Abstract
Reproductive drugs that include contraceptive and fertility drugs are used to manage reproductive health in both humans and animals. Contraceptive drugs are mainly used by humans for reversible contraception whereas fertility drugs are mainly used in animals to increase milk production, poultry products and meat production. Usage of these drugs has increased manifold in the last decade. These drugs are excreted through body fluids (mainly urine and milk) that lead to contamination of surface water, milk and animal produce. Consumption of such contaminated products or water results in reproductive disorders and different types of cancers in humans. This questionnaire-based study was designed and conducted involving gynecologists, pharmacies, medical stores and veterinarians in Patiala city and its adjoining areas in India to evaluate the quantitative and qualitative aspects of use of these drugs. A total of 150 survey points were identified with random sampling method. Data was analyzed using appropriate statistical tools. The results showed that contraceptive drugs constitute 86% of reproductive drugs usage in humans. Further, steroidal contraceptives constitute a huge 94.7% share of contraceptive drugs, and of these combined oral contraceptives have 79.79% share among which a combination is ethinylestradiol and levonorgestrel is the most popular (20.92%). The consumption of COCs is higher than that of progestin only pills (Z = 3.39) as well as estrogen only pills (Z = 4.30). In contrast, usage of non-hormonal fertility drugs (89%) dominates over the hormonal class (11%) in humans. The most widely used non-hormonal fertility drug is clomiphene citrate (73.87%). In animals, the prescription rate of hormonal fertility drugs is higher (83%) than the non-hormonal one, where in the most widely prescribed drug is buserelin acetate. These findings are in consonance with the similar studies carried out in US, Europe and Canada which suggest that reproductive drugs usage pattern is more or less similar across the globe. A careful control to discourage indiscriminate use of such drugs is the need of hour to prevent damage of environment and ultimately to the health of living beings.
Keywords: Reproductive drugs, Contraceptives, Fertility drugs, Reproductive health, Prevalence, Environment
1. Introduction
The global pharmaceuticals market ($934.8 billion in 2017) is growing with compound annual growth rate (CAGR) of 5.8%, and is estimated to reach $1170 billion in 2021. The Indian Pharmaceutical sector, which accounts for 3.1–3.6 percent of the global pharmaceutical market is expected to grow to $100 billion by 2025. The global human reproductive technologies market, which was $42.1 billion in 2015, is expected to reach $64 billion by the year 2025, and Indian reproductive technologies market is growing with CAGR about 16% contributing a significant chunk to national drug market. These data and statistics suggest that use of reproductive drugs (fertility and contraceptive) is increasing nationally as well as globally.
The use of contraceptive drugs at national level can be dated back to the year 1952, when family planning programme was launched in India. Under the influence of this program, 187 million unintended pregnancies are prevented each year (Elkalmi et al., 2015). Ministry of Health and Family Welfare (MoHFW) of Government of India has taken some initiatives to control fertility rate from 2.28 to 2.1 by 2025. Now, oral contraceptives is the most common means to control fertility rate because it does not involve any surgical intervention, and it is a reversible method of contraception. Market of contraceptive pills in India is growing exponentially due to strong economic growth, increased awareness and literacy rate, increasing number of nuclear families, increased professional commitments of the working couples and increase in availability of their “over the counter” (OTC) products.
On the contrary, infertility is becoming one of the major worldwide problem and affecting 50 and 80 million couples (Singh et al., 2017). According to a study by Ernst and Young conducted in 2015, 27.5 million couples were suffering from infertility in India. According to World Health Organisation (WHO), one in every four couples is infertile in developing countries. Various factors impelling rise in infertility include cultural shift involving decrease in marriage rate, delayed marriages (30–40 years), a shift from rural to urban societies; profession pressure induced delayed conception during advanced maternal age (35 years or older); life style changes like use of excessive protein diet and steroids by gym lovers, increasing trend towards fast food and stress induced hormonal imbalance; pathological reasons such as obesity; and hazardous habits such as smoking and alcohol consumption.
Livestock sector is one of the growing industries and contributes 4.6% in total gross domestic product. About 20.5 million people in India depend upon livestock for their livelihood. In order to get maximum returns from livestock, fertility drugs are used in dairy farms to increase milk production, in poultry to increase egg and meat production, and in fisheries to increase growth rate of fishes. Due to additive effects of increasing infertility among humans and intended fertility in livestock, the market of fertility drugs is also growing at a CARG of 4.36%. Therefore, there is significant increase in usage of both contraceptive and fertility drugs. Different types of reproductive drugs that are used among humans and animals are summarised in Table 1.
Table 1.
Different types of reproductive drugs.
| Contraceptive drugs | |
|---|---|
| Steroidal drugs | Non-steroidal drugs |
| A. Combined Oral Contraceptives (COCs): Cyproterone acetate and ethinylestradiol, Desogestrel and ethinylestradiol, Norgestrel and ethinylestradiol, Levonorgestrel and ethinylestradiol, Drospirenone and ethinylestradiol, Norethindrone and ethinylestradiol, Gestodene and ethinylestradiol | Selective Estrogen Receptor Modulators: Ormeloxifene (Centchroman) |
| B. Progestin Only Pills (POPs): Levonorgestrel, Norethisterone, Desogestrel, Medroxyprogesterone acetate | |
| C. Estrogen Only Pills: Estradiol | |
| Fertility drugs | |
| Steroidal drugs | Non-steroidal drugs |
| Testosterone, Progesterone, Human Chorionic Gonadotropin, Follicle Stimulating Hormone, Gonadotropin-releasing Hormone | Letrozole, Clomiphene citrate |
The reproductive drugs are excreted through urine and faeces as hydroxylated and methylated metabolites that may be conjugated with glucuronides and sulphates (Lai et al., 2000, Junior et al., 2010, Brito et al., 2010) and enter to municipal sewage. These drugs are not completely removed by sewage treatment plant (Zheng et al., 2008), leading to contamination of surface water as well as groundwater. Surface water is also contaminated by such drugs from excreta of fishes and farm animals (Casey et al., 2003, Hanselman et al., 2003, Kjaer et al., 2007). High content of estrogens is also found in poultry manure (Hanselman et al., 2003). Use of poultry manure and animal waste as fertilizer on agricultural land may represent an important source and sink of these drugs in the environment. Dairy milk may contain hormones, progesterone, estrogens, IGF-1 growth hormone, cortisone and other adrenal steroids, oxytocin and prolactin (Ganmaa et al., 2001, Malekinejad and Rezabakhsh, 2015). Therefore, the rampant misuse of these reproductive drugs in humans, farm animals, poultry and fisheries is responsible for pollution of surface water, groundwater and agriculture produce, meat, cattle and breast milk. Consumption of such polluted water, milk, agricultural, meat and poultry products are responsible for a number of reproductive disorders like penial and testicular weakness, alternations in steroli cell numbers, changes in levels of male reproductive hormones and permanently malformated penis among males (Atanassova et al., 1999, Fisher et al., 1999, Williams et al., 2001, Goyal et al., 2007, Mathews et al., 2009), alterations in pelvis, anococcygeus muscles and polycystic ovary among females (Lguchi et al., 1995, Sotomayor-Zarate et al., 2008), and increased risk of different types of cancers in both males and females (Outwater et al., 1997, Ganmaa and Sato, 2005). In addition to these, the adverse effects of these drugs on fishes include feminization of male fishes (Rouhani Raunkouhi et al., 2004, Tyler et al., 2005, Moura Costa et al., 2010). These data warrant a dire need to assess the impact of this invisible pollution due to these drugs through scientifically designed analytical and biological studies. In order to undertake such exhaustive experimental study, an understanding of types of drugs and their extent of usage among humans and animals. Therefore, this study has been aimed to assess the usage rate, widely used and prescribed products of contraceptive and fertility drugs among humans and animals through a market survey.
Patiala is a very important city of south-east Punjab. It is an educational hub accommodating universities, medical, engineering, ayurvedic, basic sciences and humanities colleges, numerous schools and uncountable academic and professional coaching centers. The city also houses head offices of all major state run government organization such as Punjab Pollution Control Board, Punjab Public Service Commission, Punjab State Power Corporation Limited. It is also an important healthcare stop for people of this region of Punjab.
Exhaustive review of literature on use of reproductive drugs from an environmentalist viewpoint has revealed that only limited studies are conducted in India regarding usage rate of reproductive drugs especially in Punjab, though various studies on awareness and perception regarding different contraception method are reported. According to Census 2011, population of this area is 406,192 having proportion of 215,617 males and 190,575 females. This area has significant literacy rate (76%) as well as employment rate (Pushkarna, 2017). The population is mainly urban and their increasing demand for dairy and poultry products is met by numerous dairy and poultry farms working in Patiala city as well as in rural area adjoining the city. Therefore, Patiala city and its adjoining areas are an ideal study area to analyze rate of consumption of contraceptive and fertility drugs in this region of Punjab. This study can contribute immensely in assessment of pollution caused by these drugs and provide reliable data to department of public welfare and pharmaceutical professionals in order to ensure welfare of society as a whole.
2. Material and methods
The primary data was collected through paper based survey using structured questionnaire having 6 questions both open-ended and closed-ended. It was designed to capture some of the main aspects related to usage of contraceptive and fertility drugs in Patiala city and its adjoining areas. Closed ended questions offering possibility to choose option were related to availability of drugs either for human or veterinary purposes at target places i.e. medical stores/hospitals. The open part was appraised as a valuable part of survey as it contributes to evaluate most widely used and most commonly prescribed contraceptive and fertility products.
A total of 150 survey points that included pharmacies, medical stores, physicians, gynecologists and veterinarians were identified with random sampling method. Questionnaires were provided at each survey point, and clarifications were made where ever required. The collated data was analyzed by using appropriate statistical tools.
Data was sorted in Excel spreadsheet. Z test was performed to test the mean differences in usage of reproductive drugs.
3. Results
Out of the total 150 questionnaires, 111 were completed representing a total response rate of 73.33%. Majority of participants in this study were pharmacy store professionals (80.18%) and the other were physicians (19.82%). The participants, who completed the response, are referred to as respondents hereon. The responses have shown that both hormonal and non-hormonal reproductive drugs are used among humans (Fig. 1), whereas only fertility drugs in animals (Fig. 2). In humans, the usage of contraceptive drugs was significantly higher than that of fertility drugs (Z = 3.81) (Fig. 1). A large majority of contraceptive drugs in use are found belonging to steroidal class of drugs (94.70%). In contrast, non-hormonal drugs dominate (89%) over the hormonal drugs (Z = 2.56) among the fertility drugs in use. Contrary to the higher usage of non-hormonal fertility drugs in humans, the prescription rate of hormonal drugs (83%) was much higher than the non-hormonal ones (17%) in animals (Fig. 2). The increased usage of contraceptive drugs over fertility drugs among humans can be attributed to their desire to avoid pregnancies. The factors contributing to these desires include, though not limited to, increasing professional commitments or ambitions, increasing literacy rate and awareness, and increasing number of nuclear families. On the other hand, no respondent indicated the usage of contraceptive drugs in animals. The intended use of only fertility drugs in animals can be ascribed to the need of increasing milk production among dairy animals and increasing progeny growth in poultry and fisheries for maximizing monetary benefits.
Fig. 1.
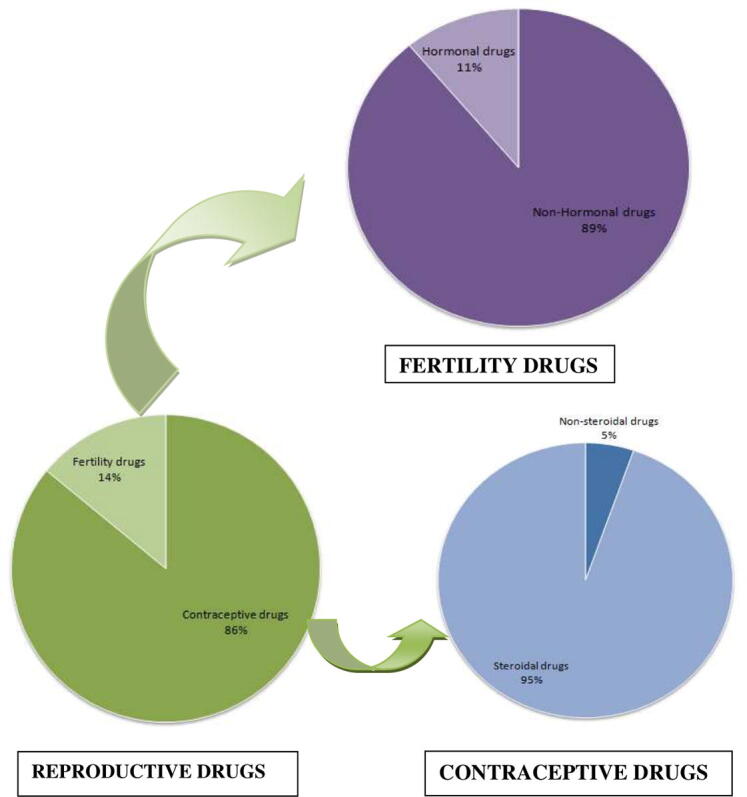
Usage pattern of reproductive drugs in humans.
Fig. 2.
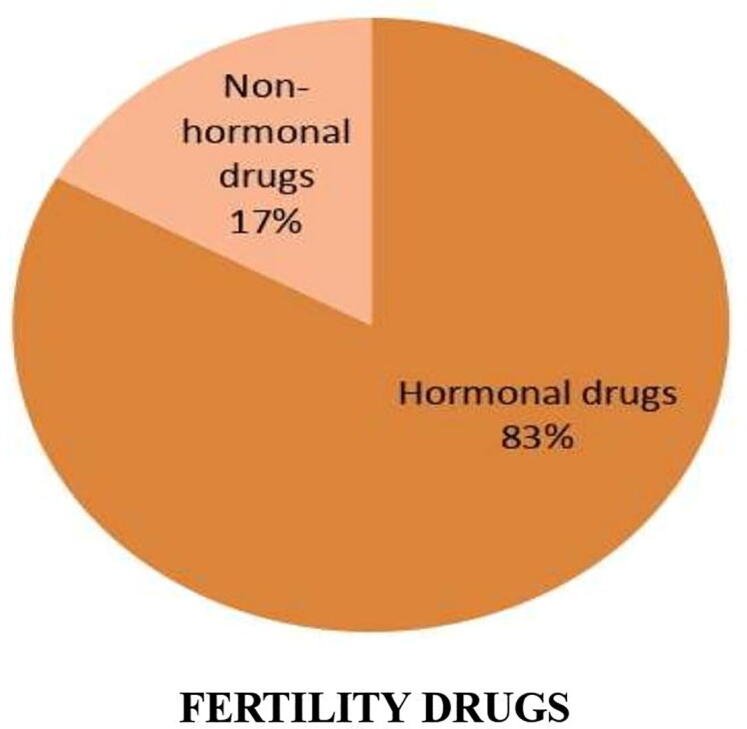
Usage of fertility drugs in animals.
3.1. Brand- wise usage of reproductive drugs in humans
3.1.1. Contraceptive products
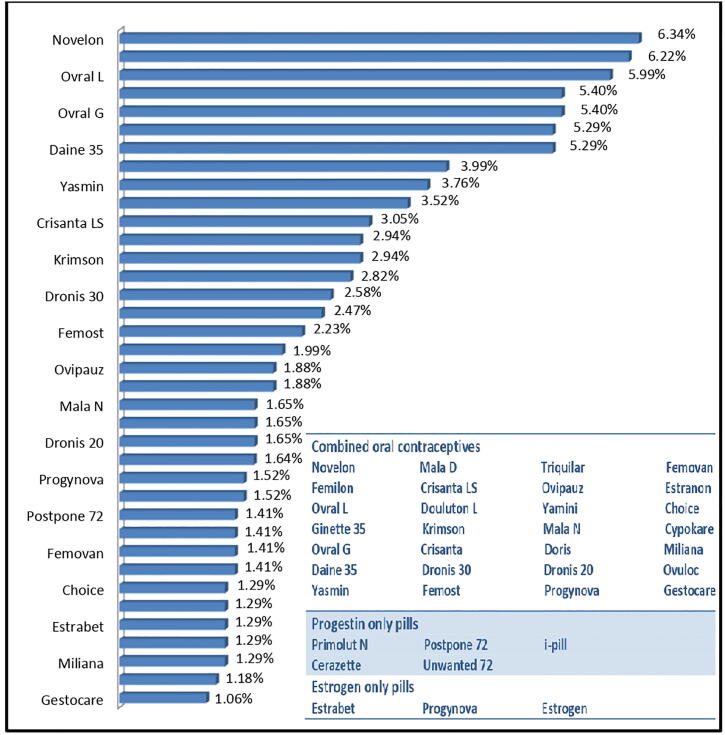
During survey, it was found that 37 brands of contraceptive drugs are available in the study area. The relative availability of these 37 brands, expressed as percent prevalence is shown in Fig. 3. This percent prevalence of different brands linearly correlated with their sale volume. The data has revealed that 28 brands belonged to COCs. Novelon, Femilon, Ovral L, Ginette 35, Ovral G, Saheli and Daine 35 are the most widely used contraceptives (prevalence ˃5%). These brands except, Saheli, belong to the COCs category, and their composition are given in Table 2. Saheli is the lone non-steroidal contraceptive among the list of 37 brands. It was first developed by Central Drug Research Institute (CDRI), Lucknow in 1967 (Kamboj et al., 2018) and approved for marketing in 1991. The Government of India included it in its National Family Planning Programme under the trade name “Chhaya” with effect from April 2016. Mala N is another COCs product, which is available only at government hospitals and community health centres, and is provided free of cost for use to the needy by the Government of India. The first COC pill named 'Envoid' came into market in 1960. In the year 1970, it has been reported that continuous use of COCs increases the risk of gall bladder disease (National Research Council Committee, 1989). Thereafter, more studies continue to pour in the literature revealing more side effects of COCs such as risk of venous thrombosis, arterial thrombosis and several types of cancers i.e. ovarian cancer, endometrial cancer and colorectal cancer (Sitruk-Ware et al., 2013, Brynhildsen, 2014). Food and drug Administration (FDA) has reported 6 deaths and 20 cases of thromoembolism .
Fig. 3.
Percent prevalence of brands of oral contraceptive pills.
Table 2.
Top seven brands of contraceptives in use.
| Brand | Composition |
|---|---|
| Novelon | Ethinylestradiol and desogestrel |
| Femilon | Ethinylestradiol and desogestrel |
| Ovral L | Ethinylestradiol and levonorgestrel |
| Ginette 35 | Ethinylestradiol and cyproterone acetate |
| Ovral G | Ethinylestradiol and norgestrel |
| Saheli | Ormeloxifene |
| Daine 35 | Ethinylestradiol and cyproterone acetate |
Progestin only pills came into existence in 1973. Progesterones used for contraception are structurally related to testosterone (such as desogestrel, levonorgestrel, gestodene, norethisterone, norgestrel), progesterone (cyproterone acetate) and spirolactones (drospirenone) (Sitruk-Ware and Anita Nath, 2010, Brynhildsen, 2014). The pill containing levonorgestrel is used for emergency contraception. In India, emergency contraceptive pill (ECP) was first introduced by Ministry of Health and Family Welfare (MoHFW) in 2002. To avoid unsafe abortions and unwanted pregnancy, ECP was declared as over the counter (OTC) product in 2005. The present study has found, five brands of testosterone derived POPs in market. The most frequently used brands are i-pill (3.99%), unwanted 72 (2.47%) and postpone 72 (1.41%). i-pill became a pioneer product in market with the help of advertising campaigns. The other factors that raise ECPs market include avoiding medical surgeries during abortion, cheap consumption, availability as OTC product and to avoid awkward visits to gynecologists. But now a days, the advertisements of ECPs is banned in order to reduce their indiscriminate usage and several side-effects among women. The remaining two brands named Primolut N (1.29%) and Cerazette (1.41%) containing norethisterone and desogestrel, respectively. These POPs have their own set of side effects such as incidence of bleeding, negative effect on high density lipoproteins (HDL). Moreover, failure rate of these pills is also more than that of COCs (Sharma et al., 2001, Sitruk-Ware et al., 2013, Harpreet Kaur et al., 2018). The metabolites of COCs and POPs are non-degradable, and contaminate surface water, ground water, soil, agricultural produce etc (Harpreet Kaur et al., 2018).
Recent developments in science have introduced new formulations of oral contraceptives i.e. estrogen only pills with a hope to overcome adverse affects of COCs and POPs on human as well as environment health. During the survey, it was found that estrogen only pills are also in use under three brand names i.e. Estrogen (1.52%), Progynova (1.52%) and Estrabet (1.29%). These estrogen only pills has fewer side effects on human health, and also their metabolites have shorter life time and are biodegradable in nature (Wu et al., 2019).
Among oral contraceptives, the most widely used are the one that are derived from combinaion ethinylestradiol with levonorgestrel (20.91%), drospirenone (17.39%), cyperoterone acetate (16.33%), and desogestrel (16.10%) (Table 3). The most popular formulation of progestin only pills is derived from levonorgestrel (7.87%). Levonorgestrel is often used in emergency contraceptive pills. The consumption of COCs is higher than that of progestin only pills (Z = 3.39) as well as of estrogen only pills (Z = 4.30), whereas the consumption of progestin only pills is not statistically different from estrogen only pills (Table 4).
Table 3.
Percentage distribution of oral contraceptive drug usage.
| Combined oral contraceptives | Percentage (%) | 95% confidence interval |
|
|---|---|---|---|
| from | to | ||
| Ethinylestradiol/levonorgestrel | 20.92 | 18.17 | 23.64 |
| Ethinylestradiol/drospirenone | 17.39 | 14.8 | 19.91 |
| Ethinylestradiol/cyproterone acetate | 16.33 | 13.84 | 18.81 |
| Ethinylestradiol/desogestrel | 16.10 | 13.6 | 18.4 |
| Ethinylestradiol/norgestrel | 5.41 | 3.89 | 6.92 |
| Ethinylestradiol/norethisterone | 2.23 | 1.23 | 3.22 |
| Ethinylestradiol/gestodene | 1.41 | 0.61 | 2.20 |
| Progestin only Pills | |||
| Levonorgestrel | 7.87 | 6.06 | 9.67 |
| Desogestrel | 1.41 | 0.61 | 2.20 |
| Norethisterone | 1.29 | 0.53 | 2.04 |
| Estrogen only Pills | |||
| Estradiol | 4.35 | 2.97 | 5.72 |
| Selective Estrogen Receptor Modulators | |||
| Ormeloxifene | 5.29 | 3.78 | 6.79 |
Table 4.
Comparison of consumption of different hormonal formulation of contraceptives.
| Comparison between Groups | Mean values | Standard deviation | Calculated Z value |
|---|---|---|---|
| Combined oral contraceptives | 24.25 | 14.39 | 3.39* |
| Progestin only pills | 14 | 4.06 | |
| Progestin only pills | 14 | 4.06 | 0.792 |
| Estrogen only pills | 12.33 | 0.984 | |
| Combined oral contraceptives | 24.25 | 14.39 | 4.30* |
| Estrogen only pills | 12.33 | 0.984 |
shows significant difference, Level of significance- 0.05.
3.1.2. Fertility products
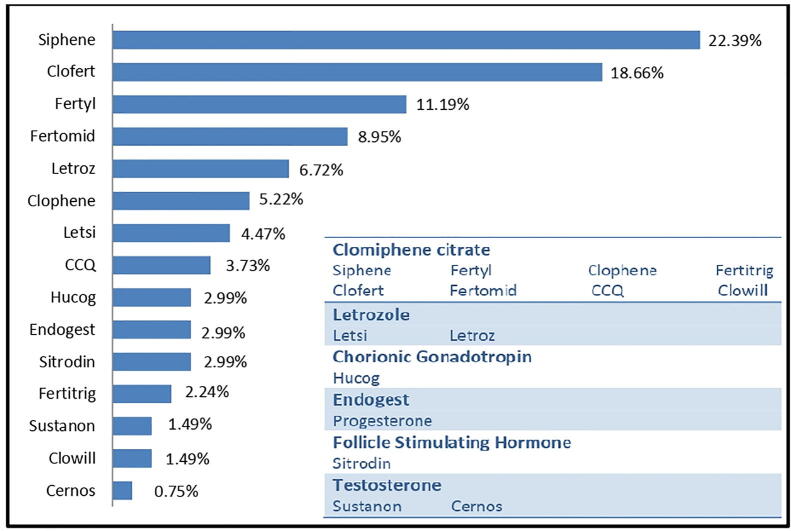
The fertility products, prescribed by gynecologists, belong to both hormonal and non-hormonal types under different brand names. Out of total 14 brands, 10 brands belong to non-hormonal, and the other to the hormonal type. Eight of the 10 non-hormonal type products are derived from clomiphene citrate, and the other are derived from letrozole. As shown in Fig. 4, four brands of clomiphene citrate formulations account for more than 60% of market share of non-hormonal type fertility pills. Siphene is the most prescribed brand (22.39% share) followed by Clofert (18.66%), Fertyl (11.19%), Fertomid (8.95%). Futher, during the survey it is found that clomiphene citrate is available in three different dosages i.e. 25, 50 and 100 mg, and amongst these clomiphene citrate 50 mg is prescribed maximally (35.82%) followed by clomiphene citrate 100 mg (26.87%) and Clomiphene citrate 25 mg (14.93%) (Table 5). Clomiphene citrate is the most prescribed drug, in concordance with studies conducted at Owaisi hospital and research centre, Hyderabad, India (Siddiqui et al., 2017) and Basaveshar hospital, Karnataka, India reported 58% and 12.76% patients received clomiphene citrate to treat fertility revealed problems (Anjanappa et al., 2015) respectively. However, there are several adverse effects reported to be associated with the use of clomiphene such as ovarian enlargement, thrombocytopenia, malignant melanoma, hepatic damage and multiple pregnancies. Letrozole, which is originally developed as an anticancer drug has emerged as an alternative to clomiphene. It is used as ovulation inducer under two brand names i.e., Letsi and Letroz, and has fewer side effects, low risk of multiple pregnancy and higher pregnancy rate in patients with polycystic ovary syndrome (PCOs) . Letrozole is currently following the clomiphene citrate closely follows clomiphene citrate 25 mg market very closely. Testosterone is the least prescribed drug, except in specific cases, to reduce androgen abuse in athletes and weightlifting communities.
Fig. 4.
Percent prevalence of brands of fertility pills (non-hormonal type).
Table 5.
Percentage distribution of fertility drugs usage.
| Fertility pills | Percentage (%) | 95% confidence interval |
|
|---|---|---|---|
| from | to | ||
| Clomiphene citrate 50 mg | 35.82 | 27.70 | 43.93 |
| Clomiphene citrate 100 mg | 26.87 | 19.36 | 34.37 |
| Clomiphene citrate 25 mg | 14.93 | 8.89 | 20.96 |
| Letrozole | 11.19 | 5.33 | 16.52 |
| Human Chorionic Gonadotropin | 2.985 | 0.53 | 5.43 |
| Follicle Stimulating Hormone | 2.985 | 0.53 | 5.43 |
| Progesterone | 2.985 | 0.53 | 5.43 |
| Testosterone | 2.24 | 0 | 4.73 |
3.2. Drugs used in animals
3.2.1. Brands of fertility drugs
Fertility drugs are used in animals in order to increase farm business profitability. There are 34 brands of fertility drugs that are mostly prescribed by Veterinarian.
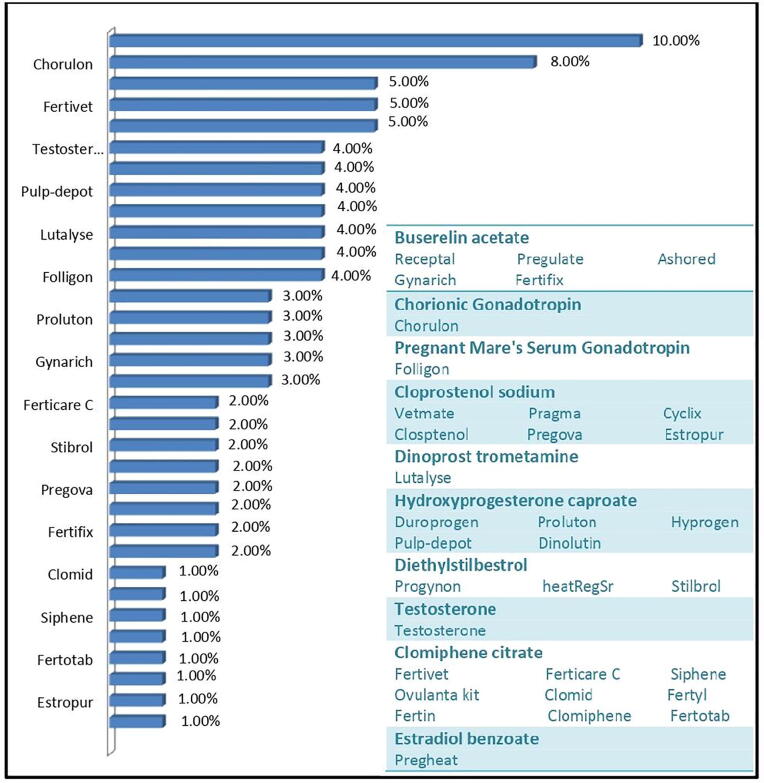
The highest number of brands belong to clomiphene citrate (9) followed by cloprostenol sodium (6), buserelin acetate (6), hydroxyprogesterone caproate (5), and diethylstilbestrol (3) (Fig. 5). Receptal and chorulon, both buserelin acetate derived products, contribute a major chunk (18%) of the veterinary fertility pills market.
Fig. 5.
Percent prevalence of brands of fertility pills (Hormonal type).
Buserelin acetate is the most widely prescribed drug (18%) for increasing fertility rate in animals. It is closely followed by cloprostenol sodium (17%), Clomiphene citrate (17%) and hydroprogesterone caproate (14%) (Table 6).
Table 6.
Percentage distribution of fertility drugs.
| Fertility pills | Percentage (%) | 95% confidence interval |
|
|---|---|---|---|
| from | to | ||
| Buserelin acetate | 18 | 10.46 | 25.53 |
| Clomiphene citrate | 17 | 9.63 | 24.36 |
| Cloprostenol Sodium | 17 | 9.63 | 24.36 |
| Hydroprogesterone Caproate | 14 | 7.19 | 20.8 |
| Diethylstilbestrol | 9 | 3.39 | 14.6 |
| Human Chorionic Gonadotropin | 8 | 2.68 | 13.3 |
| Estradiol benzoate | 5 | 0.72 | 9.27 |
| Pregnant Mare's Serum Gonadotropin | 4 | 0.15 | 7.84 |
| Testosterone | 4 | 0.15 | 7.84 |
| Dinoprost Tromethamine | 4 | 0.15 | 7.84 |
4. Discussion
The findings in the present study reveal that oral contraceptives are commonly adopted method for reversible contraception (86%) in humans. Despite several side effects, COCs are most prevalently used oral contraceptives (79.79%). These findings are found to be similar to those reported in other studies carried out in USA, Europe and Canada (Table 7). Even the drug combinations found most prevalent in this study are also reported similarly in the other countries (Table 7). These comparisons suggest that usage of OCs, and COCs in particular, is similarly across the globe. Usage of COCs to such a prevalent extent exposes the users to increased health risks. Moreover, the metabolites of COCs are excreted through urine and faeces, which lead to environmental contamination. Therefore, there is a need to control the extensive use of OCs. The various approaches to achieve this aim may be (i) sensitizing people regarding usage, advantages and disadvantages of oral contraceptives through counseling forums, seminars and media and (ii) promoting use of non-COC products have fewer side effects to human health and their metabolites have shorter life time and biodegradable in nature (Harpreet Kaur et al., 2018).
Table 7.
Comparative analysis of the findings on usage of OCs and COCs among humans in the present study and the similar studies carried out in US, Canada and Europe.
| Present study (India) | Reported studies |
|---|---|
| Usage prevalence of OCs is 86% | In US, 73% women are OCs users (Hall et al., 2012) In Europe and America, 98% women prefer OCs (Elkalmi et al., 2015) |
| COCs are maximally used OCs (79.78%) | In Canada, 99% users take COCs (Rotermann et al., 2015) |
| Ethinylestradiol and levonorgestrel combination is the most widely used COC (20.19%) | Ethinylestradiol and levonorgestrel combination is most commonly used COC in Europe (Brynhildsen, 2014) as well as in Canada (28.5%) (Rotermann et al., 2015) |
| Usage of ethinylestradiol in combination with desogestrel is 16.10%, and that with drospirenone is 17.39%. | In US, most widely used combination is of ethinylestradiol with norgestimate (27.2%) or drospirenone (Brynhildsen, 2014) In Canada, usage prevalence of ethinylestradiol with desogestrel is 17%, and that with drosperidone is 16% (Rotermann et al., 2015) |
The most prevantly used drugs for treatment of infertility in humans are non-hormonal ones (89%). It is because the hormonal therapy requires series of injection, intensive monitoring and has also some serious side effects like ovarian hyperstimulation syndrome. Clomiphene citrate is commonly used non-hormonal product (73.87%). Clomiphene and letrozole constitute > 88% share of non-hormonal fertility drugs, and out of these clomiphene alone accounts for > 73% share. It is despite of clomiphene having several side effects in comparison to latrozole, which is more effective and safer than clomiphene (Atay et al., 2006; Harira, 2018).
Fertility drugs are extensively used, or rather misused in animals to increase productivity of meat and milk. Further, Diethylstilbestrol, which is a synthetic estrogen, has been banned in United States (US) by Food and Drug Administration (FDA) since 1979 because it causes different types of reproductive disorder and cancers in present as well as in future generation (Raun et al., 2001, Harpreet Kaur et al., 2018). But it is still used in India as growth promoter in animals. It is excreted in milk as such, and consumption of such milk poses a health threat to the users. Hence, there is a dire need for the government to take strict action against misuse of such hormonal drugs in poultry as well as dairy farms, in order to reduce incidents of cancer, prematurity, reproductive disorders. In addition of this measure, Ministry of Health and Family Welfare should make amendments in Drugs and Cosmetics Act and Food Safety and Standards Authority of India (FSSAI) rules to limit the dosage and levels of these reproductive drugs in dairy and poultry farms as well as their products so that the misuse and pollution by these drugs can be curtailed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by Taif University Researches Supporting Project number (TURSP-2020/222), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Anjanappa G., Patil V.B., Vardhamane S., Jeevangi S., Kanaki A., Basavambika A. A prospective study of prescribing pattern of drugs in female infertility cases with pharmacoeconomics at a tertiary care center in India. Asian J. Pharmaceut. Health Sci. 2015;5(2):1242–1245. [Google Scholar]
- Atanassova N., Mckinnell C., Walker M., Turner K.J., Fisher J.S., Morley M., Millar M.R., Groome N.P., Sharpe R.M. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999;140(11):5364–5373. doi: 10.1210/endo.140.11.7108. [DOI] [PubMed] [Google Scholar]
- Brito D.M., Porto R.M., Vidal M.T.C., Ojeda R.S., Perez A.R. Excretion Study of Clomiphene in Human Urine. Evaluation of Endogenous Steroids Profile after Multiple Oral Doses. J. Braz. Chem. Soc. 2010;21(12):2220–2225. [Google Scholar]
- Brynhildsen J. Combined hormonal contraceptives: prescribing patterns, compliance, and benefits versus risks. Therap. Adv. Drug Saf. 2014;5(5):201–213. doi: 10.1177/2042098614548857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey F.X.M., Larsen G.L., Hakk H., Simunek J. Fate and Transport of 17β-Estradiol in Soil-Water Systems. Environ. Sci. Technol. 2003;37:2400–2409. doi: 10.1021/es026153z. [DOI] [PubMed] [Google Scholar]
- Elkalmi R.M., Khan M.U., Ahmad A., Srikanth A.B., Abdurhaman N.S., Jamshed S.Q., Awad A.I., Ab Hadi H.B. Knowledge, awareness, and perception of contraception among senior pharmacy students in Malaysia: A pilot study. J. Res. Pharm Pract. 2015;4(2):94–98. doi: 10.4103/2279-042X.155760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J.S., Turner K.J., Brown D., Sharpe R.M. Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environ. Health Perspect. 1999;107(5):397–405. doi: 10.1289/ehp.99107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganmaa D., Sato A. The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers. Med Hypotheses. 2005;65:1028–1037. doi: 10.1016/j.mehy.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Ganmaa D., Wang P.Y., Qin L.Q., Hoshi K., Sato A. Is milk responsible for male reproductive disorders? Med. Hypotheses. 2001;57(4):510–514. doi: 10.1054/mehy.2001.1380. [DOI] [PubMed] [Google Scholar]
- Goyal H.O., Braden T.D., Williams C.S., Williams J.W. Role of estrogen in induction of penile dysmorphogenesis: a review. Reprod. 2007;134:199–208. doi: 10.1530/REP-07-0207. [DOI] [PubMed] [Google Scholar]
- Hall K.S., Trussell J., Schwarz E.B. Progestin-only contraceptive pill use among women in the United States. Contraception. 2012;86(6):653–658. doi: 10.1016/j.contraception.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanselman T.A., Graetz D.A., Wilkie A.C. Manure-borne estrogens as potential environmental contaminants: a review. Environ. Sci. Technol. 2003;37(24):5471–5478. doi: 10.1021/es034410+. [DOI] [PubMed] [Google Scholar]
- Harira M. Use of Letrozole versus clomiphene-estradiol for treating infertile women with unexplained infertility not responding well to clomiphene alone, comparative study. Middle East Fertility Soc. J. 2018;23(4):384–387. [Google Scholar]
- Harpreet Kaur H., Bala M., Bansal G. Reproductive drugs and environmental contamination: quantum, impact assessment and control strategies. Environ. Sci. Pollut. Res. 2018 doi: 10.1007/s11356-018-2754-z. [DOI] [PubMed] [Google Scholar]
- Junior E.A., Luciana Fernandes Duarte L.F., Pirasol Vanunci M.L., Teixeira M.L. Bioequivalence of Two Oral Contraceptive Drugs Containing Ethinylestradiol and Gestodene in Healthy Female Volunteers. J. Bioequival. Bioavail. 2010;2(6):125–130. [Google Scholar]
- Kamboj V.P., Ray S., Anand N. Centchroman: A safe reversible postcoital contraceptive with curative and prophylactic activity in many disorders. Front. Biosci. 2018;10:1–14. doi: 10.2741/e807. [DOI] [PubMed] [Google Scholar]
- Kjaer J., Olsen P., Bach K., Barlebo H.C., Ingerslev F., Hansen H., Sorensen B.H. Leaching of estrogenic hormones from manure treated structured soils. Environ. Sci. Technol. 2007;41:3911–3917. doi: 10.1021/es0627747. [DOI] [PubMed] [Google Scholar]
- Lai K.M., Johnson K.L., Scrimshaw M.D., Lester J.N. Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environ. Sci. Technol. 2000;34:3890–3894. [Google Scholar]
- Lguchi T., Fukazawa Y., Howard A., Bern H.A. Effects of sex hormones on oncogene expression in the vagina and on development of sexual dimorphism of the pelvis and anococcygeus muscle in the mouse. Environ. Health Perspect. 1995;103:79–82. doi: 10.1289/ehp.95103s779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekinejad H., Rezabakhsh A. Hormones in Dairy Foods and Their Impact on Public Health- A Narrative Review Article. Iran J Public Health. 2015;44(6):742–758. [PMC free article] [PubMed] [Google Scholar]
- Mathews E., Braden T.D., Williams C.S., Williams J.W., Bolden-Tiller O., Goyal H.O. Mal-Development of the penis and loss of fertility in male rats treated neonatally with female contraceptive 17a-Ethinyl Estradiol: A Dose-Response study and a comparative study with a known estrogenic teratogen Diethylstilbestrol. Toxicol. Sci. 2009;112(2):331–343. doi: 10.1093/toxsci/kfp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura Costa D.D., Filipak Neto F., Costa M.D.M., Morais R.N., Garcia J.R.E., Esquivel B.M., Oliveira Ribeiro C.A. Vitellogenesis and other physiological responses induced by 17-β-estradiol in males of freshwater fish Rhamdia quelen. Comp. Biochem. Physiol C. 2010;151:248–257. doi: 10.1016/j.cbpc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee on Population, 1989. Parnell AM, editor. Contraceptive Use and Controlled Fertility: Health Issues for Women and Children Background Papers. National Academies Press (US), Washington (DC). Health Effects of Contraception. [PubMed]
- Outwater J.L., Nicholson A., Barnard N. Dairy products and breast cancer: the IGF-1, estrogens, bGH hypothesis. Med Hypothesis. 1997;48:453–461. doi: 10.1016/s0306-9877(97)90110-9. [DOI] [PubMed] [Google Scholar]
- Pushkarna M. Literacy Patterns in Punjab: Rural- Urban Differentials. J. Human. Soc. Sci. 2017;22(7):32–39. [Google Scholar]
- Raun A.P., Preston R.L. History of diethylstilbestrol use in cattle. Am. Soc. Anim. Sci. 2001;1–7 [Google Scholar]
- Rotermann M., Dunn S., Black A. Oral contraceptive use among women aged 15 to 49: Results from the Canadian Health Measures Survey. Health Rep. 2015;26(10):21–28. [PubMed] [Google Scholar]
- Rouhani Raunkouhi T., Sanderson J.T., van Holsteijn I., van Leeuwen C., Vethaak A.D., van den Berg M. Effects of natural and synthetic estrogens and various environmental contaminants on vitellogenesis in fish primary hepatocytes: Comparison of Bream (Abramis brama) and Carp (Cyprinus carpio) Toxicol. Sci. 2004;81:90–102. doi: 10.1093/toxsci/kfh176. [DOI] [PubMed] [Google Scholar]
- Sharma R.S., Rajalakshmi M., Antony Jeyaraj D. Current status of fertility control methods in India. J. Biosci. 2001;26(4):391–405. doi: 10.1007/BF02704741. [DOI] [PubMed] [Google Scholar]
- Siddiqui S., Tahniyath F., Butool A., Fatima K., Reddy R., Rozati R. A retrospective study on prescribing pattern of drugs in female infertility at a tertiary care centre in South India. Indo Am. J. Pharmaceut. Res. 2017;7(3):7861–7868. [Google Scholar]
- Singh K., Kumari R., Ranjan A., Bharti G. Analysis of causes and clinical pattern of infertility in couples coming to a tertiary care centre in Bihar, India. Int. J. Reprod. Contrac. Obstetr. Gynecol. 2017;6(6):2279–2283. [Google Scholar]
- Sitruk-Ware R., Anita Nath A. The use of newer progestins for contraception. Contraception. 2010;82:410–417. doi: 10.1016/j.contraception.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R., Anita Nath A., Mishell D.R., Jr Contraception technology: past, present and future. Contraception. 2013;87(3):319–330. doi: 10.1016/j.contraception.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor-Zarate R., Dorfman M., Paredes A., Lara H.E. Neonatal exposure to estradiol valerate programs ovarian sympathetic innvervation and follicular development in adult rat. Bio Reprod. 2008;78:673–680. doi: 10.1095/biolreprod.107.063974. [DOI] [PubMed] [Google Scholar]
- Tyler C.R., Spary C., Gibson R., Santos E.M., Shears J., Hill E.M. Accounting for differences in estrogenic responses in Rainbow trout (Oncorhynchus mykiss: Salmonidae) and Roach (Rutilus rutilus: Cyprinidae) exposed to effluents from wastewater treatment works. Environ. Sci. Techn. 2005;39:2599–2607. doi: 10.1021/es0488939. [DOI] [PubMed] [Google Scholar]
- Williams K., McKinnell C., Saunders P.T.K., Walker M., Fisher J.S., Turner K.J., Atanassova N., Sharpe R.M. Neonatal exposure to potent and environmental oestrogens and abnormalities of male reproductive system in the rat: Evidence for importance of androgen-oestrogen balance and assessment of the relevence to man. Hum Reprod. Update. 2001;7(3):236–247. doi: 10.1093/humupd/7.3.236. [DOI] [PubMed] [Google Scholar]
- Wu K., Lee T., Chen Y., Wang Y., Wang P., Yu C., Chu K., Chiang Y. Metabolites Involved in Aerobic Degradation of the A and B Rings of Estrogen. Appl. Environ. Microbiol. 2019;85(3):1–15. doi: 10.1128/AEM.02223-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Yates S.R., Bradford S.A. Analysis of steroid hormones in a typical dairy waste disposal system. Environ. Sci. Technol. 2008;42:530–535. doi: 10.1021/es071896b. [DOI] [PubMed] [Google Scholar]