Abstract
Practice guidelines for adults with obsessive-compulsive disorder (OCD) recommend augmenting serotonin reuptake inhibitors (SRIs) with exposure and ritual prevention (EX/RP). However, fewer than half of patients remit after a standard 17-session EX/RP course. We studied whether extending the course increased overall remission rates and which patient factors predicted remission. Participants were 137 adults with clinically significant OCD (Yale-Brown Obsessive Compulsive Scale [Y-BOCS] score ≥18) despite an adequate SRI trial (≥12 weeks). Continuing their SRI, patients received 17 sessions of twice-weekly EX/RP (standard course). Patients who did not remit (Y-BOCS ≤12) received up to 8 additional sessions (extended course). Of 137 entrants, 123 completed treatment: 49 (35.8%) remitted with the standard course and another 46 (33.6%) with the extended course. Poorer patient homework adherence, more Obsessive-Compulsive Personality Disorder (OCPD) traits, and the Brain-Derived Neurotrophic Factor (BDNF) Val66MET genotype were associated with lower odds of standard course remission. Only homework adherence differentiated non-remitters from extended course remitters. Extending the EX/RP course from 17 to 25 sessions enabled many (69.3%) OCD patients on SRIs to achieve remission. Although behavioral (patient homework adherence), psychological (OCPD traits), and biological (BDNF genotype) factors influenced odds of EX/RP remission, homework adherence was the most potent patient factor overall.
Keywords: Obsessive-compulsive disorder, OCD, exposure and ritual prevention, EX/RP, cognitive-behavioral therapy, CBT
INTRODUCTION
Cognitive-behavioral therapy (CBT) consisting of exposure and ritual prevention (EX/RP) is an established treatment for obsessive-compulsive disorder (OCD), both as monotherapy (Foa et al., 2005) and combined with serotonin reuptake inhibitors (SRIs) (Simpson et al., 2013, 2008). In randomized trials (Simpson et al., 2008; Simpson, Foa, et al., 2013; Simpson, Huppert, Petkova, Foa, & Liebowitz, 2006), fewer than half of patients who receive the 17-session EX/RP course achieve remission (defined as a Yale-Brown Obsessive Compulsive Scale [Y-BOCS; Goodman, Price, Rasmussen, Mazure, Delgado, et al., 1989; Goodman, Price, Rasmussen, Mazure, Fleischmann, et al., 1989] score ≤12 [Mataix-Cols et al., 2016; Simpson et al., 2006]). In clinical practice, clinicians often extend therapy when an initial course does not suffice, but little research has systematically evaluated the effects of continuing EX/RP (Foa et al., 2013; Foa et al., 2015). This paper presents data from a clinical trial that examined how extending the course of EX/RP affected overall remission rates in medicated adults with OCD, and which psychological, biological, and behavioral factors were associated with remission from a standard or extended EX/RP course.
The notion that individuals differ in the dose of EX/RP they need to achieve remission accords with a precision medicine approach, which seeks to provide each patient the right treatment at the right dose based on individual variables (Collins & Varmus, 2015). Developing a precision medicine approach for medicated patients with OCD requires identifying individual patient characteristics that define who benefits most from adding EX/RP to SRIs as well as how much (therapeutic dose) EX/RP they require. Identifying the range in EX/RP dose required for remission as well as which patient characteristics strongly predict remission is a first step.
Prior studies have investigated many factors that might predict EX/RP outcome (Knopp, Knowles, Bee, Lovell, & Bower, 2013; Maher et al., 2010, 2014; Olatunji, Davis, Powers, & Smits, 2013; Öst, Havnen, Hansen, & Kvale, 2015; Steketee, Siev, Yovel, Lit, & Wilhelm, 2019; Wheaton, Rosenfield, Foa, & Simpson, 2015), including: demographic characteristics; OCD severity; dysfunctional beliefs; degree of insight; comorbidity; treatment history; and functioning/quality of life. Some factors show small effects, but there is limited replication across studies. One notable exception has been reported: patient adherence to between-session homework strongly predicted EX/RP acute outcome in two trials (Simpson et al., 2011; Wheaton et al., 2016) as well as outcome six months later (Simpson, Marcus, Zuckoff, Franklin, & Foa, 2012).
Recent data suggest other factors may also influence EX/RP outcome. Comorbid obsessive-compulsive personality disorder (OCPD) predicted worse EX/RP outcome in one study of 49 patients with OCD (Pinto, Liebowitz, Foa, & Simpson, 2011). Change in metacognitions was associated with EX/RP response in another study of 83 OCD patients (Solem, Håland, Vogel, Hansen, & Wells, 2009; Wells, Myers, Simons, & Fisher, 2017), raising the question of whether severity of metacognitions predicts EX/RP outcome. Finally, several gene variants have been associated with abnormal fear processing in laboratory studies and with poorer outcomes in exposure-based CBT (like EX/RP). These include: 1) a polymorphism in human brain-derived neurotrophic factor (BDNF) gene rs6265 (a valine [Val] to methionine [Met] substitution [Val66Met]), linked in rodents and healthy humans to poorer extinction learning (Soliman et al., 2010) and to poorer EX/RP outcomes in OCD (Fullana et al., 2012); 2) a COMT gene rs4680 polymorphism (a Val to Met substitution), linked to extinction learning deficits in healthy humans (Lonsdorf et al., 2009) and poorer CBT outcome in panic disorder (Lonsdorf et al., 2010); and 3) the 14-repeat short (S) variant in the promoter region of the serotonin transporter (5-HTT), which yields less transcriptional activity and lower serotonin uptake than the 16-repeat long (L) variant (5-HTTLPR), correlated with increased amygdala reactivity to fearful and angry faces in healthy humans (Munafò, Brown, & Hariri, 2008), and poorer CBT outcome in PTSD (Pierce et al., 2010). If these factors strongly predicted EX/RP outcome in OCD, the data could support testing a precision medicine approach to selecting particular treatments. No prior study has examined patient EX/RP adherence, the most potent predictor found to date, and these other factors within the same sample. Moreover, few studies have focused on predictors of remission, despite the fact that this is the outcome associated with good functioning and quality of life (Farris, McLean, Van Meter, Simpson, & Foa, 2013).
To address this gap, we used data from a clinical trial funded by the National Institute of Mental Health (NIMH) that recruited 137 adults with OCD who had received an adequate SRI trial but who still had clinically significant OCD symptoms. As the preparatory phase to a double-blind discontinuation trial, patients were continued on their SRI at a stable dose and provided a standard 17-session course of EX/RP; for patients who had not yet achieved remission (defined as a Y-BOCS ≤12 consistent with [Mataix-Cols et al., 2016; Simpson et al., 2006]), the therapist could extend this course for up to 8 more sessions. This design enabled us to examine whether extending the course of EX/RP improved overall remission rates, a clinically-important question since two prior studies indicated that time alone is not sufficient (Foa et al, 2013; Foa et al. 2015). It also enabled us to study which patient characteristics were associated with remission from the standard versus extended course. Based on the literature described above, we hypothesized that patient adherence to between-session homework, OCPD traits, and the BDNF polymorphism would be associated with EX/RP outcome; we also explored metacognitions and the COMT and 5-HTT variants.
METHODS
Overview
This study, registered at clinicaltrials.gov (Identifier: NCT01686087), took place at two outpatient research clinics: The Center for OCD and Related Disorders in New York, NY and the Center for the Treatment and Study of Anxiety (CTSA) in Philadelphia, PA. It examined in a double-blind discontinuation trial whether adults with OCD on SRIs could discontinue their medication after successful EX/RP augmentation. This paper presents results from the preparatory phase, in which participants who still had clinically-significant symptoms despite an adequate SRI trial received up to 25 sessions of EX/RP. Each site’s institutional review board (IRB) reviewed and approved the study. Patients were recruited via clinician referrals, community flyers, and a study-specific website. All patients provided written informed consent before entering the study.
Participants
Eligible patients were adults (aged 18–75 years) with a principal diagnosis of OCD (≥1 year) who had received an adequately dosed (≥12 weeks) SRI but remained at least moderately symptomatic (Y-BOCS score ≥ 18 [Goodman, Price, Rasmussen, Mazure, Delgado, et al., 1989; Goodman, Price, Rasmussen, Mazure, Fleischmann, et al., 1989]). Adequate SRI dose was defined as: clomipramine ≥225 mg/d; fluoxetine ≥60 mg/d; paroxetine ≥60 mg/d; sertraline ≥200 mg/d; fluvoxamine ≥250 mg/d; citalopram ≥40 mg/d; and escitalopram ≥30 mg/d. Patients constrained by intolerable side effects to lower doses were also eligible; mean SRI doses appear in Table 1. Benzodiazepines (e.g., for sleep) and stimulants (for prior diagnosis of attention deficit hyperactivity disorder) were permitted as long as they were taken routinely.
Table 1:
Demographic and Clinical Characteristics of the Study Sample
| All (n = 137) | NY (n = 73) | PENN (n = 64) | |
|---|---|---|---|
| Demographics | |||
| Age (in years), mean (SD) | 31.8 (11.6) | 33.8 (13.1) | 29.5 (9.4) |
| Female, No. (%) | 70 (51.1) | 39 (53.4) | 31 (48.4) |
| Race, White, No. (%) | 114 (83.2) | 64 (87.7) | 50 (78.1) |
| Ethnicity, Hispanic, No. (%) | 10 (7.3) | 4 (5.5) | 6 (9.4) |
| Education, y, mean (SD) | 15.9 (2.2) | 15.6 (2.3) | 16.1 (2.0) |
| Marital status, No. (%) | |||
| Single | 96 (70.1) | 42 (57.5) | 54 (84.4) |
| Married/partnered | 36 (26.3) | 28 (38.4) | 8 (12.5) |
| Divorced/separated | 5 (3.6) | 3 (4.1) | 2 (3.1) |
| Employment, No. (%) | |||
| Employed (full or part-time) | 67 (48.9) | 39 (53.4) | 28 (43.8) |
| Student at least part-time enrollment | 36 (26.3) | 18 (24.7) | 18 (28.1) |
| Other | 34 (24.8) | 16 (21.9) | 18 (28.1) |
| Clinical Characteristics | |||
| Yale-Brown Obsessive-Compulsive Scale, mean (SD) | 26.6 (3.5) | 26.0 (3.6) | 27.2 (3.3) |
| Hamilton Depression Rating Scale, mean (SD) | 7.0 (5.0) | 6.9 (4.7) | 7.1 (5.4) |
| Quality of Life Enjoyment and Satisfaction Questionnaire-SF mean of percent maximum (SD) | 57.6 (15.5) | 59.4 (13.9) | 55.6 (17.1) |
| Age at OCD onset, y, mean (SD) | 15.3 (8.6) | 16.2 (9.2) | 14.2 (7.8) |
| Duration of OCD, y, mean (SD) | 16.0 (11.3) | 17.1 (13.1) | 14.8 (8.6) |
| Current psychiatric diagnoses, No. (%) | |||
| OCD only | 89 (65.0) | 57 (78.1) | 32 (50.0) |
| Depressive Disorder | 23 (16.8) | 7 (9.6) | 16 (25.0) |
| Any anxiety disorder | 26 (19.0) | 10 (13.7) | 16 (25.0) |
| Other | 7 (5.1) | 3 (4.1) | 4 (6.3) |
| Treatment History | |||
| Current SRI dose, mg/d, mean (SD) | |||
| Citalopram hydrobromide (n = 10) | 37.0 (10.6) | 34.0 (8.9) | 40.0 (12.2) |
| Clomipramine hydrochloride (n = 4) | 150.0 (57.7) | 100.0 (0.0) | 166.7 (57.7) |
| Escitalopram oxalate (n = 25) | 27.8 (7.6) | 26.9 (6.0) | 29.4 (10.1) |
| Fluoxetine (n = 40) | 55.8 (17.2) | 56.7 (14.9) | 54.4 (20.6) |
| Fluvoxamine (n = 16) | 234.4 (79.0) | 264.3 (47.6) | 211.1 (92.8) |
| Paroxetine hydrochloride (n = 8) | 54.8 (14.5) | 60.0 (20.0) | 51.6 (11.5) |
| Sertraline hydrochloride (n = 34) | 158.1 (49.9) | 164.7 (43.4) | 151.5 (56.2) |
| Weeks receiving SRI dose, mean (SD) | 79.4 (156.1) | 52.7 (113.2) | 110.8 (191.0) |
| Participants receiving first SRI, No. (%) | 56 (40.9) | 29 (39.7) | 27 (42.2) |
| Current adjunctive psychiatric medication, No. (%) | |||
| SRI only | 106 (77.4) | 57 (78.1) | 49 (76.6) |
| Benzodiazepines | 19 (13.9) | 12 (16.4) | 7 (10.9) |
| Stimulants | 11 (8.0) | 3 (4.1) | 8 (12.5) |
| Other | 8 (5.8) | 2 (2.7) | 6 (9.4) |
| History of any prior EX/RP sessions, No. (%) | 30 (21.9) | 18 (24.7) | 12 (18.8) |
| History of prior EX/RP session while receiving SRI | 18 (13.1) | 11 (10.9) | 7 (10.9) |
Abbreviations. EX/RP = Exposure and Ritual Prevention; No=number; SD=standard deviation; SRI = Serotonin Reuptake Inhibitor; SF=Short form
Exclusion criteria included: (1) recurrent Major Depressive Disorder (MDD; ≥3 major depressive episodes [MDE]) and/or MDE with psychotic features;1 (2) history of bipolar or psychotic disorder; (3) substance use disorder (abuse or dependence) in the past 3 months; (4) suicidal ideation; (5) medical or neurological condition requiring immediate intervention; (6) pregnancy or nursing; or (7) prior course of EX/RP (≥8 sessions delivered within 2 months) in the past 5 years.
A psychiatric interview conducted by study clinicians determined eligibility. Trained raters using the Structured Clinical Interview for DSM-IV (SCID; [First, Spitzer, Gibbon, & Williams, 1995]) confirmed diagnoses; following publication of the DSM-5 in 2013, raters confirmed all participants met DSM-5 criteria for OCD as well. Treatment history was confirmed with the referring clinician and medical record when possible.
Study Treatments
Maintenance SRI:
Patients entered the study receiving a stable SRI dose. Their study psychiatrist, with whom the patient met every four weeks, maintained this dose throughout. The first visit lasted 60 minutes; subsequent visits were 30 minutes. During visits, the study psychiatrist confirmed current SRI dose, assessed side effects, but did not conduct psychotherapy including EX/RP. SRI blood levels were measured at baseline and at the end of EX/RP treatment to confirm SRI adherence (Foglia, Birder, & Perel, 1989; Øyehaug, Terje Østensen, & Salvesen, 1982; Suckow, Zhang, & Cooper, 1992).
EX/RP Augmentation:
Study therapists provided patients manualized EX/RP (Foa, Yadin, Lichner, 2012), comprising an initial course of two introductory sessions (focused on psychoeducation and exposure planning) followed by 15 exposure sessions and between-session phone check-ins. Sessions were 90 minutes long, delivered twice-weekly. Exposure sessions included therapist-aided exposure (in which patients faced their obsessional fears for prolonged periods without ritualizing and discussed dysfunctional cognitions within the context of exposure), daily homework (≥1 hour of self-directed exposures and instructions to stop ritualizing), and education about relapse prevention. Therapists made phone calls (each lasting <20 minutes) between each session (e.g., 16 calls with the standard 17 session protocol, 24 calls for those receiving 25 sessions) to review homework progress and offer encouragement and support.
All patients were provided 17 EX/RP sessions (2 introductory, 15 exposure) over 8 weeks, the standard course used in prior randomized controlled trials (Simpson et al., 2008; Simpson, Foa et al. 2013). Patients who reported symptom improvement but had not remitted after 17 sessions (defined a priori as Y-BOCS score ≤12 based on the literature [Mataix-Cols et al., 2016; Simpson et al., 2006]) were offered 4 additional sessions; those still not remitted were offered an additional 4 sessions, for a total maximum of 25 sessions. The rationale was twofold: 1) to mimic clinical practice (in which degree of response determines treatment length); and 2) to maximize the number of patients eligible to enter the double-blind SRI discontinuation trial.
Doctoral-level therapists (Ph.D. or Psy.D) with EX/RP experience delivered EX/RP. EX/RP experts led group supervision in weekly conference calls. Sessions were audiotaped for independent review by trained raters (Master’s Level or above) who had no contact with study patients. Therapist adherence to the EX/RP manual was assessed in 95 randomly-selected sessions: 91% of the manual-prescribed therapy elements (e.g., therapist designed an in vivo exposure that addressed the patient’s identified obsessions) and 98% of the prescribed therapist factors (e.g., therapist exhibited nonjudgmental and supportive attitude) were used.
Study Assessments
Clinical Symptoms:
Independent evaluators (IEs) having no other contact with study patients and blind to study aims and design used the Y-BOCS (Goodman, Price, Rasmussen, Mazure, Delgado, et al., 1989; Goodman, Price, Rasmussen, Mazure, Fleischmann, et al., 1989) and Hamilton Depression Rating Scale (HAM-D, 17-item version [Hamilton, 1960]) to assess OCD and depressive severity, respectively. Assessments occurred at week 0 (pre-EX/RP), week 4 (midway through the standard EX/RP protocol), week 8 (after the standard EX/RP protocol); and at weeks 10 and 12 for patients who received additional sessions. IE assessments were audiotaped, with random selections sent to the IE supervisor for review. IEs re-rated a random sample of tapes and ratings were discussed in group supervision during monthly conference calls. Intraclass correlations (ICCs) among IEs were high (Y-BOCS ICC=0.94; 95% CI=0.92,0.96).
Study patients completed self-report measures: the Pathological Obsessive-Compulsive Personality Scale (POPS; [Pinto, Ansell, & Wright, 2011; Sadri, McEvoy, Pinto, Anderson, & Egan, 2018]) to assess maladaptive OCPD traits, at baseline; the Metacognitions Questionnaire (MCQ-30; [Wells & Cartwright-Hatton, 2004]) to assess beliefs about cognitions and the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF, [Ritsner, Kurs, Gibel, Ratner, & Endicott, 2005; Wyrwich et al., 2009]), at each IE assessment.
Patient EX/RP Adherence:
Therapists rated patient adherence to EX/RP homework using the Patient EX/RP Adherence Scale (PEAS), a 3-item scale designed to assess the quantity of exposures attempted, the quality of exposures attempted, and the degree of ritual prevention achieved since the prior session (Simpson et al., 2011). Each item is rated on a scale from 1 to 7, with higher scores indicating better adherence; the three scores are then averaged. An average PEAS score of 5 is considered “good”, 6 “very good”, and 7 “excellent” adherence (Simpson et al., 2011). The therapist used the PEAS to evaluate homework adherence (session 4 onward) once having assigned homework (session 3 onward). The PEAS has good psychometric properties and predicts acute and 6-month EX/RP outcome (Simpson et al., 2011, 2012). Throughout the study, bi-monthly therapist group conference calls were conducted to review PEAS ratings. Therapists at both sites formally re-rated a random sample of 65 by listening to session audiotapes. Intraclass correlations (ICCs) among therapists were high (PEAS: ICC=0.86; 95% CI=0.80, 0.91). For patients who had ≥1 PEAS (n=132), model-estimated average PEAS scores were computed using a longitudinal linear mixed effects model (including patient-specific random intercepts and random slopes) that fit all available PEAS scores up to session 17 for each patient. This method was used in a prior study (Simpson et al., 2011) to allow for computing average PEAS over 17 sessions for all participants (including those with missing PEAS data) based on each person’s own individual PEAS trajectory.
Genotyping:
A blood sample was taken from all participants and sent to the NIMH Center for Collaborative Genetic Studies in Mental Disorders at Rutgers University (http://www.rucdr.org/). a repository that supports the acquisition of genetic samples in NIMH-funded studies. DNA samples derived from whole blood or lymphoblastoid cell lines were provided by the repository to the New York site for analysis. For the BDNF and COMT genes, Taqman 5’ exonuclease assays (Assay on Demand, Life Technologies) were used to genotype DNA samples at BDNF Val66Met (rs6265) and COMT Val158Met (rs4680) sites. Genomic DNA (10 ng) was transferred to a 384-well polymerase chain reaction (PCR) plate and mixed with primer mix (containing locus-specific primers and allele-specific fluorescently labeled probes (C_11592758_10 for rs6265 and C_25746809_50 for rs4680, respectively) and a 2x TaqPath ProAmp Master Mix (containing PCR enzyme, buffer, and nucleotides). Assays performed on a Thermo Fisher QuantStudio7 Real-Time PCR System used its standardized cycling protocols, with genotype call conducted using QuantStudio Software.
The serotonin transporter gene (SLC6A4), the 5-HTTLPR insertion/deletion (“long” and “short” respectively) was analyzed following published procedures (Contreras-Sesvold, Abraham, Devaney, Harmon, & Deuster, 2015). Genotyping was performed by polymerase chain reaction (PCR) using 25 ng genomic DNA, 300 nmoles of each primer, forward 5’ GGCGTTGCCGCTCTGAATGC 3’ and reverse 5’ GAGGGACTGAGCTGGACAACCAC 3’, and GoTaq master mix kit (Promega). Thermocycler (ABI 9700) conditions were initial denaturation at 95°C (5 min), followed by 40 cycles of 95°C (30 sec), 60°C (30 sec), and 72°C (40 sec), with a final elongation step at 72°C (7 min). All experiments used a positive control and “no template” negative control. This assay yielded amplicons of 485 bp for short allele, 528 bp for long allele (see Supplemental Material). Genotype was read manually.
Statistical Analyses:
Descriptive statistics characterized demographic and clinical characteristics of the sample and EX/RP predictors. To assess change in Y-BOCS from baseline to session 17, both observed and weighted change scores were computed. Weights for each subject’s change score were determined using inverse probability weighting based on baseline demographic and clinical factors to account for dropouts with missing session 17 data. Correlations between predictors were examined. A series of multinomial logistic regression models were utilized to examine which potential predictors were associated with remission (Y-BOCS ≤12). Outcome was categorized as 3 levels: 1) Y-BOCS ≤12 by session 17 (standard course); 2) Y-BOCS ≤12 by session 25 (extended course); and 3) never achieved Y-BOCS ≤12 (no-remission). Odds-ratios were computed for all possible remission outcome contrasts: extended course remission compared to standard course remission, no-remission compared to standard course remission, and no-remission compared to extended course remission. The first model included only baseline Y-BOCS. The second, third, and fourth models all included baseline Y-BOCS along with the following: patient homework adherence, measured by the model-estimated PEAS scores (model 2); clinical measures POPS and MCQ (model 3); or genetic measures (model 4) including BDNF, COMT, and SLC6A4 genotypes. The final model (model 5) included all predictors simultaneously. Area under the receiver operating characteristics curve (AUC) was reported as a measure of model fit. Sensitivity analyses were performed to assess whether additional clinical measures (depressive severity as measured by HAM-D and quality of life as measured by Q-LES-Q-SF) improved model fit.
Fourteen patients were excluded from the logistic regression models because they either received too few sessions to predict EX/RP outcome (dropped out prior to week 4) and/or had no Y-BOCS rating other than baseline. To examine the influence of missing data due to dropout on the logistic regression, we performed a sensitivity analysis, designating dropouts as non-remitters.
All analyses were run using SAS, version 9.4. Statistical tests were two-sided with a significance level of alpha=0.05. All continuous measures were standardized to allow interpretation of estimates in standard deviation units.
RESULTS
Sample
As Figure 1 shows, 475 patients were assessed for eligibility, 219 were eligible, and 137 signed consent and were offered the standard 17-session EX/RP protocol. Of 123 who completed the protocol, all but four received between 15 and 17 sessions by week 8, and 49 remitted. Non-remitters (n=74) were offered 4 additional sessions; those still not remitted (n=39) were offered an additional 4 sessions, for a maximum of 25 total sessions. Baseline demographic and clinical characteristics did not differ between those that received up to 4 additional sessions in comparison to those that received up to 8 additional sessions.
Figure 1: Consort Diagram.
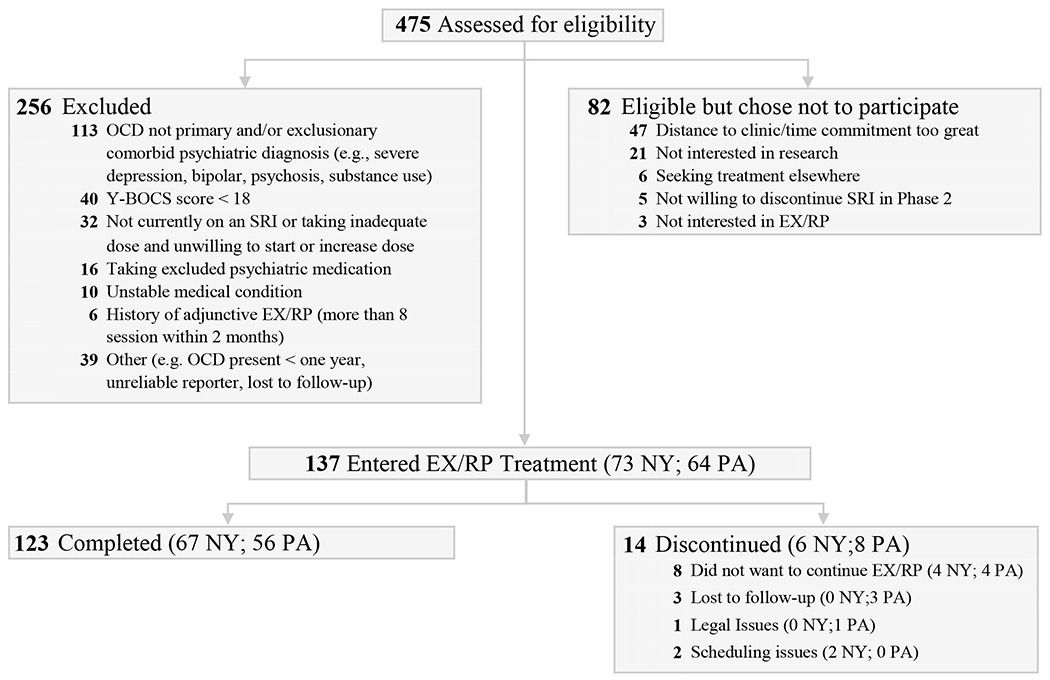
EX/RP = Exposure and Ritual Prevention; NY=New York site; OCD=Obsessive Compulsive Disorder; PA=Pennsylvania site; SRI = Serotonin Reuptake Inhibitor; Y-BOCS = Yale-Brown Obsessive Compulsive Scale
Table 1 presents demographic and clinical characteristics of the 137 patients who began EX/RP. On average, patients had had OCD for over 15 years. Despite receiving a stable SRI dose for ≥12 weeks (range 12-1128, mean weeks [SD]= 83.36 [157]), patients suffered from moderate to severe OCD symptoms (Y-BOCS mean [SD]= 26.6 [3.5]) and reported reduced quality of life (mean percent of maximum [SD]: 57.6 [15.5]); depressive severity, however, was low (7.0 [5.0]). The samples at each site were similar but one site enrolled more patients who identified as single and who were diagnosed with comorbid disorders.
Fourteen patients discontinued the study protocol (see Figure 1 for drop reasons), all but two by week 4. Baseline clinical measures did not significantly differ between the 123 patients who completed the study and the 14 who did not.
Effects of Standard versus Extended Dose of EX/RP: Descriptive Data
Patients receiving the standard 17-session EX/RP protocol showed large reductions in OCD severity measured by Y-BOCS change over time (observed mean [SD]: baseline Y-BOCS=26.6 [3.5], n=137; session 17 Y-BOCS=14.1 [5.7], n=123, mean change=−12.4 [6.1], Cohen’s d=3.5; inverse probability weighted mean session 17 Y-BOCS=14.4, weighted mean change=−11.7, Cohen’s d=3.3). After 17 EX/RP sessions, 49 (35.8%) achieved remission (Y-BOCS ≤12). Another 46 (33.6%) achieved remission with up to 8 additional sessions, based on lowest achieved Y-BOCS score. Twenty-eight (20.4%) who completed the study protocol did not achieve remission, despite receiving up to 25 sessions. Figure 2 presents observed mean Y-BOCS values at baseline and after the standard or extended course of the three remission groups (i.e., standard course remission, extended course remission, no-remission).
Figure 2: Observed OCD Severity Across Time by Remission Status.
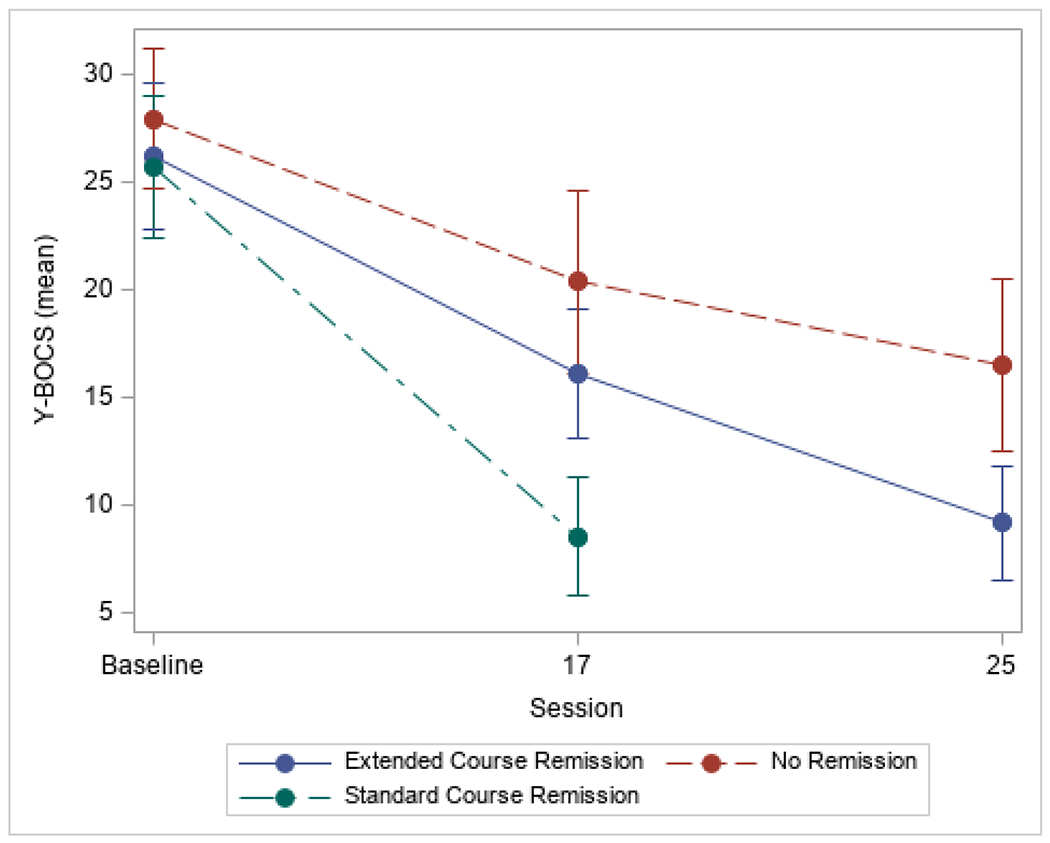
Observed OCD Severity (as measured by the Yale-Brown Obsessive Compulsive Scale [Y-BOCS]) is presented by remission status. Error bars are 1 standard deviation.
Hypothesized Predictor Variables: Descriptive Data
Table 2 shows potential outcome predictors for the entire sample, for those achieving remission by Session 17 (n=49, 35.8% of entrants) or by Session 25 (n=46, 33.6% of entrants), for those not achieving remission during the study protocol (n=28, 20.4% of entrants), and for study dropouts (n=14, 10.2% of entrants). Average patient adherence to EX/RP during the standard EX/RP course was good (mean [SD]= 5.3 [0.6]) but individuals varied (median=5.3, mean range=3.1 to 6.6). There were small but significant correlations between clinical measures: baseline Y-BOCS negatively correlated with PEAS (r =−0.21, p = 0.013), positively correlated with POPS (r = 0.20, p = 0.022), and positively correlated with MCQ (r= 0.18, p=0.040). Baseline POPS was not significantly correlated with PEAS (r=−0,12, p=0.169), The distribution of BDNF, COMT, and SLC6A4 genotypes was consistent with descriptions in the dbSNP database (build 151) for the general population (National Center for Biotechnology Information, n.d.).
Table 2:
Hypothesized Predictors of EX/RP Outcome
| By Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 137) | Standard Course Remission (n = 49; 35.8%) | Extended Course Remission (n = 46; 33.6%) | No Remission (n = 28; 20.4%) | Dropout (n = 14; 10.2%) | ||||||
| Measure | n | % or M (SD) | n | % or M (SD) | n | % or M (SD) | n | % or M (SD) | n | % or M (SD) |
| Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) | 137 | 26.6 (3.5) | 49 | 25.7 (3.3) | 46 | 26.2 (3.4) | 28 | 27.9 (3.3) | 14 | 28.1 (4.1) |
| Patient EX/RP Adherence Scale (PEAS) | 132 | 5.3 (0.6) | 49 | 5.7 (0.4) | 46 | 5.2 (0.5) | 28 | 4.8 (0.7) | 9 | 4.9 (0.6) |
| Pathological Obsessive-Compulsive Personality Scale (POPS) | 134 | 149.0 (35.8) | 47 | 138.1 (33.7) | 46 | 151.8 (32.7) | 28 | 166.7 (32.1) | 13 | 140.2 (47.2) |
| Metacognition Questionnaire (MCQ) | 135 | 72.5 (13.9) | 48 | 71.1 (11.8) | 46 | 74.9 (11.2) | 28 | 73.3 (18.1) | 13 | 67.2 (18.7) |
| Genetic Measures | ||||||||||
| BDNF | ||||||||||
| C/C (val/val) | 85 | 64.4 | 35 | 72.9 | 23 | 53.5 | 17 | 63.0 | 10 | 71.4 |
| C/T (val/met) | 41 | 31.1 | 8 | 16.7 | 19 | 44.2 | 10 | 37.0 | 4 | 28.6 |
| T/T (met/met) | 6 | 4.5 | 5 | 10.4 | 1 | 2.3 | 0 | 0.0 | 0 | 0.0 |
| COMT | ||||||||||
| A/A (met/met) | 32 | 24.2 | 13 | 27.1 | 11 | 25.6 | 5 | 18.5 | 3 | 21.4 |
| A/G (met/val) | 71 | 53.8 | 28 | 58.3 | 21 | 48.8 | 15 | 55.6 | 7 | 50.0 |
| G/G (val/val) | 29 | 22.0 | 7 | 14.6 | 11 | 25.6 | 7 | 25.9 | 4 | 28.6 |
| SLC6A4 | ||||||||||
| LL | 35 | 26.5 | 13 | 27.1 | 13 | 30.2 | 6 | 22.2 | 3 | 21.4 |
| LS | 54 | 40.9 | 22 | 45.8 | 15 | 34.9 | 7 | 25.9 | 10 | 71.4 |
| SS | 43 | 32.6 | 13 | 27.1 | 15 | 34.9 | 14 | 51.9 | 1 | 7.1 |
Abbreviations. EX/RP = Exposure and Ritual Prevention; No=number; SD=standard deviation; SRI = Serotonin Reuptake Inhibitor
Predicting Remission from Standard versus Extended Course
The multinomial logistic model that included all predictors simultaneously (model 5) had the best fit (AUC = 0.806, see Supplemental Table 1 for results from each model). As Figure 3 illustrates, patient homework adherence (on the PEAS), OCPD traits (on the POPS), and BDNF genotype each significantly estimated the course of EX/RP needed to achieve remission after a standard course. Specifically, compared to patients who remitted after the standard course, those remitting after the extended course had worse PEAS (OR = 0.26, 95% CI = [0.12,0.56], p < 0.001) and more BDNF Met allele (OR = 3.45, 95% CI = [1.14, 10.39], p = 0.028, C/T vs C/C genotype group). Additionally, compared to patients who remitted after the standard course, non-remitters had worse PEAS (OR=0.12, 95% CI = [0.05, 0.31], p < 0.001) and greater POPS (OR = 2.74, 95% CI = [1.27, 5.91], p = 0.010). Finally, compared to patients who remitted with the extended course, non-remitters with the extended course had worse PEAS (OR = 0.48, 95% CI = [0.25, 0.90], p = 0.022). Sensitivity analyses including the clinical measures HAM-D and QLESQ found neither measure to be significantly related to remission and led to only a minimal increase in model fit (AUC = 0.807). Sensitivity analyses including dropouts as non-remitters showed similar overall results (Supplemental Table 2).
Figure 3: Model-estimated Odds-ratios Predicting Remission Status after Standard or Extended Coursea,b.
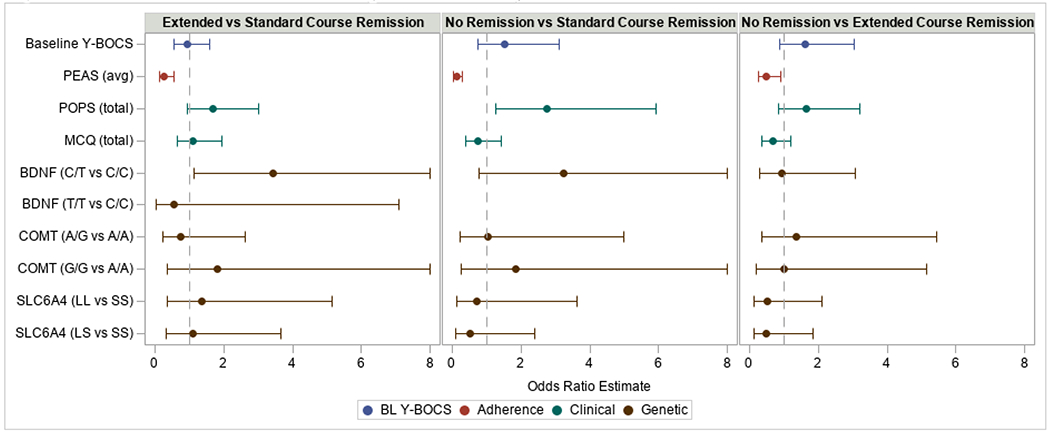
Multinomial logistic regression models examined which hypothesized predictors were associated with remission status. Odds-ratios were computed for all contrasts: extended course remission compared to standard course remission (left panel), no-remission compared to standard course remission (middle panel), and no-remission compared to extended course remission (right panel). As a result, odds-ratios greater than 1 indicate higher odds of extended course remission or no remission compared to those with standard course remission (left and middle panel, respectively) and higher odds of no remission compared to those with extended course remission (right panel) for every one standard deviation unit increase in the predictor. Hypothesized predictors included: baseline OCD severity (measured by the Yale-Brown Obsessive Compulsive Scale [Y-BOCS]), patient homework adherence (measured by the PEAS), psychological traits (measured by the POPS and MCQ), and specific genotypes (BDNF, COMT, SLC6A4). See text for details.
a Values greater than 8 are not shown. b The BDNF T/T vs C/C contrast is not estimated in the no-remission versus standard course and extended course remission comparisons because no subjects in the no-remission group had the T/T genotype. This contrast is estimated in the extended course versus standard course remission comparison, though only one subject in the extended course remission group had the T/T genotype.
DISCUSSION
This paper examined whether extending the standard course of EX/RP improves overall remission rates in medicated adults with OCD and which psychological, biological, and behavioral factors are associated with the odds of remission from a standard or extended course. There were three main findings. First, after a standard 17-session EX/RP course, 36% of medicated patients remitted (Y-BOCS≤12), consistent with data from two prior randomized trials that also recruited adults with OCD on SRIs and used the same EX/RP protocol (Simpson, Foa et al., 2013; Simpson et al. 2008). However, an additional 33.8% achieved remission with up to 8 additional EX/RP sessions, resulting in a total remission rate of 69.3%. Second, patient homework adherence, and the BDNF gene Val66Met polymorphism were significantly associated with the odds of remitting from a standard versus an extended EX/RP course, and patient homework adherence and OCPD traits were significantly associated with the odds of remitting from a standard EX/RP course versus non-remitting. Finally, the only factor that differentiated non-remitters and remitters after an extended EX/RP course was patient EX/RP homework adherence.
In clinical practice, clinicians frequently extend therapy for patients who do not achieve good outcomes after an initial treatment course, despite little systematic research testing the benefits of this. Meta-analyses of CBT variants (typically ranging from 8-21 sessions) that include EX/RP OCD protocols have not found protocol duration to be associated with treatment effect size (Olatunji et al., 2013; Öst et al., 2015). The data here therefore yield important new results demonstrating that extending EX/RP from 17 to 25 sessions, when needed, can achieve up to 69.3% remission. Given that longitudinal follow-up of the standard 17 session protocol find that patients typically plateau after treatment has concluded (Foa et al., 2013; Foa et al., 2015), this finding suggests that adding additional EX/RP sessions may enable many more patients to achieve remission. Moreover, we showed that patient homework adherence during the standard 17-session course was significantly associated with remission during the extended course. These data illustrate the value of personalizing treatment recommendations to achieve best outcomes (Collins & Varmus, 2015).
That patient homework adherence strongly predicts EX/RP remission from a standard 17-session EX/RP course corroborates findings from two prior studies with smaller samples (Simpson et al., 2011; Wheaton et al., 2016). That patient adherence during the standard course predicts who will benefit most from an extended course is new. These findings likely reflect that EX/RP is a skill-based treatment, providing new skills and recommending their use in daily life (Abramowitz, Franklin, & Cahill, 2003). Patient adherence to EX/RP homework displays motivation to learn and implement such new skills. That the PEAS, a 3-item measure of EX/RP adherence, predicts both acute (in this study) and long-term outcome (Simpson et al., 2012), underscores the value of this simple tool for tracking patient treatment progress. Indeed, since prior research found that PEAS ratings can forecast individual patient outcomes from a standard course even before mid-treatment (Wheaton et al., 2016) and herein we found that PEAS ratings during a standard course were associated with odds of remission from an extended course, we recommend its routine use in clinical practice. Important next steps include: identifying which patient characteristics predict poor adherence following the preliminary work of Maher et al. (2012); elucidating which aspects of the standard EX/RP protocol facilitate homework adherence (e.g., self-monitoring forms, between-session phone check-ins, frequency of sessions); and developing new methods that robustly enhance patient homework adherence and demonstrating that these causally maximize EX/RP outcome.
That more severe OCPD traits were associated with lower odds of remission from a standard EX/RP course (n=137) is consistent with a prior study in 49 adults with OCD. That study utilized DSM-IV OCPD criteria and found that patients who had a greater number of baseline OCPD criteria had poorer outcomes (higher post-treatment severity) (Pinto, Liebowitz, et al., 2011). Using the POPS, developed to dimensionally capture pathological OCPD traits (Pinto, Ansell, et al., 2011; Sadri et al., 2018), we found that more severe OCPD traits was associated with higher odds of not remitting, independent of patient adherence. How OCPD traits interfere with EX/RP outcome remains unclear. We speculate that the rigidity and inflexibility of OCPD interferes with learning during EX/RP, a question deserving future study. Data from a large case series suggest that OCD patients with comorbid OCPD benefit when CBT targets not only OCD but also OCPD by including techniques to address maladaptive perfectionism, cognitive flexibility, and appraisals of responsibility and their impact on compulsive behavior (Gordon, Salkovskis, & Bream, 2016).
In our study, the BDNF Val66Met variant was significantly associated with greater odds of achieving remission after an extended versus standard EX/RP course. In a prior study, OCD patients with the BDNF Met allele had poorer outcomes in exposure-based CBT than patients without that allele (Fullana et al., 2012). Because rodent and healthy human studies found that Met allele carriers displayed poorer extinction learning in a laboratory paradigm (Soliman et al., 2010), and extinction learning may play a role in the mechanisms underlying exposure-based CBT like EX/RP (Dougherty et al., 2018), this raises the question of whether this BDNF variant is not only a marker for EX/RP outcome but also a factor that may relate to its mechanism. However, as our effect was only for Met/Val carriers (n=41), our sample of Met/Met carriers was very small (n=6), and other factors (i.e., patient homework adherence) were more robust and easier to measure, our data do not support using the BDNF variants in routine clinical practice. Replication in a larger cohort and further examination of potential causal connections between BDNF variants, extinction learning deficits, and EX/RP outcomes is first warranted.
Four other factors—baseline OCD severity, depressive severity, metacognitions, and quality of life—did not robustly predict remission with EX/RP above and beyond the factors described above. That initial OCD severity (baseline YBOCS) was not a robust indicator of whether patients achieved remission (with either a standard or extended dose of EX/RP) is consistent with prior findings that initial OCD severity is not always linked to EX/RP outcomes (Knopp et al., 2013). Moreover, our data illustrate that even those with severe OCD can achieve remission with EX/RP, thus OCD severity is not a contraindication on its own to EX/RP. Depression may only affect EX/RP outcome when severe (Abramowitz, Franklin, Street, Kozak, & Foa, 2000; Steketee et al., 2019). Because our patients were receiving SRI medication and recurrent MDD was an exclusion criterion, depressive severity was low in our sample. Moreover, although quality of life predicted EX/RP outcome in a prior study (Maher et al., 2010), the predictive power in that study (measured by the incremental R2) was very small. These null results are consistent with the broader literature on predictors of EX/RP outcomes, which include many mixed results and null findings (Knopp et al., 2013). Given this, it is important for future studies to standardize measures of both predictors and outcomes so that meta-analyses can synthesize this mixed literature. Based on our data, patient adherence and OCPD traits should be included in future studies as important factors to consider in relation to EX/RP outcomes.
Several limitations merit consideration. First, our EX/RP protocol offered only up to 25 sessions. Thus, it is unclear whether further extending EX/RP might have benefitted more patients, or if gains plateau at this point. Moreover, the study design (where those who achieved remission after the standard protocol were entered into a double-blind SRI discontinuation trial and the others offered up to 8 additional EX/RP sessions) precludes addressing other questions, such as the effect of continued homework practice without additional sessions. Second, all patients continued taking SRIs while receiving EX/RP. Whether these results also apply to EX/RP monotherapy needs further study. Third, the sample, while large for OCD clinical trials, is small for genetic studies and investigated only three gene variants. Finally, like many clinical OCD trials (Williams, Powers, Yun, & Foa, 2010), the sample was primarily Non-Hispanic White.
Despite these limitations, the study offers several clinical implications. First, although some adults with symptoms despite an adequate SRI trial will attain remission after the addition of 17 EX/RP sessions, many others may remit from an extended course of up to 25 sessions. Treating to remission matters, as it is associated with improved functioning, higher quality of life (Farris et al., 2013), and better maintenance of gains (Foa et al., 2013, 2015). Second, patient homework adherence predicts remission after a standard course as well as who benefits most from an extended course. Future studies can now be designed to assess whether patient adherence behavior can classify remission status at the individual level; if so, this could inform a precision medicine approach to EX/RP. Finally, diverse factors are associated with odds of remission. In this study, key factors included patient homework adherence, OCPD personality traits and the BDNF Val66Met genotype, with patient adherence being the strongest EX/RP predictor overall. These data illustrate the importance of taking a biopsychosocial approach to precision medicine to ensure consideration of all factors predicting treatment success, including those that are easily implemented, such as monitoring patient homework adherence.
Supplementary Material
Highlights.
We tested EX/RP as an SRI augmentation strategy for adults with OCD
Extending EX/RP from 17 to 25 sessions of EX/RP enabled 69.3% of patients to achieve remission
patient adherence, OCPD traits, and BDNF genotype influenced the odds and timing of remission
Acknowledgments:
We would like to thank the patients who participated in this study, the research assistants who helped (Liza Alpert, Joseph Carpenter, Stephanie Chen, Natalie Gay, Ashley Greene, Shari Lieblich, Rachel Middleton, Julie Peterson, and Marissa Schwartz), and Drs. Lily Brown and Sapana Patel who assisted with patient assessments. We also thank Dr. David Rosenfield for consulting on an earlier version of this manuscript and Ms. Gabrielle R. Messner for her help in submission of the manuscript.
PI of grants (R01MH045436 and R01MH045404) and contributed equally
Disclosures:
During the conduct of this study and in the prior 36 months, Dr. Simpson has received research support from Biohaven, royalties from Cambridge University Press and UpToDate, Inc, and a stipend from JAMA for her role as Associate Editor at JAMA Psychiatry. Dr. Puliafico has received royalties from UpToDate, Inc. Dr. Foa has received support for research from Pfizer, Solvay, Eli Lilly, SmithKline Beecham, GlaxoSmithKline, Cephalon, Bristol Myers Squibb, Forest, Ciba Geigy, Kali-Duphar, American Psychiatric Association, NIDA, NIAAA, NIH, DOJ and DoD, speaking fees from Pfizer, GlaxoSmithKline, Forest Pharmaceuticals, American Psychiatric Association and Jazz Pharmaceuticals, consulted for Actelion Pharmaceuticals and royalties from Bantam and Oxford University Press for book sales, including a manual of cognitive behavioral therapy for OCD. She also receives payment for training she conducts on obsessive-compulsive disorder. All other authors report no financial relationships with commercial interests.
Footnotes
Conflict of interest statement
During the conduct of this study and in the prior 36 months, Dr. Simpson has received research support from Biohaven, royalties from Cambridge University Press and UpToDate, Inc, and a stipend from JAMA for her role as Associate Editor at JAMA Psychiatry. Dr. Puliafico has received royalties from UpToDate, Inc. Dr. Foa has received support for research from Pfizer, Solvay, Eli Lilly, SmithKline Beecham, GlaxoSmithKline, Cephalon, Bristol Myers Squibb, Forest, Ciba Geigy, Kali-Duphar, American Psychiatric Association, NIDA, NIAAA, NIH, DOJ and DoD, speaking fees from Pfizer, GlaxoSmithKline, Forest Pharmaceuticals, American Psychiatric Association and Jazz Pharmaceuticals, consulted for Actelion Pharmaceuticals and royalties from Bantam and Oxford University Press for book sales, including a manual of cognitive behavioral therapy for OCD. She also receives payment for training she conducts on obsessive-compulsive disorder. All other authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These exclusions follow the American Psychiatric Association Practice Guidelines for Major Depression Disorder (www.psych.org/guidelines/mdd2010), which recommend continued antidepressant medication after an initial response for ≥4-9 months and recommend maintenance antidepressant medication in specific situations (e.g., history of psychotic depression or recurrent MDD).
REFERENCES
- Abramowitz JS, Franklin ME, & Cahill SP (2003). Approaches to common obstacles in the exposure-based treatment of obsessive-compulsive disorder. Cognitive and Behavioral Practice, 10(1), 14–22. 10.1016/S1077-7229(03)80004-4 [DOI] [Google Scholar]
- Abramowitz JS, Franklin ME, Street GP, Kozak MJ, & Foa E (2000). Effects of comorbid depression on response to treatment for obsessive-compulsive disorder. Behavior Therapy, 31(3), 517–528. 10.1016/S0005-7894(00)80028-3 [DOI] [Google Scholar]
- Andersson E, Rück C, Lavebratt C, Hedman E, Schalling M, Lindefors N, … Furmark T (2013). Genetic polymorphisms in monoamine systems and outcome of cognitive behavior therapy for social anxiety disorder. PLoS ONE, 8(11). 10.1371/journal.pone.0079015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, & Varmus H (2015). A new initiative on precision medicine. New England Journal of Medicine, 372(9), 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Sesvold CL, Abraham P, Devaney JM, Harmon BT, & Deuster PA (2015). The Association of 5-HTTLPR XLL Genotype with Higher Cortisol Levels in African Americans. International Journal of Medical Genetics, 2015(L), 1–6. 10.1155/2015/123159 [DOI] [Google Scholar]
- Dougherty DD, Brennan BP, Stewart SE, Wilhelm S, Widge AS, & Rauch SL (2018). Neuroscientifically Informed Formulation and Treatment Planning for Patients with Obsessive-Compulsive Disorder: A Review. JAMA Psychiatry, 75(10), 1081–1087. 10.1001/jamapsychiatry.2018.0930 [DOI] [PubMed] [Google Scholar]
- Farris SG, McLean CP, Van Meter PE, Simpson HB, & Foa E (2013). Treatment response, symptom remission, and wellness in obsessive-compulsive disorder. The Journal of Clinical Psychiatry, 74(7), 685–690. 10.4088/JCP.12m07789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1995). Structured clinical interview for DSM-IV axis I disorders-Patient edition (SCID-I/P, Version 2.0) New York. NY: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Foa EB, Yadin E, & Lichner TK (2012). Exposure and response (ritual) prevention for obsessive compulsive disorder: Therapist guide. Oxford University Press. [Google Scholar]
- Foa E, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, … Tu X (2005). Randomized, Placebo-Controlled Trial of Exposure and Ritual Prevention, Clomipramine, and Their Combination in the Treatment of Obsessive-Compulsive Disorder. American Journal of Psychiatry, 162(1), 151–161. 10.1176/appi.ajp.162.l.151 [DOI] [PubMed] [Google Scholar]
- Foa E, Simpson HB, Liebowitz MR, Powers MB, Rosenfield D, Cahill SP, … Williams MT (2013). Six-month follow-up of a randomized controlled trial augmenting serotonin reuptake inhibitor treatment with exposure and ritual prevention for obsessive-compulsive disorder. Journal of Clinical Psychiatry, 74(5), 464–469. 10.4088/JCP.12m08017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Simpson HB, Rosenfield D, Liebowitz MR, Cahill SP, Huppert JD, … Williams MT (2015). Six-month outcomes from a randomized trial augmenting serotonin reuptake inhibitors with exposure and response prevention or risperidone in adults with obsessive-compulsive disorder. Journal of Clinical Psychiatry, 76(4), 440–446. 10.4088/JCP.14m09044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglia JP, Birder LA, & Perel JM (1989). Determination of fluvoxamine in human plasma by high-performance liquid chromatography with ultraviolet detection. Journal of Chromatography B: Biomedical Sciences and Applications, 495(C), 295–302. 10.1016/S0378-4347(00)82635-3 [DOI] [PubMed] [Google Scholar]
- Fullana MA, Alonso P, Gratacòs M, Jaurrieta N, Jiménez-Murcia S, Segalàs C, … Menchón JM (2012). Variation in the BDNF Val66Met polymorphism and response to cognitive-behavior therapy in obsessive-compulsive disorder. European Psychiatry, 27(5), 386–390. 10.1016/j.eurpsy.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, & Chamey DS (1989). The Yale-Brown Obsessive Compulsive Scale: II. Validity. Archives of General Psychiatry, Vol. 46, pp. 1012–1016. 10.1001/archpsyc.1989.01810110054008 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, … Goodman Wayne K, Price Lawrence H, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, C. D. (1989). Yale-Brown Obsessive Compulsive Scale: Development, Use, and Reliability. Archives of General Psychiatry, 46(11), 1006–1011. 10.1001/archpsyc.1989.01810110048007 [DOI] [PubMed] [Google Scholar]
- Gordon OM, Salkovskis PM, & Bream V (2016). The Impact of Obsessive Compulsive Personality Disorder on Cognitive Behaviour Therapy for Obsessive Compulsive Disorder. Behavioural and Cognitive Psychotherapy, 44(4), 444–459. 10.1017/S1352465815000582 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A Rating Scale for Depression. Journal of Neurology, Neurosurgery &Amp; Psychiatry, 23(1), 56 LP–62. 10.1136/jnnp.23.L56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp J, Knowles S, Bee P, Lovell K, & Bower P (2013). A systematic review of predictors and moderators of response to psychological therapies in OCD: Do we have enough empirical evidence to target treatment? Clinical Psychology Review, 33(8), 1067–1081. 10.1016/j.cpr.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Rück C, Bergström J, Andersson G, Öhman A, Lindefors N, & Schalling M (2010). The COMTval158met polymorphism is associated with symptom relief during exposure-based cognitive-behavioral treatment in panic disorder. BMC Psychiatry, 10. 10.1186/1471-244X-10-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, & Öhman A (2009). Genetic Gating of Human Fear Learning and Extinction: Possible Implications for Gene-Environment Interaction in Anxiety Disorder. Psychological Science, 20(2), 198–206. 10.1111/j.1467-9280.2009.02280.X [DOI] [PubMed] [Google Scholar]
- Maher MJ, Huppert JD, Chen H, Duan N, Foa E, Liebowitz MR, & Simpson HB (2010). Moderators and predictors of response to cognitive-behavioral therapy augmentation of pharmacotherapy in obsessive-compulsive disorder. Psychological Medicine, 40(12), 2013–2023. 10.1017/S0033291710000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MJ, Wang Y, Zuckoff A, Wall MM, Franklin M, Foa EB, & Simpson HB (2012). Predictors of patient adherence to cognitive-behavioral therapy for obsessive-compulsive disorder. Psychotherapy and psychosomatics, 81(2), 124–126. doi: 10.1159/000330214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Fernandez de La Cruz L, Nordsletten AE, Lenhard F, Isomura K, & Simpson HB (2016). Towards an international expert consensus for defining treatment response, remission, recovery and relapse in obsessive-compulsive disorder. World Psychiatry, 15(1), 80–81. 10.1002/wps.20299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, & Hariri AR (2008). Serotonin Transporter (5-HTTLPR) Genotype and Amygdala Activation: A Meta-Analysis. Biological Psychiatry, 63(9), 852–857. 10.1016/j.biopsych.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information, (n.d.). The Single Nucleotide Polymorphism Database (dbSNP). Retrieved from U.S. National Library of Medicine; website: https://www.ncbi.nlm.nih.gov/snp/ [Google Scholar]
- Olatunji BO, Davis ML, Powers MB, & Smits JAJ (2013). Cognitive-behavioral therapy for obsessive-compulsive disorder: A meta-analysis of treatment outcome and moderators. Journal of Psychiatric Research, 47(1), 33–41. 10.1016/j.jpsychires.2012.08.020 [DOI] [PubMed] [Google Scholar]
- Öst LG, Havnen A, Hansen B, & Kvale G (2015). Cognitive behavioral treatments of obsessive-compulsive disorder. A systematic review and meta-analysis of studies published 1993-2014. Clinical Psychology Review, 40, 156–169. 10.1016/j.cpr.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Øyehaug E, Terje Østensen E, & Salvesen B (1982). Determination of the antidepressant agent citalopram and metabolites in plasma by liquid chromatography with fluorescence detection. In Journal of chromatography (Vol. 227). 10.1016/S0378-4347(00)80362-X [DOI] [PubMed] [Google Scholar]
- Pierce KD, Schofield PR, Bryant RA, Felmingham KL, Pe Benito L, Dobson-Stone C, … Schofield PR (2010). Preliminary Evidence of the Short Allele of the Serotonin Transporter Gene Predicting Poor Response to Cognitive Behavior Therapy in Posttraumatic Stress Disorder. Biological Psychiatry, 67(12), 1217–1219. 10.1016/j.biopsych.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Pinto A, Ansell EB, & Wright AGC (2011). A new approach to the assessment of obsessive compulsive personality. Integrated paper session conducted at the annual meeting of the Society for Personality Assessment. Annual Meeting of the Society for Personality Assessment. [Google Scholar]
- Pinto A, Liebowitz MR, Foa E, & Simpson HB (2011). Obsessive compulsive personality disorder as a predictor of exposure and ritual prevention outcome for obsessive compulsive disorder. Behaviour Research and Therapy, 49(8), 453–458. 10.1016/j.brat.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner M, Kurs R, Gibel A, Ratner Y, & Endicott J (2005). Validity of an abbreviated Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q-18) for schizophrenia, schizoaffective, and mood disorder patients. In Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation (Vol. 14). 10.1007/sl1136-005-2816-9 [DOI] [PubMed] [Google Scholar]
- Sadri SK, McEvoy PM, Pinto A, Anderson RA, & Egan SJ (2018). A Psychometric Examination of the Pathological Obsessive Compulsive Personality Scale (POPS): Initial Study in an Undergraduate Sample. Journal of Personality Assessment, 3891, 1–10. 10.1080/00223891.2018.1428983 [DOI] [PubMed] [Google Scholar]
- Simpson HB, Foa E, Liebowitz MR, Huppert JD, Cahill S, Maher MJ, … Campeas R (2013). Cognitive-Behavioral Therapy vs Risperidone for Augmenting Serotonin Reuptake Inhibitors in Obsessive-Compulsive Disorder. JAMA Psychiatry, 70(11), 1190. 10.1001/jamapsychiatry.2013.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Foa E, Liebowitz MR, Ledley R, Huppert JD, Cahill S, … Franklin M (2008). A Randomized, Controlled Trial of Cognitive-Behavioral Therapy for Augmenting Pharmacotherapy in Obsessive-Compulsive Disorder. Am J Psychiatry, 165(5), 621–630. 10.1176/appi.ajp.2007.07091440.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Huppert JD, Petkova E, Foa E, & Liebowitz MR (2006). Response Versus Remission in Obsessive-Compulsive Disorder. In The Journal of clinical psychiatry (Vol. 67). 10.4088/JCP.v67n0214 [DOI] [PubMed] [Google Scholar]
- Simpson HB, Maher MJ, Wang Y, Bao Y, Foa E, & Franklin M (2011). Patient adherence predicts outcome from cognitive behavioral therapy in obsessive-compulsive disorder. Journal of Consulting and Clinical Psychology, 79(2), 247–252. 10.1037/a0022659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Marcus SM, Zuckoff A, Franklin M, & Foa E (2012). Patient adherence to cognitive-behavioral therapy predicts long-term outcome in obsessive-compulsive disorder. The Journal of Clinical Psychiatry, 73(9), 1265–1266. 10.4088/JCP.12107879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solem S, Håland ÅT, Vogel PA, Hansen B, & Wells A (2009). Change in metacognitions predicts outcome in obsessive-compulsive disorder patients undergoing treatment with exposure and response prevention. Behaviour Research and Therapy, 47(4), 301–307. 10.1016/j.brat.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, … Casey BJ (2010). A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science (New York, N.Y.), 327(5967), 863–866. 10.l126/science.l181886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee G, Siev J, Yovel I, Lit K, & Wilhelm S (2019). Predictors and Moderators of Cognitive and Behavioral Therapy Outcomes for OCD: A Patient-Level Mega-Analysis of Eight Sites. Behavior Therapy, 50(1), 165–176. 10.1016/j.beth.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Suckow RF, Zhang MF, & Cooper TB (1992). Sensitive and selective liquid-chromatographic assay of fluoxetine and norfluoxetine in plasma with fluorescence detection after precolumn derivatization. Clinical Chemistry, 35(9), 1756–1761. 10.1093/clinchem/38.9.1756 [DOI] [PubMed] [Google Scholar]
- Wells A, & Cartwright-Hatton S (2004). A short form of the metacognitions questionnaire: Properties of the MCQ-30. Behaviour Research and Therapy, 42(4), 385–396. 10.1016/S0005-7967(03)00147-5 [DOI] [PubMed] [Google Scholar]
- Wells A, Myers S, Simons M, & Fisher P (2017). Metacognitive Model and Treatment of OCD. The Wiley Handbook of Obsessive Compulsive Disorders, (December 2018), 644–662. 10.1002/9781118890233.ch36 [DOI] [Google Scholar]
- Wheaton MG, Galfalvy H, Steinman SA, Wall MM, Foa E, & Simpson HB (2016). Patient adherence and treatment outcome with exposure and response prevention for OCD: Which components of adherence matter and who becomes well? Behaviour Research and Therapy, 85, 6–12. 10.1016/j.brat.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton MG, Rosenfield D, Foa E, & Simpson HB (2015). Augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: What moderates improvement? Journal of Consulting and Clinical Psychology, 83(5), 926–937. 10.1037/ccp0000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Powers M, Yun Y-G, & Foa E (2010). Minority participation in randomized controlled trials for obsessive-compulsive disorder. Journal of Anxiety Disorders, 24(2), 171–177. 10.1016/jjanxdis.2009.ll.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrwich K, Harnam N, Revicki DA, Locklear JC, Svedsäter H, & Endicott J (2009). Assessing health-related quality of life in generalized anxiety disorder using the Quality of Life Enjoyment and Satisfaction Questionnaire. International Clinical Psychopharmacology, 24(6), 289–295. 10.1097/YIC.0b013e32832d6bf4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


