Abstract
Vitamin D deficiency and periodontitis are commonly prevalent among Saudi adults. However, the association between periodontitis and vitamin D status has not been well documented. This study aims to examine the association between periodontitis and vitamin D status among adults in the Albaha region of Saudi Arabia. A case-control study of 123 Saudi adults was conducted; 60 had severe or moderate periodontitis, and 63 were periodontally healthy. Data was collected by an online self-reported sociodemographic questionnaire. All participants then underwent a full periodontal examination. Blood samples were also provided to assess participants’ vitamin D statuses through serum levels of 25-hydroxyvitamin D (25(OH)D). A total of 60 cases and 63 controls matched for BMI (30.2 ± 4.86 kg/m2), age (40.01 ± 7.73 years), and sex (46.3% and 53.7% male and female, respectively) participated in the study. Mean levels of 25(OH)D were significantly lower in periodontitis participants than in controls (25.03 ± 8.55 ng/ml, 29.19 ± 12.82 ng/ml, p = 0.037, respectively). Lower odds of periodontitis were detected per unit of 25(OH)D level (OR 0.964, 95% CI; 0.931–0.999, p = 0.043). In conclusion, periodontitis is significantly associated with deficient and insufficient levels of vitamin D among Saudi adults in the Albaha region. Future longitudinal research with a larger sample size may be suggested to confirm these results.
Keywords: vitamin D, Periodontitis, Genetic, Epidemiology, Disease prevention, Public health
Abbreviations: 25(OH)D, Serum level of 25-hydroxyvitamin D; ESCPG, the Endocrine Society Clinical Practice Guidelines; BMI, Body Mass Index; AL, Attachment Loss; PD, Probing Depth; OR, Odd Ratio; VDR, Vitamin D Receptor
1. Introduction
Vitamin D plays a key role in several immunomodulatory, antifibrotic, antioxidant, and anti-inflammatory actions (Murdaca et al., 2019). Furthermore, serum vitamin D level, 25-hydroxyvitamin D (25(OH)D), has been revealed to be significantly associated with periodontitis and to play a crucial role in oral homeostasis. Patients with chronic periodontitis had lower levels of vitamin D compared with other periodontally healthy patients (Machado et al., 2020). Both vitamin D deficiency and periodontitis are caused by genetical and environmental factors. However, there is a lack of research on the relationship between periodontitis and vitamin D status, and a great need exists to conduct robust longitudinal research with consistent definitions of periodontitis and vitamin D (Pinto et al., 2018). According to the Institute of Medicine and the Endocrine Society Clinical Practice Guidelines (ESCPG), serum 25(OH)D is classified into three levels in relation to overall health: sufficient (≥30 ng/ml), insufficient (20–29.9 ng/ml), and deficient (<20 ng/ml) (Institute of Medicine, 2011, Holick et al., 2011).
Deficient and insufficient levels of vitamin D have been deemed to be a global community health challenge (Palacios and Gonzalez, 2014). In the United States, the rates of insufficient and deficient vitamin D among adults have been reported as 41% and 29%, respectively (Liu et al., 2018). In the United Kingdom, 55% and 92% had deficient and insufficient levels of vitamin D among adults, respectively (Darling et al., 2020). Similarly, in Saudi Arabia, the rates of deficient and insufficient levels of vitamin D were more than 44% and 45%, respectively (Kaddam et al., 2017). Additionally, vitamin D deficiency rates among Saudi pregnant women and Saudi individuals with diabetes were even greater—82.5% and 60.8%, respectively (Al-Ajlan et al., 2018, Darraj et al., 2019). One study found that the overall prevalence of 25(OH)D deficiency among different Saudi populations was 81% (Al-Daghri, 2018).
Periodontitis is not only considered a dental public health issue worldwide; it also contributes to tooth loss and poor oral health (Nazir, 2017). Patients with periodontitis have commonly demonstrated a more negative impact on their quality of life than periodontally healthy individuals (Ferreira et al., 2017). The dental literature has revealed several risk factors associated with periodontitis, including but not limited to rheumatoid arthritis, smoking, chronic disorders, chronic depression, diabetes, and osteoporosis (Eke et al., 2012, Lang et al., 2015, Rodríguez-Lozano et al., 2019). Developing countries have shown a higher prevalence of moderate and severe periodontitis than developed countries (Pihlstrom et al., 2005).
In Saudi Arabia, not only is the prevalence of periodontitis among adults significantly higher compared with other countries, but it has also been regarded as a common dental health issue (Nazir, 2017, Thomas et al., 2020). The majority of Saudi research has indeed not commonly examined the prevalence rate of periodontitis. Instead, the research direction has been shown to be focused more on exploring periodontitis among individuals in high-risk groups, such as individuals with diabetes or obesity (Bahammam, 2015, Thomas et al., 2020). Even though several studies have shown a widespread prevalence of periodontitis among the Saudi population (Nazir, 2017, Thomas et al., 2020, Bahammam, 2015); neither a systematic review nor a national survey has explored the prevalence rate of periodontitis among Saudi adults.
The lack of evidence concerning the association between vitamin D status and periodontitis in Saudi Arabia in general, and particularly in the Albaha region, together with the high prevalence of both periodontitis and vitamin D deficiency among Saudi adults, indicates an urgent need to explore this territory of research. It was hypothesized in the present study, an increase of the 25(OH)D level as periodontitis risk decrease. Consequently, the aim of this study was to examine the association between periodontitis and vitamin D status among Saudi adults in the Albaha region.
2. Materials and methods
2.1. Participants and settings
A case-control study of 123 participants was conducted at a private settings between January and November 2020. Participants were classified into two groups: group one, cases, comprising participants with severe or moderate periodontitis (n = 60), and group two, controls, comprising participants who had healthy periodontal status (n = 63). The two groups will hereafter be referred to as the cases group and the control group. All participants met four inclusion criteria: 1) they were Saudi adults who had not taken any vitamin D supplements; 2) they were not smokers and did not have chronic depression, chronic disorders, liver diseases, diabetes type 1 or type 2, osteoporosis, heart disease, or kidney problems; 3) they had 14 or more teeth at the time of their periodontal examination to ensure meaningful evaluation of the individuals’ periodontal status (Genco and Borgnakke, 2020); and 4) they agreed to participate in this study and signed the consent forms. Pregnant women and individuals undergoing orthodontic care or had previous periodontal treatments were excluded from the study sample. In order to reduce bias for individuals’ selection and maximize the sampling’s representation, the included participants in this study had different genders, ages, vitamin D status, and Body Mass Index (BMI); which emphasizes the diversity in the sample of this study.
Data collection was conducted by completing an online self-reported questionnaire to provide information on participants’ socio-demographic characteristics, including sex, age, height, and weight. The questionnaire started with introductory information about the title and purpose of this study, the confidentiality and anonymity of the participants, their rights to withdraw, and consent for their participation. After this, participants were examined to assess their periodontal status, and a venepuncture blood sample was obtained from each participant to evaluate their vitamin D status. Cases were recruited first, followed by controls, to match those covariates. The BMI was also calculated for all participants.
2.2. Ethical considerations
Ethical approval was obtained from the Deanship of Scientific Research in Albaha University, Saudi Arabia (approval number: 13-06-1440-40204617). Moreover, it can be confirmed that the study was conducted in full accordance with the Saudi Ministry of Health Ethical Standards and the World Medical Association Declaration of Helsinki.
2.3. Evaluation of vitamin D levels
To assess 25(OH)D concentration in serum, a specialized phlebotomist took 5-ml venous blood samples from each individual in both the cases and control groups. The collected samples were kept in a refrigerator until centrifugation (at 3,000 rpm for five minutes) on the same day. Extracted serum of the 25(OH)D concentration was stored at −20 °C and analysed for a maximum of four days from sample collection day. An automated enzyme linked immunosorbent assay (ELISA) analyser (Elisys Uno; Human mbH, Wiesbaden, Germany) was used according to manufacturer instructions to measure serum concentrations of 25(OH)D. This analysis method, competitive solid phase ELISA, measures the total 25(OH)D in a range of 7.6–144 ng/ml using five calibrators and two controls. All outrange results were excluded. Although the cut-off points for 25(OH)D have been debated, these values were adopted according to the guidance from the Institute of Medicine and the ESCPG (Holick et al., 2011, Al-Alyani et al., 2018, Institute of Medicine, 2011), in which the serum level is classified into three levels: sufficient (≥30 ng/ml), insufficient (20–29.9 ng/ml), and deficient (<20 ng/ml).
2.4. Examination of periodontal status
Classified and registered dental consultants with the Saudi Commission for Health Specialities examined the periodontal statuses of the participants. During the examination, attachment loss (AL) and probing depth (PD) at six sites (buccal, mesiobuccal, distobuccal, lingual, mesiolingual, and distolingual) on all teeth, omitting only the third molars, were determined. A classification from the Health and Nutrition Examination Survey, the American Academy of Periodontology Working Group, and the Centres for Disease Control and Prevention was used to categorize periodontal cases into moderate or severe periodontitis. Moderate periodontitis was recorded when ≥ 2 interproximal sites with PD ≥ 5 mm (not on the same tooth) or ≥ 2 interproximal sites with AL ≥ 4 mm (not on the same tooth) were found, while severe periodontitis was recorded when ≥ 1 interproximal sites with PD ≥ 5 mm and ≥ 2 interproximal sites with AL ≥ 6 mm (not on the same tooth) were found (Page and Eke, 2007, Eke et al., 2012). At the time of examination, the control group was periodontally healthy, with neither severe nor moderate periodontitis.
Training and calibration of the dental consultants was conducted during the pilot assessment examination of the participants’ periodontal status. As the World Health Organization recommended, a 10% of the randomly chosen cases (i.e., 13 participants) were examined and re-examined after a one week by an independent dental consultant to measure the intra-examiner consistency and agreement concerning the AL and PD evaluation (World Health Organization. 2013). Weighted Cohen's kappa was used to measure the interrater reliability. The weighted kappa values for the AL and PD items were 0.89, and 0.87, respectively, indicating high consistency and agreement between the results of the two consultants. Periodontal examination kits consisting of a community periodontal index probe, mouth mirror, face mask, tweezers, gauze, cotton, and gloves; were used. The Saudi Ministry of Health Sterilization Protocol was also followed during carrying out this study.
2.5. Data analysis
Data were analysed using the Statistical Package for the Social Sciences software version 20.0 (IBM, Armonk, NY). Frequencies and corresponding percentages were used for the descriptive analysis; additionally, the mean associated and standard deviation were reported, as appropriate. The Shapiro-Wilk test and the Student’s t-test were used to assess normality and compare means between cases and control groups, respectively. The chi-square test and p-value were used to compare the three levels of 25(OH)D (deficient, insufficient, and sufficient) between different groups. A multivariate logistic regression model was also performed to examine the association between periodontitis and 25(OH)D serum levels. Statistical significance was set at 0.05.
3. Results
A total of 60 cases and 63 controls matched for BMI (30.2 ± 4.86 kg/m2), age (40.01 ± 7.73 years), and sex (46.3% and 53.7% for males and females, respectively) participated in the present study. Our findings revealed that 29 and 31 participants in the cases group had moderate and severe periodontitis, respectively. The control group, as mentioned previously, was periodontally healthy, with neither severe nor moderate periodontitis. Significantly higher means of PD and AL levels were observed in the cases group than in the control group (p < 0.001). However, the mean levels of 25(OH)D were higher in the control group than in the cases group (p = 0.037). The characteristics of the study sample are summarized and described in Table 1.
Table 1.
Characteristics of the study sample.
| Variable | Controls (n = 63) (mean ± SD) or n (%) | Cases (n = 60) (mean ± SD) or n (%) | Mean Difference | p value |
|---|---|---|---|---|
| Age (years) | 38.05 ± 8.20 | 42.07 ± 6.54 | 4.02 | 0.004 |
| Sex (males/females) | 31 (49.2%) / 32 (50.8%) | 26 (43.3%)/ 34 (56.7%) | – | – |
| AL* | 3.45 ± 0.24 | 5.06 ± 1.14 | 1.6 | <0.001 |
| PD** | 3.85 ± 0.31 | 5.05 ± 0.53 | 1.2 | <0.001 |
| BMI (kg/m2)*** | 30.12 ± 4.53 | 30.29 ± 5.23 | 1.7 | 0.846 |
| Serum 25(OH)D (ng/ml) | 29.19 ± 12.82 | 25.03 ± 8.55 | 4.2 | 0.037 |
AL = Attachment Loss Level, **PD = Probing Depth,***BMI = Body Mass Index.
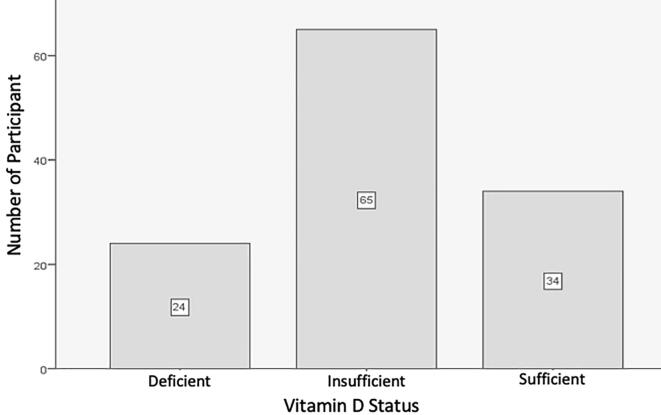
A normal distribution of vitamin D levels was observed in both the cases and control groups. Additionally, the majority of participants had either deficient or insufficient levels of vitamin D (n = 24, 19.51%, and n = 65, 52.85%, respectively). Fig. 1 describes the participants’ vitamin D status. However, not only was the proportion of participants with sufficient 25(OH)D levels drastically greater in the controls than the periodontitis subjects, but also, importantly, deficient and insufficient levels of 25(OH)D were significantly more common in the periodontitis patients than the controls (p = 0.029).
Fig. 1.
Vitamin D status of the study participants.
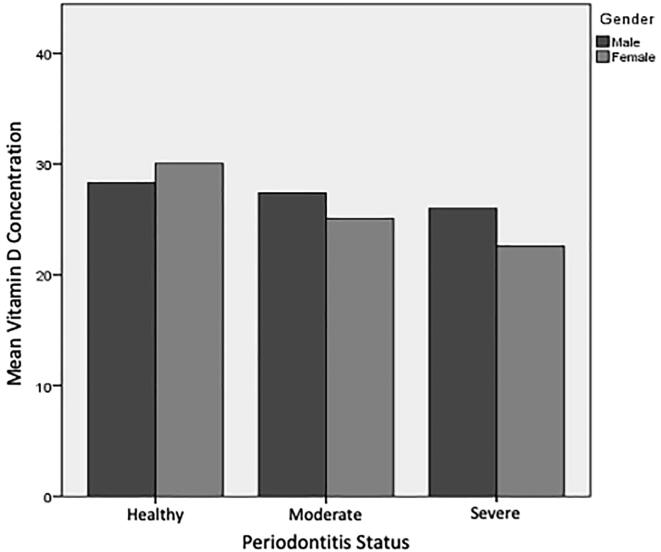
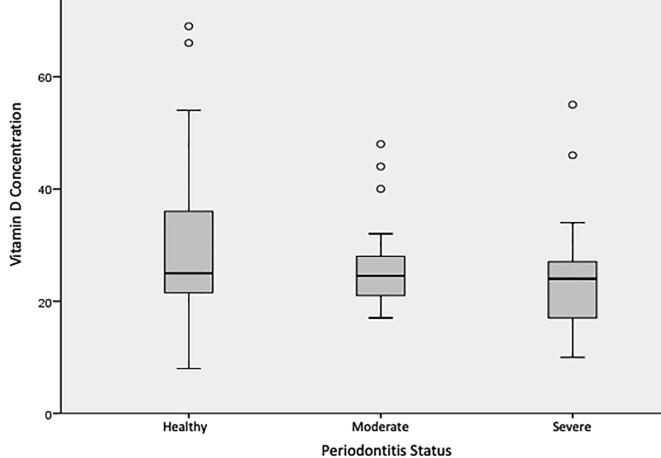
The findings of the current study revealed that for every unit rise in 25(OH)D levels, the odds of both severe and moderate periodontitis declined significantly by 3.6% (OR 0.964; 95% CI 0.931–0.999; p = 0.043). No significant association was found concerning vitamin D concentration in relation to periodontal status between males and females (see Fig. 2). Fig. 3 shows the association between periodontitis status and 25(OH)D levels of all participants, while Table 2 illustrates a comparison of periodontal status and 25(OH)D levels between periodontitis patients and controls. It can be concluded that there is a significant association between periodontitis and lower levels of 25(OH)D among Saudi adults in the Albaha region. Furthermore, the age, gender, and BMI failed to show a significant association with vitamin D status and periodontitis status.
Fig. 2.
Association of participants’ gender in relation to the mean vitamin D concentration and periodontitis status.
Fig. 3.
Association between vitamin D concentration and periodontitis status of the participants.
Table 2.
A comparison of vitamin D status in relation to periodontal status between cases and control groups.
| Category of Vitamin D Status | Controls n (%) |
Cases n (%) |
p value |
|---|---|---|---|
| Deficient 25(OH)D < 20 ng/ml |
11 (45.8%) | 13 (54.2%) | 0.029 |
| Insufficient 25(OH)D = 20 to 29.9 ng/ml |
28 (43.1%) | 37 (56.9%) | |
| Sufficient 25(OH)D ≥ 30 ng/ml |
24 (70.6%) | 10 (29.4%) |
4. Discussion
One of the key aims of the dental public health approach is to assist with oral health surveillance and community-based disease prevention. However, there is a lack of literature concerning the association between periodontitis and 25(OH)D levels among adults in Saudi Arabia, particularly those living in the Albaha region. Both periodontitis and deficient and insufficient 25(OH)D levels are not only community health challenges but also commonly prevalent among Saudi adults (Nazir, 2017, Thomas et al., 2020, Bahammam, 2015, Kaddam et al., 2017, Al-Ajlan et al., 2018, Darraj et al., 2019, Al-Daghri, 2018). Combining the vitamin D status and the periodontitis, might enable a broader approach to prevention and management of dental diseases. No studies in the literature have directly examined the association between periodontitis and vitamin D among Saudi adults. While the majority of the Saudi periodontal research has focused on exploring periodontal diseases among individuals in high-risk groups, such as individuals with diabetes or obesity (Bahammam, 2015, Thomas et al., 2020). This underscores the importance of this study to explore the association between periodontitis and vitamin D status, especially given the high prevalence rates of periodontitis amongst the Saudi population (Nazir, 2017, Thomas et al., 2020). Consequently, the present study aimed to examine the association between periodontitis and vitamin D status among Saudi adults in the Albaha region.
The findings of this study revealed that not only were lower serum levels of 25(OH)D significantly more prevalent in the cases group compared with the control group (p = 0.037), but also, for every unit of 25(OH)D serum level increase, the odds of both severe and moderate periodontitis declined significantly by 3.6% (OR 0.964; 95% CI 0.931–0.999; p = 0.043). This is consistent with another study conducted in Puerto Rico, where lower 25(OH)D levels were strongly associated with periodontitis and for every unit of 25(OH)D serum level increase, the occurrence of severe and moderate periodontitis decreased dramatically by 12% (OR 0.885; 95% CI 0.785, 0.997; p < 0.05) (Abreu et al., 2016). Our results are also similar to findings from India and Austria, where low levels of vitamin D were significantly associated with periodontitis (Bhargava et al., 2019, Laky et al., 2017). By contrast, no association was found between periodontitis severity and 25(OH)D levels among Finnish adults (Antonoglou et al., 2015). However, the overall lower 25(OH)D levels among the Finnish population was the main contributor to this lack of association.
The results of this study pointed out significant associations between periodontitis severity (AL and PD) and levels of 25(OH)D (p = <0.001) (see Table 1). Likewise, an association between periodontitis severity and 25(OH)D levels was observed among Puerto Rican adults (Abreu et al., 2016). However, Finnish adults were shown to have no association between the severity of their periodontitis (AL and PD) and 25(OH)D levels (Antonoglou et al., 2015). This lack of association might be attributed to variations in the population characteristics, including the population’s genetic profile, smoking, and the overall levels of 25(OH)D (Deng et al., 2011, Lee et al., 2015).
Evidence has demonstrated that elevated plasma vitamin D-binding protein levels are associated with severe periodontitis (Zhang et al., 2013). There is also no robust evidence to indicate either that vitamin D may prevent the development of periodontitis or that this association is causative in nature (Millen and Pavlesen, 2020, Jagelavičienė et al., 2018). However, improving vitamin D deficiency through supplementation or diet may have an enormous and valuable impact on reducing the prevalence of periodontal disease. Vitamin D supplementation as a prevention strategy may be an effective, inexpensive, and safe way to control the prevalence of periodontal diseases (Bhargava et al., 2019). Nonetheless, future clinical long-term research with a follow-up design exploring the role of vitamin D supplementation in relation to protecting periodontal tissues from inflammatory collapse may be indicated. The feasibility of prescribing vitamin D supplementation, its optimal dosage, and its impact on preventing periodontitis may also be investigated.
Population’s genetic background can be a major reason for a discrepancy in the association between vitamin D deficiency and periodontitis. The severe or chronic periodontitis observed among family members with different generation is an indicator of the genetic background effect on the predisposition of periodontal disease (Newman et al., 2011, Jilani et al., 2015). Indeed, the susceptibility of moderate and severe periodontitis has associated with several polymorphisms in Vitamin D receptor (VDR) gene that regulates the biological function of vitamin D and the expression of many other genes. Noticeably, the association of these polymorphisms in VDR gene with moderate and severe periodontitis has been found in several ethnic group such as British, Libyans, and Taiwanese populations(Jilani et al., 2015, Ho et al., 2017, De Brito Junior et al., 2004, Brett et al., 2005); yet this has not been the case amongst the Turkish population (Gunes et al., 2008).
The biological mechanism of linking 25(OH)D level with periodontitis has placed on the fact that deficiency in the 25(OH)D level contributes to progression of periodontitis, osteoporosis, reduced bone density, and causes occurrence of jawbone’s resorption (Bhargava et al., 2019). On the other hand, sufficiency of 25(OH)D level could not only reduce the risk of periodontitis; but also enhances wound healing afterward surgical periodontal care, escalates antibacterial resistance of epithelial cells in gingiva, and reduces inflammation of the gingiva (Jagelavičienė et al., 2018). Nevertheless, in order to attain the best periodontal therapy outcomes, future research may be suggested to explore and examine required concentration of the 25(OH)D level in plasma before initiating periodontal therapy.
The present study did have some limitations. First, there was a limited sample size due to the case control design, and second, the study did not include some factors associated with blood levels of 25(OH)D, such as the participants’ physical activity levels and socioeconomic status. However, the recruitment of all subjects was conducted from one outpatient setting, thus ensuring a homogeneity of sampling in terms of socioeconomic status. Finally, measures for evaluating the oral hygiene of the participants, such as plaque index, may be another omitted confounder in this study. However, AL and PD were measured for all participants. While this was a case-control study, and while it involved an inadequate sample size, it still offers insight into the association between periodontitis and 25(OH)D levels among Saudi adults in the Albaha region.
5. Conclusions
Lower levels of 25(OH)D are significantly associated with periodontitis among Saudi adults. The severity of periodontitis, including AL and PL, was also significantly associated with levels of 25(OH)D among the participants. To confirm and validate the findings of this study, a larger study with long term follow up over the country focussing on screening periodontitis patients for lower levels of 25(OH)D seems to be needed. Moreover, exploring further determinants of periodontal disease and the practicability of prescribing vitamin D supplementation, its optimal dosage, and its impacts on preventing or reducing periodontitis may also be necessary. A further study on the genetic polymorphisms in VDR genes and other related genes in relation to the risk of moderate and severe periodontitis among the Saudi population is highly recommended.
6. Availability of Data and Materials
The data supporting the findings of the article is available upon request form the main author Dr Alzahrani, email: aahalzahrani@bu.edu.sa.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the Deanship of Scientific Research at Albaha University in Saudi Arabia for funding and helping to facilitate this study during the academic year 2019/2020 (Grant number: 1439/11).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abdullah Ali H. Alzahrani, Email: aahalzahrani@bu.edu.sa.
Raed A. Alharbi, Email: ralharbi@bu.edu.sa.
Mohammed Sarhan A. Alzahrani, Email: m.sarhan@bu.edu.sa.
Ghalia Shamlan, Email: shamlana@ksu.edu.sa.
Mohammad A. Albanghali, Email: mohammad.aref@bu.edu.sa.
Abdulmajeed Abdulghani A. Sindi, Email: asindi@bu.edu.sa.
References
- Abreu O.J., Tatakis D.N., Elias-Boneta A.R. Low vitamin D status strongly associated with periodontitis in Puerto Rican adults. BMC Oral Health. 2016;16(1):89–93. doi: 10.1186/s12903-016-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ajlan A., Al-Musharaf S., Fouda M.A. Lower vitamin D levels in Saudi pregnant women are associated with higher risk of developing GDM. BMC Pregnancy Childbirth. 2018;18(1):86–92. doi: 10.1186/s12884-018-1723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alyani H., Al-Turki H.A., Al-Essa O.N. Vitamin D deficiency in Saudi Arabians: A reality or simply hype: A Meta-Analysis (2008–2015) J. Family Community Med. 2018;25(1):1–4. doi: 10.4103/jfcm.JFCM_73_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Daghri N.M. Vitamin D in Saudi Arabia: prevalence, distribution and disease associations. J. Steroid Biochem. Mol. Biol. 2018;175(1):102–107. doi: 10.1016/j.jsbmb.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Antonoglou G., Knuuttila M., Niemelä O. Low serum level of 1, 25 (Oh) 2d is associated with chronic periodontitis. J. Periodontal Res. 2015;50(2):274–280. doi: 10.1111/jre.12207. [DOI] [PubMed] [Google Scholar]
- Bahammam M.A. Periodontal health and diabetes awareness among Saudi diabetes patients. Patient Prefer Adherence. 2015;9(1):225–233. doi: 10.2147/PPA.S79543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A., Rastogi P., Lal N. Relationship between Vitamin D and chronic periodontitis. J. Oral Biol. Craniofac. Res. 2019;9(2):177–179. doi: 10.1016/j.jobcr.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett P., Zygogianni P., Griffiths G. Functional gene polymorphisms in aggressive and chronic periodontitis. J. Dent Res. 2005;84(12):1149–1153. doi: 10.1177/154405910508401211. [DOI] [PubMed] [Google Scholar]
- Darling A.L., Blackbourn D.J., Ahmadi K.R. Very high prevalence of 25-Hydroxyvitamin D deficiency in 6433 Uk South Asian Adults: Analysis of the Uk Biobank Cohort. Br. J. Nutr. 2020;125(4):448–459. doi: 10.1017/S0007114520002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darraj H., Badedi M., Poore K.R. Vitamin D deficiency and glycemic control among patients with Type 2 diabetes mellitus in Jazan City, Saudi Arabia. Diabetes Metab. Syndr. Obes. 2019;12(1):853–862. doi: 10.2147/DMSO.S203700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito Junior R.B., Scarel-Caminaga R.M., Trevilatto P.C. Polymorphisms in the Vitamin D receptor gene are associated with periodontal disease. J. Periodontol. 2004;75(8):1090–1095. doi: 10.1902/jop.2004.75.8.1090. [DOI] [PubMed] [Google Scholar]
- Deng H., Liu F., Pan Y. Bsmi, Taqi, Apai, and Foki polymorphisms in the Vitamin D receptor gene and periodontitis: A meta-analysis of 15 studies including 1338 cases and 1302 controls. J. Clin. Periodontol. 2011;38(3):199–207. doi: 10.1111/j.1600-051X.2010.01685.x. [DOI] [PubMed] [Google Scholar]
- Eke P.I., Dye B., Wei L. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Ferreira M.C., Dias-Pereira A.C., Branco-De-Almeida L.S. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal Res. 2017;52(4):651–665. doi: 10.1111/jre.12436. [DOI] [PubMed] [Google Scholar]
- Genco, R.J., Borgnakke, W.S., 2020. Diabetes as a Potential Risk for Periodontitis: Association Studies. Periodontology 2000. 83(1), 40-45. [DOI] [PubMed]
- Gunes S., Sumer A.P., Keles G.C. Analysis of Vitamin D receptor gene polymorphisms in patients with chronic periodontitis. Indian J. Med. Res. 2008;127(1):58–64. [PubMed] [Google Scholar]
- Ho Y.-P., Lin Y.-C., Yang Y.-H. Association of Vitamin D receptor gene polymorphisms and periodontitis in a Taiwanese Han population. J. Dent Sci. 2017;12(4):360–367. doi: 10.1016/j.jds.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F., Binkley N.C., Bischoff-Ferrari H.A. Evaluation, treatment, and prevention of Vitamin D deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . National Academies Press; 2011. Dietary Reference Intakes for Calcium and Vitamin D. [PubMed] [Google Scholar]
- Jagelavičienė E., Vaitkevičienė I., Šilingaitė D. The Relationship between Vitamin D and Periodontal Pathology. Medicina (Kaunas). 2018;54(3):45–53. doi: 10.3390/medicina54030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilani M., Mohamed A.A., Zeglam H.B. Association between Vitamin D receptor gene polymorphisms and chronic periodontitis among Libyans. Libyan J. Med. 2015;10(1):1–7. doi: 10.3402/ljm.v10.26771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddam I.M., Al-Shaikh A.M., Abaalkhail B.A. Prevalence of Vitamin D deficiency and its associated factors in three regions of Saudi Arabia. Saudi Med J. 2017;38(4):381–390. doi: 10.15537/smj.2017.4.18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laky M., Bertl K., Haririan H. Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clin. Oral. Invest. 2017;21(5):1553–1558. doi: 10.1007/s00784-016-1965-2. [DOI] [PubMed] [Google Scholar]
- Lang N.P., Suvan J.E., Tonetti M.S. Risk factor assessment tools for the prevention of periodontitis progression a systematic review. J. Clin. Periodontol. 2015;42(S16):S59–S70. doi: 10.1111/jcpe.12350. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Je D.I., Won S.J. Association between Vitamin D deficiency and periodontal status in current smokers. Community Dent. Oral Epidemiol. 2015;43(5):471–478. doi: 10.1111/cdoe.12173. [DOI] [PubMed] [Google Scholar]
- Liu X., Baylin A., Levy P.D. Vitamin D Deficiency and Insufficiency among Us Adults: Prevalence, Predictors and Clinical Implications. Br J Nutr. 2018;119(8):928–936. doi: 10.1017/S0007114518000491. [DOI] [PubMed] [Google Scholar]
- Machado V., Lobo S., Proença L. Vitamin D and periodontitis: A systematic review and meta-analysis. Nutrients. 2020;12(8):1–18. doi: 10.3390/nu12082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen A.E., Pavlesen S. Could vitamin D influence risk for periodontal disease—to “D” or Not to “D”? Curr. Oral Health Rep. 2020;7(1):98–111. doi: 10.1007/s40496-020-00253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaca G., Tonacci A., Negrini S. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun. Rev. 2019;18(9) doi: 10.1016/j.autrev.2019.102350. [DOI] [PubMed] [Google Scholar]
- Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- Newman, M.G., Takei, H., Klokkevold, P.R., et al. 2011. Carranza's Clinical Periodontology, Elsevier health sciences.
- Page R.C., Eke P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007;78(7):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- Palacios C., Gonzalez L. Is Vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014;144(1):138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(94):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Pinto J., Goergen J., Muniz F. Vitamin D levels and risk for periodontal disease: A systematic review. J. Periodontal Res. 2018;53(3):298–305. doi: 10.1111/jre.12531. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lozano B., González-Febles J., Garnier-Rodríguez J.L. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: A case-control study. Arthritis Res. Ther. 2019;21(1):27–38. doi: 10.1186/s13075-019-1808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.T., Thomas T., Ahmed M. Prevalence of periodontal disease among obese young adult population in Saudi Arabia—a cross-sectional study. Medicina. 2020;56(4):197–202. doi: 10.3390/medicina56040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2013. World Health Organization, Oral health surveys: basic methods. WHO Library Cataloguing-in-Publication Data.
- Zhang X., Meng H., Sun X. Elevation of Vitamin D-binding protein levels in the plasma of patients with generalized aggressive periodontitis. J. Periodontal Res. 2013;48(1):74–79. doi: 10.1111/j.1600-0765.2012.01505.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the article is available upon request form the main author Dr Alzahrani, email: aahalzahrani@bu.edu.sa.