Abstract
Tinosporide and 8-hydroxytinosporide isolated from Tinospora cordifolia were evaluated for acetylcholinesterase (AChE) and butylcholinesterase (BuChE) inhibitory activities. The structure of the compound was confirmed by spectroscopic analysis, whereas cholinesterase inhibition was investigated by Ellman method using donepezil as standard drug and the data were presented as IC50 (μg/ml ± SEM). Furthermore, donepezil, tinosporide and 8-hydroxytinosporide were executed for docking analysis. The results from the isolated compounds TC-16R confirmed as tinosporide promisingly inhibited AChE with IC50 value of 13.45 ± 0.144, whereas TC-19R confirmed as 8-hydroxytinosporide moderately inhibited AChE with IC50 value of 46.71 ± 0.511. In case of BuChE inhibition, the IC50 values were found to be 408.50 ± 17.197 and 317.26 ± 6.918 for tinosporide and 8-hydroxytinosporide, respectively. The in silico studies revealed that the ligand tinosporide fit with the binding sites and inhibited AChE. Overall, the study findings suggested that tinosporide would be a complementary noble molecule of donepezil which is correlated with its pharmacological activity through in vitro studies, while 8-hydroxytinosporide modestly inhibited BuChE and the results are very close to the standard donepezil.
Keywords: Tinosporide, Acetylcholinesterase, Butyrylcholinesterse, ADMET analysis, Molecular docking
1. Introduction
As a multifactorial syndrome having different relatable proteins that are responsible for the etiology, Alzheimer's disease (AD) is a degenerative disorder which progressively causes the loss of basal forebrain neurons, ultimately lower the cortical and hippocampal level of acetylcholine (ACh) (Melnikova, 2007). AD occurs due to the cholinesterase induced declination of neurotransmitters most commonly to the people in their mid-60s. Enzymes involved in breaking down of neurotransmitters like ACh and butyrylcholine (BuCh) are respectively called acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) (Yusoff et al., 2014). The therapeutic use of acetylcholinesterase inhibitors (AChEI) has been provided by observing a correlation between cholinergic dysfunction and AD severity (Sabbagh, 2009). The inhibition of AChE has been proven as a successful way to relieve some cognitive and behavioral symptoms of the AD and therefore, several AChE inhibitors, such as tacrine (Davis et al., 1992), galanthamine (Thomsen and Kewitz 1990), huperzine A (Xu et al., 1995) and donepezil (Courtney, Farrell et al. 2004) have been used mainly for the clinical treatment of AD. However, the level of cognitive decline is associated with these drugs and further brain studies of the advanced stage age and AD have consistently experienced damage and anomalies specially in basal forebrain projections.
With the development of “cholinergic hypothesis”, the current pharmacotherapy for AD aims to increase the levels of ACh through the AChE and BuChE inhibition. As BuChE is capable of compensating for missing AChE catalytic functions in the synaptic cleft with the increase in activity by 30–60% during AD (Jhee et al., 2002, Mesulam et al., 2002, Darvesh et al., 2003, Roger and Roger, 2007, Kamal et al., 2008), cholinesterases (ChEs) inhibition using a dual inhibitor result in increased levels of ACh in the brain, which provide more successful clinical efficacy of AD (Basiri et al., 2013, Khoobi et al., 2013). Search for newer molecules from natural products has gained much attention by the researchers’ worldwide despite of having synthetic drugs available for the symptomatic treatment of AD.
Majority of the secondary metabolites with potential AChE and BuChE inhibitory activity are alkaloids followed by terpenes, sterols, flavanoids, and glycosides and compounds having potential anticholinesterase activity are isolated from Buxaceae, Amaryllidaceae, Lycopodiaceae, Lamiaceae, Chenopodiaceae, Papaveraceae, Apocynaceae, and Labiatae species (Ata et al., 2010, Ahmed et al., 2013). Variety of secondary metabolites show AChE activity include vasicinone, vasicine, harmine, deoxyvasicinone, deoxyvasicine, harmaline, harmol, and harmane (Zhao, Ding et al. 2013). For a large number of enzyme-ligand complexes, it is determined by X-ray crystallography that the three-dimensional structure of AChE, reveals two main binding sites: the catalytic active site (CAS), comprising the Ser-His-Glu catalytic triad, and the peripheral anionic site (PAS), connected by a deep, hydrophobic gorge (Sussman et al., 1991, Bourne et al., 1999, Kryger et al., 2000).
In this study, two secondary metabolites, named tinosporide and 8-hydroxytinosporide were isolated from Tinospora cordifolia and identified them by 1D and 2D NMR spectroscopy using 1H, 13C, HSQC, HMBC, COSY, and NOESY. In vitro studies were carried out to develop these two metabolites as potential anticholinesterase agents by determining the inhibitory activities using Ellman’s colorimetric method. Finally, with the help of molecular docking, we have explored the ability of the metabolites as potent inhibitors of AChE and elucidated the possible mechanism of action through in silico study.
2. Experimental
2.1. Instrumentations
The column was initially packed with fine vacuum liquid chromatography (VLC) grade silica (Kiesel gel 60H), then some column and VLC fractions were further fractionated by gel permeation chromatography using Sephadex-LH20. The 1H and 13C NMR spectra were recorded with Bruker instrument (Rheinstetten, Germany) spectrometer using deuterated chloroform (CDCl3) as a solvent. Reaction progress and the product mixtures were routinely checked by thin-layer chromatography (TLC) on Merck silica gel 60F254 (Darmstadt, Germany) aluminum plates.
2.2. General procedure for the isolation of tinosporide and 8-hydroxytinosporide
The methanolic extract (1 gm) of the stem of T. cordifolia (Willd.) Hook. f. and Thoms. (Family: Menispermaceae) were subjected to vacuum liquid chromatography (VLC) and gravity column chromatography. The column was packed with fine VLC grade silica (Kiesel gel 60H, Merck). The ethyl acetate extract was adsorbed with column grade silica and then added to VLC column. The column with the extract was first washed with 100% petroleum ether and the polarity of the eluent was increased by using dichloromethane, ethyl acetate and methanol in appropriate amount. Finally the column was washed with 100% methanol.
After initial screening by thin layer chromatography, ethyl acetate soluble VLC fractions were further fractionated by gravity column chromatography. The VLC column fraction further on gravity column eluted with toluene and ethyl acetate in 40:60% yielded white residues. Upon repeated washing with hexane–ethyl acetate afforded compound coded as TC-16R (6 mg) on TLC followed by 1% vanillin-sulfuric acid spray, it showed purple color with the Rf value of 0.78 [mobile phase- toluene: ethyl acetate (4:1)]. On the other hand VLC fraction on gravity column eluted with 30:70% toluene: ethyl acetate provided compound coded as TC-19R (5.5 mg) which on TLC followed by 1% vanillin-sulfuric acid spray showed purple color and the Rf value was 0.59 [mobile phase: toluene: ethyl acetate (4:1)]. The isolated pure compounds were then characterized by extensive spectroscopic studies like 1H NMR, 13C NMR, HSQC, HMBC, 1H–1H COSY and 1H–1H NOESY experiments. Compound TC-16R was identified as tinosporide and TC-19R was identified as 8-hydroxytinosporide.
2.3. Chemicals and reagents
Tris-HCl and bovine serum albumin were from Merck, Germany and, 5́-dithio-bis-(2-nitro) benzoic acid (DTNB), acetylcholineiodide, butyrylcholine iodide, Triton X-100, BCA (bicinchoninic acid) kit, and trichloroacetic acid were purchased from Sigma-Aldrich, Germany. As a kind gift from Incepta Pharmaceuticals Ltd., donepezil was collected. Other chemicals required were collected from local sources.
3. Methods
3.1. In vitro AChE inhibition assay
With minor modification using acetylthiocholine iodide as a substrate, inhibition of AChE was assessed following Ellman method (Ellman, Courtney et al. 1961; Rashedul Islam 2019). Rat brains were homogenized in a homogenizer with 5 volumes of a homogenization buffer [10 mM Tris-HCl (pH 7.2), as a source of AChE, that contained 1 M NaCl, 50 mM MgCl2 and 1% Triton X-100]. The mixture was then centrifuged at 10,000 rpm for 15 min and the supernatant was used as an enzyme source. The process of enzyme preparation was carried out at 4 °C and the protein concentration was measured using the BCA (bicinchoninic acid kit) using BSA as a protein standard. To carry out the reaction, each tested compound or standard (500 µl) was mixed with an enzyme solution (500 µl) and incubated at 37 °C for 15 min, and then Ellman’s reaction mixture [(3.5-ml0.5 mM acetylthiocholine, 1 mM 5, 5́-dithio-bis (2-nitro benzoic acid) in a 50 mM sodium phosphate buffer (pH 8.0)] were added. Immediately after adding Ellman's reaction mixture, the rate of hydrolysis by acetylcholinesterase were monitored spectrophotometrically reading absorbance at 405 nm. From the absorbance of control and test sample, AChE inhibition was calculated and expressed in percentage (Rashedul Islam, 2019).
3.2. In vitro BuChE inhibition assay
BuChE assay was performed by following the AChE inhibition assay method, using butyrylthiocholine iodide as a substrate. Nevertheless, human blood was homogenized in a homogenizer as a source of the enzyme with 5 volumes of a homogenization buffer [10 mM Tris-HCl (pH 7.2), that contained 1 M NaCl, 50 mM MgCl2 and 1% Triton X-100, and centrifuged at 10000 rpm for 15 min. The resulting supernatant was used as an enzyme. BuChE inhibition was calculated and expressed in percent as mentioned above for AChE inhibition assay.
4. In silico molecular docking analysis
To explore intermolecular interactions, docking studies were performed for tinosporide and 8-hydroxytinosporide. The interacted molecular properties were then compared with standard donepezil. Docking of the two compounds were subjected against AChE and BuChE. Docking studies were performed using software (AutoDock vina) and docking server (Mcule). Followed by, each docking job was carried out for “Docking score” comparison studies. Receptor-ligand interaction studies were performed through different visualization tools like PyMOL, Discovery Studio Visualizer, and PyRx to catch up the best output.
The crystal structure of AChE (4pqe) and BuChE (6esy), were downloaded from the RCSB protein databank (PDB). All the crystallographic water molecules, as well as all heteroatoms were removed as they may interfere in binding. Proteins were prepared by adding polar hydrogens as well Gasteiger charge using Auto Dock Tools 1.5.6. The crystal structure of PDB ID: 4pqe and 6esy were used as the target for the docking studies. Selected structures were also prepared for docking studies by adding polar hydrogen and Gasteiger charge. Finally, the energy of candidates was minimized for docking purpose.
4.1. ADMET analysis
Tinosporide and 8-hydroxytinosporide were subjected to ADMET studies to find the best pharmacokinetic profile with lower toxicity. The ultimate objective of the in silico ADMET studies was to accurately predict the in vivo pharmacokinetics of these lead molecules (Gleeson et al., 2011, Foster et al., 2014). In this study, our goal was to focus on the physicochemical properties, lipophilicity, water solubility, pharmacokinetics, drug-likeness (Lipinski’s rule of five, Ghose rules etc.) of the tested compounds (Gleeson, Anne et al. 2011). Moreover, AMES toxicity, acute oral toxicity, carcinogenicity, etc. were also evaluated using admetSAR and SwissADME servers.
4.2. Statistical analysis
The data analyzed by one-way ANOVA with p < 0.05 and p < 0.01 were considered statistically significant. The results presented as mean ± SEM (standard errors) of the triplicate experiment. The IC50 values of the compounds were evaluated by non-linear regression analysis using GraphPad Prism Data Editor for Windows, Version 6.0 (GraphPad Software Inc., San Diego, CA).
5. Results
5.1. NMR spectroscopic data and identification of the isolated compounds
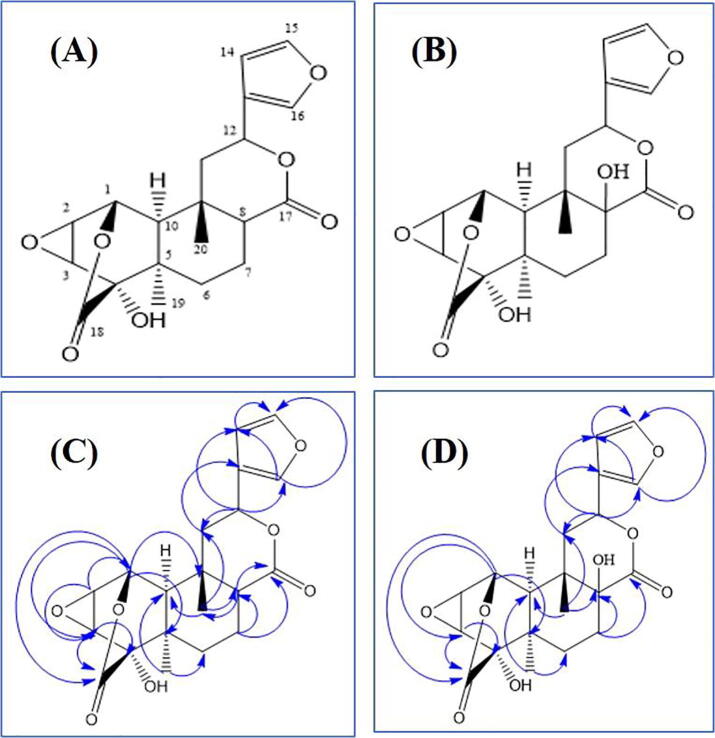
The isolated compound TC-16R was obtained as white residue. The 1H NMR spectrum of compound TC-16R displayed signals at a δ6.65 (br s), δ7.62 (br s) and δ6.69 (br s) that was assigned to the protons of the β-substituted furan moiety. Two tertiary methyl groups resonating at δ1.48 and δ1.29 were observed as singlets. The signals at δ5.96 (1H, dd, J = 12.2, 4.1 Hz), was assigned to the C12 proton, which bear the β-substituted furan moiety. C-11 methylene protons attributed signals with aliphatic region at δ2.46 (1H, dd, J = 14.7, 4.1 Hz) and δ2.29 as multiplate. The HOMO-COSY correlation profile displayed two multiplets at δ2.88 and 2.03 were assigned to protons at C-7. The experience of anisotropic effect of the lactone ring had made this downfield signal at 2.88 which was allocated to an axial proton on C-7. The signals at δ5.23 (d, 2.6 Hz), δ3.97 (dd, 2.6, 4.3) and δ4.01 (d, 4.3 Hz) were given to protons on C-1, C-2 and C3 of ring A. Furthermore, the spectrum exhibited two oxymethine protons at δ 5.23 (d, J = 2.6 Hz, δc 71, H-1) and δ 5.96 (dd, J = 12.2, 4.1 Hz, δc 70.9, H-12), as well as a number of methylenes and methines appearing in the high field region from δ2.31 to 2.88. The 13C NMR spectrum showed 20 carbons including two carbonyl carbons at δc172.5 and δc173.8. The presence of two oxymethine protons together with two carbonyl protons suggested the presence of two lactone rings which was further substantiated by HMBC experiment. Location of two lactone groups and furan ring was established by HMBC experiment as H-1 and H-8 protons revealed 3 J and 2 J correlations to δc172.5 and 173.8, and H-12 proton showed 2 J and 3 J correlations to C-13 (δc125.9) and C-16 (δc140.3), respectively. 1H–1H COSY and 1H–1H NOESY experiments showed the expected correlation. On the basis of the spectroscopic data (Table 1), compound TC-16R was identified as tinosporide (Fig. 1). Compound TC-19R also exhibited a similar proton and carbon NMR profile, except the presence of carbon bearing the tertiary hydroxyl group (Table 1, Fig. 1).
Table 1.
1H NMR, 13C NMR and HMBC data for Compounds TC-16R identified as tinosporide and TC-19R identified as 8-hydroxy tinosporide.
| Sl. |
TC-16R |
TC-19R |
||||
|---|---|---|---|---|---|---|
| δC | δH | HMBC | δC | δH | HMBC | |
| 1 | 71.0 | 5.23 d (2.6) | 34.9 (C-9),41.6 (C-5), 52.1 (3), 50.0 (2), 172.5 (C-18) | 70.9 | 5.37br s | 41.6 (C-5), 52.3 (3), 172.6 (C-18) |
| 2 | 50.0 | 3.97 dd (2.6, 4.3) | 52.1 (3), 71.0 (1) | 50.2 | 4.10 m | – |
| 3 | 52.1 | 4.01 d (4.3) | 50.1 (2), 81.3 (C-4), 172.5 (C-18), 71.0 (1) | 52.3 | 4.03br s | 50.2 (2), 81.6 (C-4), 172.6 (C-18) |
| 4 | 81.3 | – | – | 81.6 | – | – |
| 5 | 41.6 | – | – | 41.6 | – | – |
| 6 | 26.5 | 1.99 m, 1.75 m | 17.0 (C-7),23.3 (C-19), 41.6 (C-5), 81.3 (4) | 27.7 | 2.04 m | 23.7 (C-19),27.2 (C-7), 41.6 (C-5), 47.4 (C-10), 72.8 (C-8) |
| 7 | 17.0 | 2.88 m, 2.03 m | 26.5 (C-6), 34.9 (C-9), 44.0 (C-8), 173.8 (C-17), | 27.2 | 3.43 m, 2.03 m | 27.7 (C-6), 40.0 (C-9), 72.8 (C-8), 174.3 (C-17), |
| 8 | 44.0 | 2.55 dd (11.1, 1.5) | 17.0 (C-7), 26.5 (C-6), 27.9 (C-20), 34.9 (C-9), 46.3 (C-10), 173.8(C-17) | 72.8 | – | – |
| 9 | 34.9 | – | – | 40.0 | – | – |
| 10 | 46.3 | 2.31 s | 23.3 (C-19), 27.9 (C-20), 34.9 (C-9), 41.6 (C-5), 50.0 (C-2), 81.3 (C-4) | 47.4 | 2.41 s | 21.0 (C-20), 23.7 (C-19), 27.7 (C-6), 36.3 (C-11), 40.0 (C-9), 41.6 (C-5), 50.2 (C-2), 70.9 (C-1), 81.6 (C-4) |
| 11 | 41.2 | 2.46 dd (14.7, 4.1) 2.01 m |
27.9 (C-20), 34.9 (C-9), 44.0 (C-8), 46.3 (C-10), 70.9 (C-12),125.9 (C-13) | 36.3 | 2.80dd (14.5, 11.6) 2.32 dd (14.5, 5.5) |
21.0 (C-20), 40.0 (C-9), 47.4 (C-10), 71.4 (C-12), 72.8 (C-8), 126.5 (C-13) |
| 12 | 70.9 | 5.96 dd (12.2, 4.1) | 41.2 (C-11), 109.1 (C-14), 125.9 (C-13), 140.3 (C-16) | 71.4 | 6.00 dd (11.6, 5.5) | 36.3 (C-11), 109.3 (C-14), 126.5 (C-13), 140.3 (C-16) |
| 13 | 125.9 | – | – | 126.5 | – | – |
| 14 | 109.1 | 6.65 br s | 125.9 (C-13), 140.3 (C-16), 144.1 (C-15) | 109.3 | 6.69br s | 126.5 (C-13), 140.3 (C-16), 144.1 (C-15) |
| 15 | 144.1 | 7.62 br s | 109.1(C-14), 125.9 (C-13), 140.3 (C-16) | 144.1 | 7.59br s | 109.3(C-14), 126.5 (C-13), 140.3 (C-16) |
| 16 | 140.3 | 7.69 br s | 109.1(C-14), 125.9 (C-13), 144.1 (C-15) | 140.3 | 7.71br s | 109.3(C-14), 126.5 (C-13), 144.1 (C-15) |
| 17 | 173.8 | – | – | 174.3 | – | – |
| 18 | 172.5 | – | – | 172.6 | – | – |
| 19 | 23.3 | 1.48, 3H s | 26.5 (C-6), 41.6 (C-5), 46.3 (C-10), 81.3 (C-4) | 23.7 | 1.64, 3H s | 27.7 (C-6), 41.6 (C-5), 47.4 (C-10), 81.6 (C-4) |
| 20 | 27.9 | 1.29, 3H s | 34.9 (C-9), 41.2 (C-11), 44.0 (C-8), 46.3 (C-10), | 21.0 | 1.53, 3H s | 36.3 (C-11), 40.0 (C-9), 47.4 (C-10), 72.8 (C-8) |
Fig. 1.
Structure and key HMBC correlations of tinosporide (A, C) and 8-hydroxytinosporide (B, D).
5.2. In vitro AChE and BuChE inhibitory activity
The inhibitory effects of the tested compounds are shown in Table 2. From the obtained data, it was observed that tinosporide showed promising activity in inhibiting AChE with the IC50 value of 13.45 ± 0.144 (μg/ml ± SEM), whereas 8-hydroxytinosporide exhibited moderate activities with the value of 46.71 ± 0.511 (μg/ml ± SEM) (Table 2). None of them showed any significant BuChE inhibitory activity.
Table 2.
AChE and BuChE inhibitory activity of secondary metabolites tinosporide and 8-hydroxytinosporide obtained from T. cordifolia where donepezil was used as standard drug.
| Tested compounds | AChE inhibition (IC50 (μg/ml) ± SEM) |
BuChE inhibition (IC50 (μg/ml) ± SEM) |
|---|---|---|
| Donepezil | 6.31 ± 0.089 | 11.93 ± 0.129 |
| Tinosporide | 13.45 ± 0.144 | 408.50 ± 17.197 |
| 8-Hydroxytinosporide | 46.71 ± 0.511 | 317.26 ± 6.918 |
5.3. In silico molecular docking analysis for AChE and BuChE
To investigate the interaction of in vivo studied ligands, interactions with the respective target macromolecule in silico molecular studies were carried out (Fig. 2). The protein AChE crystal structure comprises of chain A and BuChE crystal structure comprises of both chain A and B in its structure were docked using AutoDock Vina and Mcule along with the standard drug donepezil with target protein chain. The subsequent binding energy was found to be −8.3 kcal/mol and 8.2 kcal/mol for AChE, and 9.9 kcal/mol and −9.3 kcal/mol for BuChE target, respectively thus having < 200 RMSD value (Fig. 2, Table 3). Whereas in between bioactive molecule 8-hydroxytinosporide and AChEdocked conformation, the ligand generously embedded in the binding pocket of AChE attaining binding energy of −9.0 kcal/mol (AutoDock vina) and −8.7 kcal/mol (Mcule) and for BuChE, the binding energy was of −9.5 kcal/mol (AutoDock vina) and −9.9 kcal/mol (Mcule) correspondingly having < 2.00 RMSD value. Elsewhere, the ligand tinosporide appeared to be embedded in the deeper core of the protein binding pocket seems like in the heart of the pocket having a binding energy of −8.7 kcal/mol (AutoDock vina) and 9.0 kcal/mol (Mcule), whereas with BuChE, the values were −8.8 kcal/mol (AutoDock vina) and −9.4 kcal/mol (Mcule) with the same RMSD limit (Table 3). Post-docked binding mode of the selected compound exposed to be smoothly embedded in the target protein binding pocket (Fig. 3, and Fig. 4).
Fig. 2.
Structure of target molecule (A) AChE, (B) BuChE, (C) donepezil, (D) tinosporide and (E) 8-hydroxytinosporide.
Table 3.
Consensus docking affinity score of ligands against two target proteins.
| Target | Ligand | Binding affinity (kcal/mol) |
||
|---|---|---|---|---|
| AutoDock Vina | Mcule | Consensus Docking | ||
| AChE | Donepezil | −8.3 | −8.2 | −8.2 |
| Tinosporide | −8.7 | −9.0 | −8.8 | |
| 8-hydroxytinosporide | −9.0 | −8.7 | −8.8 | |
| BuChE | Donepezil | −9.9 | −9.3 | −9.6 |
| Tinosporide | −8.8 | −9.4 | −9.1 | |
| 8-hydroxytinosporide | −9.5 | −9.9 | −9.7 | |
Fig. 3.
3D and 2D interaction of donepezil, tinosporide, 8-hydroxytinosporide with AChE.
Fig. 4.
3D and 2D interaction of donepezil, tinosporide, 8-hydroxytinosporide with BuChE.
6. Discussion
According to the NMR spectroscopic data isolated compound TC-16R was confirmed as tinosporide. On the other hand, isolated compound TC-19R exhibited a similar proton and carbon NMR profiles. On careful comparison of the NMR data of TC-16R and TC-19R, it is clear that TC-19R was similar in all respects to TC-16R, except the presence of carbon bearing the tertiary hydroxyl group. From the literature reports we could find that 6-OH and 8-OH substituted tinosporide have been isolated from T. cordifolia (Sivasubramanian, Gadepalli Narasimha et al. 2013). Based on the spectroscopic data and by comparison with published literature, the compound TC-19R was confirmed as 8-hydroxytinosporide (Table I, Fig. 1).
Choline esters, such as ACh and BuCh, play an important role in the transmission of nerve impulse, are lysed by cholinesterase enzyme. For this reason inhibition of cholinesterases has become an important target for the treatment of neurodegenerative disorders, including AD (Reisberg, Doody et al. 2003). In this study, these two isolated secondary metabolites were assessed in vitro for AChE and BuChE inhibitory activities as described under experimental section and none of them were reported with any significant BuChE inhibitory effects.
The molecular interaction profile of donepezil with acetylcholinesterase includes a single H-bond with TYR A: 72 having 2.84 Å bonding distance, pi-sigma bond with TYR A: 341 along with many other Van der Waals, pi-pi T-shaped, pi-pi stacked interactions with ASN A: 283, PHE A: 295, PHE A: 338, VAL A: 294, TYR A: 124, TYR A: 337, TYR A: 72, TRP A: 286. However, molecular interaction profile of donepezil with BuChE includes three C-H bonds with TRP A: 82, PRO A: 285, SER A:287 having 3.74 Å, 3.37 Å, 3.75 Å bonding distance individually, pi-sigma bond with TRP A: 82 along with many other Van der Waals, pi-pi T-shaped, pi-pi stacked and pi-alkyl interactions with GLY A: 121, THR A: 122, LEU A: 125, THR A: 120, SER A: 198, PHE A: 398, TRP A: 231, GLY A:117, HIS A: 438, GLY A: 116, GLY A: 439, GLU A: 197, TYR A: 128, GLY A: 115, PHE A: 329, TRP A: 82, LEU A: 286 (Fig. 4, Table 4). Ligand-protein interaction analysis was performed based on electrostatic interactions and hydrogen bonds. Observed interactions most possibly are responsible for contributing to the binding affinity of donepezil with the target as well prevail its pharmacologic effect against Alzheimer's disease.
Table 5.
ADMET analysis data.
|
Molecule → Parameters ↓ |
Donepezil | Tinosporide | 8-hydroxytinosporide |
|---|---|---|---|
| MR | 115.31 | 198.19 | 166.61 |
| TPSA | 38.77 | 32.23 | 35.5 |
| MLOGP | 3.06 | 3.32 | 2.97 |
| GI absorption | High | High | High |
| BBB permeant | Yes | Yes | Yes |
| Lipinski #violations | 0 | 0 | 0 |
| Ghose #violations | 0 | 1 | 0 |
| Veber #violations | 0 | 0 | 1 |
| Bioavailability Score | 0.55 | 0.30 | 0.27 |
| PAINS #alerts | 0 | 0 | 0 |
| Leadlikeness #violations | 2 | 3 | 1 |
| Synthetic Accessibility | 3.62 | 2.55 | 3.38 |
| Ames Mutagenesis | Negative | Negative | Negative |
| Acute oral toxicity | III | III | III |
| Eye Irritation | – | – | – |
| hERG | + | – | – |
Besides, compare to that of donepezil, the molecular interaction of 8-hydroxytinosporide with AChE claimed two H-bond with catalytic site residue ASN A: 533 and GLN A: 403 having 2.06 Å, 2.23 Å bonding distance as well a single C-H bond with HIS A: 405 along with many other Van der Waals, pi-alkyl, alkyl interactions with binding pocket catalytic residue THR A: 238, ASN A: 233, PRO A: 410, GLU A: 313, LEU A: 540, PRO A: 235, TRP A: 532. During the comparison with donepezil, the molecular interaction of 8-hydroxytinosporide with BuChE claimed two H-bond with catalytic site residue SER A: 198 and HIS A: 438 having 2.06 Å, 2.23 Å bonding distance as well a single C-H bond with GLY A: 116 along with many other Van der Waals, pi-alkyl, alkyl interactions with binding pocket catalytic residues SER A: 287, TYR A: 332, SER A: 79, ASP A: 70, TRP A: 430, GLY A: 70, TYR A: 440, THR A: 120, GLY A: 117, PRO A: 285, TRP A: 82, HIS A: 438, ALA A: 328 (Fig. 4, Table 4).
While another ligand tinosporide moleculer interaction with AChE showed two H-bond with ARG A: 296, TRP A: 532 having 2.73 Å, 2.22 Å bonding distance along with many other Van der Waals, pi-alkyl, pi-cation, alkyl interactions with binding pocket catalytic residues ASN A: 233, GLN A: 413, PRO A: 637, VAL A: 370, PRO A: 368, LEU A: 536, HIS A: 405, PRO A: 235, ARG 296, HIS A: 405, TRP A: 532, LEU A: 540. Additionally with BuChE, tinosporide moleculer interaction showed three H-bond with PRO A: 285, SER A: 198, GLY A: 116 having 2.44 Å, 2.14 Å, and 3.05 Å bonding distance GLY A: 117, ALA A: 199, GLU A: 197, TYR A: 128, THR A: 120, ASP A: 70, TYR A: 332, GLY A: 115, TRP A: 82, PHE A: 329, HIS A: 438. (Fig. 3, Table 4).
AChEIs also inhibit BuChE because AChE and BuChE both share 65% amino acid sequence similarity even though being encoded by different genes on human chromosomes (Inestrosa and Alarcón 1998). Functionally, both enzymes hydrolyze acetylcholine efficiently but at a different rate even at the same temperature and pH. But AChE has higher hydrolytic acetylcholine activity than BuChE (Houghton, Ren et al. 2006). In this study, we observed optimum affinity of tinosporide and 8-hydroxytinosporide towards AChE and BuChE, respectively in a manner similar to that of standard drug donepezil. In the analysis of ligand interaction, the ligand tinosporide comparatively showed a maximum number of interactions with amino acid residues including five (05) types of non-covalent interactions than the other two. On the other hand, 8-hydroxytinosporide appeared to be in ligand interaction mostly the same phenomena of donepezil having five (05) types of interactions along with H-bonds. From the literature review, non-covalent interactions of a drug must place with the protein to reduce the ability of a drug to dissociate from its binding site through enhancing binding affinity and binding constant (Zhou et al., 2012, Tang et al., 2017). Therefore, such interactions greatly impact in drug design, prominently in the synthesis of many organic molecules (Schneider and Böhm, 2002, Cockroft and Hunter, 2007, Mitra and Dash, 2018). A satisfactory number of Van der Waals interactions in both ligands predicts their moderate solubility in lipid. Besides, 1.72–2.85 Å range of H-bond distances predicts good docking simulation results (Schneider and Böhm 2002) in which the requirements were desirably achieved by both of the experimental ligands than donepezil.
Compounds under investigation satisfy all the characteristics of CNS acting drugs like good brain penetration and other potential toxicity profiles (Table 5). These compounds have the possibility to be the best drug-like characteristics. Further on, both test ligands fulfill the criteria of Lipinski's Rule of Five, confer that both are perfect for the orally active drug (Table 5). As a result, overall study findings suggest that the ligand 8-hydroxytinosporide would be the best choice for the treatment of cognitive impairment ruling as a potent inhibitor of AChE as a complementary noble molecule of donepezil which is correlated with its pharmacological activity through in vitro studies, while 8-hydroxytinosporide modestly inhibited BuChE and results are very close to the standard donepezil.
7. Conclusion
The study involved the isolation of metabolites from T. cordifolia and their evaluation of potential role in AD. AChE and BuChE inhibitory activity of tinosporide and 8-hydroxytinosporide obtained from methanol extract of T. cordifolia has been investigated. Tinosporide exhibited promising anti-AChE activity. The docking study findings suggest that the ligand tinosporide would be a lead for the development of drugs to be used in the treatment of cognitive impairment ruling as a potent inhibitor of AChE enzyme as a complementary noble molecule of donepezil. Furthermore, the results of 8-hydroxytinosporide with standard donepezil suggest that it would be the choice as lead molecule for development of BuChE inhibitors to be used in the BuCh related disorders which is correlated with its activity through in vitro studies. These preliminary data suggest that these two natural metabolites might be used as lead to develop new drugs for AD and also warrant us for further in vivo experiments using animal model.
Author contributions
Abdul Mazid and Monira Ahsan designed and coordinated the research, Mohiminul Adib isolated the metabolites and Rashedul Islam carried out in vitro experiments. Arifur Rahman did in silico studies, Mahmud Hossain helped in data analysis, Mustafizur Rahman, Sultan M. Alshehri and Mohsin Kazi helped in drafting, review and editing the manuscript. All authors have gone through the final manuscript and approved it.
Acknowledgments
Acknowledgement
The authors are thankful to the Researcher Supporting Project (number RSP-2020/146) at King Saud University, Riyadh, Saudi Arabia, and the article processing charge (APC) is also supported by RSP. Authors are also thankful to Department of Marine Biotechnology, University of Science and Technology, South Korea, for the recording the NMR spectra of the isoalated compounds and Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Bangladesh for providing laboratory support.
Declaration of Competing Interest
Authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed F., Ghalib R.M., Sasikala P., Ahmed K.K. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013;7(14):121–130. doi: 10.4103/0973-7847.120511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ata A., Iverson C.D., Kalhari K.S., Akhter S., Betteridge J., Meshkatalsadat M.H., Orhan I., Sener B. Triterpenoidal alkaloids from Buxus hyrcana and their enzyme inhibitory, anti-fungal and anti-leishmanial activities. Phytochemistry. 2010;71(14):1780–1786. doi: 10.1016/j.phytochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Basiri A., Murugaiyah V., Osman H., Kumar R.S., Kia Y., Awang K.B., Ali M.A. An expedient, ionic liquid mediated multi-component synthesis of novel piperidone grafted cholinesterase enzymes inhibitors and their molecular modeling study. Eur. J. Med. Chem. 2013;67:221–229. doi: 10.1016/j.ejmech.2013.06.054. [DOI] [PubMed] [Google Scholar]
- Bourne Y., Grassi J., Bougis P.E., Marchot P. Conformational flexibility of the acetylcholinesterase tetramer suggested by x-ray crystallography. J. Biol. Chem. 1999;274(43):30370–30376. doi: 10.1074/jbc.274.43.30370. [DOI] [PubMed] [Google Scholar]
- Cockroft S.L., Hunter C.A. Chemical double-mutant cycles: dissecting non-covalent interactions. Chem. Soc. Rev. 2007;36(2):172–188. doi: 10.1039/b603842p. [DOI] [PubMed] [Google Scholar]
- Courtney, C., Farrell, D., Gray, R., Hills, R., Lynch, L., Sellwood, E., Edwards, S., Hardyman, W., Raftery, J., Crome, P., Lendon, C., Shaw, H., Bentham, P., A.D.C. Group, 2004. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet 363(9427): 2105–2115. [DOI] [PubMed]
- Darvesh S., Hopkins D.A., Geula C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003;4(2):131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- Davis K.L., Thal L.J., Gamzu E.R., Davis C.S., Woolson R.F., Gracon S.I., Drachman D.A., Schneider L.S., Whitehouse P.J., Hoover T.M., Morris J.C., Kawas C.H., Knopman D.S., Earl N.L., Kumar V., Doody R.S. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer's disease. N. Engl. J. Med. 1992;327(18):1253–1259. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Foster D.C.D., Conn, P.J., Rook, J., 2014. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer's disease and schizophrenia. Neuropsychiatr Dis Treat 10: 183–191. [DOI] [PMC free article] [PubMed]
- Gleeson M.P., Anne H., Supa H. In-silico ADME models: a general assessment of their utility in drug discovery applications. Curr. Top. Med. Chem. 2011;11(4):358–381. doi: 10.2174/156802611794480927. [DOI] [PubMed] [Google Scholar]
- Houghton P.J., Ren Y., Howes M.-J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006;23(2):181–199. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]
- Inestrosa N.C., Alarcón R. Molecular interactions of acetylcholinesterase with senile plaques. J. Physiol.-Paris. 1998;92(5):341–344. doi: 10.1016/S0928-4257(99)80002-3. [DOI] [PubMed] [Google Scholar]
- Jhee S.S., Shiovitz T., Hartman R.D., Messina J., Anand R., Sramek J., Cutler N.R. Centrally acting antiemetics mitigate nausea and vomiting in patients with Alzheimer's disease who receive rivastigmine. Clin. Neuropharmacol. 2002;25(2):122–123. doi: 10.1097/00002826-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Kamal M.A., Klein P., Luo W., Li Y., Holloway H.W., Tweedie D., Greig N.H. Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental alzheimer therapeutic, dihydrobenzodioxepine cymserine. Neurochem. Res. 2008;33(5):745–753. doi: 10.1007/s11064-007-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoobi M., Alipour M., Moradi A., Sakhteman A., Nadri H., Razavi S.F., Ghandi M., Foroumadi A., Shafiee A. Design, synthesis, docking study and biological evaluation of some novel tetrahydrochromeno [3′,4′:5,6]pyrano[2,3-b]quinolin-6(7H)-one derivatives against acetyl- and butyrylcholinesterase. Eur. J. Med. Chem. 2013;68:291–300. doi: 10.1016/j.ejmech.2013.07.045. [DOI] [PubMed] [Google Scholar]
- Kryger G., Harel M., Giles K., Toker L., Velan B., Lazar A., Kronman C., Barak D., Ariel N., Shafferman A., Silman I., Sussman J.L. Structures of recombinant native and E202Q mutant human acetylcholinesterase complexed with the snake-venom toxin fasciculin-II. Acta Crystallographica Section D. 2000;56(11):1385–1394. doi: 10.1107/s0907444900010659. [DOI] [PubMed] [Google Scholar]
- Melnikova I. Therapies for Alzheimer's disease. Nat. Rev. Drug Discovery. 2007;6(5):341–342. doi: 10.1038/nrd2314. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M., Guillozet A., Shaw P., Levey A., Duysen E.G., Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110(4):627–639. doi: 10.1016/s0306-4522(01)00613-3. [DOI] [PubMed] [Google Scholar]
- Mitra S., Dash R. Structural dynamics and quantum mechanical aspects of shikonin derivatives as CREBBP bromodomain inhibitors. J. Mol. Graph. Model. 2018;83:42–52. doi: 10.1016/j.jmgm.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Rashedul Islam M.A., Monira Ahsan Md., Mustafizur Rahman Md., Mazid Abdul. Cholinesterase and glycation inhibition assay of several metabolites obtained from plant and fungi. Dhaka Univ. J. Pharm. Sci. 2019;18(1):31–38. [Google Scholar]
- Reisberg B., Doody R., Stöffler A., Schmitt F., Ferris S., Möbius H.J. Memantine in moderate-to-severe Alzheimer's disease. N. Engl. J. Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- Roger B., Roger L. Executive dyscontrol in dementia, with emphasis on subcortical pathology and the role of butyrylcholinesterase. Curr. Alzheimer Res. 2007;4(3):277–293. doi: 10.2174/156720507781077313. [DOI] [PubMed] [Google Scholar]
- Sabbagh M.N. Drug development for Alzheimer's disease: where are we now and where are we headed? Am. J. Geriatric Pharmacotherapy. 2009;7(3):167–185. doi: 10.1016/j.amjopharm.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G., Böhm H.-J. Virtual screening and fast automated docking methods. Drug Discovery Today. 2002;7(1):64–70. doi: 10.1016/s1359-6446(01)02091-8. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian A., Gadepalli Narasimha K.K., Rathnasamy R., Campos A.M.F.O. A new antifeedant clerodane diterpenoid from Tinospora cordifolia. Nat. Prod. Res. 2013;27(16):1431–1436. doi: 10.1080/14786419.2012.722088. [DOI] [PubMed] [Google Scholar]
- Sussman J.L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253(5022):872. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Tang Z., Roberts C.C., Chang C.A. Understanding ligand-receptor non-covalent binding kinetics using molecular modeling. Front. Biosci. 2017;22:960–981. doi: 10.2741/4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen T., Kewitz H. Selective inhibition of human acetylcholinesterase by galanthamine in vitro and in vivo. Life Sci. 1990;46(21):1553–1558. doi: 10.1016/0024-3205(90)90429-u. [DOI] [PubMed] [Google Scholar]
- Xu S.S., Gao Z.X., Weng Z., Du Z.M., Xu W.A., Yang J.S., Zhang M.L., Tong Z.H., Fang Y.S., Chai X.S. Efficacy of tablet huperzine-A on memory, cognition, and behavior in Alzheimer's disease. Zhongguo Yao Li Xue Bao. 1995;16(5):391–395. [PubMed] [Google Scholar]
- Yusoff M., Hamid H., Houghton P. Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules. 2014;19(1):1201–1211. doi: 10.3390/molecules19011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Ding K.-M., Zhang L., Cheng X.-M., Wang C.-H., Wang Z.-T. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β-Carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J. Chem. 2013;2013 [Google Scholar]
- Zhou P., Huang J., Tian F. Specific noncovalent interactions at protein-ligand interface: implications for rational drug design. Curr. Med. Chem. 2012;19(2):226–238. doi: 10.2174/092986712803414150. [DOI] [PubMed] [Google Scholar]