Abstract
Sandhoff disease (SD) is caused by decreased function of the enzyme β-N-acetylhexosaminidase, resulting in accumulation of GM2 ganglioside in tissues. Neural tissue is primarily affected and individuals with the infantile form of the disease generally do not survive beyond 4 years of age. Current treatments address neurometabolic deficits to improve lifespan, however, this extended lifespan allows clinical disease to become manifest in other tissues, including the musculoskeletal system. The impact of SD on bone and joint tissues has yet to be fully determined. In a feline model of infantile SD, animals were treated by intracranial injection of adeno-associated virus vectors to supply the central nervous system with corrective levels of hexosaminidase, resulting in a twofold to threefold increase in lifespan. As treated animals aged, signs of musculoskeletal disease were identified. The present study characterized bone and joint lesions from affected cats using micro-computed tomography and histology. All affected cats had similar lesions, whether or not they were treated. SD cats displayed a significant reduction in metaphyseal trabecular bone and markedly abnormal size and shape of epiphyses. Abnormalities increased in severity with age and appear to be due to alteration in the function of chondrocytes within epiphyseal cartilage, particularly the articular-epiphyseal complex. Older cats developed secondary osteoarthritic changes. The changes identified are similar to those seen in humans with mucopolysaccharidoses. Statement of clinical significance: the lesions identified will have significant implications on the quality of life of individuals whose lifespans are extended due to treatments for the primary neurological effects of SD.
Keywords: animal models, bone development, chondrocytes, gangliosidosis, growth plate
1 |. INTRODUCTION
Lysosomal storage disorders (LSDs) encompass a group of rare diseases that are characterized by abnormal accumulation of metabolites in many different cell types as a result of defects in lysosome function. The majority of these disorders occur due to autosomal recessive inheritance and result in deficiencies or defects in enzymes, membrane transporters, or accessory proteins required by the lysosome to recycle certain molecules, resulting in abnormal accumulation of “storage” material and subsequent cellular malfunction. The GM2 gangliosidoses, a subgroup of LSDs that includes Sandhoff disease (SD; OMIM #268800) and Tay-Sachs disease (TSD; OMIM #272800), are caused by decreased function of the enzyme β-N-acetylhexosaminidase (Hex, EC3.2.1.52) resulting in an accumulation of its primary substrate, GM2 ganglioside.1 In humans, GM2 ganglioside is catabolized only by the A isoenzyme of Hex (HexA, a heterodimer of α and β subunits), while other uncharged substrates can be degraded by the B isoenzyme (HexB, a homodimer of β subunits). Disease-causing mutations in Hex α-subunits reduce or eliminate HexA activity and cause TSD, while defective β-subunits affect both HexA and HexB, producing SD.2 Therefore, mutations in either subunit result in HexA deficiency leading to an accumulation of primarily GM2 ganglioside predominantly in the nervous system and accumulation of other substrates in peripheral tissues. Affected individuals present with significant clinical, physical, and cognitive deficiencies that are similar for both TSD and SD. Clinical severity is classified according to age of onset as infantile, juvenile, or adult onset. The life expectancy of infantile-onset patients is only 4 years of age without treatment.3 Although rodent models are invaluable for developing a treatment for the GM2 gangliosidoses, cats provide certain experimental advantages over rodents including a brain size and ganglioside metabolism that are more similar to that of humans.4,5
Treatments to improve the central nervous system (CNS) deficiencies have been explored in affected cats with gangliosidoses.2,6–8 This naturally-occurring model was first reported in 19779 and has been continuously maintained as a research colony.2 The mutation is a 25 base pair inversion at the extreme 3′ terminus of the beta-subunit (HexB) coding sequence, and results in animals with this mutation having less than 3% of normal Hex activity and approximately 15% of normal protein levels.5,10 Compared with other animal models of the disease, the degree of GM2/GA2 accumulation in cats with SD is more comparable to human patients.10 The use of intracranial adeno-associated virus (AAV) vector gene therapy has successfully increased lifespan and improved overall quality of life in affected cats, providing a model for treating this disease in affected children.2,7,8 Animals utilized in the current study included unaffected controls (no deficiency in Hex) as well as affected individuals (known deficiency in Hex). Male and female affected cats received an intracranial injection of AAV vector at approximately 1 month of age, using a treatment approach that has been previously described in detail.2,8,11 This increased the age at which the cats reached humane endpoints for euthanasia due to neurological disease from an average of 4.4 months (range: 3.9–5 months) in untreated cats to an average of 20.9 months of age (range: 9.9–36.2 months) in treated cats.
Although AAV-treated SD cats live longer and have diminished CNS disease, they develop musculoskeletal abnormalities as they age.11 The impact of Hex deficiency on bone and joint tissues has not been thoroughly explored, although radiographic changes secondary to SD have been reported in the axial skeleton and some long bones in cats with infantile SD.11 Osteoarticular changes are part of the disease presentation in several classifications of mucopolysaccharidoses (MPSs) patients.12–14 Therapies to reverse subsequent damage have recently been investigated but are largely unsuccessful.13 However, gene therapies have been shown to ameliorate clinical signs in animal models.15 MPSs are a type of LSD caused by deficiencies in lysosomal enzymes involved in the glycosaminoglycan (GAG) breakdown pathway, resulting in their storage within several cell types.12 It is well known that GAGs are required for a multitude of normal bone and joint functions. Thus, it is not unexpected that this disorder would be associated with bone and joint abnormalities. However, the role that substrates of the Hex enzyme play in normal functional processes within bones and joints is unclear.
The purpose of the current study was to characterize the morphology of the bone and joint lesions that previously have been noted clinically11 in SD cats in which the lifespan was increased with AAV treatment. All AAV-treated SD cats exhibited clinical signs of elbow and hip dysplasia, bilateral weakness in fore- and hindlimbs, and luxations of the hip and patella. These were recognized as early as 4 months of age and increased in severity until the cats were euthanized.11 Based on these previous observations within this feline model, we hypothesized that the mutation resulting in the neurological signs associated with SD would also result in skeletal manifestations. With advancements in treatments aimed to improve CNS deficiencies and to prolong lifespan of affected individuals, which previously has not been possible, it is important to consider the longer-term musculoskeletal sequelae of this disease, which may dramatically impact the quality of life of these individuals and which, thus far, remain resistant to this method of treatment.
2 |. METHOD
2.1 |. Study design and tissue collection
A feline model of SD was used in the current study.9,16 Animals were bred in-house, and the study was approved by the Auburn University Institutional Animal Care and Use Committee. All husbandry and treatment descriptions have been previously described.8 Cats were treated via lateral ventricular and bilateral thalami injections with AAVrh8 vectors expressing feline Hex α and β subunits in a 1:1 ratio as previously published7,8 at approximately 1 month of age, as part of another study examining the effects of treatment on neurological and other outcomes. Therefore, treatment groups were based on endpoints evaluated in those studies. For the current study, bone samples from 26 cats (18 females, 8 males, age range: 3.9–37.2 months), including affected cats (treated with AAV and untreated) and unaffected controls (all untreated) were obtained (Table 1). Due to the known changes that occur in bone with age, the cats evaluated by micro-computed tomography (μCT) were divided into three groups based on age, one of which was skeletally mature (>9 months of age17; outlined in detail in Table 1). The age groups included the following: group 1: 3.9–4.3 months; group 2: 5.2–6.2 months; and group 3: 9.9–37.2 months. All affected cats in group 3 received AAV treatment (to allow them to reach these older ages); all affected cats in groups 1 and 2 were untreated.
TABLE 1.
Groups of samples based on age ranges of cats
| Groupa | Age range | Total N | Affected | Unaffected controls |
|---|---|---|---|---|
| Group 1 | 3.9–4.3 mo | 8 | 1 male, 3 females | 1 male, 3 females |
| Group 2 | 5.2–6.2 mo | 9 | 2 females | 1 male, 6 females |
| Group 3 | 9.9–37.2 mo | 9 | 4 males, 3 females | 1 male, 1 female |
All cats in groups 1 and 2 were untreated. All affected cats in group 3 were treated by gene therapy.
Investigators performing the analyses in the current study were blinded to treatment group. All cats that were euthanized at an age younger than 6.2 months in the current study were untreated, including both unaffected control and affected cats. Long bones (femora, tibiae, humeri, radii, ulnae) were dissected free from soft tissue at necropsy, fixed in 10% neutral-buffered formalin, and stored in 70% ethanol for evaluation with μCT and histology. Samples from all cats were blinded for subsequent analyses.
2.2 |. Micro-computed tomography
Five regions of interest (ROI) were identified for μCT evaluation. These included (a) distal femur, chosen because patellar luxation was one of the signs clinically identified in adult treated cats, the stifle (knee) joint is a common location for skeletal disorders including osteoarthritis, and the distal and midshaft femur are accepted ROIs in long bones for evaluating trabecular and cortical bone, respectively18; (b) the proximal humerus, chosen as a second site for trabecular bone evaluation; (c) the proximal femur, chosen to evaluate the changes within the femoral head that may have contributed to clinical signs of hip dysplasia; (d) the proximal tibiae, chosen because the stifle joint is a predilection site for osteoarthritis; and (e) intact elbow joints, chosen for several reasons. First, the cats displayed clinical signs of elbow dysplasia with age and radiographic findings indicated abnormal joint spaces.11 Second, in dogs, ununited anconeal processes and medial coronoid process fragmentation are well-defined developmental precursors to elbow dysplasia.3,19 Finally, the elbow joint contains numerous physes and apophyses in close proximity to one another, allowing a unique opportunity to evaluate potential delays in endochondral ossification in multiple locations within one μCT scan.
The ROIs in the femur and humerus were determined to be: (a) the distal 30% of the femur extending to the distal physis and proximal 30% of the humerus extending to the proximal physis for trabecular bone and (b) a region at midshaft in both bones for cortical bone. The proximal femora were scanned from the most proximal aspect of the greater trochanter to the distal aspect of the lesser trochanter. The proximal epiphyses of the tibiae were scanned to the distal end of the tibial tuberosity. The elbow joints contained the distal 25% of the humerus and proximal 25% of the radius and ulna, as measured distally from the proximal end of the olecranon.
Samples were placed in holders for scanning by μCT (Scanco model 40; Scanco Medical AG, Basserdorf, Switzerland) in appropriate fluid (70% ethanol) to appropriately evaluate density and bone volume (BV). The samples were scanned at 55 kV, 0.3-second integration time, with a 30 μm voxel size in plane and a 30 μm slice thickness. The proper thresholds for 3D reconstructions to qualitatively evaluate overall bone morphology and quantitatively evaluate trabecular and cortical bone were tested. The same thresholds were used throughout the study. For trabecular bone, BV (mm3), total volume (TV, mm3), BV/TV, bone mineral density (mg HA/cc), trabecular number (1/mm), trabecular thickness (mm), trabecular spacing (mm), connectivity density (Conn.D, 1/mm3), structural model index (SMI), bone surface (BS, mm), and degree of anisotropy (DA) were evaluated. For cortical bone, cortical area (Ct.Ar, mm3), cortical thickness (mm), and cortical porosity (%) were evaluated. Established nomenclature was used for all variables.18
2.3 |. Histology
Following the μCT studies, distal femora from eleven cats, including six unaffected controls (four females and two males) and five affected cats (two males, three females) were prepared for histology. Animals ranged from 5 to 6 months (including two affected cats and four unaffected controls) and 21 to 37 months (including three affected cats and two unaffected controls). Only the three affected cats from the oldest group (group 3) had been treated with an intracranial injection of AAV. All samples were decalcified in 10% ethylenediaminetetraacetic acid. Locations for histological evaluation were chosen based on the μCT findings in these animals and included sections cut in the following locations/planes: (a) through the center of the weight-bearing area of the femoral condyles, perpendicular to the articular cartilage, in a plane that extended through the proximal limit of the trochlea; and (b) in an axial plane (perpendicular to the articular cartilage) through the proximal and distal thirds of the trochlea. Samples were routinely processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin.
2.4 |. Statistical analysis
Data were tested for normality and parametric t tests (significance determined to be P < .05) were conducted on various parameters between affected cats and unaffected controls (SPSS v.21, SPSS Inc, Chicago, IL).
3 |. RESULTS
3.1 |. Gross skeletal appearance
Overall morphology of the bones was determined via 3D reconstructions of the distal femur, proximal humerus, proximal femur, proximal tibia, and elbow. All affected cats exhibited skeletal abnormalities, primarily involving the epiphyses, which increased in severity with increased age. Although there were changes in bone morphology, there were no significant differences in long bone length in any of the groups.
In groups 1 and 2, the distal epiphysis of the femur and proximal epiphysis of the humerus in the affected cats were much smaller (less than half the size) than the corresponding sites in the unaffected control animals and exhibited marked surface pitting (Figure 1). In addition, the patellar grooves were less developed and the growth plates were irregularly widened in affected animals (Figure 1C). This phenotype was similar across the age groups. However, the femoral condyles and patellar groove were so poorly developed in affected animals that they were barely distinguishable in the youngest group (group 1). Similarly, the greater and lesser trochanters of the proximal femur and the greater and lesser tubercles of the humerus and humeral head (Figure 1) were grossly underdeveloped in affected animals. General retardation of development was also noted in the greater trochanter of the proximal femur (data not shown), which develops by a separate apophysis.
FIGURE 1.
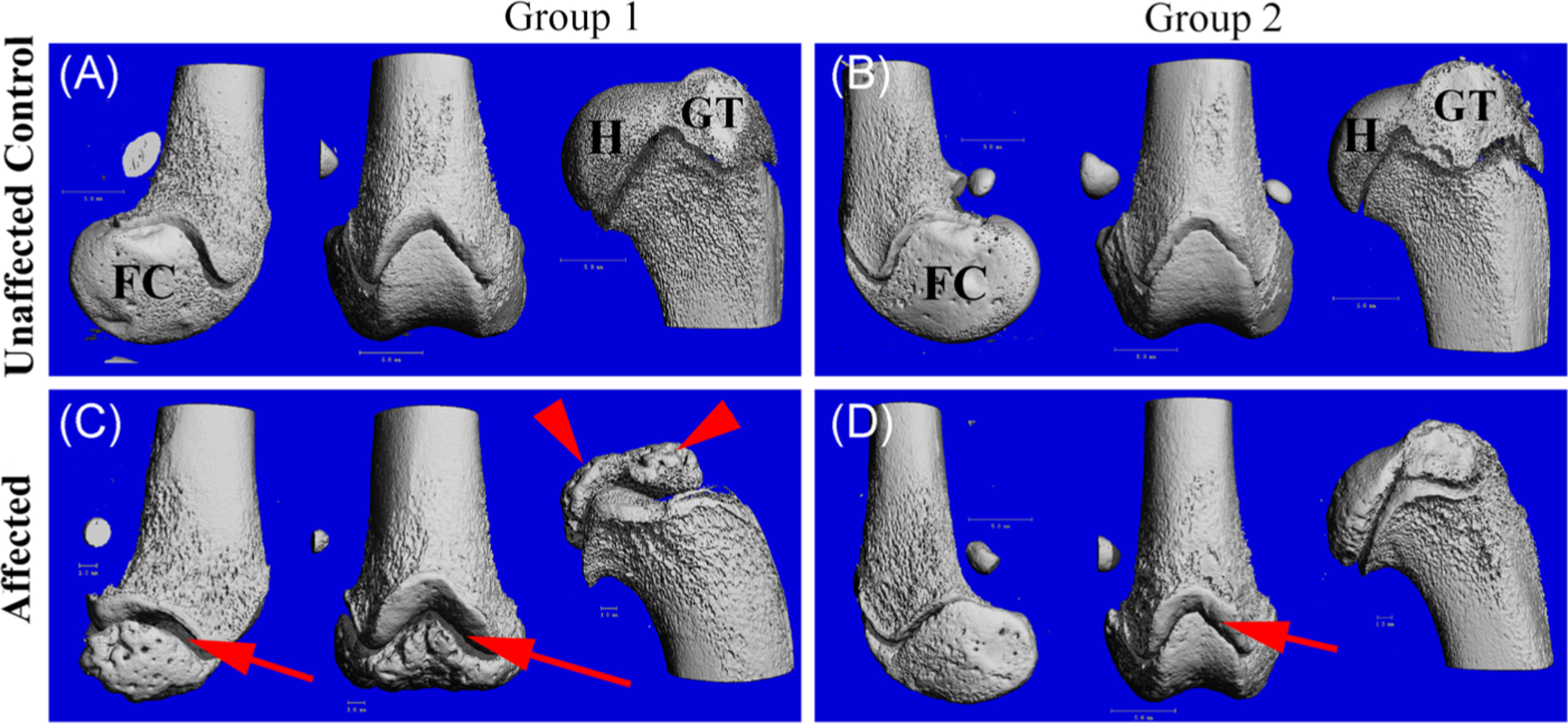
3D reconstruction of μCT scans of the medial distal femur (left), cranial femur (center), and lateral proximal humerus (right) from a 3.9 months old unaffected control (A), 5 months old unaffected control (B), 3.9 months old affected (C), and 5 months old affected (D). Arrows indicate widened distal physes compared to controls; arrow heads indicate underdeveloped humeral head and greater tubercle, which is more visually apparent in group 1 than group 2. Distal femoral epiphysis also is remarkably decreased in size compared with that of unaffected control animals. Both humeral and femoral epiphyses contain numerous pits over the surface of the bone from affected cat (C). μCT, micro-computed tomography; FC, femoral condyle; GT, greater tubercle; H, humeral head
The articulating surface of the distal femur in the older affected cats (group 3) had a roughened appearance (Figure 2F–I). In addition, periarticular osteophytes were present in some samples. One adult affected cat (aged 30.8 months) had particularly severe lesions, including several large cavitations that extended from the surface into the underlying bone, creating a cystic appearance (Figure 2I). This cat also had a large abaxial periarticular osteophyte in the medial joint compartment (Figures 2F–H and 5D). In addition, growth plates appeared to persist in the older affected cats, even in the second oldest animal in the study (36.2 months) compared to unaffected controls.
FIGURE 2.
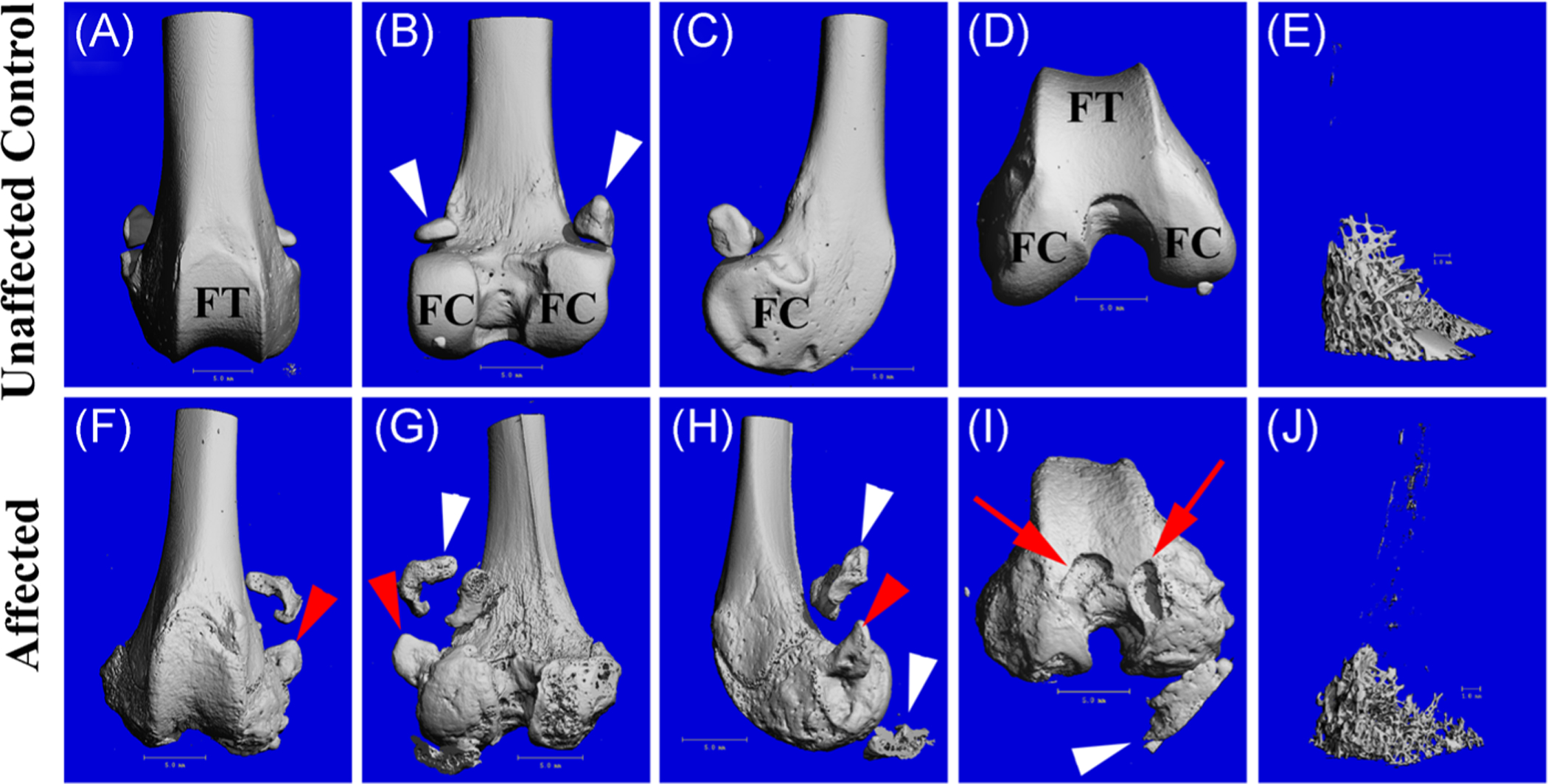
3D reconstruction of μCT scans of the distal femur showing cranial, caudal, medial, and distal views, as well as isolated metaphyseal trabecular bone from (A-E) a 29.7 months old unaffected control cat and (F-J) a 30.8 months old affected cat, demonstrating osteopenia of the trabecular bone (J). Red arrow heads indicate a periarticular osteophyte on the medial aspect of the femur of the affected cat; red arrows indicate subchondral cavitations; white arrow heads indicate sesamoid bones embedded into the gastrocnemius muscle (likely displaced in the affected animal due to tissue preparation) and remnants of a calcified meniscus. μCT, micro-computed tomography; FC, femoral condyle; FT, femoral trochlea
FIGURE 5.
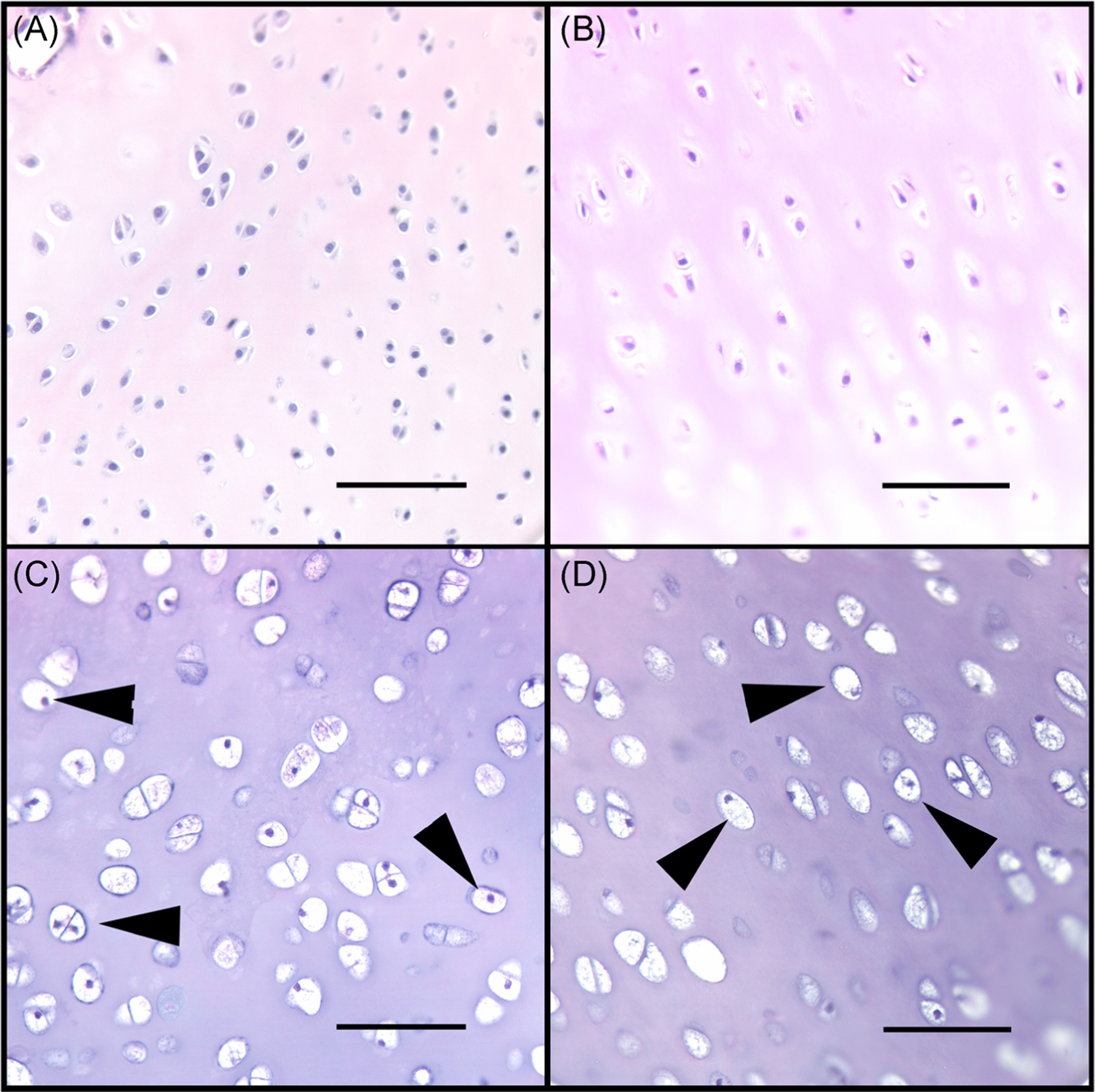
Histological sections from the articular cartilage of distal femur of representative cats: (A) a 5 months old unaffected control; (B) a 37.2 months old unaffected control; (C) a 5 months old affected; and (D) a 30.8 months old affected cat demonstrating diffusely vacuolated chondrocytes in (C) and (D) (arrow heads). Bar = 75 μm
The proximal humeri in the older affected cats (group 3) had similar changes as the femora, including roughened articulating surfaces on the humeral heads and small periarticular osteophytes surrounding the humeral heads in affected adult animals (data not shown). Intact elbow joints in the older affected cats had periarticular osteophytes on the proximal radius and ulna and roughened articular surfaces (Figure 3). As previously reported radiographically,11 the lateral joint space within the elbow was slightly larger in the affected cats than in unaffected controls (Figure 3D,E), however this change was not quantified. As noted in the femora, physes in other long bones, including distal humerus and proximal radius and ulna, persisted in affected adults but were ossified in unaffected control cats older than 29 months (data not shown). Evidence of periarticular osteophytes were present in the distal humerus of affected adult cats as well.
FIGURE 3.
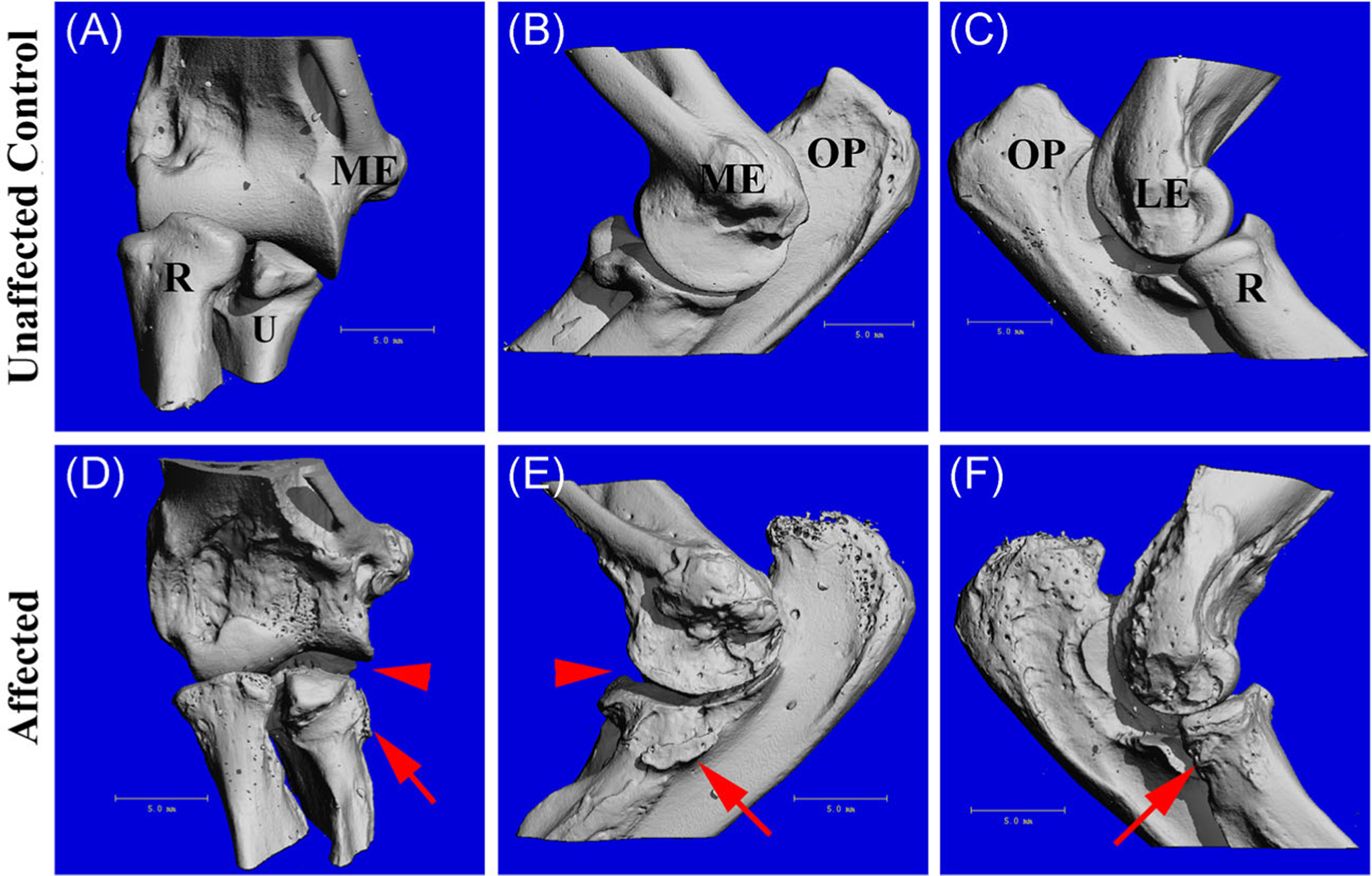
3D reconstruction of μCT scans of the intact elbow joint (cranial, lateral, and medial views) from a 29.7 months old unaffected control (A-C) and a 20.8 months old affected cat (D-F). Arrow heads indicate widening of the lateral joint space and arrows indicate periarticular osteophytes on the proximal radius and ulna. Last, the distal epiphysis of the humeri and the proximal epiphyses of the radii and ulnae of the affected animals (D) were reduced in size compared to the controls (A). μCT, micro-computed tomography; LE, lateral epicondyle; ME, medial epicondyle; OP, olecranon process; R, radius; U, ulna
3.2 |. μCT morphometry
3.2.1 |. Trabecular architecture
Overall, affected cats had lower BV/TV than unaffected controls. (Table 2 and Figure 4) In group 3 (oldest animals), the affected cats had significantly lower BV/TV in the femora and humeri (P = .011 and P = .004, respectively) (Table 2 and Figure 4). In group 2, BV/TV was significantly lower in the femora and humeri (femora, P = .001; humeri, P = .011) (Table 2 and Figure 4). However, in group 1 (youngest animals), BV/TV was not significantly different in the femur or humerus between the affected cats and unaffected control cats (Table 2). BS was also decreased in the affected cats (Table 2 and Figure 4). In groups 2 and 3, it was significantly decreased in the femur (P < .001 and P = .002, respectively) and humerus (P < .001 and P = .004, respectively) (Figure 4). Similar to BV/TV, BS was not significantly different between affected cats and unaffected control cats in group 1 (Table 2).
TABLE 2.
Trabecular bone morphology data; mean (SD)
| BV, mm3 | BV/TV | Conn.D, 1/mm3 | SMI | Bone mineral density (mg HA/cc) | BS, mm | DA | ||
|---|---|---|---|---|---|---|---|---|
| Femur | Group 1 | |||||||
| Control | 34.493 (38.456) | 0.077 (0.065) | 8.253 (7.177) | 2.340 (0.733) | 1.988 (0.118) | 678.213 (648.119) | 1.763 (0.160) | |
| Affected | 17.671 (9.242) | 0.056 (0.031) | 6.182 (4.340) | 2.731 (0.344) | 1.988 (0.094) | 421.274 (228.520) | 1.901 (0.110) | |
| Group 2 | ||||||||
| Control | 94.131 (10.240) | 0.157 (0.029) | 15.612 (2.667) | 1.637 (0.280) | 2.021 (0.038) | 1609.412 (150.233) | 1.663 (0.119) | |
| Affected | 13.863* (2.284) | 0.0322* (0.006) | 2.805* (1.018) | 2.731* (0.034) | 2.067 (0.009) | 312.203* (58.408) | 1.886 (0.099) | |
| Group 3 | ||||||||
| Control | 80.100 (19.678) | 0.091 (0.027) | 2.573 (0.408) | 0.931 (0.423) | 2.250 (0.066) | 1259.953 (592.524) | 1.620 (0.014) | |
| Affected | 15.835* (6.102) | 0.033* (0.020) | 1.703 (1.134) | 2.399* (0.383) | 2.247 (0.047) | 310.164* (99.182) | 1.446 (0.096) | |
| Humerus | Group 1 | |||||||
| Control | 42.546 (51.524) | 0.084 (0.067) | 7.967 (7.226) | 2.702 (0.625) | 2.133 (0.081) | 912.076 (966.777) | 1.749 (0.248) | |
| Affected | 16.418 (0.802) | 0.049 (0.002) | 4.165 (0.786) | 3.064 (0.014) | 2.135 (0.046) | 409.966 (29.962) | 1.703 (0.014) | |
| Group 2 | ||||||||
| Control | 67.604 (8.513) | 0.127 (0.025) | 14.863 (2.922) | 2.411 (0.188) | 2.010 (0.040) | 1467.376 (107.754) | 1.784 (0.052) | |
| Affected | 19.143* (4.198) | 0.052* (0.005) | 4.619* (0.576) | 2.942* (0.178) | 2.084 (0.028) | 473.462* (76.006) | 1.791 (0.151) | |
| Group 3 | ||||||||
| Control | 79.949 (20.029) | 0.087 (0.019) | 2.023 (0.313) | 1.435 (0.087) | 2.328 (0.036) | 1168.292 (479.173) | 1.662 (0.019) | |
| Affected | 11.632* (5.335) | 0.030* (0.012) | 1.740 (1.290) | 2.449* (0.357) | 2.320 (0.095) | 221.471* (84.976) | 1.552 (0.070) |
Note: Mean values of affected groups indicated by * significantly differ from unaffected controls with P < .05.
Abbreviations: BS, bone surface; BV, bone volume; Conn.D, connectivity density; DA, degree of anisotropy; SD, standard deviation; SMI, structural model index; TV, total volume.
FIGURE 4.

Bone volume/total volume (BV/TV) and bone surface (BS) of trabecular bone within the distal femur and proximal humerus from affected cats and unaffected controls in groups 2 and 3. *P < .05 when compared to the unaffected control group, and **P < 0.01 when compared to the unaffected control group
Significant differences in trabecular architecture were only identified in the distal femur of group 2, in which the affected cats had significantly thinner trabeculae when compared to unaffected controls (P = .01) (Table 2). Conn.D (the number of connected trabeculae) was significantly lower only in group 2 as well, both in the femur (P < .001) and humerus (P = .006) (Table 2). SMI, however, was higher (indicating the presence of trabeculae that are cylindrical rods vs parallel plates) in the affected cats compared to the unaffected controls (Table 2). This difference was significant in the femur and humerus in both group 3 (P = .002, P = .013, respectively) and group 2 (P = .002, P = .019, respectively) (Table 2). Finally, DA was significantly lower in the affected cats (P = .045) of group 3, indicating that the trabeculae were more isotropic (Table 2). No differences were found in group 1 in any of the above parameters (Table 2). Last, no significant differences in bone mineral density were identified in any of the groups (Table 2).
3.2.2 |. Cortical geometry
Few significant differences were detected in cortical bone although a significant reduction in Ct.Ar was identified in the femur and humerus (P < .001 and P = .010, respectively) in affected compared to unaffected control cats in group 2.
3.3 |. Histology
In sections from the youngest affected cats (<4.3 months of age), all chondrocytes were diffusely vacuolated (Figure 5C). The articular-epiphyseal cartilage complex (cartilage present at the ends of growing long bones) was greatly thickened (~1500 microns in affected vs ~500 microns in controls), due to marked retention of epiphyseal cartilage (Figure 6C). Multiple cartilage canal vessels were present in the retained epiphyseal cartilage of affected cats (Figure 7A) but were absent in unaffected control cats. Multiple variably-sized foci of retained cartilage also were present within the bone trabeculae throughout the epiphyseal/metaphyseal bone of affected cats (Figure 7B) but were absent in unaffected controls. In young animals, the bone marrow exhibited decreased cellularity in affected cats compared with unaffected controls (Figure 6C).
FIGURE 6.
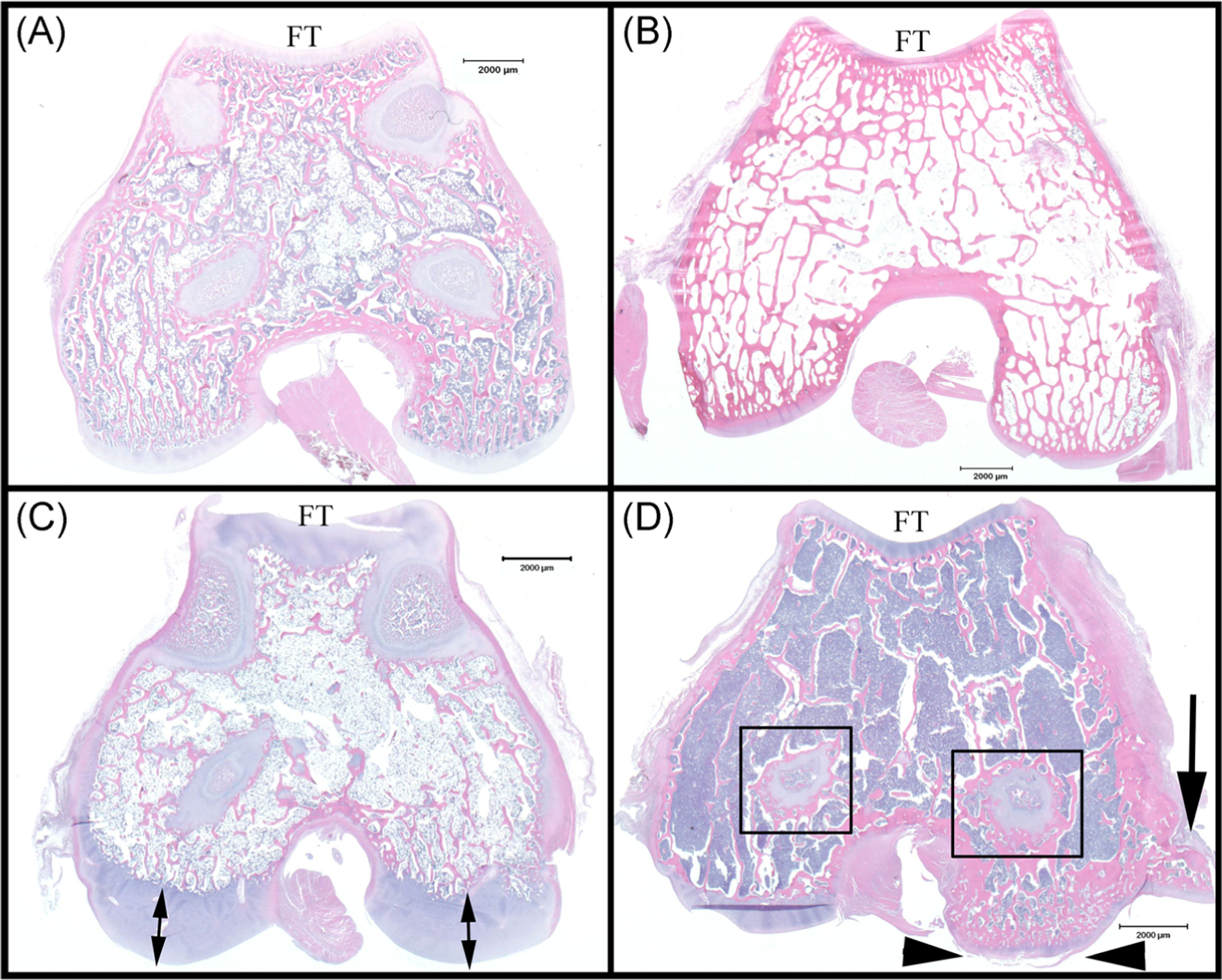
Histological sections from distal femur of representative cats: (A) a 5 months old unaffected control; (B) a 37.2 months old unaffected control; (C) a 5 months old affected cat demonstrating reduced trabecular bone, increased thickness of articular-epiphyseal complex (double arrows), and bone marrow hypocellularity when compared to (A); and (D) a 30.8 months old affected cat demonstrating reduced trabecular bone, bone marrow hypercellularity, retained growth plate cartilage (boxes), and lesions associated with osteoarthritis (arrow heads indicate articular cartilage fibrillation, arrow indicates periarticular osteophyte) when compared to (B). Bar = 2000 μm. FT, femoral trochlea
FIGURE 7.
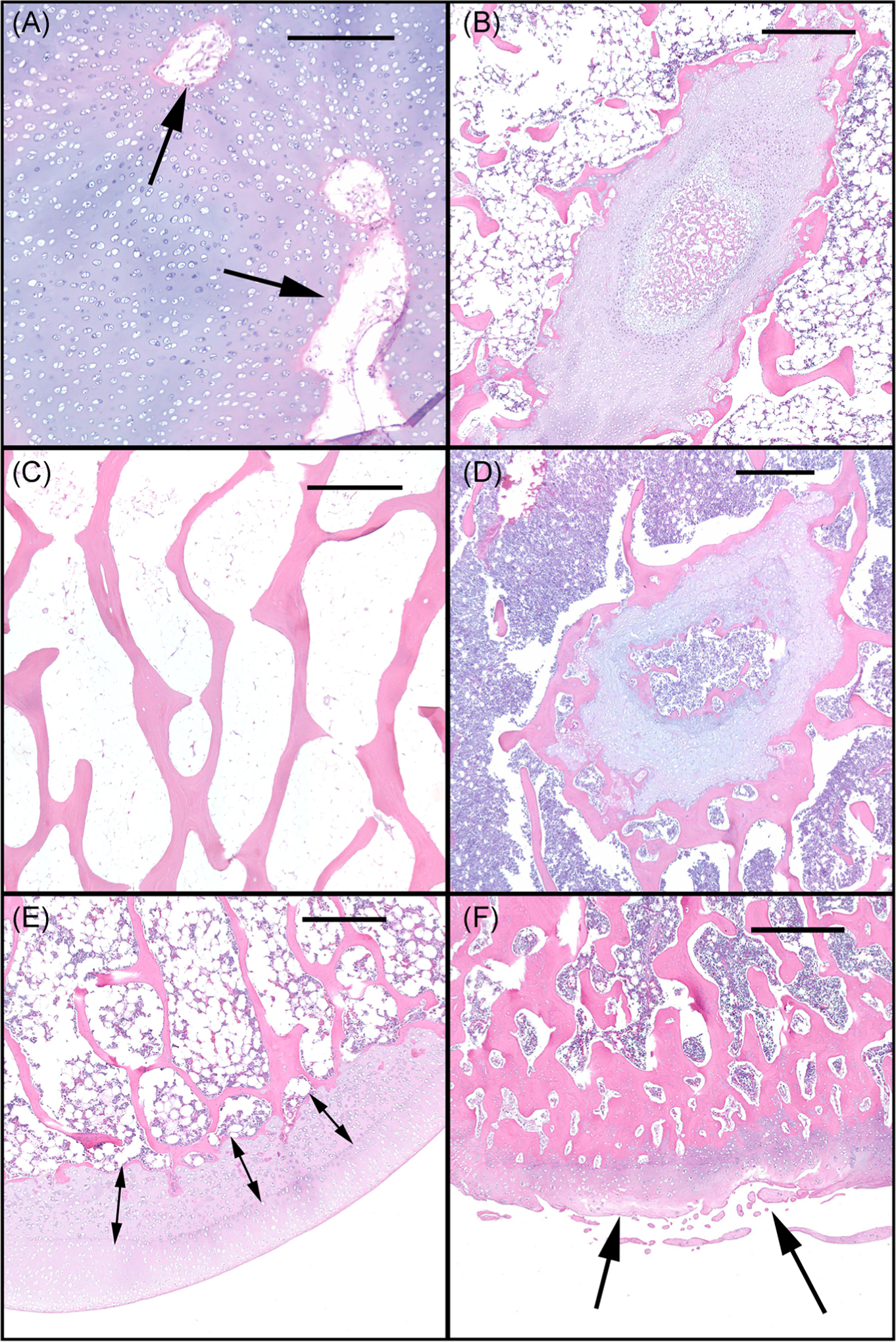
Histological sections from distal femora of representative cats: (A) a 5 months old affected cat with retained cartilage canal vessels (arrows) and (B) retained epiphyseal (growth plate) cartilage; (C) a 37.2 months old unaffected control cat demonstrating normal epiphyseal trabecular bone; (D) a 30.8 months old affected cat demonstrating retained growth plate cartilage within trabecular bone; (E) a 21.3 months old affected cat exhibiting retained and thickened calcified cartilage/deep epiphyseal cartilage (arrows); (F) a 30.8 months old affected cat demonstrating osteoarthritis lesions characterized by areas of articular cartilage fibrillation/degeneration (arrows). Bar in (A) = 300 μm, bars in B-F = 500 μm
The older affected cats (treated by gene therapy) exhibited retained growth plate cartilage (absent in unaffected controls; Figures 6B and 6D) and, similar to the younger affected cats, all chondrocytes were diffusely vacuolated (Figure 5D). The calcified cartilage/deep epiphyseal cartilage was retained and thickened (Figure 7E), sometimes forming large plugs that extended into the subchondral bone and corresponded to the surface cavitations noted on μCT (Figure 2I). These areas of retained cartilage also contained multifocal areas of matrix degeneration. Retained foci of cartilage within trabecular bone were present (Figure 7D), but were much reduced in number compared with those present in sections from the younger animals. Lesions of osteoarthritis, characterized by areas of articular cartilage fibrillation/degeneration accompanied by chondrocyte clones as well as the presence of periarticular osteophytes, were present in the femoral condyles of the oldest affected animal (30.8 months of age) (Figure 6D). Unlike the observation in the young cats, the cellularity of the bone marrow in older affected cats was moderately to markedly increased compared with that of unaffected controls (Figures 6B and 6D).
All affected cats exhibited a decreased amount of trabecular bone that was particularly evident in the younger cats, and the shape of the distal femur was altered compared with unaffected controls (Figures 4A and 6). However, the organization of the trabeculae, particularly the arrangement of the lamellae and haversian systems, appeared normal. The distal femora of the unaffected control cats in group 3 exhibited normal histological features (Figure 6B).
4 |. DISCUSSION
The results from this study reveal significant effects of SD on bone and joint morphology, particularly in the metaphyseal and epiphyseal regions of long bones, in this feline model. Based on our results, we hypothesize that these abnormalities are due to accumulated storage product (visualized as diffused cytoplasmic vacuolation) interfering with chondrocyte function, resulting in failure of endochondral ossification within the physes, epiphyses, and apophyses of long bones resulting in misshapen and underdeveloped epiphyses. These abnormalities likely resulted in the clinical signs identified in the affected animals that lived to be older than 12 months due to AAV treatment. The significant changes in trabecular morphology within the metaphyseal region of these bones appear to be a result of the changes identified in the epiphyseal cartilage, which indicate an altered endochondral ossification process. Some of these changes, including thickened growth plates and cytoplasmic vacuolation of chondrocytes, are similar to those found in mouse models of MPSs.20 These changes in the bone and joint tissue in the current feline SD model are progressive and are present prior to the age of onset of clinical signs, which also is similar to MPS mouse models.20 The current study focuses on changes in the long bones, though it has been previously shown that other bone sites may be affected as well.11 To our knowledge, impacts on skeletal development have not been documented in humans affected with either SD or TSD. However, these changes may be overlooked in patients, particularly children, when the neurodegenerative changes are far more clinically pressing. In addition, humans mature much more slowly than cats, so the chronic changes identified in the older cats likely would not be present until children were young adults. These results present a novel impact of GM2 gangliosidoses on the skeleton that likely is present in humans as well, and should be a consideration when treatments that may ameliorate the neurodegeneration lead to increased lifespan in human patients.
A substantial reduction in the amount of trabecular bone was identified in affected cats in all three groups via μCT analyses, and confirmed histologically. This included a reduction in BV/TV, interpreted as osteopenia, which would have a negative impact on bone strength and also has been identified in patients with MPS.21,22 Changes in trabecular morphometry were also identified which may indicate changes in bone remodeling kinetics between the groups. No significant changes in trabecular bone were identified in the youngest age group, indicating that the decrease in trabecular bone may also be due to an impact on normal bone remodeling. Because the animals were not administered fluorochrome labels prior to euthanasia, it was not possible to characterize potential changes in remodeling kinetics in the current study. Despite the morphological changes in trabecular bone, no changes were identified in the bone matrix of the trabeculae, and lamellar arrangement appeared normal. This differs from previous reports of skeletal changes in some animal models of MPS resulting from progressive accumulation of GAGs.23 The authors are unsure of why the bone matrix was not affected, and further directed research in this area is warranted. Last, no substantial changes were identified in midshaft cortical bone regions, indicating that the bony changes secondary to SD do not appear to affect the primary ossification center. Although physes were retained in the older animals, no effect on long bone growth was noted.
Diffuse, severe vacuolation of chondrocytes within the articular-epiphyseal complex was noted in affected animals strongly suggesting the accumulation of gangliosides in these cells and their interference with normal cell function. Coupled with the retention of cartilage canal vessels and foci of retained cartilage within the trabecular bone, these changes indicate a failure of endochondral ossification. Affected adult cats also had physes that persisted well past the age of normal ossification.
The decreased marrow cellularity in affected young cats was a relatively subtle finding, although increased marrow cellularity in the affected adult cats was a striking change. These findings could not be explained with the available tissues from the present study and will require additional study. Because only the distal femora were evaluated histologically in the present study, it is unclear whether the bone marrow changes were also present in other sites.
While the majority of changes identified in these tissues impacted epiphyseal growth and development, secondary osteoarthritis-like changes were also an important finding in the older cats affected with SD in the current study. This change is likely due to the instability created within the joint following the abnormal development of the epiphyses and articulating surfaces of the long bones; however, it is possible that accumulation of gangliosides in articular chondrocytes resulted in premature degeneration of this tissue. Osteoarthritis is a prevalent disease, affecting 22.7% of adults in the United States,24 and often results in loss of function and reduction in mobility. Therefore, premature osteoarthritis would have a substantial impact on the quality of life in long-term survivors of SD as treatments to improve the neurological effects are identified and enhanced.
Previous work in this model demonstrated that AAV gene therapy, initiated at approximately 1 month of age, greatly reduced the CNS alterations associated with SD, including GAG content within the brain. However, gene therapy had less impact on GAG content in most peripheral tissues, at least by 16 weeks posttreatment.11 This indicates that current intracranial approaches to treat SD will likely have a modest effect on the musculoskeletal changes identified in the present study. Treatment of bony changes may be enhanced by initiation of treatment early in life. As emerging therapies demonstrate efficacy in clinical trials, newborn screening for TSD and SD will become available.
In the current study, significant changes in trabecular bone were not recognized prior to the age of 4 months. However, the overall morphology of the long bone epiphyses, as well as changes identified via histopathology, were present in the youngest animals in the study, indicating that changes occur shortly after, or perhaps prior to, birth. Current treatment focusing on the skeletal changes associated with MPSs are initiated in newborn individuals, as this has been shown to provide the best opportunity to affect the disease progression.20 These MPS treatments include enzyme replacement therapies (ERTs) which are administered intravenously and have systemic effects; however, likely only a small fraction of the treatment reaches bony tissue, and none is able to reach avascular articular cartilage. Epiphyseal cartilage, however, has a substantial blood supply in young individuals and, thus, may be amenable to intravenous therapy. This is evidenced by the fact that in other animal models of MPS sub-types in which ERT (including large doses continued for extended timeframes) was administered in newborns, the skeletal abnormalities associated with MPS disease were ameliorated.25–28 In fact, one of the changes noted histologically in the present study was a retention of cartilage canal blood vessels in the affected animals at an age in which these vessels were absent in the unaffected controls. Given the reduced vascular supply to bone and cartilage, therapies are now starting to focus on bone-targeting ERT therapies, such as compounds bound to hydroxyapatite, to enhance their effect in bone and joint tissue.20 Newborn, bone-targeting ERT may be a potential method of treating the skeletal abnormalities associated with SD that have been identified in the current study. Also, intravenous gene therapy has been shown to treat both CNS and peripheral tissues in MPS diseases, making it a possible mode of treating skeletal pathology.29
This study is not without some limitations, mainly a lack of specifically age-matched groups. The animals from this study were initially utilized to evaluate neurological development of the disease and the effect of the intracranial treatment administered. Only later in the study, when the treated animals began to live past a year of age, did the skeletal defects cause clinical signs, thereby prompting the current investigation. To address this limitation, the animals were assigned to groups based on similar ages and skeletal maturity. In addition, the main finding from this study was that SD results in abnormal skeletal anatomy, probably via alterations in chondrocyte function, a result that is not entirely dependent on specifically age-matched groups. Groups 1 and 2 included animals that were affected with SD but were not treated. Samples from those animals exhibited significant morphological differences compared to the unaffected control animals. In addition, sample numbers in all of the groups are very small, which is not uncommon in studies utilizing large animal models. However, statistical significance was still achieved between groups due to the dramatic effect seen on bone development in this model.
As newer and more effective treatment modalities for LSDs such as SD continue to be discovered and developed, resulting in increased lifespans of affected patients, it is likely that previously unrecognized skeletal sequelae that can have a tremendous impact on quality of life will be identified. It is crucial that continued collaborative and multidisciplinary long-term research studies on the systemic effects of these new treatment options be conducted in order to identify and address these potentially significant sequelae in human patients.
ACKNOWLEDGMENTS
This study was funded by Louisiana Board of Regents Traditional Enhancement Grant, NIH #NS064096, National Tay-Sachs & Allied Diseases Association, Cure Tay-Sachs Foundation, and Scott-Ritchey Research Center.
Funding information
Louisiana Board of Regents; National Institutes of Health, Grant/Award Number: NS064096; National Tay-Sachs and Allied Diseases Association; Cure Tay-Sachs Foundation; Auburn University Scott-Ritchey Research Center
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Cachon-Gonzalez MB, Zaccariotto E, Cox TM. Genetics and therapies for GM2 gangliosidosis. Curr Gene Ther. 2018;18:68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradbury AM, Cochran JN, McCurdy VJ, et al. Therapeutic response in feline sandhoff disease despite immunity to intracranial gene therapy. Mol Ther. 2013;21:1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scriver CR. The metabolic and molecular bases of inherited disease. 7th ed. New York: McGraw-Hill, Health Professions Division; 1995. [Google Scholar]
- 4.Baek RC, Martin DR, Cox NR, Seyfried TN. Comparative analysis of brain lipids in mice, cats, and humans with Sandhoff disease. Lipids. 2009;44:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawson CA, Martin DR. Animal models of GM2 gangliosidosis: utility and limitations. Appl Clin Genet. 2016;9:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCurdy VJ, Johnson AK, Gray-Edwards HL, et al. Sustained normalization of neurological disease after intracranial gene therapy in a feline model. Sci Transl Med. 2014;6:231ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCurdy VJ, Rockwell HE, Arthur JR, et al. Widespread correction of central nervous system disease after intracranial gene therapy in a feline model of Sandhoff disease. Gene Ther. 2015;22:181–189. [DOI] [PubMed] [Google Scholar]
- 8.Rockwell HE, McCurdy VJ, Eaton SC, et al. AAV-mediated gene delivery in a feline model of Sandhoff disease corrects lysosomal storage in the central nervous system. ASN Neuro. 2015;7. 10.1177/1759091415569908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cork LC, Munnell JF, Lorenz MD, Murphy JV, Baker HJ, Rattazzi MC. GM2 ganglioside lysosomal storage disease in cats with beta-hexosaminidase deficiency. Science. 1977;196:1014–1017. [DOI] [PubMed] [Google Scholar]
- 10.Martin DR, Krum BK, Varadarajan GS, Hathcock TL, Smith BF, Baker HJ. An inversion of 25 base pairs causes feline GM2 gangliosidosis variant. Exp Neurol. 2004;187:30–37. [DOI] [PubMed] [Google Scholar]
- 11.Gray-Edwards HL, Brunson BL, Holland M, et al. Mucopolysaccharidosis-like phenotype in feline Sandhoff disease and partial correction after AAV gene therapy. Mol Genet Metab. 2015; 116(1–2):80–7. [DOI] [PubMed] [Google Scholar]
- 12.Giugliani R, Federhen A, Rojas MV, et al. Mucopolysaccharidosis I, II, and VI: brief review and guidelines for treatment. Genet Mol Biol. 2010;33:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opoka-Winiarska V, Jurecka A, Emeryk A, Tylki-Szymanska A. Osteoimmunology in mucopolysaccharidoses type I, II, VI and VII. Immunological regulation of the osteoarticular system in the course of metabolic inflammation. Osteoarthritis Cartilage. 2013;21:1813–1823. [DOI] [PubMed] [Google Scholar]
- 14.Tomatsu S, Montano AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931–945. [DOI] [PubMed] [Google Scholar]
- 15.Visigalli I, Delai S, Politi LS, et al. Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood. 2010;116: 5130–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cork LC, Munnell JF, Lorenz MD. The pathology of feline GM2 gangliosidosis. Am J Pathol. 1978;90:723–734. [PMC free article] [PubMed] [Google Scholar]
- 17.DeLaurier A, Jackson B, Pfeiffer D, Ingham K, Horton MA, Price JS. A comparison of methods for measuring serum and urinary markers of bone metabolism in cats. Res Vet Sci. 2004;77:29–39. [DOI] [PubMed] [Google Scholar]
- 18.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. [DOI] [PubMed] [Google Scholar]
- 19.Hornof WJ, Wind AP, Wallack ST, Schulz KS. Canine elbow dysplasia. The early radiographic detection of fragmentation of the coronoid process. Vet Clin North Am Small Anim Pract. 2000;30:257–66. [PubMed] [Google Scholar]
- 20.Tomatsu S, Almeciga-Diaz CJ, Montano AM, et al. Therapies for the bone in mucopolysaccharidoses. Mol Genet Metab. 2015;114:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin HY, Shih SC, Chuang CK, Chen MR, Niu DM, Lin SP. Assessment of bone mineral density by dual energy X-ray absorptiometry in patients with mucopolysaccharidoses. Orphanet J Rare Dis. 2013;8:71–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polgreen LE, Thomas W, Fung E, et al. Low bone mineral content and challenges in interpretation of dual-energy X-ray absorptiometry in children with mucopolysaccharidosis types I, II, and VI. J Clin Densitom. 2014;17:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomatsu S, Montano AM, Ohashi A, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–824. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) 2013. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation–United States, 2010–2012. MMWR Morb Mortal Wkly Rep 62:869–873. [PMC free article] [PubMed] [Google Scholar]
- 25.Dierenfeld AD, McEntee MF, Vogler CA, et al. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci Transl Med. 2010;2:60ra89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawley AC, Niedzielski KH, Isaac EL, Davey RC, Byers S, Hopwood JJ. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J Clin Invest. 1997;99:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogler C, Sands MS, Galvin N, et al. Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease. J Inherit Metab Dis. 1998;21:575–586. [DOI] [PubMed] [Google Scholar]
- 28.Dickson PI, Hanson S, McEntee MF, et al. Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol Genet Metab. 2010;101:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruzo A, Marco S, Garcia M, et al. Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Hum Gene Ther. 2012;23:1237–1246. [DOI] [PubMed] [Google Scholar]


