Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a severe clinical phenotype of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that remains poorly understood.
Methods
Hospitalized children <18 years of age with suspected coronavirus disease 2019 (COVID-19) (N = 53) were recruited into a prospective cohort study; 32 had confirmed COVID-19, with 16 meeting the US Centers for Disease Control criteria for MIS-C. Differences in nasopharyngeal viral ribonucleic acid (RNA) levels, SARS-CoV-2 seropositivity, and cytokine/chemokine profiles were examined, including after adjustments for age and sex.
Results
The median ages for those with and without MIS-C were 8.7 years (interquartile range [IQR], 5.5–13.9) and 2.2 years (IQR, 1.1–10.5), respectively (P = .18), and nasopharyngeal levels of SARS-CoV-2 RNA did not differ significantly between the 2 groups (median 63 848.25 copies/mL versus 307.1 copies/mL, P = .66); 75% of those with MIS-C were antibody positive compared with 44% without (P = .026). Levels of 14 of 37 cytokines/chemokines (interleukin [IL]-1RA, IL-2RA, IL-6, IL-8, tumor necrosis factor-α, IL-10, IL-15, IL-18, monocyte chemoattractant protein [MCP]-1, IP-10, macrophage-inflammatory protein [MIP]-1α, MCP-2, MIP-1β, eotaxin) were significantly higher in children with MIS-C compared to those without, irrespective of age or sex (false discovery rate <0.05; P < .05).
Conclusions
The distinct pattern of heightened cytokine/chemokine dysregulation observed with MIS-C, compared with acute COVID-19, occurs across the pediatric age spectrum and with similar levels of nasopharyngeal SARS-CoV-2 RNA.
Keywords: coronavirus, COVID-19, MIS-C, SARS-CoV-2, viral RNA
This is a prospective cohort study, where similar levels of nasopharyngeal viral RNA were found in children with MIS-C and acute COVID-19, despite finding significantly elevated cytokine/chemokine levels in MIS-C patients, after adjusting for age and sex.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the novel coronavirus first identified in Wuhan, China in 2019, maintains high levels of circulation with more than 155 000 000 cases worldwide as of May 6, 2021 [1–3]. Initial reports from China of SARS-CoV-2 infection in children described a mild disease consisting mainly of fever, upper respiratory tract illness, and gastrointestinal symptoms [4, 5]. Later, case series from Europe described a syndrome of systemic inflammation akin to Kawasaki disease (KD) [6] with features of toxic shock, referred to as pediatric multisystem inflammatory syndrome [7]. Similar cases were subsequently identified in the United States and named multisystem inflammatory syndrome in children (MIS-C) [8–10]. A similar inflammatory syndrome associated with SARS-CoV-2 was recognized in adults and referred to as multisystem inflammatory syndrome in adults [11].
Multisystem inflammatory syndrome in children is reported as largely postinfectious and mostly in older children (median age 8 years) [12] with the acute phase showing elevations in proinflammatory cytokines (interleukin [IL]-1β, IL-6, IL-8, IL-10, IL-17, and interferon [IFN]-γ) [13, 14]. Although several studies have reported elevations in cytokines with MIS-C, overlapping those seen in severely affected adult patients [15], few pediatric studies have adjusted for age and sex, or have examined quantitative differences in nasopharyngeal virus shedding in acute coronavirus disease 2019 (COVID-19) compared with MIS-C to establish the interaction between the patterns of cytokine elevations and disease phenotype.
Further knowledge regarding the patterns of cytokine elevation and disease phenotype while adjusting for factors such as age and sex, as well as the viral ribonucleic acid (RNA) levels of children with acute COVID-19 and MIS-C, will help to further understand the immunopathogenesis of SARS-CoV-2 infection and MIS-C. From a prospective cohort study established at an urban Children’s Center at the outset of the US pandemic, we report the quantitative viral RNA and cytokine/chemokine levels of children hospitalized with COVID-19 across the age-spectrum, comparing those with acute COVID-19 and MIS-C.
METHODS
Study Participants
Children admitted with suspected SARS-CoV-2 infection to the Johns Hopkins Hospital, an academic medical center in Baltimore, Maryland, were prospectively enrolled into a cohort study of children and adults with suspected COVID-19, “Clinical Characterization Protocol for Severe Emerging Infections”. Data from the study participants enrolled from April 10, 2020 to July 10, 2020 are summarized.
Informed Consent and Sample Acquisition
The study was approved by the Johns Hopkins Medicine Institutional Review Board. After informed consent, blood samples were collected in coordination with routine medical care, and/or remnant blood and nasopharyngeal samples were retrieved from the clinical laboratory. Participants transferred from outside hospitals did not have remnant samples available.
Clinical Data
Review of medical records was performed by manual chart extraction for demographic and clinical data. Participants were considered to have confirmed SARS-CoV-2 infection if they had either of the following: positive nucleic acid test (NAT); negative NAT with positive serology; or negative NAT and serology, but history of exposure to a confirmed COVID-19 contact within 1 month of admission. Participants were defined as having MIS-C using the Centers for Disease Control and Prevention (CDC) case definition: individuals <21 years with fever, laboratory evidence of inflammation, severe illness requiring hospitalization, involvement of greater than 2 organ systems, no alternative plausible diagnoses, and positive for current/recent SARS-CoV-2 infection by real-time polymerase chain reaction (RT-PCR), serology, or antigen test; or exposure to a suspected/confirmed COVID-19 case within 4 weeks of symptom onset [10].
Virologic Diagnosis and Viral Ribonucleic Acid Quantitation
Infection with SARS-CoV-2 was based on positive NAT by any of the 7 clinically validated RT-PCR assays available at the Johns Hopkins Hospital. The SARS-CoV-2 viral RNA levels were quantified using digital droplet PCR (Bio-Rad, Hercules, CA) on RNA extracted from remnant viral transport media (VTM). Primers targeting the nucleocapsid regions N1 and N2 were used. The estimated limit of quantitation was 1.25 copies per milliliter of VTM.
Serological Testing
Serologic testing was performed in participants either as part of clinical care or on archived serum using the EUROIMMUN (Mountain Lakes, NJ) ELISA detecting immunoglobulin (Ig)G targeting the S1 domain of the SARS-CoV-2 spike protein with a limit of detection of 1.23 units. Seropositivity is reported as a unitless ratio by dividing the optical density by the assay calibrator [16].
Cytokine/Chemokine Profiling
Serum cytokines/chemokines were measured using the Meso Scale Discovery V-plex 30 kit, which allows quantification of 21 cytokines (granulocyte-macrophage colony-stimulating factor [GM-CSF], IFN-γ, IL-10, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IL-1α, IL-1β, IL-2, IL-23p40, IL-4, IL-5, IL-6, IL-7, IL-8, IL-8(HA), tumor necrosis factor [TNF]-α, TNF-β, vascular endothelial growth factor [VEGF]) and 9 chemokines (eotaxin, eotaxin-3, IP-10, monocyte chemoattractant protein [MCP]-1, MCP-4, MDC, macrophage-inflammatory protein [MIP]-1α, MIP-1β, TARC), to which 7 additional cytokines (IL-18, IL-1RA, IFN-λ, IL-23, IL-2RA, IFN-α2, TNF-β) and 1 chemokine (MCP-2) were added. Assays were performed according to the manufacturer’s protocol, and data were acquired on a MESO QuickPlex SQ 120. Testing was only performed on participants with freshly collected blood on Day 0 of study enrollment (N = 19). In one participant who succumbed to SARS-CoV-2 infection, a remnant sample was used.
Statistical Analyses
Comparison of the distribution of categorical variables between those with acute COVID-19 and MIS-C was performed using Fisher’s exact test, and comparisons of continuous variables were evaluated using non-parametric rank-sum test. Logistic regression analyses were used for odds ratios and confidence intervals (CIs). Data were analyzed using R; significance was determined by P < .05. To evaluate differences in length of stay, viral load (log-transformed), IgG titer, and cytokines/chemokines (log-transformed) between children with acute COVID-19 and MIS-C, after adjusting for age and sex, a linear regression was fitted for each of these dependent variables using disease status, age, and sex as independent variables. P values for disease status were obtained to indicate whether each dependent variable was statistically different between acute COVID-19 and MIS-C status. For cytokines, P values for disease status were converted to Benjamini-Hochberg false discovery rates (FDRs) [17]. A significant association between cytokines and disease status was determined by FDR <0.05. We have also applied similar analyses to compare the cytokine/chemokines between MIS-C and unconfirmed and between COVID-19 and unconfirmed. Similarly, cytokines associated with age or sex were identified by linear regression using disease status, age, or sex as independent variables; a relaxed FDR of <0.25 was used to determine significance for age or sex, due to the sample size. To test whether the association between cytokine and disease status was different for different age or sex, linear regression was fitted using disease status, age, sex, and interaction between disease status and age or sex as independent variables. A significant interaction was determined by a relaxed FDR of <0.25, due to sample size. Figures were generated with GraphPad Prism 8.4.3 and R.
RESULTS
Demographics of the Overall Cohort
Of the 53 participants, 32 met study criteria with confirmed SARS-CoV-2 infection (Supplemental Figure 1). The median age was 7.4 years (interquartile range [IQR], 1.6–13.9 years, range: 13 days–20 years); 25% were black and 50% were Hispanic (Table 1). The cohort was evenly divided between males and females. For the 21 children without confirmed SARS-CoV-2 infection, the median age was 3.45 years (IQR, 1.4–6.19 years), which was not significantly different from those with confirmed infection (P = .241); their clinical and laboratory data are summarized in Supplemental Table 1.
Table 1.
Demographic, clinical, virologic and immunologic characteristics of children with acute COVID-19 or with MIS-C
| No. (%) | ||||
|---|---|---|---|---|
| Total Cohort with confirmed COVID-19, N = 32 | Participants with MIS-C, N = 16 | Participants with acute COVID-19, N = 16 | P value | |
| Median Age, years (IQR) | 7.4 (1.6–13.9) | 8.7 (5.5–13.9) | 2.2 (1.1–10.5) | .18 |
| Race | .56 | |||
| Black | 8 (25) | 3 (19) | 5 (31) | |
| White | 6 (19) | 2 (13) | 4 (25) | |
| Asian | 2 (6) | 1 (6) | 1 (6) | |
| Other | 16 (50) | 10 (63) | 6 (38) | |
| Hispanic Ethnicity | 16 (50) | 10 (63) | 6 (37) | .29 |
| Male Sex | 16 (50) | 9 (56) | 7 (44) | .72 |
| Positive SARS-CoV-2 NAT | 20 (63) | 8 (50) | 12 (75) | .27 |
| Viral load, copies/mL (N1) | 563.2(0–1998353.95) | 63848.25(461.38–1254399.25) | 307.1(0–3546348.75) | .656 |
| Viral load, copies/mL (N2) | 736.3(0–2019419.8) | 64281.3(520.12–1287013.95) | 325.6(0–3447867.2) | .656 |
| Serology | ||||
| IgG positive | 19 (59) | 12 (75) | 7 (44) | .026 |
| Not done | 6 (19) | 0 (0) | 6 (38) | |
| Median IgG titer, units (IQR) | 5.58 (0.28–9.71) | 6.75 (0.84–10.88) | 2.98 (0.28–5.76) | .241 |
| Chronic Conditions a | 13 (41) | 5 (31) | 8 (50) | .47 |
| BMI (kg/m2) ≥ 85th percentile for age and sex [10] | .61 | |||
| BMI unavailable or <2 years old | 10 (31) | 2 (13) | 8 (50) | |
| Yes | 17 (53) | 10 (63) | 7 (44) | |
| No | 5 (16) | 4 (25) | 1 (66) | |
| Immunocompromised b | 4 (13) | 1 (6) | 3 (19) | .60 |
| Admitted | 30 (94) | 16 (100) | 14 (88) | .48 |
| PICU admission | 17 (53) | 12 (75) | 5 (31) | .032 |
| Oxygen Requirement | 9 (28) | 5 (31) | 4 (25) | 1.00 |
| Intubated | 1 (6) | 1 (6) | 0 (0) | 1.00 |
| Vasopressors | 6 (19) | 6 (38) | 0 (0) | .018 |
| Treatment c | 14 (44) | 13 (81) | 1 (6) | <.001 |
| IVIG | 11 (34) | 11 (69) | 0 (0) | |
| Steroids | 6 (19) | 6 (38) | 0 (0) | |
| Remdesivir | 2 (6) | 2 (13) | 0 (0) | |
| Convalescent plasma | 1 (6) | 0 (0) | 1 (6) | |
| Echocardiogram | <.001 | |||
| Abnormal | 9 (28) | 9 (56) | 0 (0) | |
| Normal | 6 (19) | 4 (25) | 2 (12) | |
| Not Done | 17 (53) | 3 (19) | 14 (88%) | |
| Median length of stay, days (IQR) | 5.5 (3.0–7.8) | 6.0 (3.0–8.3) | 5 .0 (3.3–6.8) | .835 |
| Re-Admitted within 30 days | 3 (9) | 0 (0) | 3 (19) | .23 |
| Death | 1 (6) | 1 (6) | 0 (0) | 1.00 |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; IQR, interquartile range; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children; NAT, nucleic acid test; PICU, pediatric intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aChronic conditions include the following (patients may have had more than 1 condition): participants with MIS-C: chromosomal/genetic syndromes (N = 3); developmental delay (N = 2); cerebral palsy (N = 1); chronic lung disease of prematurity (N = 1); history of prematurity (N = 1); asthma (N = 1). Participants with acute COVID-19: chromosomal/genetic syndromes (N = 2); congenital heart disease (N = 2); chronic lung disease (N = 1); prematurity (N = 2); gastroesophageal reflux disease (N = 2); sickle cell disease (N = 2); asthma (N = 1); type 2 diabetes mellitus (N = 1).
bImmunocompromised conditions include the following: participants without MIS-C: myelodysplastic syndrome (N = 1); hematopoietic stem cell transplantation (N = 1); liver transplant recipient (N = 1).
cPatients may have received more than 1 treatment.
Demographic, Clinical, and Laboratory Features by Disease Status
Fifty percent (N = 16) met CDC criteria for MIS-C (Supplemental Figure 1). The median ages were 8.72 years (IQR, 5.45–13.93 years; range, 3 months–17.5 years) and 2.24 years (IQR, 1.09–10.5 years; range, 13 days–20 years) for children with MIS-C and acute COVID-19, respectively (P = .175). A higher proportion (63%) of children with MIS-C were Hispanic compared with those without (37.5%). Fifty-six percent of children with MIS-C were male. Body mass index percentile was not significantly different between children with and without MIS-C. Children with MIS-C were significantly more likely to present with gastrointestinal (88% versus 44%, P = .023), mucocutaneous (63% versus 13%, P = .009), and musculoskeletal symptoms (31% versus 0%, P = .043) compared with those acute COVID-19. The median duration of symptoms before presentation was similar in both groups (3 versus 4 days, P = .494) as was the median length of stay (6 versus 5 days, P = .835) (Table 1 and Supplemental Table 2). This finding was the same even after adjusting for age and sex, although the median length of stay for those with MIS-C and acute COVID-19 was significantly longer than the median length of stay for those with unconfirmed SARS-CoV-2 infection (3 days; P = .004 and P = .003, respectively) (Supplemental Figure 2).
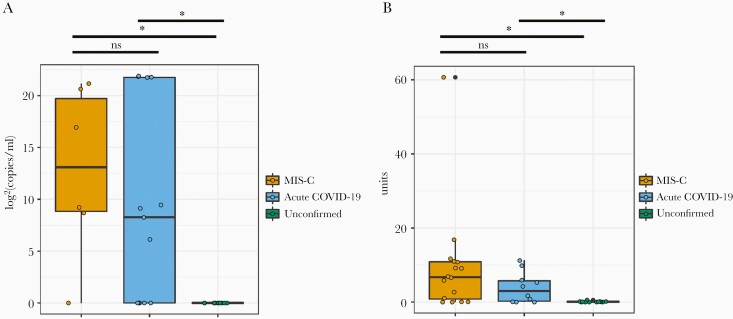
Nucleic acid test was positive in 50% of children with MIS-C and 75% of those with acute COVID-19 (P = .27). The median viral load in the children with MIS-C was 63 848.25 copies/mL (IQR, 461.38 to >1 254 000; range, <1.25 to >1 254 000) and 307.1 copies/mL (IQR, <1.25 to >1 254 000; range, <1.25 to >1 254 000) in those with acute COVID-19 (P = .66) (Figure 1A). More MIS-C patients were seropositive for SARS-CoV-2 when compared with those with acute COVID-19 (75% versus 44%, P = .026) (Figure 1B).
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nasopharyngeal viral ribonucleic acid (RNA) copy number and antibody status in children with acute coronavirus disease 2019 (COVID-19), with multisystem inflammatory syndrome in children (MIS-C), and those without confirmed SARS-CoV-2 infection. Statistically significant values are denoted with a star (*). The dark line indicates the median. (A) Nasopharyngeal SARS-CoV-2 viral RNA [log2(copies/mL)] to N1 of SARS CoV-2 in children with MIS-C (N = 6), with acute COVID-19 (N = 13), and unconfirmed (N = 11). Similar values were seen for N2 (data not shown). Values at zero denote those that are below the limit of quantitation (<1.25 copies/mL) by ddPCR. (B) Plasma immunoglobulin G concentrations (absorbance units) in children with MIS-C (N = 15), with acute COVID-19 (N = 10), and unconfirmed (N = 12). Values at zero denote those that are below the limit of detection (<1.23 absorbance units) of the EUROIMMUN ELISA.
Overall, MIS-C patients had higher acuity than those with acute COVID-19 (Table 1). Fifty-six percent (N = 9 of 13) of MIS-C patients had abnormal echocardiograms showing the following: decreased left ventricular function/ventricular strain (N = 5); coronary artery dilatation (N = 4); and one child with coronary artery dilatation also had a pericardial effusion. Two children without MIS-C received echocardiograms, which were normal. One participant, a female adolescent, succumbed to MIS-C after 5 days of hospitalization from cardiogenic shock. This participant, who was previously reported, was 15 years old and presented early in the pandemic in May 2020, with a 1-week history of worsening epigastric pain without nausea or diarrhea. She progressively developed worsening hypovolemic shock and left ventricular dysfunction requiring vasopressors. An autopsy revealed diffuse lymphoplasmacytic inflammatory infiltrate in the septum of the heart and inflammation of the small arterioles of the heart. There was no evidence of inflammation in the lungs. Two NATs for SARS-CoV-2 were negative at presentation, but a third NAT on hospital day 5 was positive, and replication-competent virus was recovered in a research laboratory. Her SARS-CoV-2 serology was reported on hospital day 5 as positive.
Laboratory Findings
Laboratory tests on admission are summarized in Supplemental Table 3. As in other studies, the children with MIS-C had significantly lower absolute lymphocyte counts ([ALC] median, 770; IQR, 395–1645), platelet counts (median, 171K; IQR, 85–253K), and significantly higher C-reactive protein (CRP) levels on admission than those without (12.2 versus 0.95 mg/dL, P = .001). Participants who presented with an ALC <1500 or platelet count <250K were more likely to have MIS-C (odds ratio [OR] = 9.53, 95% CI = 2.54–41.56 and OR = 3.8, 95% CI = 1.08–14.88, respectively). In our study, cardiac biomarkers, such as troponin and pro-brain natriuretic protein, did not differ significantly between the 2 groups.
Cytokine/Chemokine Profiles and Associations, After Adjusting for Age and Sex
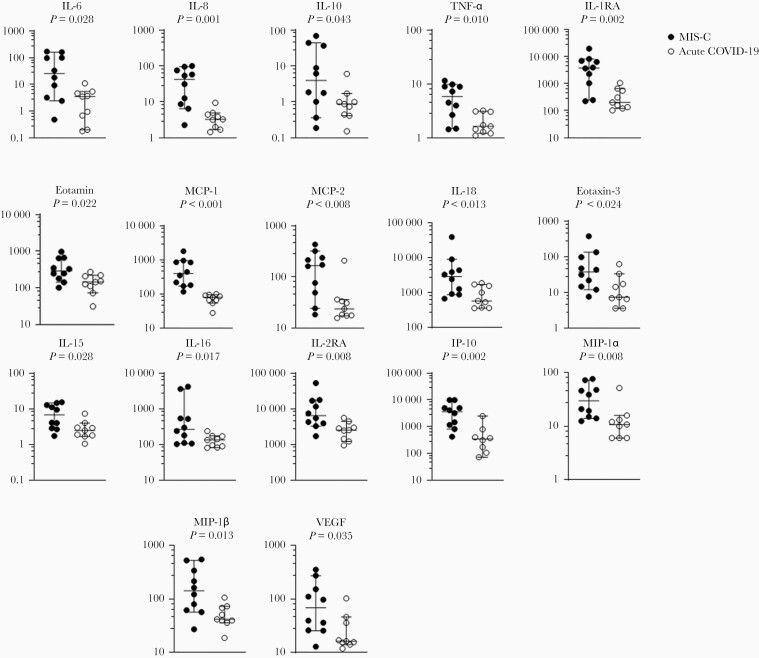
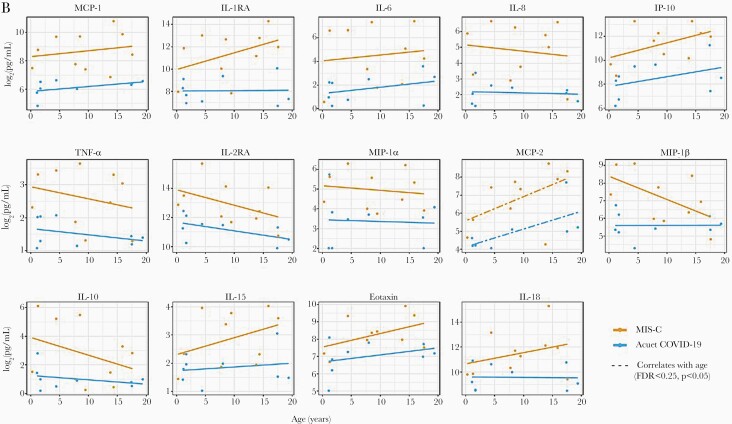
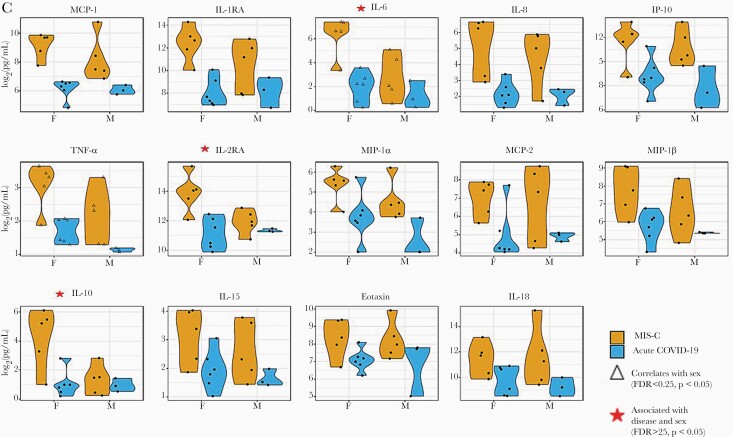
Of the 37 cytokines and chemokines measured, 10 cytokines (IL-10, IL-15, IL-16, IL-18, IL-1RA, IL-2RA, IL-6, IL-8, TNF-α, VEGF) and 7 chemokines (eotaxin, eotaxin-3, IP-10, MCP-1, MCP-2, MIP-1α, MIP-1β) were significantly higher (P < .05) in children with MIS-C compared with those with acute COVID-19 (Figure 2A, Supplemental Table 4). After adjusting for age and sex, 8 of the cytokines (IL-1RA, IL-2RA, IL-6, IL-8, TNF-α, IL-10, IL-15, IL-18) and 6 of the chemokines (MCP-1, IP-10, MIP-1α, MCP-2, MIP-1β, eotaxin) were significantly elevated in MIS-C patients (FDR <0.05, P < .05). No significant differences in cytokine levels were observed with age and disease status (FDR >0.25, P > .05) (Figure 2B) (Supplemental Table 5). Regardless of disease status, MCP-2 was found to be positively correlated with age (FDR <0.25, P < .05). Independent of disease status, females with SARS-CoV-2 infection had significantly higher levels of IL-6 and TNF-α compared with males (FDR <0.25, P < .05). Levels of IL-6, IL-10, and IL-2RA also tended to be higher in females compared with males with MIS-C (FDR >0.25, P < .05) (Figure 2C, Supplemental Table 5); however, no significant differences in cytokines were observed when sex and disease status were combined (FDR >0.25, P > .05). Comparisons between the participants with confirmed SARS-CoV-2 infection to those unconfirmed are summarized in Supplemental Figure 3 and Supplemental Table 6.
Figure 2.
(A–C) Cytokine differences between those with acute coronavirus disease 2019 (COVID-19) and those with multisystem inflammatory syndrome in children (MIS-C). (A) Seventeen statistically significant cytokine/chemokine differences between children with acute COVID-19 and with MIS-C. Testing was performed on admitted pediatric patients with MIS-C (N = 10) and with acute COVID-19 (N = 9). Median days to sample collection was 1 day after admission in both groups (P = .898). All values are picogram per milliliter. (B and C) Correlations in cytokine/chemokine profiles with MIS-C status and associations with age and sex. All fourteen 14 cytokines/chemokines displayed had statistically significant correlation with MIS-C status, independent of age or sex (false discovery rate [FDR] <0.05, P < .05). (B) Cytokines/chemokines with significant correlation with age, independent of disease status, are denoted by a dashed line (FDR <0.25, P < .05). (C) Cytokines/chemokines with significant correlation with sex, independent of disease status, are denoted by an open triangle (∆) (FDR <0.25, P < .05). Cytokines/chemokines which showed a notable association with disease status and sex, together, are denoted with a red star ( ) (FDR > 0.25, P < .05).
) (FDR > 0.25, P < .05).
Discussion
In this prospective cohort study established early in the US pandemic among children hospitalized with COVID-19 at a single urban center, we evaluated the presence of viral RNA as well as cytokine/chemokine profiles in pediatric patients infected with SARS-CoV-2, comparing acute COVID-19 with MIS-C, including those with clinical signs and symptoms of acute COVID-19 but with unconfirmed SARS-CoV-2 infection. Our cohort of children with MIS-C had significantly higher odds of lymphopenia (10-fold) and thrombocytopenia (4-fold) and higher levels of CRP, confirming appropriate disease stratification of the participants during the early phases of the pandemic in the United States. We found similar levels of SARS-CoV-2 RNA in the nasopharynx at presentation among those with acute COVID-19 and those with MIS-C despite significant differences in the cytokine/chemokine levels of these patients, and seropositivity status, after adjusting for age and sex. Some MIS-C participants had levels of nasopharyngeal viral RNA above the upper limit of the assay (>1 254 000 copies/mL) at presentation.
Given the age- and sex-related differences in immune responses to viral infections in children, cytokine and chemokine differences were also examined after adjusting for disease status. Except for MCP-2, which showed a positive correlation with increasing age, no age-related differences were observed in this small cohort. Female sex was found to be associated with higher levels of IL-6 and TNF-α with SARS-CoV-2 infection. However, explorations of the associations between cytokine/chemokine elevations and the combined factors of either age and disease status or sex and disease status showed no significant association, and only trends in elevations of IL-6, IL-10, and IL-2RA were seen as a function of female sex and disease status, which would require studies in larger cohorts.
In general, MIS-C is considered a postinfectious process [12, 18–21], and indeed 75% of the children with MIS-C in our cohort were seropositive on admission compared with 44% of those with acute COVID-19. It is interesting to note that 50% of the children with MIS-C in our cohort had a positive NAT with viral loads similar to those with acute COVID-19, and the median copy number was higher in the MIS-C group. The current CDC definition of MIS-C includes SARS-COV-2 positivity by RT-PCR as criteria for diagnosis. In addition, previous studies and larger cohorts have evaluated the detection of viral RNA by NAT in patients with MIS-C and have noted as many as half or more of patients admitted with suspected or confirmed MIS-C in their cohorts, to have tested positive [8, 22]. However, few studies have looked at the viral load in these patients to quantify the viral RNA detected to aid with distinguishing low-level virus shedding from possible replicating virus. In one study of 18 patients with MIS-C, 2 (11%) had quantifiable viral RNA in the nasopharynx [23], indicating that a proportion of children with MIS-C can be identified with nucleic acid testing and can still have quantifiable levels of SARS-CoV-2 viral RNA present at the time of presentation. Although viral culture was not routinely performed in our study or the Yonker et al [23] study to assess infectiousness, studies have found correlations between cycle thresholds in real-time PCR-based NATs with recovery of replication competent virus [24, 25]. It is interesting to note that 1 patient in our cohort, who succumbed to MIS-C, did have replication competent virus recovered [26]. Inclusion of viral load quantitation and its correlation with replicating virus may inform the immunopathogenesis and management of MIS-C, as well as expand the possibility for consideration of antivirals and neutralizing antibodies in the acute management of MIS-C.
Regardless of age or sex, after evaluating an extensive panel of cytokines and chemokines, we observed 14 inflammatory cytokines (IL-1RA, IL-2RA, IL-6, IL-8, TNF-α, IL-10, IL-15, IL-18) and chemokines (MCP-1, IP-10, MIP-1α, MCP-2, MIP-1β, eotaxin) that were significantly elevated in correlation with MIS-C compared with acute COVID-19, despite similar duration of symptoms, length of hospital stay, and nasopharyngeal viral RNA levels, highlighting the unique host inflammatory responses responsible for MIS-C. Our findings of cytokine/chemokine dysregulation with MIS-C are consistent with previous reports [18, 22, 27–29]. In one study, 16 children with MIS-C, compared with acute COVID-19, had significantly higher levels of 7 cytokines (IL-8, IL-6, IFN-γ, IL-17A, IL-6, TNF-α, IP-10) [30]; and elevated levels of 6 cytokines (IL-1β, IL-6, IL-8, IL-10, IL-17, IFN-γ) were observed in a cohort of 25 children with MIS-C [13]. Likewise, Cheung et al [31] also reported, in 8 children with MIS-C, elevated levels of IL-2R, IL-18, and CXCL 9 levels and mildly increased IFN and IL-8 in less than half, but normal levels of TNF-α. Compared with these studies, ours included a broader panel of 37 cytokines/chemokines to compare between the 2 disease phenotypes (hospitalized children with acute COVID-19 and MIS-C), which was also used to study the pathobiology of severe SARS-CoV-2 infection in adults at the same urban care center [15]. In the adult study, IL-18, IL-6, and TNF-ɑ were significantly elevated in adults with severe COVID-19, highlighting similarities in innate immune response pathways in severe COVID-19 in both children and adults [15].
The 14 cytokines/chemokines we identified that distinguished between MIS-C and acute COVID-19 reflect innate immune system activation, particularly of the monocyte/macrophage lineage [32], which are important for generating the adaptive immune responses, especially against viral pathogens [33]. It is interesting to note that significant elevation of IL-6, TNF-ɑ, IP-10, MCP-1, MCP-2, MIP-1α, and MIP-1β seen in MIS-C patients is also reported in macrophage activation syndrome [34]. Together with the high SARS-CoV-2 RNA detected in the nasopharynx of patients with MIS-C, these findings warrant further exploration. Improved understanding of the host-specific cytokine/chemokine dysregulation implicated in the immunopathogenesis of SARS-CoV-2 infection may also aid in identifying targeted immunotherapeutic approaches as it has with KD [35]. For example, targeted anticytokine therapy has been used in the management of KD, with previous studies exploring anti-TNF-α for those at high risk for coronary artery aneurysm development, as well as current American Heart Association guidelines recommending infliximab or anakinra for intravenous immunoglobulin-resistant disease [6].
In regards to age and sex correlations, our small sample size revealed primarily exploratory findings. The proinflammatory chemokine MCP-2 was found to be positively correlated with age, irrespective of MIS-C status. Because this cytokine was also separately noted to be associated with disease status, irrespective of age or sex, it could raise the possibility of MCP-2 as an important biomarker of MIS-C. Monocyte chemoattractant protein-2 stimulates and attracts granulocytes and mononuclear cells, and it previously was studied as a biomarker for tuberculous disease where a simple enzyme-linked immunosorbent assay-based assay was used to accompany a clinically used IFN-γ assay (QuantiFERON TB Gold In-Tube) in efforts to increase sensitivity of detecting clinically severe patients [36]. Regarding sex, male adults with COVID-19 generally have more severe outcomes [37], although one study demonstrated that higher levels of innate immune-mediated cytokines (IL-8 and IL-18) in women can be associated with more severe disease progression [38]. We noted higher levels of IL-6 and TNF-α in females compared with males with SARS-CoV-2 infection, independent of disease status, and higher levels of IL-6, IL-10, and IL-2RA in females with MIS-C compared with males, although these findings are limited by the small sample size. Sex differences in cytokine responses to SARS-CoV-2 infection in children have not been reported and warrant further investigation in larger cohorts of children.
There are several limitations to this study. First, this was a single-center, observational cohort, and our data could be influenced by selection bias that reflects the demographic of our patient population, which is predominantly black and Hispanic. It should be noted, however, that Baltimore’s population primarily consists of individuals who identify as black (63%), and those identifying as Hispanic account for only ~6% of the population [39]. It remains unclear why our cohort was so disproportionately Hispanic, but national reports share similar findings of higher prevalence of MIS-C in Hispanic children [40], and further studies investigating the disparities in this disease are necessary [41]. Additional limitations include the small sample size due to the relatively mild disease course of SARS-CoV-2 infection in children, rarity of MIS-C, and stringent SARS-CoV-2 diagnostic criteria [42] to eliminate confounders, thus excluding 21 suspected but unconfirmed cases in our cohort. In these 21 children presenting with an unspecified viral infection, cytokine dysregulation was also not observed, highlighting the unique host response to SARS-CoV-2 in the subset of children who develop MIS-C associated with high risk for cardiovascular compromise and currently unknown long-term sequelae.
Conclusions
In conclusion, our prospective cohort study in which participants were sequentially enrolled in real-time during the pandemic further characterizes the unique cytokine/chemokine profiles of children with MIS-C compared with acute COVID-19, irrespective of age or sex, along with information on nasopharyngeal viral RNA levels in MIS-C. In addition, potential sex differences in cytokines with SARS-CoV-2 infection were observed, and these, taken together, add to the understanding of this severe clinical phenotype arising from SARS-CoV-2 infection in children [8, 18, 22, 26, 28] with possible implications on approaches to diagnosis and management.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the children and their families for their contribution to this study, the participant recruitment and follow-up team (Thuy Anderson, Aleisha-Collinson-Streng, RN, Bonnie Addison) and JHU ReVision (Elizabeth Partan and Ashley Curran). We also acknowledge Joseph Szewczyk, Ya Hui Chen, and Adit Dhummakupt for their assistance with the quantitative droplet digital PCR assay. We thank Johns Hopkins University, Division of Pediatric Infectious Diseases, Johns Hopkins Children’s Center and Pediatric Emergency Department and Johns Hopkins University for their financial support of this study.
Financial support. This work is funded by the National Institutes of Health (NIH) (Grant Number NIH5T32AI052071; to N. P. A. and J. H. R.); NIH Grant Numbers 5T32HD044355-17 (to T. K.), R01HG009518 (to W. Z. and H. J.), UM1AI0686, UM1 AI068613-14, R01 DA045556-04S1, and 3U54HL143541-02S2 (to H. M.); The Bauernschmidt Committee of the Eudowood Board at Johns Hopkins School of Medicine (to N. P. A.); The Johns Hopkins University Center for AIDS Research (Grant Number P30AI094189; to D. P.); the International Maternal Pediatric Adolescent Clinical Trials Laboratory Center (Grant Number UM1AI106716; to D. P.); the Bill & Melinda Gates Foundation (Grant Number 134582; to A. L. C.); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant Number K12-HD000850; to J. H. R.); and also supported by a Johns Hopkins University Provost Research Grant.
Potential conflicts of interest. D. P. has served as a Scientific Advisor for Merck & Co. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Novel Coronavirus (2019-nCoV) SITUATION REPORT - 1. 2020:1–5. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed 13 October 2020.
- 3.Johns Hopkins University and The Johns Hopkins Coronavirus Resource Center (CRC). Available at: https://coronavirus.jhu.edu/map.html. Accessed 4 October 2020.
- 4.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020; 145:e20200702.32179660 [Google Scholar]
- 5.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team . SARS-CoV-2 infection in children. N Engl J Med 2020; 382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–99. [DOI] [PubMed] [Google Scholar]
- 7.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020; 79:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome (MIS-C). Available at: https://www.cdc.gov/mis-c/cases/index.html. Accessed 5 October 2020.
- 11.Morris SBS N, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators . Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 2020; 26:1701–7. [DOI] [PubMed] [Google Scholar]
- 14.Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team . The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183:968–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaba AH, Zhou W, Hsieh LL, et al. Differential cytokine signatures of SARS-CoV-2 and influenza infection highlight key differences in pathobiology.Clin Infect Dis 2021; ciab376. doi: 10.1093/cid/ciab376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caturegli G, Materi J, Howard BM, Caturegli P. Clinical validity of serum antibodies to SARS-CoV-2: a case-control study. Ann Intern Med 2020; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statis Soc B 1995; 57:289–300. [Google Scholar]
- 18.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahase E. Covid-19: cases of inflammatory syndrome in children surge after urgent alert. BMJ 2020; 369:m1990. [DOI] [PubMed] [Google Scholar]
- 20.Alexandre Belot DL-B. Multisystem inflammatory syndrome in children in the United States. N Engl J Med 2020. doi: 10.1056/NEJMc2026136 [DOI] [PubMed] [Google Scholar]
- 21.Okba NMA, Muller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis 2020; 26:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team . Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonker LM, Neilan AM, Bartsch Y, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr 2020; 227:P45–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis 2020; 39:1059–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis 2020; 71:2663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaudry JT, Dietrick B, Lammert DB, et al. Fatal SARS-CoV-2 inflammatory syndrome and myocarditis in an adolescent: a case report. Pediatr Infect Dis J 2021; 40:e72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142:429–36. [DOI] [PubMed] [Google Scholar]
- 28.Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest 2020; 130:5967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeBiasi RL, Song X, Delaney M, et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J Pediatr 2020; 223:199–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce CA, Preston-Hurlburt P, Dai Y, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med 2020; 12:eabd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020; 324:294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera PY, Lichy JH, Waldmann TA, Perera LP. The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect 2012; 14:247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014; 5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eloseily EM, Cron RQ. Macrophage activation syndrome. Microbiome in Rheumatic Diseases and Infection. In: Ragab G, Atkinson T, Stoll M, eds. The Microbiome in Rheumatic Diseases and Infection. Cham: Springer; 2018:151–82. [Google Scholar]
- 35.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol 2020; 72:1791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruhwald M, Bodmer T, Maier C, et al. ; TBNET . Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J 2008; 32:1607–15. [DOI] [PubMed] [Google Scholar]
- 37.Meng Y, Wu P, Lu W, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog 2020; 16:e1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; 588:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United States Census Bureau. Quick Facts; Baltimore City, Maryland; USA. Available at: https://www.census.gov/quickfacts/geo/chart/baltimorecitymarylandcounty/AGE295219. Accessed 4 October 2020.
- 40.Godfred-Cato S, Bryant B, Leung J, et al. ; California MIS-C Response Team . COVID-19-associated multisystem inflammatory syndrome in Children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page KR, Flores-Miller A. Lessons we’ve learned — covid-19 and the undocumented Latinx community. N Engl J Med 2020. doi: 10.1056/NEJMp2024897 [DOI] [PubMed] [Google Scholar]
- 42.Levin M. Childhood multisystem inflammatory syndrome — a new challenge in the pandemic. N Engl J Med 2020; 383:393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.