Abstract
Luffa echinata Roxb. is one of the neglected medicinal plants. It is an important source of bioactive metabolites and used in several Ayurvedic formulations. In the present analysis, mature leaves and fruits were extracted with acetone, ethanol, acetonitrile, methanol and water. Phytochemicals like total phenolic (TPC), flavonoid (TFC), tannin (TTC), alkaloid (TAC) and terpenoid (TTEC) content were analysed. Further, antioxidant (AOX) activities like 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, 2,2′-azino-bis-(3-ethyl) benzothiazoline-6-sulfonic acid (ABTS) radical scavenging, metal chelating activity (MC), ferric reducing antioxidant power (FRAP) and phosphomolybdenum assay (PMA) were studied. Highest TPC and TFC (189.57 ± 1.9 mg TAE/g extract, 30.48 ± 0.7 mg CE/g extract, respectively) were reported from acetone extract of the leaves. Ethanolic fruit extract showed the highest TTC (13.79 ± 0.2 mg CE/g extract). Acetone and acetonitrile fruit extract revealed maximum TTEC (602.79 ± 3.5 mg UAE/g extract) and moderate TAC (19.96 ± 0.9 mg GE/g extract), respectively. In AOX, highest DPPH (50.52 ± 0.03 mg AAE/g extract) and ABTS (26.78 ± 0.03 mg TE/g extract) radical scavenging reported in methanolic extract of fruit; however, acetone extract of leaf showed highest FRAP (376.89 ± 1.95 mg Fe(II)/g extract) and PMA (326.54 ± 4.73 mg AAE/g extract). In contrast, aqueous extract of leaf and fruit revealed highest metal chelating activity (41.67 ± 0.49 mg EDTA/g extract). In anti-diabetic studies, acetonitrile extract of leaves and fruits exhibited appreciable inhibition of α-amylase (83.33%) and α-glycosidase (77.42%) enzymes. Similarly, acetyl cholinesterase (AChE) inhibition was highest in water (88.91%) and acetone (81.87%) extracts of leaf and fruits. Fruit extracts showed potent anticancer activity against breast (MCF-7) and colon (HT-29) cancer cell lines (LC50 329.36 and 385.17 µg/mL, respectively). RP-HPLC analysis revealed highest cucurbitacin B (CuB) (196.24 ± 1.4 mg/g DW), followed by cucurbitacin I (CuI) and cucurbitacin E (CuE) in the fruits (57.14 ± 4.9 and 2.03 ± 0.03 mg/g DW, respectively). RP-HPLC analysis of extracts revealed presence of gallic acid (GA), catechin (CA), vanillic acid (VA), chlorogenic acid (CHLA) and coumaric acid (COA), in which highest GA found in the fruits (1.26 ± 0.07 mg/g DW). Liquid chromatography and mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC–MS) analysis revealed presence of bioactive compounds from various groups. Based on the present findings, it was revealed that the fruit and leaf of L. echinata can be used as potent bioresource for natural antioxidants, anti-diabetic, and anticancer drug.
Keywords: Luffa echinata, Antioxidants, Cucurbitacin, LC-MS, HPLC, Phytochemicals
1. Introduction
The plant kingdom is blessed with large amount of biologically active compounds like phenolics, flavonoids, terpenes, ascorbate acid, tocopherols, carotenoids are collectively called as an antioxidant. All the plant parts like root, stem, leaf, fruit, seed and flowers show presence of naturally occurring antioxidants (Gámez-Meza et al., 2009). These antioxidants have ability to protect plant cell from the damage caused by unstable molecule called as reactive oxygen species (ROS). ROS are a group of compounds which are derived from metabolism of oxygen led to biochemical and physiological lesions and often results in metabolic impairment and finally cell death (Li et al., 2011). They also act as mediator of many diseases including cancer, premature ageing, prostaglandin-mediated inflammatory processes, hypertension and heart disease (Souri et al., 2007). Synthetic antioxidants like butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), tertiary butylated hydroquinones and gallic acid esters have been suspected to cause or promote negative health effects (Patel et al., 2018).
Family Cucurbitaceae Juss. contain high economic value as it is a major source of food and possess pharmacological properties like antiulcer, antidiabetic, analgesic, nephro-protective and anticancer (Patel et al., 2020). In India, 31 genera and 94 species of family cucurbitaceae were reported (Renner and Pandey, 2013). Ribosome inactivating proteins (MAP30, luffin A and B) and anti-HIV activity have been reported in some species, hence these plants serve as an important source of anti-retroviral drugs (Modi and Kumar, 2013). Terpenoids are versatile group from bioactive compounds widely used in pharmaceutical, cosmetic and food industries (Jaeger and Cuny, 2016). Terpenoids are used not only to treat various health related issues but also in flavours, fragrances, spices as well as food additives (Schempp et al., 2017). Members of Cucurbitaceae contain cucurbitacins that possess keto-, hydroxy- and acetoxy-groups and are broadly divided into twelve categories (A-T). Since ancient times, cucurbitacins are used in folk medicines due to broad applicability in pharmacological activities like anticancer, cytotoxic, anti-inflammatory, anti-fertility, diabroticites and cardiovascular protective. It is a good inhibitor of JAK2/STAT3; a common oncogenic signalling pathway (Abdelwahab et al., 2011, Eyol et al., 2016). Cucurbitacins have chemo-preventive role against several human cancer cell lines including breast, lung, uterine, cervix, brain, liver, skin and prostate cancers. Some toxic effects like increase in the capillary permeability irritate the intestinal mucosa, and strongly increase intestinal motility have been reported (Eyol et al., 2016).
Luffa echinata Roxb. is commonly called as bitter sponge gourd or Bristly luffa and used in traditional Ayurvedic medicines. The plant possesses various medicinal properties such as laxative, analgesic and used to treat bronchitis, piles, jaundice, and vaginal discharge. Fruits are effective in biliary, intestinal colic and putrid fever. Whole plant is also used as chronic bronchitis, anti-helminthic, emetic, stomachic, nephritis and abortifacient (Kirtikar and Basu, 1933, Nadkarni and Nadkarni, 1976, Kumar et al., 2012). Many reports showed the potency of fruit against jaundice and also exhibited diuretic and antihypertensive effects (Modi and Kumar, 2013). Pharmacological studies reported that fruits and seeds are anti-hepatic and anti-anthelmintic (Murthy et al., 2011). Ethanolic extract revealed presence of alkaloids, carbohydrates, proteins, glycosides, flavonoids, sterols, triterpenes, flavones, reducing sugars and tannins (Kailasiya et al., 2011). Potent bioactive compounds like CuB, eletarin (CuE), eletarin-2-glucoside, isocucurbitacin B, β-sitosterol, echinatol-A and -B, chrysirol-7-glucoside, chrysirol-7-epiglucoside, echinatol A, echinatol B, echinatin have been reported from the fruits (Seshadri and Vydeeswaran, 1971, Ahmed et al., 2001). Datiscacin (cucurbitin-20-acetate); a bitter compound known to have antitumor activity has been reported from L. echinata (Ahmad et al., 1994). Similarly, flavonoids (luteolin-7-glucoside and chrysoeriol-7-glucoside), triterpenes, fatty acids, and saponin (gypsogennin) have been also isolated from seeds (Modi and Kumar, 2013).
Extraction must be nontoxic, eco-friendly, fast and cost effective. Both conventional and non-conventional extraction methods are preferred in which microwave assisted extraction (MAE) is widely used technique for the extraction of bioactive compounds. In this method, solvent and sample mixture heated by using energy of microwave radiation that increase diffusivity of phytochemicals. MAE has several advantages over the classical extraction methods. It consumes less time and quantity of solvent for extraction (Aires, 2017). Hence, MAE was preferred in the present investigation.
Cancer is one of the most distractive common diseases leading to loss of many life forms. It has been estimated that total death due to cancer will rise to 10 million in 2020 and over 16 million in 2050 (Jemal et al., 2011). To overcome this problem several treatments like chemotherapy, radiotherapy and synthetic drugs are preferred but showed several ill effects on human health. Hence, there is an urgent need to focus on alternative therapies which cure cancer (Ochwang'i et al., 2014). Diabetes is also one of severe life threatening disease. It was estimated that by the year 2030, total 7.8% of world population will be affected by this disease (Whiting et al., 2011). Diabetes leads to cardiovascular diseases, premature death, kidney failure and depression. Hence, it is the need of time to investigate natural α-amylase and α-glucosidase inhibitors (Patel et al., 2020). Alzheimer's disease (AD) is considered as one of most common form of dementia, and characterized by slow degradation of neurological functions. Acetylcholine esterase inhibitors (AChEIs) have ability to increase acetylcholine in between synaptic region, resulting into restoration of deficient cholinergic neurotransmission (Ghane et al., 2018, Attar and Ghane, 2019). Several compounds from the genus Luffa have been identified as AChEIs (Feitosa et al., 2011).
Literature survey revealed that very little information available on the potent bioactivities from L. echinata. Hence, in present investigation, mature leaf and fruits were sequentially extracted using several solvents to investigate in vitro antioxidant, anti-diabetic, acetylcholine esterase inhibitory and anticancer potential. In addition, potent bioactive compounds were separated and identified using RP-HPLC, GC–MS, and LC-MS.
2. Materials and methods
2.1. Preparation of extract
Mature leaves and fruits of L. echinata were collected in November 2017, from Shahada town of Nandurbar district, Maharashtra, India. Location lies in between 1°32′34.6″N and 74°29′33.1″E. Plant materials were dried in hot air oven for 72 h at 60 °C and powdered by using electric mill. The powdered material (5 g) was sequentially extracted with 50 mL of respective solvents (acetone, acetonitrile, ethanol, methanol and water) using microwave oven (Samsung CE1350L, Thailand at 900 W power) for period of 180 s. Extracts were centrifuged at 6000 rpm for 10 min, and supernatants were collected in petridish. After complete evaporation, the residue was re-dissolved in 4 mL of respective solvent (acetone, acetonitrile, ethanol, methanol and water), filtered using bacterial filter (6 μm), stored at 4 °C and used for further analysis.
2.2. Determination of total phenolic content (TPC)
TPC was determined according to the method adopted by Patel et al. (2020) with minor modifications. Briefly, the sample (40 µL from 5 mg/mL stock) and standard tannic acid were introduced into a 5 mL test tube. Pre-diluted (1:10) 1 mL Folin reagent and 0.8 mL of sodium carbonate (7.5% w/v) were added, mixed and incubated for 60 min at room temperature. Post-incubation, absorption of reaction mixture was taken at 765 nm using UV–Vis Spectrophotometer (Jasco, V-730, Japan). TPC was expressed as mg of tannic acid equivalents (TAE) per gram extract.
2.3. Determination of total flavonoid content (TFC)
TFC was determined as per our earlier protocol (Ghane et al., 2018). Plant extract (50 µL) was treated with 75 µL of (5% w/v) NaNO2, after 6 min incubation, 150 µL (10% w/v) AlCl3 was added. Post-incubation, 75 µL of distilled water was used to make up volume and finally 800 µL 1 M NaOH was added. Absorbance of the reaction mixture was taken immediately at 510 nm. Standard catechin was used to plot calibration curve and results were expressed as mg of catechin equivalent (CE)/g of plant extract.
2.4. Determination of total tannin content (TTC)
The vanillin-HCl method with minor modifications was used to determine total tannin content (Attar and Ghane, 2017). Briefly, plant extract or standard catechin (100 µL) and 1 mL reagent (4% vanillin and 8% conc. HCl; 1:1 in methanol) were mixed and incubated at room temperature for 20 min and absorbance were taken at 500 nm. All the results were expressed as mg catechin equivalent (CE)/g extract.
2.5. Total terpenoid content (TTEC)
To determine TTEC, the method adopted by earlier researchers was used (Patel et al., 2018). In test tube, 20 µL plant extract or standard ursolic acid (5–40 µg) and 150 µL acidic vanillin reagent (5 g vanillin in 100 mL glacial acetic acid) was taken and mixed. Perchloric acid (500 µL) was added to the reaction mixture and heated in water bath for 45 min at 60 °C. Further, all the reaction mixtures were placed on an ice bath and brought to room temperature and 2.25 mL GAA was added. Absorbance was taken at 548 nm and results were expressed as mg ursolic acid equivalent (UAE)/g extract.
2.6. Total alkaloid content (TAC)
TAC was determined as per method reported by Ghane et al. (2018). Briefly, 69.8 mg of bromocresol green was dissolved in 3 mL 2 N NaOH and 5 mL distilled water, heated and diluted for 1000 mL using distilled water. Plant extract (100 µL from 5 mg/mL stock), 1 mL bromocresol green and 1 mL sodium phosphate buffer was mixed and reaction mixture was extracted using 2 mL chloroform and absorbance of chloroform layer was measured at 470 nm. Galanthamine (20–120 µL) was used as standard and content were expressed as mg galanthamine equivalent (GE)/g extract.
2.7. Antioxidant assay
2.7.1. DPPH radical scavenging activity (DPPH)
The activity was performed as per Patel et al. (2020). Stock solution of DPPH was prepared by dissolving 25 mg DPPH in 1000 mL methanol and kept it in refrigerator until further use. Working solution of DPPH and 30 µL (5 mg/mL stock) plant extract or standard ascorbic acid (mg/mL) were taken in test tube. This reaction kept at dark for 30 min and absorbance of reaction was measured at 515 nm. Control was prepared by using 50 μL methanol in place of the plant sample. Results were expressed in mg of ascorbic acid equivalent (AAE)/g extract.
2.7.2. Ferric reducing antioxidant property assay (FRAP)
FRAP assay was performed according Patel et al. (2020). Briefly, FRAP reagent was prepared by mixing acetate buffer (pH 3.6), 10 µM TPTZ in 40 µM HCl, and 20 µM FeCl3 at ratio of 10:1:1 (v/v/v). Plant extract (20 µL from 5 mg/mL stock) and 1 mL reagent was mixed, incubated at 37 °C in water bath for 30 min, and absorbance was read at 593 nm. Standard FeSO4·7H2O (20–120 mg/mL) was used to plot curve. Results were expressed in mg Fe (II) equivalent/g extract.
2.7.3. Phosphomolybdate assay (PMA)
The method given by Prieto et al. (1999) was adopted with some minor modifications. In this assay, 20 µL plant extract (5 mg/mL stock) or standard ascorbic acid (20–120 µg/mL) was mixed with phosphomolybdate reagent (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). Then reaction mixture was incubated in water bath at 90 °C for 90 min, cooled to room temperature and absorbance was measured at 695 nm. The antioxidant capacity was expressed as mg of ascorbic acid equivalents (AAE)/g extract.
2.7.4. Metal chelating activity (MC)
To determine total antioxidant capacity of L. echinata extracts, metal chelating assay was performed as per our previous reports (Attar and Ghane, 2019). In brief, 50 µL of 2 mM FeCl2 was added to 40 µL (5 mg/mL stock) extract or standard EDTA (20–120 μg/mL). The reaction was started by the addition of 100 µL of 5 mM ferrozine solution and 650 µL distilled water was added to make up the volume. The mixture was vigorously shaken and left to stand at room temperature for 10 min. The absorbance of the solution was thereafter measured at 562 nm. Na2-EDTA was used as positive control. The results were expressed as mg EDTA equivalent (EE)/g extract.
2.7.5. ABTS radical scavenging activity (ABTS)
ABTS radical cation decolourization assay performed according to our earlier protocols (Patel et al., 2018). ABTS radical cation (ABTS) was produced by reacting ABTS solution with 2.45 mM potassium persulfate (1:1 ratio) and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. ABTS solution was diluted in methanol (1:89 ratio) to obtain an absorbance 0.7 (±0.02) at 734 nm. In test tube, 1 mL ABTS solution and 20 µL of extract (5 mg/mL stock) or standard was taken, incubated at 30 °C and absorbance taken at 730 nm exactly after 30 min using methanol as blank. All the solutions were used on the day of preparation. The results were expressed as mg trolox equivalent (TE)/g extract.
2.8. Anti-diabetic activity
2.8.1. α-Amylase inhibition activity
This activity was performed by using earlier published protocols with some minor modifications (Ghane et al., 2018). Plant extract (100 µL) was mixed with 111 µL α-amylase solution (18 units) and 0.02 M sodium phosphate buffer (pH 6.9) and final volume was made to 1 mL. This reaction mixture was then incubated at 25 °C for 10 min, 500 µL 1% starch solution was added and reaction mixture was incubated 25 °C about 30 min. Post incubation, 0.5 mL dinitrosalicylic acid reagent (1 g 3, 5-dinitrosalicylic acid in 20 mL of 2 M NaOH + 30 g Rochelle salt + 50 mL distilled water, all the contents were dissolved and final volume made up to 100 mL with distilled water) was used for interruption of the reaction. All the test tubes were incubated in boiling water bath (100 °C, 5 min) and cooled to room temperature. Finally, reaction mixture was diluted 5 times with distilled water and absorbance was taken at 540 nm. Control was prepared by using sodium phosphate buffer. Acarbose was used as a positive control. α-Amylase inhibition was calculated on a percent basis.
2.8.2. α-Glucosidase inhibition activity
α-Glucosidase inhibition activity was studied as per Ghane et al. (2018). Briefly, 20 µL plant extract was mixed with 100 µL α-glucosidase solution (1 U/mL), 0.1 mol/L phosphate buffer (pH 6.9) and volume made to 500 µL. After 5 min of pre-incubation at 25 °C, 100 µL p-nitrophenyl-α-D-glucopyranoside (5 mmol/L) solution was added and reaction mixture was incubated (10 min, 25 °C). Reaction was terminated by adding 1 mL of 0.1 M Na2CO3 and absorbance was measured at 405 nm. The control was without any extract and acarbose was used as a positive control. Inhibition of α-glucosidase was calculated on a percent basis.
2.9. Acetylcholine esterase (AChE) inhibitory activity
AChE inhibition was studied as per Ghane et al. (2018). Known volume of plant extract (10 µL) was mixed with 15 µL (0.04 unit) of AChE solution. Sodium phosphate buffer (0.1 M pH 8.0) was added to make 800 μL reaction volume and incubated for 15 min at room temperature. Post-incubation, 60 μL of 0.5 mM 5,5-dithiobis- (2-nitrobenzoic acid) (DTNB) was added and reaction was initiated by adding 20 μL acetyl thiocholine iodide (0.71 mM). After 30 min of incubation, absorbance of the reaction was measured at 412 nm on UV–visible spectrophotometer. Galanthamine hydrobromide was used as a standard control. The control was prepared as above except plant extract. AChE inhibitory activity was calculated on a percent basis.
2.10. Anticancer activity
Anticancer activity was studied as per Patel et al. (2020). Breast (MCF-7) and colon (HT-29) cancer cell lines were grown on RPMI 1640 medium which contain 10% fetal bovine serum and 2 mM L-glutamine. All the selected cells were inoculated into 96 well microtiter plates (100 µL) and plates were incubated at 37 °C temperature, 5% CO2, 95% air and 100% relative humidity for 24 h. All the extracts were solubilized in dimethyl sulfoxide (100 μg/mL) and diluted using distilled water to obtain 1 mg/mL stock. Extracts were diluted to 100–800 μg/mL with the medium and 10 µL of these different drug dilutions were added to the appropriate microtiter wells. Post-inoculation, all the plates were incubated for 48 h, and 50 µL cold TCA was used to stop the reaction and incubated for 60 min at 4 °C. Further, supernatant was discarded and washed with tap water. Sulforhodamine B (0.4%, 50 µL) solution was mixed in each plate and incubated for 20 min. Washing five times with residual dye was removed from well 1% acetic acid followed by air drying. Bound stain was then eluted with 10 mM trizma base and absorbance was measured at 540 and 690 nm. Percent growth was calculated and expressed as ratio between extract well absorbance to control well absorbance multiplied by 100 and results were expressed as % control growth, total growth inhibition (TGI) and lethal concentration 50 (LC50).
2.11. Analysis of cucurbitacins, phenolics and other metabolites by RP-HPLC, GC–MS and HR-LCMS
2.11.1. RP-HPLC analysis of cucurbitacins
Three tetracyclic tri-terpenes viz. cucurbitacin I (CuI), cucurbitacin B (CuB) and cucurbitacin E (CuE) were quantified using RP-HPLC apparatus equipped with quaternary pump, autosampler, Hiber C18 column (5 μm, 250–4.6 mm) and UV detector (UV 2070) (Jasco Model No. LC-2000 Plus). Mobile phase consists of acetonitrile, water and methanol (32:35:33, v/v/v) and flow rate was maintained at 1 mL/min (Patel et al., 2020). Injection volume was kept at 20 μL. All the peaks were monitored at 230 nm with 40 min as run time. For identification of compounds; standard cucurbitacins (25–400 μg/mL) were injected and retention time was compared with samples. ChromNAV software system was used for data processing. All the experiment performed in triplicate for assessing suitability of system and amount of cucurbitacins were expressed in milligram per gram of dry weight (mg/g DW).
2.11.2. RP-HPLC analysis of phenolics
RP-HPLC analysis of phenolics was performed using Jasco chromatographic system equipped with quaternary pump, autosampler and UV detector. For separation of phenolics Hiber C18 column (5 μm, 250–4, 6 mm) was used. Mobile phase was water: acetonitrile: glacial acetic acid (90:5:5) at 0.9 mL/min flow rate with 20 μL injection volume. All the peaks were monitored at 280 nm and 50 min as run time (Attar and Ghane, 2017). All the peaks were confirmed by comparing retention time of samples with those of respective standards (gallic acid; GA, catechin; CA, vanillic acid; VA, chlorogenic acid; CHLA and coumaric acid; COA). Further, verification was done by spiking the samples with known concentration of the respective standard. A standard curve of selected phenolics with five different concentrations (30–150 µg/mL) was prepared and results were expressed as micrograms per gram of dry weight (mg/g DW). Analysis was done in triplicate to assess suitability. All the experimental solutions were filtered through 0.22 µm nylon syringe filter before injection.
2.11.3. Identification of major metabolites using GC–MS
Analysis of bioactive compounds from methanol extract of fruits was done using GC–MS instrument (Model QP 2010 series, Shimadzu, Tokyo, Japan) equipped with RTX-1 fused silica capillary column (30 m length, 0.25 mm id, and 0.25 µm thickness) and model AOC-20i autosampler. Pure helium gas (99.99%) was used as carrier at a flow rate of 1.5 mL/min. The injector temperature was maintained at 280 °C and samples were injected through split injection mode. Ion source temperature was 230 °C and interface temperature was fixed to 250 °C. The oven temperature was programmed from 50 °C (isothermal for 2 min), with increase of 10 °C/min to 280 °C. Mass spectra were taken in range of 36 to 800 m/z at a rate of 3.0 scan/ s. The relative percentage amount of each component was calculated by comparing its average peak area to total areas. The mass detector was used in this analysis was GC–MS library (NIST 11).
2.11.4. Identification of major metabolites using LC–MS
Major metabolites were identified from the methanolic fruit extract using HPLC-ESI-MS-NEG-PHENOMENEX in negative ionization mode. System was equipped with binary pump, auto sampler, thermostated column compartment and iFunnel quadrapole time-of-flight spectrometer (Q-TOF). Compound separation was achieved on zorbax eclipse C18 column (4.6 × 250 mm, 5 μm particle size) at 25 °C temperature. In the present study, two mobile phases were used wherein first system consist of mixture of acetonitrile: methanol: water (32:35:33) at a flow rate of 1 mL/min. Second solvent system with 0.1% (v/v) formic acid (A), and acetonitrile (B) was used in gradient elution. Gradient was initiated 80% A and 20% B to 30% B (after 10 min), followed by 40% B (40 min), 60% B (60 min), 90% B (80 min) and finally returned to the initial conditions. Solvent system B injected with flow rate of 0.8 mL/min. Mass spectrometer was operated in range of 100–1000 m/z. N2 was used as nebulizer, drying and collision gas. Drying gas flow rate was 8 lit/min at 325 °C and nebulizer gas at 25 psi with fragmentor voltage 150 V. For data analysis, mass hunter qualitative analysis software package (Agilent Technologies) was used. Detected compounds were validated on the basis of molecular formula, molecular mass, retention time and m/z ratio. For the authentication of compounds, all the details were compared with published database and METLIN personal metabolite database.
2.12. Statistical analysis
The results are presented as mean ± standard error (SE) of at least three independent replicates. Statistical analysis was performed using Analysis of Variance (ANOVA) and the significances of the differences between sample mean were determined using Duncan's multiple range test (DMRT) using SPSS software ver. 16. Statistical significance was set at a level of p < 0.05. Principal component analysis (PCA) was performed using Minitab ver. 18 software.
3. Result
3.1. Phytochemical analysis
In phytochemicals, TPC, TFC, TTC, TAC and TTEC were determined and results are depicted in Table 1. TPC was dependent on the plant parts and solvents used in the present study and values ranged between 189.56 and 38.70 mg TAE/g extract. Acetone extract of leaves (189.56 ± 1.97 mg TAE/g extract) and methanol extract of fruits (104.85 ± 0.61 mg TAE/g extract) exhibited highest TPC, while aqueous extract of both leaf and fruit denoted lowermost phenolic content (58.04 ± 0.86 and 38.69 ± 1.84 mg TAE/g extract, respectively). In TFC, acetone extract of leaf and fruit represent inflated TFC (30.48 ± 0.72 and 11.27 ± 0.50 mg CE/g extract, respectively). Alike TPC, least TFC was noted in aqueous extract of leaf and fruits (1.81 ± 0.52 and 0.057 ± 0.02 mg CE/g extract, respectively). In case of TTC, all the analysed extracts failed to show significant tannin content (13.78 to 2.80 mg CE/g extract), of which, methanol extract of leaf and fruit exhibited the highest TTC (13.78 ± 0.2 and 7.87 ± 0.15 mg CE/g extract, respectively) (Table 1). Highest TTEC was documented from acetone extract of leaf and fruit (541.0 ± 2.23 and 602.7 ± 3.5 mg UAE/g extract, respectively). Similarly, acetone leaf extract and acetonitrile fruit extract revealed highest TAC (18.99 ± 0.8 and 19.96 ± 0.9 mg GE/g extract, respectively) (Table 1).
Table 1.
Total phenolic content (TPC), total flavonoid content (TFC), total tannin content (TTC), total terpenoid content (TTEC), total alkaloid content (TAC), α-amylase, α-glucosidase and acetylcholine esterase inhibitory activities from leaves and fruits of Luffa echinata.
| Solvent | Plant part | Phytochemicals Bioactivities |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TPCa | TFCb | TTCb | TTECc | TACd | α-amylasee | α-glucosidasee | Acetylcholine esterasef | ||
| Acetone | Leaf | 189.57 ± 1.9a | 30.48 ± 0.7a | 8.18 ± 0.1b | 541.09 ± 2.2b | 18.99 ± 0.8a | 53.17 ± 3.9d | 15.11 ± 0.7 fg | 55.14 ± 6.1de |
| Fruit | 95.72 ± 5.8d | 11.28 ± 0.5c | 5.76 ± 0.4d | 602.79 ± 3.5a | 8.33 ± 0.5d | 76.59 ± 3.5ab | 60.66 ± 1.0c | 81.87 ± 0.9a | |
| Acetonitrile | Leaf | 70.87 ± 1.4f | 13.55 ± 0.3b | 13.11 ± 0.07a | 305.12 ± 2.8c | 16.67 ± 0.1b | 83.33 ± 3.9a | 64.65 ± 1.5b | 63.60 ± 3.7bc |
| Fruit | 81.30 ± 3.9e | 8.29 ± 0.7d | 2.80 ± 0.3f | 173.33 ± 2.0d | 19.96 ± 0.9a | 6.35 ± 1.5f | 77.42 ± 1.4a | 47.43 ± 0.9e | |
| Ethanol | Leaf | 101.67 ± 1.9 cd | 6.64 ± 0.2e | 4.09 ± 0.2e | 84.96 ± 2.9f | 7.75 ± 0.5 cd | 76.59 ± 3.5ab | 24.18 ± 1.3e | 52.57 ± 2.7e |
| Fruit | 79.28 ± 2.1e | 2.69 ± 0.2f | 3.71 ± 0.07e | 84.65 ± 0.9f | 5.81 ± 0.3d | 54.37 ± 1.1d | 40.20 ± 1.2d | 48.94 ± 0.3e | |
| Methanol | Leaf | 145.43 ± 2.1b | 13.96 ± 0.8b | 13.79 ± 0.2a | 112.25 ± 2.2e | 9.50 ± 0.5c | 61.51 ± 1.1 cd | 10.31 ± 1.3 h | 71.15 ± 1.05b |
| Fruit | 104.86 ± 0.6c | 6.42 ± 0.1e | 7.88 ± 0.1b | 89.30 ± 4.1f | 7.56 ± 0.6 cd | 67.86 ± 0.3bc | 40.46 ± 0.9d | 48.49 ± 0.7e | |
| Water | Leaf | 58.04 ± 0.8 g | 1.81 ± 0.5f | 8.18 ± 0.2b | 22.95 ± 4.0 h | 6.40 ± 0.6d | ND | 16.73 ± 0.09f | 88.91 ± 0.9a |
| Fruit | 38.70 ± 1.8 h | 0.06 ± 0.02 g | 7.12 ± 0.3c | 39.07 ± 1.8 g | 8.14 ± 0.8c | 18.65 ± 3.5e | 11.79 ± 1.5gh | 62.70 ± 1.3 cd | |
Values were the means of three replicates ± Standard Error (SE). Mean value with different alphabets in column were showed statistically significant differences (p < 0.05) according to Duncan multiple range test.
(mg TAE/g extract), b(mg CE/g extract), c(mg UAE/g extract), d(mg GE/g extract). TAE: Tannic acid equivalent, CE: Catechin equivalent, UAA: Ursolic acid equivalent, GE: Galantamine equivalent. e % inhibition at standard acarbose at 100 μg – 36.84%, facetylcholine esterase inhibition at standard galanthamine (3 μg) − 32.41%, ND – Not defined.
3.2. Antioxidant assays
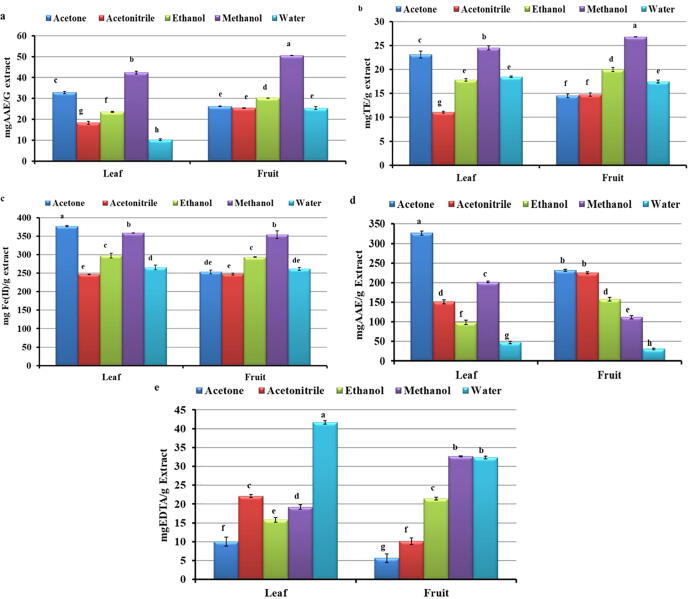
Antioxidant potential from the leaf and fruit extracts of L. echinata was estimated by measuring DPPH, ABTS, FRAP, PMA, and MC activities (Fig. 1). Among tested extracts, methanol leaf and fruit extracts showed promising DPPH radical scavenging activity (Fig. 1a) (42.35 ± 0.74 and 50.52 ± 0.03 mg AAE/g extract, respectively). In contrast, least responses were noted from aqueous extracts of leaf and fruit (10.22 ± 0.43 and 25.35 ± 0.69 mg AAE/g extract, respectively). In ABTS radical scavenging activity, methanolic extract of leaf and fruit exhibited remarkable activity (24.50 ± 0.39 and 26.78 ± 0.03 mg TE/g extract, respectively), whereas lowest activity was found in acetonitrile leaf and acetone fruit extract (11.07 ± 0.18 and 14.48 ± 0.39 mg TE/g extract respectively) (Fig. 1b). Promising FRAP activity was observed in all the tested extracts wherein highest reduction was reported in acetone leaf extract (376.89 ± 1.95 mg Fe (II)/g extract), followed by methanol fruit extract (353.98 ± 10.1 mg Fe (II)/g extract) (Fig. 1c). Leaves showed comparatively higher PMA values than the fruits, wherein acetone extract showed highest (326.54 ± 4.73 mg AAE/g extract) activity than water (47.26 ± 3.22 mg AAE/g extract) extract (Fig. 1d). Similarly, acetone fruit extract delivered highest activity (231.51 ± 2.94 mg AAE/g extract) than water (30.67 ± 1.89 mg AAE/g extract). MC activity was found superior in aqueous extract of leaves and methanol extract from the fruit (41.67 ± 0.49 and 32.64 ± 0.19 mg EDTA/g extract, respectively) (Fig. 1e). However, lowest activity was reported from acetone extract of leaves and fruits (10.05 ± 1.20 and 5.64 ± 1.15 mg EDTA/g extract, respectively).
Fig. 1.
a: 2, 2-diphenyl-1-picrylhydrazyl radical scavenging activity, b: 2,2′-azino-bis-(3-ethyl) benzothiazoline-6-sulfonic acid radical scavenging activity, c: Ferric reducing antioxidant power assay, d: Phosphomolybdenum assay, e: Metal chelating activity of leaf and fruit extract of Luffa echinata Note: Values are means of three replicates ± standard error. Bars having different alphabets showed statistically significant differences (P < 0.05) according to Duncan’s multiple range test.
3.3. Chemometric analysis
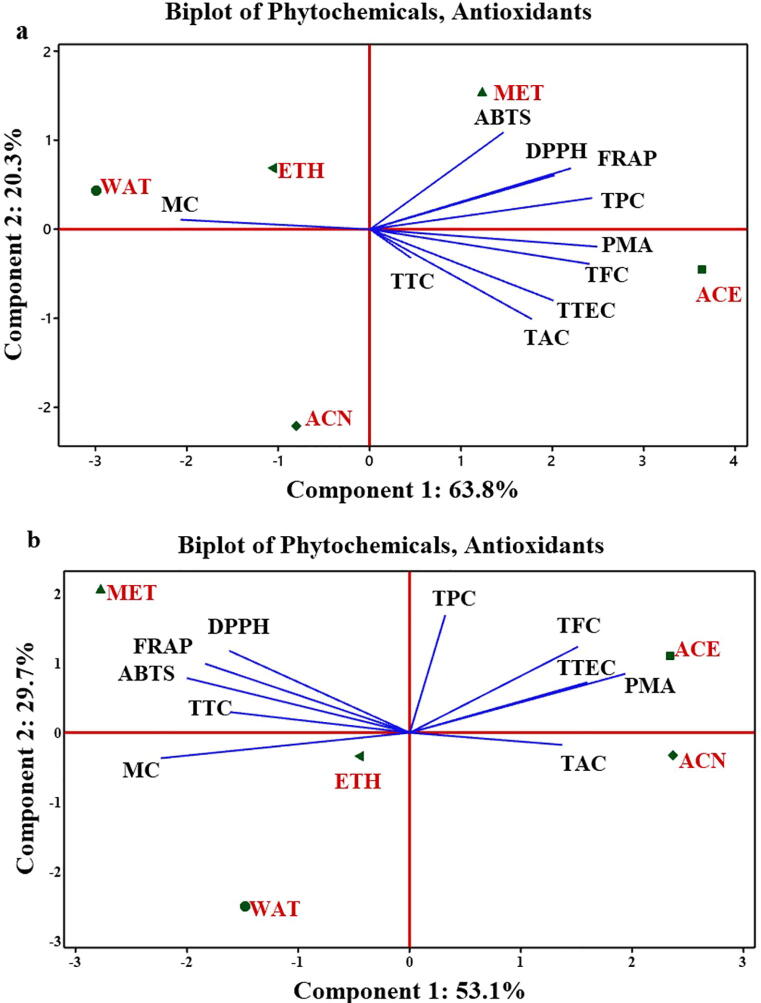
Relationship between phytochemicals and antioxidant activities from leaf and fruit extracts was studied (Fig. 2). Multivariate analysis of leaf extract revealed 84.1% total variability wherein component 1 contributed 63.8% variability. Among all the variables studied, TPC, TFC, TAC, TTEC, DPPH, ABTS, FRAP and PMA enjoyed positive plane of component 1 and exhibited largest distribution with the coefficients 0.381, 0.377, 0.278, 0.315, 0.317, 0.230, 0.345 and 0.390, respectively (Fig. 2a). Similarly, component 2 was mainly dominated by DPPH, ABTS, FRAP and TPC with the loading values 0.299, 0.538, 0.338 and 0.174, respectively. From Fig. 2a, it was cleared that only methanol solvent along with DPPH, ABTS, FRAP and TPC occupied positive plane of both the components. Similarly, variables from the fruits extracts were subjected to PCA and it was found that component first explained 53.1% variability out of 82.9% (Fig. 2b). Component 1 was dominated mainly due to TFC (0.284), TAC (0.258), TTEC (0.300), PMA (0.365) and TPC (0.060). The variables like TPC (0.568), TFC (0.415), TTC (0.100), TTEC (0.243), DPPH (0.395), ABTS (0.264), FRAP (0.333), and PMA (0.284) enjoyed positive side of component 2. In both the components, strong relationship between the phytochemicals (TPC, TFC, TTC and TTEC) and antioxidant activities (DPPH, FRAP, ABTS and PMA) was observed from methanol and acetone extracts of the fruits (Fig. 2b).
Fig. 2.
(a) Principal components analysis (scores and loading plots, biplot) based on different phytochemical compounds analyzed in five different leaf extract of Luffa echinata and their antioxidant activities (DPPH, ABTS, FRAP, PMA and MC). TPC: total phenolics content; TFC: total flavonoid content, TTC: total tannin content, TTEC: total terpenoid content, TAC: total alkaloid content. (b) Principal component analysis (scores and loading plots, biplot) based on different phytochemical compounds analyzed in five different fruit extract of Luffa echinata and their antioxidant activities (DPPH, ABTS, FRAP, PMA and MC). TPC: total phenolics content; TFC: total flavonoid content, TTC: total tannin content, TTEC: total terpenoid content, TAC: total alkaloid content.
3.4. Bioactivities
Anti-diabetic, anti-acetylcholine esterase (AChE) and anticancer activities were performed and results are presented in Table 1, Table 2. Anti-diabetic activity of the selected extracts was performed by α-amylase and α-glucosidase inhibition and showed significant variation in the results. Acetonitrile leaf and acetone fruit extracts showed highest α-amylase inhibitory activity (83.33 ± 3.96 and 76.58 ± 3.57%, respectively), while aqueous extract of leaf and fruit showed poor activity (Table 1). Acetonitrile extract of leaves and fruits revealed the highest inhibition of α-glucosidase enzyme (64.64 ± 1.55 and 77.41 ± 1.43%, respectively). In contrast, acetone extract of leaves and aqueous fruit extract explained the lowermost inhibitory activity (15.11 ± 0.76 and 11.79 ± 1.55%, respectively) (Table 1). Strong AChE inhibition was reported in aqueous leaf extract (88.91 ± 0.90%) and acetone fruit extract (81.87 ± 0.90%). Anticancer activity was evaluated against human breast (MCF-7) and colon (HT-29) cancer cell lines and results are presented in Table 2 and supplementary Figure 1. Growth inhibition was directly proportional to concentration of extract tested. Fruit extract of L. echinata showed highest cytostatic (TGI-161.58 µg/mL) and cytotoxic (LC50 329.36 µg/mL) activity against MCF-7 cell line when compared to standard adriamycin (GI50 < 10 µg/mL, TGI 104.27 µg/mL, LC50 385.17 µg/mL). Similarly, efficient activity reported against HT-29 cell line. The extract also showed remarkable anticancer activity (GI50 < 10 µg/mL, TGI-3.95 µg/mL, LC50 159.98 µg/mL) against adriamycin (GI50 < 10, TGI 0.880, LC50 63.20 µg/mL) (Table 2; Supplementary Fig. 1).
Table 2.
In vitro anticancer activity of Luffa echinata fruit against human cancer cell lines.
| MCF-7 |
HT-29 |
|||||
|---|---|---|---|---|---|---|
| GI50 (µg/mL) | TGI (µg/mL) | LC50 (µg/mL) | GI50 (µg/mL) | TGI (µg/mL) | LC50 (µg/mL) | |
| Standard adriamycin | <10 | 104.27 | 385.17 | <10 | 0.880 | 63.20 |
| Luffa echinata fruit | <10 | 161.58 | 329.36 | <10 | 3.95 | 159.98 |
TGI - concentration of drug causing total inhibition of cell growth, LC50 -concentration of drug causing 50% cell death, GI50 - concentration of drug cause 50% of maximal inhibition of cell proliferation.
3.5. Detection of cucurbitacins, phenolics and other bioactives
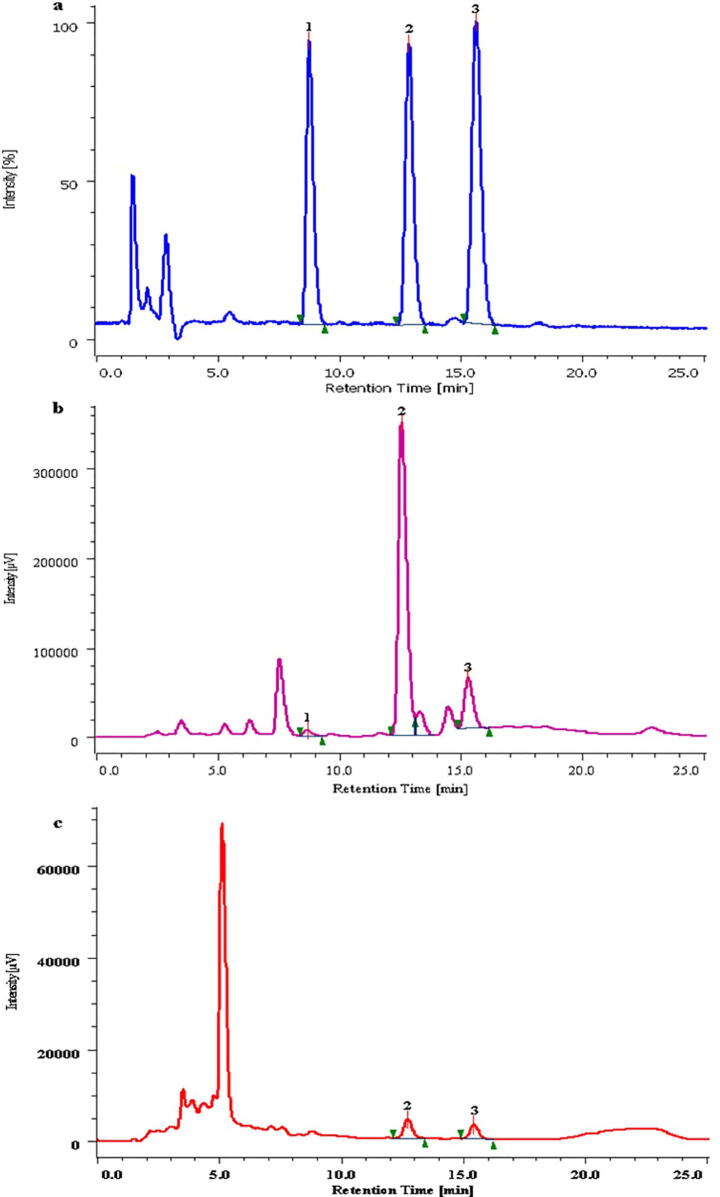
Important bioactive metabolites were separated by using RP-HPLC, GC–MS, and LC-MS. RP-HPLC analysis revealed presence of three cucurbitacins (CuI, CuB and CuE) (Fig. 3, Supplementary Fig. 2). Fruits extracts divulged presence of all the three cucurbitacins; wherein, CuB (196.24 ± 1.4 mg/g DW) content was highest followed by CuE (57.14 ± 4.9 mg/g DW) and CuI (2.03 ± 0.03 mg/g DW) (Fig. 3b). Similarly, leaf extract showed presence of only two cucurbitacins viz. CuB (2.19 ± 0.17 mg/g DW) and CuE (2.97 ± 0.54 mg/g DW) (Fig. 3c). Fruit extract revealed presence of phenolics where GA (1.26 ± 0.07 mg/g DW) was found in highest quantities followed by CA (0.40 ± 0.04 mg/g DW), VA (0.14 ± 0.01 mg/g DW), CHLA (0.039 ± 0.006 mg/g DW) and COA (0.005 ± 0.00 mg/g DW). Content of all the phenolics noted comparatively less from the leaves wherein GA was observed in higher (1.26 ± 0.07 mg/g DW) quantities (Supplementary Table 1 and Supplementary Fig. 3). By using GC–MS, bioactive metabolites were separated and identified from methanol extract. In the present study, total 24 compounds belong to fatty acids, phenols, lipids, volatiles, aldehyde and triterpenes were detected and identified on the basis of chromatogram peak area, molecular weight, molecular formula, and derived compounds. Compounds such as 9,12-octadecadienoic acid, 18-nonadecenoic acid, hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester, n-hexadecanoic acid, octadecanoic acid, squalene were identified (Supplementary Table 1). LC-MS analysis revealed presence of total 30 major compounds from the fruit extract (Table 3, Supplementary Fig. 4.). Detected compounds were mainly from terpenoids (6), phenolics (2), flavonoids (6), alkaloids (3), and fatty acids (13) categories. Several important metabolites like cinnacassiol E (398.19 g/mol), cinncassiol C3 (382.19 g/mol), hymenoxon (282.14 g/mol), clerodin (434.23 g/mol), [6]-shogaol, maritimetin, gartanin, rotenone, hispidulin, berbamunine, bebeerines, daphnoline, dimethyl adipate, traumatic acid, palmitic acid and petroselinic acid were reported (Table 3, Supplementary Fig. 4).
Fig. 3.
RP-HPLC chromatogram (a) Std. 1-CuI, 2-CuB, 3-CuE; (b) Cucurbitacins in L. echinata fruit; (c) Cucurbitacins in L. echinata leaf.
Table 3.
The compounds detected in fruit extract of Luffa echinata by LCMS–ESI-Q-TOF-MS.
| Sr. No. | Category | Name of Compound | Molecular Formula | RT | M/Z | Mass |
|---|---|---|---|---|---|---|
| 1 | Triterpenoids | Cinncassiol Ea | C20H30O8 | 10.8 | 397.1851 | 398.1924 |
| 2 | Hymenoxona | C15H22O5 | 12.58 | 281.1386 | 282.146 | |
| 3 | Ibuprofen acyl glucuronidea | C19H26O8 | 12.65 | 381.1538 | 382.1612 | |
| 4 | Cinncassiol C3b | C20H30O7 | 4.59 | 381.1918 | 382.199 | |
| 5 | Clerodinb | C24H34O7 | 4.86 | 433.2229 | 434.2301 | |
| 6 | Nigakilactone Eb | C24H34O8 | 7.71 | 449.2182 | 450.2255 | |
| 7 | Phenols | [6]-Shogaola | C17H24O3 | 13.36 | 275.1642 | 276.1713 |
| 8 | Maritimetina | C15H10O6 | 20.1 | 285.0391 | 286.0464 | |
| 9 | Flavonoids | Gartanina | C23H24O6 | 34.22 | 395.1502 | 396.1573 |
| 10 | Rotenonea | C23H22O6 | 34.81 | 393.1346 | 394.1416 | |
| 11 | Apiinb | C26H28O14 | 3.28 | 563.1402 | 564.1476 | |
| 12 | Meloside Ab | C27H30O15 | 3.31 | 593.151 | 594.1583 | |
| 13 | Galanginb | C15H10O5 | 6.52 | 269.0457 | 270.053 | |
| 14 | Hispidulinb | C16H12O6 | 6.73 | 299.0564 | 300.0637 | |
| 15 | Alkaloids | Berbamuninea | C36H40N2O6 | 26.63 | 595.2819 | 596.2885 |
| 16 | Bebeerinea | C36H38N2O6 | 28.38 | 593.2663 | 594.2733 | |
| 17 | Daphnolinea | C35H36N2O6 | 30.82 | 579.2506 | 580.2576 | |
| 18 | Fatty acid | Methyl N-(a-methylbutyryl)glycinea | C9H16O4 | 17.41 | 187.0971 | 188.1044 |
| 19 | Dimethyl adipatea | C8H14O4 | 15.14 | 173.0815 | 174.0887 | |
| 20 | Traumatic Acida | C12H20O4 | 24.1 | 227.1278 | 228.1353 | |
| 21 | Chaulmoogric acida | C18H32O2 | 42.73 | 279.2343 | 280.2416 | |
| 22 | Di(2-ethylhexyl) adipatea | C22H42O4 | 43.04 | 369.3007 | 370.308 | |
| 23 | Palmitic acida | C16H32O2 | 46.44 | 255.2335 | 256.2407 | |
| 24 | Petroselinic acida | C18H34O2 | 47.03 | 281.2496 | 282.2569 | |
| 25 | 2-Isopropylmalic acidb | C7H12O5 | 5.22 | 175.0614 | 176.0687 | |
| 26 | 3-Hydroxyisoheptanoic acidb | C7H14O3 | 5.52 | 145.0869 | 146.0941 | |
| 27 | 9S,12S,13S-trihydroxy-10E-octadecenoic acidb | C18H34O5 | 7.84 | 329.2338 | 330.241 | |
| 28 | Traumatic Acidb | C12H20O4 | 9.73 | 227.1293 | 228.1367 | |
| 29 | (±)9,10-DiHOMEb | C18H34O4 | 27.42 | 313.2392 | 314.2465 | |
| 30 | Sebacic acidb | C10H18O4 | 29.92 | 201.1135 | 202.1208 |
Isocratic system (acetonitrile: methanol: water; 32:35:33).
Gradient system (0.01% formic acid: acetonitrile; 65:35).
4. Discussion
Luffa echinata is rich source of bioactive compounds and hence used in pharmaceutical and cosmetic industries (Kirtikar and Basu, 1933). The presence of massive and important bioactivities relies on bioactive composites particularly phytochemicals and antioxidants. For that purpose validation and documentation of bioactive metabolites is very much essential (Ghane et al., 2018, Attar and Ghane, 2019, Patel et al., 2020). Phytochemical analysis of L. echinata exhibited diversified components from various extracts of leaf and fruits. Acetone and methanol extracts were found superior for phytochemical extraction, suggesting a greater content of polar than non polar compounds. This investigation reported reliable amount of phenolics, flavonoids, tannins, terpenoids and alkaloids from the leaves and fruits of L. echinata. Findings support the results of Modi et al., 2011, Attar and Ghane, 2019, who observed similar trend in seeds of Lagenaria. Presence of secondary metabolites always linked to potential biological effects. Phenolics are type of secondary metabolite which contribute in defence mechanism of plant and protect plant from stress conditions (Patel et al., 2018). Flavonoids are radical scavenging molecules and known for hydrolytic and oxidative enzymes inhibition and anti-inflammatory action (Atanassova et al., 2011). Acetone was found to be significant solvent for phytoconstituent extraction; these results agreed with Attar and Ghane (2019). Tannins are the antimicrobial and anticarcinogenic agent used in treatment of ulcerated tissue (Patel et al., 2018). Terpenoids are the most diverse and largest producing metabolites in plants that play overpriced role during growth and development (Tholl, 2015). Cucurbitaceae members are known for tetracyclic triterpenes referred as cucurbitacin (Patel et al., 2020). Except aqueous extract, all the tested extracts showed reliable amount of terpenoids wherein fruit performed better. Attar and Ghane (2019) reported similar trend in acetone extracts of epicarp, mesocarp and seeds of L. siceraria (325.24, 365.36, 384.19 mg UAE/g extract, respectively). Alkaloids are pharmaceutically and cosmetically very important and possesses anti-parasitic, anti-plasmodial, anticorrosive, anti-oxidative antibacterial, and insecticidal activities (Kurek, 2019). Acetone and acetonitrile extracts of leaf and fruit were found to be the best for alkaloid extraction. Ghane et al. (2018) revealed highest alkaloid content from leaves of different Crinum species. Presence of different types of bioactive phytoconstituents are known for offering imperative biological activities in plant system.
Diabetes is considered as one of the most common disease in 21st century and seventh leading cause for death (WHO, Diabetes). Due to imbalance between blood sugar absorption and insulin secretion type 2 diabetes (T2D) disease is caused, postprandial hyperglycemia play major role in development of T2D. The disease is regulated by altering plasma glucose level (Baron, 1998). Amylases are the enzymes which catalyses hydrolysis of starch into sugars, inhibition of such enzyme delay carbohydrate digestion resulting in decrease in glucose absorption and decreasing in post-prandial hyperglycemia (Sales et al., 2012). α-Glucosidase; a carbohydrate hydrolyzing enzyme found responsible for increasing blood glucose level. Practice of diet or drug which delay the production and absorption of glucose is one of the therapeutic approaches for decreasing postprandial hyperglycemia (Tiwari and Rao, 2002). Analysed extract denoted significant antidiabetic activity wherein acetonitrile and acetone extracts found to be the best. Hence, polar solvents could be preferred to separate antidiabetic drugs from L. echinata. Kushawaha et al. (2016) determined similar findings from hot water extract of Cucurbita maxima seeds. Acetylcholinesterase inhibitors are the medications that prevent the breakdown of acetylcoline in human body. Acetylcholine is one of the chemicals responsible for communication in between nerve cells and brain. Its reduced level initiates symptoms of Alzheimer’s disease (Khadri et al., 2010). Leaf and fruit extracts showed presence of significant AChE inhibitory activity. Similar findings have been reported by Ghane et al. (2018) in different species of Crinum. Anticancer activity of methanolic fruit extract was tested against human breast (MCF-7) and colon (HT-29) cancer cell lines and findings showed presence of promising anticancer compounds in L. echinata. Patel et al. (2020) reported higher inhibitory activity from the methanolic fruit extract of D. palmatus when tested against MCF-7 and HT-29 cell lines (LC50 44.27 and 68.31 µg/mL, respectively) that could be due to the presence of cucurbitacins.
Antioxidants are responsible for detoxification of reactive oxygen intermediates in plant system (Patel et al., 2018, Patel et al., 2020). Therefore, improved antioxidant status can minimize the risk of developing free radical induced disease. Antioxidants are determined spectrophotometrically by exploiting ability to reduce fluorescent or oxidizing agent, and this change in colour is correlated with antioxidant activity (Siddeeg et al., 2020). Antioxidants are commonly screened by using DPPH, ABTS, FRAP, phosphomolybdate, metal chelating etc. assays (Ghane et al., 2018, Patel et al., 2018, Attar and Ghane, 2019, Patel et al., 2020). DPPH is a stable free radical which accepts electrons and hydrogen radical from antioxidant compound. A solution of DPPH radicals is converted into DPPH-H (diphenylhydrazine) molecules having low colour intensity. The discolouration of DPPH solution due to the extract represents radical scavenging activity (Aksoy et al., 2013). Similar findings were reported in L. cylindrica and Lagenaria siceraria (Sharma et al., 2012, Attar and Ghane, 2019). ABTS cation radical was developed by reaction of potassium per sulphate with ABTS salt. Loss of nitrogen atom from ABTS responsible for the formation of free radicals. Nitrogen quenches hydrogen atom and results into decolourization of solution. In FRAP assay, antioxidants react with a ferric tripyridyltriazine (Fe3+-TPTZ) complex which produces blue coloured ferrous tripyridyltriazine (Fe2+-TPTZ) and its reducing potential is measured at 593 nm (Patel et al., 2018, Attar and Ghane, 2019). In phosphomolybdate assay, there was production of green phosphate/Mo(V) complex due to presence of antioxidant and phosphate ions were reduced. By using spectrophotometer green phosphate/Mo(V) complex was measured (Prieto et al., 1999). Metal ions like iron which stimulates lipid peroxidation by Fenton reaction as well as responsible for decomposition of lipid hydro peroxides into peroxyl and alkoxyl radicals that can perpetuate the chain reaction. Chelating activity is significant which reduces the concentration of the transition metal that catalyzes lipid peroxidation (Mohan et al., 2012). Highest DPPH, ABTS, FRAP, PMA, and MC were recorded from methanol, acetone and aqueous extracts and hence could be the best solvents for maximizing the recovery of antioxidants (Ghane et al., 2018, Patel et al., 2018, Attar and Ghane, 2019). Antioxidant activities showed positive correlation with analysed phytochemicals, this might indicate that these phytochemicals are main contributor to antioxidant activity in the examined extracts (Attar and Ghane, 2019).
Further, correlation between phytochemicals and bioactivities from five extracting solvents was determined by principal component analysis (PCA). PCA is the most common statistical method used to analyse relationship between numbers of variables to compress original data into small factors with minimum loss of information. Data with suppressed or hidden information of all the variables increase the efficiency of statistical technique and computed variables could be intercorrelated in PCA (Garciaa et al., 2019). From the PCA data, it could be inferred that methanol and acetone extracts found responsible for the potent antioxidant activities. These solvents could be used for the extraction of natural antioxidants from L. echinata. Our results agreed with Attar and Ghane (2019), who tested fruit parts of L. siceraria for determination of variation in the chemical profile. Ghane et al. (2018) observed similar pattern of attraction in between phytochemicals and antioxidants of methanol extract of Crinum species. Similarly, Gupta et al. (2018) demonstrated variation in chemical profile of different plant parts of Citrullus colocynthis.
HPLC analysis confirmed the presence of three tetracyclic triterpenes i.e. cucurbitacins in methanolic extract of leaves and fruits. Fruits represented all the three cucurbitacins (CuI, CuB and CuE), while leaf acquired only CuB and CuE. Similarly fruit extract acquire phenolics (GA, CA, VA, CHLA and COA) in significant amount. Terpenes as well as phenolics are also known to possess promising antioxidant potential (Celaya et al., 2016, Attar and Ghane, 2018, Patel et al., 2020). Additionally, GC–MS and LC-MS analysis revealed presence of compounds like octadecadienoic acid, nonadecenoic acid, hexadecanoic acid, squalene, cinncassiol E, clerodin, galangin, hispidulin, maritimetin, and bebeerines etc. were known to possess anticancer, antidiabetic, antimicrobial, antioxidant, anti-inflammatory, antimutagenic activities (De Freitas et al., 2017, Kou et al., 2018, Oetari et al., 2019, Yin et al., 2008). Compounds detected in RP-HPLC, GC–MS and LC-MS analysis could be responsible to exhibit potent bioactivities in L. echinata.
5. Conclusion
Present study revealed that fruit and leaf extract of L. echinata exhibited promising antioxidant activities that could be due to the presence of phenolics, flavonoids and terpenoids. Acetone and methanol were found to be the best solvents for maximizing the recovery of antioxidants. Acetone and acetonitrile extracts revealed promising antidiabetic (α-amylase, α-glucosidase inhibitory) and anti-acetylcholine esterase activities. Methanol extract of fruit was found effective against tested cancer cell lines viz. MCF-7 and HT-29. RP-HPLC study showed presence of tetracyclic tri-terpenes called cucurbitacins viz. CuI, CuB and CuE and five phenolics (GA, CA, VA, CHLA and COA). In LC-MS and GC–MS analysis also revealed diverse array of compounds belongs to terpenoids, phenolics, alkaloids and fatty acids. All the detected compounds could be responsible for the potent antioxidant, antidiabetic, anti-acetylcholine esterase and anticancer activities. We conclude that fruits of L. echinata can be used for the management of diabetes, neurological disorder and cancer through development of novel drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are thankful to Science and Engineering Board (SERB), New Delhi, India for financial assistance (Sanction no. SB/EMEQ-460/2014). DST-FIST and UGC-DRS-SAP programs are duly acknowledged. We are also grateful to Anti-cancer drug screening facility (ACDSF), Advanced Centre for Treatment, Research & Education in Cancer, (ACTREC), Tata Memorial Centre, Mumbai, India for their help in anticancer activities.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.03.050.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abdelwahab S.I., Hassan L.E., Sirat H.M., Yagi S.M., Koko W.S., Mohan S., Taha M.M., Ahmad S., Chuen C.S., Narrima P., Rais M.M., Hadi A.H. Anti-inflammatory activities of cucurbitacin E isolated from Citrullus lanatus var. citroides: Role of reactive nitrogen species and cyclooxygenase enzyme inhibition. Fitoterapia. 2011;82:1190–1197. doi: 10.1016/j.fitote.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Ahmad M.U., Huq M.E., Sutradhar R.K. Bitter principles of Luffa echinata. Phytochem. 1994;36:421–423. doi: 10.1016/S0031-9422(00)97088-2. [DOI] [Google Scholar]
- Ahmed B., Alam T., Khan S.A. Hepatoprotective activity of Luffa echinata fruits. J. Ethnopharmacol. 2001;76:187–189. doi: 10.1016/s0378-8741(00)00402-5. [DOI] [PubMed] [Google Scholar]
- Aires, A., 2017. Phenolics in foods: extraction, analysis and measurements. In: Phenolic compounds - Natural sources, importance and applications, pp. 61-88.10.5772/66889.
- Aksoy L., Kolay E., Yasin A., Aslan Z., Kargıoglu M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 2013;20:235–239. doi: 10.1016/j.sjbs.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassova M., Georgieva S., Ivancheva K. Total phenolic and total flavonoid contents, antioxidant capacity and biological contaminants in medicinal herbs. J. Chem. Technol. Metall. 2011;46:81–88. [Google Scholar]
- Attar U.A., Ghane S.G. Phytochemicals, Antioxidant activity and phenolic profiling of Diplocyclos palmatus (L.) C. Jeffery. Int. J. Pharm. Pharm. Sci. 2017;9:101–106. doi: 10.22159/ijpps.2017v9i4.16891. [DOI] [Google Scholar]
- Attar U.A., Ghane S.G. Optimized extraction of anti-cancer compound – cucurbitacin I and LC–MS identification of major metabolites from wild Bottle gourd (Lagenaria siceraria (Molina) Standl.) S. Afr. J. Bot. 2018;119:181–187. doi: 10.1016/j.sajb.2018.09.006. [DOI] [Google Scholar]
- Attar U.A., Ghane S.G. In vitro antioxidant, antidiabetic, antiacetylcholine esterase, anticancer activities and RP-HPLC analysis of phenolics from the wild bottle gourd (Lagenaria siceraria (Molina) Standl.) S. Afr. J. Bot. 2019;125:360–370. doi: 10.1016/j.sajb.2019.08.004. [DOI] [Google Scholar]
- Baron A.D. Postprandial hyperglycaemia and alpha-glucosidase inhibitors. Diabetes Res. Clin. Pract. 1998;40:S51–S55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Celaya L.S., Viturro C.I., Silva L.R., Moreno S. Natural antioxidants isolated from Schinus areira leaves by ultrasound-assisted extraction. Int. J. Food Stud. 2016;5:167–179. doi: 10.7455/ijfs.v5i2.329. [DOI] [Google Scholar]
- De Freitas M., De Miranda M.B., De Oliveira D.T., Vieira-Filho S.A., Caligiorne R.B., De Figueiredo S.M. Biological activities of Red Propolis: A review. Recent Pat Endocr. Metab. Immune Drug Discov. 2017;11:3–12. doi: 10.2174/1872214812666180223120316. [DOI] [PubMed] [Google Scholar]
- Eyol E., Tanrıverdi Z., Karakuş F., Yılmaz K., Ünüvar S. Synergistic anti-proliferative effects of cucurbitacin I and irinotecan on human colorectal cancer cell lines. J. Clin. Exp. Pharmacol. 2016;6:219. doi: 10.4172/2161-1459.1000219. [DOI] [Google Scholar]
- Feitosa C.R.S., Silva R.C., Braz-Filho R., Menezes J.E.S.A., Siqueira S.M.C., Monte F.J.Q. Characterization of chemical constituents of Luffa operculata (Cucurbitaceae) Am. J. Anal. Chem. 2011;02:989–995. doi: 10.4236/ajac.2011.28116. [DOI] [Google Scholar]
- Gámez-Meza N., Noriega-Rodríguez J.A., Leyva-Carrillo L., Ortega-García J., Bringas-Alvarado L., García H.S., Medina-Juárez L.A. Antioxidant activity comparison of thompson grape pomace extract, rosemary and tocopherols in soybean oil. J. Food Process. Preserv. 2009;33:110–120. doi: 10.1111/j.1745-4549.2008.00285.x. [DOI] [Google Scholar]
- Garciaa D.P., Caraschi J.C., Ventorim G., Henrique F., Vieira A., Protásio T.P. Assessment of plant biomass for pellet production using multivariate statistics (PCA and HCA) Renew. Energy. 2019;139:796–805. doi: 10.1016/j.renene.2019.02.103. [DOI] [Google Scholar]
- Ghane S.G., Attar U.A., Yadav P.B., Lekhak M.M. Antioxidant, anti-diabetic, acetylcholinesterase inhibitory potential and estimation of alkaloids (lycorine and galanthamine) from Crinum species: An important source of anticancer and anti-Alzheimer drug. Ind. Crops Prod. 2018;125:168–177. doi: 10.1016/j.indcrop.2018.08.087. [DOI] [Google Scholar]
- Gupta, S.C., Tripathi, T., Paswan, S.K., Agarwal, A.G., Rao, C.V., Sidhu, O.P., 2018. Phytochemical investigation, antioxidant and wound healing activities of Citrullus colocynthis (bitter apple). Asian Pac. J. Trop. Biomed. 8, 418-424. 10.4103/2221-1691.239430.
- Jaeger R., Cuny E. Terpenoids with special pharmacological significance: A review. Nat. Prod. Commun. 2016;11:1373–1390. doi: 10.1177/1934578X1601100946. [DOI] [PubMed] [Google Scholar]
- Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. “Global cancer statistics”, CA Cancer. J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kailasiya D., Jain S.K., Alok S., Verma M., Kanaujia V. Phytochemical screening on the aerial part of the Luffa echinata Linn. Int. J. Pharm. Sci. Res. 2011;2:2446–2450. doi: 10.13040/IJPSR.0975-8232. [DOI] [Google Scholar]
- Khadri A., Neffati M., Smiti S., Falé P., Lino A.R.L., Serralheiro M.L.M., Araújo M.E.M. Antioxidant, anti-acetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT-Food Sci. Tech. 2010;43:331–336. doi: 10.1016/j.lwt.2009.08.004. [DOI] [Google Scholar]
- Kirtikar, K.R., Basu, B.D., 1933. Indian medicinal plants. Lalit Mohan Basu and Co. Allahabad, India. Vol 2, 2nd Ed., p.1125.
- Kou X., Wang X., Ji R., Liu L., Qiao Y., Lou Z., Ma C., Li S., Wang H., Ho C.T. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018;9:1310–1327. doi: 10.1039/c7fo01354j. [DOI] [PubMed] [Google Scholar]
- Kumar D., Kumar A., Prakash O. Potential antifertility agents from plants: a comprehensive review. J. Ethnopharmacol. 2012;140:1–32. doi: 10.1016/j.jep.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Kurek J. Introductory Chapter: Alkaloids - their importance in nature and for human life, alkaloids - their importance in nature and human life. Joanna Kurek, IntechOpen. 2019 doi: 10.5772/intechopen.85400. [DOI] [Google Scholar]
- Kushawaha D.K., Yadav M., Chatterji S., Srivastava A.K., Watal G. α-Amylase and α-Glucosidase inhibitory activity assessment of Cucurbita maxima seeds – a LIBS based study. Int. J. Phytomedicine. 2016;8:312–318. doi: 10.5138/09750185.1906. [DOI] [Google Scholar]
- Li P., Huo L., Su W., Lu R., Deng C., Liu L., Deng Y., Guo N., Lu C., He C. Free radical-scavenging capacity, antioxidant activity and phenolic content of Pouzolzia zeylanica. J. Serb. Chem. Soc. 2011;76:709–717. doi: 10.2298/JSC100818063L. [DOI] [Google Scholar]
- Modi A., Kumar V. Luffa echinata Roxb.-A review on its ethanomedicinal, phytochemical and pharmacological perspective. Asian Pac. J. Trop. Dis. 2013;4(Suppl 1):S7–S12. doi: 10.1016/S2222-1808(14)60409-6. [DOI] [Google Scholar]
- Modi A., Kumar V., Jain P.K., Jain S.K., Uplanchiwar V.P. Evaluation of antioxidant activity of flavonoid and phenolic contents of Luffa echinata Roxb. fruits and Nyctanthus arbor-tristis leaves. Int. J. Phytopharm. 2011;1:8–14. doi: 10.7439/ijpp.v1i1.158. [DOI] [Google Scholar]
- Mohan S.C., Balamurugan V., Salini S.T., Rekha R. Metal ion chelating activity and hydrogen peroxide scavenging activity of medicinal plant Kalanchoe pinnata. J. Chem. Pharm. Res. 2012;4:197–202. [Google Scholar]
- Murthy P.K., Joseph S.K., Murthy P.S. Plant products in the treatment and control of filariasis and other helminth infections and assay systems for antifilarial/anthelmintic activity. Planta Med. 2011;77:647–661. doi: 10.1055/s-0030-1250452. [DOI] [PubMed] [Google Scholar]
- Nadkarni, K.M., Nadkarni, A.K., 1976. Indian materia medica. Popular Parkashan, Mumbai, India, Vol. 31, 3rd Ed., p. 268.
- Ochwang'i D.O., Kimwele C.N., Oduma J.A., Gathumbi P.K., Mbaria J.M., Kiama S.G. Medicinal plants used in treatment and management of cancer in Kakamega County Kenya. J. Ethnopharmacol. 2014;151:1040–1055. doi: 10.1016/j.jep.2013.11.051. [DOI] [PubMed] [Google Scholar]
- Oetari R.A., Hasriyani H., Prayitno A., Sahidin S. Gartanin compounds from extract ethanol pericarp mangosteen (Garcinia mangostana Linn.) Open Access Maced. J. Med. Sci. 2019;7:3891–3895. doi: 10.3889/oamjms.2019.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.B., Attar U.A., Ghane S.G. Antioxidant potential of wild Lagenaria siceraria (Molina) Standl. Thai J. Pharm. Sci. 2018;42:90–96. [Google Scholar]
- Patel S.B., Attar U.A., Sakate D.M., Ghane S.G. Efficient extraction of cucurbitacins from Diplocyclos palmatus (L.) C. Jeffrey: Optimization using response surface methodology, extraction methods and study of some important bioactivities. Sci. Rep. 2020;10:2109. doi: 10.1038/s41598-020-58924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Renner S.S., Pandey A.K. The Cucurbitaceae of India: Accepted names, synonyms, geographic distribution and information on images and DNA sequences. Phytokeys. 2013;20:53–118. doi: 10.3897/phytokeys.20.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales P.M., Souza P.M., Simeoni L.A., Silveira D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012;15:141–183. doi: 10.18433/j35s3k. [DOI] [PubMed] [Google Scholar]
- Schempp F.M., Drummond L., Buchhaupt M., Schrader J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J. Agric. Food. Chem. 2017;66:2247–2258. doi: 10.1021/acs.jafc.7b00473. [DOI] [PubMed] [Google Scholar]
- Seshadri T.R., Vydeeswaran S. Chemical examination of Luffa echinata. Phytochem. 1971;10:667–669. doi: 10.1016/S0031-9422(00)94717-4. [DOI] [Google Scholar]
- Sharma N.K., Sangh P., PriyankaJha K.K., Singh H.K., Shrivastava A.K. Free radical scavenging activity of methanolic extract of Luffa cylindrica leaves. Int. J. Green Pharm. 2012;6 doi: 10.22377/ijgp.v6i3.267. [DOI] [Google Scholar]
- Siddeeg, A., AlKehayez, N.M., Abu-Hiamed, H.A., Al-Sanea, E.A., AL-Farga, A.M., 2020. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci. https://doi.org/10.1016/j.sjbs.2020.11.064. [DOI] [PMC free article] [PubMed]
- Souri E., Amin G., Farsam H., Jalalizadeh H., Barezi S. Screening of thirteen medicinal plant extracts for antioxidant activity. Iran. J. Pharm. Sci. 2007;7:149–154. doi: 10.22037/IJPR.2010.758. [DOI] [Google Scholar]
- Tholl, D., 2015. Biosynthesis and biological functions of terpenoids in plants. In: Schrader, J., Bohlmann, J. (Eds.) Biotechnology of Isoprenoids. Advances in Biochemical Engineering/Biotechnology, vol 148. Springer, Cham. https://doi.org/10.1007/10_2014_295 [DOI] [PubMed]
- Tiwari A.K., Rao J.M. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr. Sci. 2002;83:30–38. https://www.jstor.org/stable/24106071 [Google Scholar]
- Whiting D.R., Gurriguata L., Weil C., Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). Diabetes. https://www.who.int/ Home/Newsroom/Fact sheets/Detail/Diabetes/ (accessed 18 November 2020).
- Yin J., Xing H., Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.