Abstract
Screening can decrease the burden of breast, cervical, and colorectal cancers. The COVID-19 pandemic led many countries to suspend cancer screening services as part of their response to the pandemic. The International Cancer Screening Network (ICSN) carried out an online survey to assess the effects of the first wave of the COVID-19 pandemic on cancer screening. A 33-item survey was distributed to 834 email addresses to gather information about settings and assess decision-making processes that led to cancer screening suspension. Information about communication, impact on resources, and patient follow-up was collected. Quantitative data was analyzed as frequencies overall and by setting, while a comment section under each survey item captured nuanced details. Responses were recategorized into 66 settings, representing 35 countries. Most settings suspended cancer screening services (n = 60, 90.9%) in March 2020 (n = 45, 68.2%), guided by a government decision (n = 51, 77.3%). Few settings made the decision whether to suspend services based on a preparedness plan (n = 17, 25.8%). In most settings, professionals were reassigned (n = 41, 62.1%) and infrastructure repurposed (n = 35, 53.0%). The first wave of the COVID-19 pandemic has had profound effects on cancer screening worldwide, including the suspension of services in almost all settings. Most settings were unprepared to deal with the scale of the pandemic but demonstrated flexibility in the response. These results contribute to inform, through experiences and lessons learned, the next steps for the global cancer screening community to further evaluate the impact of COVID-19 and prepare for future disruptions.
Keywords: Cancer screening, COVID-19, Health care services, Disruption
1. Background
The rise of COVID-19 to a pandemic level in March 2020 brought immediate challenges to cancer screening services. The International Cancer Screening Network (ICSN), a consortium of cancer screening practitioners (including researchers, evaluators, implementers, policy makers, and program staff) received early reports about disruptions to cancer screening services as governments and health authorities in many countries mandated the suspension of non-emergency medical procedures. Cancer screening services are an essential component of the cancer control continuum, with established evidence of reductions in the burden of breast, cervical, and colorectal cancers when these services are adequately implemented. In Europe, following the decades-long experience in cancer screening of some countries, recommendations to set up breast, cervical and colorectal cancer screening programs through an organized, population-based approach with appropriate quality assurance at all levels were progressively implemented across the region after 2003 (Arbyn et al., 2010; European Colorectal Cancer Screening Guidelines Working Group et al., 2013; Perry et al., 2008; von Karsa et al., 2008). In the United States, the U.S. Preventive Services Task Force (Siu, 2016; Preventive Services Task Force et al., 2016; Preventive Services Task Force et al., 2018) put forward evidence-based recommendations, which along with guidelines from organizations such as the American Cancer Society (Smith et al., 2019), support screening for breast, cervical, and colorectal cancer as effective means for cancer prevention and early detection.
Previous experiences with major disruptions, such as armed conflicts or the Ebola outbreak in 2014, have demonstrated that important health gains could be lost in a short period of time (Delamou et al., 2017; El Saghir, Soto Pérez de Celis, and Fares, 2018; Kieny, Evans, Schmets, and Kadandale, 2014). Both high-income countries (HICs) and low- and middle-income countries (LMICs) are susceptible to these disruptions and losses, and the negative effects of the COVID-19 pandemic on availability of resources for cancer control and research, as they are diverted to the emergency response, may exacerbate these common challenges (DeBoer, Fadelu, Shulman, and Van Loon, 2020). For example, concerns have been expressed about the disruption of programs on HPV vaccination and cervical cancer screening, which have made great strides in the last decade (Arbyn, Bruni, Kelly, et al., 2020; Rahman, Gultekin, and Lassi, 2020). Public health leaders have called for countries to maintain sufficient resources to sustain cervical cancer screening, as one of the main targets of the WHO Global Strategy for Cervical Cancer Elimination launched in November 2020 (Canfell et al., 2020; WHO, 2020a).
The immediate effects of the first wave of the COVID-19 pandemic, which affected most countries between February and July 2020 (Carroll et al., 2020; Salyer et al., 2021), on cancer screening services are still largely unexplored. To address this, the ICSN leadership conducted a survey within its network to understand how settings represented by its members were responding to the pandemic. The purpose of the survey was to capture the details and consequences of decisions to suspend cancer screening services and understand the effects of the first wave of the COVID-19 pandemic on cancer screening worldwide. The experiences and lessons learned could inform the evaluation of COVID-19 impact on cancer screening and help planning for future pandemics.
2. Methods
Between April and early May 2020, the ICSN Steering Committee developed a survey instrument drawing on the expertise of its 18 members. The instrument was developed in English only, and covered diverse aspects of cancer screening services, from delivery to program management and resource allocation. Survey items were formulated and pilot tested for clarity and completeness within the Steering Committee, leading to further refinement.
The study proposal was submitted for review by the research ethics committee of the Radboud university medical center, Nijmegen, The Netherlands. The committee granted a waiver to this study, as it did not fall within the remit of the Medical Research Involving Human Subjects Act in the Netherlands.
2.1. Survey instrument
The final survey instrument included 33 items, divided in two parts. The first part captured general characteristics, such as respondent contact information, setting description, and details about the screening services provided. The second part focused on the effects of the first wave of COVID-19 on the setting, gathering information about the status and process of decision-making regarding cancer screening services, communication with clients and patients, health professionals and other stakeholders (i.e., non-governmental organizations, cancer charities, advocacy groups), and impact on resources and patient follow-up. Most questions were open-ended, with comment boxes allowing the respondents to elaborate on their answers and provide as much nuanced information as possible. Several questions allowed more than one response, anticipating, for example, that some respondents could answer for more than one cancer program. When respondents selected multiple options, they were encouraged to explain their selection in the comment section. In addition, respondents were invited to share documents, reports or websites describing how decisions were made in their settings or any guidance regarding the suspension and resumption of cancer screening services. An online survey was created through the SurveyMonkey® platform (https://www.surveymonkey.com/) and the full questionnaire is available in Appendix 1.
2.2. Sample and survey implementation
The survey was distributed to 834 unduplicated email addresses from 69 countries included in the ICSN contact list formed by ICSN meeting attendees, individuals who participated in ICSN working groups or otherwise requested subscription to the list. Through this sample, we attempted to capture information from professionals working in different areas of cancer screening, including research, implementation, service delivery, management and quality assurance, opening it to a wide variety of perspectives about how the pandemic affected screening services. The survey remained open between 12 May and 12 July 2020, with reminders sent out at the second and sixth weeks.
2.3. Data analysis
During preliminary analysis of the responses, we observed that certain settings were represented by more than one respondent. A setting was defined as a unit of analysis that could represent a region, a program, a facility, a research project, or an expert group. For example, six individuals responded for the Dutch national cancer screening program, and two individuals responded for the Parkland Health and Hospital System in Dallas, Texas, United States. Considering the complexity and variety of cancer screening systems around the world and that screening services sometimes are organized and delivered differently within the same country, two researchers (D.M.P.P., M.J.M.B) independently mapped the individual sets of responses to defined settings and compiled the individual-level responses into a new unique set of responses when appropriate. A third researcher (K.M.E.) assessed the final list of settings and resolved any discrepancies between the two mappings. Differences in individual-level responses among two or more respondents for the same setting were resolved by combining all selected options in the new unique set if more than one option was allowed for a given question. However, for discrepancies in the responses that only allowed one response option, the survey respondents' comments were used to decide the most logical response. About one-third of the 17 settings with more than one respondent across 10 dichotomous questions required resolving individual-level differences based on their comments.
We generated frequency distributions for each survey item. For questions that allowed for more than one response option, the proportions add to more than 100%. Furthermore, the survey items and results were grouped based on relevant themes identified from a literature review of the interruption of cancer screening services (Puricelli Perin et al., 2021) in previous disasters (Table 1 ). In this review, information from the selected studies were thematically analyzed following six stages, i.e., familiarization with the data, coding, developing themes, reviewing themes, defining and naming themes, and final analysis (Braun and Clarke, 2006). Four main thematic categories - coordination, communication, resource availability and patient follow-up – were recognized by all co-authors as key for the organization and delivery of cancer screening services. Then, during the survey analysis, results were organized under those four thematic categories. Moreover, we used excerpts of the responses provided in the comment section under each survey item to further elaborate on these results, highlighting and clarifying some of our findings. Shared documents were read in their completeness, and their main points summarized for the purposes of this analysis.
Table 1.
Thematic categories (Puricelli Perin et al., 2021) which the ICSN COVID-19 survey responses were grouped under.
| Themes | Purpose | Total number of survey questions |
|---|---|---|
| Coordination | Describe the process through which the decision whether to suspend cancer screening was made at different levels of decision-making (e.g., geographical, organizational) and the plans for resuming services. | 7 |
| Communication | Understand the means by which the decision whether to suspend cancer screening was disseminated within and across the health system. | 4 |
| Resource availability | Assess how the allocation of personnel and infrastructure to the COVID-19 response affected the delivery of cancer screening services. | 3 |
| Patient follow-up | Assess the outcome of the decision whether to suspend cancer screening at patient-level. | 1 |
3. Results
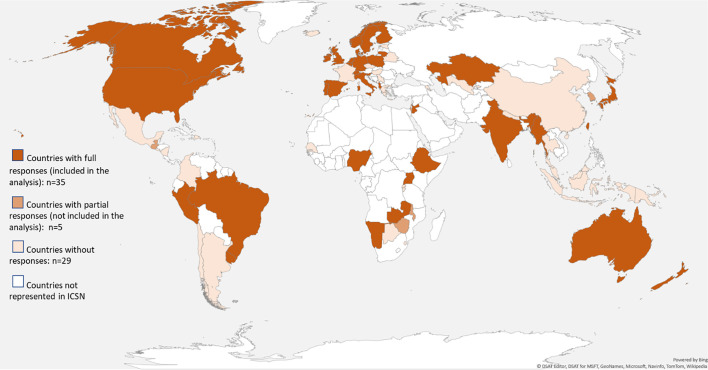
Of the 113 participants from 40 countries who responded, 98 from 35 countries completed the full questionnaire (12% individual-level and 51% country-level response rates). The 98 responses were further categorized into 66 unique settings (Fig. 1 ), and the remaining 15 partial responses were not included in this analysis as they did not provide information about the status of cancer screening services in their setting.
Fig. 1.
Countries reached in the ICSN COVID-19 survey.
3.1. Setting characteristics
Fifty-four of 66 settings (81.8%) reported organized cancer screening programs (Miles, Cockburn, Smith, and Wardle, 2004), defined as including all the standard components of organized screening (i.e., invitation, delivery management, screening registration, and evaluation). In addition, 19 settings (28.8%) reported opportunistic cancer screening (with no centralized invitation/outreach, delivery, or evaluation), 9 settings reported (13.6%) case finding (detection that occurs in the course of daily clinical care in response to signs or symptoms of cancer), and 12 settings reported (18.2%) pilot research projects (research that is being performed to understand the feasibility or effectiveness of screening in a setting) (Table 2 ).
Table 2.
General characteristics of the screening services provided among the identified settings and effects of the first wave of the COVID-19 pandemic.
| Settings / n. of respondents |
Type of screening delivery |
Screening decisions |
Cancer sites |
Screening suspended |
Research/pilots stopped |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
O |
Op |
C |
P |
Ot |
O |
L |
R |
N |
Ot |
Cx |
CRC |
B |
L |
Ot |
Y/N |
Y/N/? |
||
| TOTAL (%) | 66 settings / 98 respondents (100%) | 54 (81.8%) | 19 (28.8%) | 9 (13.6%) | 12 (18.2%) | 4 (6.1%) | 33 (50.0%) | 11 (16.7%) | 28 (42.4%) | 34 (51.5%) |
3 (4.5%) | 42 (63.6%) | 39 (59.1%) | 51 (77.3%) | 14 (21.2%) | 7 (10.6%) | Y = 60 (90.9%) | Y = 43 (65.2%) |
| 1 | Albania / 1 | O | N | Cx | Y | Y | ||||||||||||
| 2 | Australia / 3 | O | Op | C | Ot | O | R | N | CRC | N | N | |||||||
| 3 | Belgium - Flanders / 3 | O | O | R | N | Cx | CRC | B | Y | Y | ||||||||
| 4 | Brazil / 4 | Op | L | R | N | Cx | B | Y | Y | |||||||||
| 5 | Canada – Ontario / 3 | O | Op | C | P | O | L | R | N | Cx | CRC | B | L | Ot | Y | Y | ||
| 6 | Canada – Alberta / 1 | O | Op | R | N | Cx | CRC | B | Y | Y | ||||||||
| 7 | Denmark / 3 | O | R | N | Cx | CRC | B | N | N | |||||||||
| 8 | Denmark – Copenhagen / 1 | O | C | P | O | L | R | N | B | N | N | |||||||
| 9 | Ethiopia – Oromia / 1 | O | Op | C | P | O | L | R | N | Cx | Y | ? | ||||||
| 10 | Ethiopia – Addis Ababa /1 | O | Op | P | N | Cx | Y | Y | ||||||||||
| 11 | Finland / 1 | O | L | Cx | B | Y | N | |||||||||||
| 12 | Germany / 1 | O | N | B | Y | Y | ||||||||||||
| 13 | India – Tamil Nadu / 1 | O | Op | O | Cx | B | Ot | Y | Y | |||||||||
| 14 | Ireland / 3 | O | O | N | Cx | CRC | B | Y | Y | |||||||||
| 15 | Italy – Veneto / 1 | O | R | Cx | CRC | B | Y | Y | ||||||||||
| 16 | Italy – Piedmont / 3 | O | R | Cx | CRC | B | Y | Y | ||||||||||
| 17 | Italy – ONS / 1 | O | N | Cx | CRC | B | Y | Y | ||||||||||
| 18 | Italy – Lombardy / 1 | O | R | Cx | CRC | B | Y | Y | ||||||||||
| 19 | Italy – Tuscany / 1 | O | R | Cx | CRC | B | Y | Y | ||||||||||
| 20 | Japan / 2 | O | Op | R | Cx | CRC | B | L | Ot | Y | Y | |||||||
| 21 | Jordan / 1 | O | Op | O | L | R | N | B | Y | ? | ||||||||
| 22 | Kazakhstan / 1 | O | N | Cx | CRC | B | Y | Y | ||||||||||
| 23 | Lithuania / 1 | Ot | N | Ot | Y | ? | ||||||||||||
| 24 | Myanmar / 1 | Op | C | N | Cx | B | Y | Y | ||||||||||
| 25 | Namibia / 1 | O | N | Cx | B | Y | ? | |||||||||||
| 26 | Netherlands / 6 | O | N | Cx | CRC | B | Y | Y | ||||||||||
| 27 | New Zealand / 2 | O | O | R | N | Cx | CRC | B | Y | N | ||||||||
| 28 | Nigeria - Gombe / 1 | Op | O | Cx | B | Ot | Y | Y | ||||||||||
| 29 | Norway / 2 | O | O | L | R | N | B | Y | Y | |||||||||
| 30 | Peru / 1 | O | O | N | Cx | CRC | B | Y | Y | |||||||||
| 31 | Poland / 1 | O | Op | O | N | CRC | Y | Y | ||||||||||
| 32 | Portugal / 1 | O | R | B | Y | Y | ||||||||||||
| 33 | Slovenia / 4 | O | N | Cx | CRC | B | Y | Y | ||||||||||
| 34 | Spain – Catalonia / 3 | O | O | L | R | Cx | CRC | B | Y | Y | ||||||||
| 35 | Spain – Navarra / 1 | O | R | CRC | B | Y | Y | |||||||||||
| 36 | Spain – Valencia / 2 | O | R | CRC | B | Y | N | |||||||||||
| 37 | Spain – Basque Country / 1 | O | R | CRC | Y | Y | ||||||||||||
| 38 | Sweden / 1 | O | R | Cx | B | Y | N | |||||||||||
| 39 | Switzerland – Graubünden / 1 | O | N | CRC | Y | N | ||||||||||||
| 40 | Switzerland – Vaud / 1 | O | R | CRC | B | Y | ? | |||||||||||
| 41 | Switzerland – Valais / 1 | O | R | B | Y | ? | ||||||||||||
| 42 | Switzerland – Fribourg / 1 | O | O | R | CRC | B | Y | Y | ||||||||||
| 43 | Switzerland – Geneva / 1 | O | O | L | N | CRC | B | Y | N | |||||||||
| 44 | Switzerland – Thurgau / 1 | O | O | R | B | Y | N | |||||||||||
| 45 | Taiwan / 2 | O | O | N | Cx | CRC | B | Ot | N | N | ||||||||
| 46 | Uganda / 1 | Op | C | P | O | Y | Y | |||||||||||
| 47 | UK – NHSE targeted lung health check program / 1 | O | N | L | Y | Y | ||||||||||||
| 48 | UK – Liverpool health lung project / 1 | P | Ot | L | Y | Y | ||||||||||||
| 49 | UK – UK National Screening Committee / 1 | O | N | Ot | Cx | CRC | B | Y | Y | |||||||||
| 50 | UK – Yorkshire lung screening trial / 1 | P | L | L | Y | Y | ||||||||||||
| 51 | UK – Scotland / 1 | O | N | Cx | CRC | B | Y | Y | ||||||||||
| 52 | Uruguay / 1 | P | N | Cx | CRC | B | Y | Y | ||||||||||
| 53 | USA – Marshfield clinic health system, WI / 1 | O | Op | O | Cx | CRC | B | L | Y | Y | ||||||||
| 54 | USA – Kaiser Permanente Washington, WA / 1 | O | Op | C | Ot | O | Cx | CRC | B | L | Y | Y | ||||||
| 55 | USA – Parkland health and hospital system, Dallas, TX / 2 | Op | C | P | Ot | O | Cx | CRC | B | L | Ot | Y | Y | |||||
| 56 | USA – New Jersey Cancer education and early detection program, NJ / 1 | O | O | Cx | CRC | B | L | Y | ? | |||||||||
| 57 | USA – Mass general hospital, Boston, MA / 1 | Op | O | Cx | CRC | B | L | Y | Y | |||||||||
| 58 | USA – Nebraska health and human Services, Lincoln, NE / 1 | O | O | L | R | Cx | CRC | B | Y | ? | ||||||||
| 59 | USA – Federally qualified health centers and community clinics, Texas / 1 | O | O | CRC | B | Y | N | |||||||||||
| 60 | USA – University of Kentucky Healthcare, Lexington, KY / 1 | O | P | O | L | Y | Y | |||||||||||
| 61 | USA – Kaiser Permanente, San Francisco Bay Area, CA / 1 | O | O | Cx | CRC | B | L | Y | Y | |||||||||
| 62 | USA – Ohio State University Cancer center, Columbus, OH / 1 | O | O | Cx | CRC | B | L | Y | Y | |||||||||
| 63 | USA – Presbyterian healthcare systems, Albuquerque, NM / 1 | Op | O | B | L | Y | N | |||||||||||
| 64 | USA – NCI moonshot® Cancer cures “accelerated control of cervical Cancer” / 2 | O | Op | P | O | R | N | Ot | Cx | Y | Y | |||||||
| 65 | Zambia – JSI-DISCOVER-health project / 1 | C | O | N | Cx | B | N | |||||||||||
| 66 | Zambia - CIDRZ / 1 | O | P | O | N | Cx | N | N | ||||||||||
Abbreviations: Screening modality: O - Organized screening program, Op - Opportunistic screening, C – Case finding, P – Pilot project, Ot - Other.
Screening decisions: O - Organization/practice; L - Local (city, county, metropolitan area, etc.); R - Regional (state, province, region, etc.); N – National; Ot - Other.
Cancer site: Cx – cervical; CRC – colorectal; B – breast; L - lung; Ot – Other.
Screening suspended/Research/pilots stopped: Y - Yes; N - No;? - I don't know.
Forty-three settings (65.2%) covered more than one cancer screening site, including 12 (18.2%) with two disease sites, 21 (31.8%) with three sites and 10 (15.2%) with four or more sites. Breast cancer was represented in 51 settings (77.3%), cervical cancer in 42 settings (63.6%), colorectal cancer in 39 (59.1%), and lung cancer in 14 (21.2%). Other screening sites included liver cancer (1 setting), stomach cancer (1 setting), oral cancer (2 settings), and prostate cancer (3 settings). Thirty-four settings (51.5%) reported that the general decisions related to cancer screening were made at the national level, 28 (42.4%) at the regional level, 11 (16.7%) at the local level (city, county or metropolitan area), and 33 settings (50.0%) at the level of the organization or practice.
Sixty-five settings (97.0%) reported that the COVID-19 pandemic had an impact on cancer screening services, while cancer screening services were suspended in 60 settings (90.9%) over 31 countries and 43 settings (65.2%) reported that cancer screening research or pilot programs had stopped within their setting. Among the six settings where cancer screening was not suspended, five reported other types of impact. For example, in one setting where fecal immunochemical test kits continued to be mailed, there was a backlog of participants with positive results with delayed access to a colonoscopy due to the suspension of elective procedures. Another setting reported that even though mammography screening continued, there was a reduction in the number of invitations to ensure adequate distancing in the waiting areas and attendance also decreased due to safety concerns.
3.2. Coordination across and beyond the health sector to decide whether to suspend services
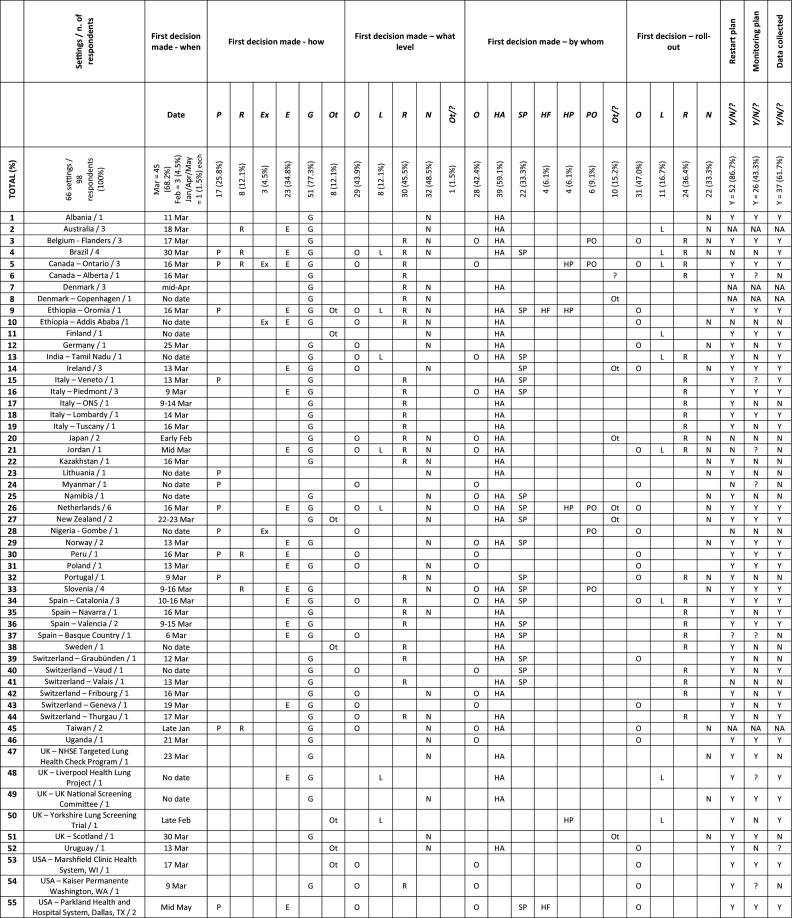
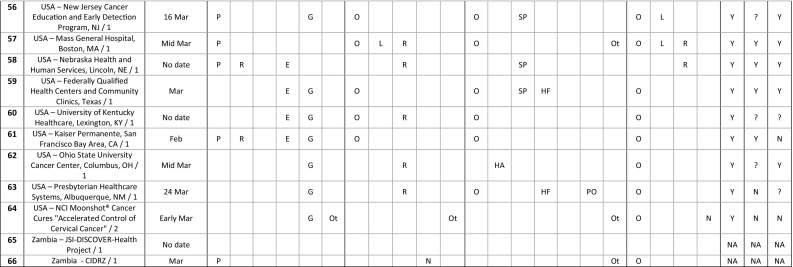
The decision whether to suspend cancer screening services was made in March 2020 in 45 settings (68.2%). Three settings (4.5%) decided in February, one in January, one in April, and one in May, while no date was given for 15 settings (Table 3 ). It was guided by a government decision in 51 settings (77.3%), expert opinion in 23 settings (34.8%), and following a preparedness plan in 17 settings (25.8%). The decision was taken after a systematic review of the literature in 8 settings (12.1%), and in 3 settings (4.5%), it was also based on earlier experience, including an outbreak of Lassa fever and the 2003 SARS epidemic. Some comments illustrate these decision-making processes:
“A guidance document was produced […] to provide recommendations for a systematic approach in determining priority for consultation and treatment of patients with cancer, as well as cancer screening, […] during the time of a pandemic. This guideline was developed through expert consultation and […] recommended that all routine screening be deferred during the COVID-19 pandemic […] Shortly after, [the government] issued a directive that all non-essential and elective healthcare services should be ceased or reduced to minimal levels.”
“[…] government decided centrally after consultation with professionals and suspended non-emergency care including cancer screening.”
“The fast rise of COVID-19 […] put the country in […] alarm. All the activities, not urgent or essential, were forbidden. The technical decisions about the suspension were decided by experts.”
Table 3.
Issues around coordination of cancer screening services in response to the first wave of the COVID-19 pandemic.
Abbreviations: Decision made – how: P - Following a preparedness plan; R - Based on a review of scientific evidence; Ex - Based on earlier experience; E - Guided by expert opinion; G - Guided by government decision; Ot - Other.
First decision made – what level: O - Organization/practice; L - Local (city, county, metropolitan area, etc.); R - Regional (state, province, region, etc.); N - National; Ot - Other;? - I don't know.
First decision made – by whom: O - Organization/practice leadership HA - Health authority; SP - Screening program director; HF - Healthcare facility; HP - Healthcare professional; PO - Professional organization/society; Ot - Other;? - I don't know.
First decision – roll-out: O - Within the organization/practice; L - Locally (city, county, metropolitan area, etc.); R - Regionally (state, province, region, etc.); N - Nationally.
Restart plan/Monitoring plan/Data collected: Y - Yes; N - No;? - I don't know; NA - Not applicable.
Understanding that several sequential decisions at different levels of governance may have taken place during this process, the first decision whether to suspend cancer screening services was made at the national level in 32 settings (48.5%), at the regional level in 30 (45.5%), at the local level in 8 (12.1%), and at the organization or practice in 29 (43.9%). In addition, the first decision regarding screening was rolled-out nationally in 22 settings (33.3%), regionally in 24 (36.4%), locally in 11 (16.7%), and within the organization or practice in 31 (47.0%). Finally, the decision whether to suspend cancer screening services was made by the health authority in 39 settings (59.1%), the organization or practice leadership in 28 settings (42.4%), the screening program director in 22 settings (33.3%), the professional organization or society in 6 settings (9.1%), the healthcare facility in 4 settings (6.1%), and the healthcare professional in 4 settings (6.1%). This decision-making process often involved several levels of governance and different authorities as highlighted by the following comments:
“Following the announcement of the country going into a state of emergency and social distancing measures announced by the Minister of Health, the departmental head as well as the screening program director had to suspend mass screening campaigns in line with the social distancing measures implemented.”
“For those few practices suspending screening activity before the general recommendations, the decision was made by the organization/practice leadership. The general suspension recommendation was made by the screening program coordinator.”
“Individual closures were either done by the facility […] due to decision of regional health authority to […] postpone all none critical services to keep capacity free for emergencies […] or by the screening director of the unit himself […] Suspension of screening invitation on national level was decided by national health authority”.
Of the 60 settings that suspended cancer screening services, 52 (86.7%) reported that a plan was being developed or already in place at the time of responding to the survey for how to restart these services, while 26 settings (43.3%) were developing or already had a plan in place to monitor the impact of the temporary suspension of cancer screening services. Seven settings shared publicly available documents and the central concerns driving these plans were to establish protocols that could ensure safe delivery of services for clients and health personnel, and to devise prioritization strategies for dealing with the backlog in cancer screening. Finally, 37 settings (61.7%) were collecting data to assess the impact of the cancer screening suspension.
3.3. Communication about the suspension within the health system and with the public
Three separate survey items assessed the communication of the decision whether to suspend cancer screening services relative to clients or patients, to health professionals, and to other stakeholders (Table 4 ). Thirty-six settings (54.5%) communicated the decision to clients or patients directly through electronic means (e.g, phone, email, SMS, voice messages). Thirty-two settings (48.5%) communicated this information indirectly through mass media (e.g., television, radio, social media campaigns) and 15 settings (22.7%) communicated directly through mailed letter. Among the 16 settings (24.2%) that reported ‘other’, some examples included communication through health care providers, clinics, pharmacies, and community outreach. In 7 settings (10.6%), the respondents did not know how the decision was communicated to clients or patients. Of the 6 settings where screening was not suspended, 3 reported that communicating the decision was deemed unnecessary although updates were posted online, as illustrated below:
“No formal communication as there was no change to invitations for screening.”
“As the decision was to continue screening there was no need to communicate a change”.
“Updates posted on the national cancer screening website […Program] updated their local website.”
Table 4.
Assessment of communication around cancer screening services, resource availability and patient follow-up in the aftermath of the first wave of COVID-19.
| Settings / n. of respondents |
Communication to clients/patients |
Communication to professionals |
Communication to stakeholders |
Infrastructure repurposed |
Professionals reassigned |
Percentage reassigned |
Patient follow-up |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | DE | IM | Ot/? | TD | PO | IM | Ot/? | Y/N/? | Y/N/? | Y/N/? | %/? | VC | VD | Both | Ot/? | ||
| TOTAL (%) | 66 settings / 98 respondents (100%) | 15 (22.7%) | 36 (54.5%) | 32 (48.5%) | 23 (34.8%) | 57 (86.4%) | 14 (21.2%) | 11 (16.7%) | 5 (7.6%) | Y = 34 (51.5%) | Y = 35 (53.0%) | Y = 41 (62.1%) | 25 (37.9%) | 26 (39.4%) | 11 (16.7%) | 2 (3.0%) | |
| 1 | Albania / 1 | IM | Ot | TD | ? | N | N | NA | VD | ||||||||
| 2 | Australia / 3 | Ot | TD | PO | IM | Y | Y | N | NA | Both | |||||||
| 3 | Belgium - Flanders / 3 | IM | TD | PO | IM | Y | N | N | NA | VD | |||||||
| 4 | Brazil / 4 | DM | DE | IM | Ot | TD | Y | N | Y | 26–100% | VC | ||||||
| 5 | Canada – Ontario / 3 | DM | DE | IM | Ot | TD | PO | IM | Y | Y | Y | ? | Both | ||||
| 6 | Canada – Alberta / 1 | IM | TD | ? | Y | Y | ? | VC | |||||||||
| 7 | Denmark / 3 | IM | Ot | TD | PO | Y | Y | Y | 1–25% | VC | |||||||
| 8 | Denmark – Copenhagen / 1 | Ot | TD | Y | N | N | NA | VC | |||||||||
| 9 | Ethiopia – Oromia / 1 | Ot | TD | Ot | Y | Y | Y | 51–75% | VD | ||||||||
| 10 | Ethiopia – Addis Ababa /1 | Ot | TD | ? | Y | ? | ? | VD | |||||||||
| 11 | Finland / 1 | ? | TD | ? | Y | ? | ? | VC | |||||||||
| 12 | Germany / 1 | DM | DE | IM | Ot | TD | IM | ? | Y | N | NA | VC | |||||
| 13 | India – Tamil Nadu / 1 | Ot | TD | Y | Y | Y | 76–100% | VD | |||||||||
| 14 | Ireland / 3 | DM | DE | IM | Ot | TD | PO | Y | Y | Y | 26–50% | VC | |||||
| 15 | Italy – Veneto / 1 | DE | TD | ? | Y | Y | ? | VC | |||||||||
| 16 | Italy – Piedmont / 3 | DE | TD | N | ? | Y | 26–50% | VC | |||||||||
| 17 | Italy – ONS / 1 | DM | DE | TD | Y | Y | Y | 1–25% | Both | ||||||||
| 18 | Italy – Lombardy / 1 | DE | TD | Y | Y | Y | 51–75% | Both | |||||||||
| 19 | Italy – Tuscany / 1 | DE | TD | ? | Y | Y | 1–25% | VC | |||||||||
| 20 | Japan / 2 | DM | DE | IM | TD | IM | N | N | N | NA | Both | ||||||
| 21 | Jordan / 1 | DE | IM | TD | PO | IM | Y | N | Y | 1–25% | VD | ||||||
| 22 | Kazakhstan / 1 | IM | IM | Y | N | N | NA | VD | |||||||||
| 23 | Lithuania / 1 | IM | TD | Y | N | ? | None | VC | |||||||||
| 24 | Myanmar / 1 | IM | PO | Y | Y | Y | 1–25% | VD | |||||||||
| 25 | Namibia / 1 | ? | TD | ? | N | Y | 26–50% | VD | |||||||||
| 26 | Netherlands / 6 | DM | DE | IM | TD | PO | IM | Y | Y | Y | 1–25% | Both | |||||
| 27 | New Zealand / 2 | DE | IM | TD | IM | Y | Y | N | NA | Both | |||||||
| 28 | Nigeria - Gombe / 1 | DE | PO | Y | Y | Y | 1–25% | VD | |||||||||
| 29 | Norway / 2 | DE | IM | TD | ? | N | ? | ? | VC | ||||||||
| 30 | Peru / 1 | DE | IM | Ot | TD | Y | Y | Y | 1–25% | VD | |||||||
| 31 | Poland / 1 | DE | TD | N | N | ? | ? | Ot | |||||||||
| 32 | Portugal / 1 | DM | DE | TD | Y | Y | N | NA | VD | ||||||||
| 33 | Slovenia / 4 | DM | DE | IM | TD | IM | Y | Y | Y | 1–100% | Both | ||||||
| 34 | Spain – Catalonia / 3 | DM | DE | IM | Ot | TD | Y | Y | Y | 26–100% | Both | ||||||
| 35 | Spain – Navarra / 1 | DE | N | N | Y | 76–100% | VC | ||||||||||
| 36 | Spain – Valencia / 2 | DE | Ot | TD | N | Y | Y | 76–100% | VD | ||||||||
| 37 | Spain – Basque Country / 1 | Ot | ? | ? | ? | Y | 1–25% | VD | |||||||||
| 38 | Sweden / 1 | IM | TD | PO | ? | N | ? | ? | VC | ||||||||
| 39 | Switzerland – Graubünden / 1 | DM | TD | Y | N | N | NA | ||||||||||
| 40 | Switzerland – Vaud / 1 | DM | DE | TD | N | N | N | NA | VC | ||||||||
| 41 | Switzerland – Valais / 1 | DE | TD | N | N | Y | 26–50% | VC | |||||||||
| 42 | Switzerland – Fribourg / 1 | DE | TD | N | N | N | NA | VC | |||||||||
| 43 | Switzerland – Geneva / 1 | IM | Ot | TD | Y | N | N | NA | VC | ||||||||
| 44 | Switzerland – Thurgau / 1 | DM | DE | TD | PO | N | N | N | NA | VC | |||||||
| 45 | Taiwan / 2 | DE | IM | TD | Y | N | Y | 1–25% | VC | ||||||||
| 46 | Uganda / 1 | IM | TD | Y | Y | Y | 1–25% | VD | |||||||||
| 47 | UK – NHSE targeted lung health check program / 1 | ? | TD | Y | N | Y | ? | VD | |||||||||
| 48 | UK – Liverpool health lung project / 1 | DM | DE | TD | ? | Y | Y | ? | VD | ||||||||
| 49 | UK – UK National Screening Committee / 1 | ? | TD | ? | Y | ? | ? | VD | |||||||||
| 50 | UK – Yorkshire lung screening trial / 1 | DE | TD | Y | N | Y | 1–25% | VC | |||||||||
| 51 | UK – Scotland / 1 | IM | ? | ? | Y | Y | ? | Ot | |||||||||
| 52 | Uruguay / 1 | IM | PO | ? | N | N | NA | VD | |||||||||
| 53 | USA – Marshfield clinic health system, WI / 1 | DE | IM | TD | ? | Y | Y | ? | VD | ||||||||
| 54 | USA – Kaiser Permanente Washington, WA / 1 | DM | DE | TD | ? | Y | Y | ? | VD | ||||||||
| 55 | USA – Parkland health and hospital system, Dallas, TX / 2 | ? | TD | N | ? | Y | ? | VD | |||||||||
| 56 | USA – New Jersey Cancer education and early detection program, NJ / 1 | DE | IM | TD | Y | Y | Y | ? | VC | ||||||||
| 57 | USA – Mass general hospital, Boston, MA / 1 | IM | TD | ? | Y | Y | 26–50% | VD | |||||||||
| 58 | USA – Nebraska health and human Services, Lincoln, NE / 1 | DE | Ot | Y | Y | Y | ? | Both | |||||||||
| 59 | USA – Federally qualified health centers and community clinics, Texas / 1 | DE | TD | Ot | Y | Y | Y | ? | VD | ||||||||
| 60 | USA – University of Kentucky Healthcare, Lexington, KY / 1 | ? | TD | ? | ? | Y | ? | VD | |||||||||
| 61 | USA – Kaiser Permanente, San Francisco Bay Area, CA / 1 | ? | TD | PO | N | N | Y | None | VC | ||||||||
| 62 | USA – Ohio State University Cancer center, Columbus, OH / 1 | DE | TD | Y | N | N | NA | VD | |||||||||
| 63 | USA – Presbyterian healthcare systems, Albuquerque, NM / 1 | DE | IM | TD | ? | N | N | NA | VC | ||||||||
| 64 | USA – NCI moonshot® Cancer cures “accelerated control of cervical Cancer” / 2 | IM | TD | Y | Y | Y | 1–25% | Both | |||||||||
| 65 | Zambia – JSI-DISCOVER-health project / 1 | ||||||||||||||||
| 66 | Zambia - CIDRZ / 1 | IM | TD | PO | IM | Y | Y | Y | 1–25% | VC | |||||||
Abbreviations Communicated to clients/patients – how: DM - Directly through mailed letter; DE - Directly through electronic means (phone email SMS voice messages etc.); IM - Indirectly through mass media (TV radio social media campaigns etc.); Ot - Other;? - I don't know.
Communicated to professionals: TD - Top-down approach - communicated directly by responsible institute/director; PO - Indirectly through professional organization; IM - Indirectly through mass media (TV, radio, social media campaigns, etc.); Ot - Other;? - I don't know.
Communicated to stakeholders/Infrastrcture repurposed/Professionals reassigned: Y - Yes; N - No;? - I don't know.
Percentage reassigned -? - I don't know; NA – not applicable.
Clients / patients follow-up: VC - Most follow up visits continue to take place; VD - Most follow up visits have been delayed; Ot - Other;? - I don't know.
Communication to health professionals occurred in a top-down approach in 57 settings (86.4%), indirectly through professional organizations in 14 settings (21.2%) and indirectly through mass media in 11 settings (16.7%). In 2 settings (3.0%), respondents were unaware of how the decision was communicated to health professionals. Finally, 3 settings (4.5%) reported ‘other’, exemplified by a bottom-up approach where program managers communicated the decision to the leadership at the clinic.
Thirty-four settings (51.5%) communicated the decision to other stakeholders, including non-governmental organizations (NGOs), cancer charities, and advocacy groups. Twenty-nine settings (43.9%) reported that the decision whether to suspend cancer screening elicited a response from citizens, advocacy groups and other stakeholders (i.e., politicians, health care providers and the media). Reported reactions included concerns about delays in cancer screening and follow up, as well as when and how services would resume. Initially, the need for suspending services was often understood by the diverse stakeholders, but soon after there was a pressure to resume screening from the media, advocacy groups and the general population. Some respondent comments illustrate this disposition:
“Several subjects contacted the screening call centers to get information about the planned procedures for restarting.”
“In recent weeks, due to the lack of a re-start date, there have been an increasing number of complaints, queries, parliamentary questions, politician and journalist queries.”
Moreover, in some settings, there were comments about stakeholders who disagreed with the suspension of cancer screening services, clients demanding to receive screening, and health care providers providing services before the program officially restarted.
3.4. Resource availability and follow-up
Cancer screening infrastructure was repurposed to support the COVID-19 pandemic response in 35 settings (53.0%), and professionals were re-trained or reassigned to help with the response in 41 settings (62.1%). Among these 41 settings, 5 (12.2%) reported that more than 50% of cancer screening professionals were reassigned to the pandemic response; 19 settings (46.3%), reported that up to 50% of professionals were reassigned; and 13 settings (31.7%) were not able to provide this information (Table 4). One setting reported that professionals were re-trained but not reassigned.
Most follow-up visits after a positive cancer screening examination continued to take place in 25 settings (37.9%) and were delayed in another 26 settings (39.4%). Eleven settings (16.7%) reported both occurrences, exemplified by the following statement:
“[the health organization] recommended that individuals with abnormal screening results highly suspicious for cancer continue to receive follow up, though the timing of follow-up visits may be impacted by local resource availability. In addition, […] follow up could be delayed for individuals with results that were abnormal but not highly suspicious.”
In addition, prioritization strategies for follow up of those with a positive screen were implemented in some settings and at times differed according to cancer site:
“Further diagnostics in women with low-grade lesions […] were cancelled between [dates] and restarted at [date]. However, diagnostics and treatment of women with high-grade lesions, follow-up after treatment and management of pregnant women with cervical lesions were not interrupted.”
“[…] In breast cancer screening, most of follow-up visits continue taking place. In colorectal cancer screening, most of the colonoscopies were delayed […]”.
Finally, follow-up visits were also affected by the availability of resources, the patient's decision, and broader constraints brought on by the pandemic:
“Continuity of follow-up visits was guaranteed as per policy, but with significant delays […] due to the disruption of hospital services and/or patients' preference”.
“[…] As a result, follow-up for people who had results highly suspicious for cancer […] continued across the province; however, there was local variability in follow-up depending on local resource capacity.”
“Patients had no transport to get to us. Public means suspended.”
4. Discussion
The first wave of the COVID-19 pandemic, which started showing its effects by February 2020, led almost all of the 66 settings to suspend cancer screening services already in March 2020, and most stopped research or pilot programs as well. Cancer sites, types of screening delivery, and decision-making processes and governance varied across the settings. Even when the first wave of the COVID-19 pandemic did not lead to the suspension of cancer screening services, its effects were felt in service delays, health and broader infrastructure constraints (i.e., lack of personnel and supplies at the health facility, lack of transportation for patients to reach services) or the patients themselves not willing to attend screening during the pandemic. In parallel, a WHO survey implemented between May and July 2020, about the continuity of essential health services during the pandemic found that, among the 105 responding countries, 55% experienced disruptions in cancer diagnosis and treatment (WHO, 2020b). In addition, our results were complementary to the findings from a survey carried out by the International Agency for Research on Cancer (IARC) between August and September 2020. Most of the 18 settings targeted by the IARC survey were not captured by ours, and similarly 14 of the 18 IARC settings (77.8%) reported that cancer screening had been suspended (Villain et al., 2021).
More often the suspension of cancer screening was guided by government decision and implemented at the national, regional, and organizational levels through health authorities and organizational leadership. Few settings made their decision about the suspension of cancer screening services based on expert opinion, and even fewer followed a preparedness plan or based their decision-making on a review of the scientific literature. More notably, less than a handful of settings considered previous experiences when making their decisions. This is in line with the results of a recent systematic literature review focused on disaster management scenarios, which identified only 11 studies relevant to cancer screening interruptions (Braun and Clarke, 2006). We recognize that the scale of the COVID-19 pandemic is unprecedented since mass screening services have been implemented around the world. However, an opportunity exists to apply previously learned knowledge from localized occurrences such as the Great East Japan Earthquake in 2011 (Kodama et al., 2014; Miki, Tase, Tokunaga, Yaegashi, and Ito, 2020a; Ozaki et al., 2017), or Hurricane Katrina in southern USA in 2005 (Lobato et al., 2007; Nogueira, Sahar, Efstathiou, Jemal, and Yabroff, 2019), or outbreaks such as Ebola in 2014 (Traore, Kourouma, Bah, and Keita, 2020) or SARS in 2003 (Naylor, Chantler, and Griffiths, 2004). In addition, the limited number of studies, especially on the effects of systemic disruptions to cancer screening, should encourage researchers to evaluate and share findings related to the impact of the ongoing COVID-19 pandemic and to prepare for future ones.
Only 7 settings shared plans to restart cancer screening services and monitor the impact of COVID-19 on them, but prioritization of people to be screened and safety during the delivery of the screening services were main concerns among those shared. It was not clear if most settings were ready to restart these services any time soon, considering that they were facing the first wave of the COVID-19 pandemic by the time of the survey. In the beginning of 2021, as the COVID-19 pandemic continues to challenge health care around the world, some settings have already restarted their services (Dinmohamed et al., 2020; Yong et al., 2020a). However, it is not clear that the same circumstances that led to the suspension of services during the first wave of the pandemic led to similar responses in subsequent waves. Future research should assess and evaluate the approaches used to restart cancer screening services and their outcomes, and how they differed in the recurrent waves of the pandemic. In addition, it will be crucial to exchange information and provide guidance on planning for future pandemics based on the lessons learned during the COVID-19 pandemic.
Only a few settings that continued offering cancer screening services reported no communication with clients or patients, although even in these settings communication with healthcare professionals and other stakeholders about the decision did happen. The importance of communicating about the decision-making process with the various stakeholders was highlighted by respondents' comments regarding inquiries from the general public, representatives of advocacy groups and the media about the status of the cancer screening services. Moreover, there are indications that communication efforts may contribute to maintaining confidence and recovering participation in cancer screening services once they are reestablished (Gorji, Jafari, Heidari, and Seifi, 2018; Larsen, Svanholm, and Andersen, 2016; Miki, Tase, Tokunaga, Yaegashi, and Ito, 2020b).
Most settings saw cancer screening infrastructure repurposed and cancer screening professionals were often reassigned to the COVID-19 response efforts. This impact in resource availability is commonly encountered in disaster scenarios, and preparedness frameworks are important measures to address the issue. For example, the Sendai Framework for Disaster Risk Reduction (The United Nations Office for Disaster Risk Reduction, 2015) offers strategies to ensure the resilience of the health system and adequate resource allocation in preparation for scenarios like the ongoing COVID-19 pandemic, so that essential health services are not interrupted. In addition, there are opportunities for settings with diverse levels of resources, based in HICs or LMICs, to learn from each other, as optimization of resources and use of resource-stratified strategies (i.e., based on the resources available for a specific setting) become important elements for restarting services while ensuring quality and effectiveness of cancer screening (DeBoer, Fadelu, Shulman, and Van Loon, 2020).
Follow-up visits after a positive cancer screening examination were delayed in at least one-third of the settings. Although prioritization strategies were put in place to ensure that people who screened positive could be properly assessed after the suspension of cancer screening services, other factors such as lack of resources or patients' fear of infection may play an important part in the delays. The effects of these delays should be measured and evaluated going forward, but some model estimations provide an alarming picture of cancer outcomes in the years to come. For example, in the UK, where the number of patients referred for cancer diagnosis decreased by 76% in April 2020 compared to pre-COVID-19 levels, one study estimated increases in the number of deaths up to 5 years after diagnosis for breast, colorectal, and lung cancers due to diagnostic delays (Maringe et al., 2020). In Canada, researchers projected for the period of 2020–2029 that a six-month interruption in breast cancer screening could lead to 670 more late-stage cancer cases, and 250 excess breast cancer deaths in the country; while for colorectal cancers, a six-month interruption could increase incidence by 2200 cases and mortality by 960 deaths if services are immediately restored to full capacity (Yong et al., 2020b). In Australia, model estimates projected 90 excess deaths and $12 million excess healthcare costs over 5 years for a three-month delay in diagnosis and treatment, and 350 excess deaths and $46 million in extra costs over the same period for a six-month delay (Degeling, Baxter, Emery, et al., 2020).
Our study has some limitations. We sought to rapidly assess the effects of the first wave of the COVID-19 pandemic on cancer screening globally and did not validate the survey instrument. However, survey development was guided by the expertise of the 18 ICSN Steering Committee members, who represent many cancer screening settings in different countries. Moreover, to capture as much information as possible, the survey was open to the full network and not targeted to managers of cancer screening services. Although this approach provided a variety of perspectives, often more than one person responded for the same setting, making a direct analysis of frequency distributions impractical. The researchers addressed this issue by recategorizing the responses into unique settings, combining the results and resolving any discrepancies based on the respondents' comments and their knowledge of the setting. Considering the complexity, fragmentation and diversity of cancer screening services across settings, as well as their interplay across various levels of governance and decision-making processes, it was not possible to determine who offered the most accurate responses or how representative these were of the population that they covered. However, the researchers did draw on their expertise and full assessment of the data to generate a comprehensive picture of how the first wave of the COVID-19 pandemic affected cancer screening services. Finally, as respondents were able to answer for any cancer sites that were relevant to their settings, we could not assess characteristics and effects of the COVID-19 pandemic specific to each cancer screening site.
5. Conclusions
The COVID-19 pandemic had profound effects on cancer screening services worldwide, including their suspension in almost all settings included in this study. Although most settings were not prepared to deal with the scale of the COVID-19 pandemic, they demonstrated flexibility in supporting the COVID-19 response. Moreover, there is an opportunity to continue learning from the efforts to sustain and restore cancer screening in the ongoing pandemic and develop preparedness plans addressing key components, such as coordination across and beyond the health sector, open communication within the health system and with the public, resource availability and patient follow-up. The results reported here contribute to inform the next steps for assessing and understanding the long-term impact of COVID-19 on cancer screening, while aligning with the efforts of other groups such as the COVID-19 and Cancer Global Modelling Consortium (https://ccgmc.org/), and international bodies such as the WHO and IARC. ICSN plans to engage its members and the international community through follow up surveys and working groups to monitor and measure the effects of this pandemic, so that cancer screening practitioners can better respond to its ongoing needs, mitigate its impact on cancer outcomes, and prepare for future health care disruptions.
Funding
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024, Task Order No. 75N91019F00129 (D.M.P.P.) and under Grant No. UM1CA221940 (A.K.) and UM1CA222035 (A.K.).
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention or the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgments
The authors would like to express their gratitude to the ICSN members, who took time from their already overstretched schedules due to the COVID-19 pandemic, to respond to the survey and provide insights into the effects of this public health emergency in their settings. Furthermore, we would like to thank the ICSN Steering Committee members for their contributions to the development of the questionnaire.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2021.106642.
Appendix A. Supplementary data
Supplementary material
References
- Arbyn M., Anttila A., Jordan J., Ronco G., Schenck U., Segnan N., Wiener H., Herbert A., von Karsa L. European guidelines for quality assurance in cervical cancer screening. Second edition--summary document. Ann Oncol. 2010 Mar;21(3):448–458. doi: 10.1093/annonc/mdp471. PMID: 20176693; PMCID: PMC2826099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbyn M., Bruni L., Kelly D., et al. Tackling cervical cancer in Europe amidst the COVID-19 pandemic. Lancet Public Health. 2020;5(8) doi: 10.1016/S2468-2667(20)30122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- Canfell K., Kim J.J., Brisson M., Keane A., Simms K.T., Caruana M., Burger E.A., Martin D., DTN Nguyen, Bénard É., Sy S., Regan C., Drolet M., Gingras G., Laprise J.F., Torode J., Smith M.A., Fidarova E., Trapani D., Bray F., Ilbawi A., Broutet N., Hutubessy R. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020 Feb 22;395(10224):591–603. doi: 10.1016/S0140-6736(20)30157-4. Epub 2020 Jan 30. PMID: 32007142; PMCID: PMC7043006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll W.D., Strenger V., Eber E., Porcaro F., Cutrera R., Fitzgerald D.A., Balfour-Lynn I.M. European and United Kingdom COVID-19 pandemic experience: The same but different. Paediatr Respir Rev. 2020 Sep;35:50–56. doi: 10.1016/j.prrv.2020.06.012. Epub 2020 Jul 4. PMID: 32709461; PMCID: PMC7334652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer R.J., Fadelu T.A., Shulman L.N., Van Loon K. Applying lessons learned from low-resource settings to prioritize cancer care in a pandemic. JAMA Oncol. 2020 Sep 1;6(9):1429–1433. doi: 10.1001/jamaoncol.2020.2976. PMID: 32761149. [DOI] [PubMed] [Google Scholar]
- Degeling K., Baxter N.N., Emery J., et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Pre-print: medRxiv. 2020 doi: 10.1101/2020.05.30.20117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamou A., Delvaux T., El Ayadi A.M., Beavogui H., Okumura J., Van Damme W., et al. Public health impact of the 2014–2015 Ebola outbreak in West Africa: seizing opportunities for the future. BMJ Glob. Health. 2017;2 doi: 10.1136/bmjgh-2016-000202. e000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinmohamed A.G., Cellamare M., Visser O., de Munck L., Elferink M.A.G., Westenend P.J., Wesseling J., Broeders M.J.M., Kuipers E.J., Merkx M.A.W., Nagtegaal I.D., Siesling S. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J Hematol Oncol. 2020 Nov 4;13(1):147. doi: 10.1186/s13045-020-00984-1. PMID: 33148289; PMCID: PMC7609826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Saghir N.S., Soto Pérez de Celis E., Fares J.E. Sullivan R. cancer care for refugees and displaced populations: Middle East conflicts and global natural disasters. Am Soc Clin Oncol Educ Book. 2018 May 23;38:433–440. doi: 10.1200/EDBK_201365. PMID: 30231320. [DOI] [PubMed] [Google Scholar]
- European Colorectal Cancer Screening Guidelines Working Group, von Karsa L., Patnick J., Segnan N., Atkin W., Halloran S., Lansdorp-Vogelaar I., Malila N., Minozzi S., Moss S., Quirke P., Steele R.J., Vieth M., Aabakken L., Altenhofen L., Ancelle-Park R., Antoljak N., Anttila A., Armaroli P., Arrossi S., Austoker J., Banzi R., Bellisario C., Blom J., Brenner H., Bretthauer M., Camargo Cancela M., Costamagna G., Cuzick J., Dai M., Daniel J., Dekker E., Delicata N., Ducarroz S., Erfkamp H., Espinàs J.A., Faivre J., Faulds Wood L., Flugelman A., Frkovic-Grazio S., Geller B., Giordano L., Grazzini G., Green J., Hamashima C., Herrmann C., Hewitson P., Hoff G., Holten I., Jover R., Kaminski M.F., Kuipers E.J., Kurtinaitis J., Lambert R., Launoy G., Lee W., Leicester R., Leja M., Lieberman D., Lignini T., Lucas E., Lynge E., Mádai S., Marinho J., Maučec Zakotnik J., Minoli G., Monk C., Morais A., Muwonge R., Nadel M., Neamtiu L., Peris Tuser M., Pignone M., Pox C., Primic-Zakelj M., Psaila J., Rabeneck L., Ransohoff D., Rasmussen M., Regula J., Ren J., Rennert G., Rey J., Riddell R.H., Risio M., Rodrigues V., Saito H., Sauvaget C., Scharpantgen A., Schmiegel W., Senore C., Siddiqi M., Sighoko D., Smith R., Smith S., Suchanek S., Suonio E., Tong W., Törnberg S., Van Cutsem E., Vignatelli L., Villain P., Voti L., Watanabe H., Watson J., Winawer S., Young G., Zaksas V., Zappa M., Valori R. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45(1):51–59. doi: 10.1055/s-0032-1325997. Epub 2012 Dec 4. PMID: 23212726; PMCID: PMC4482205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorji H.A., Jafari H., Heidari M., Seifi B. Cancer patients during and after natural and man-made disasters: A systematic review. Asian Pac J Cancer Prev. 2018 Oct 26;19(10):2695–2700. doi: 10.22034/APJCP.2018.19.10.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny M.-P., Evans D.B., Schmets G., Kadandale S. Health-system resilience: reflections on the Ebola crisis in western Africa. Bull. World Health Organ. 2014;92:850. doi: 10.2471/BLT.14.149278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y., Oikawa T., Hayashi K., Takano M., Nagano M., Onoda K., Yoshida T., Takada A., Hanai T., Shimada S., Shimada S., Nishiuchi Y., Onoda S., Monma K., Tsubokura M., Matsumura T., Kami M., Kanazawa Y. Impact of natural disaster combined with nuclear power plant accidents on local medical services: a case study of Minamisoma municipal general hospital after the great East Japan earthquake. Disaster Med Public Health Prep. 2014 Dec;8(6):471–476. doi: 10.1017/dmp.2014.112. Epub 2014 Nov 27. PMID: 25427564. [DOI] [PubMed] [Google Scholar]
- Larsen M.B., Svanholm H., Andersen B. An adverse event in a well-established cervical cancer screening program: an observational study of 19,000 females unsubscribed to the program. J Healthc Leadersh. 2016 Oct 27;8:61–69. doi: 10.2147/JHL.S114462. PMID: 29355205; PMCID: PMC5741009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato M.N., Yanni E., Hagar A., Myers C., Rue A., Evans C., Lambert L.A. Olney RS; Louisiana OPH-CDC newborn screening assessment team. Impact of hurricane Katrina on newborn screening in Louisiana. Pediatrics. 2007 Oct;120(4):e749–e755. doi: 10.1542/peds.2006-3616. 17908732 [DOI] [PubMed] [Google Scholar]
- Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., Rachet B., Aggarwal A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020 Aug;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. (Epub 2020 Jul 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y., Tase T., Tokunaga H., Yaegashi N., Ito K. Cervical cancer screening rates before and after the Great East Japan Earthquake in the Miyagi Prefecture, Japan. PLoS One. 2020 Mr 11;15(3) doi: 10.1371/journal.pone.0229924. e0229924. PMID: 32160221; PMCID: PMC7065810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y., Tase T., Tokunaga H., Yaegashi N., Ito K. Cervical cancer screening rates before and after the Great East Japan Earthquake in the Miyagi Prefecture, Japan. PLoS One. 2020 Mar 11;15(3) doi: 10.1371/journal.pone.0229924. e0229924. PMID: 32160221; PMCID: PMC7065810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A., Cockburn J., Smith R.A., Wardle J. A perspective from countries using organized screening programs. Cancer. 2004 Sep 1;101(5 Suppl):1201–1213. doi: 10.1002/cncr.20505. PMID: 15316915. [DOI] [PubMed] [Google Scholar]
- Naylor C.D., Chantler C., Griffiths S. Learning from SARS in Hong Kong and Toronto. JAMA. 2004 May 26;291(20):2483–2487. doi: 10.1001/jama.291.20.2483. 15161900 [DOI] [PubMed] [Google Scholar]
- Nogueira L.M., Sahar L., Efstathiou J.A., Jemal A., Yabroff K.R. Association between declared hurricane disasters and survival of patients with lung cancer undergoing radiation treatment. JAMA. 2019 Jul 16;322(3):269–271. doi: 10.1001/jama.2019.7657. PMID: 31310288; PMCID: PMC6635902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki A., Nomura S., Leppold C., Tsubokura M., Tanimoto T., Yokota T., Saji S., Sawano T., Tsukada M., Morita T., Ochi S., Kato S., Kami M., Nemoto T., Kanazawa Y., Ohira H. Breast cancer patient delay in Fukushima, Japan following the 2011 triple disaster: a long-term retrospective study. BMC Cancer. 2017 Jun 19;17(1):423. doi: 10.1186/s12885-017-3412-4. PMID: 28629330; PMCID: PMC5477136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry N., Broeders M., de Wolf C., Törnberg S., Holland R., von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition--summary document. Ann. Oncol. 2008 Apr;19(4):614–622. doi: 10.1093/annonc/mdm481. Epub 2007 Nov 17. PMID: 18024988. [DOI] [PubMed] [Google Scholar]
- Preventive Services Task Force U.S., Bibbins-Domingo K., Grossman D.C., Curry S.J., Davidson K.W., Epling J.W., Jr., García F.A.R., Gillman M.W., Harper D.M., Kemper A.R., Krist A.H., Kurth A.E., Landefeld C.S., Mangione C.M., Owens D.K., Phillips W.R., Phipps M.G., Pignone M.P., Siu A.L. Screening for colorectal Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016 Jun 21;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- Preventive Services Task Force U.S., Curry S.J., Krist A.H., Owens D.K., Barry M.J., Caughey A.B., Davidson K.W., Doubeni C.A., Epling J.W., Jr., Kemper A.R., Kubik M., Landefeld C.S., Mangione C.M., Phipps M.G., Silverstein M., Simon M.A., Tseng C.W., Wong J.B. Screening for cervical Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018 Aug 21;320(7):674–686. doi: 10.1001/jama.2018.10897. PMID: 30140884. [DOI] [PubMed] [Google Scholar]
- Puricelli Perin D.M., Christensen T., Burón A., Haas J.S., Kamineni A., Pashayan N., Rabeneck L., Smith R.A., Elfström M., Broeders M.J.M., International Cancer Screening Network ICSN Interruption of cancer screening services due to COVID-19 pandemic: lessons from previous disasters. Prev Med. Rep. 2021;23 doi: 10.1016/j.pmedr.2021.101399. Epub 2021 May 17. PMID: 34026465; PMCID: PMC8126519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.S., Gultekin M., Lassi Z.S. Effective approaches towards eliminating cervical cancer from low-and middle-income countries amid COVID-19 pandemic. Int. J. Gynecol. Cancer. 2020;30:1848–1849. doi: 10.1136/ijgc-2020-002013. [DOI] [PubMed] [Google Scholar]
- Salyer S.J., Maeda J., Sembuche S., Kebede Y., Tshangela A., Moussif M., Ihekweazu C., Mayet N., Abate E., Ouma A.O., Nkengasong J. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet. 2021 Mar 24 doi: 10.1016/S0140-6736(21)00632-2. S0140–6736(21)00632–2. Epub ahead of print. PMID: 33773118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu A.L. U.S. Preventive Services Task Force. Screening for breast Cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2016 Feb 16;164(4):279–296. doi: 10.7326/M15-2886. (Epub 2016 Jan 12) [DOI] [PubMed] [Google Scholar]
- Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D., Wender R.C. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019 May;69(3):184–210. doi: 10.3322/caac.21557. Epub 2019 Mar 15. PMID: 30875085. [DOI] [PubMed] [Google Scholar]
- The United Nations Office for Disaster Risk Reduction . 2015. Sendai Framework for Disaster Risk Reduction 2015–2030. New York: United Nations.https://www.undrr.org/publication/sendai-framework-disaster-risk-reduction-2015-2030 Available from: (Accessed 31 January 2021) [Google Scholar]
- Traore B., Kourouma M., Bah M., Keita M. What Is the impact of the ebola virus disease outbreak on cancer management in Guinea? JCO Glob Oncol. 2020 Jun;6:913–918. doi: 10.1200/GO.20.00101. PMID: 32589461; PMCID: PMC7328109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain P., Carvalho A.L., Lucas E., Mosquera I., Zhang L., Muwonge R., Selmouni F., Sauvaget C., Basu P., IARC COVID-19 Impact Study Group Cross-sectional survey of the impact of the COVID-19 pandemic on cancer screening programs in selected low- and middle-income countries: Study from the IARC COVID-19 impact study group. Int J Cancer. 2021 Feb 3 doi: 10.1002/ijc.33500. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Karsa L., Anttila A., Ronco G., Ponti A., Malila N., Arbyn M., Segnan N., Castillo-Beltran M., Boniol M., Ferlay J., Hery C., Sauvaget C., Voti L., Autier P. European Commission; Luxembourg: 2008. Cancer Screening in the European Union. Report on the Implementation of the Council Recommendation on Cancer Screening.http://ec.europa.eu/health/ph_determinants/genetics/documents/cancer_screening.pdf Available from: [Google Scholar]
- WHO . World Health Organization; Geneva: 2020. Global strategy to accelerate the elimination of cervical cancer as a public health problem.https://www.who.int/publications/i/item/9789240014107 52 p. License: CC BY-NC-SA 3.0 IGO. Available from. (accessed 31 January 2021) [Google Scholar]
- WHO . World Health Organization; 2020. Pulse survey on continuity of essential health services during the COVID-19 pandemic.https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1 License: CC BY-NC-SA 3.0 IGO. Available from: (Accessed 31 January 2021) [Google Scholar]
- Yong J.H., Mainprize J.G., Yaffe M.J., Ruan Y., Poirier A.E., Coldman A., Nadeau C., Iragorri N., Hilsden R.J., Brenner D.R. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2020 Nov 26 doi: 10.1177/0969141320974711. 969141320974711. Epub ahead of print. PMID: 33241760; PMCID: PMC7691762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J.H., Mainprize J.G., Yaffe M.J., Ruan Y., Poirier A.E., Coldman A., Nadeau C., Iragorri N., Hilsden R.J., Brenner D.R. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2020 Nov 26 doi: 10.1177/0969141320974711. 969141320974711. Epub ahead of print. PMID: 33241760; PMCID: PMC7691762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material