreased ACE2 in sputum with inhaled corticosteroid use in asthma, and downregulation of ACE2 induced by inhaled corticosteroids at the gene and protein levels in COPD, have raised the question of whether inhaled corticosteroid use exerts a protective effect.19, 20, 21 The OpenSafely collaborative analysed an asthma cohort and a separate COPD cohort, and reported that inhaled corticosteroid use was not protective for survival from COVID-19.23 Indeed, they reported worse outcomes associated with high-dose inhaled corticosteroid use in asthma, and all inhaled corticosteroid use in COPD. However, further analyses revealed considerable residual confounding, thought to be due to the severity of the underlying respiratory disease, which could not be fully determined in their cohorts.23 The findings of the OpenSafely collaborative with regard to the association between asthma and COVID-19 outcomes are potentially in keeping with the present study; although we could not analyse our results according to inhaled corticosteroid dose, we found that survival was reduced in patients with more severe disease (who are more likely to be using high-dose inhaled corticosteroids). Others have postulated that the timing of inhaled corticosteroid use might be critical in terms of the effect of inhaled corticosteroids on SARS-CoV-2 infection versus the effect on the host response to infection.18 In this regard, our study participants reported using their medication within 2 weeks of admission (ie, at the time of severe COVID-19). Pointedly, participants in the OpenSafely study could be prescribed inhaled corticosteroid any time within 4 months before entering the study cohort. Our study also comprised a different population—ISARIC CCP-UK only includes patients admitted to hospital with COVID-19—whereas the OpenSafely study included the general population, those with and those without SARS-CoV-2 infection. Two studies in the USA based on electronic medical records only from patients with asthma (n=1526 and n=1827) addressed the risk of admission to hospital for COVID-19;14, 22 Wang and colleagues also analysed critical care admission and deaths in patients admitted to hospital for COVID-19 in China.34 Both US studies found that inhaled corticosteroid use was not associated with the risk of admission to hospital, but Wang and colleagues found a non-significant association between inhaled corticosteroid use and reduced risk of critical care admission and improved survival. A study in South Korea comprising 218 patients with asthma who were admitted to hospital with COVID-19 found no significant association between mortality and any asthma medications in patients admitted to hospital with COVID-19.35
To date, there are no published observational data analysing the impact of different inhaled corticosteroids on SARS-CoV-2 infection. In-vitro data indicate that ciclesonide can suppress SARS-CoV-2 replication.36 However, due to the small number of patients prescribed ciclesonide inhalers in the UK, this analysis did not have sufficient power to assess its association with mortality.
This study has various limitations. This dataset is not currently linked to primary care or secondary care records, and where data were obtained from self-reporting (eg, medication and diagnoses of conditions, including asthma), they could not be confirmed. This approach could have resulted in misclassification of the prescriptions and diagnoses. We also did not have detailed information about the severity of patients' underlying respiratory conditions, except for information about medications they were on within 2 weeks of, and including at the time of, admission. Information about the dose of inhaled corticosteroids was often missing, such that this variable was not used. We assumed patients were using their prescribed medications on admission to hospital, but this might not have been the case for some patients. For this reason, we were unable to classify the severity of asthma, except to assume that patients on three maintenance asthma medications in the 2 weeks before admission had more severe asthma than other patients. We did not know the reasons why any oral corticosteroids might have been prescribed before hospital admission. Conditions not included on the study case report forms could not be evaluated separately, including atopy, and COPD and bronchiectasis, which were probably included in the “chronic pulmonary disease (no asthma)” category. Physiological measurements for clinical severity were limited to those included in the NEWS2 score and were only available on admission. Finally, we were interested in the outcome of in-hospital mortality (incorporating palliative discharge); as a result, some patients who were discharged and died in the community could have been missed from the analysis. We used discharge as an absorbing state; considering all these patients as being alive, discharge is associated with an outcome (ie, is informative) and therefore should not be used as a censoring event in this analysis. This is a reasonable assumption given our methods of data collection, but it is a limitation.
In conclusion, chronic respiratory disease was the most prevalent comorbidity in the ISARIC CCP-UK cohort. However, there was a disparity between different respiratory conditions and the level of in-hospital care received. Patients with chronic pulmonary disease had a high level of mortality, with a prevalence of 40% for in-hospital death. Of patients with asthma, only those with severe asthma had increased mortality compared to those without an underlying respiratory condition. Patients with asthma (aged ≥50 years) had a lower mortality risk if they had used inhaled corticosteroids within 2 weeks of admission. These results confirm that many patients with existing respiratory conditions are at high risk and should continue to take precautions against exposure to SARS-CoV-2. The role of inhaled corticosteroids in COVID-19 remains unclear, but inhaled corticosteroids protect against exacerbations of respiratory disease and might therefore protect against severe COVID-19.
Data sharing
ISARIC4C welcomes applications for data and material access through our Independent Data and Material Access Committee. Data collected for the study, including individual anonymised participant data and a data dictionary defining each field in the set will be available, including the ISARIC WHO CCP-UK study protocol and consent forms. Data will be shared after approval of the proposal and with a signed data access agreement.
Acknowledgments
Acknowledgments
This work uses data provided by patients and collected by the UK National Health Service (NHS) as part of their care and supports #DataSavesLives. We are grateful to the 2648 front-line NHS clinical and research staff and volunteer medical students who collected these data in challenging circumstances; and grateful for the generosity of the participants and their families for their individual contributions in these difficult times. We also acknowledge the support of Jeremy J Farrar and Nahoko Shindo. This work is supported by grants from the National Institute for Health Research (NIHR award CO-CIN-01), the Medical Research Council (grant MC_PC_19059) and the NIHR Health Protection Research Units in Emerging and Zoonotic Infections at the University of Liverpool and in Respiratory Infections at Imperial College London in partnership with Public Health England. We acknowledge support from the Liverpool School of Tropical Medicine and the University of Oxford (NIHR award 200907), the NIHR Biomedical Research Centre at Imperial College London, the Wellcome Trust, and Department for International Development (215091/Z/18/Z), and the Bill and Melinda Gates Foundation (OPP1209135). We thank the Liverpool Experimental Cancer Medicine Centre for providing infrastructure support (grant reference: C18616/A25153). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Contributors
PJMO, PC, CIB, BJL, SLJ, JKB, JD, GC, JSN-V-T, and MGS were responsible for conceptualisation of the study. TMD and CIB were responsible for curation of the data. TMD, CIB, PC, EMH, ABD, BJL, and SLJ were responsible for the formal analysis and methodology. PC, PJMO, ABD, CIB, EMH, and MGS were responsible for study supervision. CIB and TMD wrote the first draft of the manuscript. PC, ABD, BJL, SLJ, EMH, MGS, JSN-V-T, and PJMO were responsible for writing, reviewing, and editing the manuscript. JKB, GC, PJMO, and MGS were responsible for acquisition of funding. TMD, CIB, ABD, and EMH had access to the raw data, and TMD and CIB have verified the data used in the study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
BJL has received funding from AstraZeneca, Chiesi, and Teva for research, consulting, advisory boards, and giving talks, from Novartis Glenmark, Cipla, and Vectura for consulting, and from Sanofi for research and consulting. SLJ reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis, outside of the submitted work. SLJ is the Asthma UK clinical chair (grant CH11SJ) and an NIHR Emeritus senior investigator and is funded in part by European Research Council Advanced Grant 788575. JKB reports grants from the UK Medical Research Council. MGS reports grants from the Department of Health and Social Care, the National Institute for Health Research, the UK Medical Research Council, and the Health Protection Research Unit in Emerging and Zoonotic Infections at the University of Liverpool, UK, during the conduct of the study, and from Integrum Scientific (Greensboro, NC, USA) outside of the submitted work. PJMO reports personal fees from Janssen and Pfizer, and collaborative grant funding from GSK within a UK Medical Research Council consortium and is an NIHR Senior Investigator. ABD reports grants from the Department of Health and Social Care during the conduct of the study; and grants from Wellcome Trust outside of the submitted work. JSN-V-T reports grants from the Department of Health and Social Care during the conduct of the study and is seconded to the Department of Health and Social Care. CIB, TMD, PC, JD, EMH, and GC report no competing interests.
Contributor Information
ISARIC investigators:
Beatrice Alex, Benjamin Bach, Wendy S Barclay, Debby Bogaert, Meera Chand, Graham S Cooke, Ana da Filipe, Tom Fletcher, Christoper A Green, Ewen M Harrison, Julian A Hiscox, Antonia Ying Ho, Peter W Horby, Samreen Ijaz, Saye Khoo, Paul Klenerman, Andrew Law, Wei Shen Lim, Alexander J Mentzer, Laura Merson, Alison M Meynert, Mahdad Noursadeghi, Shona C Moore, Massimo Palmarini, William A Paxton, Georgios Pollakis, Nicholas Price, Andrew Rambaut, David L Robertson, Clark D Russell, Vanessa Sancho-Shimizu, Janet T Scott, Thushan de Silva, Louise Sigfrid, Tom Solomon, Shiranee Sriskandan, David Stuart, Charlotte Summers, Richard S Tedder, Emma C Thomson, AA Roger Thompson, Ryan S Thwaites, Lance CW Turtle, Maria Zambon, Hayley Hardwick, Chloe Donohue, Ruth Lyons, Fiona Griffiths, Wilna Oosthuyzen, Lisa Norman, Riinu Pius, Cameron J Fairfield, Stephen R Knight, Kenneth A Mclean, Derek Murphy, Catherine A Shaw, Jo Dalton, Michelle Girvan, Egle Saviciute, Stephanie Roberts, Janet Harrison, Laura Marsh, Marie Connor, Sophie Halpin, Clare Jackson, Carrol Gamble, Gary Leeming, Andrew Law, Murray Wham, Sara Clohisey, Ross Hendry, James Scott-Brown, William Greenhalf, Victoria Shaw, Sara McDonald, Seán Keating, Katie A. Ahmed, Jane A Armstrong, Milton Ashworth, Innocent G Asiimwe, Siddharth Bakshi, Samantha L Barlow, Laura Booth, Benjamin Brennan, Katie Bullock, Benjamin WA Catterall, Jordan J Clark, Emily A Clarke, Sarah Cole, Louise Cooper, Helen Cox, Christopher Davis, Oslem Dincarslan, Chris Dunn, Philip Dyer, Angela Elliott, Anthony Evans, Lorna Finch, Lewis WS Fisher, Terry Foster, Isabel Garcia-Dorival, William Greenhalf, Philip Gunning, Catherine Hartley, Rebecca L Jensen, Christopher B Jones, Trevor R Jones, Shadia Khandaker, Katharine King, Robyn T. Kiy, Chrysa Koukorava, Annette Lake, Suzannah Lant, Diane Latawiec, Lara Lavelle-Langham, Daniella Lefteri, Lauren Lett, Lucia A Livoti, Maria Mancini, Sarah McDonald, Laurence McEvoy, John McLauchlan, Soeren Metelmann, Nahida S Miah, Joanna Middleton, Joyce Mitchell, Shona C Moore, Ellen G Murphy, Rebekah Penrice-Randal, Jack Pilgrim, Tessa Prince, Will Reynolds, P. Matthew Ridley, Debby Sales, Victoria E Shaw, Rebecca K Shears, Benjamin Small, Krishanthi S Subramaniam, Agnieska Szemiel, Aislynn Taggart, Jolanta Tanianis-Hughes, Jordan Thomas, Erwan Trochu, Libby van Tonder, Eve Wilcock, J. Eunice Zhang, Lisa Flaherty, Nicole Maziere, Emily Cass, Alejandra Doce Carracedo, Nicola Carlucci, Anthony Holmes, Hannah Massey, Kayode Adeniji, Daniel Agranoff, Ken Agwuh, Dhiraj Ail, Ana Alegria, Brian Angus, Abdul Ashish, Dougal Atkinson, Shahedal Bari, Gavin Barlow, Stella Barnass, Nicholas Barrett, Christopher Bassford, David Baxter, Michael Beadsworth, Jolanta Bernatoniene, John Berridge, Nicola Best, Pieter Bothma, David Brealey, Robin Brittain-Long, Naomi Bulteel, Tom Burden, Andrew Burtenshaw, Vikki Caruth, David Chadwick, Duncan Chambler, Nigel Chee, Jenny Child, Srikanth Chukkambotla, Tom Clark, Paul Collini, Catherine Cosgrove, Jason Cupitt, Maria-Teresa Cutino-Moguel, Paul Dark, Chris Dawson, Samir Dervisevic, Phil Donnison, Sam Douthwaite, Ingrid DuRand, Ahilanadan Dushianthan, Tristan Dyer, Cariad Evans, Chi Eziefula, Chrisopher Fegan, Adam Finn, Duncan Fullerton, Sanjeev Garg, Sanjeev Garg, Atul Garg, Effrossyni Gkrania-Klotsas, Jo Godden, Arthur Goldsmith, Clive Graham, Elaine Hardy, Stuart Hartshorn, Daniel Harvey, Peter Havalda, Daniel B Hawcutt, Maria Hobrok, Luke Hodgson, Anil Hormis, Michael Jacobs, Susan Jain, Paul Jennings, Agilan Kaliappan, Vidya Kasipandian, Stephen Kegg, Michael Kelsey, Jason Kendall, Caroline Kerrison, Ian Kerslake, Oliver Koch, Gouri Koduri, George Koshy, Shondipon Laha, Steven Laird, Susan Larkin, Tamas Leiner, Patrick Lillie, James Limb, Vanessa Linnett, Jeff Little, Michael MacMahon, Emily MacNaughton, Ravish Mankregod, Huw Masson, Elijah Matovu, Katherine McCullough, Ruth McEwen, Manjula Meda, Gary Mills, Jane Minton, Mariyam Mirfenderesky, Kavya Mohandas, Quen Mok, James Moon, Elinoor Moore, Patrick Morgan, Craig Morris, Katherine Mortimore, Samuel Moses, Mbiye Mpenge, Rohinton Mulla, Michael Murphy, Megan Nagel, Thapas Nagarajan, Mark Nelson, Igor Otahal, Mark Pais, Selva Panchatsharam, Hassan Paraiso, Brij Patel, Natalie Pattison, Justin Pepperell, Mark Peters, Mandeep Phull, Stefania Pintus, Jagtur Pooni, Frank Post, David Price, Rachel Prout, Nikolas Rae, Henrik Reschreiter, Tim Reynolds, Neil Richardson, Mark Roberts, Devender Roberts, Alistair Rose, Guy Rousseau, Brendan Ryan, Taranprit Saluja, Aarti Shah, Prad Shanmuga, Anil Sharma, Anna Shawcross, Jeremy Sizer, Manu Shankar-Hari, Richard Smith, Catherine Snelson, Nick Spittle, Nikki Staines, Tom Stambach, Richard Stewart, Pradeep Subudhi, Tamas Szakmany, Kate Tatham, Jo Thomas, Chris Thompson, Robert Thompson, Ascanio Tridente, Darell Tupper-Carey, Mary Twagira, Andrew Ustianowski, Nick Vallotton, Lisa Vincent-Smith, Shico Visuvanathan, Alan Vuylsteke, Sam Waddy, Rachel Wake, Andrew Walden, Ingeborg Welters, Tony Whitehouse, Paul Whittaker, Ashley Whittington, Meme Wijesinghe, Martin Williams, Lawrence Wilson, Sarah Wilson, Stephen Winchester, Martin Wiselka, Adam Wolverson, Daniel G Wooton, Andrew Workman, Bryan Yates, and Peter Young
Supplementary Material
References
- 1.Johnston SL. Asthma and COVID-19: is asthma a risk factor for severe outcomes? Allergy. 2020;75:1543–1545. doi: 10.1111/all.14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NHS Who's at higher risk from coronavirus. 2020. https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/whos-at-higher-risk-from-coronavirus/
- 3.Wark PAB, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha A, Dunning J, Tunstall T, et al. Patterns of systemic and local inflammation in patients with asthma hospitalised with influenza. Eur Respir J. 2019;54 doi: 10.1183/13993003.00949-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8:436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panettieri RA, Carson J, Horton D, Barrett E, Roy J, Radbel J. Asthma and COVID: what are the important questions? J Allergy Clin Immunol Pract. 2020;8:2487–2488. doi: 10.1016/j.jaip.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JM, Koh HY, Moon SY, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146:790–798. doi: 10.1016/j.jaci.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Jr, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146:327. doi: 10.1016/j.jaci.2020.06.001. 29.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307. doi: 10.1016/j.jaci.2020.06.010. 14.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovinsky-Desir S, Deshpande DR, De A, et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146:1027. doi: 10.1016/j.jaci.2020.07.026. 34.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55 doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finney LJ, Glanville N, Farne H, et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. bioRxiv. 2020 doi: 10.1101/2020.06.13.149039. published online June 15. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milne S, Li X, Yang CX, et al. Inhaled corticosteroids downregulate SARS-CoV-2-related gene expression in COPD: results from a RCT. medRxiv. 2020 doi: 10.1101/2020.08.19.20178368. published online Aug 22. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters MC, Sajuthi S, Deford P, et al. COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Foer D, Bates DW, Boyce JA, Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020;146:808–812. doi: 10.1016/j.jaci.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickware C, Robinson J. Asthma inhaler stocks low after COVID-19 linked demand spike, government says. Pharm J. 2020 https://www.pharmaceutical-journal.com/news-and-analysis/news/asthma-inhaler-stocks-low-after-covid-19-linked-demand-spike-government-says/20207873.article?firstPass=false published online April 2. [Google Scholar]
- 25.Bloom CI, Saglani S, Feary J, Jarvis D, Quint JK. Changing prevalence of current asthma and inhaled corticosteroid treatment in the UK: population-based cohort 2006–2016. Eur Respir J. 2019;53 doi: 10.1183/13993003.02130-2018. [DOI] [PubMed] [Google Scholar]
- 26.Qin L, Jing X, Qiu Z, et al. Aging of immune system: immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging. 2016;8:848–859. doi: 10.18632/aging.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.British Society for Immunology The ageing immune system and COVID-19. Nov 10, 2020. https://www.immunology.org/sites/default/files/BSI_Ageing_COVID-19_Report_Nov2020_FINAL.pdf
- 28.Nicolau DV, Bafadhel M. Inhaled corticosteroids in virus pandemics: a treatment for COVID-19? Lancet Respir Med. 2020;8:846–847. doi: 10.1016/S2213-2600(20)30314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler MW, O'Reilly A, Dunican EM, et al. Prevalence of comorbid asthma in COVID-19 patients. J Allergy Clin Immunol. 2020;146:334–335. doi: 10.1016/j.jaci.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beurnier A, Jutant E-M, Jevnikar M, et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56 doi: 10.1183/13993003.01875-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs M, Van Eeckhoutte HP, Wijnant SRA, et al. Increased expression of ACE2, the SARS-CoV-2 entry receptor, in alveolar and bronchial epithelium of smokers and COPD subjects. Eur Respir J. 2020;56 doi: 10.1183/13993003.02378-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YJ, Park J-Y, Lee HS, et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. 2020 doi: 10.1183/13993003.02226-2020. published online Sept 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuyama S, Kawase M, Nao N, et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95:e01648–e01720. doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ISARIC4C welcomes applications for data and material access through our Independent Data and Material Access Committee. Data collected for the study, including individual anonymised participant data and a data dictionary defining each field in the set will be available, including the ISARIC WHO CCP-UK study protocol and consent forms. Data will be shared after approval of the proposal and with a signed data access agreement.