Keywords: oxidative stress, nutraceuticals, astaxanthin, superoxide dismutase (SOD), neurodegeneration, Alzheimer's disease
Abstract
Oxidative stress, the imbalance of the antioxidant system, results in an accumulation of neurotoxic proteins in Alzheimer's disease (AD). The antioxidant system is composed of exogenous and endogenous antioxidants to maintain homeostasis. Superoxide dismutase (SOD) is an endogenous enzymatic antioxidant that converts superoxide ions to hydrogen peroxide in cells. SOD supplementation in mice prevented cognitive decline in stress-induced cells by reducing lipid peroxidation and maintaining neurogenesis in the hippocampus. Furthermore, SOD decreased expression of BACE1 while reducing plaque burden in the brain. Additionally, Astaxanthin (AST), a potent exogenous carotenoid, scavenges superoxide anion radicals. Mice treated with AST showed slower memory decline and decreased depositions of amyloid-beta (Aβ) and tau protein. Currently, the neuroprotective potential of these supplements has only been examined separately in studies. However, a single antioxidant cannot sufficiently resist oxidative damage to the brain, therefore, a combinatory approach is proposed as a relevant therapy for ameliorating pathological changes in AD.
Research in context
1. Systematic review. The authors conducted an electronic search across PubMed, Medline, PsycINFO, ScienceDirect, Google Scholar, Embase library databases for English, peer-reviewed, articles and reviews published after 1964 using the following MeSH terms: Alzheimer's Disease (AD) AND Models, Reactive Oxygen Species (ROS) AND/OR Oxidative Stress (OS) AND Astaxanthin (AS) AND models, Superoxide dismutase (SOD) AND models. Case reports were excluded. The results were further screened by title and abstract for studies performed in rats, mice and humans, at which time full-text articles were screened for eligibility.
2. Interpretation. Based on previous studies, an integrated hypothesis is presented, explaining the neuroprotective role of antioxidants in the AD. This proposal provides evidence for the combinatory treatment, of endogenous and exogenous antioxidants simultaneously, for the prevention and treatment of AD.
3. Future directions. Future trials should consider administering combinations rather than single antioxidants to facilitate redox cycling as well as maximize bioavailability, efficiency to different cellular compartments and establish the regimens for practical interventions at each stage of AD. The oral bioavailability of AST is limited by its solubility in the gut and lipid-based formulations of AST have been proposed as possible alternatives.
1. Objectives
This review aims to synthesize scientific findings on the neuroprotective and therapeutic role of two specific substances—astaxanthin (AST) and superoxide dismutase (SOD)—that each play a role in the endogenous and exogenous pathways of the antioxidant system against oxidative stress (OS). The combination of such substances will be beneficial for patients suffering from Alzheimer's disease (AD).
2. Background
Patients with AD, along with their families and caregivers, are faced with devastating health and financial consequences. The number of people living with this disease is projected to increase as the elderly population grows older and life expectancy increases, which will eventually create a strain on the economy [1]. Since OS in the brain increases over time and AD is a significant age-dependent disorder of the brain, the development of novel antioxidant-regulating treatments is crucial for preventing and preserving cognitive function in this population [2,3]. Thus, reducing the levels of ROS is an essential strategy for AD treatment.
2.1. Generation of free radicals and oxidative stress
Reactive oxygen species (ROS) are produced by living organisms due to normal cellular metabolism processes like cellular oxidation, cell regulation and signalling [4], and formed by the cells of aerobic organisms such as the electron transport chain, macrophages and peroxisomes [5]. At low to moderate concentrations, ROS can be beneficial and regulate cellular processes such as hormonal regulation and intracellular secondary messaging [6,7]. At higher concentrations, they can adversely modify cellular lipids, protein expression and DNA, eventually causing cell death [8–10]. Free radicals are highly reactive, inorganic and unstable molecules or atoms that have lost one electron, making their outer valance shell incomplete [11]. As a result, these incomplete and intermediate oxygen-carrying metabolites known as oxyradicals aggressively search for their remaining electron in other molecules and, once paired, continue to produce more free radicals [7,12]. In turn, this creates a chain reaction of more unstable free radicals interacting with other molecules leading to more complex and toxic mechanisms [5]. Of these, the most damaging radical in many tissues is the superoxide ion (O−2) [13], which is produced mainly in the respiratory chain of the mitochondria [14] and is the primary source of all radical-induced toxicity [15,16]. O−2 is the sequential reduction of molecular oxygen via step-wise addition of electrons, as it can readily produce the hydroxide ion (OH−) free radical, which can later cross the cell membrane and cause severe molecular damage known as lipid peroxidation [17]. OH− causes loss of functional integrity of the cell and membrane receptors, alters membrane permeability and increases membrane rigidity, thus decreasing membrane fluidity [18,19]. If the integrity of the cell is not maintained, homeostasis of the cell may be disrupted with little to no chance of reversal. Homeostasis within a biological system is maintained through the balance of antioxidation and oxidation systems [20]. A disruption of this harmony results in a relative deficiency of the antioxidant system, with the favour of ROS, creating an environment known as OS. The persistence of this ongoing rise in ROS molecules leads to adverse modifications in cell components and is marked by lipid peroxidation, high levels of oxidized proteins and oxidative modifications in mitochondrial DNA [21–23]. OS is thought to be involved in the pathophysiology of several chronic diseases like diabetes [7,11,24], macular degeneration [25] and cancer [26,27].
2.2. Oxidative stress and Alzheimer's disease
Oxygen is essential for sustaining life, but it does come with a cost as it is also a threat to biological systems. Particularly, the brain accounts for 20% of all the oxygen consumed by the body [28,29] while only weighing 2% of the total body weight [30]. These features make it highly susceptible to oxidative damage due to a high concentration of easily oxidizable polyunsaturated fatty acids, iron and metals, as well as the neuronal metabolic rate which mediates the continuous production of ROS [31]. Neurons are vulnerable to free radicals as there is a rather scarce amount of antioxidant enzymes compared to other organs. For example, catalase (CAT) in the brain is 10–20% of that found in liver and heart [32,33], as well as a high content of methyl ions in specific brain areas [34].
Evidence has consistently supported the involvement of OS as a major contributing factor in physiological ageing and the progression of multiple neurodegenerative pathologies [35–37]. Specifically, though the exact causes and mechanisms underlying AD progression remain unclear and multiple factors have been analysed, OS seems to be a leading contender and has gained importance with the progression of this disease [38,39]. This has been confirmed by the increase in markers of OS such as carbonyls, malondialdehydes and 4-hydroxynonenal [40,41]. Free radical damage in the ageing brain influences Aβ toxicity and tauopathy [42], which are responsible for impairment in memory, thinking and language abilities in AD patients [31,43].
2.3. Impact of oxidative stress on neurons
AD is a neurodegenerative disorder characterized by progressive memory loss and disorientation, with extracellular depositions of Aβ protein (senile plaques) and intracellular fibrillary deposits of hyperphosphorylated tau (P-tau) protein (neurofibrillary tangles, NFT). These neuropathological features can eventually lead to neuronal death and brain atrophy [44,45] with the loss of neuronal synapses leading to cognitive impairment [46,47]. Aβ results when there is abnormal cleavage of amyloid precursor protein (APP) by β- and γ-secretases [48,49]. The increase of soluble Aβ creates plaques and a toxic environment to neurons, leading to a decrease in the number and plasticity of synapses [50] and, consequently, initiating the formation of NFT [51]. Accumulation of Aβ causes a loss and malformation of spines due to spine turnover in dendrites [52,53], causing characteristic behavioural and cognitive deficits. It has been demonstrated through cell culture models, transgenic mouse models [21] and post-mortem brains of AD [54] that the abundance of ROS and neuronal oxidation [55–57] activates signalling pathways that alter APP or tau processing [58]. For example, a high concentration of OS stimulates c-Jun amino terminal kinase and p38 MAPK, which increases the expression of β-secretase [59], leading to Aβ deposition [60], while the activation of glycogen synthase kinase 3 (GSK-3β) triggers tau phosphorylation and formation of NFT. Also, in vitro and in vivo studies have shown that OS affects Aβ production and oligomerization, which generates free radicals and, in turn, causes APP processing to create more free radicals, leading to a vicious cycle of OS furthering the neurodegenerative process [61,62].
3. Supporting the hypothesis
3.1. Antioxidant system: endogenous and exogenous
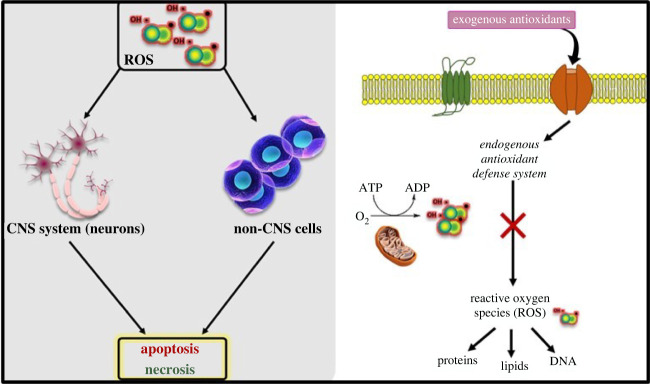
An ideal, physiologically functioning antioxidant system is a defense mechanism against ROS (figure 1) and is made up of two critical components—the endogenous antioxidants and the exogenous ones. Enzymatic and non-enzymatic endogenous antioxidants include SOD, CAT, glutathione peroxidase (GPX) and glutathione, which are made by the body and are therefore highly potent in repairing free radical damage as they initiate cell regeneration, work in membrane domains and act intracellularly to impact gene expression [64]. Studies have shown that enhanced endogenous antioxidant activity against ROS is directly achieved by the activation of the Nrf2/ARE signalling pathway (nuclear erythroid 2-related factor 2/antioxidant response element) which regulates the expression of these enzymes and is a key mediator in OS [64]. Exogenous antioxidants such as vitamin C, vitamin E, carotenoids and polyphenol are assimilated through diet, undergo a nutritional process and are responsible for repairing free radical damage extracellularly. This can be accomplished at a high kinetic rate by transferring one electron to reduce free radicals [65], quench oxygen singlets, stimulate cell regeneration [66,67] and sequester transition metals through a chelation process [68].
Figure 1.
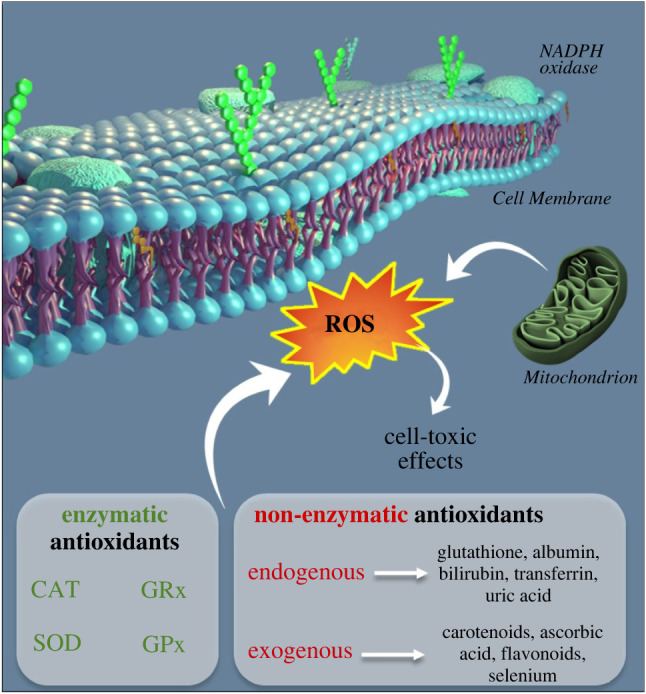
Enzymatic and non-enzymatic classification of antioxidants in the cytosol of cells, acting on the formation of ROS that illicit cell toxic effects.
Additionally, dietary exogenous antioxidants such as carotenoids and polyphenols impact the proper utility of these enzymatic endogenous antioxidants as they act as enzymatic cofactors [69] in maintaining or re-establishing redox homeostasis in the cell [70]. Studies have demonstrated that there is a potent synergism effect between exogenous and endogenous after revealing that the total moles of radicals neutralized and the velocity by which they reacted to remove free radicals increased due to this combination. This is relevant from a biological perspective given that a faster-marked reaction rate equates to less cellular targets damaged [71]. Thus, dietary exogenous antioxidants play a key role in reinforcing and replenishing the endogenous antioxidant enzymes to eliminate excess oxygen metabolites. Hence, an interactive and often synergistic action occurs between endogenous and exogenous antioxidants to maintain balance with ROS (figure 2) [7,72].
Figure 2.
The interplay of exogenous antioxidants and endogenous antioxidants to reduce ROS levels.
3.2. Astaxanthin
AST is a red xanthophyll carotenoid present in freshwater areas and produced by marine microorganisms such as bacteria, yeasts and fungi whereby the richest source is in the microalga Haematococcus pluvialis [1,73,74]. As a result, AST is consumed by fish such as salmon and trout, giving these organisms a dark red-orange pigment [75,76]. To maintain appreciable levels of AST, it is advised that AST be taken in supplement form (e.g. 4–20 mg), to obtain the beneficial effects rather than through diet alone, which would equate to 600–2000 t of salmon [77]. The unique configuration and size of AST—two hydroxylated ionone rings at both ends of the long carbon chain—allows the molecule to vertically bond to the polar heads of phospholipids and become easily incorporated into the membrane [78]. The shape of AST allows for increased bioavailability and quick absorption into lipids, prevents lipid peroxidation, and increases the stability and integrity of cell membranes [75]. Consequently, after consumption, AST is readily passed through the gastrointestinal (GI) tract into the blood and crosses the blood–brain barrier, eventually treating the brain using AST as a treatment of AD [79–81].
By contrast, comparative studies have shown that AST is 6000 times more potent than vitamin C and 100 times more potent than vitamin E in neutralizing toxic ROS without forming pro-oxidants, commonly known as a negative side effect among many other carotenoids [82,83]. The powerful antioxidant potential of AST is due to its ketone-bearing ionone rings which attract ROS into its polyene backbone. In addition, its ability to freely donate an electron and form chemical bonds with free radicals helps in scavenging free radicals and quenching singlet oxygen particles [84–86]. When a cell is stressed, AST inhibits phosphorylated extracellular regulated protein kinase/extracellular regulated protein kinase ratio (p-ERK/ERK) [87], which increases the concentration of haem oxygenase-1 (HO-1) due to the activation of Nrf2/ARE, consequently promoting the expression of SOD and other endogenous antioxidant enzymes [8,88,89]. These mechanisms of action are of importance given that ROS increases with normal brain ageing [90] and is further increased in AD. Therefore, additional antioxidants need to be in place to keep OS to a minimum.
3.2.1. AST: in vivo studies
AST's ability to ameliorate the cognitive decline in normal ageing and decrease the pathophysiology of AD have been investigated by researchers in animal and humans [91,92]. The supplement showed promotion of neuronal survival when differentiated pheochromocytoma (PC12) were subjected to Aβ [93,94] in a mouse model of AD. Extrapolated studies revealed that AST was able to protect neuroblastoma cells from amyloid toxicity by upregulating the HO-1 anti-oxidative enzyme of the haem pathway [95]. Another study demonstrated that the supplement decreased the amount of apoptotic-related mediators such as caspase 3,9, cytochrome C and protected L-glutamate induced cell death in PC12 cells through the Bcl-2/Bax apoptotic pathway [96–98]. These findings were replicated by Lobos et al., and the multipotent nature of the nutraceutical was further shown when primary hippocampal neurons were seen to be protected against amyloid-produced ROS [78]. Densitometry and western blot analysis showed that the expression of P-tau protein was inhibited with the use of AST in transgenic mice [99]. In a recent study, Hongo and colleagues [100] investigated the effects of AST intake on the cognitive and pathological progression of AD. They used AppNl-G-f mice which carry three APP knockout mutations with familial AD causing elevated levels of Aβ42 and observed decreased amyloid deposition, a decline in P-tau-positive areal fraction in the hippocampus (p < 0.001), increased activation of microglial in the area of amyloid deposition (p < 0.001) and a significantly higher mean Parvalbumin-positive (PV+) neuron density (p = 0.019) in the AST group over the control group. The Barnes maze test was later used to assess memory function in these mice and results indicated a significant difference in the number of visits to the goal region in the control-fed mice as opposed to the AST-fed mice (p = 0.0247). In another experiment, the nutraceutical was shown to enrich spatial learning and memory skills by reducing the number of reference memory errors and working memory errors made by APP/PS1 transgenic mice in a radial 8-arm maze apparatus and Morris water maze test (p < 0.05) [100]. Overall, AST has a multitude of neuroprotective effects, from reducing oxidative brain dysfunctions and preventing cellular toxicity and neuronal apoptosis to promoting survival in adult hippocampal neurogenesis and improving spatial memory [101,102].
3.2.2. Implication of astaxanthin in clinical studies
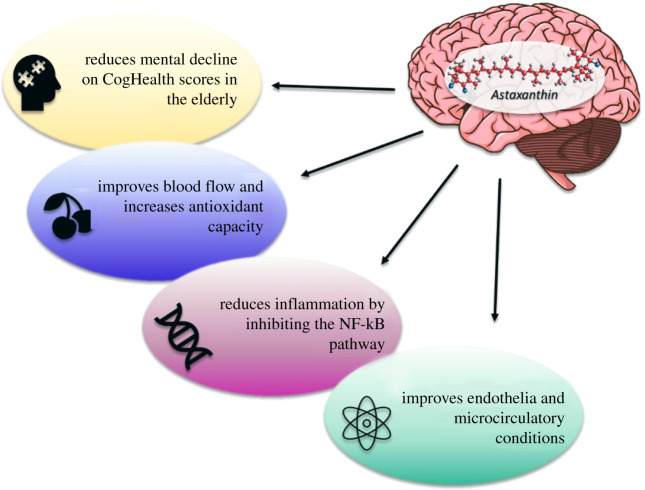
Some preliminary work has begun to examine the therapeutic effects of AST supplementation on humans based on results gathered from in vivo trials. A total of 96 healthy subjects ranging from 45 to 64 years of age, with self-reported complaints of age-related forgetfulness, were recruited for a randomized, double-blind, placebo-controlled human trial. It was discovered that AST supplementation, either in the 6 µg d−1 or 12 µg d−1 group, improved the performance of individuals to a greater degree than the placebo group [103]. Scores in memory and thinking were administered by the CogHealth tests while the executive function was tested using the Groton Maze Learning test, a maze learning paradigm. Repeated trials allowed for the assessment of learning and revealed that the nutraceutical improved cognitive function in aged individuals who had no underlying conditions and participants made fewer errors in the maze test. Subjects who were designated under the high-dose category expressed faster reaction times in the computerized, card-based design test. The efficacy of AST in improving cognitive function was demonstrated in an open-label trial with male participants aged 50–69 with symptoms of mild cognitive deficiencies who were treated with doses of 20 mg d−1 for 12 weeks [104]. The CogHealth test battery was used to assess neurocognitive functioning while the P300 was used to measure recognition and selective attention in the context of decision making [105]. Results showed an increase in brain function—cognition, attention, memory, information processing—compared to baseline values (figure 3). Dietary supplementation with AST has been shown to improve psychomotor speed, which is an indication of mental and physical coordination, comprehension and ability to perform complex tasks efficiently and accurately [106,107].
Figure 3.
Effects of Astaxanthin on neuronal functions, including the reduction of mental decline in ageing populations, improving blood flow and increasing antioxidant activity, reducing inflammation and inhibiting the NF-kB pathway and improving microcirculatory conditions.
Elevated lipid oxidation [108,109] and phospholipid hydroperoxide (PLOOH) are present in abnormally high levels in erythrocytes of AD patients. However, in a double-blind human trial with participants 50–69 years of age, it was noted that after a 12-week AST diet, plasma and erythrocyte concentrations of PLOOH decreased compared to the control group, which confirms that AST improves antioxidant levels in cells [110]. OS influences the pathogenesis of neuronal loss in neurodegenerative diseases, especially AD, and, as a result, the neuroprotective capability of this substance is of value for co-treatment in the prevention of these diseases [111].
3.3. Superoxide dismutase
As previously mentioned, O−2 is the most common ROS [26,112]. SOD is a metalloenzyme that forms the first-line antioxidant defense mechanism and is one of the major enzymatic components to detoxify superoxide radicals [113]. ROS production is a cascade effect that initially begins with the production of O−2 but can be neutralized by SOD as means of protecting the cells [114]. This renders the potentially harmful O−2 less hazardous [115–117]. By removing O−2, SODs decrease the risk of OH• formation via the Haber–Weiss-type reaction which has a 10 000-fold faster rate than spontaneous dismutation [119] and instead, reacts in the presence of Fe2+ through the Fenton reaction to form hydrogen peroxide (H2O2) and oxygen (O2). This enzyme is unique in that its activity determines the concentrations of O−2 and H2O2, the two Haber–Weiss reaction substrates, and is therefore likely to be central in the antioxidant defence mechanism [118,120]. SOD can eliminate O−2 rapidly due to its ability to convert a second-order reaction to a first-order reaction [121,122]. However, if the concentration of free radicals overwhelms the capacity of the enzyme, the O−2 can combine with NO to form peroxy-nitrite or undergo the Fenton reaction to form OH• radicals [123], which is a stronger oxidant and far more damaging than O−2.
SOD consists of three types of isoforms that are found in mammalian cells: copper/zinc SOD (CuZn-SOD), which is a cytoplasmic enzyme; manganese SOD (Mn-SOD, SOD2), which is a mitochondrial matrix enzyme; and extracellular SOD (EC-SOD, SOD3) [124]. Converging evidence confirms that most of the proteins associated in the pathogenesis of AD have direct involvement with mitochondrial enzyme SOD–MnSOD [125]. Observations from studies have shown that SOD knockout mice accelerate Aβ plaque deposition [126], increase tau phosphorylation [127] and worsen behavioural deficits [128], all suggesting that SOD plays a pivotal role in human ageing and AD. Unfortunately, it has been found that the SOD molecule is deactivated and does not become bioavailable as it passes through the GI tract once it encounters acids and enzymes [129,130]. As a result, scientists have worked around this problem by having SOD coupled with a protective protein derived from wheat, which can then sustain the gastric acids and be delivered in full form and absorbed into the bloodstream, thus effectively enhancing the body's own primary defence system [131,132]. Research has shown that oral SOD is linked with the induction of endogenous antioxidant enzymes' expression of CAT, GPX and further SOD [133,134]. These specific enzymes decrease in concentration and the production of free radicals increases with age [135], and it has been shown that this supplement could increase and strengthen the body's endogenous supply, increase the activities of all three enzymes and establish a reduction in neuronal damage in AD patients.
3.3.1. SOD: In vivo studies
The most abundant ROS in cells influencing synaptic plasticity, memory function and neuronal death is the superoxide radical [136]. SOD plays a protective role in neurodegeneration and has been shown to protect the ageing brain against human APP (hAPP)/Aβ-induced impairments after learning that inactivating one SOD allele in human hAPP transgenic mice led to a depletion of microtubule-associated protein 2 (neuronal dendritic marker) in the hippocampus and neocortex, decreased astrocytosis, promoted cerebrovascular amyloid gliosis and plaque-dependent neuritic dystrophy. In regard to behavioural issues, the lack of one SOD allele led to alterations in behaviour with lower anxiety levels and reduced disinhibition. Previous work conducted on mice with SOD deficiency in Tg2576 AD hastened the process of Aβ aggregation [137]. Other studies revealed that mice overexpressing SOD showed a reduction in plaque production and less memory impairments [138]. SOD supplementation was able to contrast the observed exacerbation of all AD-like features as well as counteract the manifestation of cognitive impairments by effectively responding to oxidative injuries [139,140]. OS affects AβPP processing at least in part by upregulating β-site AβPP cleavage enzyme BACE1 (beta-site APP cleaving enzyme 1) [141]. SOD treatment significantly downregulated the BACE1 enzyme in Tg19959 mice and brain levels of Aβ were decreased. SOD mimetics given to ageing mice for 6 months resulted in decreased lipid peroxidation, nucleic oxidation and ROS levels, and improved age-related decline in performance during fear conditioning tasks [142]. This study showed that age-dependent OS increases resulted in learning and memory deficits due to damage to the hippocampus and amygdala and could be significantly reduced by chronic treatment of SOD, indicating that it is oxidative load in aged mice that accounts for 50–60% of the variance in learning ability [56,143]. Antioxidant treatment, especially EUK-207, which is a SOD mimetic, inhibited the progression of tau phosphorylation and, in effect, decreased the disease symptoms in 3X-Tg-AD, an aggressive mouse model of AD [144].
3.3.2. Implication of SOD in clinical studies
Information from animal studies has been applied to clinical studies and similar outcomes have been seen in humans. A randomized clinical study of 20 healthy volunteers was exposed to pure oxygen (2.5 absolute atmospheres) for 60 min to induce cellular OS. They were then orally treated with Gliosidin (SOD supplement) and their SOD enzymatic activities were analysed in erythrocytes. It was found that SOD played a critical role in protecting DNA from cellular damage due to hyperbaric oxygen [131]. A double-blind, placebo-controlled pilot study was conducted using the melon juice concentrate, 10 mg Extramel (140 IU SOD per capsule) in 70 healthy volunteers between the ages of 30 and 55 years (mean = 40.26 years) with a BMI from 17 to 42, who felt daily stress and fatigue. Participants reported a reduction of stress and physical fatigue, as well as a significant improvement in cognitive performance on psychometric scales: Ferreri Anxiety Rating Diagram (p = 0.032), Cohen Perceived Stress scale (p = 0.01), 12-Item Short Form Survey (p = 0.049) after four weeks of oral supplementation [145]. A similar study was carried out by Carillon et al. [133], showing that three months of Extramel treatment improved the score on the Stroop test (27.9%; p < 0.001) which is used to measure cognitive flexibility, processing speed and overall executive function.
4. Testing the hypothesis
Oxidative damage is not just a by-product or end product of AD but is the direct initiation in the process of neurodegeneration [146]. Accumulation of amyloid in AD changes the expression of antioxidant genes, which further adds to the oxidative damage, free radicals and neuronal dysfunction [147]. Although the aetiology of AD is multifactorial, the combination of genetic and environmental factors, which includes nutrition, plays a central role in the onset and progression of the disease. Nutritional intervention can present a relevant route to achieve beneficial effects in AD treatment, at least in combination with pharmaceutical therapy. The administration of exogenous antioxidants is beneficial in treating the side effects of OS by compensating the inefficacy of the endogenous defense systems through inhibiting the intricate network of oxidative damage pathways and enhancing the systemic antioxidant response [20]. AST acts through exogenous antioxidant mechanisms as well as stimulating endogenous anti-oxidative enzymes [148,149,153], which is of importance given that, with age, the concentration of endogenous antioxidant enzymes decreases along with the efficiency and activity of these enzymes. Previous studies have shown that oral supplementation of SOD was found to promote the circulation, in blood and brain, of endogenous enzymes such as SOD and CAT [154]. This promoting effect will go on to combat the OS occurring in the cell and serve as neuroprotection. Substantial evidence discussed above suggests that the two nutraceutical molecules, AST and SOD, can synergistically work together as each plays a role in the exogenous and endogenous antioxidant system. Thus, it is predicted that AST and SOD will exhibit stronger anti-oxidative activity and provide pleiotropic functions than AST or SOD alone in improving learning and memory abilities in different degrees across AD patients. However, to date no experimental trials have been conducted to verify this dual combination. Future trials should consider administering combinations rather than single antioxidants to facilitate redox cycling as well as maximize bioavailability efficiency to different cellular compartments and establish the regimens for practical interventions at each stage of AD.
4.1. Limitations
The need to find and develop innovative delivery systems is of necessity when it comes to AST, due to its low bioavailability, poor water solubility and susceptibility to heat stress [150–152]. There have been some promising suggestions to address this drawback and, recently, AST has been formulated with lipid-based carriers such as oil-loaded solid lipid nanoparticles, constructed lipid carriers and cyclodextrin. These novel delivery systems have been shown to increase its stability and thus potentiate its antioxidant capacity [150,155,156]. Unfortunately, more experimentation is required to develop an appropriate and potent delivery system to precisely study AST's multi-target neuroprotective effects [157]. Other challenges include developing standardized, precise and definitive biomarkers of OS that can be used as early detection for AD [158,159]. From there, experiments need to be examined to validate if a causal relationship exists and whether those markers respond to antioxidant intervention [160]. Further studies should be conducted in order to determine whether the simultaneous ingestion of nutraceuticals contributes to an overall practical and beneficial healthcare strategy in the treatment of AD. Importantly, an optimum combination of SOD and AST in terms of doses is an area that still needs to be analysed, as the removal of many ROS by supplementation of antioxidants may cause ‘anti-oxidative stress’, which can be detrimental to neuron physiology by disrupting cell signalling pathways in the brain and worsening the disease [161–163].
4.2. Translational concerns
Despite clear implications that oxidative damage is a key factor in the pathophysiology of AD and literature suggesting the therapeutic nature of AST and SOD in targeting ROS/antioxidant imbalance, there seems to be a translational problem. Improper design of human intervention studies [164,165] such as a low number of recruited participants [145], short duration of treatment and analysis of end points, which are related to pharmacokinetic and pharmacodynamic constraints, can all prevent the possibility to detect potential improvements in cognitive function in these patients. The lack of efficient animal models to mimic the overall pathophysiological conditions in the pathogenesis of AD in humans could also be a reason for low clinical applications [166]. There is also a concern to determine different doses depending on gender, age, underlying health issues in accordance with AD, previous drug use, social habits, baseline nutritional levels of patients, genotype and biochemical status, which could explain inter-individual differences in terms of bioavailability [167]. Decreased rates of success in clinical trials could be the result of supplements being administered in patients with advanced stages of AD, while most of the in vivo studies have been performed at earlier stages, and therefore a very large time gap exists, possibly decades, from pre-clinical signs to the clinical onset of AD [168,169]. Therefore, these supplements should be tested in earlier phases of the disease to uncover their therapeutic potential. Lastly, the most crucial factor is the brain's complexity. OS causes neuronal dysfunction inducing compensatory responses and a change in neural circuitry [170]. This scenario complicates the efficacy of antioxidant therapy due to the regeneration of the lost neuronal network that is found in the ageing brain.
5. Conclusion
The prevalence and high mortality of AD presents medical and financial burdens on society, specifically the patients' caregivers. An important feature of ageing is the weakening of the biological antioxidant system defence system such as the loss of endogenous antioxidant enzymes like SOD, as well as the increasing levels of ROS, creating a state of OS in the brain. Many AD histopathological studies confirm disruption of the redox homeostasis in the brain as playing a fundamental role in amyloid plaque formation and hyperphosphorylation of tau protein [171], which contribute to impairments in cognition [172]. Focusing on the development and utilization of antioxidant therapies can assist in reducing toxic depositions. Various clinical and basic research studies have provided support for antioxidant treatment in AD, and it is likely that a single antioxidant may not be sufficiently resistant to oxidative damage given that OS is modulated by a complex system of endogenous and exogenous antioxidants [78]. The integrated approach of AST and SOD antioxidant therapy, along with first-line synthetic drugs, is suggested to provide a promising natural treatment alternative in delaying the progression of AD. Future research can elucidate the role of both these compounds, which can provide insight into providing safe and potent neuroprotective agents that could improve the quality of life and life expectancy of these patients.
Acknowledgements
V.B. would like to thank Lucshnavy Balendra for her unwavering support and steadfast encouragement. Special appreciation goes to J.C.L. This work was performed with resources from the non-government/non-profit organization Indian Scientific Education and Technology Foundation, Lucknow, India.
Data accessibility
This article does not contain any additional data.
Authors' contributions
V.B. is responsible for conceptualization, literature review, synthesis and writing. S.K.S. performed editing and proofreading of the manuscript.
Competing interests
We declare we have no competing interests
Funding
We received no funding for this study.
Financial disclosure
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- 1.Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. 2017. Neuroprotective mechanisms of Astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience 39, 19-32. ( 10.1007/s11357-017-9958-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, van der Flier WM. 2015. Alzheimer's disease first symptoms are age dependent: evidence from the NACC dataset. Alzheimer's Dementia 11, 1349-1357. ( 10.1016/j.jalz.2014.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowell ER, et al. 2007. Sex differences in cortical thickness mappedF in 176 healthy individuals between 7 and 87 years of age. Cerebral cortex 17, 1550-1560. ( 10.1093/cercor/bhl066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47-95. ( 10.1152/physrev.00018.2001) [DOI] [PubMed] [Google Scholar]
- 5.Kurutas EB. 2016. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 15, 71. ( 10.1186/s12937-016-0186-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Autréaux B, Toledano MB. 2007. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813-824. ( 10.1038/nrm2256) [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44-84. ( 10.1016/j.biocel.2006.07.001) [DOI] [PubMed] [Google Scholar]
- 8.Kansanen E, Jyrkkänen HK, Levonen AL. 2012. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Rad. Biol. Med. 52, 973-982. ( 10.1016/j.freeradbiomed.2011.11.038) [DOI] [PubMed] [Google Scholar]
- 9.Young IS, Woodside JV. 2001. Antioxidants in health and disease. J. Clin. Pathol. 54, 176-186. ( 10.1136/jcp.54.3.176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu BP, Suescun EA, Yang SY. 1992. Effect of age-related lipid peroxidation on membrane fluidity and phospholipase A2: modulation by dietary restriction. Mech. Ageing Dev. 65, 17-33. ( 10.1016/0047-6374(92)90123-U) [DOI] [PubMed] [Google Scholar]
- 11.Aruoma O, Neergheen VS, Bahorun T, Jen L. 2006. Free radicals, antioxidants and diabetes: Embryopathy, retinopathy, neuropathy, nephropathy and cardiovascular complications. Neuroembryol. Aging 4, 117-137. ( 10.1159/000109344) [DOI] [Google Scholar]
- 12.Rammal H, Bouayed J, Soulimani R. 2010. A direct relationship between aggressive behavior in the resident/intruder test and cell oxidative status in adult male mice. Eur. J. Pharmacol. 627, 173-176. ( 10.1016/j.ejphar.2009.11.001) [DOI] [PubMed] [Google Scholar]
- 13.Sauer H, Wartenberg M, Hescheler J. 2001. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. physiol. Biochem. 11, 173-186. ( 10.1159/000047804) [DOI] [PubMed] [Google Scholar]
- 14.Grivennikova VG, Vinogradov AD. 2006. Generation of superoxide by the mitochondrial Complex I. Biochim. et Biophys. Acta 1757, 553-561. ( 10.1016/j.bbabio.2006.03.013) [DOI] [PubMed] [Google Scholar]
- 15.Singh PP, Mahadi F, Roy A, Sharma P. 2009. Reactive oxygen species, reactive nitrogen species and antioxidants in etiopathogenesis of diabetes mellitus type-2. Indian J. Clin. Biochem. 24, 324-342. ( 10.1007/s12291-009-0062-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turrens JF. 2003. Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335-344. ( 10.1113/jphysiol.2003.049478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliwell B, Gutteridge JM. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219, 1-14. ( 10.1042/bj2190001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. 2004. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Phys. India 52, 794-804. [PubMed] [Google Scholar]
- 19.Xiao M, Zhong H, Xia L, Tao Y, Yin H. 2017. Pathophysiology of mitochondrial lipid oxidation: role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Rad. Biol. Med. 111, 316-327. ( 10.1016/j.freeradbiomed.2017.04.363) [DOI] [PubMed] [Google Scholar]
- 20.Bouayed J, Bohn T. 2010. Exogenous antioxidants–double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longevity 3, 228-237. ( 10.4161/oxim.3.4.12858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gómez-Ramos A, Díaz-Nido J, Smith MA, Perry G, Avila J. 2003. Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. J. Neurosci. Res. 71, 863-870. ( 10.1002/jnr.10525) [DOI] [PubMed] [Google Scholar]
- 22.Di Meo S, Reed TT, Venditti P, Victor VM. 2016. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longevity 2016, 1245049. ( 10.1155/2016/1245049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. 2008. Nucleic acid oxidation in Alzheimer disease. Free Rad. Biol. Med. 44, 1493-1505. ( 10.1016/j.freeradbiomed.2008.01.002) [DOI] [PubMed] [Google Scholar]
- 24.Lenzen S. 2008. Oxidative stress: the vulnerable beta-cell. Biochem. Soc. Trans. 36, 343-347. ( 10.1042/BST0360343) [DOI] [PubMed] [Google Scholar]
- 25.Beatty S, Koh H, Phil M, Henson D, Boulton M. 2000. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 45, 115-134. ( 10.1016/S0039-6257(00)00140-5) [DOI] [PubMed] [Google Scholar]
- 26.Salman KA, Ashraf S. 2015. Reactive oxygen species: a link between chronic inflammation and cancer. Asia-Pacific J. Mol. Biol. Biotechnol. 22, 42-49. [Google Scholar]
- 27.Toyokuni S. 2008. Molecular mechanisms of oxidative stress-induced carcinogenesis: from epidemiology to oxygenomics. IUBMB Life 60, 441-447. ( 10.1002/iub.61) [DOI] [PubMed] [Google Scholar]
- 28.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. 1997. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 17, 2653-2657. ( 10.1523/JNEUROSCI.17-08-02653.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu BP. 1994. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 74, 139-162. ( 10.1152/physrev.1994.74.1.139) [DOI] [PubMed] [Google Scholar]
- 30.Bigos KL, Hariri AR. 2007. Neuroimaging: technologies at the interface of genes, brain, and behavior. Neuroimaging Clin. North Am. 17, 459-467. ( 10.1016/j.nic.2007.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang WJ, Zhang X, Chen WW. 2016. Role of oxidative stress in Alzheimer's disease. Biomed. Rep. 4, 519-522. ( 10.3892/br.2016.630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, Weber JR. 2002. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Invest. 109, 19-27. ( 10.1172/JCI12035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalaria RN. 1999. Microglia and Alzheimer's disease. Curr. Opin. Hematol. 6, 15-24. ( 10.1097/00062752-199901000-00004) [DOI] [PubMed] [Google Scholar]
- 34.Floyd RA, Carney JM. 1992. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann. Neurol. 32, S22-S27. ( 10.1002/ana.410320706) [DOI] [PubMed] [Google Scholar]
- 35.Chinta SJ, Andersen JK. 2008. Redox imbalance in Parkinson's disease. Biochim. Biophys. Acta 1780, 1362-1367. ( 10.1016/j.bbagen.2008.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD. 2016. The role of reactive oxygen species in the pathogenesis of Alzheimer's disease, Parkinson's disease, and Huntington's disease: a mini review. Oxid. Med. Cell. Longevity 2016, 8590578. ( 10.1155/2016/8590578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meraz-Ríos MA, Franco-Bocanegra D, Toral Rios D, Campos-Peña V. 2014. Early onset Alzheimer's disease and oxidative stress. Oxid. Med. Cell. Longevity 2014, 375968. ( 10.1155/2014/375968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. 2018. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 14, 450-464. ( 10.1016/j.redox.2017.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marlatt M, Lee HG, Perry G, Smith MA, Zhu X. 2004. Sources and mechanisms of cytoplasmic oxidative damage in Alzheimer's disease. Acta Neurobiol. Exp. 64, 81-87. [DOI] [PubMed] [Google Scholar]
- 40.Perluigi M, Sultana R, Cenini G, Di Domenico F, Memo M, Pierce WM, Coccia R, Butterfield DA. 2009. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer's disease: role of lipid peroxidation in Alzheimer's disease pathogenesis. Proteomics 3, 682-693. ( 10.1002/prca.200800161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 1997. 4-hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J. Neurochem. 68, 2092-2097. ( 10.1046/j.1471-4159.1997.68052092.x) [DOI] [PubMed] [Google Scholar]
- 42.Floyd R, Hensley K. 2002. Oxidative stress in brain aging: implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 23, 795-807. ( 10.1016/S0197-4580(02)00019-2) [DOI] [PubMed] [Google Scholar]
- 43.Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. 2005. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann. Neurol. 58, 730-735. ( 10.1002/ana.20629) [DOI] [PubMed] [Google Scholar]
- 44.Butterfield DA, Drake J, Pocernich C, Castegna A. 2001. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 7, 548-554. ( 10.1016/S1471-4914(01)02173-6) [DOI] [PubMed] [Google Scholar]
- 45.Schachter AS, Davis KL. 2000. Alzheimer's disease. Dialogues Clin. Neurosci. 2, 91-100. ( 10.31887/DCNS.2000.2.2/asschachter) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeKosky ST, Scheff SW. 1990. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 27, 457-464. ( 10.1002/ana.410270502) [DOI] [PubMed] [Google Scholar]
- 47.Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. 1994. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci. Lett. 174, 67-72. ( 10.1016/0304-3940(94)90121-X) [DOI] [PubMed] [Google Scholar]
- 48.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. 1990. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science 248, 1122-1124. ( 10.1126/science.2111583) [DOI] [PubMed] [Google Scholar]
- 49.Selkoe DJ. 1998. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 8, 447-453. ( 10.1016/S0962-8924(98)01363-4) [DOI] [PubMed] [Google Scholar]
- 50.Rajmohan R, Reddy PH. 2017. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer's disease neurons. J. Alzheimer's Dis. 57, 975-999. ( 10.3233/JAD-160612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardy JA, Higgins GA. 1992. Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184-185. ( 10.1126/science.1566067) [DOI] [PubMed] [Google Scholar]
- 52.Meyer-Luehmann M, et al. 2008. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature 451, 720-724. ( 10.1038/nature06616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai J, Grutzendler J, Duff K, Gan WB. 2004. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 7, 1181-1183. ( 10.1038/nn1335) [DOI] [PubMed] [Google Scholar]
- 54.Nunomura A, et al. 2001. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 60, 759-767. ( 10.1093/jnen/60.8.759) [DOI] [PubMed] [Google Scholar]
- 55.Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. 2005. Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol. Med. 11, 164-169. ( 10.1016/j.molmed.2005.02.008) [DOI] [PubMed] [Google Scholar]
- 56.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. 2003. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl Acad. Sci. USA 100, 8526-8531. ( 10.1073/pnas.1332809100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda A, Smith MA, Avilá J, Nunomura A, Siedlak SL, Zhu X, Perry G, Sayre LM. 2000. In Alzheimer's disease, heme oxygenase is coincident with Alz50, an epitope of tau induced by 4-hydroxy-2-nonenal modification. J. Neurochem. 75, 1234-1241. ( 10.1046/j.1471-4159.2000.0751234.x) [DOI] [PubMed] [Google Scholar]
- 58.Wu Z, Zhao Y, Zhao B. 2010. Superoxide anion, uncoupling proteins and Alzheimer's disease. J. Clin. Biochem. Nutr. 46, 187-194. ( 10.3164/jcbn.09-104-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR. 2004. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J. Alzheimer's Dis. 6, 659-681. ( 10.3233/JAD-2004-6610) [DOI] [PubMed] [Google Scholar]
- 60.Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, Smith MA. 2000. Neuronal oxidative stress precedes amyloid-beta deposition in Down Syndrome. J. Neuropathol. Exp. Neurol. 59, 1011-1017. ( 10.1093/jnen/59.11.1011) [DOI] [PubMed] [Google Scholar]
- 61.Cras P, Smith MA, Richey PL, Siedlak SL, Mulvihill P, Perry G. 1995. Extracellular neurofibrillary tangles reflect neuronal loss and provide further evidence of extensive protein cross-linking in Alzheimer disease. Acta Neuropathol. 89, 291-295. ( 10.1007/BF00309621) [DOI] [PubMed] [Google Scholar]
- 62.Ramassamy C, et al. 1999. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer's disease is related to the apolipoprotein E genotype. Free Rad. Biol. Med. 27, 544-553. ( 10.1016/S0891-5849(99)00102-1) [DOI] [PubMed] [Google Scholar]
- 63.Nguyen T, Nioi P, Pickett CB. 2009. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 284, 13 291-13 295. ( 10.1074/jbc.R900010200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasconcelos AR, Dos Santos NB, Scavone C, Munhoz CD. 2019. Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front. Pharmacol. 10, 33. ( 10.3389/fphar.2019.00033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prior RL, Wu X, Schaich K. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agricult. Food Chem. 53, 4290-4302. ( 10.1021/jf0502698) [DOI] [PubMed] [Google Scholar]
- 66.Andre CM, Larondelle Y, Evers D. 2010. Dietary antioxidants and oxidative stress from a human and plant perspective: a review. Curr. Nutr. Food Science 6, 2-12. ( 10.2174/157340110790909563) [DOI] [Google Scholar]
- 67.Biehler E, Bohn T. 2010. Methods for assessing aspects of carotenoid bioavailability. Curr. Nutr. Food Sci. 6, 44-69. ( 10.2174/157340110790909545) [DOI] [Google Scholar]
- 68.Nguyen T, Sherratt PJ, Pickett CB. 2003. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Ann. Rev. Pharmacol. Toxicol. 43, 233-260. ( 10.1146/annurev.pharmtox.43.100901.140229) [DOI] [PubMed] [Google Scholar]
- 69.Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. 2018. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 63, 68-78. ( 10.1016/j.advms.2017.05.005) [DOI] [PubMed] [Google Scholar]
- 70.Achike FI, Murugan DD. 2020. Quercetin and antioxidant potential in diabetes. In Diabetes (ed. Preedy VR), pp. 293-302. New York, NY: Academic Press. [Google Scholar]
- 71.Noguer M, Cerezo AB, Moyá L, Troncoso AM, García-Parrilla MC. 2014. Synergism effect between phenolic metabolites and endogenous antioxidants in terms of antioxidant activity. Adv. Chem. Eng. Sci. 4, 258-265. ( 10.4236/aces.2014.42029) [DOI] [Google Scholar]
- 72.Bouayed J, Rammal H, Soulimani R. 2009. Oxidative stress and anxiety: relationship and cellular pathways. Oxid. Med. Cell. Longevity 2, 63-67. ( 10.4161/oxim.2.2.7944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galasso C, Corinaldesi C, Sansone C. 2017. Carotenoids from marine organisms: biological functions and industrial applications. Antioxidants (Basel, Switzerland) 6, 96. ( 10.3390/antiox6040096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan JP, Peng J, Yin K, Wang JH. 2011. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 55, 150-165. ( 10.1002/mnfr.201000414) [DOI] [PubMed] [Google Scholar]
- 75.Guerin M, Huntley ME, Olaizola M. 2003. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 21, 210-216. ( 10.1016/S0167-7799(03)00078-7) [DOI] [PubMed] [Google Scholar]
- 76.Kidd P. 2011. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Alter. Med. Rev. 16, 355-364. [PubMed] [Google Scholar]
- 77.Seabra LMJ, Pedrosa LFC. 2010. Astaxanthin: structural and functional aspects. Revista de Nutrição 23, 1041-1050. ( 10.1590/S1415-52732010000600010) [DOI] [Google Scholar]
- 78.Lobos P, Bruna B, Cordova A, Barattini P, Galáz JL, Adasme T, Hidalgo C, Muñoz P, Paula-Lima A. 2016. Astaxanthin protects primary hippocampal neurons against noxious effects of Aβ-oligomers. Neural Plasticity 2016, 3456783. ( 10.1155/2016/3456783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gualtieri CT, Johnson LG. 2005. Neurocognitive testing supports a broader concept of mild cognitive impairment. Am. J. Alzheimer's Dis. Other Dem. 20, 359-366. ( 10.1177/153331750502000607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakagawa K, Kiko T, Miyazawa T, Carpentero Burdeos G, Kimura F, Satoh A, Miyazawa T. 2011. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 105, 1563-1571. ( 10.1017/S0007114510005398) [DOI] [PubMed] [Google Scholar]
- 81.Petri D, Lundebye AK. 2007. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp. Biochem. Physiol. Part C 145, 202-209. ( 10.1016/j.cbpc.2006.12.008) [DOI] [PubMed] [Google Scholar]
- 82.Goto S, Kogure K, Abe K, Kimata Y, Kitahama K, Yamashita E, Terada H. 2001. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 1512, 251-258. ( 10.1016/S0005-2736(01)00326-1) [DOI] [PubMed] [Google Scholar]
- 83.Nishida Y, Yamashita E. 2007. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 11, 16-20. [Google Scholar]
- 84.Dose J, Matsugo S, Yokokawa H, Koshida Y, Okazaki S, Seidel U, Eggersdorfer M, Rimbach G, Esatbeyoglu T. 2016. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 17, 103. ( 10.3390/ijms17010103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galasso C, Orefice I, Toscano A, Vega Fernández T, Musco L, Brunet C, Sansone C, Cirino P. 2018. Food modulation controls astaxanthin accumulation in eggs of the sea urchin Arbacia lixula. Mar. Drugs 16, 186. ( 10.3390/md16060186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson H, Braun CL, Ernst H. 2008. The chemistry of novel xanthophyll carotenoids. Am. J. Cardiol. 101(10A), 50D-57D. ( 10.1016/j.amjcard.2008.02.008) [DOI] [PubMed] [Google Scholar]
- 87.Fakhri S, Abbaszadeh F, Dargahi L, Jorjani M. 2018. Astaxanthin: a mechanistic review on its biological activities and health benefits. Pharmacol. Res. 136, 1-20. ( 10.1016/j.phrs.2018.08.012) [DOI] [PubMed] [Google Scholar]
- 88.Dani C, et al. 2004. Role of heme oxygenase and bilirubin in oxidative stress in preterm infants. Ped. Res. 56, 873-877. ( 10.1203/01.PDR.0000145281.12853.9E) [DOI] [PubMed] [Google Scholar]
- 89.Araujo JA, Zhang M, Yin F. 2012. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 3, 119. ( 10.3389/fphar.2012.00119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. 2014. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar. Drugs 12, 128-152. ( 10.3390/md12010128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen X, Huang A, Hu J, Zhong Z, Liu Y, Li Z, Pan X, Liu Z. 2015. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: involvement of the Akt/GSK-3β pathway. Neuroscience 303, 558-568. ( 10.1016/j.neuroscience.2015.07.034) [DOI] [PubMed] [Google Scholar]
- 92.Wu H, Niu H, Shao A, Wu C, Dixon BJ, Zhang J, Yang S, Wang Y. 2015. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs 13, 5750-5766. ( 10.3390/md13095750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang CH, Chen CY, Chiou JY, Peng RY, Peng CH. 2010. Astaxanthine secured apoptotic death of PC12 cells induced by beta-amyloid peptide 25–35: its molecular action targets. J. Med. Food 13, 548-556. ( 10.1089/jmf.2009.1291) [DOI] [PubMed] [Google Scholar]
- 94.Otton R, Marin DP, Bolin AP, Santos R, Polotow TG, Sampaio SC, de Barros MP. 2010. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem. Biol. Inter. 186, 306-315. ( 10.1016/j.cbi.2010.05.011) [DOI] [PubMed] [Google Scholar]
- 95.Wang HQ, Sun XB, Xu YX, Zhao H, Zhu QY, Zhu CQ. 2010. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 1360, 159-167. ( 10.1016/j.brainres.2010.08.100) [DOI] [PubMed] [Google Scholar]
- 96.Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. 2018. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res. Bullet. 143, 217-224. ( 10.1016/j.brainresbull.2018.09.011) [DOI] [PubMed] [Google Scholar]
- 97.Masoudi A, Dargahi L, Abbaszadeh F, Pourgholami MH, Asgari A, Manoochehri M, Jorjani M. 2017. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav. Brain Res. 329, 104-110. ( 10.1016/j.bbr.2017.04.026) [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Wang W, Wang H, Cui X, Zhang L. 2015. Astaxanthin protects PC12 cells from glutamate-induced neurotoxicity through multiple signaling pathways. J. Funct. Foods 16, 137-151. ( 10.1016/j.jff.2015.04.008) [DOI] [Google Scholar]
- 99.Che H, et al. 2018. Effects of astaxanthin and docosahexaenoic-acid-acylated astaxanthin on Alzheimer's disease in APP/PS1 double-transgenic mice. J. Agricult. Food Chem. 66, 4948-4957. ( 10.1021/acs.jafc.8b00988) [DOI] [PubMed] [Google Scholar]
- 100.Hongo N, Takamura Y, Nishimaru H, Matsumoto J, Tobe K, Saito T, Saido TC, Nishijo H. 2020. Astaxanthin ameliorated parvalbumin-positive neuron deficits and Alzheimer's disease-related pathological progression in the hippocampus of AppNL-G-F/NL-G-F mice. Front. Pharmacol. 11, 307. ( 10.3389/fphar.2020.00307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X, Shibata T, Hisaka S, Osawa T. 2009. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 1254, 18-27. ( 10.1016/j.brainres.2008.11.076) [DOI] [PubMed] [Google Scholar]
- 102.Xue Y, Qu Z, Fu J, Zhen J, Wang W, Cai Y, Wang W. 2017. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bullet. 131, 221-228. ( 10.1016/j.brainresbull.2017.04.019) [DOI] [PubMed] [Google Scholar]
- 103.Katagiri M, Satoh A, Tsuji S, Shirasawa T. 2012. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: a randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 51, 102-107. ( 10.3164/jcbn.D-11-00017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Satoh A, et al. 2009. Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich Haematococcus pluvialis extract. J. Clin. Biochem. Nutr. 44, 280-284. ( 10.3164/jcbn.08-238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Polich J, Ehlers CL, Otis S, Mandell AJ, Bloom FE. 1986. P300 latency reflects the degree of cognitive decline in dementing illness. Electroencephalogr. Clin. Neurophysiol. 63, 138-144. ( 10.1016/0013-4694(86)90007-6) [DOI] [PubMed] [Google Scholar]
- 106.Murakami K, et al. 2011. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid β protein oligomerization and memory loss in mouse model of Alzheimer disease. J. Biol. Chem. 286, 44 557-44 568. ( 10.1074/jbc.M111.279208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terry RD, Pena C. 1965. Experimental production of neurofibrillary degeneration. 2. Electron microscopy, phosphatase histochemistry and electron probe analysis. J. Neuropathol. Exp. Neurol. 24, 200-210. ( 10.1097/00005072-196504000-00003) [DOI] [PubMed] [Google Scholar]
- 108.Kawamoto EM, et al. 2005. Oxidative state in platelets and erythrocytes in aging and Alzheimer's disease. Neurobiol. Aging 26, 857-864. ( 10.1016/j.neurobiolaging.2004.08.011) [DOI] [PubMed] [Google Scholar]
- 109.Kawamoto EM, Vasconcelos AR, Degaspari S, Böhmer AE, Scavone C, Marcourakis T. 2013. Age-related changes in nitric oxide activity, cyclic GMP, and TBARS levels in platelets and erythrocytes reflect the oxidative status in central nervous system. Age (Dordrecht, Netherlands) 35, 331-342. ( 10.1007/s11357-011-9365-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyazawa T, Nakagawa K, Kimura F, Satoh A, Miyazawa T. 2011. Plasma carotenoid concentrations before and after supplementation with astaxanthin in middle-aged and senior subjects. Biosci. Biotechnol. Biochem. 75, 1856-1858. ( 10.1271/bbb.110368) [DOI] [PubMed] [Google Scholar]
- 111.Cho KS, Shin M, Kim S, Lee SB. 2018. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longevity 2018, 4120458. ( 10.1155/2018/4120458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drew B, Leeuwenburgh C. 2002. Aging and the role of reactive nitrogen species. Ann. New York Acad. Sci. 959, 66-81. ( 10.1111/j.1749-6632.2002.tb02084.x) [DOI] [PubMed] [Google Scholar]
- 113.Gonzalez FJ. 2005. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat. Res. 569, 101-110. ( 10.1016/j.mrfmmm.2004.04.021) [DOI] [PubMed] [Google Scholar]
- 114.Liguori I, et al. 2018. Oxidative stress, aging, and diseases. Clin. Interventions Aging 13, 757-772. ( 10.2147/CIA.S158513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dringen R, Pawlowski PG, Hirrlinger J. 2005. Peroxide detoxification by brain cells. J. Neurosci. Res. 79, 157-165. ( 10.1002/jnr.20280) [DOI] [PubMed] [Google Scholar]
- 116.Fridovich I. 1995. Superoxide radical and superoxide dismutases. Ann. Rev. Biochem. 64, 97-112. ( 10.1146/annurev.bi.64.070195.000525) [DOI] [PubMed] [Google Scholar]
- 117.Ighodaro OM, Akinloye OA. 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54, 287-293. ( 10.1016/j.ajme.2017.09.001) [DOI] [Google Scholar]
- 118.Alfadda AA, Sallam RM. 2012. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 936486. ( 10.1155/2012/936486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bolisetty S, Jaimes EA. 2013. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int. J. Mol. Sci. 14, 6306-6344. ( 10.3390/ijms14036306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seifried HE, Anderson DE, Fisher EI, Milner JA. 2007. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 18, 567-579. ( 10.1016/j.jnutbio.2006.10.007) [DOI] [PubMed] [Google Scholar]
- 121.Fridovich I. 1974. Superoxide dismutases. Adv. Enzymol. Related Areas Mol. Biol. 41, 35-97. ( 10.1002/9780470122860.ch2) [DOI] [PubMed] [Google Scholar]
- 122.Fridovich I. 1975. Superoxide dismutases. Ann. Rev. Biochem. 44, 147-159. ( 10.1146/annurev.bi.44.070175.001051) [DOI] [PubMed] [Google Scholar]
- 123.Imlay JA, Linn S. 1988. DNA damage and oxygen radical toxicity. Science 240, 1302-1309. ( 10.1126/science.3287616) [DOI] [PubMed] [Google Scholar]
- 124.Valentine JS, Doucette PA, Zittin Potter S. 2005. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Ann. Rev. Biochem. 74, 563-593. ( 10.1146/annurev.biochem.72.121801.161647) [DOI] [PubMed] [Google Scholar]
- 125.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. 2003. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell Biol. 161, 41-54. ( 10.1083/jcb.200207030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li F, et al. 2004. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J. Neurochem. 89, 1308-1312. ( 10.1111/j.1471-4159.2004.02455.x) [DOI] [PubMed] [Google Scholar]
- 127.Melov S, et al. 2007. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE 2, e536. ( 10.1371/journal.pone.0000536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Esposito L, et al. 2006. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J. Neurosci. 26, 5167-5179. ( 10.1523/JNEUROSCI.0482-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Regnault C, Soursac M, Roch-Arveiller M, Postaire E, Hazebroucq G. 1996. Pharmacokinetics of superoxide dismutase in rats after oral administration. Biophar. Drug Disposition 17, 165-174. () [DOI] [PubMed] [Google Scholar]
- 130.Vouldoukis I, Conti M, Krauss P, Kamaté C, Blazquez S, Tefit M, Mazier D, Calenda A, Dugas B. 2004. Supplementation with gliadin-combined plant superoxide dismutase extract promotes antioxidant defences and protects against oxidative stress. Phytother. Res. 18, 957-962. ( 10.1002/ptr.1542) [DOI] [PubMed] [Google Scholar]
- 131.Muth CM, Glenz Y, Klaus M, Radermacher P, Speit G, Leverve X. 2004. Influence of an orally effective SOD on hyperbaric oxygen-related cell damage. Free Rad. Res. 38, 927-932. ( 10.1080/10715760412331273197) [DOI] [PubMed] [Google Scholar]
- 132.Naito Y, et al. 2005. Reduction of diabetes-induced renal oxidative stress by a cantaloupe melon extract/gliadin biopolymers, oxykine, in mice. BioFactors (Oxford, England) 23, 85-95. ( 10.1002/biof.5520230204) [DOI] [PubMed] [Google Scholar]
- 133.Carillon J, Rouanet JM, Cristol JP, Brion R. 2013. Superoxide dismutase administration, a potential therapy against oxidative stress related diseases: Several routes of supplementation and proposal of an original mechanism of action. Pharm. Res. 30, 2718-2728. ( 10.1007/s11095-013-1113-5) [DOI] [PubMed] [Google Scholar]
- 134.Romao S. 2015. Therapeutic value of oral supplementation with melon superoxide dismutase and wheat gliadin combination. Nutrition (Burbank, Los Angeles County, Calif.) 31, 430-436. ( 10.1016/j.nut.2014.10.006) [DOI] [PubMed] [Google Scholar]
- 135.Sohal RS, Weindruch R. 1996. Oxidative stress, caloric restriction, and aging. Science (New York, N.Y.) 273, 59-63. ( 10.1126/science.273.5271.59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Resende R, Moreira PI, Proença T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. 2008. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Rad. Biol. Med. 44, 2051-2057. ( 10.1016/j.freeradbiomed.2008.03.012) [DOI] [PubMed] [Google Scholar]
- 137.Murakami K, Shimizu T, Irie K. 2011. Formation of the 42-mer amyloid β radical and the therapeutic role of superoxide dismutase in Alzheimer's disease. J. Amino Acids 2011, 654207. ( 10.4061/2011/654207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Massad CA, Washington TM, Pautler RG, Klann E. 2009. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 106, 13 576-13 581. ( 10.1073/pnas.0902714106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fleming JL, Phiel CJ, Toland AE. 2012. The role for oxidative stress in aberrant DNA methylation in Alzheimer's disease. Curr. Alzheimer Res. 9, 1077-1096. ( 10.2174/156720512803569000) [DOI] [PubMed] [Google Scholar]
- 140.Niedzwiecki MM, et al. 2013. Blood glutathione redox status and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Epigenetics 8, 730-738. ( 10.4161/epi.25012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hampel H, et al. 2020. The β-secretase BACE1 in Alzheimer's disease. Biol. Psychiatry 89, 745-756. ( 10.1016/j.biopsych.2020.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clausen A, Doctrow S, Baudry M. 2010. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol. Aging 31, 425-433. ( 10.1016/j.neurobiolaging.2008.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. 2001. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 21, 8348-8353. ( 10.1523/JNEUROSCI.21-21-08348.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clausen A, Xu X, Bi X, Baudry M. 2012. Effects of the superoxide dismutase/catalase mimetic EUK-207 in a mouse model of Alzheimer's disease: protection against and interruption of progression of amyloid and tau pathology and cognitive decline. J. Alzheimer's Dis. 30, 183-208. ( 10.3233/JAD-2012-111298) [DOI] [PubMed] [Google Scholar]
- 145.Milesi MA, Lacan D, Brosse H, Desor D, Notin C. 2009. Effect of an oral supplementation with a proprietary melon juice concentrate (Extramel) on stress and fatigue in healthy people: a pilot, double-blind, placebo-controlled clinical trial. Nutr. J. 8, 40. ( 10.1186/1475-2891-8-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aliev G, Obrenovich ME, Reddy VP, Shenk JC, Moreira PI, Nunomura A, Zhu X, Smith MA, Perry G. 2008. Antioxidant therapy in Alzheimer's disease: theory and practice. Mini Rev. Med. Chem. 8, 1395-1406. ( 10.2174/138955708786369582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O'Neill RD, Fillenz M, Sundstrom L, Rawlins JN. 1984. Voltammetrically monitored brain ascorbate as an index of excitatory amino acid release in the unrestrained rat. Neurosci. Lett. 52, 227-233. ( 10.1016/0304-3940(84)90166-6) [DOI] [PubMed] [Google Scholar]
- 148.Curtain CC, et al. 2001. Alzheimer's disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J. Biol. Chem. 276, 20 466-20 473. ( 10.1074/jbc.M100175200) [DOI] [PubMed] [Google Scholar]
- 149.Haider S, Saleem S, Perveen T, Tabassum S, Batool Z, Sadir S, Liaquat L, Madiha S. 2014. Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age (Dordrecht, Netherlands) 36, 9653. ( 10.1007/s11357-014-9653-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu X, McClements DJ, Cao Y, Xiao H. 2016. Chemical and physical stability of astaxanthin-enriched emulsion-based delivery systems. Food Biophys. 11, 302-310. ( 10.1007/s11483-016-9443-6) [DOI] [Google Scholar]
- 151.Mercke Odeberg J, Lignell A, Pettersson A, Höglund P. 2003. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 19, 299-304. ( 10.1016/S0928-0987(03)00135-0) [DOI] [PubMed] [Google Scholar]
- 152.Taksima T, Limpawattana M, Klaypradit W. 2015. Astaxanthin encapsulated in beads using ultrasonic atomizer and application in yogurt as evaluated by consumer sensory profile. LWT Food Sci. Technol. 62, 431-437. ( 10.1016/j.lwt.2015.01.011) [DOI] [Google Scholar]
- 153.Puertas MC, Martínez-Martos JM, Cobo MP, Carrera MP, Mayas MD, Ramírez-Expósito MJ. 2012. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp. Gerontol. 47, 625-630. ( 10.1016/j.exger.2012.05.019) [DOI] [PubMed] [Google Scholar]
- 154.Al-Amin MM, Akhter S, Hasan AT, Alam T, Nageeb Hasan SM, Saifullah AR, Shohel M. 2015. The antioxidant effect of astaxanthin is higher in young mice than aged: a region specific study on brain. Metabolic Brain Dis. 30, 1237-1246. ( 10.1007/s11011-015-9699-4) [DOI] [PubMed] [Google Scholar]
- 155.Pan L, Wang H, Gu K. 2018. Nanoliposomes as vehicles for astaxanthin: characterization, in vitro release evaluation and structure. Molecules (Basel, Switzerland) 23, 2822. ( 10.3390/molecules23112822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zuluaga M, Gueguen V, Letourneur D, Pavon-Djavid G. 2018. Astaxanthin-antioxidant impact on excessive reactive oxygen species generation induced by ischemia and reperfusion injury. Chem. Biol. Inter. 279, 145-158. ( 10.1016/j.cbi.2017.11.012) [DOI] [PubMed] [Google Scholar]
- 157.Fakhri S, Aneva IY, Farzaei MH, Sobarzo-Sánchez E. 2019a. The neuroprotective effects of astaxanthin: therapeutic targets and clinical perspective. Molecules (Basel, Switzerland) 24, 2640. ( 10.3390/molecules24142640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chang YT, et al. 2014. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer's disease: a systematic review. BioMed Res. Int. 2014, 182303. ( 10.1155/2014/182303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frijhoff J, et al. 2015. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 23, 1144-1170. ( 10.1089/ars.2015.6317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ribou AC. 2016. Synthetic sensors for reactive oxygen species detection and quantification: a critical review of current methods. Antioxid. Redox Signal. 25, 520-533. ( 10.1089/ars.2016.6741) [DOI] [PubMed] [Google Scholar]
- 161.Dundar Y, Aslan R. 2000. Antioxidative stress. Eastern J. Med. 5, 45-47. [Google Scholar]
- 162.Poljsak B, Milisav I. 2012. The neglected significance of ‘antioxidative stress'. Oxid. Med. Cell. Longevity 2012, 480895. ( 10.1155/2012/480895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ristow M. 2014. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med. 20, 709-711. ( 10.1038/nm.3624) [DOI] [PubMed] [Google Scholar]
- 164.Aisen PS, et al. 2017. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimer's Res. Ther. 9, 60. ( 10.1186/s13195-017-0283-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. 2006. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 69, 443-449. ( 10.1021/np050354+) [DOI] [PubMed] [Google Scholar]
- 166.Halliwell B. 2013. The antioxidant paradox: less paradoxical now? Br. J. Clin. Pharmacol. 75, 637-644. ( 10.1111/j.1365-2125.2012.04272.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Galan P, et al. 2005. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 59, 1181-1190. ( 10.1038/sj.ejcn.1602230) [DOI] [PubMed] [Google Scholar]
- 168.Sperling RA, et al. 2011. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia 7, 280-292. ( 10.1016/j.jalz.2011.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wojsiat J, Zoltowska KM, Laskowska-Kaszub K, Wojda U. 2018. Oxidant/antioxidant imbalance in Alzheimer's disease: therapeutic and diagnostic prospects. Oxidat. Med. Cell. Longevity 2018, 6435861. ( 10.1155/2018/6435861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gandhi S, Abramov AY. 2012. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longevity 2012, 1-11. ( 10.1155/2012/428010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sadhukhan P, Saha S, Dutta S, Mahalanobish S, Sil PC. 2018. Nutraceuticals: an emerging therapeutic approach against the pathogenesis of Alzheimer's disease. Pharmacol. Res. 129, 100-114. ( 10.1016/j.phrs.2017.11.028) [DOI] [PubMed] [Google Scholar]
- 172.Finkel T, Holbrook NJ. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239-247. ( 10.1038/35041687) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any additional data.