Abstract
Antimicrobial resistance patterns among different Escherichia coli isolates in the Kingdom of Saudi Arabia. This study aimed to investigate the patterns of antimicrobial resistance in E. coli isolated from different samples, and to identify potential pathogenic isolates in Riyadh, Kingdom of Saudi Arabia (KSA). In total, 51 bacterial isolates were recovered from 113 samples of human urine, food (raw meat, raw chicken, raw egg surface, and fresh vegetables), water, and air. Twenty-four E. coli isolates were tested for susceptibility to 26 antibiotics. The air sample isolates were most resistant to amoxicillin, ampicillin, amoxicillin/clavulanic acid, amoxicillin/sulbactam, piperacillin/tazobactam, cefalotin, cefuroxime, cefoxitin, cefixime, nitrofurantoin, and trimethoprim/sulfamethoxazol. The isolates from vegetable samples were resistant to amoxicillin, ampicillin, amoxicillin/clavulanic acid, amoxicillin/sulbactam, cefalotin, cefuroxime, cefoxitin, and cefixime. By contrast, the isolates from the water samples were resistant only to amoxicillin and ampicillin. The isolates from the human urine samples were most frequently resistant to norfloxacin (80%) followed by amoxicillin and ampicillin (70%), trimethoprim/sulfamethoxazole (55%), ciprofloxacin and ofloxacin (50%), cefalotin (30%), cefuroxime, cefixime and cefotaxime (25%), ceftazidime, ceftriaxone, cefepime and aztreonam (20%), amoxicillin/clavulanic acid, piperacillin/tazobactam and gentamicin (10%), and amoxicillin/sulbactam and cefoxitin (5%). Almost all (23/25, 95.8%) (n = 23) of the isolates were multi-drug resistant (MDR) (i.e., resistant to 3 or more classes of antibiotics), and 16.7% (n = 4) of those were positive for extended spectrum β-lactamase (ESBL). Of the 4 ESBL-producers, 3 were positive for blaCTX-M-15 and blaCTX-M1group, 2 were positive for blaCMY-2, and 1 each was positive for blaCTX-M-2 group, blaSHV, and blaOXA-47. The quinolone resistance gene qnrS was detected in 25% (n = 6) of the E. coli strains isolated from urine (N = 5) and air (N = 1) samples. The considerable number of antimicrobial resistance genes detected among E. coli isolates tested here is alarming and should raise public health concern.
Keywords: Escherichia coli; Antimicrobial resistance; Human urine; Food, Water; Air; Riyadh
1. Introduction
The drastic increase in the prevalence of bacterial antibiotic resistance has become a major health concern worldwide (Toval et al., 2014). The frequent exposure of humans to antimicrobial agents contributes considerably to the spread of both antimicrobial resistance and the development of antimicrobial-resistant bacteria. For instance, antimicrobial-resistant Escherichia coli isolates arising in antibiotic-treated animals can infect humans. The increased consumption of plant-based diet has been linked to increased numbers of human infections and outbreaks (Berg et al., 2014) as fruits and vegetables could act as reservoirs for pathogens or opportunistic pathogens. Furthermore, antimicrobial-resistant bacteria can also be transmitted to humans via the food chain and water, further affecting human health.
According to the European Union, the rate of human deaths related to antibiotic resistant bacterial infection was estimated at 25,000 per year, where two-thirds of these infections were due to Gram-negative bacteria (ECDC, 2015). Among the potentially most pathogenic bacteria encountered in the environment are coliform bacteria, in particular E. coli, a common Gram-negative coliform bacterium found in the intestinal flora of humans and other warm-blooded animals, is considered the most common cause of nosocomial and community-acquired infections (Van den Bogaard and Stobberingh, 2000). E. coli can easily spread via various environmental sources, including food, water, and soil; thus, its presence is widely used as an indicator of fecal contamination.
There are different routes through which humans can be infected or colonized by bacteria, including occupational exposure, physical contact, or food consumption. Consuming food from animal sources is an key source of antibiotic-resistant pathogens (von Baum and Marre, 2005). Some studies have established relationships between increased antibiotic-resistant E. coli isolates isolated from humans and food sources (Manges et al., 2007, Johnson et al., 2007, Ramchandani et al., 2005, Voltattoni et al., 2002). For example, studies have reported the presence of E. coli in chicken, mutton, beef, turkey, pork, ice cream, vegetables, fruit juice, cheese, yogurt, among others (Senkel et al., 2003, Tambekar et al., 2006, Badri et al., 2009, Badri et al., 2009, Apun et al., 2011, Rahimi and Chaleshtori, 2011, Adzitey et al., 2012). To better assess public health risks due to antimicrobials in the environment, a clear understanding of resistance ecology is required. E. coli can pose a serious clinical threat due to its ability to acquire antibiotic resistance. E. coli is most frequently associated with diarrhea and other enteric diseases and is one of the main causes of nosocomial and urinary tract infections (Qadri et al. 2005). E. coli is the main water quality control organism determinator. While most E. coli isolates are generally harmless, some have virulence genes that can lead to life-threatening diseases. Although most E. coli infections are treatable, the rate of death due to E. coli infections is increasing, mainly due to higher incidence of antibiotic resistance. Based on their pathogenicity, E. coli isolates are divided into six categories: enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC), diffusely adhering E. coli (DAEC), enterotoxigenic E. coli (ETEC), and Shiga toxin-producing E. coli (STEC) (Allocati et al., 2013).
Antibiotics are widely used in treating bacterial infections both in humans and animals, and they are applied as a prophylactic agent and growth promoter in animal feed. Thus, continuous misuse of available antibiotics supports the emergence of new antibiotic-resistant isolates. Importantly, newly arising resistance genes can be transmitted to other populations (Davies and Davies, 2010) via horizontal gene transfer (HGT) between Enterobacteriacae. Given its ease of growth, the cost-effectiveness of handling it, and its mechanisms of resistance gene acquisition and preservation, E. coli has been widely studied for monitoring antibiotic resistance trends in both humans and animals (Aarestrup et al., 1998, Van den Bogaard and Stobberingh, 2000).
The aim of this study was to evaluate the patterns of antimicrobial susceptibility of E. coli isolates isolated from various sources, including humans, water, air, and food, as well as to identify potential pathogenic isolates.
2. Materials and methods
2.1. Sample collection
The food samples used in this study were collected from local retailers and supermarkets in Riyadh, Kingdom of Saudi Arabia, from January to April 2019. The samples were derived from different sources, including raw meat, raw chicken, raw egg surface, and fresh vegetables, comprising 13 samples of locally produced meat and 30 types of fresh, locally produced vegetables (eggplant, capsicum, carrot, lettuce, and radish). All samples were collected aseptically and refrigerated. Later, samples were analyzed within 24 hrs. Water samples were collected from different regions in Riyadh. A total of 30 tap water samples were collected from January to April 2019 at different locations of use in the community. At each location, a 500-ml water sample was collected aseptically and kept in cooler bags, then transported to the laboratory within 3 to 4 h. Human urine samples were collected from 20 healthy adult volunteers (females) in sterile urine containers and brought immediately to the laboratory for culture and further experimental work.
2.2. Microbiological analysis
Food samples were analyzed in a safety cabinet and cut using sterile scalpels. Ten grams of the sample were excised and transferred into a sterile homogenizer flask containing 45 ml of nutrient broth (Oxoid, Manchester, UK). The mixture was incubated for 15 min at room temperature. For isolation, 9 ml of buffered peptone water (Oxoid) was added to 1 ml of each sample and incubated at 37 °C overnight. Then, 1 ml of the buffered peptone water was plated on eosin methylene blue (EMB) agar (Oxoid) and incubated aerobically at 37 °C for 18–24 h. Suspected E. coli colonies (metallic green colonies on EMB agar) were selected for further investigation.
Monitoring for airborne microbial contamination was performed via a sedimentation technique that uses open Petri dishes containing different culture media. The plates were distributed throughout rooms, bathrooms, and laboratories of Princess Nourah bint Abdulrahman University (PNU) at various distances from the floor. Immediately after sample collection, the plates were taken to the laboratory of Microbiology, Faculty of Science, for further bacteriological analysis.
Urine samples (1 ml) were added to tubes containing 10 ml of buffered peptone water (Oxoid) and cultured at 37 °C for 12 h. The bacterial suspension was then streaked onto EMB agar plates. After incubation at 37 °C for 18–24 h, metallic green colonies colonies were chosen for further biochemical identification.
2.3. Bacterial identification
For each sample, one bacterial isolate showing the expected morphology was chosen from the selective medium (EMB) for identification. Bacteria were preserved at −80 °C.
Biochemical identification of the bacteria was performed using a VITEK 2-compact 15 automated identification system (bioMérieux, France), following the manufacturer’s instructions. Briefly, bacterial suspensions were prepared at McFarland standard of 0.5–0.63 using a VITEK 2 DensiChek instrument (bioMérieux, France), and utilized Gram-negative (GN) cards for Gram-negative bacteria.
2.4. Antimicrobial susceptibility tests
The VITEK 2-compact 15 system was used to perform the antimicrobial susceptibility testing for all isolates. The GPS-500 test cards were used to test for the following antimicrobials: ampicillin, cefuroxime, cefotaxime, ciprofloxacin, cefotiam, imipenem, gentamicin, piperacillin, ofloxacin, nitrofurantoin, trimethoprim/sulfamethoxazole, tetracycline, ampicillin/sulbactam, and piperacillin/tazobactam. In order to identify ESBL production, Cefotaxime and Ceftazidime with and without clavulanic acid were used. Antibiotic susceptibility to chloramphenicol was determined by the disk diffusion method on Mueller-Hinton (MH) agar. The minimum inhibitory concentration (MIC) for all E. coli isolates was determined via the double broth dilution method according to the Clinical Laboratory Standards Institute (CLSI) guidelines. E. coli ATCC 25922 was used as a control strain.
2.5. DNA extraction
All identified E. coli isolates were cultured overnight in 5 ml of LB medium, and genomic DNA was extracted from each sample using the Invitrogen Pure link genomic DNA mini kit following the manufacturer’s instructions (Life Science Technology, USA). The extracted DNA was stored at −20 °C for further use.
2.6. Detection of antibiotic resistance genes via PCR
PCR was used to screen for isolates displaying resistance phenotypes by detecting genes conferring resistance to β-lactams (blaCTX-M, blaSHV, blaTEM, blaOXA-47, blaOXA-1 group, blaCMY-2, blaNDM-1, rmtB, rmtC, armA), and quinolones (qnrA, qnrB, qnrS). The primer sets used for each gene are shown in Table 1. The selected genes were among the most prevalent genes detected in the environment and clinics E. coli isolates in previous studies. PCR reactions were first performed with each set of primers separately in a total volume of 25 µl, including 12.5 µl of GoTaq green Master Mix (Promega, USA), 0.125 µl of each set of primers, 9.5 µl of DNAse and RNAse-free water, and 3 µl of DNA template. Amplification reactions were carried out in a Genepro thermocycler (Bioer, China) as follows: 94 °C for 2 min, 35 cycles consisting of 94 °C for 1 min, the Tm of each set of primers for 30 sec (Table 1), 72 °C for 1 min, followed by a final extension step at 72 °C for 10 mins. Amplified samples were analyzed via electrophoresis in 1.5% ethidium bromide-stained agarose gels. All amplicon bands were compared to the Solis Biodyne 100-bp DNA ladder (Switzerland). We used E. coli ATCC 25922 as a positive control.
Table 1.
Primers (Talukdar et al., 2013) used for resistance gene detection.
| Resistance gene | Nucleotide sequence (5’-3’) |
|---|---|
| blaTEM 1150 | TCGGGGAAATGTGCGCG TGCTTAATCAGTGAGGACCC |
| blaSHV 885 | CACTCAAGGATGTATTGTG TTAGCGTTGCCAGTGCTCG |
| blaCTX-M-1group 866 | GGTTAAAAAATCACTGCGTC TTGGTGACGATTTTAGCCGC |
| blaCTX-M-2 group 866 | ATGATGACTCAGAGCATTCG TGGGTTACGATTTTCGCCGC |
| blaCTX-M-8 group 688 |
TCGCGTTAAGCGGATGATGC AACCCACGATGTGGGTAGC |
| blaCTX-M-9 group 857-870 |
ATGGTGACAAAGAGAGTGCA CCCTTCGGCGATGATTCTC |
| blaCTX-M-15 996 | CACACGTGGAATTTAGGGACT GCCGTCTAAGGCGATAAACA |
| blaOXA-1 group 813 | ACACAATACATATCAACTTCGC AGTGTGTTTAGAATGGTGATC |
| blaOXA-47 591- 609 | TCAACTTTCAAGATCGCA GTGTGTTTAGAATGGTGA |
| blaCMY-2 556 1007- 1143 |
GACAGCCTCTTTCTCCACA TGGAACGAAGGCTACGTA |
| blaNDM-1 465 | GGTTTGGCGATCTGGTTTTC CGGAATGGCTCATCACGATC |
| rmtB | GCTTTCTGCGGGCGATGTAA ATG CAA TGC CGC GCT CGTAT |
| rmtC | CGA AGA AGT AAC AGC CA AG ATC CCA ACA TCT CTC CCA CT |
| armA | ATT CTG CCT ATC CTA ATTGG ACC TAT ACT TTA TCG TCGTC |
| qnrA | AGAGGATTTCTCACGCCAGG TGCCAGGCACAGATCTTGAC |
| qnrB | GGMATHGAAATTCGCCACTG TTTGCYGYYCGCCAGTCGAA |
| qnrS 428 | GCAAGTTCATTGAACAGGGT TCTAAACCGTCGAGTTCGGCG |
2.7. Ethical approval
This study was conducted in accordance with the Declaration of Princess Nourah University and was approved by the Institutional Review Board (approval number 17-0208).
3. Results
3.1. Isolation of E. coli from samples
Approximately 21.2% (n = 113) of the examined samples yielded positive growth for E. coli as follows: 2.4% were isolated from food samples, 2.4% from the vegetables, 2.4% from the air and 2.4% of the water samples. All of the human urine samples (83.3%) were positive for E. coli (Table 2).
Table 2.
Presence of E. coli isolates in positive cultures.
| Isolated E. coli strains | Food (raw meat) | Fresh vegetables | Water | Air | Urine |
|---|---|---|---|---|---|
| Number of bacteria | 1 | 1 | 1 | 1 | 20 |
| % | 4.2 | 4.2 | 4.2 | 4.2 | 83.3 |
3.2. Antibiotic resistance phenotypes of E. coli isolates
Antibiotic susceptibility tests to 26 antibiotics were performed via the disc diffusion method for 24 E. coli isolates. The isolate from air sample revealed resistance to amoxicillin, ampicillin, amoxicillin/clavulanic acid, amoxicillin/sulbactam, piperacillin/tazobactam, cefalotin, cefuroxime, cefoxitin, cefixime, nitrofurantoin, and trimethoprim/sulfamethoxazole. The isolates from fresh vegetables showed resistance to amoxicillin, ampicillin, amoxicillin/clavulanic acid, amoxicillin/sulbactam, cefalotin, cefuroxime, cefoxitin, and cefixime. Isolates from the water samples showed resistance only to amoxicillin and ampicillin. Urine sample isolates were the most resistant to norfloxacin (80%) followed by amoxicillin, ampicillin (70%), trimethoprim/sulfamethoxazole (55%), ciprofloxacin and ofloxacin (50%), cefalotin (30%), cefuroxime, cefixime and cefotaxime (25%), ceftazidime, ceftriaxone, cefepime and aztreonam (20%), piperacillin/tazobactam, amoxicillin/clavulanic acid, and gentamicin (10%), and amoxicillin/sulbactam and cefoxitin (5%). Four isolates were positive for ESBL.
Table 2 presents the presence of antimicrobial resistance between the E. coli isolates isolated from urine, fresh vegetables, water, and air. Overall, 17 (70.8%) of the isolates were resistant to at least 8 of 26 antibiotics examined, 8 (33.3%) had intermediate susceptibility, and 1 (4.2%) was pan-sensitive. For isolates isolated from humans (n = 20), 20 (100%) were susceptible to all tested antibiotics, while 16 (80%) were resistant to norfloxacin,14 (70%) were resistant to amoxicillin and ampicillin, 11 (55%) were resistant to trimethoprim/sulfamethoxazole, 10 (50%) were resistant to ofloxacin and ciprofloxacin, 6 (30%) were resistant to cefalotin, 5 (25%) were resistant to cefuroxime, cefixime and cefotaxime, 4 (20%) were resistant to ceftazidime, cefepime, ceftriaxone, and aztreonam, 2 (10%) were resistant to piperacillin/tazobactam, amoxicillin/clavulanic acid, and gentamicin, and 1 (5%) was resistant to amoxicillin/sulbactam and cefoxitin. Among the isolates recovered from the air, 1 (100%) was susceptible to all antibiotics tested, 1 (100%) was resistant to amoxicillin, amoxicillin/clavulanic acid, ampicillin, piperacillin/tazobactam, amoxicillin/sulbactam, cefoxitin, cefalotin, cefixime, cefuroxime, nitrofurantoin, and trimethoprim/sulfamethoxazole (Table 3). Among the isolates recovered from fresh vegetables, 1 (100%) was susceptible to some of antibiotics tested, 1 (100%) was resistant to amoxicillin, ampicillin, amoxicillin/clavulanic acid, amoxicillin/sulbactam, cefalotin, cefuroxime, cefoxitin, and cefixime (Table 3). Among the isolates recovered from water, 1 (100%) was resistant only to amoxicillin and ampicillin (Table 3).
Table 3.
Antibiotic susceptibility patterns of E. coli isolates.
| Antibiotic | R% n |
I% n |
S% n |
|---|---|---|---|
| Amoxicillin | (70.8)17 | 0 | (29.2)7 |
| Ampicillin | (70.8)17 | 0 | (29.2) 7 |
| Amoxicillin/clavulanic Acid | (16.7)4 | (16.7) 4 | (66.7) 16 |
| Amoxicillin/Sulbactam | (12.5) 3 | 0 | (87.5) 21 |
| Piperacillin/Tazobactam | (12.5) 3 | (12.5) 3 | (75) 18 |
| Cefalotin | (33.3) 8 | (12.5) 3 | (54.2) 13 |
| Cefuroxime | (29.2) 7 | 0 | (70.8)17 |
| Cefoxitin | (12.5) 3 | (4.2) 1 | (83.3) 20 |
| Cefixime | (29.2) 7 | 0 | (70.8)17 |
| Cefotaxime | (20.3) 5 | 0 | (79.2) 19 |
| Ceftazidime | (16.7) 4 | (4.2) 1 | (79.2) 19 |
| Ceftriaxone | (20.3) 5 | 0 | (79.2) 19 |
| Cefepime | (16.7) 4 | 0 | (83.3) 20 |
| Aztreonam | (16.7) 4 | 0 | (83.3) 20 |
| Imipenem | 0 | 0 | (100) 24 |
| Meropenem | 0 | 0 | (100) 24 |
| Amikacin | 0 | (4.2) 1 | (95.8) 23 |
| Gentamicin | (8.3) 2 | 0 | (91.7) 22 |
| Ciprofloxacin | (41.7) 10 | 0 | (58.3) 14 |
| Norfloxacin | (66.7) 16 | – | – |
| Ofloxacin | (41.7)10 | 0 | (58.3) 14 |
| Doxycyclin | – | – | – |
| Minocycline | – | – | – |
| Tigecycline | 0 | 0 | (100) 24 |
| Nitrofurantoin | (4.2) 1 | (12.5) 3 | (83.3) 20 |
| Trimethoprim/Sulfamethoxazole | (50) 12 | 0 | (50) 12 |
R: Resistant, I: Intermediate, S: Susceptible.
3.3. Detection of antibiotic resistance genes in E. coli isolates
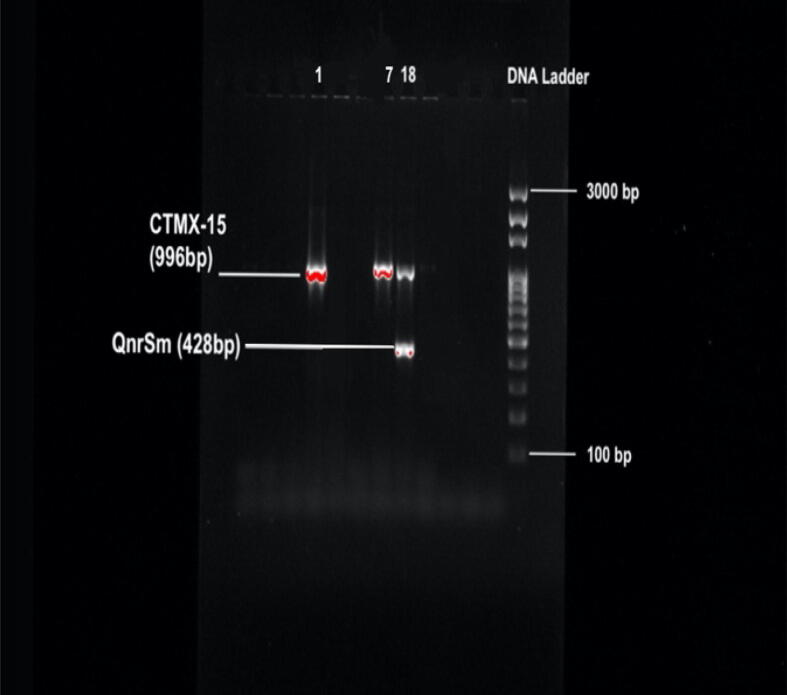
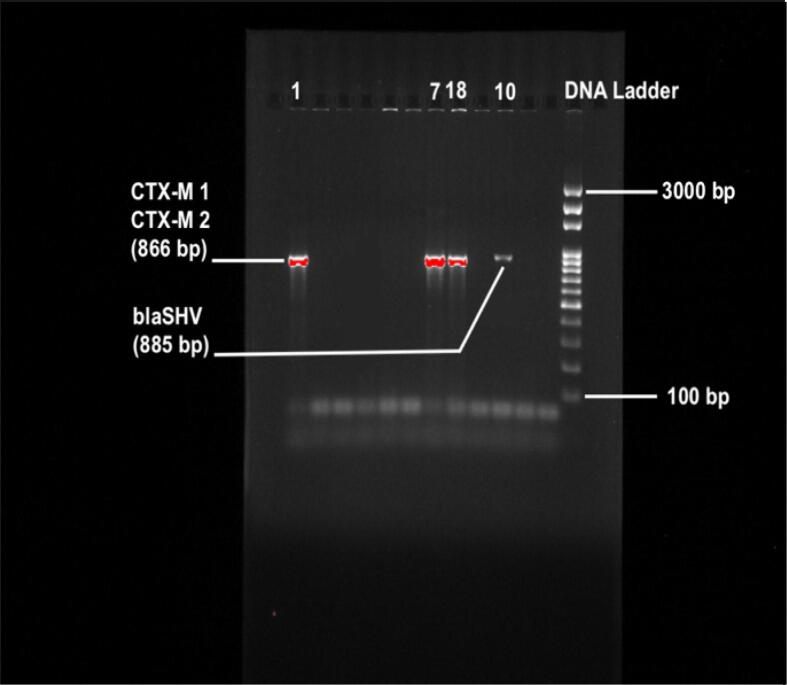
We found that of the 24 isolated E. coli isolates, 7 (29%) were positive for the blaCTX-M-15 and blaCTX-M-1-group-specific gene. We next validated the presence of blaCTX-M-15 by sequencing the PCR product. Eight (33%) isolates were positive for the blaCTX-M-2-group-specific gene, while none of the isolates were positive for the blaCTX-M-8-group-specific genes or blaCTX-M-9-group. Approximately 12.5% (n = 3) and 50% (n = 12) of the isolates were positive for blaSHV and blaTEM, respectively. Among the carbapenemase resistance genes, blaOXA-1-group was detected in 4% (n = 1), while blaOXA-47 was detected in 8% (n = 2) of the isolates. We did not detect any isolates with the metallo-β-lactamase gene blaNDM-1. 54% (n = 13) of the isolates were positive for the β-lactamase blaCMY-2 gene. We found qnrS, quinolone resistance gene, in 25% (n = 6) of the isolates (Table 4, Table 5) (Fig. 1, Fig. 2).
Table 4.
Distribution of resistance genes in 24 isolated E. coli isolates.
| Gene | % of gene resistance in 24 identified E. coli |
|---|---|
| blaTEM | 12 (50%) |
| blaSHV | 3 (12.5%) |
| blaCTX-M-1group | 7 (29%) |
| blaCTX-M-2 group | 8 (33%) |
| blaCTX-M-8 group | 0 |
| blaCTX-M-9 group | 0 |
| blaCTX-M-15 | 7 (29%) |
| blaOXA-1 group | 1 (4%) |
| blaOXA-47 | 2 (8%) |
| blaCMY-2 | 13 (54%) |
| blaNDM-1 | 0 |
| rmtB | 0 |
| rmtC | 0 |
| armA | 0 |
| qnrA | 0 |
| qnrB | 0 |
| qnrS | 6 (25%) |
Table 5.
Occurrence of resistance genes in E. coli isolated from different samples.
| Antibiotic resistance pattern | Number | % Percentage of Resistance | Strain number | ESBL gene(s) |
|---|---|---|---|---|
| – | – | – | 2 20 M4 21 |
|
| Nor** | 1 | 4.2 | 9, 11 | |
| Amx, Amp | 2 | 8.3 | 5, 17 W | |
| Cip, Nor, Ofl, | 3 | 12.5 | 4 17 |
|
| Amx, Amp, Cef, TrSu | 4 | 16.7 | 6 14 19 |
|
| Amx, Amp, Cip, Nor, Ofl | 5 | 20.9 | 12 | |
| Amx, Amp, Cip, Nor, Ofl, TrSu | 6 | 25 | 3 13 15 |
|
| Amx, Amp, Gen, Cip, Nor, Ofl, TrSu | 7 | 29.2 | 16 | |
| Amx, Amp, AmCl, AmSu, Cef, Cur, Cox, Cix | 8 | 33.3 | Q14 | |
| Amx, Amp, AmCl, AmSu, Cef, Cur, Cox, Cix, Nir, TrSu | 10 | 41.7 | 13A | |
| Amx, Amp, Cef, Cur, Cix, Cot, Cta, Ctr, Cep, Azt, Nor, TrSu | 12 | 50 | 10* | blaSHV |
| Amx, Amp, Cef, Cur, Cix, Cot, Cta, Ctr, Cep, Azt, Cip, Nor, Ofl | 13 | 54.2 | 1* | blaCTX-M-1group, blaCTX-M-15, blaCMY-2 |
| Amx, Amp, Cef, Cur, Cix, Cot, Cta, Ctr, Cep, Azt, Cip, Nor, Ofl, TrSu | 14 | 58.3 | 7* | blaCTX-M-1group, blaCTX-M-2 group, blaCTX-M-15, blaCMY-2 |
| Amx, Amp, AmCl, PiTa, Cef, Cur, Cix, Cot, Cta, Ctr, Cep, Azt, Nor, TrSu | 14 | 58.3 | 18* | blaTEM, blaCTX-M-1group, blaCTX-M-15, blaOXA-47, qnrS |
| Amx, Amp, AmCl, AmSu, PiTa, Cef, Cur, Cix, Cot, Ctr, Gen, Cip, Nor, Ofl, TrSu | 15 | 62.5 | 8 |
* ESBL (+) strains; **Amx, Ampicillin; Amp, Ampicillin; AmCl , Amoxicillin/clavulanic Acid; AmSu, Amoxicillin/Sulbactam; PiTa, Piperacillin/Tazobactam; Cef, Cefalotin; Cur, Cefuroxime; Cox, Cefoxitin; Cix, Cefixime; Cot, Cefotaxime; Cta, Ceftazidime; Ctr, Ceftriaxone; Cep, Cefepime; Azt, Aztreonam; Imi, Imipenem; Mer, Meropenem; Ami, Amikacin; Gen, Gentamicin; Cip, Ciprofloxacin; Nor, Norfloxacin; Ofl, Ofloxacin; Dox, Doxycycline; Min, Minocycline; Tig, Tigecycline; Nir, Nitrofurantoin; and TrSu, Trimethoprim/Sulfamethoxazole;. (1–20) urine samples, (21) ATCC 25922, (M) meat samples, (W) water samples, and (A) air samples.
Fig. 1.
Image of ESBL genes, confirmed in ESBL-producing E. coli strains (1, 7, 18 numbered strains) on an agarose gel. Gene Ruler DNA ladder was used as size marker.
Fig. 2.
Image of ESBL genes, confirmed in ESBL-producing E. coli strains (1, 7, 10 numbered strains) on an agarose gel. Gene Ruler DNA ladder was used as size marker.
4. Discussion
The accurate and rapid detection of antibiotic resistance is extremely important in treating and preventing infections. Recently, the existence of genetic similarity was found between ESBL E. coli isolates, indicating the ability of these isolates to be transferred form one source to another, for example, from vegetables to humans, potentially causing serious infections (Cetinkaya et al., 2014.). Frequent use of antibiotics has contributed to increased resistance to the most common standard antibiotics and to increased genetics-based resistance or multidrug resistance among bacterial isolates. Saudi Arabia is facing several challenges that can encourage the emergence and spread of antibiotic resistance. In general, antibiotics are used to treat both human and animal infections and as sub-therapeutics in food animals for promoting growth. When use of these antibiotics becomes the norm in human and animal medicine, multigene resistance may emerge and develop, which can, in turn, be shared among the bacterial population ultimately causing public health problems (Davies and Davies, 2010, Frank et al., 2011, Ukah et al., 2018). Humans can be exposed to antibiotic-resistant bacteria through contaminated water and food samples (Robinson et al., 2016); thus, the environment is an essential factor in the emergence, persistence, and transmission of antibiotic resistant bacteria, indicating that the health of animals, humans, and the environment are closely linked. The increasing threat of antibiotic resistant bacteria may be associated with enhanced virulence and pathogenicity (Guillard et al., 2016, Roux et al., 2015), and with increased antibiotic resistance, increased virulence may naturally evolve (Guillard et al., 2016). Therefore, when controlling the spread of antibiotic resistance, we must also control the spread of virulence (Meredith et al., 2017).
Data from the present work indicated that human urine isolates were more commonly resistant to tetracycline, ampicillin, and trimethoprim/sulfamethoxazole than to other drugs, consistent with (Jakobsen et al., 2010), Uysal and Durak, 2012, and Melo et al. (2015). Similarly, Pires et al., 2007, Uysal et al., 2013, and Vranic and Uzunovic (2016) reported that ampicillin resistance was most common followed by trimethoprim/sulfamethoxazole However, the spread of this antibiotic resistance in E. coli food isolates noted in the latter study was lower than that documented by Altalhi et al., 2000, Van et al., 2007, Van et al., 2008, where resistance rates close to 100% were reported. It is believed that the overuse of antimicrobial agents in animal diets and the selective pressure that these drugs put on microorganisms it is suspected that isolates obtained from animal-based food products could have more complex resistance profiles than isolates from other food sources.
In contrast, others have reported lower proportions of multidrug-resistant E. coli in food isolates than in human isolates (Thorsteinsdottir et al. 2010). In 2011, a study reported that in the Saudi Arabian region of Najran, reported, 31.1% of urine E. coli isolates were resistant to multiple drugs (Masoud et al., 2011). In another study, 36.7% of the E. coli isolates were found to be resistant to carbenicillin, amoxicillin, ampicillin, and piperacillin (Halawani, 2011). Kresken and colleagues (2014) reported that 42.9% of 499 E. coli urine sample isolates were found resistant to amoxicillin then amoxicillin/clavulanic acid (AMC) (32.7%) and followed by trimethoprim/sulfamethoxazole (SXT) (30.9%). Another study found that 87.9% of 366 E. coli urine sample isolates were resistant to nalidixic acid, 82.7% were resistant to ciprofloxacin, and 81.4% were resistant to trimethoprim (Fennell et al., 2012). Similarly, Uysal et al. (2018) reported that 77.92% were multidrug resistant (i.e., resistant to more than 2 antibiotics), in particular to cephalothin (54.63%), followed by tetracycline (53.6%) and nalidixic acid (44.32%).
Among the ESBL producers, we found three positive isolates for resistance genes with the clinically significant class A β-lactamases, which includes blaCTX-M-1-group and blaCTX-M-15. With the beginning of 21 century, blaCTX-M-15-producing E. coli isolates have emerged and spread worldwide and are now considered an important source of human nosocomial, urinary tract, and blood infections (Oteo et al., 2010, Pitout, 2010). There is a reported increase in the prevalence of CTX-M-type β-lactamases in Enterobacteriaceae, and it is now more prevalent than the TEM and SHV types in some geographic locations (Falagas and Karageorgopoulos, 2009). The TEM and SHV types have mostly been found in environmental and clinical samples, e.g, from animal farms and estuarine waters (Henriques et al., 2006, Hiroi et al., 2011). Interestingly, the majority of isolates identified in our study possess blaTEM, while one isolate possess blaSHV. Additionally, various enterobacterial species, including E. coli, are known to possess the qnr genes (quinolone resistance genes) (Poirel et al., 2008, Robicsek et al., 2006, Takasu et al., 2011). Reports have indicated that the qnr genes, especially qnrB, are mainly found in clinically important K. pneumoniae isolates and other Enterobacteriaceae species in Asian countries (Shin et al., 2008, Teo et al., 2009); however, nonclinical sources of qnr genes have also been found in E. coli isolates from livestock, swine, and poultry (Ma et al., 2009, Yue et al., 2008). Additionally, a qnr gene (qnrS) was identified in isolates from the River Seine in Paris and a Swiss lake containing Aeromonas (a water-borne bacterial species) (Cattoir et al., 2008) (Picao et al., 2008). The results of our current study indicated that 6 isolates were positive for plasmid-mediated qnrS-type qnr genes. Isolate 18, which carried qnrS, co-harbored different classes of β-lactamase resistance genes, including blaOXA-47, blaCTX-M-1-group, and blaCTX-M-15, was resistant to 14 antibiotics, excluding ciprofloxacin. Conversely, isolate 15, which carried qnrS, blaTEM, and co-harbored blaCTX-M-2 was resistant to 6 antibiotics, including ciprofloxacin. Therefore, in parallel to pervious work, we conclude that qnrS alone may not grant resistance to fluroquinolones (Cattoir et al., 2008).
5. Conclusion
The prevalence of pathogenic E. coli in various food and water sources highlights the need for cost-effective, accurate, and rapid identification systems to decrease the public’s exposure to E. coli infection.
This study provides important information about the antimicrobial susceptibility patterns in E. coli from the Riyadh Region in Saudi Arabia and reveals the prevalence of ESBL production in the region. The present data and antimicrobial resistance profiles for E. coli in Riyadh will aid local clinicians in recommending the optimal antimicrobial drug regimens to treat E coli infections. There is an ongoing need to mitigate the spread of antimicrobial resistance determinants in pathogenic bacteria, which can be supported via the judicious use of antimicrobial agents. Furthermore, surveillance studies must be conducted to monitor the current patterns of antibiotic resistance among bacteria. Of the E. coli isolates tested in this study, 70.8% were antibiotic resistant, primarily to amoxicillin and ampicillin for the isolates from human, fresh vegetables, water, and air, and norfloxacin for human isolates. Furthermore, a high percentage of multidrug resistance was observed in all of the human isolates.
Data availability
The data used to support the findings of this research are included in the article. If there are any specifics needed, please contact the corresponding author.
CRediT authorship contribution statement
Kawther Aabed: Conceived and designed the experiments and was responsible for the visualization, supervision, project administration, and funding acquisition; Kawther Aabed, Nadine Moubayed, and Saleha Alzahrani: Performed the experiments; Kawther Aabed and Nadine Moubayed Analyzed the data, prepared the original draft, and reviewed and edited the final version. All authors have read and agreed to the publication of the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University through the Fast-track Research Funding Program. I would like to express my special thanks and gratitude to Ms. Lama Bahammam, Ms. Maha Almotiri, Ms. Mashael Alamri, Ms. Mody Alsubaiei, Ms. Mona Alanazi, Ms. Reem Alshenaifi, Ms. Reema Alreshidi, Ms. Rehab Alshammari, and Ms. Shahad Albawardi for their help in this study. Without their assistance and dedicated involvement in every step throughout the process, this research would have never been accomplished.
References
- Aarestrup F.M., Bager F., Jensen N.E., Madsen M., Meyling A. Resistance to antimicrobial agents used for animal therapy in pathogenic-, zoonotic- and indicator bacteria isolated from different food animals in Denmark: a baseline study for the Danish Integrated Antimicrobial Resistance Monitoring Programme (DANMAP) APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 1998;106 doi: 10.1111/j.1699-0463.1998.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Adzitey F., Liew C.Y., Aronal A.P., Huda N. Isolation of Escherichia coli from ducks and duck related samples. Asian J. Anim. Vet. Adv. 2012;7(4):351–355. doi: 10.3923/ajava.2012.351.355. [DOI] [Google Scholar]
- Allocati N., Masulli M., Alexeyev M.F., Ilio C.D. Escherichia coli in Europe: an overview. Int. J. Environ. Res. Public Health. 2013;10(12):6235–6254. doi: 10.3390/ijerph10126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altalhi A.D., Gherbawy Y.A., Hassan S.A. Antibiotic resistance in Escherichia coli isolated from retail raw chicken meat in Taif, Saudi Arabia. Foodborne Pathog. Dis. 2000;7:281285. doi: 10.1089/fpd.2009.0365. [DOI] [PubMed] [Google Scholar]
- Apun K., Kho K.L., Chong Y.L., Hashimatul F.H., Abdullah M.T., Rahman M.A. Detection of Escherichia coli O157:H7 in wildlife from disturbed habitats in Sarawak, Malaysia. Res. J. Microbiol. 2011;6(2):132–139. doi: 10.3923/jm.2011.132.139. [DOI] [Google Scholar]
- Badri S., Fassouane A., Filliol I., Hassar M., Cohen N. Clonal analysis of Escherichia coli strains isolated from food by pulsed-field gel electrophoresis. Internet J. Food Safety. 2009;11:44–49. [Google Scholar]
- Berg G., Erlacher A., Smalla K., Krause R. Vegetable microbiomes: is there a connection among opportunistic infections, human health and our ’gut feeling’?”. Microbial. Biotechnol. 2014;7(6):487–495. doi: 10.1111/1751-7915.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoir V., Poirel L., Aubert C., Soussy C.J., Nordmann P. Unexpected occurrence of plasmid-mediated quinolone resistance determinants in environmental Aeromonas spp. Emerg. Infect. Dis. 2008;14:231–237. doi: 10.3201/eid1402.070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetinkaya F., Mus T., Yibar A., Guclu N., Tavsanli H., Cibik R. Prevalence, serotype identification by multiplex polymerase chain reaction and antimicrobial resistance patterns of Listeria monocytogenesisolated from retail foods. J. Food Saf. 2014;34:42–49. [Google Scholar]
- Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC and EMEA Technical Report. The bacterial challenge: time to react. http://ecdc.europa.eu (Internet). 2009 (access: April 15, 2015).
- Falagas M.E., Karageorgopoulos D.E. Extended-spectrum beta-lactamaseproducing organisms. J. Hospital Infect. 2009;73:345–354. doi: 10.1016/j.jhin.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Fennell J., Vellinga A., Hanahoe B., Morris D., Boyle F., Higgins F. Increasing prevalence of ESBL production among Irish clinical Enterobacteriaceae from 2004 to 2008: an observational study. BMC Infect. Dis. 2012 doi: 10.1186/1471-2334-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C., Werber D., Cramer J.P. Epidemic profile of Shiga-toxin–producing Escherichia coli O104:H4 outbreak in Germany preliminary report. N. Engl. J. Med. 2011 doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- Guillard T., Pons S., Roux D., Pier G.B., Skurnik D. Antibiotic resistance and virulence: understanding the link and its consequences for prophylaxis and therapy. BioEssays. 2016;38:682–693. doi: 10.1002/bies.201500180. [DOI] [PubMed] [Google Scholar]
- Halawani E.M. Beta-lactam antibiotic resistance in Escherichia coli commensal faecal flora of healthy population in Taif, Saudi Arabia. African J. Microbiol. Res. 2011;5:73–78. [Google Scholar]
- Henriques I.S., Fonseca F., Alves A., Saavedra M.J., Correia A. Occurrence and diversity of integrons and beta-lactamase genes among ampicillin-resistant isolates from estuarine waters. Res. Microbiol. 2006;157:938–947. doi: 10.1016/j.resmic.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Hiroi M., Harada T., Kawamori F., Takahashi N., Kanda T. A survey of betalactamase-producing Escherichia coli in farm animals and raw retail meat in Shizuoka Prefecture, Japan. Jpn. J. Infect. Dis. 2011;64:153–155. [PubMed] [Google Scholar]
- Jakobsen L., Kurbasic A., Skjøt-Rasmussen L., Ejrnaes K., Porsbo L., Pedersen K. Escherichia coli Isolates from Broiler Chicken Meat, Broiler Chickens, Pork, and Pigs Share Phylogroups and AntimicrobialResistance with Community-Dwelling Humans and Patientswith Urinary Tract Infection. Foodborne Pathog. Dis. 2010;7:537–547. doi: 10.1089/fpd.2009.0409. [DOI] [PubMed] [Google Scholar]
- Johnson J.R., Sannes M.R., Croy C. Antimicrobial drug-resistant escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002–2004. Emerg. Infect. Dis. 2007;13:838–846. doi: 10.3201/eid1306.061576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresken M., Pfeifer Y., Hafner D., Wresch R., Körber-Irrgang B., Party Working. Antimicrobial Resistance’ of the Paul-Ehrlich-Society for C. Occurrence of multidrug resistance to oral antibiotics among Escherichia coli urine isolates from outpatient departments in Germany: extended-spectrum β-lactamases and the role of fosfomycin. Int. J. Antimicrob. Agents. 2014;44:295–300. doi: 10.1016/j.ijantimicag.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Ma J., Zeng Z., Chen Z., Xu X., Wang X. High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac(69)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrobial. Agents Chemother. 2009;53:519–524. doi: 10.1128/AAC.00886-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manges A.M., Smith S.P., Lau B.J. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case-control study. Foodborne Pathogs. Dis. 2007;4:419–431. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- Masoud E., Mahdy M., Esmat A. Bacterial prevalence and resistance to antimicrobial agents in Southwest, Saudi Arabia. Egypt. Acad. J. Biolog. Sci. 2011;3:105–111. [Google Scholar]
- Melo D.B., Menezes A.P., Reis J.N., Guimarães A.G. Antimicrobial resistance and genetic diversity of Escherichia coli isolated from humans and foods. Brazil. J. Microbiol. 2015;46(4):1165–1170. doi: 10.1590/S1517-838246420130874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith S., Brooks B.D., Brooks A.E. The complex relationship between virulence and antibiotic resistance. Genes. 2017;8:1–3. doi: 10.3390/genes8010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteo J., Perez-Vazquez M., Campos J. Extended-spectrum beta-lactamase producing Escherichia coli: changing epidemiology and clinical impact. Curr. Opin. Infect. Dis. 2010;23:320–326. doi: 10.1097/qco.0b013e3283398dc1. [DOI] [PubMed] [Google Scholar]
- Picao R.C., Poirel L., Demarta A., Silva C.S., Corvaglia A.R. Plasmid-mediated quinolone resistance in Aeromonas allosaccharophila recovered from a Swiss lake. J. Antimicrobial. Chemother. 2008;62:948–950. doi: 10.1093/jac/dkn341. [DOI] [PubMed] [Google Scholar]
- Pires M.C.S., Frota K.S., Martins Junior P.O. Prevalence and bacterial susceptibility of community acquired urinary tract infection in University Hospital of Brasília, 2001 to 2005. Rev. Soc. Bras. Med. Trop. 2007;40:643–647. doi: 10.1590/s0037-86822007000600009. [DOI] [PubMed] [Google Scholar]
- Pitout J.D.D. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70:313–333. doi: 10.2165/11533040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Poirel L., Cattoir V., Nordmann P. Is plasmid-mediated quinolone resistance a clinically significant problem? Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2008;14:295–297. doi: 10.1111/j.1469-0691.2007.01930.x. [DOI] [PubMed] [Google Scholar]
- Qadri F., Svennerholm A.M., Faruque A.S., SackEnterotoxigenic R.B. Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 2005;18 (3:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi, E., Chaleshtori, S.S., and Parsaei, P. 2011. Prevalence and antimicrobial resistance of Escherichia coli O157 isolated from traditional cheese, ice cream and yoghurt in Iran. Afr.
- Ramchandani M., Manges A.R., DebRoy C. Possible animal origin of humanassociated multidrug-resistant, uropathogenic Escherichia coli. Clinic. Infect. Dis. 2005;40:251–257. doi: 10.1086/426819. [DOI] [PubMed] [Google Scholar]
- Robicsek A., Jacoby G.A., Hooper D.C. The worldwide emergence of plasmidmediated quinolone resistance. Lancet Infect. Dis. 2006;6:629–640. doi: 10.1016/S1473-3099(06)70599-0. [DOI] [PubMed] [Google Scholar]
- Robinson T.P., Wertheim H.F., Kakkar M., Kariuki S., Bu D., Price L.B. Animal production and antimicrobial resistance in the clinic. Lancet. 2016;387(10014):1–3. doi: 10.1016/S0140-6736(15)00730-8. [DOI] [PubMed] [Google Scholar]
- Roux D., Danilchanka O., Guillard T., Cattoir V., Aschard H., Fu Y., Skurnik D. Fitness cost of antibiotic susceptibility during bacterial infection. Sci. Trans. Med. 2015;7:297. doi: 10.1126/scitranslmed.aab1621. [DOI] [PubMed] [Google Scholar]
- Senkel I.A., Jr, Jolbitado B., Zhang Y., White D.G., Ayers S., Meng J. Isolation and characterization of Escherichia coli recovered from Maryland apple cider and the cider production environment. J. Food Prot. 2003;66(12):2237–2244. doi: 10.4315/0362-028x-66.12.2237. PMID:14672219. [DOI] [PubMed] [Google Scholar]
- Shin J.H., Jung H.J., Lee J.Y., Kim H.R., Lee J.N. High rates of plasmidmediated quinolone resistance QnrB variants among ciprofloxacin-resistant Escherichia coli and Klebsiella pneumoniae from urinary tract infections in Korea. Microbial. Drug Resistance. 2008;14:221–226. doi: 10.1089/mdr.2008.0834. [DOI] [PubMed] [Google Scholar]
- Takasu H., Suzuki S., Reungsang A., Pham H.V. Fluoroquinolone (FQ) contamination does not correlate with occurrence of FQ-resistant bacteria in aquatic environments of Vietnam and Thailand. Microbes Environ./JSME. 2011;26:135–143. doi: 10.1264/jsme2.me10204. [DOI] [PubMed] [Google Scholar]
- Talukdar P.K., Rahman M., Rahma M., Nab A., Islam Z., Hoque M.M. Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. PLOS One. 2013;8(4):e61090. doi: 10.1371/journal.pone.0061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambekar D.H., Hirulkar N.B., Kalikar M.V., Patil Y.S., Gulhane S.R. Prevalance of thermotolerant Escherichia coli in drinking water and its multidrug resistance. Res. J. Microbiol. 2006;1(5):458–462. doi: 10.3923/jm.2006.458.462. [DOI] [Google Scholar]
- Teo J.W., Ng K.Y., Lin R.T. Detection and genetic characterisation of qnrB in hospital isolates of Klebsiella pneumoniae in Singapore. Int. J. Antimicrobial Agents. 2009;33:177–180. doi: 10.1016/j.ijantimicag.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Thorsteinsdottir T.R., Haraldsson G., Fridriksdottir V. Prevalence and genetic relatedness of antimicrobial resistant Escherichia coli isolated from animals, foods and humans in Iceland. Zoonoses Public Hlth. 2010;57:189–196. doi: 10.1111/j.1863-2378.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- Toval F., Köhler C.D., Wagenlehner F., Mellmann A., Fruth A., Schmidt M.A. Characterization of Escherichia coli isolates from hospital inpatients or outpatients with urinary tract infection. J. Clin. Microbiol. 2014;52(2):407–418. doi: 10.1128/JCM.02069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukah U.V., Glass M., Avery B., Daignault D., Mulvey M.R., Reid-Smith R.J., Parmley E.J., Portt A., Boerlin P., Manges A.R. Risk factors for acquisition of multidrug-resistant Escherichia coli and development of community-acquired urinary tract infections. Epidemiol. Infect. 2018;146(1):46–57. doi: 10.1017/S0950268817002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal A., Durak Y., Arslan U. Characterization of escherichia coli strains isolated from well waters: Molecular typing by pulsed-field gel electrophoresis, antibiotic resistance patterns and plasmid profiles. Fresenius Environ. Bullet. 2013;22(12):3525–3533. [Google Scholar]
- Uysal A., Gunes E., Arslan E., Durak Y. Characterization of uropathogenic Escherichia coli stains: antibiotic resistance patterns, detection of Esbl genes and interactions by lytic phages. Fresenius Environ. Bullet. 2018;27:402–414. [Google Scholar]
- Uysal A., Durak Y. Pulsed-field gel electrophoresis typing, antibiotic resistance, and plasmid profiles of Escherichia coli strains isolated from foods. Can. J. Microbiol. 2012;58(11):1278–1287. doi: 10.1139/w2012-108. [DOI] [PubMed] [Google Scholar]
- Van den Bogaard A.E., Stobberingh E.E. Epidemiology of resistance to antibiotics: links between animals and humans. Int. J. Antimicrobial Agents. 2000;14(4):327–335. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- Van T.T.H., Moutafis G., Tran L.T. Antibiotic resistance in food-borne bacterial contaminants in Vietnam. Appl. Environ. Microbiol. 2007;73:7906–7911. doi: 10.1128/AEM.00973-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T.T.H., Chin J., Chapman T. Safety of raw meat and shellfish in Vietnam: an analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 2008;124:217–223. doi: 10.1016/j.ijfoodmicro.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Voltattoni P., Hofer C., Redolfi A.L. Identificación de virotipos de Escherichia coli aislados de alimentos listos para el consumo. Rev. Invest. Salud. 2002;5:75–83. [Google Scholar]
- von Baum H., Marre R. Antimicrobial resistance of Escherichia coli and therapeutic implications. Int. J. Med. Microbiol. 2005;295:503–511. doi: 10.1016/j.ijmm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Vranic S.M., Uzunovic A. Antimicrobial resistance of Escherichia coli strains isolated from urine at outpatient population. Mater Sociomed. 2016;28(2):121–124. doi: 10.5455/msm.2016.28.121-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Jiang H.X., Liao X.P., Liu J.H., Li S.J. Prevalence of plasmid-mediated quinolone resistance qnr genes in poultry and swine clinical isolates of Escherichia coli. Veterinary Microbiol. 2008;132:414–420. doi: 10.1016/j.vetmic.2008.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this research are included in the article. If there are any specifics needed, please contact the corresponding author.