Abstract
Background
Monoclonal antibody treatment may prevent complications of coronavirus disease 2019 (COVID-19). We sought to quantify the impact of bamlanivimab monoclonal antibody monotherapy on hospitalization and mortality among outpatients at high risk of COVID-19 complications.
Methods
In this observational study we compared outpatients who received bamlanivimab monoclonal antibody from December 9, 2020 to March 3, 2021 to nontreated patients with a positive polymerase chain reaction or antigen test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the same period who were eligible for monoclonal antibody treatment. The primary outcome was 28-day hospitalization or all-cause mortality, and the secondary outcome was hospitalization or emergency department visit without hospitalization. The risk-adjusted odds of study outcomes comparing bamlanivimab treated and untreated patients was determined using 1:5 propensity matching and multivariable logistic regression.
Results
Among 232 patients receiving bamlanivimab matched with 1160 comparator patients, the mean age was 67 years, 56% were female, and 196 (14%) of patients experienced hospitalization or mortality. After adjustment for propensity to receive treatment, bamlanivimab treatment was associated with a significantly reduced risk-adjusted odds of hospitalization or mortality within 28 days (odds ratio [OR], 0.40; 95% confidence interval [95% CI], 0.24–0.69; P < .001). Bamlanivimab treatment was also associated with a significantly lower risk adjusted odds of hospitalization or emergency department visit without hospitalization (OR, 0.54; 95% CI, 0.35–0.82; P = .004). The results were most strongly associated with patients age 65 years and older.
Conclusions
Bamlanivimab monoclonal antibody monotherapy was associated with reduced hospitalizations and mortality within 28 days among outpatients with mild to moderate COVID-19.
Use of bamlanivimab monotherapy for outpatients with mild to moderate COVID-19 infection was associated with reductions in hospitalizations and mortality within 28 days. Benefit was strongest in those age 65 years or older
Keywords: bamlanivimab, COVID-19, monoclonal antibodies, SARS-CoV-2
Monoclonal antibodies (mAb) bind to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein and block viral entry into host cells, neutralizing the virus [1–5]. Between November 2020 and February 2021, 4 mAbs provided as 3 treatments received US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for treatment of patients with mild to moderate coronavirus disease 2019 (COVID-19) within 10 days of symptom onset: 700 mg bamlanivimab (LY-coV555; Eli Lilly), 1400 mg etesevimab (LY-CoV016; Eli Lilly), 1200 mg casirivimab (REGN10933; Regeneron), and 1200 mg imdevimab (REGN10987; Regeneron). Several clinical trials are currently evaluating mAb for prevention or treatment of COVID-19. However, real-world data are limited, and the role of mAb for patients with COVID-19 remains controversial [3, 5, 6].
Use of mAb therapy is low in the United States despite widespread drug availability due to lack of robust efficacy data, operational challenges with outpatient infusions, and patient access issues [7]. Our health system established a mAb program in November 2020 to decrease COVID-19-related complications for patients with mild to moderate illness. In its implementation, particular care was taken to expand access for underserved patients. Initially, only bamlanivimab monotherapy was available; the evaluation and distribution process is described elsewhere [8]. As of April 16, 2021, only combination monoclonal antibody therapy is now authorized for use in the United States. However there remains limited real-world evidence beyond the initial clinical trials showing benefit of any mAb treatment whether it be bamlanivimab monotherapy or combination mAb therapy. Knowledge gained from an evaluation of initial bamlanivimab monotherapy remains vital to informing providers about the role of COVID-19 mAbs in general and guiding future investigations and clinical decisions.
This study quantifies the risk-adjusted association between bamlanivimab monotherapy treatment and no bamlanivimab treatment on hospitalizations, mortality, and emergency department (ED) visits among outpatients at high risk of progressing to severe COVID-19. We also explored whether patient age, body mass index (BMI), and timing of treatment relative to initial diagnosis modified the association between mAb treatment and outcome.
METHODS
This study was approved by the UPMC Quality Improvement Review Committee (Project ID 2882 and Project ID 3116).
Patient Consent Statement
All data extraction and analysis were done in an aggregated and deidentified fashion. This was performed under a Quality Improvement approval and individual patient consent was not required.
Study Setting
UPMC is a 40-hospital integrated healthcare system providing care principally within central and western Pennsylvania (USA). After the November 2020 EUA was granted for 700 mg of bamlanivimab infused once, UPMC established 16 outpatient infusion centers across all served geographical areas. In addition, infusions occurred in UPMC Senior Communities (ie, long-term care facilities), patient homes (via collaboration with a home infusion company), and behavioral health units. Patients were referred via the electronic medical record (EMR) systems for UPMC providers and by paper order for non-UPMC prescribers. A centralized team with pharmacists and physicians reviewed orders daily to confirm criteria for receipt (Supplemental Table 1). Decentralized nursing teams then contacted and scheduled eligible patients for infusions.
We used the EMR to access all key clinical data including detailed sociodemographic and medical history data, diagnostic and clinical tests conducted, surgical and other treatment procedures performed, prescriptions ordered, and billing charges on all outpatient and in-hospital encounters, with diagnoses and procedures coded based on the International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10, respectively). We linked the deidentified primary data sources using common variables within the UPMC data systems aggregated in its Clinical Data Warehouse [9].
Study Population
The study population was derived from patients who received bamlanivimab treatment from December 9, 2020 to March 3, 2021. During this time period at UPMC, 700 mg of bamlanivimab was used exclusively and was administered over 1 hour per the initial EUA directives. Patients were candidates for bamlanivimab therapy based on criteria initially derived from and later matching EUA criteria including the following: recently diagnosed mild to moderate COVID-19 (with a positive polymerase chain reaction or antigen test for SARS-CoV-2 virus); symptom onset within 10 days before mAb treatment; body mass at least 40 kg; age ≥65 years; or a medical condition conferring high risk of COVID-19 progression to severe disease and/or hospitalization (Supplemental Table 1) [10]. A mild to moderate COVID-19 definition was consistent with EUA language and included patients not requiring hospitalization for COVID-19 or requiring new or increased oxygenation support. Patients were included if they completed bamlanivimab treatment and had at least 28 calendar days of follow-up, or if they experienced one of the study outcomes of interest within 28 days after treatment. No patients who met EUA eligibility for infusion were excluded simply due to any individual patient characteristics.
We also derived a comparator group from the same at-risk population by identifying nonhospitalized patients with a positive polymerase chain reaction or antigen test for SARS-CoV-2 during the same time period who were eligible for mAb treatment based on EUA criteria but were not treated.
For treated patients, the 28-day follow-up period began on the day of their treatment. For comparator patients, the follow-up period began 2 days after their SARS-CoV-2 test result date, which corresponded to the earliest time from test positivity to initiation of treatment for treated patients.
Study Outcomes
The primary outcome was a composite of hospitalization or all-cause mortality during the follow-up windows. We assessed in-hospital mortality using the discharge disposition of “Ceased to Breathe” sourced from the inpatient EMR and out-of-hospital deaths from the Social Security Death Index. The secondary outcome was hospitalization or 28-day ED visit without hospitalization. To understand the contribution of individual elements of the primary and secondary composite outcomes, we also studied the frequency of individual events: ED visit without hospitalization, hospitalization, and mortality within 28 days.
Statistical Methods
In this observational study of outpatients eligible to receive bamlanivimab for COVID-19, we created within age strata propensity scores for receipt of mAb, matched those receiving bamlanivimab treatment to eligible patients not receiving treatment in a 1:5 ratio, and then calculated the odds ratios (OR) of study outcomes.
First, we selected from the at-risk comparator population individuals matched by propensity score (Supplemental Figure 1) [11, 12]. Age is a strong predictor for complications of COVID-19, and the prevalence of high-risk medical conditions vary substantially by patient age [13, 14]. We anticipated that differences in patient profiles between treated and nontreated patients would vary by age because of the age-specific criteria for bamlanivimab contained in the EUA. Moreover, immunosenescence occurs with aging, so we anticipated age to be a potential effect modifier in the relationship between mAb receipt and study outcomes [15]. Therefore, we planned a priori to select nontreated patients using propensity scores within age strata consistent with the EUA criteria: less than 55 years, 55 years to less than 65 years, and 65 years of age and older [10]. Patients receiving bamlanivimab treatment and the selected comparator patients across all age strata were then combined to constitute the study population.
Propensity scores were derived using logistic regression models fit from covariates in age-stratified groups with treatment with mAb as the response variable and forward stepwise selection of measured pretreatment explanatory variables at P < .15 (Supplemental Table 2). We included variables deemed biologically relevant into all models before stepwise selection. We used 1:5 propensity score matching with a maximum propensity score probability difference of 0.01 to construct matched treated and nontreated groups within age strata (Supplemental Figures 1 and 2). We did not impute missing values for variables used in deriving the propensity scores, but we did compare characteristics of patients with propensity scores (ie, full covariate data) to those without propensity scores.
We compared characteristics and unadjusted outcomes of treated versus nontreated patients using Student t tests for continuous variables and χ 2 tests for categorical variables. The time at risk for nontreated patients was estimated starting at the time they would have received bamlanivimab had they been referred for care, conservatively using 2 days after SARS-CoV-2 testing. We compared the distribution of time from beginning of follow-up to study outcome among patients receiving treatment and those who did not, both in the matched and at-risk populations (Supplemental Table 3). For the propensity-matched analysis of primary and secondary outcomes, as well as individual elements of the composite outcomes, we fit unconditional logistic regression models with bamlanivimab receipt as the primary exposure.
We also performed 1 sensitivity and 3 exploratory analyses. First, we used the propensity score (ie, predicted probability of being treated with bamlanivimab) as a continuous variable to control for confounding and evaluate study outcomes in the unmatched cohort of patients who did and did not receive bamlanivimab. Second, because we postulated that treatment may have differential effects in potentially immunosenescent older population, we evaluated the association between bamlanivimab and study outcomes in the predefined age strata of the propensity score-matched cohort. Third, in the unmatched cohort, we tested for an interaction between treatment and BMI because in one clinical trial higher BMI was associated with a more pronounced treatment effect [16]. Fourth, to identify a potential benefit of prompt (versus delayed) administration of bamlanivimab, among study patients receiving it, we explored the frequency of treatment outcomes in 4 categories of time from diagnosis to treatment: 0 to 2 days, 3 to 4 days, 5 to 7 days, and 8 to 10 days. Treatment effects are reported as risk-adjusted OR with 95% confidence interval (CI). We set the alpha error at 0.05 for calculation of adjusted OR. All analyses were performed using the SAS System (SAS Institute, Cary, NC), version 9.4. Methods and results are reported in accordance with The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement (see Supplemental Table 4) [17].
RESULTS
Study Population
Among 636 patients who received bamlanivimab during the study period, 463 (72.8%) patients achieved a study outcome or completed 28-day follow-up. The nonhospitalized at-risk population who met EUA criteria and did not receive bamlanivimab treatment included 11 311 patients, 10 438 (92.2%) of whom achieved study outcome or had sufficient follow-up time. The unmatched populations were significantly different on most characteristics with patients receiving bamlanivimab demonstrating higher frequencies of these medical conditions (Table 1). After propensity score matching within age strata, 232 patients receiving mAb treatment and 1160 patients not receiving bamlanivimab were included in the study population. The mean age of the 1392 patients in this cohort was 67 years and 56% were female. After propensity matching, almost all matched and selected unmatched variables did not differ statistically and with minimal clinical differences between patients who received versus did not receive bamlanivimab treatment (Table 1). The distribution of propensity scores in the unmatched and matched nontreated and treated groups are depicted in Supplemental Figure 2.
Table 1.
Comparison of Characteristics of Unmatched and Propensity-Matched Patients
| Characteristic | Age Group Matching* | Unmatched | Propensity Matched | ||||
|---|---|---|---|---|---|---|---|
| Treated | Not Treated | Treated | Not Treated | ||||
| (N = 463) | (N = 10438) | P Value | (N = 232) | (N = 1160) | P Value | ||
| Age, mean (SD) | A, B, C | 66.3 (14.4) | 55.8 (18.9) | <.001 | 67.3 (13.0) | 67.1 (13.4) | .91 |
| Female sex (No.), % | A, B, C | (247) 53.3 | (6219) 59.6 | .008 | (124) 53.4 | (650) 56.0 | .47 |
| Black race (No.), % | B, C | (26) 5.7 | (752) 7.2 | .22 | (12) 5.2 | (70) 6.0 | .61 |
| Charlson comorbidity index score, mean (SD) | A, B, C | 1.7 (1.8) | 0.9 (1.3) | <.001 | 1.6 (1.7) | 1.5 (1.7) | .46 |
| Pittsburgh as city of residence (No.), % | C | (79) 26.2 | (1778) 17.0 | <.001 | (64) 27.6 | (281) 24.2 | .28 |
| Allegheny as county of residence (No.), % | A, B, C | (150) 49.7 | (3345) 32.1 | <.001 | (119) 51.3 | (560) 48.3 | .40 |
| History of morbid obesity (No.), % | A, B, C | (65) 27.4 | (2668) 25.6 | .52 | (61) 26.3 | (278) 24.0 | .45 |
| History of end stage renal disease (No.), % | A, B | (7) 3.0 | (95) 0.9 | .001 | (6) 2.6 | (31) 2.7 | .94 |
| History of adrenal insufficiency (No.), % | A | (18) 7.6 | (384) 3.7 | .002 | (18) 7.8 | (75) 6.5 | .47 |
| History of irritable bowel syndrome (No.), % | A | (23) 9.7 | (651) 6.2 | .03 | (22) 9.5 | (103) 8.9 | .77 |
| History of cirrhosis (No.), % | B | (12) 3.0 | (92) 0.9 | <.001 | (7) 3.0 | (17) 1.5 | .10 |
| History of obstructive sleep apnea (No.), % | C | (124) 30.5 | (2007) 19.2 | <.001 | (70) 30.2 | (292) 25.2 | .11 |
| History of asthma (No.), % | C | (171) 42.1 | (3859) 37.0 | .04 | (104) 44.8 | (501) 43.2 | .65 |
| History of COPD (No.), % | C | (101) 24.9 | (1881) 18.0 | <.001 | (60) 25.9 | (285) 24.6 | .68 |
| History of GERD (No.), % | C | (112) 47.3 | (3554) 34.1 | <.001 | (110) 47.4 | (541) 46.6 | .83 |
| History of atrial fibrillation (No.), % | C | (61) 15.0 | (620) 5.9 | <.001 | (34) 14.7 | (133) 11.5 | .17 |
| History of hyperlipidemia (No.), % | C | (179) 75.5 | (5474) 24.5 | <.001 | (176) 75.9 | (854) 73.6 | .48 |
| History of major bleed (No.), % | C | (74) 31.2 | (2145) 20.6 | <.001 | (73) 31.5 | (348) 30.0 | .66 |
| History of vascular disease (No.), % | C | (22) 9.3 | (400) 3.8 | <.001 | (20) 8.6 | (104) 9.0 | .87 |
| Prednisone (No.), % | A | (18) 7.6 | (1307) 12.5 | .02 | (17) 7.3 | (119) 10.3 | .17 |
| DOACS (No.), % | B | (28) 11.8 | (581) 5.6 | <.001 | (26) 11.2 | (119) 10.3 | .67 |
| Hydroxychloroquine (No.), % | B | (9) 3.8 | (163) 1.6 | .007 | (9) 3.9 | (31) 2.7 | .32 |
| Immunomodulators (No.), % | B | (9) 3.8 | (168) 1.6 | .009 | (8) 3.4 | (25) 2.2 | .24 |
| Statins (No.), % | B | (140) 59.1 | (3983) 38.2 | <.001 | (136) 58.6 | (654) 56.4 | .53 |
| Current tobacco use (No.), % | Not matched | (17) 7.2 | (962) 9.3 | .27 | (16) 6.9 | (81) 7.0 | .95 |
| Alcohol use (No.), % | Not matched | (126) 53.8 | (5107) 51.3 | .45 | (124) 54.1 | (551) 48.1 | .10 |
| Illicit drug use (No.), % | Not matched | (7) 1.5 | (283) 2.7 | .12 | (6) 2.6 | (28) 2.4 | .88 |
| History of diabetes (No.), % | Not matched | (123) 30.3 | (2342) 22.4 | <.001 | (76) 32.8 | (369) 31.8 | .78 |
| History of hypertension (No.), % | Not matched | (277) 68.2 | (5310) 50.9 | <.001 | (171) 73.7 | (792) 68.3 | .10 |
| History of coronary artery disease (No.), % | Not matched | (79) 19.5 | (1281) 12.3 | <.001 | (46) 19.8 | (250) 21.5 | .56 |
| History of congestive heart failure (No.), % | Not matched | (55) 13.5 | (692) 6.6 | <.001 | (34) 14.7 | (148) 12.7 | .43 |
| History of pulmonary hypertension (No.), % | Not matched | (20) 4.9 | (194) 1.9 | <.001 | (15) 6.5 | (44) 3.8 | .07 |
| ACE inhibitors (No.), % | Not matched | (52) 21.9 | (1937) 18.6 | .19 | (50) 21.5 | (257) 22.2 | .84 |
| Antidepressants (No.), % | Not matched | (77) 32.5 | (3452) 33.1 | .85 | (75) 32.3 | (393) 33.9 | .65 |
| Beta blockers (No.), % | Not matched | (90) 38.0 | (2567) 24.6 | <.001 | (86) 37.1 | (397) 34.2 | .41 |
| Corticosteroids (No.), % | Not matched | (156) 38.4 | (4685) 44.9 | .01 | (86) 37.1 | (476) 41.0 | .26 |
| TNF inhibitors (No.), % | Not matched | (0) 0.0 | (102) 1.0 | .18 | (0) 0.0 | (19) 1.6 | .06 |
| Asthma biologics (No.), % | Not matched | (0) 0.0 | (20) 0.2 | >.99 | (0) 0.0 | (4) 0.3 | >.99 |
| History of chronic kidney disease (No.), % | Not matched | (18) 4.4 | 188 (1.8) | .001 | (9) 3.9 | (53) 4.6 | .73 |
| History of sickle cell disease (No.), % | Not matched | (0) 0.0 | (0) 0.0 | >.99 | (0) 0.0 | (0) 0.0 | >.99 |
| History of cancer (No.), % | Not matched | (84) 20.7 | (1465) 14.0 | <.001 | (63) 27.2 | (277) 23.9 | .32 |
| History of chemotherapy (No.), % | Not matched | (31) 6.7 | 434 (4.2) | .01 | (19) 8.2 | (78) 6.7 | .40 |
| History of bone marrow transplant (No.), % | Not matched | 2 (0.4) | (23) 0.2 | .29 | 2 (0.9) | (1) 0.1 | .07 |
| History of stem cell receipt (No.), % | Not matched | 2 (0.4) | (7) 0.1 | .05 | 2 (0.9) | (0) 0.0 | .03 |
| History of transplant (No.), % | Not matched | 12 (2.6) | (56) 0.5 | <.001 | (3) 1.3 | (14) 1.2 | >.99 |
Abbreviations: ACE, angiotensin-converting enzyme; COPD, chronic obstructive pulmonary disease; DOACS, direct oral anticoagulants; GERD, gastroesophageal reflux disease; SD, standard deviation; TNF, tumor necrosis factor.
*A, age <55 years; B, age 55 to <65 years; C, age 65 years and older.
NOTE: Missing values exist for some variables within the nonmatched cohort.
Primary and Secondary Outcomes
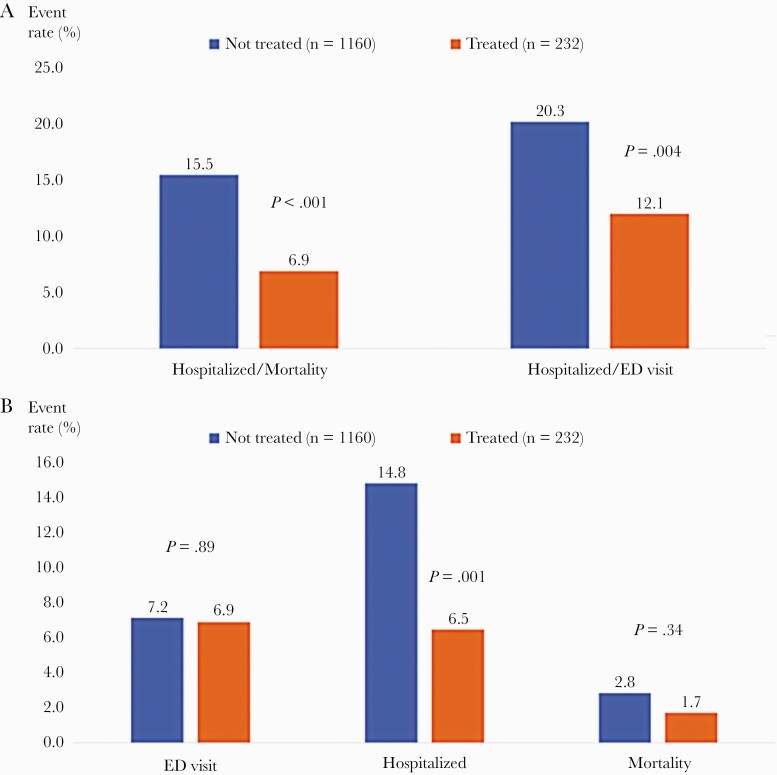
Among the 232 propensity-matched patients receiving bamlanivimab treatment, 15 (6.4%) were hospitalized, 4 (1.7%) died, and 16 (6.9%) had an ED visit without hospitalization for any reason. Among the 1160 propensity-matched patients not receiving bamlanivimab treatment, 172 (14.8%) were hospitalized, 33 (2.8%) died, and 83 (7.2%) had an ED visit without hospitalization (Table 2, Figure 1b). The primary outcome of hospitalization or mortality occurred in 6.9% (16 of 232) of patients receiving bamlanivimab and 15.5% (180 of 1160) of patients not receiving bamlanivimab. The secondary outcome of hospitalization or ED visit without hospitalization occurred in 12.1% (28 of 232) of patients receiving bamlanivimab and 20.3% (235 of 1160) of patients not receiving bamlanivimab (Figure 1a). In the propensity-matched analyses, patients receiving bamlanivimab had an estimated 60% lower risk-adjusted odds of hospitalization or mortality (OR, 0.40; 95% CI, 0.24 to 0.69) and estimated 46% lower risk-adjusted odds of hospitalization or ED visit without hospitalization (OR, 0.54; 95% CI, 0.35 to 0.82) (Table 2).
Table 2.
Primary and Secondary Outcomes From Propensity-Matched Models Stratified by Age
| Outcome All Patients | Number of Events | 28-Day Event Rate (%) | Odds Ratio Estimates | ||||
|---|---|---|---|---|---|---|---|
| Treated (n = 232) | Not Treated (n = 1160) | Treated | Not Treated | Odds Ratio | (95% CI) | P Value | |
| Hospitalization or mortality | 16 | 180 | 6.9 | 15.5 | 0.40 | (0.24–0.69) | <.001 |
| Hospitalization or ED visit without hospitalization | 28 | 235 | 12.1 | 20.3 | 0.54 | (0.35–0.82) | .004 |
| ED visit without hospitalization | 16 | 83 | 6.9 | 7.2 | 0.96 | (0.55–1.67) | .89 |
| Hospitalization | 15 | 172 | 6.5 | 14.8 | 0.40 | (0.23–0.69) | .001 |
| Mortality | 4 | 33 | 1.7 | 2.8 | 0.60 | (0.21–1.71) | .34 |
| Age <55 years | (n = 41) | (n = 205) | |||||
| Hospitalization or mortality | 2 | 17 | 4.9 | 8.3 | 0.57 | (0.13–2.55) | .46 |
| Hospitalization or ED visit without hospitalization | 7 | 26 | 17.1 | 12.7 | 1.42 | (0.57–3.53) | .45 |
| ED visit without hospitalization | 6 | 14 | 14.6 | 6.8 | 2.34 | (0.84–6.50) | .10 |
| Hospitalization | 2 | 15 | 4.9 | 7.3 | 0.65 | (0.14–2.95) | .58 |
| Mortality | 0 | 2 | 0.0 | 1.0 | ----- | ----- | ----- |
| Age 55 to <65 years | (n = 33) | (n = 165) | |||||
| Hospitalization or mortality | 2 | 22 | 6.1 | 13.3 | 0.42 | (0.09–1.88) | .26 |
| Hospitalization or ED visit without hospitalization | 2 | 33 | 6.1 | 20.0 | 0.26 | (0.06–1.13) | .07 |
| ED visit without hospitalization | 1 | 19 | 3.0 | 11.5 | 0.24 | (0.03–1.86) | .17 |
| Hospitalization | 2 | 22 | 6.1 | 13.3 | 0.42 | (0.09–1.88) | .26 |
| Mortality | 0 | 2 | 0.0 | 1.2 | 0.0 | ----- | ----- |
| Age 65 years and older | (n = 158) | (n = 790) | |||||
| Hospitalization or mortality | 12 | 141 | 7.6 | 17.8 | 0.38 | (0.20–0.70) | .002 |
| Hospitalization or ED visit without hospitalization | 19 | 176 | 12.0 | 22.3 | 0.48 | (0.29–0.79) | .004 |
| ED visit without hospitalization | 9 | 50 | 5.7 | 6.3 | 0.89 | (0.43–1.86) | .76 |
| Hospitalization | 11 | 135 | 7.0 | 17.1 | 0.36 | (0.19–0.69) | .002 |
| Mortality | 4 | 29 | 2.5 | 3.7 | 0.68 | (0.24–1.97) | .48 |
Abbreviations: CI, confidence interval; ED, emergency department.
Figure 1.
Frequency of 28-day study outcomes among propensity-matched patients receiving and not receiving bamlanivimab monoclonal antibody treatment. (a) depicts the frequency of 28-day hospitalization or mortality 408 (primary outcome) and hospitalization or emergency department (ED) visit without hospitalization (secondary outcome) among the matched patients receiving bamlanivimab monoclonal antibody treatment (orange bars) versus those not receiving bamlanivimab monoclonal antibody treatment (blue bars). (b) depicts the frequency of the individual elements of the composite primary and secondary outcomes. P values are from the matched cohort logistic regression models.
Sensitivity and Exploratory Analyses
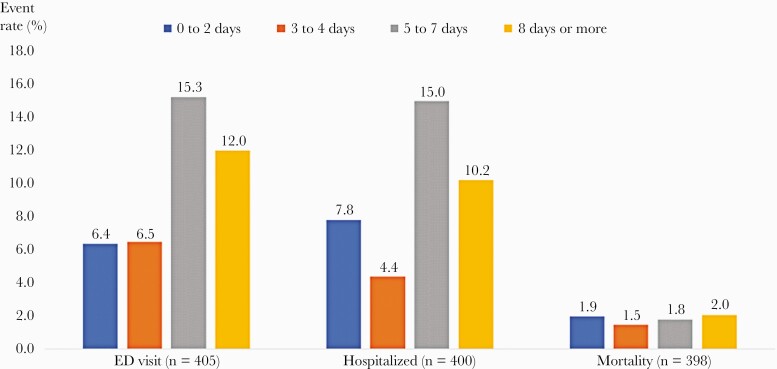
In an unmatched cohort of patients receiving bamlanivimab treatment and an at-risk population of patients not receiving the treatment, adjusted for propensity to receive bamlanivimab, bamlanivimab was associated with a 0.42 (95% CI, 0.25 to 0.73) lower odds of hospitalization or mortality and a 0.60 (95% CI, 0.40 to 0.91) lower odds of hospitalization or ED visit (Supplemental Table 5). A post hoc parallel conditional logistic regression analysis yielded virtually identical results (Supplemental Table 6). In the age-stratified matched analysis, bamlanivimab treatment resulted in a 0.57 (95% CI, 0.13 to 2.55), 0.42 (95% CI, 0.09 to 1.88), and 0.38 (95% CI, 0.20 to 0.70) lower odds of the primary outcome in the <55 years, 55 to <65 years, and 65 years and older age groups, respectively (Table 2). In propensity score-adjusted analyses for the 28-day rate of hospitalization or mortality, there was no differential treatment effect by BMI for the association between bamlanivimab treatment and the primary outcomes (P values for the interaction term of mAb × BMI were 0.67, 0.78, 0.43, and 0.83 for all those age <55 years, 55 to less than 65 years, and 65 years and older, respectively. Supplemental Figure 3 depicts the frequency of the primary outcome among study population aged 65 years and older, by treatment received and BMI category. Figure 2 indicates that among patients in the study population receiving bamlanivimab treatment, those who received their treatment within 4 days of their positive SARS-CoV-2 test result had lower 28-day rates of ED visit without hospitalization and hospitalization than patients who received their treatment 5 days or more after their positive SARS-CoV-2 test result. Rates of mortality were low and similar by timing of treatment.
Figure 2.
Frequency of 28-day study outcomes among patients receiving bamlanivimab monoclonal antibody treatment, stratified by timing of treatment. ED, emergency department.
DISCUSSION
In a propensity-matched cohort study, bamlanivimab treatment among outpatients with mild to moderate COVID-19 was associated with a significantly reduced risk-adjusted odds of hospitalization or death at 28 days. These results were robust (1) to sensitivity analyses including an unmatched analysis controlled by propensity to receive treatment and (2) in age strata of the matched cohort.
In a previous randomized clinical trial, 700 mg bamlanivimab in outpatients with mild to moderate COVID-19 showed improvement of symptoms at day 11 and fewer hospitalizations and ED visits at day 29 compared with placebo [3, 18]. A post hoc analysis of those 65 or older or with BMI of 35 kg/m2 or greater in this trial demonstrated a larger potential benefit, 2.7% vs 13.5% [3]. In this study, we identified a similar association between bamlanivimab monotherapy and hospitalization or mortality, extending the trial findings to a generalizable population.
This study affirms the benefit of bamlanvimab treatment and expands our understanding of where the benefit may be greatest. Cumulatively, our report shows benefit for patients across several possible outcomes, and we consider this highly relevant from a clinical standpoint. Rates of hospitalizations and ED visits in patients aged 65 years or older were higher than observed in the clinical trial, yet a reduction in primary and secondary outcomes was nonetheless observed [3]. Our exploratory analysis of benefit based on time to therapy warrants further investigation into how promptly this therapy should be administered for maximal benefit. Unlike the previous trial, this study did not identify an association between BMI and response to mAb therapy [18]. Future studies should continue to explore medical conditions and patient populations that may most benefit from mAb for the treatment of COVID-19.
Prevention of hospitalization is a highly relevant clinical metric for patients with COVID-19. Before mAb, no outpatient strategies resulted in decreased rates of hospitalization or death. These data support health systems’ investment of resources in developing infrastructure for mAb infusions. In addition, the potential benefits of mAb could be explored in patients cared for in the ED and those early in hospital admission without severe disease.
There are several limitations of our study. First, given the observational design, there were substantial differences between bamlanivimab treated and untreated populations. We mitigated measured confounding with propensity score modeling and a sensitivity analysis using propensity-score adjusted unmatched patients not receiving mAb treatment. Although we may have missed some visits to non-UPMC facilities during the follow-up period in both groups, we did capture all deaths by obtaining information from the Social Security Death Index. Second, we cannot reliably distinguish the presence, extent, or severity of COVID-19 symptoms, all of which may impact effectiveness of mAb treatment. Third, SARS-CoV-2 viral loads were not measured in any biologic site as part of routine practice and the clinical relevance of this is unknown.
Finally, during the time of this study, we used bamlanivimab monotherapy exclusively, so we are unable to comment on any comparison to the other available monoclonal antibodies for treatment of mild to moderate COVID-19 infection. On March 24, 2021, the US Department of Health and Human Services announced they would no longer supply sites with bamlanivimab alone due to concern about increased rates of resistant variants, and on April 16, 2021, the EUA for bamlanivimab monotherapy was rescinded by the FDA [19]. Many of our patients received bamlanvimab when the rate of resistant variants in this country was low, thus explaining why we still saw benefit with bamlanivimab monotherapy. More generally, however, our findings provide early, real-world experience, which suggests that neutralizing mAbs that efficiently target common circulating strains of SARS-CoV-2 can lead to improved clinical outcomes. Our findings provide encouragement to providers still hesitant to provide COVID-19 mAbs in general and encourage further evaluation into the best patient groups to gain benefit from these treatments. It will be critical to show similar results for the other available mAb therapies and to define where each therapy may be best used, including possibly any continued role for bamlanivimab monotherapy. Likewise, it will be helpful to better define effectiveness by time to infusion from date of symptom onset. This would identify when patients receive the most benefit and inform operational considerations such as actual need for 7 days per week availability and other considerations.
CONCLUSIONS
In this propensity-matched observational study, bamlanivimab monotherapy was associated with reduced hospitalizations and mortality within 28 days among patients with mild to moderate COVID-19. This effect was most pronounced among those 65 years or older.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the clinical staff of the UPMC monoclonal antibody infusion centers as well as the support and administrative staff behind this effort, including but not limited to the following personnel: Debbie Albin, Jennifer Dueweke, Robert Shulik, Amy Lukanski, Rozalyn Russell, Debra Rogers, Jesse Duff, Kevin Pruznak, Jennifer Zabala, Trudy Bloomquist, Daniel Gessel, LuAnn King, Jonya Brooks, Libby Shumaker, Betsy Tedesco, Sarah Sakaluk, Kathleen Flinn, Susan Spencer, Le Ann Kaltenbaugh, Michelle Adam, Meredith Axe, Melanie Pierce, Debra Masser, Theresa Murillo, Sherry Casali, Jim Krosse, Jeana Colella, Rebecca Medva, Jessica Fesz, Ashley Beyerl, Jodi Ayers, Hilary Maskiewicz, Mikaela Bortot, Amy Helmuth, Heather Schaeffer, Janice Dunsavage, Erik Hernandez, Ken Trimmer, Sheila Kruman, Teressa Polcha, and their entire teams. We also thank the US Federal Government and Pennsylvania Department of Health for the provision of monoclonal antibody treatment.
Author contributions. J. R. B., E. K. M., R. J. W., O. C. M., D. M. Y., D. A. N., D. T. H., D. C. A., and G. M. S. contributed to conception or design. J. R. B., E. K. M., O. C. M., K. C., and M. S. contributed to data collection. J. R. B., E. K. M., R. J. W., O. C. M., K. E. K., and G. M. S. contributed to data analysis and interpretation. J. R. B., E. K. M., K. E. K., and G. M. S. contributed to drafting the article. All authors critically revised the article. All authors gave final approval of the version to be published.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Jones BE, Brown-Augsburger PL, Corbett KS, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021; 13:eabf1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen MS, Nirula A, Mulligan M, et al. For BLAZE-2 study team. Bamlanivimab prevents COVID-19 morbidity and mortality in nursing home setting. Abstract 121. Conference on Retroviruses and Opportunistic Infections Annual Meeting. March 6-10, 2021. Boston MA USA.
- 3. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate covid-19: a randomized clinical trial. JAMA 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Webb BJ, Buckel W, Vento T, et al. Real-world effectiveness and tolerability of monoclonal antibodies for ambulatory patients with early COVID-19. medRxiv. doi: 10.1101/2021.03.15.21253646. [DOI] [PMC free article] [PubMed]
- 6. Kumar RN, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for COVID-19: a case-control study. Clin Infect Dis 2021; doi: 10.1093/cid/ciab305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ault A. Rollout of COVID Monoclonal Antibodies Lacked Unified Plan: Expert Panel. Available at: https://www.medscape.com/viewarticle/945223. Accessed 23 March 2021.
- 8. Bariola JR, McCreary EK, Khadem T, et al. Establishing a distribution network for COVID-19 monoclonal antibody therapy across a large health system during a global pandemic. Open Forum Infect Dis 2021; doi: 10.1093/ofid/ofab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reitz KM, Marroquin OC, Zenati MS, et al. Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes. JAMA Surg 2020; 155:e200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19; November 9, 2020.. (NEWS RELEASE). Available at: https://bit.ly/2HesBBs. Accessed 13 November 2020.
- 11. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 13. Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med 2021; 203:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bajaj V, Gadi N, Spihlman AP, et al. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol 2020; 11:571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med 2020; 173:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee. The REporting of studies conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen P, Nirula A, Heller B, et al. ; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services. Update on COVID-19 variants and impact on bamlanivimab distribution. Available at: https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Bamlanivimab/Pages/default.aspx. Accessed 22 April 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.