Abstract
Scutellaria barbata is a perennial herb which was vastly prescribed in Chinese medicine to treat inflammations, infections and it is also used a detoxifying agent. We synthesized silver nanoparticles with Scutellaria barbata extract and characterized the nanoparticles with UV–Vis spectroscopic analysis, TEM, AFM, FTIR and XRD. The biofilm inhibiting property of synthesized silver nanoparticles were examined with XTT reduction assay and the antimicrobial property was examined with well diffusion method. The silver nanoparticles were also coated with cotton fabrics and their efficacy against antimicrobials was analyzed to prove its application. The cytotoxic property of synthesized silver nanoparticles was examined with L929 fibroblast cells using MTT assay. Finally we analyzed the wound healing property of synthesized silver nanoparticles with wound scratch assay. The result of our UV–Vis spectroscopic analysis confirms Scutellaria barbata aqueous extract reduced silver ions and synthesized silver nanoparticles. The characterization studies TEM, AFM, FTIR and XRD confirms the synthesized silver nanoparticles are in ideal shape and size to be utilized as a drug. The XTT reduction assay proves silver nanoparticles effectively inhibits the biofilm formation in both resistant and sensitive strains. Antimicrobial sensitivity tests confirms synthesized silver nanoparticles and cotton coated synthesized silver nanoparticles both are effective against gram positive, gram negative and fungal species. Further the results of MTT assay confirms the synthesized silver nanoparticles are non toxic and finally the wound healing potency of the nanoparticles was confirmed with wound scratch assay. Over all our results authentically confirms the silver nanoparticles synthesized with Scutellaria barbata aqueous extract is potent wound healing drug.
Keywords: Green synthesis, Silver nanoparticles, Scutellaria barbata, Antimicrobial, Wound healing drug
1. Introduction
Older population is prone to many risks one among them is chronic wounds, a silent killer. Not only older populations are prey to non healing chronic wounds but also the persons suffering with various life style disorders such diabetes, nephropathies, cardiovascular diseases etc (Järbrink et al., 2017). A non healing chronic wound impairs the productivity of the individual and also creates a heavy economic burden to the country (Augustin et al., 2014). In diabetic patients chronic wounds had lead to 70% of limb amputation and overall 85% of ulcers proceed to amputations in patients. The amputated patient’s 5 year mortality is 40–70% and it is higher in patient’s undergone major amputation (Lindholm and Searle, 2016, Gonzalez et al., 2016). Skin is the largest organ of the body which plays critical role in maintaining the body homeostasis and prevents the body from pathogenic microbes, UV rays, toxins etc. (Percival et al., 2015). Disturbing the skin integrity leads to wounds which normally heals within three months. The wounds which don’t heal within three months are called chronic wounds and it occurs due to numerous reasons (Järbrink et al., 2017). One of the major reasons for non healing of wounds are the microbial infections at the site of wound (Leaper et al., 2015). The drugs available in the market mostly targets promoting the wound healing capacity whereas fails to effectively inhibit the colonization of microbes.
The most common drugs used to treat wounds in developing countries are cetrimide solution, sodium hypochloride, chlorhexidine etc., all these drugs are proven to be least efficient and also render side effects on prolonged usage (Moore, 1992, Toppo and Pawar, 2015). Silver is one of the oldest metals which documented to be used as wound healing in 69BC. Silver possess antimicrobial property whereas ionization of silver is required to increase its efficacy hence in late 1960′s silver was combined with antibiotics such as sulphadiazine and used for treating wounds but these products produced higher resistance rate and bone marrow toxicity (Trop et al., 2006, Atiyeh et al., 2007). Invention of nanoparticles changed the usage of silver in the medical field, silver nanoparticles used in various areas of biomedical field (Toppo and Pawar, 2015). The stability, catalytic property, electric conductivity and antibacterial activity of silver nanoparticles promote the usage in treating various diseases (Mooney et al., 2006, Nakkala et al., 2014). The antimicrobial potency of silver nanoparticles increased the usage of silver nanoparticles in wound dressing, drug carriers and also in artificial implantation (Haider, 2015, Liao et al., 2019 Jan 21).
Eco friendly green synthesis of nanoparticles is biocompatible, cost effective, less time and energy consuming and it also non toxic compared to the physical and chemical methods. Silver nanoparticles were synthesized by using various biological sources such as plant extracts, bacteria, fungi, algae (Ahmed et al., 2016 Jan, Daphne et al., 2018). Plant extracts are very potent reducing agents which reduces the noble metals ions and synthesis metal nanoparticles (Peralta-Videa et al., 2016). Scutellaria barbata is a perennial herb which is prescribed in traditional Chinese and Korean medicine to treat diseases such as inflammation, microbial infections, hematuria, tumor and also acts as a detoxifying agent (Zhang et al., 2018). Scutellaria barbata consists of more than 203 bioactive compounds which includes diterpenoids, flavonoids, triterpenoids, lignans, phenyl propanoids (Wang et al., 2020). These bioactive compounds acts a reducing agents in synthesis of silver nanoparticles and also promotes the wound healing capacity of the drug. Scutellaria barbata has demonstrated potent anticancer (Lu et al., 2020, Chen et al., 2017), and neuroprotective (Cheng et al., 2020) activities. Numerous studies demonstrated that the Scutellaria barbata exhibited the effective antimicrobial activities towards pathogenic microbes (Tang et al., 2016, Yu et al., 2004, Tsai et al., 2018). Though, the formulation metallic silver nanoparticles and its effectiveness on cotton fabric coating was not studied yet. Hence in the present study we synthesized silver nanoparticles in eco friendly way using aqueous extract of Scutellaria barbata and assessed its antimicrobial efficacy and wound healing potency.
2. Materials & methods
2.1. Chemicals & reagents
Silver nitrate, XTT, MTT, Menadione was purchased from Sigma Aldrich, USA. Sabouraud-Dextrose Agar and Mueller–Hinton agar and the bacterial strains were procured HiMedia, USA. Eagle's Minimum Essential Medium, dimethyl sulfoxide, Trypsin-EDTA solution, antibiotic–antimycotic solution, FBS were purchased from Gibco, USA.
2.2. Preparation of Scutellaria barbata extract
Fresh Scutellaria barbata plants were collected from local market in Jinan city, China. The plant was authenticated by the botanist, Jinan University Jinan City, Shandong Province, China. The voucher specimen was stored in a herbarium of the department. The plant collection has followed by WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants washed with distilled water and shade dried. The shade dried plants were finely powdered and the powder was used for the further analysis. Aqueous extract of Scutellaria barbata was prepared by mixing 100gm of Scutellaria barbata powder in 500 ml of deionized water. The mixture was placed in shaker for 10 min followed boiling at 90 °C for 30 min. The mixture was cooled and filtered using Whatman filter paper, the extract was utilized for the synthesis of silver nanoparticles.
2.3. Synthesis of Scutellaria barbata silver nanoparticles (Sb-AgNP)
To 90 ml of 1 mM silver nitrate solution 10 ml of Scutellaria barbata aqueous extract was added and the mixture was incubated in dark chamber for 30 min. The synthesis of silver nanoparticles by reduction of Ag+ to Ag0 was observed by the color change from colorless solution to brown colored solution.
2.4. Characterization of Sb-AgNP
2.4.1. UV–Visible spectroscopic analysis
The synthesis of Sb-AgNP was confirmed using UV–Visible spectrophotometer (Shimadzu UV-1800, Japan) at a resolution of 1 nm, spectral range 200–800 nm.
2.4.2. Transmission electron microscopic analysis
The size and shape of synthesized Sb-AgNP were analyzed with high resolution transmission electron microscopy (JOEL-JSM-100CX-TEM). Sb-AgNP were placed on to the carbon coated copper grid and incubated under vacuum desiccator overnight. The Sb-AgNP supplemented carbon coated copper grid was placed on the specimen holder and the micrograph of Sb-AgNP was analyzed at an accelerating voltage of 200 kV.
2.4.3. Atomic force microscopy analysis
The surface morphology and the particle size of Sb-AgNP was analyzed using enviroscope scanning microscope (VEECO Digital Instruments, CA). 0.05 mg/ml of Sb-AgNP was diluted in deionized water and the mixture was spread on to the mica sheet and the sample was dried with nitrogen gas. The nanoparticles were scanned using Arrow-NCR etched silicon tips in an intermittent contact at a resonance frequency of 300 kH under normal atmospheric pressure.
2.4.4. X ray diffraction analysis
The structure of Sb-AgNP was assessed using XRay diffractometer (XRD-6000, Shimadzu). The samples were analyzed at 2ϴ from angle of 30° to 80° at a speed of 0.041°/minute. The time constant was maintained for 2 s.
2.4.5. Fourier Transform Infrared (FTIR) Spectroscopy analysis
The biomolecules on the surface of Sb-AgNP were examined using Fourier Transform Infra Red Spectrophotometer (FTIR Spectrum 2000, Perkin Elmer, USA). The synthesized silver nanoparticles were dispersed in deionized water and centrifuged 10,000 rpm for 10 min. The rinsed pellets were lyophilized and 1 mg of lyophilized Sb-AgNP powder was mixed with 100 mg of potassium bromide, placed on the sample holder. The mixture was then examined at spectroscopic range of 4000–5000 cm−(Järbrink et al., 2017 Jan 24) and about 50 scans were performed at resolution of 4 cm/scan. The images were then assessed with WINFIRST software.
2.5. Antimicrobial property of Sb-AgNP
2.5.1. XTT reduction assay
The antimicrobial activity of Sb-AgNP was analyzed with XTT reduction assay (Pierce et al., 2008). 1 × 106/ml of resistant and sensitive candida strains were individually seeded onto the sterile 96 well flat bottomed culture plates and incubated for 24 h. After 24 h incubations the candid biofilms were treated with 30 µg/ml of Sb-AgNP, standard antifungal drug fluconazole and the plates were incubated for 4 h at 37 °C. The media was then removed and the wells were rinsed thrice with distilled water to remove non adherent cells. The treated and untreated biofilms were subjected XTT reduction assay. XTT solution was prepared by dissolving 1 mg of XTT in 1 ml of PBS and the solution was filtered and stored. 0.4 mM menadione solution was freshly prepared before the assay. To the wells 158 µl of PBS, 40 µl of XTT and 2 µl of menadione was added and the plates were incubated in dark for 2 h. After incubation period 100 µl solution from each well was transferred to a new 96 well plates and the absorbance of the solution was measured 492 nm using microtitre plate reader.
2.5.2. Zone of inhibition assay
Most commonly infection causing gram positive bacteria Staphylococcus aureus (ATCC 23235), Streptococcus pyogenes (ATCC 19615), gram negative bacteria Escherichia coli (ATCC 25922), Pseudomonas aerogenesa (ATCC 15442) were cultured on a Muller Hinton agar. The bacterial and fungal cultures were treated with different concentrations of Sb-AgNP (20, 40 and 60 µg/ml) and the plates were incubated for 24 h at 35 °C. The zone of inhibition of each wells were measured and the readings were recorded.
2.5.3. Minimum inhibitory concentration assay
The minimum inhibitory concentration of synthesized Sb-AgNP was analyzed using broth micro dilution technique (Singh et al., 2013). The bacterial organisms were seeded on to 96 well plate along with different concentration of Sb-AgNP ranging from (20, 40 and 60 µg/ml) and the plates were incubated for 24 h at 37 °C. After 24 h incubation period the turbidity of was measured at 600 nm using microplate reader.
2.6. Sb-AgNP embedding on cotton fabrics
Sterile cotton cloth was washed thoroughly using non ionic detergent, the cloth was rinsed several times with hot deionized water to ensure nil impurities in the cotton cloth. The cotton cloth was embedded with Sb-AgNP by soaking in solution consisting 1 mg of lypholized Sb-AgNP powder in 100 ml of deionized water. The mixed cotton fabrics were prepared by soaking the cotton cloth in solution consisting of 10 ml of Scutellaria barbata aqueous extract and 90 ml of 1 mM silver nitrate solution. The soaked fabrics were subjected ultra sonication for 30 min. The fabrics were then removed rinsed with deionized water and dehydrated at 37 °C. The Sb-AgNP embedded cotton fabrics were then utilized to assess the zone of inhibition against skin infection causing organisms.
2.7. Culturing of L929 fibroblast cells
The murine L929 fibroblast cells were procured for ATCC and the cells were cultured with Eagle's Minimum Essential Medium in 5% CO2 incubator at 37 °C. Upon obtaining 80% confluence the cells were subcultured using Trypsin-EDTA solution and utilized for further studies.
2.8. Cytotoxicity assay
The cytotoxicity of Sb-AgNP against fibroblast cells were analyzed using MTT assay. L929 fibroblast cells were seeded on to 24 h cell culture plate and incubated for 24 h in 5% CO2 incubator at 37 °C. After incubation period the cells were then treated with different concentrations of Sb-AgNP (control, 2.5, 5, 7.5, 10 and 15 µg/ml) and further incubated for 24 h in 5% CO2 incubator at 37 °C. The cells were then rinsed with PBS and incubated with serum free medium containing 10 µl MTT (0.5/ml) for 4 h in dark. The insoluble formazon crystals formed were dissolved with 100 µl of DMSO solution and the absorbance of each sample was spectroscopic analyzed at 545 nm using microplate reader.
2.9. Wound scratch assay
The wound healing property of Sb-AgNP was examined in vitro condition using wound scratch assay (Liang et al., 2007). L929 fibroblast cells were seeded on to 6 well culture plate and incubate until it reaches 90% confluency. Upon obtaining 90% confluence the plates were scratched vertically using a sterile micropipette. The breadth of the scratch was uniformly maintained in all the wells. The scratched cells debris was rinsed with fresh EMEM medium and the cells were treated with 5 and 7.5 µg of Sb-AgNPs. The cells were then incubated for 24 h at 37 °C in 5% CO2 incubator. After incubation period the closure of wound was microscopically viewed and the images were photographed. The relative migration ratio (RMR) of the cells were measured through the below mentioned formula
| RMR = ([A0-A1]/A0) X 100, |
where A0 – Area of scratch made initially, A1 – Area of scratch after 24 h incubation
2.10. Statistical analysis
All the experiments were performed in triplicates and the results were statistically analyzed using GraphPad Prism statistical software. The data were statistically assessed with One Way ANOVA followed by post hoc test Newman-Keuls test. p < 0.05 were considered to be statistically significant.
3. Results
3.1. Characterization of Sb-AgNP
3.1.1. UV–Visible spectroscopic analysis of Sb-AgNP
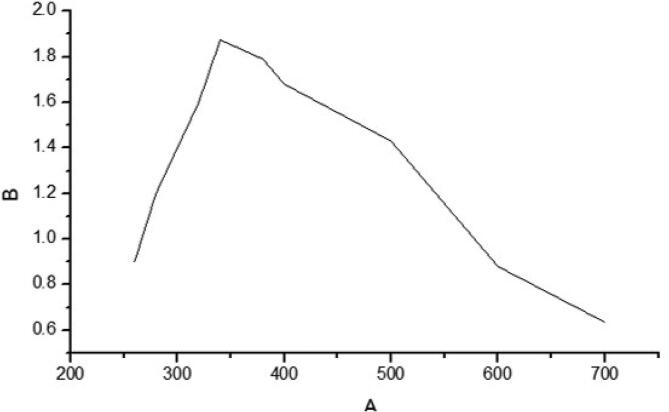
The synthesis of silver nanoparticles was confirmed with UV spectroscopic analysis and the result was depicted in Fig. 1. The maximum absorbance peak was observed at 400 nm which confirms Scutellaria barbata aqueous extract reduced Ag+ to Ag0 thereby synthesized silver nanoparticles.
Fig. 1.
Characterization of Sb-AgNP. UV–Visible Spectroscopic analysis of Sb-AgNP. Silver nanoparticles were synthesized by reducing Ag+ to Ag0 using Scutellaria barbata aqueous extract. Maximum absorbance peak was observed at 380 nm.
3.1.2. Transmission electron microscopic analysis of Sb-AgNP
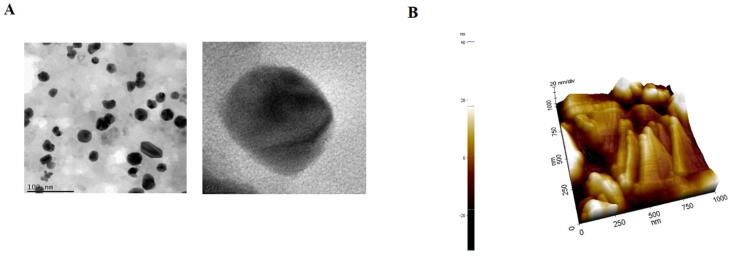
Fig. 2A illustrates the high resolution TEM images of Sb-AgNP, the synthesized silver nanoparticles were mostly spherical in shape with the size ranging from 20 to 40 nm.
Fig. 2.
Characterization of Sb-AgNP. 2A. Transmission Electron microscopic analysis of Sb-AgNP. 2B. Atomic Force Microscopy Analysis of Sb-AgNP.
3.1.3. Atomic force microscopy analysis of Sb-AgNP
The surface morphology of synthesized SB-AgNP was analyzed with atomic force microscopy and the image was represented in Fig. 2B. The sample were scanned in tapping mode with a scanning are of 1X1 which displays a uniformly dispersed nanoparticles with a size ranging of 30–40 nm. The AFM images shown spherical topology of silver nanoparticles which correlates with the images of TEM.
3.1.4. X Ray diffraction analysis of Sb-AgNP
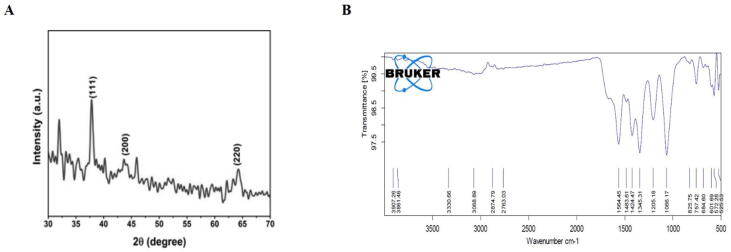
The X-ray diffraction pattern of Sb-AgNP was examined with X-Ray difractometer and the XRD patterns were depicted in Fig. 3A. Sb-AgNP shown typical peaks at 38.05°, 44.32°, 64.43° which were assigned to (1 1 1), (2 0 0) and (2 2 0) planes. This confirms the presence of silver ions and crystalline nature of the silver nanoparticles. No peaks representing the presence of impurities were observed.
Fig. 3.
Characterization of Sb-AgNP. 3A. X Ray Diffraction analysis of Sb-AgNP. XRD patterns at corresponding diffraction signals at (1 1 1), (2 0 0) and (2 2 0) planes 3B. Fourier Transform Infrared (FTIR) Spectroscopy analysis of Sb-AgNP.
3.1.5. Fourier Transform Infrared (FTIR) Spectroscopy analysis of Sb-AgNP
The functional groups present in synthesized Sb-AgNP were assessed using Fourier Transform Infrared Spectroscopy and the FTIR spectrums were depicted in the Fig. 3B. The spectrum of Sb-AgNPs shown absorbance peaks at 525, 572, 601, 684, 757, 825, 1066,1205, 1345, 1424, 1483,1564, 2763, 2874, 3068, 3330, 3861, 3907 cm−1 indicating the presence of biomolecule which acted as capping agent and stabilizer for nanoparticles. It also confirms the presence of alkenes, carbonyl, carboxylic and hydroxyl groups in the synthesized Sb-AgNP.
3.2. Antimicrobial property of Sb-AgNP
3.2.1. Biofilm inhibition potency of Sb-AgNP
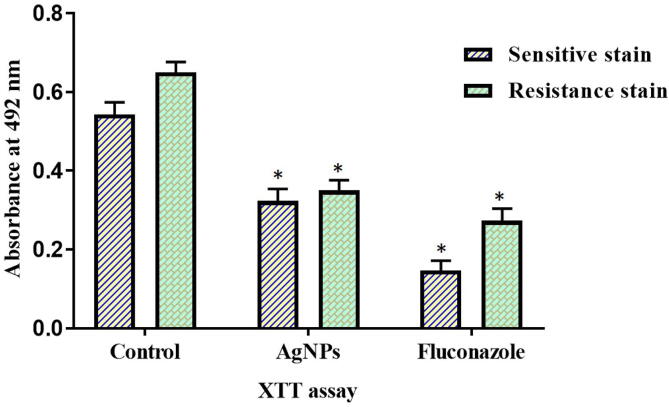
Fig. 4 depicts the biofilm inhibitory potency of Sb-AgNP analyzed with XTT reduction assay. Compared to control group the Sb-AgNP treated groups inhibited the growth of both sensitive and resistant strain. Even though the biofilm inhibition potency of Sb-AgNP is lesser than the standard drug fluconazole, Sb-AgNPs inhibited 60% of biofilm formation.
Fig. 4.
Antimicrobial property of Sb-AgNP. The biofilm inhibitory potency of Sb-AgNP analyzed with XTT reduction assay. The assay was performed with both resistant and sensitive strain. Experiment was repeated thrice and the data were statistically analyzed with GraphPad Prism software. The statistical significant was considered to be *p ≤ 0.05.
3.2.2. Antimicrobial potency of Sb-AgNP
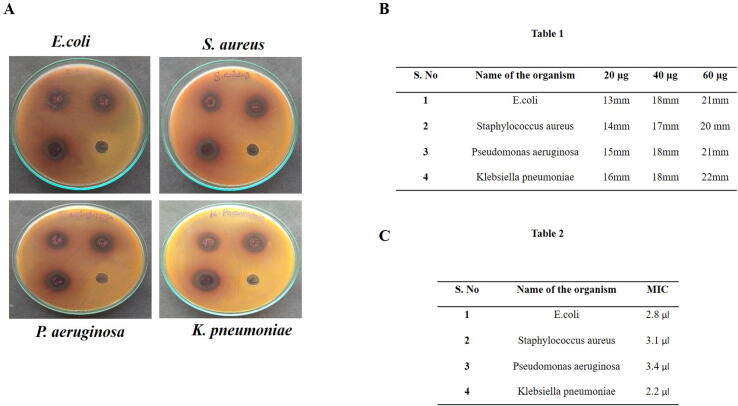
The synthesized Sb-AgNPs potentially inhibited the growth of gram positive and gram negative species (Fig. 5A). 60 µg/ml of Sb-AgNP shown increased zone of inhibition for Staphylococcus aureus (20 mm), Klebsiella pneumoniae (22 mm), Escherichia coli (21 mm), and Pseudomonas aeruginosa (21 mm) (Fig. 5B- Table 1). The least minimum inhibitory concentration for Sb-AgNP was obtained with Klebsiella pneumoniae which was about 2.2 µl and the highest minimum inhibitory concentration was observed in Pseudomonas aeruginosa which was 3.1 µl (Fig. 5C -Table 2).
Fig. 5.
A. Antimicrobial potency of Sb-AgNP. The antimicrobial potency of synthesized Sb-AgNP examined with well diffusion method. Gram positive, gram negative bacteria and fungi species were used to assess antimicrobial potency Sb-AgNP. B. Table1. Zone of Inhibition of synthesized Sb-AgNP against bacterial species such as Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli and Pseudomonas aerogenesa were measured and tabulated. C. Table 2. Minimum inhibitory concentration of Sb-AgNP against bacterial species were measured and tabulated.
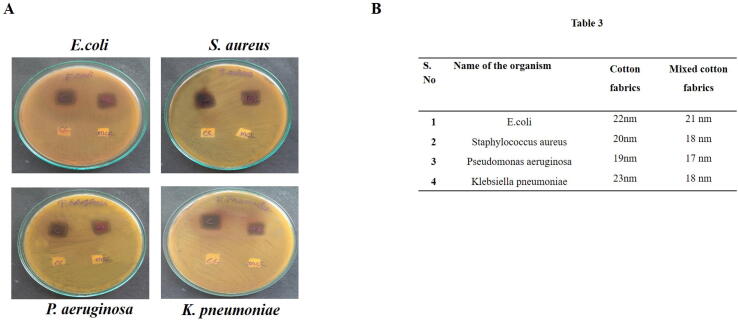
Fig. 6A exemplifies the antimicrobial potency of Sb-AgNP coated cotton fabrics. No growth was observed in Sb-AgNP coated cotton fabrics. Sb-AgNP coated cotton fabrics shown increased zone of inhibition compared to Scutellaria barbata aqueous extract and Sb-AgNP. Pseudomonas aeruginosa exhibited increased sensitive towards the Sb-AgNP coated cotton fabrics (Fig. 6B- table 3).
Fig. 6.
A. Antimicrobial potency of Sb-AgNP coated cotton fabrics. A. The antimicrobial potency of synthesized Sb-AgNP coated cotton fabrics was examined with well diffusion method. B. Table 3. Minimum inhibitory concentration of Sb-AgNP coated cotton fabrics were measured and tabulated.
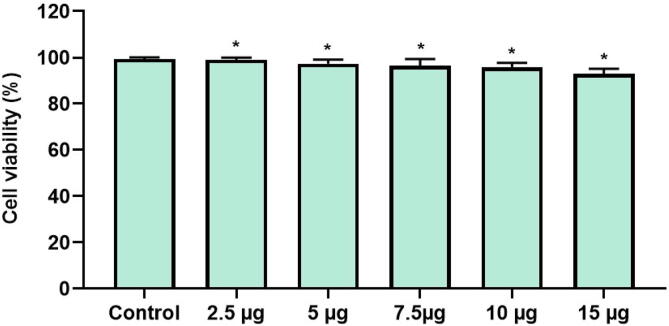
3.3. Cytotoxicity of Sb-AgNP
Fig. 7 depicts the results of cytotoxicity assay done on Sb-AgNP treated L929 fibroblast. Sb-AgNP doesn’t shown cytotoxicity against the fibroblast cells even at the highest concentration of 15 µg/ml.
Fig. 7.
Cytotoxic property of Sb-AgNP against L929 fibroblast cells. L929 fibroblast cells were treated with different concentration of synthesized Sb-AgNP and the cytotoxic potency was assessed with MTT assay. Experiment was repeated thrice and the data were statistically analyzed with GraphPad Prism software. The statistical significant was considered to be *p ≤ 0.05.
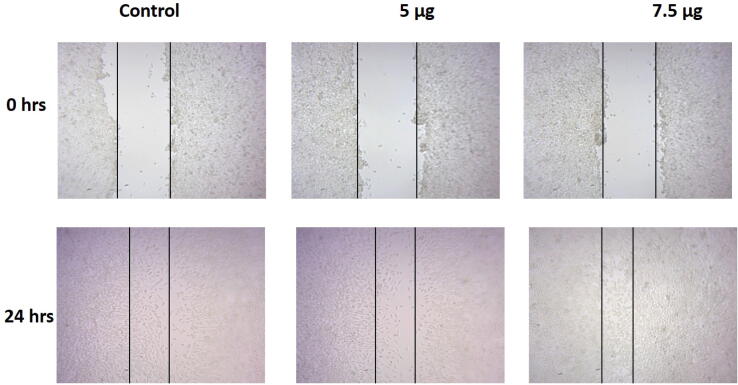
3.4. Wound healing property of Sb-AgNP
The wound healing property of Sb-AgNP was assessed with wound scratch assay and the results were illustrated in Fig. 8. Both the 5 µg and 7.5 µg Sb-AgNP treatment decreased the migration of L929 fibroblast cells. 7.5 µg Sb-AgNP treatment significantly decreased the migration of L929 fibroblast cells compared to 5 µg Sb-AgNP treated L929 fibroblast cells.
Fig. 8.
Wound healing property of Sb-AgNP on L929 fibroblast cells. The wound healing property of Sb-AgNP against L929 fibroblast cells was assessed with wound scratch assay.
4. Discussion
More than 6 million people in United States were reported to be suffering with chronic ulcers and most of them are older population and people with life style disorders (Powers et al., 2016). Amputation is the worst outcome of untreated chronic wounds and it is estimated by the year 2030 15% of American diabetic population are prone to risk of amputation (Han and Ceilley, 2017). It not only causes physical and mental stress to the patients but also impairs the productivity and increases the economic burden. Approximately 3 million pounds/year were spent by UK government for treating patients with chronic wounds (Posnett and Franks, 2008, McLister et al., 2016). Pharmaceutical companies earn profits more than 25,000 billion dollars per year in manufacturing wound care management medicine alone (Murray et al., 2019). Hence it is need of today to discover a cost effective potent drug to heal chronic wound without recurrence and rendering any side effects.
Wound dressing with efficient antimicrobial drugs prevents the colonization of microorganisms in wounds and also inhibits the biofilm formation (Clinton and Carter, 2015). The available anti biofilms drugs are costly and they are prone to antibiotic resistance hence a novel anti biofilm drug which possess antimicrobial property along with wound healing potency is need of today. Nanoparticles are one such potent agent which possess antimicrobial potency and effectively inhibits biofilm formation (Ramasamy and Lee, 2016, Simões et al., 2018), hence synthesis of wound healing drug using nanoparticles will be more effective in treating non healing chronic wounds. Silver nanoparticles are utilized as regeneration materials, drug carriers, biomaterial coaters, antimicrobial agents, wound dressing material (Liao et al., 2019 Jan 21, Baygar et al., 2019). In the present study we synthesized silver nanoparticles using Scutellaria barbata plant extract which is a perennial herb prescribed in traditional Chinese medicine to treat various diseases.
The surface plasmon resonance peak of Sb-AgNP was observed at 400 nm which correlates with the UV–Vis spectroscopic analysis of previous studies synthesized silver nanoparticles with plant extracts (Krithiga et al., 2015, Pirtarighat et al., 2019). The antimicrobial property of a nanoparticle depends on the size, shape, surface charge and concentration of the nanoparticles (Burdușel et al., 2018 Aug 31). Therefore we assessed the shape, size of the synthesized Sb-AgNP using transmission electron microscopic analysis, atomic force microscopic analysis, XRD analysis. Our characterization analysis results confirm the synthesized silver nanoparticles are with ideal shape and size to be utilized as drug. TEM analysis results shows Sb-AgNP are spherical in shape and dispersed without any agglomeration. Capping agents such as polyethylene glycol, chitosan, polymers act as capping agents and prevent agglomeration during synthesis of nanoparticles (Pillai and Kamat, 2004, Bai et al., 2007). In the present study the bioactive components present in aqueous Scutellaria barbata extract acted as capping agent and prevented the agglomeration of silver nanoparticles. The presence of bioactive components was confirmed with FTIR analysis which shows absorbance peaks ranging between 500 and 400 cm−1.
The intrinsic anti pathogenic effect exhibited by silver nanoparticles against the microorganism biofilm plays a critical role in inhibiting the wound infection. Therefore we assessed the potency of synthesized Sb-AgNP to inhibit the formation of biofilm using XTT reduction assay. Our results shows Sb-AgNP effectively inhibited the biofilm formation in both sensitive and resistant strains. The bactericidal activity of Sb-AgNP may be due to the silver cations which would have bound to thiol group of bacterial protein thereby disturbing the bacterial cells and inducing death (Radzig et al., 2013). Further we analyzed the potency of Sb-AgNP against gram positive, negative bacterial and fungal species. The green synthesized Sb-AgNP shown antimicrobial activity against most commonly skin infection causing gram positive bacteria such as Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa. Compared to the aqueous extract of Scutellaria barbata, the Sb-AgNP are more potent against skin infection causing microbial species.
Wound healing management deals with preventing the wound site from microbial infections and promoting the tissue wound healing process. Direct application of silver nanoparticles as ointments on wounds are not much effective hence incorporating the silver nanoparticles with naturally derived materials such as bacterial cellulose, chitosan, cotton fabrics are proven to be more effective on wound healing management (Ballottin et al., 2017 Jun, Su et al., 2017). The silver nanoparticles possess the property of binding to the biomaterials there by exerts antimicrobial property for a prolonged period (Paladini et al., 2015). In the present study we coated the synthesized Sb-AgNP on to cotton fabric and examined its efficacy against skin infection causing microbes. The Sb-AgNP coated cotton fabric inhibited the growth of Staphylococcus aureus; Klebsiella pneumoniae; Escherichia coli, and Pseudomonas aeruginosa. Our results correlates with the previous studies where the green synthesized silver nanoparticles treated fabrics exerted antimicrobial activity against E. coli, S. aureus (El-Rafie et al., 2012, Nateghi and Shateri-Khalilabad, 2015, Ullah et al., 2014). The silver nanoparticles get oxidized in aqueous environment and releases Ag+ ions. These silver ions bind with thiol group of bacterial proteins and disrupt the bacterial cells it also generates free radicals which causes pores on the cell wall of microbe thereby causes cell death (Chen et al., 2009, Kim et al., 2011). This may be the reason for inhibition of microbes treated with Sb-AgNP, the silver ions would have generated free radicals which disrupted the microbial causing microbial cell death.
Both the Sb-AgNPs and Sb-AgNPs coated cotton fabric shown potent antimicrobial activity against skin infection causing microorganism. Hence we further analyzed the cytotoxicity effect of Sb-AgNP on fibroblast cells. The MTT results of our study confirm even at higher dosage of 15 µg/ml Sb-AgNPs doesn’t impart any adverse cytotoxic effect on murine fibroblast L929 cells. The results of our study correlate with the study of Maghimaa and Alharbi (2020) where the silver nanoparticles synthesized with Curcuma longa extract doesn’t impart any cytotoxic effect on murine fibroblast L929 cells. The results of our wound scratch assay proves Sb-AgNP possess wound healing property the silver nanoparticles induced the proliferation, differentiation and migration of L929 fibroblast cells thereby reducing the size of wound created. The results confirm Sb-AgNP and cotton coated Sb-AgNP possess antimicrobial activity and also wound healing potency.
5. Conclusion
In the present study we synthesized cost effective eco friendly silver nanoparticles using Scutellaria barbata aqueous extract. The characterization studies with TEM, AFM, XRD and FTIR confirms the synthesized silver nanoparticles satisfy the properties to be an ideal drug. The XTT reduction assay proves Sb-AgNP effectively inhibits the biofilm formation and the antimicrobial sensitivity test confirms both Sb-AgNP and cotton coated Sb-AgNP possesses antimicrobial property against skin infection causing microbes. Sb-AgNP is non cytotoxic to L929 fibroblast cells and it also induced the differentiation and migration of fibroblast cells thereby effectively decreased the wound size. Overall our results authentically confirms Sb-AgNP and cotton coated Sb-AgNP possess potent wound healing property hence in future cotton coated Sb-AgNP can be utilized as ideal dressing material for wound healing treatment. Although, additional future studies still required to elucidate the clear mechanisms of Sb-AgNP coated cotton fabrics.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiyeh B.S., Costagliola M., Hayek S.N., Dibo S.A. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33(2):139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Augustin M., Brocatti L.K., Rustenbach S.J., Schäfer I., Herberger K. Cost-of-illness of leg ulcers in the community. Int Wound J. 2014;11(3):283–292. doi: 10.1111/j.1742-481X.2012.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Li Y., Du J., Wang S., Zheng J., Yang Q., Chen X. One-pot synthesis of polyacrylamide-gold nanocomposite. Mater. Chem. Phys. 2007;106:412–415. [Google Scholar]
- Ballottin D., Fulaz S., Cabrini F., Tsukamoto J., Durán N., Alves O.L., Tasic L. Antimicrobial textiles: biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;1(75):582–589. doi: 10.1016/j.msec.2017.02.110. [DOI] [PubMed] [Google Scholar]
- Baygar T., Sarac N., Ugur A., Karaca I.R. Antimicrobial characteristics and biocompatibility of the surgical sutures coated with biosynthesized silver nanoparticles. Bioorg Chem. 2019;86:254–258. doi: 10.1016/j.bioorg.2018.12.034. [DOI] [PubMed] [Google Scholar]
- Burdușel A.C., Gherasim O., Grumezescu A.M., Mogoantă L., Ficai A., Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials (Basel). 2018;8(9):681. doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.C., Kao C.P., Chiu M.M., Wang S.H. The anticancer effects and mechanisms of Scutellaria barata D. Don on CL1-5 lung cancer cells. Oncetarget. 2017;8(65):109340–109357. doi: 10.18632/oncotarget.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Qiao X., Qiu X., Chen J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009;44:1076–1081. [Google Scholar]
- Cheng J.J., Guo Q., Wu X.G., Ma S., Gao Y., Zhen S.Y. Scutellaria barbata flavonoids improve the composited Aβ-induced abnormal changes of glial cells in rats’ brain. Comb Chem High Throughput Screen. 2020 doi: 10.2174/1386207323666201209092358. [DOI] [PubMed] [Google Scholar]
- Clinton A., Carter T. Chronic wound biofilms: pathogenesis and potential therapies. Lab Med. 2015;46(4):277–284. doi: 10.1309/LMBNSWKUI4JPN7SO. [DOI] [PubMed] [Google Scholar]
- Daphne J., Francis A., Mohanty R., Ojha N., Das N. Green synthesis of antibacterial silver nanoparticles using yeast isolates and its characterization. Res J Pharm Technol. 2018;11(1):83–92. [Google Scholar]
- El-Rafie M.H., Shaheen T.I., Mohamed A.A., Hebeish A. Bio-synthesis and applications of silver nanoparticles onto cotton fabrics. Carbohydr. Polym. 2012;90(2):915–920. doi: 10.1016/j.carbpol.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.C., Costa T.F., Andrade Z.A., Medrado A.R. Wound healing - a literature review. An Bras Dermatol. 2016;91(5):614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A, Kang IK. Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive review. Adv Mater Sci Eng. 2015; 165257.
- Han G., Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbrink K., Ni G., Sönnergren H., Schmidtchen A., Pang C., Bajpai R., Car J. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6(1):15. doi: 10.1186/s13643-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Sh., Lee H.S., Ryu D.S., Choi S.J., Lee D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011;39:77–85. [Google Scholar]
- Krithiga N., Rajalakshmi A., Jayachitra A. Green synthesis of silver nanoparticles using leaf extracts of clitoria ternatea and solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. J. Nanosci. 2015 [Google Scholar]
- Leaper D., Assadian O., Edmiston C.E. Approach to chronic wound infections. Br J Dermatol. 2015;173(2):351–358. doi: 10.1111/bjd.13677. [DOI] [PubMed] [Google Scholar]
- Liang C.C., Park A.Y., Guan J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Liao C., Li Y., Tjong S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int J Mol Sci. 2019;20(2):449. doi: 10.3390/ijms20020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm C., Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016 Jul;13(Suppl 2):5–15. doi: 10.1111/iwj.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, L., Zhan, S., Liu, X., Zhao, X., Lin, X., Xu, H., 2020. Antitumor effects and the compatibility mechanisms of herb pair Scleromitrion diffusum (Wild.) R. J. Wang-Scutellaria barbata D. Don. Front Pharmacol. 2020; 11: 292. [DOI] [PMC free article] [PubMed]
- Maghimaa M., Alharbi S.A. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B. 2020;204(111806) doi: 10.1016/j.jphotobiol.2020.111806. [DOI] [PubMed] [Google Scholar]
- McLister A., McHugh J., Cundell J., Davis J. New developments in smart bandage technologies for wound diagnostics. Adv Mater. 2016;28(27):5732–5737. doi: 10.1002/adma.201504829. [DOI] [PubMed] [Google Scholar]
- Mooney E.K., Lippitt C., Friedman J. Silver dressings [safety and efficacy reports] Plastic & Reconstruct Sur. 2006;117(2):666–669. doi: 10.1097/01.prs.0000200786.14017.3a. [DOI] [PubMed] [Google Scholar]
- Moore D. Hypochlorites: a review of the evidence. J Wound Care. 1992;1(4):44–53. doi: 10.12968/jowc.1992.1.4.44. [DOI] [PubMed] [Google Scholar]
- Murray R.Z., West Z.E., Cowin A.J., Farrugia B.L. Development and use of biomaterials as wound healing therapies. Burns Trauma. 2019;25(7):2. doi: 10.1186/s41038-018-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakkala J.R., Mata R., Gupta A.K., Sadras S.R. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur J Med Chem. 2014;6(85):784–794. doi: 10.1016/j.ejmech.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Nateghi M.R., Shateri-Khalilabad M. Silver nanowire-functionalized cotton fabric. Carbohydr Polym. 2015;6(117):160–168. doi: 10.1016/j.carbpol.2014.09.057. [DOI] [PubMed] [Google Scholar]
- Paladini F., Picca R.A., Sportelli M.C., Cioffi N., Sannino A., Pollini M. Surface chemical and biological characterization of flax fabrics modified with silver nanoparticles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;52:1–10. doi: 10.1016/j.msec.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Peralta-Videa J.R., Huang Y., Parsons J.G. Plant-based green synthesis of metallic nanoparticles: scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol Environ Eng. 2016;11:4. [Google Scholar]
- Percival S.L., Suleman L., Vuotto C., Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol. 2015;64(Pt 4):323–334. doi: 10.1099/jmm.0.000032. [DOI] [PubMed] [Google Scholar]
- Pierce C.G., Uppuluri P., Tristan A.R., Wormley F.L., Jr, Mowat E., Ramage G., Lopez-Ribot J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008;3(9):1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai Z.S., Kamat P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B. 2004;108:945–951. [Google Scholar]
- Pirtarighat S., Ghannadnia M., Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chemist. 2019;9:1–9. [Google Scholar]
- Posnett, J., Franks, P.J., 2008. The burden of chronic wounds in the UK. Nurs Times. 2008;104(3):44–5. [PubMed]
- Powers, J.G., Higham, C., Broussard, K., Phillips, T.J., 2016. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016; 74(4): 607-25; 625-6. [DOI] [PubMed]
- Radzig M.A., Nadtochenko V.A., Koksharova O.A., Kiwi J., Lipasova V.A., Khmel I.A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids Surf B Biointerfaces. 2013;1(102):300–306. doi: 10.1016/j.colsurfb.2012.07.039. [DOI] [PubMed] [Google Scholar]
- Ramasamy M., Lee J. Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. Biomed Res Int. 2016;1851242 doi: 10.1155/2016/1851242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões D., Miguel S.P., Ribeiro M.P., Coutinho P., Mendonça A.G., Correia I.J. Recent advances on antimicrobial wound dressing: a review. Eur. J. Pharm. Biopharm. 2018;127:130–141. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Singh R., Wagh P., Wadhwani S., Gaidhani S., Kumbhar A., Bellare J., Chopade B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013;8:4277–4290. doi: 10.2147/IJN.S48913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C.H., Kumar G.V., Adhikary S., Velusamy P., Pandian K., Anbu P. Preparation of cotton fabric using sodium alginate-coated nanoparticles to protect against nosocomial pathogens. Biochem. Eng. J. 2017;117:28–35. [Google Scholar]
- Tang Q.L., Kang A.R., Lu C.X. Phytochemical analysis, antibacterial activity and mode of action of the methanolic extract of Scutellaria barbata against various clinically important bacterial pathogens. Int. J. Pharmacol. 2016;12(2):116–125. [Google Scholar]
- Toppo F.S., Pawar R.S. Novel drug delivery strategies and approaches for wound healing managements. J Crit Rev. 2015;2:12–20. [Google Scholar]
- Trop M., Novak M., Rodl S., Hellbom B., Kroell W., Goessler W. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60(3):648–652. doi: 10.1097/01.ta.0000208126.22089.b6. [DOI] [PubMed] [Google Scholar]
- Tsai C.C., Lin C.S., Hsu C.R., Chang C.M., Chang W., Lin L.W., Hung C.H., Wang J.L. Using the Chinese herb Scutellaria barbata against extensively drug-resistant Acinetobacter baumannii infections: in vitro and in vivo studies. BMC Complement Altern Med. 2018;18(1):96. doi: 10.1186/s12906-018-2151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah N., Yasin S., Abro Z., Liu L., Wei Q. Mechanically robust and antimicrobial cotton fibers loaded with silver nanoparticles: synthesized via Chinese holly plant leaves. Int. J. Text Sci. 2014;3:1–5. [Google Scholar]
- Wang L., Chen W., Li M., Zhang F., Chen K., Chen W. A review of the ethnopharmacology, phytochemistry, pharmacology, and quality control of Scutellaria barbata D. Don. J. Ethnopharmacol. 2020;23(254) doi: 10.1016/j.jep.2019.112260. [DOI] [PubMed] [Google Scholar]
- Yu, J., Lei, J., Yu, H., CAI, X., Zou, G., 2004. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochem. 2004;65(7):881–884. [DOI] [PubMed]
- Zhang H., Jin B., Bu J., Guo J., Chen T., Ma Y., Tang J., Cui G., Huang L. Transcriptomic insight into terpenoid biosynthesis and functional characterization of three diterpene synthases in Scutellaria barbata. Molecules. 2018;23(11):2952. doi: 10.3390/molecules23112952. [DOI] [PMC free article] [PubMed] [Google Scholar]